Abstract
Chemical genomics is a powerful approach to dissect processes that may be intractable using conventional genetics because of gene lethality or redundancy. Recently, a link has been established between endomembrane trafficking and gravitropism. To understand this link, we screened a library of 10,000 diverse chemicals for compounds that affected the gravitropism of Arabidopsis seedlings positively or negatively. Sixty-nine of 219 compounds from the primary screen were retested, and 34 of these were confirmed to inhibit or enhance gravitropism. Four of the 34 compounds were found to cause aberrant endomembrane morphologies. The chemicals affected gravitropism and vacuole morphology in concert in a tissue-specific manner, underscoring the link between endomembranes and gravitropism. One of the chemicals (5403629) was structurally similar to the synthetic auxin 2,4-dichlorophenoxy acetate, whereas the other three chemicals were unique in their structures. An in vivo functional assay using the reporter β-glucuronidase under the auxin-inducible DR5 promoter confirmed that the unique compounds were not auxins. Interestingly, one of the unique chemicals (5850247) appeared to decrease the responsiveness to auxin in roots, whereas another (5271050) was similar to pyocyanin, a bacterial metabolite that has been suggested to target the endomembranes of yeast. These reagents will be valuable for dissecting endomembrane trafficking and gravitropism and for cognate target identification.
Keywords: chemicals, GFP, gravitropism, trafficking, vacuoles
The endomembrane system of plants is complex genetically and functionally, which is perhaps a reflection of their sessile lifestyle. Although plants contain many of the same organelles and trafficking components as animals and fungi, the plant secretory system bears responsibility for additional functions, such as the storage of proteins, ions, and metabolites and the manufacture of cell-wall precursors (1). In addition, unlike in yeast, the plant cell vacuole is essential for viability (2). There is also an emerging body of evidence demonstrating that plant trafficking pathways are necessary for proper development and signal transduction processes (3).
Classical mutant screens have shown that the endomembrane system is intimately involved in the plant gravitropic response. The signal transduction processes mediating this response are poorly understood, but mutations in endomembrane system components, such as DET3 and the SNARE proteins VTI11 and SYP22, result in agravitropic phenotypes (4-7). Furthermore, the plant hormone auxin plays a role in gravitropism (8).
Many genes that encode endomembrane system components are essential for plants. For example, T-DNA insertions in many plant endomembrane system genes are lethal (2, 9, 10), although point mutations for a few are viable and exert clear phenotypes (7). The development of the TILLing process permits the screening of ethyl methane sulfonate (EMS) point mutations in a specific gene (11); however, for unknown genes, the only approach for examining vacuolar and endomembrane morphology and function has been forward genetics to isolate EMS point mutants. With this approach, we isolated >200 putative mutants with altered vacuole morphology using tonoplast-localized GFP as a marker. Unfortunately, half of these mutants died before setting seed (12). One of our long-term goals is to develop tools to dissect endomembrane trafficking and vacuole biogenesis where loss-of-function mutation is lethal.
Chemical genomics provides a means to circumvent problems of lethality and redundancy. This approach is possible because advances in synthetic chemistry techniques, such as split-pool synthesis, have made it possible to generate diverse libraries of small organic molecules (13, 14). The concurrent development of high-throughput screening technologies and genomics has permitted the use chemical libraries in place of mutagenesis for phenotypic screens (15). Once a compound is found that results in a desirable phenotype, it can be applied at sublethal doses at any point in development. The earliest chemical genomics studies used Saccharomyces cerevisiae and mammalian tissue culture (16, 17), but the model plant Arabidopsis thaliana has been a recent focus (18, 19). With a wide array of genetic tools available, Arabidopsis offers unique opportunities to identify the cognate targets of chemicals in a multicellular organism (20, 21).
We are interested in identifying novel components of endomembrane trafficking in plants. To avoid the problem of lethality, a chemical genomics approach was used. We took advantage of the link between the endomembrane system and gravitropism to screen a 10,000-member library for small compounds that caused aberrant gravitropic responses. We then focused on compounds that inhibited gravitropism via the endomembrane system by examining vacuole morphology. Among the many compounds that affected gravitropism, we found four that affected endomembrane system morphology and describe them here.
Methods
Plant Materials and Growth Conditions. A. thaliana ecotype Columbia (Col-0) was used in all experiments. Vacuoles were visualized by using a line containing a 35S::GFP:δ-TIP construct (22). Seeds were sterilized and sown on 1× or 0.5× Murashige and Skoog (MS) media (Invitrogen) containing 0.8 or 0.6% Phytagar (Invitrogen). Seeds were stratified in darkness for 48 h at 4°C. For gravitropism, plates were incubated in the light for 4-14 h to induce germination then vertically in darkness at 22°C. For all other experiments, plates were incubated in the light (16 h per day) at 22°C. Seeds of the DR5::GUS reporter line were generously provided by Gloria Muday (Wake Forest University, Winston-Salem, NC). ABD2-GFP seeds were kindly provided by Elison Blancaflor (Noble Foundation, Ardmore, OK).
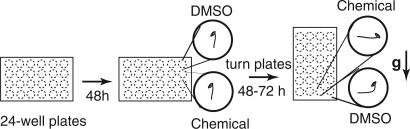
Chemical Library Screen. The 96-well format DIVERSet library (ChemBridge, San Diego) contained 10,000 small organic molecules; 0.1 mg of each compound was dissolved in 20 μl of DMSO, then diluted 1:5 in water to a concentration of 2-4 mM in 20% DMSO. The library was rearrayed to a 24-well format by using a Bio-Tek (Winooski, VT) Precision 2000 liquid-handling robot. For screening, 10 μl of each chemical was added to 390 μl of MS media agar to a final concentration of 50-100 μM (25 μg/ml) in 0.5% DMSO. Approximately 12 sterilized Arabidopsis seeds were sown per well, then grown vertically in the dark. Growth was monitored by using control plates without chemicals. Typically, plates were reoriented 90° at 48 h after stratification, then gravistimulated in the dark for 24-72 h before scoring.
