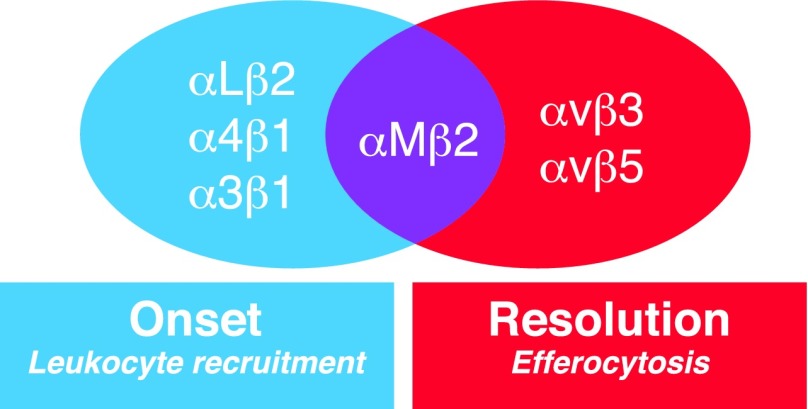
Review of integrins in leukocyte recruitment and resolution of acute inflammatory response.
Keywords: Del-1, efferocytosis, adhesion, neutrophils
Abstract
Integrins constitute a large group of adhesion receptors that are formed as heterodimers of α and β subunits. Their presence and activation status on the surface of leukocytes modulate a broad spectrum of processes in inflammation and immunity. This mini review critically outlines research advances with regard to the function of leukocyte integrins in regulating and integrating the onset and resolution of acute inflammation. Specifically, we summarize and discuss relevant, current literature that supports the multifunctional role of integrins and their partners. The latter include molecules that physically associate with integrins or regulate their activity in the context of the following: 1) leukocyte recruitment to an inflamed tissue, 2) recognition and phagocytosis of apoptotic neutrophils (efferocytosis), and 3) egress of efferocytic macrophages from the inflamed site to lymphoid tissues. The understanding of the fine-tuning mechanisms of the aforementioned processes by integrins and their functional partners may enable the design of therapeutic tools to counteract destructive inflammation and promote more efficient resolution of inflammation.
Introduction
Acute inflammation, comprising initial recruitment of leukocytes—especially neutrophils—to the inflamed site, followed by termination of the inflammatory process through the clearance of apoptotic inflammatory cells, is a tightly regulated process [1, 2]. An optimal fine-tuning of initiation and resolution of acute inflammation ensures, on the one hand, the proper response to inflammatory or infectious agents and on the other hand, the preservation of tissue function and integrity. Insufficient leukocyte recruitment may lead to uncontrolled infections and in extreme situations—as in leukocyte adhesion deficiency—may lead to severe immunopathology [3]. On the other hand, impaired resolution of acute inflammation results in perpetuation of inflammation and transition to a chronic inflammatory status [4].
Integrins are cell surface heterodimeric receptors that regulate cell-to-cell and cell-to-extracellular matrix interactions. The integrin family comprises 24 members in mammals, formed by the combination of 18 α and 8 β subunits [5–7]. They play a critical role in a wide range of important immune processes, including but not limited to leukocyte trafficking, phagocytosis of pathogens, innate immune signaling, and the formation and function of the immune synapse [7, 8]. Herein, we review studies on the involvement of integrins in the acute inflammatory process, focusing on the role of these receptors in both the initial recruitment of leukocytes into the sites of inflammation and the clearance of apoptotic cell material in the context of resolution of inflammation (Figs. 1 and 2).
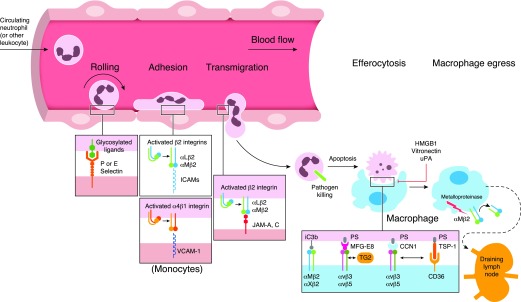
Figure 1. The contribution of integrins to the shaping of several steps of acute inflammatory responses, including leukocyte recruitment or phagocytosis of apoptotic neutrophils.
Figure 2. Integrin-dependent mechanisms drive the onset and resolution of acute inflammation.
Interaction between selectins and their glycosylated ligands promotes leukocyte rolling to endothelium. As a next step, leukocyte adhesion is mediated by the interaction among αMβ2, αLβ2, and α4β1 integrins with their counter-receptors (ICAMs, VCAM-1). The binding of β2 integrins to ICAMs or JAMs regulates transmigration that occurs mainly paracellularly. Integrins αvβ3/5 play a major role in the clearance of apoptotic neutrophils by phagocytes (efferocytosis). TSP-1, CCN1, and MFG-E8 act as bridging molecules that regulate recognition of PS that is exposed on apoptotic cells. Negative regulators of αvβ3-dependent efferocytosis include HMGB1, Vitronectin, and uPA. The β2 integrins αMβ2 and αXβ2 promote efferocytosis of apoptotic cells coated with the complement activation product iC3b. In the course of inflammation resolution, β2 integrins are also involved in macrophage egress to draining lymph nodes.
