Abstract
Proton-pump inhibitors (PPIs) are a widely prescribed class of medications used to treat acid-related disorders and use has significantly increased over the last few decades. PPIs are often inappropriately prescribed and since they have been on the market, a number of post-marketing studies have been published demonstrating associations between longer duration of PPI therapy and a number of adverse effects that are a concern in older adults. The objective of this review is to discuss the existing literature of potential adverse effects with long-term PPI use in older adults and to summarize the implications in clinical practice. A PubMed search was conducted to identify studies evaluating the potential long-term adverse effects of PPI therapy in older adults, and publications were selected based on relevant criteria. PPIs have been associated with an increased risk of a number of adverse effects including osteoporotic-related fractures, Clostridium difficile infection, community-acquired pneumonia, vitamin B12 deficiency, kidney disease, and dementia, demonstrated by a number of case-control, cohort studies, and meta-analyses. Older adults should be periodically evaluated for the need for continued use of PPI therapy given the number of potential adverse effects associated with long-term use.
Keywords: aged, bone fractures, Clostridium difficile, dementia, kidney diseases, long-term adverse effects, osteoporosis, pneumonia, proton-pump inhibitors, vitamin B 12 deficiency
Introduction
Proton-pump inhibitors (PPIs) are a class of medications that work to decrease gastric acid via inhibition of the parietal cell H+/K+ ATP pump and are United States Food and Drug Administration (US FDA)-approved for the treatment of a variety of acid-related conditions, including duodenal ulcers, gastric ulcers, erosive esophagitis, gastroesophageal reflux disorder (GERD), Helicobacter pylori eradication and pathological hypersecretory conditions such as Zollinger–Ellison syndrome. The use of PPIs has significantly increased since the first PPI, omeprazole, was introduced in the late 1980s. In a US study estimating the prevalence of ambulatory care visits in which patients used PPIs, it was found that PPIs were used in 4% of visits in 2002 and 9.2% of visits in 2009 (p < 0.001).1 It is important to note that 46.7% of patients taking PPIs were 65 years of age and older, a significant number given there were 919 million ambulatory visits in 2009. Additionally, the majority of cases indicated questionable PPI use with 62.9% of patients having no documented gastrointestinal complaints, gastrointestinal diagnoses, or concomitant high-risk medications necessitating use of a PPI.1 In a study conducted in Sweden in 2010, it was found that long-term PPI use, defined as three or more PPI prescriptions dispensed in 1 year, occurs in one in nine older adults.2 Additionally, there was no indication for PPI use identified in approximately 40% of older adult long-term PPI users.2 Overall, the prevalence of PPI use has increased across the globe, and it is especially concerning that unnecessary PPI use is so high.
Awareness of potential adverse effects due to PPIs has increased since the PPIs first came to market. The US FDA issued safety warnings for the potential increased risk of osteoporosis-related fractures and Clostridium difficile infection (CDI) associated with PPI therapy in 2010 and 2012, respectively.3,4 Additionally, the American Geriatrics Society (AGS) 2015 Updated Beers Criteria recommends avoiding scheduled use of PPIs for >8 weeks in older adults, except in high-risk patients, due to the potential risk of bone loss and fractures and risk of CDI.5 Similarly, the START/STOPP criteria recommends discontinuing or dose reducing PPI therapy in older adults who have been on therapy for >8 weeks for uncomplicated peptic ulcer disease or erosive peptic esophagitis.6 However, there are a number of other adverse effects that have surfaced over the last two decades, including increased risk of community-acquired pneumonia (CAP), vitamin B12 deficiency, and more recently dementia and kidney disease. While there have been several reviews published on the adverse effects of PPIs, none have specifically addressed the older adult population. Furthermore, the more recently emerging adverse effects such as kidney disease and dementia have not been previously addressed in current published reviews. These potential adverse effects are of particular concern in older adults because this population is already at an increased risk of these conditions and is more likely to suffer from significant morbidity and mortality as a result. In this review, we discuss the existing literature and implications of long-term PPI therapy in older adults.
Methods
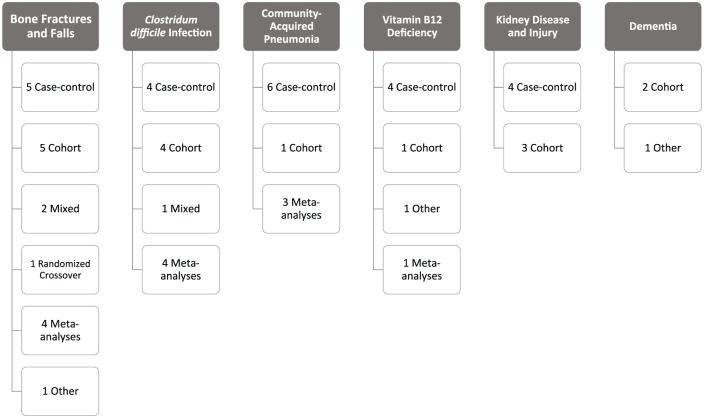
A PubMed search was conducted in October 2016 to identify studies from 1990 to 2016 evaluating the potential adverse effects of long-term PPI therapy in older adults. The following Medical Subject Heading terms were used: proton-pump inhibitors AND the following terms (separately): long-term adverse effects, aged, vitamin B12 deficiency, pneumonia, dementia, kidney diseases, Clostridium difficile, osteoporosis, fractures, accidental falls. Additionally, the following search terms were also used: acid-lowering therapy, acid-suppressive therapy, community-acquired pneumonia, chronic kidney disease, and acute interstitial nephritis. All searches were combined to comprise the full literature search. Since we found limited published reports regarding risks of vitamin B12 deficiency, kidney disease, and dementia, bibliographies were also reviewed for inclusion of additional studies for those conditions. Case-control studies, prospective and retrospective cohort studies, cohort with nested case-control studies, and meta-analyses were included. All single case reports, all cross-sectional studies, and observational studies primarily including adults <60 years of age were excluded. Studies not published in English and not conducted in humans were also excluded. One animal study was included for dementia as there are limited data in humans currently. Study titles and abstracts were reviewed and publications were selected based on the following criteria: the majority of the study population was >60 years of age or the mean age was >60 years of age; and the study evaluated the association between PPI therapy and vitamin B12 deficiency, CAP, CDI, dementia, kidney disease, or bone fractures. Studies that also evaluated histamine-2-receptor antagonists (H2RAs) in addition to PPI therapy or as a comparator to PPI therapy were also included. The threshold of age 60 was chosen over the Medicare-defined age of 65 years because several studies report age in categories of 10-year increments (i.e. 50–59 years, 60–69 years, etc.). For the adverse events of CDI and bone fractures, we chose to limit the number of included studies due to the vast amount of studies that have been conducted in this area. Studies included for these adverse events represented a mix of case-control, cohort, meta-analyses, and additional studies exploring the potential mechanism by which PPIs could cause these adverse events. A summary of the included study types for each adverse effect can be found in Figure 1.
Figure 1.
Summary of included study types for each adverse event.
Results
Bone fractures and falls
Overview
Osteoporosis is a condition that primarily affects older adults and causes >8.9 million fractures each year worldwide.7 It is estimated that almost 75% of hip, spine, and distal forearm fractures occur in the elderly age group of 65 years and older.8 Additionally, sustaining a hip fracture carries significant consequences including loss of function and independence, transition to living in a nursing home, and death. Sustaining a hip fracture has been associated with excess mortality in older adults, ranging from 8.4–36% in the first year after fracture.9 A number of observational studies have been published that have demonstrated an association between use of PPIs and risk of bone fractures. Because of the substantial number of publications that have demonstrated this risk, the US FDA issued a warning in 2010 on all PPIs stating that patients should use the lowest dose and shortest duration of PPI therapy due to an increased risk for osteoporosis-related fractures.3 The results of studies that reported an adjusted odds ratio (OR) or hazard ratio (HR) are summarized in Table 1.
Table 1.
Literature summary of studies that reported an adjusted odds ratio or hazard ratio.
