Abstract
Glaucoma is a leading cause of irreversible blindness worldwide. Primary open-angle glaucoma (POAG), the most common type, is a complex inherited disorder that is characterized by progressive retinal ganglion cell death, optic nerve head excavation, and visual field loss. The discovery of a large, and growing, number of genetic and chromosomal loci has been shown to contribute to POAG risk, which carry implications for disease pathogenesis. Differential gene expression analyses in glaucoma-affected tissues as well as animal models of POAG are enhancing our mechanistic understanding in this common, blinding disorder. In this review we summarize recent developments in POAG genetics and molecular genetics research.
Keywords: glaucoma, POAG, GWAS, linkage, association, differential expression, genetics, endophenotype
1. Introduction of Primary Open-Angle Glaucoma
Glaucoma is a heterogeneous group of optic neuropathies that are characterized by the progressive loss of retinal ganglion cells (RGC), optic atrophy, and visual field loss (Allingham et al., 2009; Allingham and Shields, 2011). Glaucoma is the most common cause of irreversible blindness that affects more than 70 million people worldwide (Quigley, 2011; Quigley and Broman, 2006; Tham et al., 2014). Primary open-angle glaucoma (POAG) is the most common type of glaucoma that is defined by characteristic glaucomatous retinal, optic nerve, and clinical findings without an identifiable secondary cause. Risk factors for POAG include increasing age, elevated intraocular pressure (IOP), and a positive family history. POAG has been identified in all populations studied to date. The prevalence of POAG varies between populations, but is notably more common in persons of African and Hispanic descent (Budenz et al., 2012a; Budenz et al., 2012b; Chopra et al., 2008; Emanuelli et al., 2005; Memarzadeh et al., 2010; Quigley et al., 2001; Rodriguez et al., 2002; Tielsch et al., 1991). Elevated IOP is arguably the most important risk factor for developing POAG, and is often used to categorize patients into “high” or “normal” tension subtypes (HTG or NTG, respectively). While a cut-off between HTG and NTG may be useful for clinical or research purposes, the specific threshold (≥ 22 mmHg or ≤21 mmHg) is arbitrary. IOP is the only modifiable risk factor that is lowered with medications or surgeries to treat glaucoma patients in the clinic to delay the progression of vision loss due to glaucoma. While higher IOP confers greater risk for glaucoma, glaucoma can develop at a statistically normal or elevated IOP. POAG frequently remains undiagnosed or unrecognized by patients until visual field loss is clinically advanced.
POAG is a complex inherited trait. In this article, we will present an updated, comprehensive review of the genetic developments in POAG.
2. Genetic Approaches used in Glaucoma Research
Investigations into the inheritance of POAG have benefitted greatly from the development and integration of many genetic approaches to identify regions in the genome that are associated with a specific phenotype, or in some cases determine the specific genetic variants that are responsible. Family-based genetic linkage analysis was the first used to identify chromosomal locations for Mendelian forms of POAG. This approach has led to the identification of many POAG-associated loci and, less often, specific causative genes. Genetic mutations for POAG have been identified in three genes – myocilin (MYOC), optineurin (OPTN), and TANK-binding kinase 1 (TBK1) (Fingert et al., 2017; Fingert et al., 2011; Rezaie et al., 2002; Sarfarazi et al., 1998; Sheffield et al., 1993; Stone et al., 1997).
Next generation, high-throughput DNA sequencing technology is increasingly being used to sequence the complete coding sequence of the human genome (exome) or the human genome in its entirety (Zheng et al., 2015). These technologies offer a powerful approach to identify causal genetic variants for many rare and common genetic disorders, including POAG.
The genome-wide association studies (GWAS), based on high-throughput DNA genotyping, are currently the most powerful, broadly used approach to identify genomic regions associated with a specific disease or phenotype. GWAS utilize up to several million single nucleotide polymorphisms (SNPs) from an individual sample for analysis. The number of subjects in clinical datasets for GWAS may number from hundreds to hundreds of thousands. This approach has been used to identify the location of thousands of inherited disorders or phenotypes (Welter et al., 2014). In ophthalmology, the first successful GWAS identified the association of complement factor H (CFH) with age-related macular degeneration (AMD) (Edwards et al., 2005; Hageman et al., 2005; Haines et al., 2005; Klein et al., 2005). Similarly, GWAS have been used to identify many genetic associations for POAG (Bailey et al., 2016; Burdon et al., 2011; Chen et al., 2014; Gharahkhani et al., 2014; Hysi et al., 2014; Lu et al., 2013; Springelkamp et al., 2014; Springelkamp et al., 2017; Springelkamp et al., 2015b; Thorleifsson et al., 2010; Wiggs et al., 2012).
In addition, the analysis of DNA copy number variants (e.g. genomic deletion and duplication) and differential expression analysis of mRNA and proteins have added to our understanding of human genetic disorders. Altogether, these technological innovations have advanced our understanding of POAG genetic etiology.
3. Genetic Linkage Analyses of POAG
Genetic linkage analysis is a family-based method used to localize chromosomal regions that are associated with a specific phenotype within related family groups. Genetic linkage is used for Mendelian traits, or traits with high heritability that are typically caused by mutations in a single gene. Genetic linkage analyses have been used to identify many genomic loci associated with POAG, designated GLC1A through GLC1P, and in some cases the gene within the locus (Table 1, Figure 1). Genes identified in these loci are discussed below.
Table 1.
POAG chromosomal Loci identified through linkage analysis
NTF4 gene in the GLC1O locus was evaluated as a candidate gene for POAG without family-based studies or linkage analysis.