For the secondary screen, seeds germinated on 0-, 0.25-, 0.5-, 1-, 5-, and 10-μg/ml concentrations of relevant chemicals were scored for gravitropism, as described. All compounds are identified by using numbers assigned by ChemBridge. For the tertiary screen, seeds of the 35S::GFP:δ-TIP line were germinated on chemicals, incubated in the light, and viewed by using a Leica TCS SP2 laser-scanning confocal microscope (Leica, Wetzlar, Germany) 7 days after stratification. Before tertiary screening, compounds 5403629, 5271050, 5850247, 6220480, and 6241121 were reexamined by MS analysis at the University of California, Riverside, MS Facility. Substructure searches for analogs used the Hit2Lead database (Hit2Lead.com; ChemBridge). Analogs were from ChemBridge. For visualizing seedling DR5::GUS expression, staining was as described (23).
Quantification of the Inhibition of the Gravitropic Response. Twenty-five to 30 seeds were sown per well of a four-well plate (Nalge Nunc, Model 176597). Wells contained DMSO or chemicals in DMSO in 0.5× MS media. At least six concentrations of each chemical were examined. After stratification and light, the plates were incubated in darkness for 4 days. The seedlings were then gravistimulated for 24 h, imaged (Model 2450 scanner; Epson, Long Beach, CA), and root and hypocotyl angles from vertical were measured (scion image software; Scion, Frederick, MD). Approximate chemical concentrations yielding half-maximal inhibition (IC50s) of curvature were derived from inhibition curves. Data are the average of at least duplicate experiments (30-50 seedlings total per data point). To assess affects on hypocotyl and root growth, lengths were measured with the scion image multiline tool and expressed as a percent of control seedling growth. Because IC50 values were derived from inhibition curves, growth at doses slightly greater than IC50 are reported in several cases.
Reversibility and Inducibility Experiments. Experiments were as in the secondary screen, except that four-well culture plates were used. For inducibility, 3-day-old vertically grown seedlings were transferred to a plate with chemical and grown for an additional 24 h. Transfer was performed under a green safelight (Corning green filter no. 4010), and the seedlings were gravistimulated for 24-48 h. For reversibility, 3-day-old seedlings germinated on chemical were transferred to a plate without chemical and gravistimulated. Chemicals 5403629 and 5271050 were at 3.3 and 3.7 μM, respectively, whereas chemical 5850247 was at 35.3 μM. For reversibility of vacuole morphology, 7-day-old seedlings on 3.3 μM chemical 5403629, 3.7 μM 5271050, 35 μM 5850247, or 30 μM 6220480 were transferred to MS media without chemical and viewed for vacuole morphology up to 7 days later.
DR5::GUS Assays. DR5::GUS seedlings were grown for 7 days without chemicals. To examine induction, seedlings were transferred to MS media containing chemicals, incubated for 5 h, then stained for β-glucuronidase (GUS) activity. To examine effects on auxin transport or induction, seedlings were preincubated with chemicals; the auxin transport inhibitor 2,3,5-triiodobenzoic acid (TIBA); or DMSO (0.5%) for 14 h. A 1 mm, a MS media agar plug containing 10 μM indole-3 acetic acid (IAA) was then placed at the tip of the root, and the seedlings were incubated for 5 additional hours before staining for GUS activity. For induction experiments, 6-day-old seedlings were preincubated for 14 h in the presence of chemical and then transferred to plates containing 1 μM IAA for 5 h before GUS staining.
Results
Screening a Diverse Chemical Library for Effectors of Gravitropic Response. To identify protein trafficking components involved in the gravitropic response, we screened A. thaliana (ecotype Columbia-0) seedlings on a diverse chemical library composed of small synthetic organic molecules. Seeds were germinated in 24-well plates, each well of which contained a different compound from the library (Fig. 1). After gravistimulation, wells in which the majority of seedlings failed to bend or displayed an enhanced gravitropic response were scored as “hits.” In our primary screen of 10,000 compounds, we scored 219 chemicals as hits (Fig. 5A and Supporting Text, which are published as supporting information on the PNAS web site). Examples of phenotypes observed included both inhibition and enhancement of the gravitropic response (Fig. 5B).
Fig. 1.
Screen for chemicals that affected gravitropism. The chemical library was screened in a 24-well format, and seedlings were scored for gravitropic response after reorientation. Chemicals dissolved in 20% DMSO were added to wells. Control wells contained an equivalent concentration of the solvent. The gravity vector is indicated by an arrow next to the g (on the right).
To prioritize the 219 chemicals from the primary screen for further characterization, we used several criteria (Fig. 5A). One hundred ninety-nine compounds appeared to inhibit gravitropism, whereas 20 appeared to enhance the response. The compounds were further classified as structurally similar or dissimilar to known auxins. For example, compounds such as 5403629 (Fig. 3A) had substructures similar to the synthetic auxin 2,4-dichlorophenoxy acetate (2,4-D) and thus might be metabolized in planta to an active auxin. It is well established that the auxin family of hormones can influence growth and tropic responses. Our objective was to identify unique nonauxin-like compounds to increase the probability of discovering novel components of the endomembrane system; therefore, many auxin-like compounds were not studied further.