| Study | Study design | Population | Mean age (years) | % Older adults* | Outcomes | Risk estimate (95% CI) |
|---|---|---|---|---|---|---|
| Bone fractures and falls | ||||||
| Chiu and colleagues10 | Case-control | Taiwan; Taiwan National Health Insurance Research database | 75 | NR | Risk of hip fracture with <29 defined daily doses of PPI | Adjusted OR 1.04 (0.73–1.49) |
| Risk of hip fracture with 29–70 defined daily doses of PPI | Adjusted OR 1.67 (1.11–2.51) |
|||||
| Risk of hip fracture with >71 defined daily doses of PPI | Adjusted OR 2.51 (1.77–3.55) |
|||||
| Eom and colleagues11 | Meta-analysis | Europe, Canada, United States, Taiwan | NR | NA | Risk of fracture with use of PPI | Pooled OR 1.29 (1.18–1.41) |
| Risk of facture with use of H2RA | Pooled OR 1.10 (0.99–1.23) |
|||||
| Ngamruengphong and colleagues12 | Meta-analysis | Europe, Canada, United States; observational study populations | NR | NA | Risk of hip fracture with use of PPI | Pooled OR 1.25 (1.14–1.37) |
| Risk of vertebral fracture with use of PPI | Pooled OR 1.50 (1.32–1.72) |
|||||
| Risk of wrist/forearm fracture with use of PPI | Pooled OR 1.09 (0.95–1.24) |
|||||
| Pouwels and colleagues13 | Case-control | Netherlands; PHARMO Record Linkage System | 75.7 cases; 75.3 controls |
NR | Risk of hip/femur fracture with current use of PPI | Adjusted OR 1.20 (1.04–1.40) |
| Ye and colleagues14 | Meta-analysis | Europe, Canada, United States; observational study populations | NR | NA | Risk of hip fracture with use of PPI | Pooled OR 1.24 (1.15–1.34) |
| Khalili and colleagues15 | Prospective cohort | United States; women enrolled in Nurses’ Health Study | 67.0 PPI users; 66.6 nonusers |
NR | Risk of hip fracture with use of PPI for at least 2 years | Adjusted HR 1.36 (1.12–1.65) |
| Yu and colleagues16 | Meta-analysis | United States, Canada, Europe, Taiwan | NR | NA | Risk of hip fracture with use of PPI | Adjusted OR 1.30 (1.19–1.43) |
| Risk of hip fracture with use of H2RA | Adjusted OR 1.10 (0.94–1.30) |
|||||
| Cea-Soriano and colleagues17 | Cohort with nested case-control | United Kingdom; Health Improvement Network Database | 68.6 men 69.1 women |
73% | Risk of falls with current use of PPI | Adjusted OR 0.95 (0.89–1.02) |
| Risk of falls with current use of H2RA | Adjusted OR 1.01 (0.90–1.14) |
|||||
| Fraser and colleagues18 | Prospective cohort | Canada; community-dwelling | 67.6 PPI users; 61.9 nonusers |
NR | Risk of first nontraumatic fracture with use of PPI | Adjusted HR 1.40 (1.11–1.77) |
| Lee and colleagues19 | Case-Control | Korea; Korean Health Insurance Review and Assessment Service database | 77.7 | 100% | Risk of hip fracture with PPI use | Adjusted OR 1.34 (1.24–1.44) |
| Risk of hip fracture with PPI use not on bisphosphonate treatment | Adjusted OR 1.30 (1.19–1.42) |
|||||
| Risk of hip fracture with PPI use and on bisphosphonate treatment | Adjusted OR 1.71 (1.31–2.23) |
|||||
| Adams and colleagues20 | Case-control | United States; Males in Kaiser Permanente healthcare system | NR | 85.3% | Risk of hip fracture with use of omeprazole in men | Adjusted OR 1.13 (1.01–1.27) |
| Ding and colleagues21 | Retrospective cohort | United States; pharmacy claims data, survey data, Medicare data | 79 | 100% | Risk of any fracture with use of PPI | Adjusted HR 1.27 (1.12–1.43) |
| Risk of hip fracture with use of PPI | Adjusted HR 1.32 (1.01–1.71) |
|||||
| Risk of vertebral fracture with use of PPI | Adjusted HR 1.69 (1.26–2.27) |
|||||
| Lewis and colleagues22 | Prospective cohort – Study 1 | Australia; patients enrolled in Calcium Intake Fracture Outcome study | 79.9 | 100% | Risk of falls and fracture-related hospitalizations with long-term use of PPI | Adjusted OR 2.17 (1.25–3.77) |
| Lewis and colleagues22 | Prospective cohort – Study 2 | Australia; outpatient | 76.7 | 100% | Risk of self-reported falling with long-term use of PPI | Adjusted OR 1.51 (1.00–2.27) |
| Clostridium difficile infection | ||||||
| Cunningham and colleagues23 | Case-control | United Kingdom; inpatient | NR | NR | Risk of CDI with use of PPI in preceding 8 weeks | Unadjusted OR 2.5 (1.5–4.2) |
| Dial and colleagues24 | Retrospective cohort | Canada; inpatient | NR | NR | Risk of CDAD with use of PPI | Adjusted OR 2.1 (1.2–3.5) |
| Dial and colleagues24 | Case-control | Canada; inpatient | 75.5 cases; 73 controls |
NR | Risk of CDAD with use of PPI | Adjusted OR 2.7 (1.4–5.2) |
| Dial and colleagues25 | Case-control | United Kingdom; General Practice Research Database | 71 | 74% | Risk of community-acquired CDAD with use of PPI | Adjusted RR 2.9 (2.4–3.4) |
| Risk of community-acquired CDAD with use of H2RA | Adjusted RR 2.0 (1.6–2.7) |
|||||
| Lowe and colleagues26 | Nested case-control | Canada; outpatient | 78.7 cases; 78.0 controls |
100% | Risk of hospitalization for CDAD with outpatient use of PPI in preceding 90 days | Adjusted OR 0.9 (0.8–1.1) |
| Aseeri and colleagues27 | Case-control | United States; inpatient | NR | 58.5% | Risk of CDAD with use of PPI | Adjusted OR 3.6 (1.7–8.3) |
| Linsky and colleagues28 | Retrospective cohort | United States; inpatient and outpatient | 74 | NR | Risk of recurrent CDI with use of PPI | Adjusted HR 1.42 (1.10–1.83) |
| Deshpande and colleagues29 | Meta-analysis | Canada, United Kingdom, United States, South Korea; inpatient, outpatient, nursing home | NR | NA | Risk of CDI with use of PPI | Pooled OR 2.15 (1.81–2.55) |
| Janarthanan and colleagues30 | Meta-analysis | United States, Europe, Canada, South Korea; inpatient and community | NR | NA | Risk of CDAD with use of PPI | Pooled RR 1.69 (1.40–1.97) |
| Kwok and colleagues31 | Meta-analysis | United States, Europe, Canada, India, South Korea; inpatient and outpatient | NR | NA | Risk of CDI with use of PPI | Pooled OR 1.74 (1.47–2.85) |
| Risk of CDI with use of H2RA | Pooled OR 0.71 (0.53–0.97) |
|||||
| Risk of CDI with concomitant use of PPI and antibiotic | Pooled OR 1.96 (1.03–3.70) |
|||||
| Tleyjeh and colleagues32 | Meta-analysis | United States, Europe, Canada, Asia; inpatient and outpatient | NR | NA | Risk of CDI with use of PPI | Adjusted OR 1.51 (1.26–1.83) |
| Freedberg and colleagues33 | Retrospective cohort | United States; inpatient | 64 | NR | Risk of recurrent CDI in inpatients with incident CDI and concurrent use of PPI | Adjusted HR 0.82 (0.58–1.16) |
| McDonald and colleagues34 | Retrospective cohort | Canada; inpatient | 71.5 PPI users (median); 69.5 nonusers (median) |
NR | Risk of CDI recurrence within 90 and days with continuous use of PPI | Adjusted HR 1.5 (1.1–2.0) |
| CAP | ||||||
| Laheij and colleagues35 | Nested case-control | Netherlands; Integrated Primary Care Information research database | NR | 58.1% | Risk of CAP with current use of PPI | Adjusted OR 1.73 (1.33–2.25) |
| Risk of CAP with current use of H2RA | Adjusted OR 1.59 (1.14–2.23) |
|||||
| Risk of CAP with current use of PPI and H2RA | Adjusted OR 1.76 (1.18–2.61) |
|||||
| Gulmez and colleagues36 | Case-control | Denmark; inpatient | 55.5 | 59.2% | Risk of CAP with current use of PPI | Adjusted OR 1.5 (1.3–1.7) |
| Risk of CAP with past use of PPI | Adjusted OR 1.2 (0.9–1.6) |
|||||
| Risk of CAP with recent initiation of PPI | Adjusted OR 5.0 (2.1–11.7) |
|||||
| Sarkar and colleagues37 | Nested case-control | United Kingdom; General Practice Research Database | 73.5 cases; 49.5 controls |
NR | Risk of CAP with current long-term use of PPI | Adjusted OR 1.02 (0.97–1.08) |
| Risk of CAP with PPI initiated in previous 2 days | Adjusted OR 6.53 (3.95–10.80) |
|||||
| Risk of CAP with PPI initiated in previous 7 days | Adjusted OR 3.79 (2.66–5.42) |
|||||
| Risk of CAP with PPI initiated in previous 14 days | Adjusted OR 3.21 (2.46–4.18) |
|||||
| Dublin and colleagues38 | Nested case-control | United States; inpatient and outpatient | 77 | 100% | Risk of CAP with current PPI or H2RA use | Adjusted OR 1.03 (0.86–1.24) |
| Johnstone and colleagues39 | Meta-analysis | Canada, Europe; inpatient and outpatient | NR | NA | Risk of CAP with use of PPI | Pooled OR 1.36 (1.12–1.65) |
| Eom and colleagues11 | Meta-analysis | Canada, United States, Europe, Australia; inpatient and outpatient | NR | NA | Risk of CAP with use of PPI | Adjusted OR 1.27 (1.11– 1.46) |
| Risk of CAP with use of H2RA | Adjusted OR 1.22 (1.09–1.36) |
|||||
| Meijvis and colleagues41 | Case-control | Netherlands; inpatient cases and population controls | 62 | NR | Risk of CAP with current PPI exposure | Adjusted OR 1.6 (1.2–2.2) |
| Risk of CAP with initiation of PPI in last 30 days | Adjusted OR 3.1 (1.4–7.1) |
|||||
| de Jager and colleagues42 | Prospective cohort | Netherlands; emergency room patients | NR | 46% non-PPI users; 69% PPI users | Risk of oropharyngeal flora as cause of CAP with PPI therapy | Adjusted OR 2.0 (1.22–3.72) |
| Hermos and colleagues43 | Nested case-control | United States; veterans | 65.8 | NR | Risk of CAP with current PPI exposure | Adjusted OR 1.29 (1.15–1.45) |
| Lambert and colleagues44 | Meta-analysis | United States, Canada, Europe, Asia; inpatient and outpatient | NR | NA | Risk of CAP with PPI therapy | Pooled OR 1.49 (1.16–1.92) |
| Risk of hospitalization for CAP with PPI therapy | Pooled OR 1.61 (1.12–2.31) |
|||||
| Vitamin B12 deficiency | ||||||
| Mitchell and Rockwood45 | Prospective cohort | Canada; outpatient and institutionalized patients with cognitive impairment | 78.7 | 100% | Risk of initiation of vitamin B12 supplementation with PPI/H2RA use at baseline | Adjusted OR 2.61 (1.31–5.23) |
| Force and colleagues46 | Case-control | United States; Medicaid patients | 71.2 | NR | Risk of initiation of vitamin B12 supplementation with ⩾10 of 12 months of PPI/H2RA | Unadjusted OR 1.82 (1.08–3.09) |
| Valuck and Ruscin47 | Case-control | United States; university-based geriatric outpatient | 82 | 100% | Risk of vitamin B12 deficiency with past use of PPI/H2RA | Adjusted OR 2.00 (0.87–4.37) |
| Risk of vitamin B12 deficiency with current use <12 months of PPI/H2RA | Adjusted OR 1.01 (0.47–2.28) |
|||||
| Risk of vitamin B12 deficiency with current use ⩾12 months of PPI/H2RA | Adjusted OR 4.45 (1.47–13.34) |
|||||
| Cotter and O’Keeffe48 | Case-control | United Kingdom, inpatient | 80 | NR | Risk of vitamin B12 deficiency with current use of PPI | Unadjusted OR 0.92 (0.53–1.60) |
| Lam and colleagues49 | Case-control | United States; inpatient and outpatient | NR | 67.2% | Risk of vitamin B12 deficiency with ⩾2-year supply of PPI | Adjusted OR 1.65 (1.58–1.73) |
| Risk of vitamin B12 deficiency with ⩾2-year supply of H2RA | Adjusted OR 1.25 (1.17–1.34) |
|||||
| Jung and colleagues50 | Meta-analysis | United States, Canada, and Ireland; inpatient and outpatient | NR | NA | Risk of vitamin B12 deficiency with ⩾10 months of PPI/H2RA | Pooled OR 1.83 (1.36–2.46) |
| Kidney disease and injury | ||||||
| Leonard and colleagues51 | Case-control | United Kingdom, General Practice Research Database | 60.2 AIN cases; 60.0 AIN controls 68.6 AKI cases; 66.9 AKI controls |
NR | Risk of AIN with use of PPI | Adjusted OR 3.20 (0.80–12.79) |
| Risk of AKI with use of PPI | Adjusted OR 1.05 (0.97–1.14) |
|||||
| Antoniou and colleagues52 | Prospective cohort | Canada; outpatients hospitalized for AKI | 74 years (median) |
100% | Risk of AKI with use of PPI | Unadjusted HR 2.52 (2.27–2.79) |
| Risk of AIN with use of PPI | Unadjusted HR 3.00 (1.47–6.14) |
|||||
| Lazarus and colleagues53 | Prospective cohort | United States; Atherosclerosis Risk in Communities study | 62.8 PPI users 63.1 H2RA users 62.5 nonusers |
NR | Association between PPI use and Incident CKD | Adjusted HR 1.50 (1.14–1.96) |
| Xie and colleagues54 | Prospective cohort | United States, Veterans Affairs database | 56.85 PPI users; 55.40 H2RA users |
NR | Association between PPI use and incident eGFR <60 ml/min/1.73 m2 | Adjusted OR 1.22 (1.18–1.26) |
| Association between PPI use and incident CKD | Adjusted OR 1.28 (1.23–1.34) |
|||||
| Dementia | ||||||
| Haenisch and colleagues55 | Prospective cohort | Germany; elderly primary care patients | 79.6 | 100% | Risk of any dementia with use of PPI | Adjusted HR 1.38 (1.04–1.83) |
| Risk of Alzheimer’s disease with use of PPI | Adjusted HR 1.44 (1.01–2.06) |
|||||
| Gomm and colleagues56 | Prospective cohort | Germany; inpatient and outpatient claims data | 83.8 | 100% | Risk of incident dementia with use of PPI | Adjusted HR 1.44 (1.36–1.52) |
Older adults defined as age 60 years and older.