Figure 1.
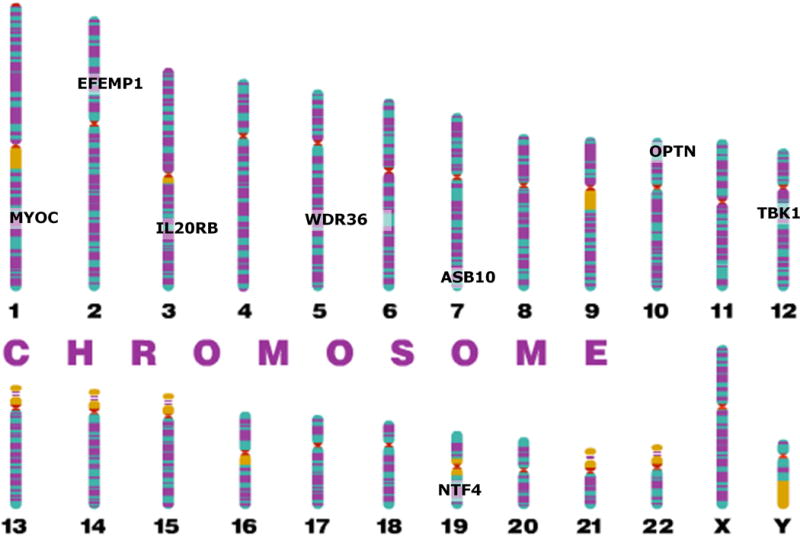
Genome plot of Mendelian genes for POAG that have been identified through genetic linkage analysis.
3.1 MYOC
Mutations in the MYOC gene, located in chromosome 1q24, were first identified in families with autosomal dominant POAG linked to the GLC1A locus (Sheffield et al., 1993; Stone et al., 1997). Mutations in MYOC are primarily associated juvenile-onset POAG (Fingert, 2011; Fingert et al., 2002; Hewitt et al., 2008). The phenotype for glaucoma with MYOC-associated mutations is generally severe, typically IOP is highly elevated and surgical management is often required for treatment (Fingert et al., 1999). MYOC-associated glaucoma accounts for 3–5% of POAG cases worldwide (Liu and Allingham, 2011). The MYOC gene contains three exons and encodes a protein with 504 amino acids (OMIM 601652). Most mutations are located in the third exon, which codes for the olfactomedin-like domain. The crystal structure of the olfactomedin (OLF) domain shows that it is a member of a small protein family characterized by a high affinity calcium binding site with a five-bladed β-propeller structure (Donegan et al., 2015; Donegan et al., 2012). Several MYOC mutations are located within or near this binding site (Donegan et al., 2012). Although MYOC is widely expressed in ocular and non-ocular tissues, glaucoma is the only reported association with a clinical disease (Kwon et al., 2009).
Over 100 POAG-associated mutations have been identified in the MYOC gene (www.myocilin.com summary data by December 18, 2016). The most prevalent mutation produces a premature termination of the myocilin protein (Q368X). This mutation causes an older-adult age of onset form of glaucoma that is clinically less severe than that seen with other MYOC mutations (Allingham et al., 1998; Alward et al., 1998; Fingert, 2011; Kwon et al., 2009). Genetic screening and counseling should be considered for affected family members in families where MYOC-associated glaucoma has been found. Since mutations are autosomal dominant, highly penetrant, clinically severe, and usually present early in life, carriers can be identified in early stages of glaucoma or screening can be initiated in those without evidence of disease (Gharahkhani et al., 2015; Hewitt et al., 2008; Souzeau et al., 2013; Souzeau et al., 2014; Souzeau et al., 2015).
The primary effect of pathogenic mutations in MYOC, which is invariably associated with elevated IOP, appears to be on the trabecular meshwork (TM) and aqueous humor outflow function. Reduced, complete loss (haploinsufficiency), or overexpression of MYOC protein does not cause glaucoma in animal models (Gould et al., 2004a; Kim et al., 2001; Kwon et al., 2009; Resch and Fautsch, 2009; Tamm, 2002; Zillig et al., 2005). The lack of a discernable ocular phenotype in these transgenic mice suggests that myocilin is not required for normal IOP or normal ocular morphology and therefore, haploinsufficiency is not the primary mechanism for POAG patients with MYOC mutations. Instead, pathology from these mutations in humans is likely caused by gain of function. Transgenic mice with the human or mouse Tyr437His develop features of glaucoma including elevated IOP, progressive RGC loss and axonal degeneration (Chou et al., 2014; Gould et al., 2006; Senatorov et al., 2006; Zhou et al., 2008; Zillig et al., 2005; Zode et al., 2012; Zode et al., 2011). Transgenic mice do not secrete myocilin into the aqueous humor; rather abnormal myocilin accumulates intracellularly. In addition to RGC loss, these mice demonstrate function loss measured by pattern electroretinogram (PERG) (Chou et al., 2014; Zhou et al., 2008).
Secretion of myocilin protein into the aqueous humor is significantly reduced in patients with POAG-associated mutations in MYOC (Jacobson et al., 2001; Resch and Fautsch, 2009; Resch et al., 2010). MYOC may enter the aqueous humor in MYOC-containing exosomes, which may help regulate aqueous outflow in the TM (Dismuke et al., 2015; Hardy et al., 2005; Hoffman et al., 2009; Liu et al., 2016; Perkumas et al., 2007; Stamer et al., 2011). It has been proposed that glaucoma-associated MYOC protein interferes with protein trafficking, receptor-mediated endocytosis, programmed cell death, myelination of the optic nerve, and stress to the endoplasmic reticulum (ER) (Koch et al., 2014; Kwon et al., 2013; Kwon et al., 2014; McKay et al., 2013; Zode et al., 2011).