INTEGRINS CONTROL LEUKOCYTE RECRUITMENT DURING THE INITIATION PHASE OF INFLAMMATION
Upon initiation of infection or inflammation, neutrophils are the first cells recruited to the afflicted site, followed by other inflammatory cell populations. The whole process in regulating inflammatory cell recruitment is highly complex and designated as the leukocyte adhesion cascade [5, 9] (Fig. 2). This cascade consists of sequential adhesive interaction events between inflamed endothelial cells and leukocytes that usually take place in postcapillary venules. The first step of leukocyte adhesion cascade depends on different selectins [10] and their glycosylated ligands, such as P-selectin glycoprotein ligand 1 [11–13]; these interactions mediate rolling of leukocytes and may trigger activation of signaling in leukocytes [13]. Additionally, rolling enables the interaction of leukocytes with chemokines exposed on the luminal surface of endothelial cells [14]. Chemokine- and selectin-driven signaling in leukocytes cooperatively induces activation of integrins [15]. In particular, inside-out signaling induced by chemokines [14] or selectin-dependent activation of Src family kinases, spleen tyrosine kinase, and ITAM domain-containing adaptor proteins [13, 16] leads to enhanced activity of leukocyte integrins, which includes a shift to a high-affinity conformation for effective binding of their ligands on the endothelial cell surface [17].
The recognition by activated integrins of their endothelial ligands (counter-receptors) allows the next steps of the cascade to take place, particularly leukocyte slow rolling, firm adhesion, and postadhesion strengthening [5, 18]. Following leukocyte arrest, the binding of integrin heterodimers to the endothelial cell surface results in their clustering, which may support adhesion strengthening, even under shear flow conditions [19]. The most prominent members of the integrin family that mediate leukocyte-endothelial interactions are the β2 integrins LFA-1 (αLβ2; CD11a/CD18), Mac-1 (αMβ2; CD11b/CD18), and the β1 integrin VLA-4 (α4β1; CD49d/CD29) [5] (Fig. 1). LFA-1 binding to endothelial ICAM-1 [18] is involved in neutrophil adhesion to the vascular endothelium, whereas the VLA-4 binding to VCAM-1 is important for monocyte endothelial adhesion [7, 18]. Interestingly, the LFA-1/ICAM-1 interaction is inhibited by an endothelial cell-secreted protein, Del-1 [7, 20, 21]. Of note, this molecule functions as an endogenous inhibitor of leukocyte adhesion; its absence or down-regulation has been associated with dysregulated inflammatory responses in several disease models [20, 22–24], whereas enhanced endothelial Del-1 expression can ameliorate acute inflammatory responses [25]. Besides Del-1, other endogenous modulators of leukocyte recruitment have been implicated in this process. Deficiency in GDF-15, a molecule that belongs to the TGF-β superfamily, leads to increased neutrophil recruitment in the infarcted myocardium of mice [7, 26]. Mechanistically, GDF-15 was shown to inhibit chemokine-induced β2 integrin activation by regulating the activity of the GTPases cell division control protein 42 homolog and Ras-related protein 1 [26]. Annexin A1 has also been demonstrated to act as an endogenous anti-inflammatory molecule [7]. The interaction of this protein with formyl peptide receptor 2 resulted in blockade of leukocyte recruitment during atherosclerotic lesion formation [27].
Integrin binding to their endothelial ligands induces outside-in signaling that further promotes the strengthening of adhesion [5]. Subsequently, leukocytes move slowly (a process termed crawling or locomotion) on the endothelial cell surface while searching for an appropriate site for extravasation. Leukocyte crawling is mediated by the β2 integrins and predominantly Mac-1 [28, 29]. Indeed, Mac-1-deficient neutrophils showed little, if any, locomotion on endothelial cells [28]. Moreover, the blocking of LFA-1 inhibited the patrolling behavior of monocytes and resulted in their release from the vessel wall [30]. Locomotion is thought to enable leukocytes to identify the proper site for TEM, which mainly takes place at interendothelial junctions (“paracellular TEM”), whereas transcellular migration of leukocytes through the endothelial cell body has been identified as a rather rare event [31–33]. LFA-1 and Mac-1 interactions with ICAM-1, ICAM-2, and the JAM family members JAM-A and JAM-C are critically involved in TEM [34–37] (Fig. 2). In addition, PECAM-1 (CD31) and CD99 contribute to leukocyte TEM through interendothelial junctions [38, 39]. Moreover, the leukocyte paracellular migration is regulated by vascular endothelial-cadherin-dependent contacts among endothelial cells [40].
After crossing the endothelial barrier, leukocytes migrate through the basal membrane [41] and interact with vascular pericytes. Specifically, the interaction of integrins LFA-1 and Mac-1 with ICAM-1 enables neutrophil crawling onto pericytes in the context of extravasation through the venular wall [42]. Finally, α3β1 (CD49c/CD29) integrin and its interacting ligands were recently shown to promote neutrophil extravasation in sepsis [43, 44].