AIN, acute interstitial nephritis; AKI, acute kidney injury; CAP, community-acquired pneumonia; CI, confidence interval; CDI, Clostridium difficile infection; CDAD, Clostridium difficile-associated diarrhea; eGFR, estimated glomerular filtration rate; H2RA, histamine-2 receptor antagonist; HR, hazard ratio; NA, not applicable; NR, not reported; OR, odds ratio; PPI, proton-pump inhibitor; RR, risk ratio.
Case-control studies
A number of case-control studies have identified an association between use of PPIs and risk of bone fracture in older adults. The dose and duration of PPI therapy that increases osteoporotic fracture risk is not entirely clear as the studies that have been published differ in their findings. In a case-control study conducted in Taiwan using a health insurance database, the investigators defined exposure to PPI therapy using the World Health Organization classification of defined daily doses (DDDs) where the higher the DDD, the greater the exposure to the drug.10 The study found an increased risk of hip fracture in patients prescribed >28 DDDs of a PPI compared with no use of PPIs, and the risk increased with increasing DDDs (29–70 DDDs, adjusted OR 1.67 and >70 DDDs, adjusted OR 2.51).10 The adjusted ORs accounted for potential confounding factors including use of anticoagulants, antipsychotics, sedatives, and diuretics. The authors also adjusted for various health conditions and found slightly less pronounced ORs, but still significant. These results suggest that a dose–response relationship may exist. Pouwels and colleagues used data from the Dutch PHARMO record linkage system and included 6763 cases with a first hip/femur fracture and 26,341 matched controls.13 Current use of PPI therapy was associated with an increased risk in hip/femur fracture [adjusted OR 1.20; 95% confidence interval (CI) 1.04–1.40], but the risk was diminished with increasing duration of use.13 The reported analyses were adjusted for use of several pharmacologic agents including: anxiolytics/hypnotics within the prior 3 months, antacids other than PPIs and H2RAs, hormone replacement therapy, beta-blockers, antidiabetic medications, antipsychotics, antidepressants, anticonvulsants, two or more nonsteroidal anti-inflammatory drugs (NSAIDs) dispensed, disease-modifying antirheumatic drugs, and average daily dose of oral corticosteroids in the 6 months prior to the index date.13 Several health conditions were also accounted for including history of diseases of the esophagus/stomach/duodenum, diabetes mellitus, rheumatoid arthritis, inflammatory bowel disease, anemia, mental disorders, endocrine disorders, congestive heart failure, cerebrovascular disease, and chronic obstructive pulmonary disease.13 In contrast, a case-control study that evaluated PPI use and risk of hip fracture specifically in men aged 45 years and older showed an increased risk with longer duration of PPI use.20 This study also found an increased risk of hip fracture in patients who had the greatest medication adherence and patients who recently initiated PPI therapy in the last 7 days.20 Greater adherence and longer duration indicates increased exposure to PPI therapy and increased exposure is likely to have a more consistent detrimental effect on the bone. It is unclear why recent PPI use would increase risk of hip fracture.
A few studies have evaluated the association between PPI therapy and risk of bone fracture when patients are on certain concomitant medications. Lee and colleagues identified 24,710 cases and 98,642 matched controls using a Korean health insurance database and found an increased risk of hip fracture in PPI users compared with nonusers (OR 1.34; 95% CI 1.24–1.44).19 Interestingly, when the study participants were stratified based on bisphosphonate use, there was a statistically significant difference in risk of hip fracture among bisphosphonate users (OR 1.71; 95% CI 1.31–2.23) compared with bisphosphonate nonusers (OR 1.30; 95% CI 1.19–1.42).19 This difference may indicate that PPIs are associated with an increased risk in hip fracture due to an interaction with bisphosphonate therapy; however, the pharmacologic interaction between PPIs and bisphosphonates is not entirely clear.19 Another case-control study sought to evaluate the interaction between PPI and histamine-1-receptor antagonists (H1RAs) on fracture risk.57 The authors hypothesized that H1RAs would reduce the effect of PPIs on the bone. They found an increased risk of all fractures (OR 1.08; 95% CI 1.05–1.11) and of hip fracture (OR 1.13; 95% CI 1.05–1.21) in PPI users.57 However, there was actually a decreased risk of overall fracture in patients who used both PPIs and H1RAs (OR 0.92; 95% CI 0.87–0.98). The interaction was not significant for hip fracture though.57 Additional studies are needed to further evaluate the hypothesis that histamine release secondary to PPI exposure may play a role in the risk of bone fracture.
Cohort studies
In addition to several case-control studies demonstrating an association between PPI exposure and fracture risk, a number of cohort studies have also demonstrated similar findings. In the Canadian Multicenter Osteoporosis study, a cohort of 9423 patients was formed and followed for 10 years.18 The primary outcome was incident nontraumatic fracture. After adjusting for several potential confounders including age, sex, body mass index, prior nontraumatic fracture, femoral neck T-score, corticosteroid use, alcohol intake, and activity levels, PPI use was associated with a 40% increased risk of nontraumatic fracture (HR 1.40; 95% CI 1.11–1.77).18 Of note, PPI use was evaluated as a time-dependent variable, and the difference in fracture risk between those exposed to PPI therapy and those unexposed increased as treatment time continued.18 Khalili and colleagues performed a prospective cohort study and found that women who regularly used PPI therapy for at least 2 years had an increased risk of hip fracture (adjusted HR 1.36; 95% CI 1.12–1.65) after adjusting for risk factors including body mass index, physical activity, and intake of calcium. Additionally, longer use was associated with an increasing risk of hip fracture: adjusted HR of 1.42 for 4 years of PPI use and adjusted HR of 1.55 for 6–8 years of PPI use.15 Furthermore, the risk for hip fracture was greater among current and former smokers who used PPIs (HR 1.51; 95% CI 1.20–1.91).15
Ding and colleagues performed a retrospective cohort study to examine the relationship between PPI adherence and fracture risk in the elderly and used administrative pharmacy claims data, survey data, and Medicare data.21 PPI adherence was measured by the proportion of days covered (PDC) which is calculated based on the patient’s fill history, where higher values indicate better adherence. The authors classified adherence into three different categories: PDC > 0.80 (high adherence), 0.40–0.79 (intermediate adherence), and <0.40 (low adherence). PPI use was associated with a greater fracture risk compared with no PPI use and a gradient in fracture risk was observed when the results were stratified based on PPI adherence. Those with highest adherence had the greatest risk (HR 1.46; p < 0.0001). Those with intermediate adherence also had a significantly increased risk of fracture (HR 1.30; p = 0.02), but lower than high adherence, and the HR was not significant for those with low adherence to PPI therapy.21 This study adds to the growing body of evidence that the level of PPI exposure may pose a greater risk of osteoporotic fracture.
Additional studies
The mechanism by which PPI therapy increases risk of fracture is not fully elucidated. It is unclear whether PPI therapy increases risk of fracture due to impaired calcium absorption, decreased bone mineral density, or increased risk of falls. A few studies have examined the association between PPI therapy and some of these variables in order to better understand the risk of bone fracture from a mechanistic standpoint. Targownik and colleagues published a study in 2010 that sought to determine the relationship between chronic PPI use and bone mineral density (BMD) loss over time.58 Data were collected from the Manitoba Bone Mineral Density database and the study included a cross-sectional case-control portion and a longitudinal portion. In the case-control portion, cases were patients who had confirmed osteoporosis at the hip or lumbar vertebrae evidenced by a T-score below −2.5 and controls had a normal BMD. PPI use was not associated with having osteoporosis at either the hip or the lumbar spine.58 In the longitudinal portion of the study, the change in BMD was measured among PPI users and nonusers. There was not a significant decrease in BMD observed that could be attributed to PPI use.58 A similar study was conducted in 2012 using the Canadian Multicenter Osteoporosis study data set. Study participants underwent BMD testing at baseline, at 5 years, and again at 10 years.59 The investigators did not find a significant acceleration in BMD loss associated with PPI use after 5 and 10 years of follow up. However, PPI use was associated with a lower BMD at the baseline visit at the total hip and femoral neck, but not the lumbar spine. Of note, <1% of the study population used PPIs continuously over the 10-year follow-up period, so the study may not have been powered to detect a change in BMD that was clinically significant.59 A third study examined the association between PPI use and BMD in older adults, but more specifically looked at the differences between cortical and trabecular BMD and cortical and trabecular cross-sectional area.60 The investigators found that PPI users had a lower trabecular BMD than nonusers (180.5 versus 207.9; p-value = 0.001).60 PPI exposure was determined by patient self-report of using a PPI over the last 15 days. The other parameters of cortical BMD, trabecular cross-sectional area, and cortical cross-sectional area were not statistically different in PPI users versus nonusers.60 In contrast with the 2010 and 2012 studies discussed above, this study included an older population (mean age = 75.7 years) but was limited by its design, as BMD was not measured over time while patients were chronically taking PPIs. These findings suggest that PPIs may increase risk of fracture via their effects specifically on trabecular bone as remodeling in trabecular bone is faster than in other areas and these changes may be an early step in the process of osteoporosis development;60 however, further studies are needed to confirm these findings.
Cea-Soriano and colleagues investigated whether acid-lowering therapy use was associated with an increased risk for falls as an attempt to describe the mechanism by which acid-lowering therapy may increase risk for fracture. Data were collected from the UK Health Improvement Network in patients aged 40–89 years.17 There was no relationship between current PPI use (adjusted OR 0.95; 95% CI 0.89–1.02) or H2RAs (adjusted OR 1.01; 95% CI 0.90–1.14) and falls after adjustment for confounders, including number of physician visits; specialist referrals and hospital admission in the prior year; presence of GERD; Charlson comorbidity index; and use of antidepressants.17 In contrast with the findings of Cea-Soriano and colleagues, a study conducted in Australia by Lewis and colleagues detected a significant association between PPI use and falls in older women at high risk of falls. This study consisted of a post-hoc analysis of a longitudinal prospective cohort followed by a replication prospective study. In the analysis, PPI therapy for duration of at least 1 year was associated with increased risk of fracture-related hospitalizations (adjusted OR 2.17; 95% CI 1.25–3.77).22 In the replication study, long-term PPI therapy (>1 year duration) was associated with an increased risk of self-reported falls (adjusted OR 1.51; 95% CI 1.00–2.27; p = 0.049) after adjusting for fall risk factors and vitamin D therapy.22 Interestingly, in the replication study, the investigators also found that objective clinical measurements such at the Timed Up and Go and Romberg eyes closed tests were impaired in long-term PPI users and these results were statistically significant.22 Due to the differences in study designs and study populations in these studies evaluating the association between PPI use and falls, it is difficult to conclude whether the increased risk of fractures associated with PPI therapy is due to increased fall risk. More research is needed to further examine the association between PPI use and risk for falls in older adults.