Mutations of MYOC have been reported in juvenile open-angle glaucoma and primary congenital glaucoma (Chakrabarti et al., 2005; Kaur et al., 2011; Kaur et al., 2005; Mookherjee et al., 2012; Vincent et al., 2002). MYOC may interact with CYP1B1 through a digenic mechanism with CYP1B1 acting as a modifier for MYOC (Kaur et al., 2011). It has been reported that CYP1B1 mutations with <10% enzymatic activity may increase the level of endogenous myocilin in human trabecular meshwork cells due to the lack of 17β estradiol metabolizing activity with CYP1B1 mutation (Mookherjee et al., 2012). However, the interaction between MYOC and CYP1B1 with POAG pathogenesis remains poorly understood.
3.2 OPTN and TBK1
OPTN and TBK1 are discussed together since they share cellular, molecular, and biological pathways. In addition, genetic variants in both of these genes cause NTG, and in some cases they are both associated with degenerative disorders of the central nervous system.
OPTN is located in the POAG-linked locus GLC1E on chromosome 10p13. Clinically, mutations in OPTN lead to NTG. This contrasts sharply with patients who have MYOC- associated POAG, which is consistently hypertensive (Ariani et al., 2006; Charlesworth et al., 2006; Hauser et al., 2006b; Rezaie et al., 2002). Among all OPTN mutations, E50K has been most clearly shown to cause glaucoma (Aung et al., 2005). POAG patients harboring the E50K mutation have an earlier age of onset, more advanced optic nerve cupping, and more frequently reqire surgical intervention (Aung et al., 2005).
OPTN mutations have been reported in patients with amyotrophic lateral sclerosis (ALS), which has prompted broad interest in the functional role of OPTN in neural disorders (Cirulli et al., 2015; Maruyama et al., 2010). OPTN is involved in membrane trafficking, protein secretion, cell division, autophagy, and host defense against pathogens (Kachaner et al., 2012; Wild et al., 2011). OPTN interacts with many other proteins, such as TBK1, RAB8 (Rab protein 8), myosin VI, transferrin receptor, and autophagosomal protein LC3. OPTN inhibits TNFα-induced NF-κB activation and affects apoptotic threshold (Kachaner et al., 2012; Morton et al., 2008; Sahlender et al., 2005; Tumbarello et al., 2012; Zhu et al., 2007). In addition, OPTN is an autophagy receptor that functions in removal of damaged mitochondria and proteins (Heo et al., 2015; Lazarou et al., 2015; Richter et al., 2016; Shen et al., 2015; Slowicka et al., 2016; Wong and Holzbaur, 2014).
Glaucoma-associated mechanisms caused by the E50K OPTN mutation are under intense study. Interaction of OPTN with TBK1 may be enhanced by overexpression of E50K (Minegishi et al., 2013; Morton et al., 2008). In addition, a wide variety of molecular and cellular effects have been reported (Chi et al., 2010a; Gao et al., 2014; Kryndushkin et al., 2012; Minegishi et al., 2013; Nagabhushana et al., 2010; Park et al., 2010; Shen et al., 2015; Shim et al., 2016; Vaibhava et al., 2012). However, it is unclear whether these effects result from overexpression of OPTN or from the mutation itself (Minegishi et al., 2016). An important step forward has been the development of an E50K transgenic mouse model that demonstrates an age-dependent decrease in RGCs and visual function in the absence of elevated IOP (Tseng et al., 2015).
TBK1 is located in the GLC1P POAG linkage locus on chromosome 12q14. TBK1 encodes a serine/threonine kinase. It plays an essential role in the regulation of inflammatory responses to foreign agents (Fingert, 2011). A duplication of the TBK1 gene has been identified in approximately 1% of normal tension POAG cases (Awadalla et al., 2015; Fingert et al., 2014; Fingert et al., 2011; Kawase et al., 2012; Liu et al., 2014b; Ritch et al., 2014; Seo et al., 2013). As discussed above, OPTN interacts with TBK1 and may share a common pathway in glaucoma pathogenesis (Heo et al., 2015; Morton et al., 2008). Like OPTN, mutations in TBK1 have been identified in patients with CNS disorders including ALS and frontotemporal dementia (Bettencourt and Houlden, 2015; Cirulli et al., 2015; Freischmidt et al., 2015; Gijselinck et al., 2015). RGC-like neurons derived from a patient with TBK1-associated NTG reportedly have increased levels of LC3-II protein and activation of LC3-II, which suggests that autophagy may be dysregulated in patients with TBK1-associated NTG (Tucker et al., 2014). This observation is consistent with reports that TBK1 and OPTN regulate autophagy of damaged mitochondria (Matsumoto et al., 2015; Moore and Holzbaur, 2016; Pilli et al., 2012; Richter et al., 2016). Transgenic mice that have a duplication of the human TBK1 gene develop progressive RGC loss at 3 months of age and are normotensive (Fingert et al., 2017). However, it remains to be determined if or how abnormalities in autophagy contribute to NTG pathogenesis in patients with genetic variants in TBK1 and OPTN (Pilli et al., 2012; Tucker et al., 2014).