THE ROLE OF INTEGRINS IN THE REGULATION OF RESOLUTION OF INFLAMMATION
The ideal outcome of an acute inflammatory response is its timely termination, mediated by a process termed resolution of inflammation, which promotes restoration of tissue homeostasis and prevents progression toward an uncontrolled chronic inflammatory state [45, 46]. Resolution of acute neutrophilic inflammation is an active process that is coordinated by the interplay of multiple mechanisms [47, 48]. These include inhibition of neutrophil recruitment, promotion of neutrophil apoptosis, and clearance of apoptotic neutrophils by macrophages, as well as egress of macrophages from the inflamed site to the lymphatics [46, 48]. Key molecules involved in these homeostatic processes are endogenously produced proresolving lipid mediators [45, 46], as well as integrins, the importance of which will be discussed below.
Efferocytosis
The engulfment of apoptotic neutrophils by macrophages is termed efferocytosis and constitutes a hallmark in the resolution of inflammation [49]. As will become evident below, efferocytosis serves more than mediating waste disposal (removal of apoptotic cell material). A plethora of interactions between apoptotic neutrophils and phagocytic receptors expressed by tissue macrophages facilitate the phagocytic uptake and clearance of apoptotic material. The sum of all these interactions contributes to the formation of the so-called phagocytic or engulfment synapse [49, 50]. PS represents a major “eat-me” signal that is exposed on the outer membrane surface of apoptotic neutrophils [51] and interacts with different groups of phagocytic receptors, either directly or indirectly, with the help of opsonins acting as bridging molecules [50] (Fig. 2). Efferocytosis induces transcriptomic changes in macrophages, thus reprogramming them toward a resolving phenotype [46]. The resulting resolving macrophage releases immunomodulatory molecules, such as TGF-β. Moreover, the process of efferocytosis activates the transcription factors liver X receptors, peroxisome proliferator-activated receptors, and retinoid X receptors, which in turn, further promote efferocytosis and the emergence of the resolving phenotype by up-regulating the expression of efferocytic receptors, relevant bridging molecules, and other proresolution molecules [52].
Besides their important role in leukocyte recruitment, integrins also contribute to the engulfment process in efferocytosis [53] (Fig. 1). Specifically, integrins αvβ3 and αvβ5 that are present in macrophages and dendritic cells participate in the clearance of apoptotic cells [49] (Figs. 1 and 2). These integrins interact indirectly with PS via bridging molecules. Such integrin-interacting bridging molecules include the “find-me” signal TSP-1, which mediates PS binding to macrophages in a manner that is dependent on the cooperation of the αvβ3 integrin with the scavenger receptor CD36 [54]. Furthermore, the bridging molecule MFG-E8 (also designated lactadherin) has been shown to promote efferocytosis via simultaneous binding of its Arg-Gly-Asp motif to the αvβ3 integrin and of its discoidin--like domains to PS [55]. Importantly, deficiency of MFG-E8 in mice resulted in defective apoptotic cell clearance linked with development of autoimmune manifestations [56]. In the same context, TG2 has been described as a coreceptor for the αvβ3 integrin that forms a complex with MFG-E8 and PS on apoptotic cells, thereby facilitating apoptotic cell recognition [57].
Further molecules have also been shown to modulate αvβ3-dependent efferocytosis. Along this line, administration of CCN1 (also designated cysteine-rich protein 61), a protein associated with extracellular matrix, enhances neutrophil efferocytosis in the setting of wound healing. A mutated version of CCN1 that cannot interact with αvβ3 has been associated with defective efferocytosis [58]. On the other hand, the chromatin-associated protein HMGB1, which is secreted extracellularly by damaged necrotic cells and functions as a proinflammatory danger signal [59], has been reported to interfere with αvβ3-, but not αvβ5-dependent efferocytosis [60]. In addition, ligation of the αvβ3 integrin by vitronectin results in decreased clearance of apoptotic neutrophils, thus impairing resolution of acute inflammation [61]. Vitronectin has also been implicated in the inhibitory role that uPA exerts on αvβ3-dependent efferocytosis [62].
Besides the direct participation of integrins in apoptotic cell recognition mediated by the interaction of integrins with bridging molecules, an active crosstalk of integrin-dependent functions with other efferocytic receptor pathways has been demonstrated in the context of inflammation resolution. For instance, the PS receptor T cell Ig mucin protein 4 cooperates with integrin-dependent efferocytosis by inducing integrin activation and stimulation of Src-family kinases and FAK [63]. In addition, the efferocytosis pathway mediated by the interaction of PS with MerTK via the opsonin growth arrest-specific factor-6 has been implicated in the modulation of integrin-dependent efferocytosis. Specifically, MerTK activation was associated with αvβ5 integrin-dependent efferocytic activity that was mediated by a pathway that involved activation of Src, FAK, and Rac1 GTPase and the subsequent facilitation of apoptotic cell internalization [64].
Integrin-dependent efferocytosis is negatively regulated by CD47 (integrin-associated protein) that functions as a “don’t eat-me” signal that is present in viable and nonapoptotic cells. CD47 interacts with αvβ3 and the bridging molecule TSP-1 [65]. During apoptosis, CD47 diffuses away from the lipid rafts, where it is normally clustered, and this results in impaired interactions with the inhibitory receptor signal regulatory protein α, thereby promoting effective phagocytosis of apoptotic cells [66, 67]. Except from its established role in mediating TEM in the course of the leukocyte recruitment cascade, CD31 has been implicated in the engulfment of apoptotic cells [68, 69]. This adhesion molecule can act as a don’t eat-me signal by repulsing live cells from their ingestion by phagocytes, whereas it facilitates the removal of apoptotic cells in an integrin-dependent manner [68].