Decreased intestinal calcium absorption secondary to PPI exposure has also been evaluated as a potential mechanism by which PPIs may increase the risk of bone fracture. Most of the studies that have been published have not included older adults or were in patients on dialysis. However, there is one study that evaluated 18 healthy women aged 65–89 years.61 O’Connell and colleagues performed a randomized, double-blind, placebo-controlled crossover trial to assess the impact of PPI therapy on calcium carbonate absorption.61 Study subjects took either omeprazole 20 mg once daily or placebo once daily for 7 days and were blinded to what they were receiving. The study participants also took a multivitamin containing 400 units of vitamin D and two 45Ca-labeled calcium carbonate capsules containing a total of 500 mg of elemental calcium. After a 3-week washout period, the process was repeated with the opposite study drug. Calcium absorption was evaluated for each patient while taking omeprazole and while taking placebo. The investigators found that fractional calcium absorption was decreased from 9.1% (95% CI 6.5–11.6%) while taking placebo to 3.5% (95% CI 1.6–5.5%) while taking omeprazole.61 Based on this small study, calcium absorption may be decreased in the short term due to PPI therapy, but it is unclear if this effect is maintained long term with prolonged exposure to PPI therapy. Additional studies are needed to further evaluate this association in nondialysis-dependent older adults.
Meta-analyses
Although there is significant heterogeneity among the studies that have been published evaluating the association between PPI use and bone fracture risk, a number of meta-analyses have been conducted and have had similar findings.11,12,14,16 The magnitude of risk is similar among the different meta-analyses. Eom and colleagues included 11 observational studies in their analyses and found a pooled OR of 1.29 (95% CI 1.18–1.41) for fracture associated with PPI use.11 The risk was not significant for H2RAs. They additionally noted that long-term use of PPIs was associated with risk of both any fracture and of hip fracture.11 Ngamruengphong and colleagues included 10 observational studies and there was an increased risk of hip fracture (OR 1.25; 95% CI 1.14–1.37) and increased risk of vertebral fracture (OR 1.50; 95% CI 1.32–1.72) associated with PPI use.12 However, the risk was no longer significant when stratified by long duration of use, which is likely due to heterogeneity among the studies.12 A third meta-analysis included seven studies in their analysis and found that PPI therapy was associated with an increased risk of hip fracture (OR 1.24; 95% CI 1.15–1.34).14 Lastly, Yu and colleagues included 11 studies in their meta-analysis and found similar results to the meta-analyses discussed above.16 There was an increased risk of hip fracture (risk ratio (RR) 1.30; 95% CI 1.19–1.43), increased risk of spine fracture (RR 1.56; 95% CI 1.31–1.85), and overall fracture (RR 1.16; 95% CI 1.04–1.30) associated with PPI use.16 The use of H2RAs was not significantly associated with increased fracture risk.16
Discussion
There is a substantial amount of literature that points to a linkage between PPI use and risk of osteoporotic fractures including hip fracture and spine fracture. Because there are no randomized controlled trials investigating this association and only observational studies have been conducted, causality has not been established. The mechanism for this adverse effect has not yet been fully elucidated, but has been hypothesized to be due to an increased risk of falls, or a decrease in intestinal calcium absorption secondary to PPI exposure, or due to direct effects of PPIs on BMD. More studies are needed in the older adult population on a larger scale and for a longer duration to determine the mechanism. Based on the available literature, there appears to be a 25–50% increased risk in hip fracture associated with the use of PPIs. Additionally, there may be a dose–response and duration–response relationship between PPI use and fracture risk.
Clostridium difficile infection (CDI)
Overview
Clostridium difficile is a gram-positive anaerobe that has been identified as a major cause of antibiotic-associated diarrhea. CDI can range from mild disease manifesting as diarrhea to severe disease manifesting as toxic megacolon, sepsis, and death. Older adults are disproportionately affected by CDI as patients 65 years and older represented 92% of CDI-related hospital stays in 2009 in the US.62 Age and recent antibiotic exposure are known risk factors for the development of CDI, but the use of PPIs has been studied over the last decade as another potential risk factor. In 2012, the US FDA disseminated an additional safety warning for PPIs due to a considerable amount of literature published linking PPIs to an increased risk of Clostridium difficile-associated diarrhea (CDAD).4 Several observational studies have been published to examine the association between the use of PPIs and risk of CDI and CDAD in older adults within various settings. The results of studies that reported ORs, HRs, or RRs are summarized in Table 1.
Case-control studies
Cunningham and colleagues published one of the first studies to evaluate the association between PPIs and CDAD, which was a retrospective case-control study of 170 cases identified from laboratory and infection control team records and matched to controls.23 The general patient demographics were not reported, so it is unclear whether the patient population was primarily elderly or not; however, they did find increased unadjusted odds of CDAD in patients who used PPIs within the preceding 8 weeks (OR 2.5; 95% CI 1.5–4.2).23 This risk was further increased in patients who were also using antibiotics or cytotoxic chemotherapy as these are known risk factors for development of CDAD.23 Similar findings were identified by Dial and colleagues in 2005.25 This group of investigators evaluated both PPIs and H2RAs and risk of CDAD in two population-based case-control studies in the United Kingdom. If the patients received at least one PPI or H2RA prescription in the 90 days prior to the index date, they were considered to be exposed to that drug. The investigators first identified all cases of CDAD using the General Practice Research Database (GPRD) which included hospital-acquired and community-acquired cases; in the second analysis, only community-acquired cases were included.25 In the first analysis, the authors found that the following factors were associated with an increased risk of CDAD: age > 65 years, exposure to antibiotics in the 90 days prior, and prior hospitalization. In the second analysis, the authors found an adjusted rate ratio of 2.9 (95% CI 2.4–3.4) for current PPI exposure and adjusted rate ratio of 2.0 (95% CI 1.6–2.7) for current H2RA exposure.25 Analyses were adjusted for sex, comorbidities, and coprescription with NSAIDs and aspirin. In contrast with the previous two studies, a nested case-control study conducted in Ontario, Canada did not find an association between PPI use (adjusted OR 0.9; 95% CI 0.8–1.1) and hospitalization for CDAD in older adults.26 Cases were patients aged 66 years and older who received outpatient antibiotic therapy and were then hospitalized for CDAD within 60 days.26 This study differs from the previous two studies discussed as the CDAD cases required hospitalization whereas the CDAD cases in the previous two studies were identified by toxin assay results or clinical diagnosis. Additionally, the authors of this study adjusted for different factors including use of H2RAs, chemotherapy, immunosuppressants, or systemic corticosteroids in the previous 6 months; history of diabetes mellitus, end stage renal disease (ESRD), hospitalization for cancer; and any hospitalization or outpatient claim for inflammatory bowel disease in the previous 2 years.26 These differences may be the reason for the conflicting findings. Not all patients who develop CDAD necessarily need to be admitted to the hospital, so there may be more cases of CDAD that the authors did not identify. Aseeri and colleagues also examined the association between exposure to acid-lowering therapy and CDAD, but evaluated patients who developed CDAD while they were already hospitalized.27 Over 40% of the CDAD cases identified occurred in elderly patients and the odds of CDAD diagnosis was increased with both PPI (OR 3.08; 95% CI 1.61–5.91) and H2RA exposure (OR 2.14; 95% CI 0.87–5.26), but more pronounced with PPI exposure.27 The OR remained significant and was increased (OR 3.6; 95% CI 1.73–8.26) after controlling for potential confounders including date of hospital admission, antibiotic usage, sex, age, patient location, and room type.27 A limitation of this study is the way in which exposure to acid-lowering therapy was defined. Patients were considered to be exposed to the drug (either PPI or H2RA) if they had a prescription prior to admission and continued therapy while hospitalized, or if they received the drug 3 days prior to development of CDAD in the hospital.27 This makes it difficult to determine the duration of exposure that poses a potential risk of CDAD.
Cohort studies
A cohort consisting of 1187 inpatients who had received antibiotics were followed over time for development of positive assay results for Clostridium difficile toxin. Patients were classified as PPI users, H2RA users, or nonusers at baseline.24 PPI use was significantly associated with a greater risk of CDAD (OR 2.1; 95% CI 1.2–3.5) and there were no patients who developed CDAD that were on H2RAs at baseline.24 In order to eliminate bias from potential confounders, the investigators also performed a case-control study among inpatients at a different hospital. Findings were similar in the case-control portion of the study as use of PPI therapy was significantly associated with an increased risk of CDAD (OR 2.7; 95% CI 1.4–5.2).24 Additionally, the cases were more likely to have prolonged exposure to PPI therapy, defined as >6 months, compared with controls. These findings suggest that a duration–response relationship may be present.24 A retrospective cohort study was also conducted to evaluate the association in hospitals with low rates of new CDI cases.63 The cohort consisted of patients who were admitted for at least 7 days and who were receiving antibiotics at two general hospitals in Canada that were considered to have a low prevalence of the hypertoxigenic NAP1 strain of Clostridium difficile. PPI exposure was significantly associated with an increased risk of the first episode of CDI (OR 1.96; 95% CI: 1.42–2.72).63 The reported OR was not adjusted for confounders, but in the logistic regression analysis, use of H2RAs, use of antidepressants, antibiotic days, PPI days, age, admission service, and length of stay were found to be predictors of risk.63 The authors concluded that in this setting of low CDI rates, the benefit of continued PPI therapy outweigh the risk of developing CDI because the absolute risk increase was modest (0.74 cases/100 patients in nonusers versus 1.44 cases/100 patients in PPI users).63 More recently, Lewis and colleagues published a retrospective cohort study evaluating both the rate and severity of hospital-acquired CDI associated with use of PPIs.64 Severity of infection was determined using the definitions outlined by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology guidelines. The relative risk of developing hospital-acquired CDI was 6.46 (95% CI 3.63–11.51) among PPI users. There were 22 patients in the PPI group and 2 patients in the control group who developed severe-complicated CDI yielding a relative risk of 15.32 (95% CI 3.6–65.3).64 Of note, the results reported in this analysis are unadjusted and are therefore subject to potential confounding bias.