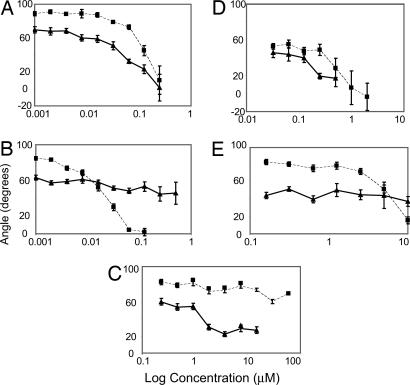
Fig. 3.
Several chemicals inhibit gravitropism in a tissue-specific manner. Dose-response experiments were done in the presence of 5403629 (A), the control auxin 2,4-D (B), 5271050 (C), 585247 (D), and 6220480 (E) at the concentrations indicated. Four-day-old seedlings grown in the presence of compounds were gravistimulated for 24 h, and the angle of curvature from a vertical position was measured. Solid lines indicate hypocotyl angles; dashed lines indicate root angles. All points are the mean ± standard error of at least duplicate experiments (n = 21-78 measurements per point). The hypocotyl and root-tip angles, respectively, of control seedlings without chemicals were: 68.0 ± 3.1, 88.3 ± 2.5 (A); 67.8 ± 3.1, 88.6 ± 2.0 (B); 68.8 ± 2.6, 79.7 ± 1.9 (C); 63.8 ± 2.3, 74.9 ± 2.9 (D); and 70.7 ± 3.6, 91.0 ± 3.9 (E). Chemical structures are shown in Table 1.
A Secondary Gravitropic Screen. To understand the chemicals in more detail, 69 of the 219 compounds from the primary screen, representing predominantly the unique chemicals but including some auxin-like compounds, were retested at several concentrations to confirm their effects on gravitropism. Twenty of the unique compounds, which included three enhancers, and 14 of the auxin-like chemicals were able to induce agravitropic or enhanced gravitropic phenotypes, confirming the activity of 34 of 69 (50%) compounds retested from the primary screen. This relatively large percentage of hits from the screen likely reflects the numerous pathways such as perception, signal transduction, and growth involved in the gravitropic response. The 34 chemicals confirmed to affect gravitropism are indicated in Table 2, which is published as supporting information on the PNAS web site.
Tertiary Screen for Chemicals That Affect Endomembrane Morphology. We germinated Arabidopsis seeds expressing a 35S::GFP:δ-TIP construct (22) on each of the 34 confirmed chemicals and examined the vacuole morphologies. Five of the 34 compounds induced dramatic changes in endomembrane system morphology or altered the localization and distribution of the GFP:δ-TIP marker. Therefore, our screen has at least a 0.05% hit rate of chemicals that affect both gravitropism and the endomembrane system. This is similar to the low frequency observed in another plant chemical genomic screen (19). In our case, the frequency may be attributable to the fact that we addressed two biological phenomena: gravitropic responses and endomembrane system morphology. The identity of one chemical (6141121) could not be confirmed by MS and was not pursued. The remaining four compounds were examined further.
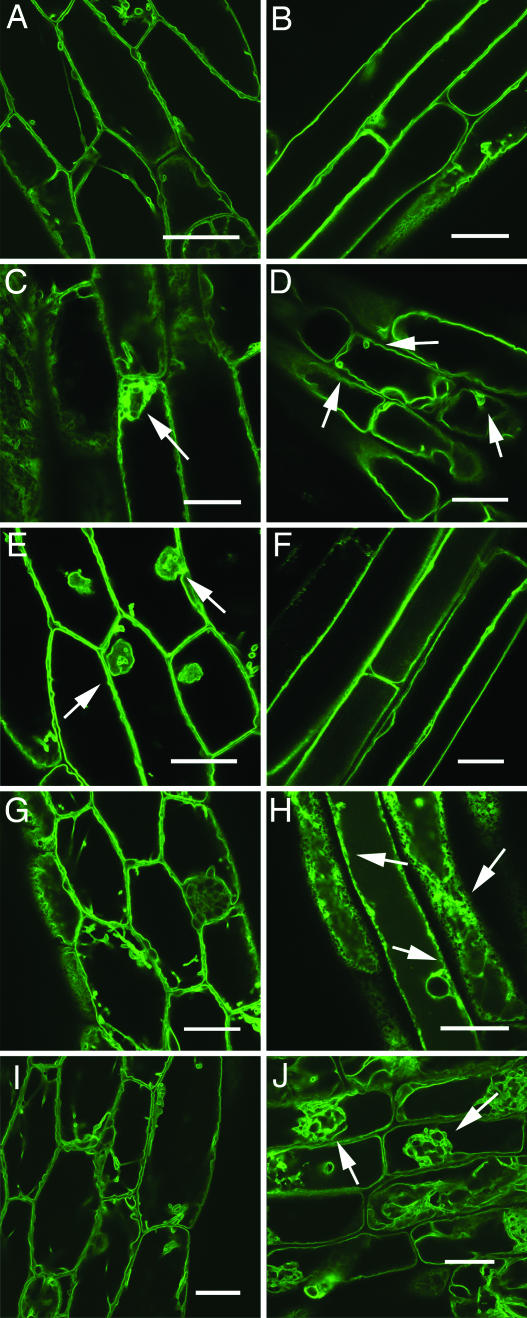
Compound 5403629 was a chlorophenoxy acetate derivative that was structurally similar to the synthetic auxin 2,4-D and resulted in vacuoles with a vesiculated appearance in both hypocotyls and roots (Fig. 2 C and D) compared with a control (Fig. 2 A and B). The auxin 2,4-D resulted in similarly aberrant vacuoles in roots (Fig. 6 A and B, which is published as supporting information on the PNAS web site), which were particularly sensitive to inhibition of gravitropism by this auxin (see below). Indole-3-acetic acid also resulted in a similar root phenotype (data not shown). The other three chemicals appeared to be structurally unique, and two resulted in intense aggregates of tonoplast in hypocotyls (5271050; Fig. 2 E and F) and roots (6220480; Fig. 2 I and J; see also Movies 1 and 2, which are published as supporting information on the PNAS web site). Compound 5850247 induced a network-like GFP:δ-TIP pattern, indicating apparent localization to the endoplasmic reticulum in root cells only (Fig. 2 G and H; see also Movie 3, which is published as supporting information on the PNAS web site), while maintaining normal vacuole morphology in those same cells (data not shown). Searches of the Hit2Lead database revealed chemicals not within our library but sharing structural similarities to 5403629, 5271050, 5850247, and 6220480. The intracellular effects of these similar compounds were examined, and five of nine resulted in endomembrane effects similar to their respective chemicals from the library (see Table 3, which is published as supporting information on the PNAS web site). Overall, the results indicate that direct chemical screening of seedlings in a moderate throughput format is a successful strategy to obtain potentially novel reagents.