β2 integrins can also act as efferocytic receptors to promote inflammation resolution. The opsonin iC3b, which is generated as a result of complement activation, binds to apoptotic cells [70]. This type of opsonization, in turn, allows their efficient recognition and uptake by the integrins Mac-1 and αΧβ2 (CD11c/CD18), thereby driving phagocytes toward an anti-inflammatory phenotype [71, 72]. ICAM-3 (CD50), a counter-receptor for β2 integrins, has been described as an efferocytic molecule [73]. Indeed, during apoptotic cell clearance, ICAM-3 acts as an eat-me signal and promotes the recognition of apoptotic cells by binding to CD14 rather than to integrins [74].
Macrophage egress
Apart from their crucial role in the regulation of efferocytic activity, integrins are also involved in other aspects of resolution of inflammation, such as macrophage egress to lymph nodes [48]. Upon clearance of apoptotic material at the site of inflammation, macrophages migrate to local lymphoid tissues [48]. Of note, the resolving phenotype of peritoneal macrophages has been associated with low levels of Mac-1 (αMβ2) integrin in a mouse model of self-limited inflammation. This specific macrophage population displayed increased egress to draining lymphatics, where, presumably, it might convey proresolution signals to adaptive immune cells [75]. Consistent with these findings, an independent study demonstrated that proteolytic shedding of the same β2 integrin promotes macrophage efflux from the inflamed site to regional lymph nodes [76] (Fig. 2). Additionally, the levels of the soluble β2 integrin, which retains ligand-binding activity and might act as soluble antagonist, were increased in the peritoneal cavity of mice during the resolution phase of inflammation [76].
Integrin regulation by inflammation-associated lipid mediators
In recent years, several lipid mediators have been increasingly appreciated as potent regulators of both the initiation and resolution of inflammatory processes. Bioactive lipids are derived from polyunsaturated fatty acids and contribute to augmentation of inflammatory responses (e.g., PGs, LTs). However, a “lipid-mediator class-switch” takes place during the resolving phase of inflammation, leading to the generation of SPMs, such as resolvins or LXs, that actively terminate neutrophil recruitment and promote neutrophil apoptosis and efferocytosis at the inflamed site [45, 77]. Additionally, the endogenous regulator Annexin A1 blocks neutrophil infiltration and promotes monocyte recruitment to the inflamed site, thus contributing to resolution of inflammation. Consistently, deficiency in Annexin A1 has been associated with sustained inflammatory responses [7, 78–80].
LTB4 is an endogenous chemotactic factor that regulates leukocyte adhesion and TEM [81–84]. Findings based on a broad set of experimental settings have underlined the involvement of LTB4 in the regulation of integrin-mediated responses in the context of inflammation. Along this line, LTB4 induced firm leukocyte adhesion [82] and increased β2 integrin avidity and affinity [85, 86]. LXs, deriving from arachidonic acid, display potent anti-inflammatory actions, such as blockade of leukocyte TEM and inhibition of chemotactic activity [87, 88]. This family of lipid mediators regulates integrin-related anti-inflammatory and proresolving functions [89, 90]. In particular, LXA4 affects leukocyte β2 integrin expression. In addition, integrin-dependent neutrophil–endothelial interactions are negatively regulated by LXA4 and LXB4 [89, 90]. Notably, LXA4 enhances efferocytic activity that is dependent on β3 and β2 integrins [91]. The impact of SPM on integrin activity is further supported by the finding that treatment of isolated neutrophils and monocytes or human whole blood with resolvins, which are derivatives from eicosapentaenoic acid and docosahexaenoic acid, was associated with decreased levels of β2 integrins [92–94]. In a similar fashion, administration of resolvins in a mouse model of peritonitis resulted in decreased β2 integrin levels in peritoneal macrophages [75]. Krishnamoorthy et al. [95] also demonstrated that resolvin D1 attenuates levels of integrin Mac-1 in human neutrophils that were treated with LTB4. The same resolvin counteracted the IL-17-mediated down-regulation of the endogenous inhibitor of leukocyte adhesion, Del-1 [96].
CONCLUDING REMARKS
As discussed in this review, integrins control multiple aspects and processes in the course of inflammation and its resolution, including the initial phase of leukocyte recruitment to the site of inflammation, the phagocytosis of apoptotic neutrophils, as well as macrophage egress to the lymph nodes. The roles of integrins and integrin-interacting partners in the modulation of the aforementioned processes are extremely complex in both spatial and temporal context, but recent advances have been quite promising. Better understanding of the mechanisms governing the onset and resolution of inflammation may contribute to the development of novel therapeutic concepts for targeting integrin signaling in inflammation-associated pathologies.
ACKNOWLEDGMENTS
This work is supported by grants from the U.S. National Institutes of Health (DE026152 to G.H. and T.C. and DE024153 to G.H.). T.C. was also supported by the European Community’s Seventh Framework Programme under Grant Agreement No. 602699 (DIREKT), Deutsche Forschungsgemeinschaft (SFB-TRR 127 Project A3 and CH279/6-2), and European Research Council (DEMETINL). I.K. was supported by the MeDDrive grant (60.400) from the Technische Universität Dresden Medical Faculty.