Meta-analyses
Several meta-analyses have also found a significant association between exposure to PPI therapy and risk of CDI .29–32 Desphande and colleagues included 30 observational studies that contained a total of 202,965 patients and reported an OR of 2.15 (95% CI 1.81–2.55). They also noted that compared with no acid suppression therapy, risk of CDI increased as level of acid suppression increased with an OR of 1.53 for H2RA therapy, OR 1.74 for daily PPI therapy, and 2.36 for more frequent PPI therapy.29 A second meta-analysis included 23 studies in total (17 case-control and 6 cohort) and found a summary risk estimate of 1.69 (95% CI 1.395–1.974) for the association between PPI exposure and CDAD among hospitalized patients.30 A third meta-analysis reported a pooled OR of 1.65 (95% CI 1.47–1.85) for the association between PPI use and CDI and included 47 observational studies.32 The authors also performed a speculative analysis that assumes the relationship between PPI exposure and CDI is a causal relationship and they reported the number needed to harm (NNH) at 1 year of 3925 in the general population. The NNH considerably decreased among hospitalized patients receiving antibiotics and PPI therapy (NNH = 50 at 2 weeks).32 Finally, Kwok and colleagues published a meta-analysis that included 42 observational studies and reported a significant association between development of CDI and PPI exposure (OR 1.74; 95% CI 1.47–2.85).31 When restricting the analysis to only studies that reported adjusted data, the pooled OR remained significant at 1.93 (95% CI 1.61–2.31). The authors also analyzed the risk of CDI associated with concomitant PPI and antibiotic use and reported an OR of 1.96 (95% CI 1.03–3.70).31 The authors noted that there was an increased risk of CDI from the interaction between PPI use and antibiotic use beyond the effects of each drug individually.31
Additional studies
There is a significant amount of literature that has demonstrated an association between PPI use and risk of incident CDI in older adults as discussed above. A number of studies have also evaluated the association between PPI therapy and recurrent CDI.28,33,34 Linsky and colleagues performed a retrospective cohort study using the New England Veterans Healthcare database and identified inpatients and outpatients with incident CDI treated by metronidazole or vancomycin.28 These patients were classified as exposed to PPI therapy if they used any PPI during the 14 days after the incident CDI diagnosis. PPI exposure during CDI treatment was significantly associated with a greater risk of recurrent CDI within 90 days (adjusted HR 1.42; 95% CI 1.10–1.83) after adjusting for covariates. Covariates adjusted for included age, initial incident CDI antibiotic treatment, additional antibiotic exposure, duration of hospital exposure, baseline comorbidities, and baseline medications. When stratifying based on age, the risk was greater in those over the age of 80 years (HR 1.86; 95% CI 1.15–3.01).28 Another retrospective cohort study conducted in Canada at two university-affiliated hospitals was published in 2015 by McDonald and colleagues34 This study identified a cohort of patients who developed healthcare-associated CDI and then followed the cohort to assess for recurrence of CDI within 15–90 days. The exposure was continuous PPI use defined as either ongoing use for at least 75% of the days in the hospital or a discharge prescription valid for beyond 90 days from the initial CDI date. Continuous PPI use was significantly associated with recurrent CDI (HR 1.5; 95% CI 1.1–2.0).34 The authors also identified that age older than 75 years was associated with an increased risk of recurrence (HR 1.5; 95% CI 1.1–2.0). Additionally, many of the patients who were prescribed PPI therapy did not have an evidence-based indication and interestingly PPIs were discontinued in only three patients with CDI.34 A third group of investigators performed a retrospective cohort analysis to examine the association between PPI use during CDI treatment and risk of recurrent CDI.33 This study only identified inpatients with incident CDI whereas Linsky and colleagues identified both inpatients and outpatients with incident CDI. There was no association between PPI exposure during CDI treatment and risk of recurrent CDI (HR 0.82; 95% CI 0.58–1.16).33 Of note, the mean age was 64 years whereas the median ages in the Linsky and colleagues and McDonald and colleagues studies were 74 years and 71.5 years respectively. Because risk of recurrent CDI is known to increase with increasing age, this may have played a role in the differing results, and Freedberg and colleagues may not have found a significant association between PPI use and recurrent CDI as they studied a younger population. Due to the observational nature of these studies, causality cannot be established. However, currently available literature suggests an association between PPI exposure and recurrent CDI within 15–90 days after initial CDI, particularly among older adults.
Discussion
Although causality cannot be established, based on the available literature it appears that an association between acid-lowering therapy and development of CDI and CDAD exists. It is hypothesized that the increased risk of CDI and CDAD in relation to PPI exposure is due to the increased gastric pH allowing the vegetative form of Clostridium difficile to survive. Survival may be due to the changes in the microbiome secondary to acid suppression, thereby limiting the barriers for Clostridium difficile survival; however, in general, spores are resistant to acid and acid suppression does not affect their survival. Thus, the exact mechanism of Clostridium difficile proliferation remains unclear, and more research is needed in this area. Based on the available studies in the older adult population, there is about a 2-fold increased risk in the development of CDI and CDAD associated with use of PPIs and a 1.5-fold increased risk in recurrent CDI associated with use of PPIs. The effect of PPI duration on this risk is unclear, but there is limited evidence that suggests that the degree of acid suppression plays a role. Additional factors such as antibiotic exposure, increasing age, and prior hospitalization also contribute to the increased risk of CDI and CDAD observed among PPI users. In older adults with multiple risk factors for CDI and CDAD, it is worth evaluating the risk versus benefit of continued PPI therapy.
Community-acquired pneumonia (CAP)
Overview
CAP is associated with significant morbidity and mortality particularly among older adults. According to the National Center for Health Statistics, influenza and pneumonia accounted for the eighth leading cause of death in 2014 among older adults.65 The incidence of pneumonia in older adults is higher compared with younger populations and the elderly are more likely to be hospitalized and die from pneumonia.66 The increased risk among older adults may be due to changes in lung physiology, reduced mucociliary clearance, greater upper airway colonization, changes in the immune system, and presence of several comorbidities.66 It is hypothesized that PPI exposure increases risk for CAP by increasing gastric pH allowing for colonization of bacteria that can then be aspirated.38 Several studies have been conducted to assess if there is an association between the risk of CAP and use of acid-lowering therapy, but the results have been inconsistent. Even within single studies, the risk of CAP differs depending on duration of PPI exposure. The results of studies in older adults that reported adjusted ORs are summarized in Table 1.
Case-control studies
Laheij and colleagues performed a nested case-control study to evaluate the association between risk of CAP and use of acid-lowering therapies.35 ORs were calculated for risk associated with any acid-lowering therapy, PPI therapy alone, H2RA therapy alone, and combined PPI and H2RA therapy. The risk of CAP was greater with PPI therapy (adjusted OR 1.73) compared with H2RA therapy (adjusted OR 1.59), and a positive dose–response relationship was observed among PPI users. Analyses were adjusted for matching factors, respiratory illness, long-term heart failure, diabetes mellitus, use of antibiotics or immunosuppressants.35 The mean duration of use of H2RA therapy was 2.8 months and the mean duration of use of PPI therapy was 5.0 months. Of note, the risk was greatest in patients who initiated PPI or H2RA therapy within the last 30 days.35 Another case-control study by Gulmez and colleagues sought to evaluate the same question of whether an association between acid-lowering therapy and development of CAP existed. An association was found with PPI therapy (adjusted OR 1.5; 95% CI 1.3–1.7), but not with H2RA therapy (adjusted OR 1.10; 95% CI 0.8–1.3]).36 ORs were adjusted for age, sex, previous discharge for CAP, chronic obstructive pulmonary disease (COPD), peptic ulcer, alcohol-related diagnoses, ischemic heart disease, liver cirrhosis, renal failure, diabetes, heart failure, stroke, and current use of corticosteroids, bronchodilators, NSAIDs, anticholinergic agents, or antipyschotics.36 Similar to the study by Laheij and colleagues, this study found the association between CAP and PPI exposure to be strongest with recent initiation of the PPI.36 The association has also been exclusively studied in veteran patients by Hermos and colleagues. This group only looked at PPI exposure and concluded that current PPI use was significantly associated with an increased risk of CAP (adjusted OR 1.29; 95% CI 1.15–1.45) and the association was stronger for patients who initiated the PPI in the prior 1–15 days compared with patients who had longer exposures. They also noted that the association was greater for patients with higher doses of PPIs, since patients who were prescribed >1 standard dose per day had a greater risk of CAP compared with patients who were prescribed <1 standard dose per day of PPI (adjusted OR 1.33; 95% CI 1.06–1.65).43 Factors that were adjusted for include the following: sex; age; diagnoses of COPD, diabetes mellitus, congestive heart failure, lung cancer, nonskin and nonlung cancer, ischemic heart disease, chronic kidney disease (CKD), chronic liver disease, dementia, alcohol/drug dependence or abuse, peptic ulcer, or reflux esophagitis; admission <90 days before end date; active use at baseline of H2RAs, systemic corticosteroids, immunomodulators/immunosuppressants, tranquilizers/sedatives, or antipsychotic agents; and antibiotic use <90 days prior to baseline.
Although the three case-control studies discussed above identified an association between PPI exposure and risk of CAP, several studies have not concluded that there was an association or were only able to conclude that there was an association with recent initiation. In a nested case-control study conducted in the United Kingdom using the GPRD, a database comprising complete and comprehensive medical records, current PPI use within 30 days of the index date was associated with an increased risk in CAP (OR 2.05; 95% CI 1.96–2.15).37 However, after adjusting for covariates, there was no association. Potential confounders that were adjusted for include current smoking, total number of general practice visits during the past year, total number of hospitalizations during the past year, COPD, asthma, myocardial infarction, congestive heart failure, chronic renal failure, cirrhosis, diabetes mellitus, stroke, history of CAP before GPRD enrollment, cancer other than basal cell carcinoma, dementia, and alcoholism. Exposure to the following drugs was also accounted for: H2RAs, anxiolytics, antidepressants, antipsychotics, antibiotics, anti-Parkinson’s drugs, barbiturates, opiates, corticosteroids, and long-term NSAIDs. Additionally, there was no association between CAP and H2RA exposure and no association between CAP and PPI exposure in adults aged 60 years or older.37 Another case-control study that was performed in the US exclusively in older adults concluded that PPI and H2RA current use was not associated with an increased risk of CAP (adjusted OR 1.03; 95% CI 0.86–1.24) in the fully adjusted analysis.38 Current use was defined as having filled two or more prescriptions that were for at least a 30-day supply within the prior 6 months of the index date and of note, the majority of the patients were on H2RA therapy. The study lacked power to rule out an increased risk of CAP when only looking at patients on PPI therapy.38
Meta-analyses
Several meta-analyses have been published that have collectively evaluated the risk of CAP with acid-lowering therapy. Johnstone and colleagues included studies that evaluated adults who took PPIs as an outpatient and found an increased risk of CAP associated with PPI use (OR 1.36; 95% CI 1.12–1.65) but was unable to definitively interpret the results as heterogeneity among the included studies was high (I2 = 92%; p < 0.001).39 Eom and colleagues included eight observational studies and found that the overall risk of CAP was higher among patients using PPIs (OR 1.27; 95% CI 1.11–1.46) and H2RAs (OR 1.22; 95% CI 1.09–1.36).40 With a 1.22 to 1.27-fold increased risk of CAP in patients taking acid-suppressive therapy, approximately 25 cases of CAP can be expected for every 1000 recipients of acid-lowering therapy.40 A third meta-analysis published in 2015 also noted an increased risk of CAP in patients prescribed PPIs (OR 1.49; 95% CI 1.16–1.92), with the highest risk in patients who initiated PPI therapy in the last 30 days.44 This study also specifically evaluated the association in older adults compared with the association in adults <65 years and found that in both groups, CAP was higher in patients exposed to PPI therapy and the risk was similar.44 Of note, this meta-analysis did not find an association between risk of CAP with H2RA therapy (OR 1.00; 95% CI 0.90–1.12).44
Additional studies
Because of the conflicting results among several case-control studies, several studies have been conducted to further examine the etiology of CAP in patients using acid-lowering therapy. It has been hypothesized that PPIs cause an increased risk of CAP due to their pH lowering effects which allows for overgrowth of gastrointestinal or oropharyngeal bacteria. Meijvis and colleagues found an increased risk of CAP in patients who recently initiated PPIs and in patients on chronic PPI therapy.41 They then looked at the causative organisms among current users, past users, and nonusers of PPIs and found that the frequency of oropharyngeal pathogens identified as the causative organism did not differ between those who recently initiated a PPI and nonusers.41 CAP was caused by gastrointestinal bacteria in only two patients who were PPI users. Therefore, Meijvis and colleagues concluded that overgrowth of gastrointestinal or oropharyngeal organisms does not fully explain the mechanism by which PPIs increase risk of CAP. In contrast, another study found that oropharyngeal organisms were more common in CAP patients using PPI therapy compared with CAP patients not using PPI therapy (OR 2.0; 95% CI 1.22–3.72).42 De Jager and colleagues also noted that CAP patients who used PPIs were more likely to be infected with Streptococcus pneumoniae compared with CAP patients who were nonusers (OR 2.3; 95% CI 1.28–3.75), while airborne pathogens were less common in PPI users.42 The authors concluded that PPI exposure is associated with an increased risk of CAP, possibly due to endogenous oropharyngeal flora.42
Discussion
The available evidence regarding an association between use of PPIs and risk of CAP is inconclusive. This is likely due to the significant heterogeneity in the studies that have been conducted. Of the studies that have demonstrated an association between PPI use and risk of CAP in older adults there appears to be approximately a 30% increased risk and the risk is greater with recent initiation of PPI therapy. It is unclear if a risk exists with prolonged use of PPI therapy and more studies are needed to come to a conclusion. The risk of CAP development due to PPI exposure is biologically plausible as an increase in gastric pH would allow for survival and growth of bacterial organisms, but the mechanism has not been definitively established at this time.