3.3 WD repeat domain 36 (WDR36)
WD repeat domain 36 (encoded by WDR36) is a nucleolar protein involved in the maturation of 18s rRNA. It is located in the POAG linkage locus GLC1G on chromosome 5q22. The role of WDR36 mutations in the pathogenesis of POAG is controversial. Mutations in WDR36 were originally reported in 1.6% to 17% of POAG patients (Monemi et al., 2005). However, subsequent studies have failed to replicate these findings. More recent studies suggest that genetic variants of WDR36 may alter POAG risk (Blanco-Marchite et al., 2011; Fan et al., 2009; Fingert et al., 2007; Frezzotti et al., 2011; Gallenberger et al., 2011; Hauser et al., 2006a; Hewitt et al., 2006; Janssen et al., 2013; Kramer et al., 2006; Miyazawa et al., 2007; Mookherjee et al., 2011; Pasutto et al., 2008; Ramdas et al., 2011a; Weisschuh et al., 2007). Knockdown of Wdr36 in zebrafish reportedly reduces levels of 18S rRNA, activates the p53 stress-response pathway, and is associated with ocular abnormalities (Skarie and Link, 2008). WDR36 mutations reportedly affect RGC axon growth that may lead to progressive peripheral retinal degeneration with normal IOP in transgenic mice (Chi et al., 2010b). WDR36-related variations in expression have been reported to delay the formation of 18S rRNA and up-regulate BAX, TP53, and CDKN1A, and lead to apoptotic cell death in human TM cells (Gallenberger et al., 2011). However, heterozygous WDR36-deficient mice do not demonstrate ocular structure abnormalities, changes in IOP, or reduced optic nerve axons (Gallenberger et al., 2014). Currently, evidence to support a major role for POAG-risk associated with the WDR36 locus is limited.
3.4 Other Genetic Loci Identified by Linkage Analysis
Several other genes, listed in Table 1, have been identified as POAG candidate genes within loci found using genetic linkage approaches. These include NTF4 (Neurotrophin 4), ASB10 (Ankyrin Repeat and SOCS Box Containing 10), EFEMP1 (EGF containing fibulin-like extracellular matrix protein 1), and IL20RB (Interleukin 20 receptor subunit beta).
NTF4 is located within the POAG linkage locus GLC1O on chromosome 19q13.33. Interestingly, NTF4 was initially evaluated as a candidate gene for POAG without family-based studies or a linkage analysis (Pasutto et al., 2009). Using a case-control POAG dataset, seven heterozygous mutations in NTF4 were found in 1.7% of POAG patients of European origin (Pasutto et al., 2009). Expression of the most prevalent NTF4 variant reportedly decreases activation of tyrosine kinase receptor B (TrkB). This has been proposed as a possible mechanism for POAG risk in patients with NTF4 variants (Pasutto et al., 2009). To date, follow-up studies in the US, India, and China have not replicated this observation (Chen et al., 2012; Huang et al., 2014; Liu et al., 2010; Rao et al., 2010).
ASB10 is located within the POAG linkage locus GLC1F on chromosome 7q36 (Murakami et al., 2010). A mutation affecting an exon splice enhancer (c.765C>T, p.Thr255Thr) has been identified in a large POAG family (Pasutto et al., 2012). Additional sequence analysis in 1172 cases and 461 controls has identified mutations in 6% POAG cases compared to 2.8% of controls. ASB10 is highly expressed in TM, RGC, and ciliary body (Pasutto et al., 2012). Knock-down of ASB10 transcription in perfused anterior segment organ culture reduced aqueous humor outflow facility by 50% (Pasutto et al., 2012). Functional studies in primary human TM cells have suggested that ASB10 may play a role in ubiquitin-mediated degradation pathways through an interaction with HSP70 and the α4 subunit of the 20s proteasome (Keller et al., 2013). The up-regulation of ASB10 expression by TNFα and IL-1α in TM cells provides further support for a role in glaucoma (Keller and Wirtz, 2017). Replication studies for the association of ASB10 variants with POAG have been mixed (Fingert et al., 2012; Micheal et al., 2015).
EFEMP1 is a juvenile-onset POAG candidate located in the GLC1H locus on chromosome 2p16. A missense mutation (p.Arg140Trp) was found in a 3-generation autosomal dominant African-American family with adult-onset, POAG (Mackay et al., 2015). EFEMP1 is expressed highly in mouse ciliary body and cornea, at lower levels in the retina. The p.Arg140Trp mutation is located in the first of six calcium-binding EGF-like domains and may lead to abnormal accumulation of the mutant protein in the cell (Mackay et al., 2015). Mutations in EFEMP1 have been associated other ocular disorders including Doyne honeycomb retinal dystrophy and Malattia Leventinese (Hulleman, 2016; Marmorstein, 2004; Stone et al., 1999; Takeuchi et al., 2010). Variants in EFEMP1 are associated with reduced optic nerve disc area (Springelkamp et al., 2015b). EFEMP1 expression in mouse retina is activated after optic nerve crush injury (Templeton et al., 2013). Expression of EFEMP1 is inhibited by TGF-β2, a cytokine that is elevated in POAG, in primary human TM cells, which suggests a potential role in glaucoma (Fuchshofer et al., 2009).