Fig. 2.
Four compounds affect vacuole morphology or targeting of the reporter molecule to the tonoplast in GFP:δ-TIP seedlings. Hypocotyls (A, C, E, G, and I) and roots (B, D, F, H, and J) were examined by laser-scanning confocal microscopy after germination and growth on the chemicals for 7 days in the light. Chemicals: (C and D) 5403629 at 6.6 μM, (E and F) 5271050 at 3.7 μM, (G and H) 5850247 at 35 μM, and (I and J) 6220480 at 30 μM final concentration. Chemical 5403629 was inhibitory to growth at the concentration tested, so seedlings were grown on medium without the chemical for 4 days then treated with the compound for 48 h. The arrows in C and D indicate vesiculations; E, aggregates; H, endoplasmic reticulum patterning; and J, aggregates. (A and B) Images of a control seedling grown in the presence of a concentration of DMSO equivalent to that of treated seedlings are shown for comparison. (Bars, 20 μm.)
An interesting question was whether the chemicals affected the morphology of only vacuoles or exerted more generalized effects. For example, actin cytoskeletal integrity has been shown to be required for normal gravitropism and statoliths positioning in some plant species (24, 25). However, our chemicals did not alter the plasma membrane localization of the styryl dye FM4-64. In addition, neither a ABD2-GFP construct that marks the actin cytoskeleton (26) (Fig. 7, which is published as supporting information on the PNAS web site) nor a chimeric gene that targeted GFP to plastids (27) displayed any apparent altered morphology (data not shown). This indicates that the effects of all four chemicals described in this study are probably vacuole- or endomembrane-specific reagents.
Potent Inhibitors of Gravitropism. Our primary screen was based on inhibition of hypocotyl gravitropism at a single concentration of chemical. To understand the effect of these four compounds on both hypocotyl and root gravitropism and to determine their relative potencies, dose-response experiments were done. The results indicated that the auxin-like chemical 5403629 inhibited both hypocotyl and root gravitropism (Fig. 3A). The auxin 2,4-D was also inhibitory to root gravitropism but was less effective on hypocotyls displaying a weak response (Fig. 3B). Chemical 5271050 inhibited hypocotyl bending; however, the inhibition was incomplete, indicating that a component of the response was not saturable (Fig. 3C). This compound had little or no effect on root bending. Given that chemicals were applied via the medium and presumably by translocation through the roots, this implied that the molecular target of 5271050 was active only in hypocotyls. The unique chemical 5850247 inhibited hypocotyl and root gravitropism (Fig. 3D). Chemical 6220480 caused weak agravitropism and a helical or spiral hypocotyl growth pattern in our primary screen (data not shown). The dose-response indicated that the chemical resulted in modest inhibition of hypocotyls bending; however, there was clear inhibition of root bending (Fig. 3E). The hypocotyl or root specificities of gravitropic inhibition were consistent with the altered vacuole morphologies in the same tissues. However, for each chemical, a much greater dose was required to observe aberrant vacuoles compared with inhibition of gravitropism (compare the doses indicated in Fig. 2 with the IC50s indicated in Table 1 and described below). Because the chemicals appear to be specific for vacuoles or endomembranes, their agravitropic effects may be evident at doses at which gross vacuole anomalies are not detected by our methods. Also, the morphological studies used light-grown seedlings, whereas the gravitropism studies required etiolated seedlings. Thus, the relative dose differences may reflect developmental differences in uptake, translocation, sequestration, or metabolism.
Table 1. Potency of chemicals and reversibility of their effects.
| Inhibition of gravitropism, IC50*
|
Inhibition of growth
|
Inhibition of hypocotyl bending
|
Vacuolar effects
|
||||
|---|---|---|---|---|---|---|---|
| Chemical | Hypocotyl | Root | Hypocotyl length†‡ | Root length†‡ | Inducible | Reversible | Reversible |
| 2,4D | NA | 0.02 M | NA | 96.8 (0.02 M) | ND | ND | ND |
| 5403629 | 0.08 M | 0.2 M | 90.4 (0.1 M) | 60 (0.3 M) | Yes | Yes | Yes |
| 5271050 | 2 M | NA | 88.7 (2 M) | NA | Yes | Yes | No |
| 5850247 | 0.2 M | 0.3 M | 93.8 (0.3 M) | 98.8 (0.3 M) | Yes | No | Yes |
| 6220480 | NA | 8 M | NA | 21.8 (10 M) | NA | NA | Yes |
NA, not applicable, because chemical has little or no effect on gravitropism. ND, not determined.