Glossary
- CCN1
CCN family member 1
- Del-1
developmental endothelial locus 1
- FAK
focal adhesion kinase
- GDF-15
growth differentiation factor 15
- HMGB1
high mobility group box 1
- iC3b
inactivated C3b
- JAM
junctional adhesion molecule
- LTB4
leukotriene B4
- LX
lipoxin
- Mac-1
macrophage-1 antigen
- MerTK
Mer tyrosine kinase
- MFG-E8
milk fat globule epidermal growth factor 8
- PS
phosphatidylserine
- SPM
specialized proresolving lipid mediator
- TEM
transendothelial migration
- TG2
transglutaminase 2
- TSP-1
thrombospondin 1
- uPA
urokinase-type plasminogen activator
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Borregaard N. (2010) Neutrophils, from marrow to microbes. Immunity 33, 657–670. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy A. D., DeLeo F. R. (2009) Neutrophil apoptosis and the resolution of infection. Immunol. Res. 43, 25–61. [DOI] [PubMed] [Google Scholar]
- 3.Moutsopoulos N. M., Konkel J., Sarmadi M., Eskan M. A., Wild T., Dutzan N., Abusleme L., Zenobia C., Hosur K. B., Abe T., Uzel G., Chen W., Chavakis T., Holland S. M., Hajishengallis G. (2014) Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci. Transl. Med. 6, 229ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugimoto M. A., Sousa L. P., Pinho V., Perretti M., Teixeira M. M. (2016) Resolution of Inflammation: what controls its onset? Front. Immunol. 7, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689. [DOI] [PubMed] [Google Scholar]
- 6.Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687. [DOI] [PubMed] [Google Scholar]
- 7.Mitroulis I., Alexaki V. I., Kourtzelis I., Ziogas A., Hajishengallis G., Chavakis T. (2015) Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol. Ther. 147, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans R., Patzak I., Svensson L., De Filippo K., Jones K., McDowall A., Hogg N. (2009) Integrins in immunity. J. Cell Sci. 122, 215–225. [DOI] [PubMed] [Google Scholar]
- 9.Nourshargh S., Alon R. (2014) Leukocyte migration into inflamed tissues. Immunity 41, 694–707. [DOI] [PubMed] [Google Scholar]
- 10.Kuwano Y., Spelten O., Zhang H., Ley K., Zarbock A. (2010) Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood 116, 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperandio M., Smith M. L., Forlow S. B., Olson T. S., Xia L., McEver R. P., Ley K. (2003) P-Selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J. Exp. Med. 197, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hidalgo A., Peired A. J., Wild M. K., Vestweber D., Frenette P. S. (2007) Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity 26, 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarbock A., Ley K., McEver R. P., Hidalgo A. (2011) Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118, 6743–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marki A., Esko J. D., Pries A. R., Ley K. (2015) Role of the endothelial surface layer in neutrophil recruitment. J. Leukoc. Biol. 98, 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mócsai A., Walzog B., Lowell C. A. (2015) Intracellular signalling during neutrophil recruitment. Cardiovasc. Res. 107, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadtmann A., Germena G., Block H., Boras M., Rossaint J., Sundd P., Lefort C., Fisher C. I., Buscher K., Gelschefarth B., Urzainqui A., Gerke V., Ley K., Zarbock A. (2013) The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. J. Exp. Med. 210, 2171–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alon R., Dustin M. L. (2007) Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity 26, 17–27. [DOI] [PubMed] [Google Scholar]
- 18.Herter J., Zarbock A. (2013) Integrin regulation during leukocyte recruitment. J. Immunol. 190, 4451–4457. [DOI] [PubMed] [Google Scholar]
- 19.Ortega-Gomez A., Salvermoser M., Rossaint J., Pick R., Brauner J., Lemnitzer P., Tilgner J., de Jong R. J., Megens R. T. A., Jamasbi J., Döring Y., Pham C. T., Scheiermann C., Siess W., Drechsler M., Weber C., Grommes J., Zarbock A., Walzog B., Soehnlein O. (2016) Cathepsin G controls arterial but not venular myeloid cell recruitment. Circulation 134, 1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi E. Y., Chavakis E., Czabanka M. A., Langer H. F., Fraemohs L., Economopoulou M., Kundu R. K., Orlandi A., Zheng Y. Y., Prieto D. A., Ballantyne C. M., Constant S. L., Aird W. C., Papayannopoulou T., Gahmberg C. G., Udey M. C., Vajkoczy P., Quertermous T., Dimmeler S., Weber C., Chavakis T. (2008) Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science 322, 1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajishengallis G., Chavakis T. (2013) Endogenous modulators of inflammatory cell recruitment. Trends Immunol. 34, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskan M. A., Jotwani R., Abe T., Chmelar J., Lim J.-H., Liang S., Ciero P. A., Krauss J. L., Li F., Rauner M., Hofbauer L. C., Choi E. Y., Chung K.-J., Hashim A., Curtis M. A., Chavakis T., Hajishengallis G. (2012) The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 13, 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi E. Y., Lim J.-H., Neuwirth A., Economopoulou M., Chatzigeorgiou A., Chung K.-J., Bittner S., Lee S.-H., Langer H., Samus M., Kim H., Cho G.-S., Ziemssen T., Bdeir K., Chavakis E., Koh J.