IL20RB is a POAG gene that is located in the GLC1C locus on chromosome 3q21. A specific IL20RB mutation T104M (rs367923973) has been identified in a large POAG family (Keller et al., 2014). Although the mutation was not present in all affected family members, there is supportive evidence that suggests its role in POAG pathogenesis. The T104M mutation lies within an active binding site of IL20RB with IL-20 subfamily cytokine members IL-19, IL-20, and IL-24 (Wirtz and Keller, 2016). IL20RB is expressed in human primary TM cells, which is upregulated in response to IL-20, IL-19, or IL-24. The expression of IL-24, but not IL-20 or IL20RB, is significantly upregulated in the retina of DBA/2J mice with moderate axon damage (Howell et al., 2011), which suggests a mechanism for IL-20 cytokines in glaucoma pathogenesis.
4. Association Studies in POAG
GWAS have advanced our understanding of the genetic architecture of numerous genetic disorders and traits. Since the first GWAS for POAG was performed in 2009 (Nakano et al., 2009), many genomic loci have been associated with POAG risk (Table 2). All reported genome-wide associations for POAG including their genomic locations are found in Figure 2. It is important to note that a large number of GWAS have been performed in populations of Asian and European derivation. However, at the time of this publication, no GWAS have been reported for POAG or other forms of glaucoma in populations of African ancestry or their diaspora, including African Americans. In addition to disease-associated studies for POAG, GWAS have been performed on quantitative traits that are associated with POAG and glaucoma. These traits include IOP, optic cup-disc ratio, optic disc area, central cornea thickness (CCT) and others. GWAS have been performed in many populations including the European, Chinese, and Japanese. POAG-associated genetic loci include CDKN2B-AS1, TMCO1, CAV1/CAV2, SIX1/SIX6, LRP12/ZFPM2, ABCA1, AFAP1, GMDS, GAS7, PMM2, ARHGEF12, TGFBR3, TXNRD3, ATXN2, FOXC1, and C12ORF23. Importantly, the first common, functional POAG-associated variant in the gene SIX6 (Asn141His) has been reported in European and Asian populations (Carnes et al., 2014; Cheng et al., 2015; Kuo et al., 2015; Skowronska-Krawczyk et al., 2015). As additional functional variants are found, a clearer picture of the underlying molecular and cellular interactions that cause POAG will emerge.
Table 2.
List of POAG-associated genomic regions identified with genome-wide association studies (GWAS). (*Asian and European ancestry)
| Nearest Gene | Leading SNP | Sample Size (case/control) | Ethnicity | POAG | Odds Ratio | Significance | References |
|---|---|---|---|---|---|---|---|
| ZP4 | rs693421 | 827/748 | Japanese | POAG | 1.35 | 4×10E-5 | (Nakano et al., 2009) |
| PLXDC2 | rs7081455 | 827/748 | Japanese | POAG | 1.49 | 1×10E-5 | |
| TMTC2 | rs7961953 | 827/748 | Japanese | POAG | 1.37 | 7×10E-5 | |
|
| |||||||
| SRBD1 | rs3213787 | 305/355 | Japanese | NTG | 2.80 | 3×10E-9 | (Meguro et al., 2010) |
| ELOVL5 | rs735860 | 305/355 | Japanese | NTG | 1.70 | 4×10E-6 | |
|
| |||||||
| CAV1/CAV2 | rs4236601 | 1263/34877 | Icelandic | POAG | 1.36 | 5×10E-10 | (Thorleifsson et al., 2010) |
|
| |||||||
| TMCO1 | rs4656461 | 892/4582 | Australian | Advanced POAG/POAG | 1.51 | 6×10E-14 | (Burdon et al., 2011) |
| CDKN2B-AS1 | rs4977756 | 892/4582 | Australian | Advanced POAG/POAG | 1.39 | 1×10E-14 | |
|
| |||||||
| CDKN2B-AS1 | rs2157719 | 3146/3487 | European American | POAG | 0.69 | 2×10E-18 | (Wiggs et al., 2012) |
| SIX1/SIX6 | rs10483727 | 3146/3487 | European American | POAG | 1.32 | 4×10E-11 | |
| CDKN2B-AS1 | rs2157719 | 720/3443 | European American | NTG | 0.58 | 1×10E-12 | |
| LRP12/ZFPM2 | Rs284489 | 720/3443 | European American | NTG | 0.62 | 9×10E-10 | |
|
| |||||||
| ABCA1 | rs2472493 | 4703/11488 | European Ancestry | Advanced POAG/POAG | 1.31 | 2×10E-19 | (Gharahkhani et al., 2014) |
| AFAP1 | rs4619890 | 4703/11488 | European Ancestry | Advanced POAG/POAG | 1.20 | 7×10E-10 | |
| GMDS | rs11969985 | 4703/11488 | European Ancestry | Advanced POAG/POAG | 1.31 | 8×10E-10 | |
|
| |||||||