Vitamin B12 deficiency
Overview
Vitamin B12 (cobalamin) is a water-soluble vitamin that is required for DNA synthesis, red blood cell formation and neurologic functioning.67 Cobalamin that comes from dietary sources is tightly protein-bound and the primary food sources of cobalamin include meat and eggs.68 The prevalence of vitamin B12 deficiency has been reported to be anywhere from 5–40% in elderly patients depending on the diagnostic criteria that is used and prevalence tends to increase with increasing age.69 The most common reason for B12 deficiency in older adults is impaired absorption secondary to decreased gastric acid secretion, as gastric acid is required to release protein-bound cobalamin. In addition to altered cobalamin metabolism, decreased acid secretion increases the pH in the small intestine which allows for bacterial overgrowth and competition for uptake of vitamin B12, further reducing cobalamin availability.70 Vitamin B12 deficiency can manifest as hematologic, neurologic, and psychiatric abnormalities which are of particular concern in older adults. Several observational and case-control studies have been conducted to evaluate the association between the use of acid-lowering therapy and vitamin B12 deficiency in older adults. Many of the studies included both PPIs and H2RAs in their analysis. Table 1 summarizes the results of the studies that reported adjusted ORs.
Case-control studies
In a case-control study conducted at Kaiser Permanente Northern California that included 25,696 cases and 184,199 controls, an increased risk for vitamin B12 deficiency (OR 1.65; 95% CI 1.58–1.73) was found to be associated with receiving a 2 or more years supply of PPI.49 Similarly, the same supply of H2RAs also significantly increased the risk of vitamin B12 deficiency (OR 1.25; 95% CI 1.17–1.34) but to a lesser extent than PPIs.49 This study evaluated confounding by contrasting ORs between models with and without each potential confounder and determined that those altering the OR by 10% or more would be included in the final model. However, of the multiple conditions and medications assessed, none met the threshold to be included in the final analysis. Another case-control study evaluated both short-term use (<12 months) and chronic use (⩾ 12 months) of PPIs and H2RAs and their association with vitamin B12 deficiency in older adults.47 The risk of vitamin B12 deficiency was significantly associated with chronic use of PPIs and H2RAs (adjusted OR 4.46; 95% CI 1.49–13.33) but it was not significantly associated with short-term use (adjusted OR 1.03; 95% CI 0.46–2.31). Analyses were adjusted for age, sex, multivitamin use, and Helicobacter pylori infection. Of note, the majority of patients in this study were taking H2RAs.47 Force and colleagues conducted a retrospective case-control study using a drug database from the State of Idaho Medicaid program and identified patients who received a first vitamin B12 injection in the preceding 12 months. They found that patients who were started on vitamin B12 injections were more likely to have received chronic acid suppression, defined as at least 10 months of treatment with full doses of PPIs or H2RAs in the year prior (OR 1.82; 95% CI 1.08–3.09).46 This study did not report adjusted results. A study conducted in Canada in outpatient and institutionalized patients with cognitive impairment found an association between PPI and H2RA use and initiation of vitamin B12 replacement even after adjusting for age, sex, and institutional residency (OR 2.61; 95% CI 1.31–5.23).45 Cotter and O’Keeffe evaluated the association between PPI use and risk of vitamin B12 deficiency among medical inpatients aged 65 years and older in a retrospective case-control study.48 There was no association between current PPI use and vitamin B12 deficiency (OR 0.92; 95% CI 0.53–1.60).48 The differing results may be due to the definition of deficiency in this study, which was a vitamin B12 level of <150 pmol/l. This threshold may be too restrictive. Additionally, the analyses were not adjusted for potential confounding factors. A meta-analysis conducted by Jung and colleagues included the studies discussed above and concluded that the use of acid-lowering therapy for at least 10 months is associated with an increased risk of vitamin B12 deficiency (OR 1.83; 95% CI 1.36–2.46).
Discussion
Although no randomized controlled trials have been conducted to confirm a causal relationship between PPI use and vitamin B12 deficiency, a significant amount of evidence points to an association. Older adults are already at an increased risk of vitamin B12 deficiency primarily due to aging-related impaired absorption; chronic use of acid-lowering therapy, including PPIs, appears to further increase this risk and is associated with initiation of vitamin B12 replacement in some studies. Only one study conducted in the United Kingdom did not find an association between long-term PPI use and decreased vitamin B12 levels. This may have been due to the small sample size compared with other studies and definition used for vitamin B12 deficiency. The studies that found a positive association between use of PPIs and vitamin B12 deficiency reported a wide range of ORs likely due to the heterogeneity among the studies conducted, but based on the meta-analysis that included several of the discussed studies, there is approximately an 80% increased risk in vitamin B12 deficiency associated with use of acid-lowering therapy. Because vitamin B12 deficiency is already more prevalent in older adults, it may be reasonable to measure vitamin B12 levels in older adults who are prescribed chronic acid suppression therapy.
Kidney disease and injury
Overview
CKD is defined as abnormalities in kidney structure or in function present for at least 3 months,71 while acute kidney injury (AKI) is defined as a sudden, temporary, and sometimes fatal loss of kidney function.72 CKD is characterized by either a glomerular filtration rate of <60 ml/min/1.73 m2 for at least 3 months or by presence of at least one marker of kidney damage such as an albumin-to-creatinine ratio of >30 mg of albumin for each gram of creatinine for at least 3 months.71 In the 2006 National Health and Nutrition Examination Survey study, the prevalence of CKD in patients aged 60 years and older increased from 18.8% in the 1988–1994 study to 24.5% in the 2003–2006 study.72 The incidence of CKD among older adults doubled between 2004 and 2008 and is increasing most rapidly among the elderly population.72 CKD can lead to the development of ESRD and mortality due to ESRD has dramatically increased over the last few decades.72 Similar to CKD, older adults are also disproportionately affected by AKI.73 A study in a Kaiser Permanente population revealed that AKI increased from 78 per 100,000 person years in patients younger than age 50 to 3545 per 100,000 person years in those aged 80 years and older.74 Acute interstitial nephritis (AIN) is a subtype of AKI that accounts for a small number of AKI cases. The most frequent cause of AIN is hypersensitivity to medications, and AIN is most common in the elderly.73 Treatment of drug-induced AIN is to remove the offending agent; however, renal recovery is variable. Although some patients fully recover after a period of time, there are a number of patients who do not fully return to their baseline renal function.75–77 A number of studies have been conducted to evaluate the association between PPI therapy and AKI and between PPI therapy and CKD. The results of the studies in older adults that reported ORs or HRs are reported in Table 1.