-Y., Boon L., Hosur K., Bornstein S. R., Meuth S. G., Hajishengallis G., Chavakis T. (2015) Developmental endothelial locus-1 is a homeostatic factor in the central nervous system limiting neuroinflammation and demyelination. Mol. Psychiatry 20, 880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin J., Maekawa T., Abe T., Hajishengallis E., Hosur K., Pyaram K., Mitroulis I., Chavakis T., Hajishengallis G. (2015) DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci. Transl. Med. 7, 307ra155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kourtzelis I., Kotlabova K., Lim J.-H., Mitroulis I., Ferreira A., Chen L.-S., Gercken B., Steffen A., Kemter E., Klotzsche-von Ameln A., Waskow C., Hosur K., Chatzigeorgiou A., Ludwig B., Wolf E., Hajishengallis G., Chavakis T. (2016) Developmental endothelial locus-1 modulates platelet-monocyte interactions and instant blood-mediated inflammatory reaction in islet transplantation. Thromb. Haemost. 115, 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kempf T., Zarbock A., Widera C., Butz S., Stadtmann A., Rossaint J., Bolomini-Vittori M., Korf-Klingebiel M., Napp L. C., Hansen B., Kanwischer A., Bavendiek U., Beutel G., Hapke M., Sauer M. G., Laudanna C., Hogg N., Vestweber D., Wollert K. C. (2011) GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med. 17, 581–588. [DOI] [PubMed] [Google Scholar]
- 27.Drechsler M., de Jong R., Rossaint J., Viola J. R., Leoni G., Wang J. M., Grommes J., Hinkel R., Kupatt C., Weber C., Döring Y., Zarbock A., Soehnlein O. (2015) Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ. Res. 116, 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillipson M., Heit B., Colarusso P., Liu L., Ballantyne C. M., Kubes P. (2006) Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 203, 2569–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenkel A. R., Mamdouh Z., Muller W. A. (2004) Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 5, 393–400. [DOI] [PubMed] [Google Scholar]
- 30.Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., Geissmann F. (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670. [DOI] [PubMed] [Google Scholar]
- 31.Muller W. A. (2016) Transendothelial migration: unifying principles from the endothelial perspective. Immunol. Rev. 273, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nourshargh S., Hordijk P. L., Sixt M. (2010) Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 11, 366–378. [DOI] [PubMed] [Google Scholar]
- 33.Woodfin A., Voisin M.-B., Beyrau M., Colom B., Caille D., Diapouli F.-M., Nash G. B., Chavakis T., Albelda S. M., Rainger G. E., Meda P., Imhof B. A., Nourshargh S. (2011) The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 12, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavakis T. (2012) Leucocyte recruitment in inflammation and novel endogenous negative regulators thereof. Eur. J. Clin. Invest. 42, 686–691. [DOI] [PubMed] [Google Scholar]
- 35.Huang M.-T., Larbi K. Y., Scheiermann C., Woodfin A., Gerwin N., Haskard D. O., Nourshargh S. (2006) ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood 107, 4721–4727. [DOI] [PubMed] [Google Scholar]
- 36.Shaw S. K., Ma S., Kim M. B., Rao R. M., Hartman C. U., Froio R. M., Yang L., Jones T., Liu Y., Nusrat A., Parkos C. A., Luscinskas F. W. (2004) Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J. Exp. Med. 200, 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostermann G., Weber K. S. C., Zernecke A., Schröder A., Weber C. (2002) JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 3, 151–158. [DOI] [PubMed] [Google Scholar]
- 38.Mamdouh Z., Chen X., Pierini L. M., Maxfield F. R., Muller W. A. (2003) Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature 421, 748–753. [DOI] [PubMed] [Google Scholar]
- 39.Schenkel A. R., Mamdouh Z., Chen X., Liebman R. M., Muller W. A. (2002) CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat. Immunol. 3, 143–150. [DOI] [PubMed] [Google Scholar]
- 40.Wessel F., Winderlich M., Holm M., Frye M., Rivera-Galdos R., Vockel M., Linnepe R., Ipe U., Stadtmann A., Zarbock A., Nottebaum A. F., Vestweber D. (2014) Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat. Immunol. 15, 223–230. [DOI] [PubMed] [Google Scholar]
- 41.Wang S., Voisin M.-B., Larbi K. Y., Dangerfield J., Scheiermann C., Tran M., Maxwell P. H., Sorokin L., Nourshargh S. (2006) Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J. Exp. Med. 203, 1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proebstl D., Voisin M.-B., Woodfin A., Whiteford J., D’Acquisto F., Jones G. E., Rowe D., Nourshargh S. (2012) Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J. Exp. Med. 209, 1219–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerman Y. V., Lim K., Hyun Y.-M., Falkner K. L., Yang H., Pietropaoli A. P., Sonnenberg A., Sarangi P. P., Kim M. (2014) Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin α3β1-dependent. Blood 124, 3515–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanian P., Mitroulis I., Hajishengallis G., Chavakis T. (2016) Regulation of tissue infiltration by neutrophils: role of integrin α3β1 and other factors. Curr. Opin. Hematol. 23, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortega-Gómez A., Perretti M., Soehnlein O. (2013) Resolution of inflammation: an integrated view. EMBO Mol. Med. 5, 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fullerton J. N., Gilroy D. W. (2016) Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 15, 551–567. [DOI] [PubMed] [Google Scholar]