Estimated concentration resulting in 50% inhibition after 24-h gravistimulation.
Lengths are reported as a percentage of the untreated controls.
Length measurements performed at the concentrations indicated.
As a measure of relative potency, we estimated the concentrations of the four chemicals resulting in 50% inhibition of gravitropism (IC50s) (Table 1). The auxin 2,4-D was the most potent inhibitor of root gravitropism. Chemicals 5403629 and 5850247 were active in the 0.2- to 0.3-μM range, with the auxin-like compound 5403629 being a particularly potent inhibitor of hypocotyl bending compared with 2,4-D. The least potent compounds were 5271050 and 6220480, which were inhibitory in the low micromolar range. None of the compounds were as active as 2,4-D, a commercial herbicide; however, given the structural diversity of the library, the four chemicals were remarkably potent.
Because gravicurvature requires growth, it was possible that several compounds were growth inhibitors whose effects on gravitropism were indirect. Thus we measured the lengths of hypocotyls and roots at or just above IC50. Compounds 5271050, 5850247, and 2,4-D did not cause any discernible inhibition of either root or hypocotyl elongation at either IC50 or slightly higher doses. Chemical 5403629 did not inhibit hypocotyl elongation at a dose slightly greater than the IC50; however, there was a 40% inhibition of root length, indicating a component of growth inhibition that could alter the gravicurvature of roots but not hypocotyls. Treatment of seedlings with the chemical 6220480 at a concentration just above IC50 causes a clear inhibition (≈80%) in root length. Therefore, the effect of chemical 6220480 on gravitropism was most likely due to growth inhibition of roots.
Reversibility and Inducibility of the Chemicals. We determined whether the inhibition of hypocotyl gravitropism by three of the compounds was reversible. Chemical 6220480 could not be examined because of its weak inhibition of hypocotyl bending. Three-day-old seedlings germinated in the presence of the chemicals were transferred to nonchemical plates and gravistimulated. The clear reversibility and inducibility of the agravitropic phenotype caused by chemical 5403629 are shown as examples (Fig. 8, which is published as supporting information on the PNAS web site). Inhibition by chemicals 5403629 and 5271050 was reversible, whereas the inhibition caused by 5850247 was not (Table 1). Reversibility indicated that the chemicals were either metabolized or compartmentalized. The nonreversibility of chemical 5850247 suggests, among other possibilities, that it may modify its molecular target irreversibly. We also determined whether a short treatment of 3-day-old seedlings germinated in the absence of compound was sufficient to inhibit gravitropism. All three chemicals induced a response with only a 24-h pretreatment, indicating that their cognate targets were available for interaction at the time of treatment.
Beyond the agravitropic phenotype, the reversibility of subcellular phenotypes was examined. Arabidopsis GFP:δ-TIP seedlings were germinated on media containing 5403629, 5271050, 5850247, or 6220480, then transferred to plates without chemical and viewed by confocal microscopy. The effects of chemicals 5403629 and 6220480 were fully reversible when observed 3 days after transfer (Table 1). Chemical 5850247 required 8 days to return to normal but was reversible. This slow reversal was consistent with the persistent inhibition of gravitropism 24 h after transfer to plates without chemical, as described above. Despite the reversibility of its gravitropic effects, the impact of chemical 5271050 on vacuole morphology was not reversible even after 8 days, indicating that, in some cases, gravitropic mechanisms can compensate for defective endomembranes.
Several Compounds Act Independently of Auxin. Our chemicals clearly affected gravitropism and vacuole morphology; however, it was important to understand functionally whether they interacted with known pathways involved in gravitropism. In particular, chemical 5403629 had an obvious structural similarity to 2,4-D, which also resulted in aberrant vacuoles. To determine whether any chemicals had auxin-like effects, an Arabidopsis line expressing the DR5::GUS transcriptional reporter construct was used. The DR5 promoter is highly responsive to auxins, and thus the GUS reporter is a sensitive indicator for the presence of intracellular auxin (28). Treatment of 7-day-old seedlings with the native auxin IAA and compound 5403629 resulted in strong GUS staining in roots, indicating auxin-induced activation of the DR5 promoter (Fig. 4A). By contrast, seedlings treated with 5271050, 5850247, and 6220480 displayed staining similar to that of the control seedlings, indicating that these chemicals did not have auxin activity. In all cases, the concentrations used were those at which vacuole phenotypes were observed (Fig. 2).
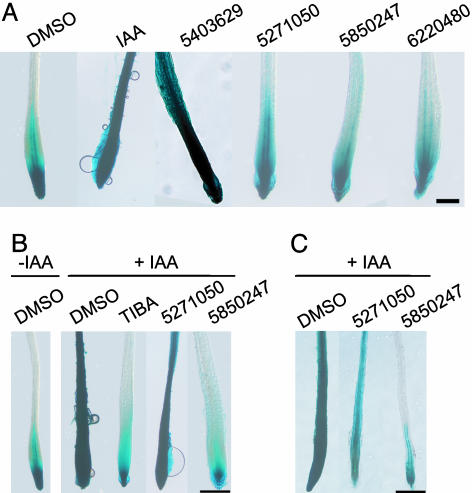
Fig. 4.