| ABCA1 | rs2472493 | 4284/95560 | Multi-ancestry* | POAG | 1.24 | 4×10E-9 | (Hysi et al., 2014) |
| TMCO1 | rs7555523 | 4284/95560 | Multi-ancestry* | POAG | 1.40 | 1×10E-16 | |
| CAV1 | rs10258482 | 4284/95560 | Multi-ancestry* | POAG | 1.20 | 6×10E-9 | |
| GAS7 | rs9913911 | 4284/95560 | Multi-ancestry* | POAG | 0.80 | 3×10E-13 | |
|
| |||||||
| ABCA1 | rs2487032 | 2906/5974 | Chinese | POAG | 0.73 | 3×10E-19 | (Chen et al., 2014) |
| ABCA1 | rs2487032 | 2147/3246 | Chinese | HTG | 0.71 | 2×10E-15 | |
| ABCA1 | rs2487032 | 759/2728 | Chinese | NTG | 0.77 | 1×10E-5 | |
| PMM2 | rs3785176 | 2906/5974 | Chinese | POAG | 1.30 | 6×10E-10 | |
| PMM2 | rs3785176 | 2147/3246 | Chinese | HTG | 1.30 | 6×10E-7 | |
| PMM2 | rs3785176 | 759/2728 | Chinese | NTG | 1.28 | 2×10E-4 | |
|
| |||||||
| ARHGEF12 | rs58073046 | 1225/4117 | European Ancestry | POAG | 1.53 | 2×10E-8 | (Springelkamp et al., 2015a) |
| ARHGEF12 | rs58073046 | 777/4117 | European Ancestry | HTG | 1.66 | 3×10E-9 | |
|
| |||||||
| CDKN2B-AS1 | rs2157719 | 9173/26780 | Multi-ancestry | POAG | 0.71 | 3×10E-33 | (Li et al., 2015) |
| CDC7-TGFBR3 | rs1192415 | 9173/26780 | Multi-ancestry | POAG | 1.13 | 2×10E-8 | |
|
| |||||||
| TXNRD2 | rs35934224 | 3853/33480 | European American | POAG | 0.78 | 4×10E-11 | (Bailey et al., 2016) |
| ATXN2 | rs7137828 | 3853/33480 | European American | POAG | 1.17 | 9×10E-10 | |
| FOXC1 | rs2745572 | 3853/33480 | European American | POAG | 1.17 | 2×10E-10 | |
| LRP12/ZFPM2 | rs284491 | 725/11145 | European American | NTG | 0.66 | 2×10E-8 | |
| CDKN2B-AS1 | rs1333037 | 725/11145 | European American | NTG | 1.67 | 1×10E-12 | |
| C12ORF23 | rs2041895 | 725/11145 | European American | NTG | 1.48 | 2×10E-8 | |
| FOXC1 | rs2317961 | 1868/31497 | European American | HTG | 0.76 | 3×10E-8 | |
| TMCO1 | rs7555523 | 1868/31497 | European American | HTG | N/A | 1×10E-12 | |
| SIX1/SIX6 | rs33912345 | 1868/31497 | European American | HTG | N/A | 2×10E-9 | |
Figure 2.
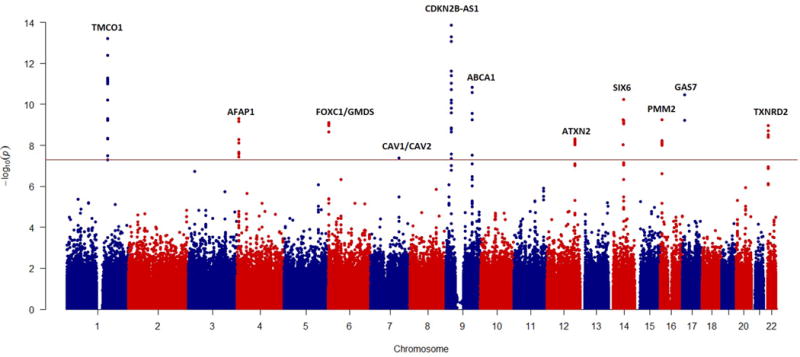
Manhattan plot of current genome-wide genetic associations for POAG (through January, 2017). The red line represents the cut-off for genome-wide significant association (5 × 10−8). This figure is was generously provided by Chiea Chuen Khor, MD, PhD at the Genome Institute of Singapore.
4.1 Caveolin 1 and 2 (CAV1/CAV2)
The CAV1 and CAV2 genes, encoding caveolins, are located on chromosome 7q31. Caveolins are involved in transcellular transport, endocytosis, mechanotransduction, cell proliferation, membrane lipid homeostasis, and signal transduction (Gu et al., 2017). The associated SNP (rs4236601), located in the intergenic region between CAV1 and CAV2, was first reported in the Icelandic population and replicated in others (Kato et al., 2013; Kim et al., 2015; Loomis et al., 2014; Micheal et al., 2014; Rong et al., 2016; Thorleifsson et al., 2010; Wiggs et al., 2011). Two reports found a stronger association in women (Loomis et al., 2014; Wiggs et al., 2011). Variants in the CAV1/CAV2 region are associated with IOP (Chen et al., 2015a; Hysi et al., 2014; Kim et al., 2015; Ozel et al., 2014; van Koolwijk et al., 2012). CAV1 and CAV2 are expressed in human and mouse retina, ciliary muscle, TM, and Schlemm canal (Gu et al., 2014; Kuehn et al., 2011; Li et al., 2012; Surgucheva and Surguchov, 2011).
CAV1 and CAV2 are expressed in TM cells cultured from POAG and non-glaucoma donor eyes (Surgucheva and Surguchov, 2011). Expression of CAV1 in these primary cells was induced by dexamethasone and inhibited by TGF-β2 (Surgucheva and Surguchov, 2011). Expression levels of both CAV1 and CAV2 are up-regulated in Schlemm canal endothelial cells derived from POAG compared to non-glaucoma donor eyes (Cai et al., 2015; Liu et al., 2013a). CAV1/CAV2 knock down has been shown to alter aqueous outflow resistance in anterior segment perfusion culture (Aga et al., 2014). Cav1 knockout mice develop elevated IOP and reduced conventional outflow facility via increased eNOS activity (Elliott et al., 2016; Gu et al., 2017; Lei et al., 2016). These data suggest that CAV1 and/or CAV2 may influence risk of POAG through an effect on IOP.