- 48.Headland S. E., Norling L. V. (2015) The resolution of inflammation: principles and challenges. Semin. Immunol. 27, 149–160. [DOI] [PubMed] [Google Scholar]
- 49.Greenlee-Wacker M. C. (2016) Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 273, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravichandran K. S. (2010) Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J. Exp. Med. 207, 1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birge R. B., Boeltz S., Kumar S., Carlson J., Wanderley J., Calianese D., Barcinski M., Brekken R. A., Huang X., Hutchins J. T., Freimark B., Empig C., Mercer J., Schroit A. J., Schett G., Herrmann M. (2016) Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 23, 962–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.A-Gonzalez N., Hidalgo A. (2014) Nuclear receptors and clearance of apoptotic cells: stimulating the macrophage’s appetite. Front. Immunol. 5, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupuy A. G., Caron E. (2008) Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J. Cell Sci. 121, 1773–1783. [DOI] [PubMed] [Google Scholar]
- 54.Savill J., Hogg N., Ren Y., Haslett C. (1992) Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 90, 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanayama R., Tanaka M., Miwa K., Shinohara A., Iwamatsu A., Nagata S. (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187. [DOI] [PubMed] [Google Scholar]
- 56.Hanayama R., Tanaka M., Miyasaka K., Aozasa K., Koike M., Uchiyama Y., Nagata S. (2004) Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304, 1147–1150. [DOI] [PubMed] [Google Scholar]
- 57.Tóth B., Garabuczi E., Sarang Z., Vereb G., Vámosi G., Aeschlimann D., Blaskó B., Bécsi B., Erdõdi F., Lacy-Hulbert A., Zhang A., Falasca L., Birge R. B., Balajthy Z., Melino G., Fésüs L., Szondy Z. (2009) Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J. Immunol. 182, 2084–2092. [DOI] [PubMed] [Google Scholar]
- 58.Jun J.-I., Kim K.-H., Lau L. F. (2015) The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat. Commun. 6, 7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bianchi M. E. (2009) HMGB1 loves company. J. Leukoc. Biol. 86, 573–576. [DOI] [PubMed] [Google Scholar]
- 60.Friggeri A., Yang Y., Banerjee S., Park Y.-J., Liu G., Abraham E. (2010) HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am. J. Physiol. Cell Physiol. 299, C1267–C1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bae H.-B., Tadie J.-M., Jiang S., Park D. W., Bell C. P., Thompson L. C., Peterson C. B., Thannickal V. J., Abraham E., Zmijewski J. W. (2013) Vitronectin inhibits efferocytosis through interactions with apoptotic cells as well as with macrophages. J. Immunol. 190, 2273–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y., Friggeri A., Banerjee S., Bdeir K., Cines D. B., Liu G., Abraham E. (2010) Urokinase-type plasminogen activator inhibits efferocytosis of neutrophils. Am. J. Respir. Crit. Care Med. 182, 1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flannagan R. S., Canton J., Furuya W., Glogauer M., Grinstein S. (2014) The phosphatidylserine receptor TIM4 utilizes integrins as coreceptors to effect phagocytosis. Mol. Biol. Cell 25, 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y., Singh S., Georgescu M.-M., Birge R. B. (2005) A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J. Cell Sci. 118, 539–553. [DOI] [PubMed] [Google Scholar]
- 65.Soto-Pantoja D. R., Kaur S., Roberts D. D. (2015) CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit. Rev. Biochem. Mol. Biol. 50, 212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lv Z., Bian Z., Shi L., Niu S., Ha B., Tremblay A., Li L., Zhang X., Paluszynski J., Liu M., Zen K., Liu Y. (2015) Loss of cell surface CD47 clustering formation and binding avidity to SIRPα facilitate apoptotic cell clearance by macrophages. J. Immunol. 195, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barclay A. N., Van den Berg T. K. (2014) The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu. Rev. Immunol. 32, 25–50. [DOI] [PubMed] [Google Scholar]
- 68.Vernon-Wilson E. F., Auradé F., Tian L., Rowe I. C. M., Shipston M. J., Savill J., Brown S. B. (2007) CD31 delays phagocyte membrane repolarization to promote efficient binding of apoptotic cells. J. Leukoc. Biol. 82, 1278–1288. [DOI] [PubMed] [Google Scholar]
- 69.Brown S., Heinisch I., Ross E., Shaw K., Buckley C. D., Savill J. (2002) Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418, 200–203. [DOI] [PubMed] [Google Scholar]
- 70.Erwig L.-P., Henson P. M. (2008) Clearance of apoptotic cells by phagocytes. Cell Death Differ. 15, 243–250. [DOI] [PubMed] [Google Scholar]
- 71.Amarilyo G., Verbovetski I., Atallah M., Grau A., Wiser G., Gil O., Ben-Neriah Y., Mevorach D. (2010) iC3b-opsonized apoptotic cells mediate a distinct anti-inflammatory response and transcriptional NF-kappaB-dependent blockade. Eur. J. Immunol. 40, 699–709. [DOI] [PubMed] [Google Scholar]