Functional assay for auxin activity and auxin transport inhibition. Arabidopsis DR5::GUS seedlings were grown in the absence of chemicals; transferred to MS media containing 0.02% DMSO or chemicals 5403629 (3.3 μM), 5271050 (3.7 μM), 5850247 (35 μM), or 6220480 (30 μM); then stained for GUS activity 5 h later. (A) IAA and chemical 5403629 displayed intense staining, indicating DR5::GUS induction, whereas staining of 5271050-, 5850247-, and 6220480-treated seedlings were similar to the control (DMSO), indicating only basal induction of DR5::GUS. (B) Seedlings were pretreated with chemicals, 0.2% DMSO, or 100 μM TIBA for 14 h as in A; however, an agar block containing IAA was placed at the root tip (+IAA samples), and seedlings were stained for GUS activity 5 h later. (C) Test for auxin responsiveness. Seedlings were pretreated with chemicals and then incubated in a 1-μM IAA solution. (Bars, 0.2 mm.)
We also did an indirect assay for auxin transport using this system. The DR5::GUS seedlings were grown for 7 days in the absence of chemicals. At day 7, seedlings were incubated on media containing chemical for 14 h. A MS media agar plug containing IAA was placed at the tip of the root, and the seedlings were incubated for 5 hours before staining (Fig. 4B). The auxin transport inhibitor TIBA was included as a positive control. Roots of seedlings preincubated with DMSO were strongly stained throughout their lengths, indicating that IAA was transported basipetally (Fig. 4B, +IAA, DMSO). The seedlings pretreated with TIBA (Fig. 4B, +IAA, TIBA) showed GUS staining near the root tip, similar to untreated controls (Fig. 4B, -IAA, DMSO), suggesting that auxin transport was inhibited. Seedlings treated with the chemicals 5403629, 5271050, and 6220480 had staining patterns similar to seedlings treated with IAA (Fig. 4B, +IAA, DMSO), indicating that these compounds did not affect auxin transport. Interestingly, seedlings treated with 5850247 exhibited a staining pattern similar to that of TIBA; however, treatment of seedlings with TIBA did not result in aberrant vacuoles (data not shown), as was the case for 5850247, indicating that the mode of action of 5850247 in apparently affecting polar auxin transport was different from that of TIBA. To clarify whether 5850247 inhibited auxin transport or caused a decrease in auxin responsiveness, seedlings were pretreated with 5850247, then incubated with IAA (Fig. 4C). Seedlings subjected to this regimen exhibited a patchy staining pattern that was greatly reduced compared with control seedlings (Fig. 4C, +IAA, DMSO; +IAA, 5271050).
Overall, we have identified four compounds that appear to target the vacuoles or endomembrane system specifically in hypocotyls, roots, or both tissues. Our results indicate that, in addition to a compound predicted to be an auxin (540369), two of our chemicals (5271050 and 6220480) affect gravitropic responses and the endomembrane system via nonauxin pathways. A third compound (5850247) may decrease auxin responsiveness via a mechanism that also affects endomembrane transport.
Discussion
There are several examples where small molecules have been used to probe gene function in the model plant Arabidopsis. Yokonolides A and B and brassinozole were isolated in forward screens for compounds that inhibit the expression of an auxin-responsive reporter construct and for compounds that inhibit the actions of cytochrome P450 proteins involved in brassinosteroid synthesis, respectively (29-31). More recently, a screen of another chemical library was focused on identifying inhibitors of auxin transcriptional activation (19). Four compounds were characterized that inhibited the induction of reporters, including DR5::GUS; however, none of our compounds resembled these four structures, indicating that we may have identified different classes of effectors. Structure searches of our library indicated that these chemicals are not contained in our collection. All of the above-mentioned screens isolated molecules with effects on hormone-signaling pathways. There are molecules, however, that induce specific effects on the secretory system. A recent screen of 4,800 chemicals yielded 14 compounds that increase secretion of carboxypeptidase Y (CPY) in yeast (18). One of these compounds, Sortin1, was shown to increase CPY secretion in Arabidopsis suspension cells and whole seedlings. Sortin1 also inhibited vacuole biogenesis and root development in Arabidopsis (18).
The gravitropic response is not well understood but involves the endomembrane system. The signal transduction pathway leading from a change in the gravity vector may be divided into signal perception, signal transduction, and signal response. The perception of gravity in plant shoots is mediated by starch-filled amyloplasts in the endodermal cell layer. The most widely accepted hypothesis is that amyloplasts act as statoliths. Plants treated with inhibitors of starch biosynthesis or that have mutations in starch biosynthesis genes have a defective gravitropic response (32, 33). The Arabidopsis shoot gravitropic response mutants sgr2-1, sgr4-1/zig-1, and sgr3-1 show improper sedimentation of amyloplasts (5-7). Two mutants that were identified in this screen for plants defective in the shoot gravitropic response have lesions in genes encoding SNARE proteins (sgr3-2 = SYP22 and sgr4-1/zig-1 = AtVTI11), and sgr2-1 encodes a phospholipase A-like protein that may contribute to the fluidity of the tonoplast (6).
During transduction of the gravistimulus, there is a measured increase in cytoplasmic Ca2+ (34, 35), and drugs that interfere with the function of calmodulin, calcium channels, or Ca2+-ATPases interfere with plant gravitropic responses (35-38), indicating that calcium acts as a second messenger in gravitropic signaling. Phosphoinositide signaling may also be involved in the gravitropic response (39). The ultimate response to a change in the gravity vector is asymmetric growth mediated by auxin gradients (40), which are established through the redistribution of auxin efflux carrier proteins. It has recently been demonstrated that the correct localization of the Arabidopsis PIN1 and PIN3 auxin efflux carriers is mediated by actin-dependent vesicle cycling (8, 41).