4.2 CDKN2B antisense RNA 1 (CDKN2B-AS1)
CDKN2B-AS1 (cyclin-dependent kinase inhibitor 2B antisense RNA 1) is located within the CDKN2B-CDKN2A gene cluster on chromosome 9p21. Sequence variants in this locus are associated with vertical cup-disc ratio and POAG-risk in multiple non-African populations worldwide (Bailey et al., 2016; Burdon et al., 2012; Burdon et al., 2011; Cao et al., 2012; Chen et al., 2015b; Fan et al., 2011; Li et al., 2015; Liu et al., 2013b; Mabuchi et al., 2012; Osman et al., 2012; Ramdas et al., 2010; Ramdas et al., 2011b; Vishal et al., 2014; Wiggs et al., 2012). The association of CDKN2B-AS1 appears to be strongest in POAG patients with normal tension, especially in females (Bailey et al., 2016; Burdon et al., 2012; Chen et al., 2015b; Ng et al., 2016; Pasquale et al., 2013a; Takamoto et al., 2012; Wiggs et al., 2012).
CDKN2B-AS1 is a long non-coding functional RNA that interacts with polycomb repressive complex -1 (PRC1) and -2 (PRC2). CDKN2B-AS1 is expressed in multiple human eye tissues including ciliary body, retina, and optic nerve (Burdon et al., 2011). The CDKN2B protein (aka p15(INK4B)) is localized to the inner retinal nuclear and ganglion cell layers and the corneal epithelium and TM in rat and human eyes (Chidlow et al., 2013). Transgenic mice that are heterozygous for a 70kb deletion of the CDKN2B-AS1 genomic region have a significant reduction of CDKN2B, but have normal RGC representation in the retina (Gao and Jakobs, 2016; Visel et al., 2010). However, mice that are homozygous for this deletion are more susceptible to RGC loss in response to elevated IOP (Gao and Jakobs, 2016). In addition to POAG, variants in the CDKN2B-AS1 locus are also associated with a variety of non-ocular disorders including myocardial infarction, intracranial aneurysm, diabetes, breast cancer, endometriosis, and gliomas (Kathiresan et al., 2009; Shete et al., 2009; Turnbull et al., 2010; Uno et al., 2010; Wrensch et al., 2009; Zeggini et al., 2007). It is not known if there is a relationship between POAG-risk and other disorders that are associated with the CDKN2B-AS1 locus.
4.3 SIX homeobox 6 (SIX6)
Sequence variants located in the SIX1-SIX6 locus (chromosome 14q23) have been associated with vertical cup-to-disc ratio (VCDR), myopia, and POAG (Burdon et al., 2015; Chen et al., 2015b; Fan et al., 2011; Osman et al., 2012; Philomenadin et al., 2015; Ramdas et al., 2010; Ramdas et al., 2011b; Sang et al., 2016; Verhoeven et al., 2013; Wiggs et al., 2012). SIX6 protein in humans is homologous to the “sine oculis” protein in Drosophila (Carnes et al., 2014; Iglesias et al., 2014). Both SIX6 genes in human and Drosophila encode homeobox proteins that play a critical role in ocular development. Mutations in SIX6 can cause anophthalmia, an ocular phenotype observed in mice and humans (Gallardo et al., 1999; Li et al., 2002; Ruf et al., 2004). SIX1 mutations lead to deafness and branchio-oto-renal syndrome in humans (OMIM 113650). Currently, it is considered that genetic variants of SIX6, a highly conserved gene, are responsible for POAG risk within this locus.
SIX6 is expressed in the developing retina, optic nerve, cornea, and TM as well as the developing and adult mouse brain (Conte et al., 2005; Gallardo et al., 1999). Further sequencing and fine mapping have identified two missense variants rs33912345 (His141Asn) and rs146737847 (Glu129Lys) in SIX6 that are highly associated with VCDR and POAG (Carnes et al., 2014; Iglesias et al., 2014).
Animal models and human studies provide support for a functional role of SIX6 variants in POAG pathogenesis. POAG-associated SIX6 coding variants affect eye size and optic nerve volume in the zebrafish model (Carnes et al., 2014; Iglesias et al., 2014) (Figure 3). In humans His141, the POAG-risk variant for SIX6, located in a highly conserved region, is associated with reduced retinal nerve fiber layer (RNFL) thickness measured by OCT. The effect on RNFL thickness is observed in European and Asian populations (Carnes et al., 2014; Cheng et al., 2015; Kuo et al., 2015). Similarly, this SIX6 variant is associated with RNFL thickness in non-glaucomatous Singapore Chinese individuals (Cheng et al., 2015). Thinner RNFL is primarily observed in the superior and inferior RNFL sectors, a pattern that is similar to that observed in human glaucoma (Carnes et al., 2014; Cheng et al., 2015; Kuo et al., 2015). A gene dosage effect has been observed in POAG and non-POAG subjects, meaning reduction in mean RNFL thickness for individuals homozygous (two copies) for the POAG-risk variant have significantly thinner RNFL thickness than persons who are heterozygous (one copy) (Cheng et al., 2015; Kuo et al., 2015). The gene dosage effect has been seen in populations of Asian and European ancestry (Cheng et al., 2015; Kuo et al., 2015).
Figure 3.