Case-control studies
Only a few observational studies have been published to evaluate the association between PPI use and AIN. Leonard and colleagues performed two retrospective case-control studies to assess the association between PPI exposure, NSAID exposure, PPI-NSAID co-exposure, the development of AIN and more broadly, development of AKI.51 Exposure was defined as having an active prescription of a PPI or NSAID on the index date. Duration of therapy prior to the index date was not reported. There was a nonsignificant increased risk for AIN for both PPI exposure (OR 3.20; 95% CI 0.80–12.79) and NSAID exposure (OR 1.90; 95% CI 0.65–5.51) after adjusting for confounding factors. There were no cases of AIN in patients who were co-exposed.51 The wide CI is likely due to a limited number of AIN cases. Additionally, the median age of the population was 60 years; significance may have been reached if an older, higher-risk study population was evaluated. When AKI was evaluated, the authors found a significant increase in risk associated with co-exposure to PPI and NSAID therapy (OR 1.33; 95% CI 1.07–1.64), but not with PPI therapy alone (OR 1.05; 95% CI 0.97–1.14). Although there was not an increase in odds of AKI associated with PPI therapy alone, an increased risk of a certain subtypes of AKI cannot be excluded as the investigators did not stratify AKI by subtype to further examine this possibility. Another case-control study used a broader diagnosis of renal disease to identify their cases because PPI-induced AIN is commonly under or misdiagnosed and has an inconsistent clinical presentation.78 The cases were patients who had renal disease which included any of the following diagnoses: hypertensive renal disease, acute glomerulonephritis, nephrotic syndrome, chronic glomerulonephritis, nephritis and nephropathy, acute renal failure, chronic renal failure, renal failure unspecified, impaired renal function disease, unspecified disorder of kidney and ureter, kidney transplant, and dialysis.78 PPI exposure was determined by claims data, and patients were considered to be exposed if they obtained at least one prescription for a PPI in the 90 days prior to the index date. PPI use was associated with an increased risk of renal disease as defined above (adjusted OR 1.72; 95% CI 1.27–2.32) after controlling for potential confounders that are known causes of AKI: diabetes, hypertension, high cholesterol, use of antibiotics, diuretics, or NSAIDs.78 Blank and colleagues performed a nested case-control study in New Zealand and found that the risk of AIN was greater with current use of PPI therapy (OR 5.16; 95% CI 2.21–12.05) relative to past use of PPI therapy and also increased with increasing age.79 Patients were considered to be current users if their last PPI prescription days’ supply extended into the 30 days prior to the index date and patients were considered to be past users if the last prescription days’ supply terminated at least 90 days prior to the index date. Adjustment for ethnicity, socioeconomic status, use of other drugs associated with increased risk of AIN in the 30 days prior to the index date, and hospital admissions in the year before the index date did not significantly alter the results.79 An additional population-based cohort study was conducted in exclusively older adults aged 66 years and older in Ontario, Canada by Antoniou and colleagues to examine the risk of AIN and AKI in patients exposed to PPIs.52 There were 290,592 patients who initiated PPI therapy during the study period and equal number of matched controls. Patients were followed over 120 days and there was a significantly increased risk in both AKI (HR 2.52; 95% CI 2.27–2.79) and AIN (HR 3.00; 95% CI 1.47–6.14) among patients who initiated PPI therapy compared with patients who did not.52
Cohort studies
In addition to the risk of AIN and AKI with PPI therapy, there has been emerging evidence to suggest there is also a risk of CKD associated with long-term PPI therapy. There have been three studies published in the last year examining the association between the risk of CKD and use of PPIs,53,54,80 but only two included primarily older adults which are discussed below. Lazarus and colleagues performed a prospective cohort study using data from the Atherosclerosis Risk in Communities study and then used data from the Geisinger Health System for a replication cohort study.53 In the initial cohort, 10,482 patients with an estimated glomerular filtration rate (eGFR) of at least 60 ml/min/1.73 m2 at baseline were followed over time to assess for development of incident CKD. In the adjusted analysis, PPI use was associated with an increased risk of incident CKD (HR 1.50; 95% CI 1.14–1.96) compared with nonusers. The association remained when PPI users were compared with H2RA users (HR 1.39; 95% CI 1.01–1.91).53 Xie and colleagues used Department of Veterans Affairs national databases as their data source.54 The investigators identified new PPI users and new H2RA users and followed them over a 5-year period to determine renal outcomes.54 The H2RA users were considered the control group. PPI users had a significantly increased risk of incident CKD (HR 1.28; 95% CI 1.23–1.34) compared with H2RA users. Additionally, PPI users had a significantly increased risk of incident eGFR <60 ml/min/1.73 m2, doubling of the serum creatinine level, eGFR decline of >30%, and ESRD.54
Discussion
It is hypothesized that the association between PPI use and CKD may be attributable to the increased risk of AIN due to PPI use. Because AIN is often unrecognized, it is possible that subclinical, undiagnosed AIN may convert to a chronic interstitial nephritis and this chronicity may lead to the development of CKD.81 Although only observational studies have been performed to examine the risk of kidney disease associated with PPI therapy in older adults, there is some evidence that links PPI exposure to an increased risk of AIN. The duration of exposure to a PPI that increases risk of AIN is not well established. There is currently less evidence that links the use of PPIs to CKD but among the studies that have been conducted, the magnitude of risk is similar (40–50% increased risk) and appears to increase with higher doses. More studies are needed at this time to confirm the relationship between PPI exposure and risk of CKD and if the underlying mechanism is related to PPI-induced AIN.
Dementia
Overview
Dementia is a clinical condition characterized by progressive cognitive decline that affects one’s ability to live independently, and predominantly affects older adults.82 Alzheimer’s disease (AD) accounts for up to 80% of dementia cases while the remaining 20% is made up of various other types of dementia including but not limited to vascular dementia, Lewy Body dementia, and mixed dementia. In 2016, an estimated 5.4 million Americans have AD and of these, 5.2 million are age 65 years and older.83 Overall, one in three elderly patients dies with AD or another type of dementia.83 Pathophysiologic characteristics of AD include accumulation of neurofibrillary tangles and amyloid plaques which lead to neuronal loss and neurodegeneration. The risk of dementia associated with use of PPI therapy has been less studied than other adverse effects. The results of studies in older adults that reported adjusted ORs are summarized in Table 1.
Cohort studies
There have been only a few manuscripts published which describe potential mechanisms by which PPIs could cause dementia, and two prospective cohort studies conducted in Germany evaluated the risk of dementia due to PPIs. Fallahzadeh and colleagues hypothesize that PPIs cross the blood–brain barrier as demonstrated in animal studies, where they can then inhibit vacuolar proton pumps on membranes of microglia.84 The inhibition of these proton pumps could result in increased lysosomal pH leading to decreased lysosomal protease function. Lysosomal proteases are responsible for digesting Aß fragments; lack of appropriate digestion may lead to increased Aß accumulation, which may contribute to the pathogenesis of AD.84 However, actual studies are needed to confirm this hypothesis. Badiola and colleagues investigated a slightly different theory by examining the effect of PPIs on amyloid metabolism in vitro and in vivo. This group specifically looked at lansoprazole and found that in amyloid cell models, lansoprazole enhances production of Aß37, Aß40, and Aß42 and lowers production of Aß38.85 The authors hypothesize that this occurs due to PPI modulation of y-secretase, an enzyme that cleaves amyloid precursor protein to the Aß species.85 The Aß42 peptide has been implicated as the main pathological species in AD.86 When Badiola and colleagues further investigated other PPIs including omeprazole, pantoprazole, and esomeprazole in vitro, there was a dose-dependent increase in Aß42 levels for all three drugs. Therefore, the authors concluded that the modulation of Aß production is a class effect.85 After this association was found, they explored the effect of lansoprazole on Aß production in the brain in mice and found that in both wildtype and triple transgenic AD mouse models that soluble Aß40 levels were significantly increased while Aß42 levels were modestly increased but did not reach statistical significance.85 Current studies demonstrate potential mechanisms indicating that PPIs may alter amyloid production and metabolism, potentially contributing to AD.
The first epidemiological study that evaluated the association between PPI use and dementia was conducted in Germany using data derived from the German Study on Aging, Cognition, and Dementia in Primary Care Patients (AgeCoDe).55 This longitudinal study included 3327 community-dwelling patients age 75 years and older and found that patients receiving PPI therapy had an increased risk for any dementia (HR 1.38; 95% CI 1.04–1.83) and of AD (HR 1.44; 95% CI 1.01–2.06).55 These results were adjusted for potential confounders including age, sex, education, ApoE4 allele status, polypharmacy and comorbidities. Duration of PPI use was not reported in the study. A similar study published by Gomm and colleagues evaluated the association between the use of PPIs and risk of dementia on a larger scale.56 This study was a prospective cohort that derived its data from the largest German statutory health insurer and included 73,679 patients aged 75 years and older. Patients were considered to be PPI users if they had at least one prescription per quarter of any of the following PPIs: omeprazole, pantoprazole, lansoprazole, esomeprazole, or rabeprazole. Regular use of PPIs was associated with a significant increased risk of incident dementia (HR 1.44; 95% CI 1.36–1.52) after controlling for potential confounders.56 The risk gradually decreased with age, which the authors noted may be due to a decreased influence of external and internal factors on dementia progression once the disease process has already been initiated.56
Discussion
Currently, there is limited evidence that suggests that PPI use increases risk of developing dementia among older adults. The two cohort studies that have been published and discussed above found a similar magnitude of risk of dementia associated with PPI use at about approximately 40% in a German population. Due to the observational nature of the studies that have been conducted, we cannot conclude with certainty that the association between PPI use and incident dementia is causal. However, from a mechanistic standpoint, it does seem conceivable that chronic PPI exposure could contribute to the pathophysiology of AD through their direct and indirect effects on Aß metabolism. Further studies are needed to explore the risk of dementia, particularly in other patient populations, in order to gain a better understanding of the potential risk.
Conclusion
This review highlights current data regarding potential adverse effects of PPIs in the older adult population. US FDA warnings currently exist for PPI use and risk of bone fractures and CDI, but current literature has identified associations between PPI use and risk of osteoporotic-related fractures, CDI, CAP, vitamin B12 deficiency, kidney disease/injury, and dementia, particularly in older adults. We do not know the exact duration of PPI therapy that would predispose older adults to some of these adverse effects. Additionally, because of the observational design of the studies that have been published, causality cannot be definitively established. However, it is very unlikely that prospective, randomized controlled trials of PPIs to verify the results of observational studies will ever be initiated. PPIs are often inappropriately prescribed and these potential adverse effects are of particular concern in the older adult population. A larger body of evidence is needed to confirm that associations between PPI use and CAP, vitamin B12 deficiency, kidney disease/injury, and dementia exist. A next step could be to conduct research to fully elucidate which patients should be targeted for PPI discontinuation and the best method to deprescribe PPIs. For now, given the potential for these risks, older adults should be periodically evaluated for the need for continued use of PPI therapy, and discontinuation or step-down to an H2RA or other treatments should be considered when appropriate.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Marina L. Maes, Department of Clinical Pharmacy, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO, USA Dr. Maes was a 4th year student at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences.
Danielle R. Fixen, Department of Clinical Pharmacy, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO, USA Dr. Maes was a 4th year student at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences.
Sunny Anne Linnebur, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, 12850 E. Montview Blvd (C238), Aurora, CO 80045, USA.
References
- 1. Rotman S, Bishoop T. Proton pump inhibitor use in the US ambulatory setting, 2002–2009. PLoS One 2013; 8: e56060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallerstedt S, Fastbom J, Linke J, et al. Long-term use of proton pump inhibitors and prevalence of disease-related and drug-related reasons for gastroprotection – a population-based study. Pharmacoepidemiol Drug Saf 2017; 26: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Food and Drug Administration. FDA drug safety communication: Possible increased risk of fractures of hip, wrist, and spine with the use of proton-pump inhibitors, http://www.fda.gov/Drugs/DrugSafety/ucm213206.htm (2010, accessed 17 October 2016).
- 4. Food and Drug Administration. FDA drug safety communication: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton-pump inhibitors (PPIs), http://www.fda.gov/Drugs/DrugSafety/ucm290510.htm (2012, accessed 17 October 2016).