The compounds described in this report appear to intersect the gravitropic signal transduction process at three different points. Compound 5403629 is a less-potent form of the synthetic auxin 2,4-D. In addition to its effects on vacuole morphology, 5403629 can induce the full range of auxin-related phenotypes, including the inhibition of hypocotyl and root growth and the induction of root hairs (M.S., C.C., and N.V.R., unpublished data). Nevertheless, its identification led to the significant observation that auxins have effects on vacuole morphology and may directly remodel endomembrane trafficking processes.
The drug 5850247 inhibits gravitropism and appears to target a membrane protein-trafficking pathway. Experiments with the auxin transport inhibitor, TIBA, indicate that 5850247 may decrease responsiveness to auxin. There are several possibilities to explain the relationship between the endomembrane phenotype and auxin responsiveness. If auxin signal transduction requires endomembrane trafficking, then the down-regulation of auxin-responsive genes could result. Alternatively, there are quality control mechanisms that are required for proper protein folding in the endoplasmic reticulum (42). Although this control has been demonstrated mainly for soluble proteins, it is possible that membrane proteins are subject to similar mechanisms. Disruption of endomembranes could directly affect these pathways and signal transduction.
Finally, the chemical 5271050 is related to the phenazine family of chemicals, including pyocyanin, which is a secreted product of Pseudomonas aureginosa and contributes to virulence in cystic fibrosis patients (43). A yeast deletion strain library was screened on pyocyanin, and a number of strains defective for protein sorting, vesicle trafficking, and V-ATPase-related genes were shown to be hypersensitive to pyocyanin (43). This suggests possible targets in plants. This hypothesis is supported by the finding that Arabidopsis seedlings exposed to phenazine display a defective vacuole phenotype similar to that observed for chemical 5271050 (M.R.-P., J.V., and N.V.R., unpublished data). Furthermore, like 5271050, phenazine causes little or no inhibition of root gravitropism (G.R.H. and N.V.R., unpublished data).
We initiated our chemical screen with the goal of assigning function to components heretofore beyond the reach of classical genetic methods and to shed light on the link between gravitropic signal transduction and the endomembrane system. We were able to identify chemicals that both inhibited the gravitropic response and impacted the morphology of, or targeting to, the tonoplast. The chemicals function in a tissue-specific manner, affecting hypocotyls, roots, or both organs in terms of gravitropic response and vacuole morphology. The correspondence between the phenotypes induced by the chemicals underscores the link between endomembrane function and gravitropic responses These chemicals will be valuable as reagents to dissect the independent and common endomembrane pathways. The cognate molecular targets of such compounds can provide valuable insight into basic mechanisms of endomembrane trafficking and their relationship to gravity sensing and response.
Supplementary Material
Acknowledgments
We are grateful to members of the Raikhel laboratory for helpful discussions. Special thanks are due to Dr. Thomas Girke, Academic Coordinator for the Bioinformatics Core at the Center for Plant Cell Biology, University of California, Riverside, for handling the compound library and performing library searches, and Ms. Jocelyn Brimo for assistance with the manuscript. This research was funded by the Office of Biological and Physical Research of the National Aeronautics and Space Administration Grant NNA04CC73G (to G.R.H. and N.V.R.).
Author contributions: M.S., M.R.-P., C.C., G.R.H., and N.V.R. designed research; M.S., M.R.-P., C.C., G.R.H., and J.V. performed research; M.S., M.R.-P., G.R.H., and N.V.R. analyzed data; and M.S., G.R.H., and N.V.R. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GUS, β-glucuronidase; TIBA, 2,3,5-triiodobenzoic acid; IAA, indole-3 acetic acid; 2,4-D, 2,4-dichlorophenoxy acetate; MS media, Murashige and Skoog media.
References
- 1.Sanderfoot, A. A. & Raikhel, N. V. (2003) The Arabidopsis Book, eds. Somerville, C. R. & Meyerowitz, E. M. (American Society of Plant Biologists, Rockville, MD).