- 72.Mevorach D., Mascarenhas J. O., Gershov D., Elkon K. B. (1998) Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 188, 2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torr E. E., Gardner D. H., Thomas L., Goodall D. M., Bielemeier A., Willetts R., Griffiths H. R., Marshall L. J., Devitt A. (2012) Apoptotic cell-derived ICAM-3 promotes both macrophage chemoattraction to and tethering of apoptotic cells. Cell Death Differ. 19, 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moffatt O. D., Devitt A., Bell E. D., Simmons D. L., Gregory C. D. (1999) Macrophage recognition of ICAM-3 on apoptotic leukocytes. J. Immunol. 162, 6800–6810. [PubMed] [Google Scholar]
- 75.Schif-Zuck S., Gross N., Assi S., Rostoker R., Serhan C. N., Ariel A. (2011) Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur. J. Immunol. 41, 366–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez I. G., Tang J., Wilson C. L., Yan W., Heinecke J. W., Harlan J. M., Raines E. W. (2012) Metalloproteinase-mediated shedding of integrin β2 promotes macrophage efflux from inflammatory sites. J. Biol. Chem. 287, 4581–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Recchiuti A., Serhan C. N. (2012) Pro-resolving lipid mediators (SPMs) and their actions in regulating miRNA in novel resolution circuits in inflammation. Front. Immunol. 3, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kolaczkowska E., Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. [DOI] [PubMed] [Google Scholar]
- 79.Galvão I., Vago J. P., Barroso L. C., Tavares L. P., Queiroz-Junior C. M., Costa V. V., Carneiro F. S., Ferreira T. P., Silva P. M. R., Amaral F. A., Sousa L. P., Teixeira M. M. (2016) Annexin A1 promotes timely resolution of inflammation in murine gout. Eur. J. Immunol. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 80.Hannon R., Croxtall J. D., Getting S. J., Roviezzo F., Yona S., Paul-Clark M. J., Gavins F. N. E., Perretti M., Morris J. F., Buckingham J. C., Flower R. J. (2003) Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB J. 17, 253–255. [DOI] [PubMed] [Google Scholar]
- 81.Oyoshi M. K., He R., Li Y., Mondal S., Yoon J., Afshar R., Chen M., Lee D. M., Luo H. R., Luster A. D., Cho J. S., Miller L. S., Larson A., Murphy G. F., Geha R. S. (2012) Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity 37, 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tager A. M., Dufour J. H., Goodarzi K., Bercury S. D., von Andrian U. H., Luster A. D. (2000) BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J. Exp. Med. 192, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsukawa A., Hogaboam C. M., Lukacs N. W., Lincoln P. M., Strieter R. M., Kunkel S. L. (1999) Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J. Immunol. 163, 6148–6154. [PubMed] [Google Scholar]
- 84.Colom B., Bodkin J. V., Beyrau M., Woodfin A., Ody C., Rourke C., Chavakis T., Brohi K., Imhof B. A., Nourshargh S. (2015) Leukotriene B4-neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo. Immunity 42, 1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friedrich E. B., Tager A. M., Liu E., Pettersson A., Owman C., Munn L., Luster A. D., Gerszten R. E. (2003) Mechanisms of leukotriene B4--triggered monocyte adhesion. Arterioscler. Thromb. Vasc. Biol. 23, 1761–1767. [DOI] [PubMed] [Google Scholar]
- 86.Goodarzi K., Goodarzi M., Tager A. M., Luster A. D., von Andrian U. H. (2003) Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat. Immunol. 4, 965–973. [DOI] [PubMed] [Google Scholar]
- 87.Takano T., Fiore S., Maddox J. F., Brady H. R., Petasis N. A., Serhan C. N. (1997) Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J. Exp. Med. 185, 1693–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197. [DOI] [PubMed] [Google Scholar]
- 89.Papayianni A., Serhan C. N., Brady H. R. (1996) Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J. Immunol. 156, 2264–2272. [PubMed] [Google Scholar]
- 90.Filep J. G., Zouki C., Petasis N. A., Hachicha M., Serhan C. N. (1999) Anti-inflammatory actions of lipoxin A(4) stable analogs are demonstrable in human whole blood: modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions. Blood 94, 4132–4142. [PubMed] [Google Scholar]
- 91.Godson C., Mitchell S., Harvey K., Petasis N. A., Hogg N., Brady H. R. (2000) Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164, 1663–1667. [DOI] [PubMed] [Google Scholar]
- 92.Spite M., Norling L. V., Summers L., Yang R., Cooper D., Petasis N. A., Flower R. J., Perretti M., Serhan C. N. (2009) Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461, 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dona M., Fredman G., Schwab J. M., Chiang N., Arita M., Goodarzi A., Cheng G., von Andrian U. H., Serhan C. N. (2008) Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 112, 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oh S. F., Dona M., Fredman G., Krishnamoorthy S., Irimia D., Serhan C. N. (2012) Resolvin E2 formation and impact in inflammation resolution. J. Immunol. 188, 4527–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C.-H., Yang R., Petasis N. A., Serhan C. N. (2010) Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 107, 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maekawa T., Hosur K., Abe T., Kantarci A., Ziogas A., Wang B., Van Dyke T. E., Chavakis T., Hajishengallis G. (2015) Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3β-C/EBPβ pathway. Nat. Commun. 6, 8272. [DOI] [PMC free article] [PubMed] [Google Scholar]