- 5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63: 2227–2246. [DOI] [PubMed] [Google Scholar]
- 6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnell O, Kanis J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17: 1726. [DOI] [PubMed] [Google Scholar]
- 8. Kanis J, Johnell O, Oden A, et al. Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 2000; 11: 669–674. [DOI] [PubMed] [Google Scholar]
- 9. Abrahamsen B, van Staa T, Ariely M, et al. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009; 20: 1633. [DOI] [PubMed] [Google Scholar]
- 10. Chiu H, Huang Y, Chang C, et al. Use of proton pump inhibitors increased the risk of hip fracture: a population-based case-control study. Pharmacoepidemiol Drug Saf 2010; 19: 1131–1136. [DOI] [PubMed] [Google Scholar]
- 11. Eom C, Park S, Myung S, et al. Use of acid-suppressive drugs and risk of fracture: a meta-analysis of observational studies. Ann Fam Med 2011; 9: 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ngamruengphong S, Leontiadis G, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol 2011; 106: 1209–1218. [DOI] [PubMed] [Google Scholar]
- 13. Pouwels S, Lalmohamed A, Souverein P, et al. Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study. Osteoporos Int 2011; 22: 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye X, Liu H, Wu C, et al. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2011; 23: 794–800. [DOI] [PubMed] [Google Scholar]
- 15. Khalili H, Huang E, Jacobson B, et al. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ 2012; 344: e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu E, Bauer S, Bain P, et al. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med 2011; 124: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cea-Soriano L, Johansson S, Garcia Rodriguez L. Risk factors for falls with use of acid-suppressive drugs. Epidemiology 2013; 24: 600–607. [DOI] [PubMed] [Google Scholar]
- 18. Fraser L-A, Leslie W, Targownik L, et al. ; CaMos Research Group. The effect of proton pump inhibitors on fracture risk: report from the Canadian Multicenter Osteoporosis Study. Osteoporos Int 2013; 24: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee J, Youn K, Choi N, et al. A population-based case-control study: proton pump inhibition and risk of hip fracture by use of bisphosphonate. J Gastroenterol 2013; 48: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 20. Adams A, Black M, Zhang J, et al. Proton-pump inhibitor use and hip fractures in men: a population-based case-control study. Ann Epidemiol 2014; 24: 286–290. [DOI] [PubMed] [Google Scholar]
- 21. Ding J, Heller D, Ahern F, et al. The relationship between proton pump inhibitor adherence and fracture risk in the elderly. Calcif Tissue Int 2014; 94: 597–607. [DOI] [PubMed] [Google Scholar]
- 22. Lewis J, Barre D, Zhu K, et al. Long-term proton pump inhibitor therapy and falls and fractures in elderly women: a prospective cohort study. J Bone Miner Res 2014; 29: 2489–2497. [DOI] [PubMed] [Google Scholar]
- 23. Cunningham R, Dale B, Undy B, et al. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect 2003; 54: 243–245. [DOI] [PubMed] [Google Scholar]
- 24. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ 2004; 171: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dial S, Delaney J, Barkun A, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005; 294: 2989–2995. [DOI] [PubMed] [Google Scholar]
- 26. Lowe D, Mamdani M, Kopp A, et al. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis 2006; 43: 1272–1276. [DOI] [PubMed] [Google Scholar]
- 27. Aseeri M, Schroeder T, Kramer J, et al. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol 2008; 103: 2308–2313. [DOI] [PubMed] [Google Scholar]
- 28. Linsky A, Gupta K, Lawler E, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile Infection. Arch Intern Med 2010; 170: 772–778. [DOI] [PubMed] [Google Scholar]
- 29. Deshpande A, Pant C, Pasupuleti V, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 225–233. [DOI] [PubMed] [Google Scholar]
- 30. Janarthanan S, Ditah I, Phil M, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012; 107: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 31. Kwok C, Arthur A, Anibueze C, et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol 2012; 107: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 32. Tleyjeh I, Bin Abdhulhak A, Muhammed R, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection: a contemporary systematic review and meta-analysis. PLoS ONE 2012; 7: e50836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Freedberg D, Salmasian J, Friedman C, et al. Proton Pump inhibitors and risk for recurrent Clostridium difficile infection among inpatients. Am J Gastroenterol 2013; 108: 1794–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McDonald E, Milligan J, Frenette C, et al. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridcium difficile infection. JAMA 2015; 175: 784–791. [DOI] [PubMed] [Google Scholar]
- 35. Laheij R, Sturkenboom M, Hassing R-J, et al. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292: 1955–1960. [DOI] [PubMed] [Google Scholar]
- 36. Gulmez S, Holm A, Frederiksen H, et al. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167: 950–955. [DOI] [PubMed] [Google Scholar]
- 37. Sarkar M, Hennessy S, Yang Y. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149: 391–398. [DOI] [PubMed] [Google Scholar]
- 38. Dublin S, Walker R, Jackson M, et al. Use of proton pump inhibitors and H2 blockers and risk of pneumonia in older adults: a population-based case-control. Pharmacoepidemiol Drug Saf 2010; 19: 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010; 31: 1165–1177. [DOI] [PubMed] [Google Scholar]
- 40. Eom C, Jeon C, Lim J, et al. Use of acid-suppresive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ 2011; 183: 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meijvis S, Cornips M, Voorn G, et al. Microbial evaluation of proton-pump inhibitors and the risk of pneumonia. Eur Respir J 2011; 38: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 42. De Jager C, Wever P, Gemen E, et al. Proton pump inhibitor therapy predisposes to community-acquired Streptococcus pneumoniae pneumonia. Aliment Pharmacol Ther 2012; 36: 941–949. [DOI] [PubMed] [Google Scholar]
- 43. Hermos J, Young M, Fonda J, et al. Risk of community-acquired pneumonia in veteran patients to whom proton pump inhibitors were dispensed. CID 2012; 54: 33–42. [DOI] [PubMed] [Google Scholar]
- 44. Lambert A, Lam J, Paik J, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS ONE 2015; 10: e0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mitchell S, Rockwood K. The association between antiulcer medication and initiation of cobalamin initiation in older persons. J Clin Epidemiol 2001; 54: 531–534. [DOI] [PubMed] [Google Scholar]
- 46. Force R, Meeker A, Cady P, et al. Increased vitamin B12 requirement associated with chronic acid suppression therapy. Ann Pharmacother 2003; 37: 490–493. [DOI] [PubMed] [Google Scholar]
- 47. Valuck R, Ruscin J. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol 2004; 57: 422–428. [DOI] [PubMed] [Google Scholar]
- 48. Cotter P, O’Keeffe S. Use of proton pump inhibitors is not associated with vitamin B12 deficiency in older hospital patients: a case control study. Eur Geriatr Med 2011; 2: 253–255. [Google Scholar]
- 49. Lam J, Schneider J, Zhao W, et al. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310: 2435–2442. [DOI] [PubMed] [Google Scholar]
- 50. Jung S, Nagaraja V, Kapur A, et al. Association between vitamin B12 deficiency and long-term use of acid-lowering agents: a systematic review and meta-analysis. Intern Med J 2015; 45: 409–416. [DOI] [PubMed] [Google Scholar]
- 51. Leonard C, Freeman C, Newcomb C, et al. Proton pump inhibitors and traditional nonsteroidal anti-inflammatory drugs and the risk of acute interstitial nephritis and acute kidney injury. Pharmacoepidemiol Drug Saf 2012; 21: 1155–1172. [DOI] [PubMed] [Google Scholar]
- 52. Antoniou T, Macdonald E, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: A population-based cohort study. CMAJ 2015; 3: E166–E171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lazarus B, Chen Y, Wilson F, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xie Y, Bowe B, Li T, et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol 2016; 27: 3153–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 2015; 265: 419–428. [DOI] [PubMed] [Google Scholar]
- 56. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73: 410–416. [DOI] [PubMed] [Google Scholar]
- 57. Abrahamsen B, Vestergaard P. Proton pump inhibitor use and fracture risk - effect modification by histamine H1 receptor blockade. Observational case-control study using National Prescription Data. Bone 2013; 57: 269–271. [DOI] [PubMed] [Google Scholar]
- 58. Targownik L, Lix L, Leung S, et al. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterol 2010; 138: 896–904. [DOI] [PubMed] [Google Scholar]
- 59. Targownik L, Leslie W, Davison K, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos). Am J Gastroenterol 2012; 107: 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maggio M, Lauretani F, Ceda G, et al. Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone 2013; 57: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. O’Connell M, Madden D, Murray A, et al. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005; 118: 778–781. [DOI] [PubMed] [Google Scholar]
- 62. DePestel D, Aronoff D. Epidemiology of Clostridium difficile infection. J Pharm Pract 2013; 26: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dalton B, Lye-Maccannell T, Henderson E, et al. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment Pharmacol Ther 2009; 29: 626–634. [DOI] [PubMed] [Google Scholar]
- 64. Lewis P, Litchfield J, Tharp J. Risk and severity of hospital-acquired Clostridium difficile infection in patients taking proton pump inhibitors. Pharmacotherapy 2016; 36: 986–993. [DOI] [PubMed] [Google Scholar]
- 65. National Center for Health Statistics. Health, United States, 2015: with special feature on racial and ethnic health disparities, https://www.cdc.gov/nchs/fastats/older-american-health.htm (2016, accessed 18 December 2016). [PubMed]
- 66. Stupka J, Mortensen E, Anzueto A, et al. Community-acquired pneumonia in elderly patients. Aging Health 2009; 5: 763–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Herbert V. Vitamin B12 in present knowledge in nutrition. 17th ed. Washington, DC: International Life Sciences Institute Press, 1996. [Google Scholar]
- 68. Howden C. Vitamin B12 levels during prolonged treatment with proton pump inhibitors. J Clin Gastroenterol 2000; 3: 29–33. [DOI] [PubMed] [Google Scholar]
- 69. Wong C. Vitamin B12 deficiency in the elderly: Is it worth screening? Hong Kong J Med 2015; 21: 155–164. [DOI] [PubMed] [Google Scholar]
- 70. Wolters M, Ströhle A, Hahn A. Cobalamin: a critical vitamin in the elderly. Prev Med 2004; 39: 1256–1266. [DOI] [PubMed] [Google Scholar]
- 71. KDIGO. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; 3: 5–14. [DOI] [PubMed] [Google Scholar]
- 72. National Institute of Diabetes and Digestive and Kidney Diseases. Kidney disease statistics for the United States, https://www.niddk.nih.gov/health-information/health-statistics/Pages/kidney-disease-statistics-united-states.aspx#1 (2012, accessed 17 October 2016).
- 73. Abdel-Kader K, Palevsky P. Acute kidney injury in the elderly. Clin Geriatr Med 2009; 25: 331–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hsu C, McCulloch C, Fan D, et al. Community-based incidence of acute renal failure. Kidney Int 2007; 72: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Geevasinga N, Coleman P, Webster A, et al. Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol 2006; 4: 597–604. [DOI] [PubMed] [Google Scholar]
- 76. Simpson I, Marshall M, Pilmore H, et al. Proton pump inhibitors and acute interstitial nephritis: report and analysis of 15 cases. Nephrology 2006; 11: 381–385. [DOI] [PubMed] [Google Scholar]
- 77. Berney-Meyer L, Hung N, Slatter T, et al. Omeprazole-induced acute interstitial nephritis: a possible Th1-Th17-mediated injury? Nephrology 2014; 19: 359–365. [DOI] [PubMed] [Google Scholar]
- 78. Klepser D, Collier D, Cochran G. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol 2013; 14: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Blank M, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton-pump inhibitor use. Kidney Int 2014; 86: 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Arora P, Gupta A, Golzy M, et al. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol 2016; 17: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Moledina D, Perazella M. PPIs and kidney disease: from AIN to CKD. J Nephrol 2016; 29: 611–616. [DOI] [PubMed] [Google Scholar]
- 82. Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and meta-analysis. Alzheimers Dement 2013; 9: 63–75. [DOI] [PubMed] [Google Scholar]
- 83. Alzheimer’s Association. 2016. Alzheimer’s disease facts and figures. Alzheimers Dement 2016; 12(4). https://www.alz.org/documents_custom/2016-facts-and-figures.pdf [DOI] [PubMed] [Google Scholar]
- 84. Fallahzadeh M, Borhani Haghighi A, Namazi M. Proton pump inhibitors: predisposers to Alzheimer disease? J Clin Pharm Ther 2010; 35: 125–126. [DOI] [PubMed] [Google Scholar]
- 85. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One 2013; 8: e58837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Younkin S. The role of Aβ42 in Alzheimer’s disease. J Physiol (Paris) 1998; 92: 289–292. [DOI] [PubMed] [Google Scholar]