- 2.Rojo, E., Gillmor, C. S., Kovaleva, V., Somerville, C. R. & Raikhel, N. V. (2001) Dev. Cell 1, 303-310. [DOI] [PubMed] [Google Scholar]
- 3.Surpin, M. & Raikhel, N. (2004) Nat. Rev. Mol. Cell Biol. 5, 100-109. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher, K., Vafeados, D., McCarthy, M., Sze, H., Wilkins, T. & Chory, J. (1999) Genes. Dev. 13, 3259-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato, T., Morita, M. T., Fukaki, H., Yamauchi, Y., Uehara, M., Niihama, M. & Tasaka, M. (2002) Plant Cell 14, 33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita, M. T., Kato, T., Nagafusa, K., Saito, C., Ueda, T., Nakano, A. & Tasaka, M. (2002) Plant Cell 14, 47-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yano, D., Sato, M., Saito, C., Sato, M. H., Morita, M. T. & Tasaka, M. (2003) Proc. Natl. Acad. Sci. USA 100, 8589-8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friml, J., Wisniewska, J., Benkova, E., Mendgen, K. & Palme, K. (2002) Nature 415, 806-809. [DOI] [PubMed] [Google Scholar]
- 9.Sanderfoot, A. A., Pilgrim, M., Adam, L. & Raikhel, N. V. (2001) Plant Cell 13, 659-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang, B. H., Rancour, D. M. & Bednarek, S. Y. (2003) Plant J. 35, 1-15. [DOI] [PubMed] [Google Scholar]
- 11.Henikoff, S., Till, B. J. & Comai, L. (2004) Plant Physiol. 135, 630-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avila, E. L., Zouhar, J., Agee, A. E., Carter, D. G., Chary, S. N. & Raikhel, N. V. (2003) Plant Physiol. 133, 1673-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balkenhohl, F., von dem Bussche-Huennefeld, C., Lansky, A. & Zechel, C. (1996) Angew. Chem. Int. Ed. Engl. 35, 2288-2337. [Google Scholar]
- 14.Oliver, S. F. & Abell, C. (1999) Curr. Opin. Chem. Biol. 3, 299-306. [DOI] [PubMed] [Google Scholar]
- 15.Engels, M. F. & Venkatarangan, P. (2001) Curr. Opin. Drug Discov. Dev. 4, 275-283. [PubMed] [Google Scholar]
- 16.Shogren-Knaak, M. A., Alaimo, P. J. & Shokat, K. M. (2001) Annu. Rev. Cell Dev. Biol. 17, 405-433. [DOI] [PubMed] [Google Scholar]
- 17.Torrance, C. J., Agrawal, V., Vogelstein, B. & Kinzler, K. W. (2001) Nat. Biotechnol. 19, 940-945. [DOI] [PubMed] [Google Scholar]
- 18.Zouhar, J., Hicks, G. R. & Raikhel, N. V. (2004) Proc. Natl. Acad. Sci. USA 101, 9497-9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong, J. I., Yuan, S., Dale, J. M., Tanner, V. N. & Theologis, A. (2004) Proc. Natl. Acad. Sci. USA 101, 14978-14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackwell, H. E. & Zhao, Y. (2003) Plant Physiol. 133, 448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng, X. S., Chan, T. F. & Zhou, H. H. (2004) Chem. Biol. 11, 609-618. [DOI] [PubMed] [Google Scholar]
- 22.Cutler, S. R., Ehrhardt, D. W., Griffitts, J. S. & Somerville, C. R. (2000) Proc. Natl. Acad. Sci. USA 97, 3718-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojas-Pierce, M. & Springer, P. S. (2003) Methods Mol. Biol. 236, 221-240. [DOI] [PubMed] [Google Scholar]
- 24.Friedman, H., Vos, J. W., Hepler, P. K., Meir, S., Halevy, A. H. & Philosoph-Hadas, S. (2003) Planta 216, 1034-1042. [DOI] [PubMed] [Google Scholar]
- 25.Mathur, J., Mathur, N., Kernebeck, B. & Hulskamp, M. (2003) Plant Cell 15, 1632-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, Y. S., Motes, C. M., Mohamalawari, D. R. & Blancaflor, E. B. (2004) Cell Motil. Cytoskeleton 59, 79-93. [DOI] [PubMed] [Google Scholar]
- 27.Kohler, R. H., Cao, J., Zipfel, W. R., Webb, W. W. & Hanson, M. R. (1997) Science 276, 2039-2042. [DOI] [PubMed] [Google Scholar]
- 28.Ulmasov, T., Murfett, J., Hagen, G. & Guilfoyle, T. J. (1997) Plant Cell 9, 1963-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi, K., Jones, A. M., Ogino, K., Yamazoe, A., Oono, Y., Inoguchi, M., Kondo, H. & Nozaki, H. (2003) J. Biol. Chem. 278, 23797-23806. [DOI] [PubMed] [Google Scholar]
- 30.Asami, T., Min, Y. K., Nagata, N., Yamagishi, K., Takatsuto, S., Fujioka, S., Murofushi, N., Yamaguchi, I. & Yoshida, S. (2000) Plant Physiol. 123, 93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asami, T., Mizutani, M., Fujioka, S., Goda, H., Min, Y. K., Shimada, Y., Nakano, T., Takatsuto, S., Matsuyama, T., Nagata, N., et al. (2001) J. Biol. Chem. 276, 25687-25691. [DOI] [PubMed] [Google Scholar]
- 32.Sack, F. D. (1991) Int. Rev. Cytol. 127, 193-252. [DOI] [PubMed] [Google Scholar]
- 33.Kiss, J. Z., Wright, J. B. & Caspar, T. (1996) Physiol. Plant 97, 237-244. [DOI] [PubMed] [Google Scholar]
- 34.Sievers, A. (1991) Am. Soc. Gravitational Space Biol. Bull. 4, 43-50. [PubMed] [Google Scholar]
- 35.Sievers, A. & Busch, M. B. (1992) Planta 188, 619-622. [DOI] [PubMed] [Google Scholar]
- 36.Bjorkman, T. & Leopold, A. C. (1987) Plant Physiol. 84, 847-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stinemetz, C. L., Kuzmanoff, K. M., Evans, M. L. & Jarrett, H. W. (1987) Plant Physiol. 84, 1337-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinclair, W., Oliver, I., Maher, P. & Trewavas, A. (1996) Planta 199, 343-351. [DOI] [PubMed] [Google Scholar]
- 39.Perera, I. Y., Heilmann, I., Chang, S. C., Boss, W. F. & Kaufman, P. B. (2001) Plant Physiol. 125, 1499-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto, K. T. (2003) Trends Plant Sci. 8, 359-360. [DOI] [PubMed] [Google Scholar]
- 41.Geldner, N., Friml, J., Stierhof, Y. D., Jurgens, G. & Palme, K. (2001) Nature 413, 425-428. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman, R. J. (2004) Trends Biochem. Sci. 29, 152-158. [DOI] [PubMed] [Google Scholar]
- 43.Ran, H., Hassett, D. J. & Lau, G. W. (2003) Proc. Natl. Acad. Sci. USA 100, 14315-14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.