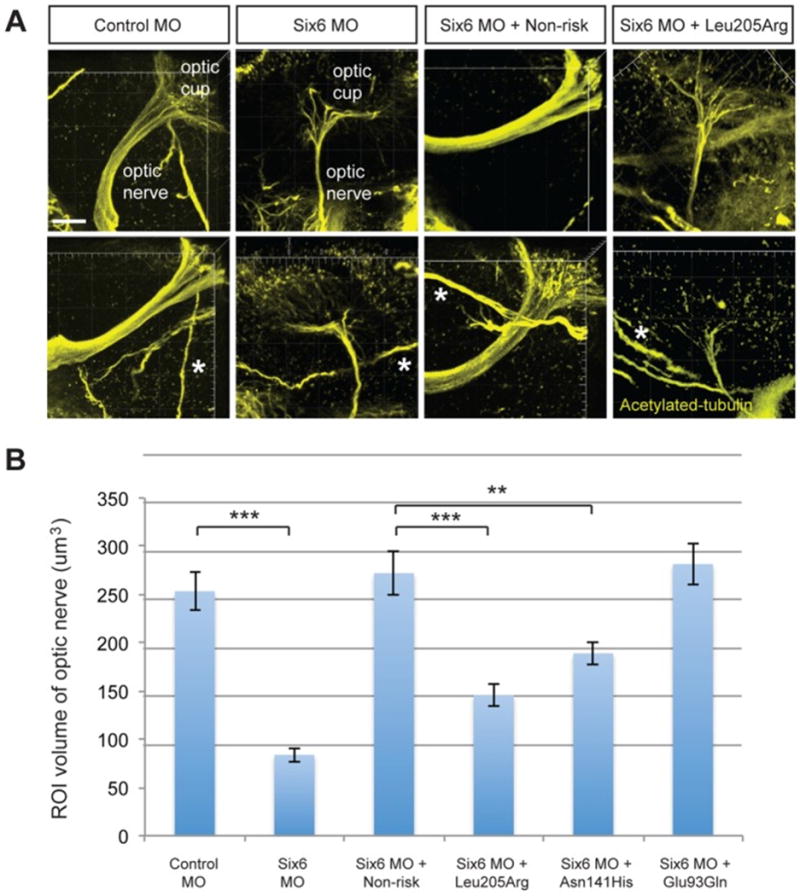
Functional evaluation of SIX6 variants on the volume of the optic nerve.
Representative whole mount images of acetylated-tubulin expression in the heads of zebrafish embryos injected with a control or six6a morpholino, rescued by co-injection with human non-risk SIX6 transcript or a transcript containing the Leu205Arg hypomorphic variant (A). Acetylated-tubulin staining is restricted primarily to axon tracts and can be used to visualize the optic nerve. Relative to the control morphants, volumetric regions of interest (ROI) along the optic nerve in six6a morphants were reduced significantly. Co-injection of human variants revealed a hypomorphic (Leu205Arg, Asn141His) or benign (Glu93Gln) role of the variants on the optic nerve (B). Sample size for all injection paradigms ranged from 7–9 and p-values are plotted for each comparison (*** p<0.001; ** p<0.01). No significant changes in the volume of other axonal tracts in the head (marked by an asterisk) were detected. Standard error of the mean is shown and white scale bars = 20 um. (Figure adapted from (Carnes et al., 2014)).
Recent evidence supports interaction between the Asn141His SIX6 variant with genes within another major POAG-risk locus, CDKN2B-AS1. Over-expression of the POAG-risk His141 SIX6 variant in cell models elevates expression of CDKN2B (aka p16(INK4A)). Further in human fetal RGCs, the rate of cellular senescence is increased in cells with the POAG-risk SIX6 variant compared to cells transfected with the protective Asn141 variant (Skowronska-Krawczyk et al., 2015). Increased expression of SIX6 and CDKN2B associated with RGC senescence was also observed in a mouse glaucoma model with elevated IOP (Skowronska-Krawczyk et al., 2015). The SIX6 His141 risk allele also leads to increased CDKN2B-AS1 expression and subsequent RGC cellular senescence in a mouse model with elevated IOP and in human POAG eyes. These data, which remain to be corroborated, suggest that SIX6 and elevated IOP play a role in RGC loss through a mechanism that includes increased expression of CDKN2B-AS1.
The protective SIX6 variant, Asn141, is found only in humans (Blanchette et al., 2004). All mammals, including mice and most vertebrates, are monomorphic for the His141 SIX6 genotype. Therefore, it is not possible to compare or analyze expression pattern and phenotype data that result from His141Asn, or any other SIX6 variant, in currently available animal models (Blanchette et al., 2004; Skowronska-Krawczyk et al., 2015). Interestingly, the His141 risk variant is essentially monomorphic in the West and South African populations, or conversely, the protective Asn141 variant is essentially absent (Liu et al., 2013b; Williams et al., 2015). While the VCDR is greater and POAG prevalence is reportedly higher in populations of West African ancestry, for reasons just cited the effect of the His141Asn SIX6 variant in these populations is challenging to test (Girkin, 2008; Girkin et al., 2010; Knight et al., 2012). Current preliminary evidence, based on population genetics, suggests that the protective Asn141 variant arose in East Africa prior to Man’s migration out of the continent. This supports observed Asn141 population allele frequencies (Tishkoff and Allingham, unpublished data). It is currently unknown if the protective Asn141 variant has experienced positive selection pressure. Regardless, the presence of this common, functional variant provides a valuable new tool to explore molecular mechanisms responsible for POAG risk and protection.
4.4 Transmembrane and coiled-coil domains 1 (TMCO1)
TMCO1, an ER transmembrane protein, is evolutionally conserved and plays an active role in maintaining ER Ca2+ storage (Wang et al., 2016; Zhang et al., 2010). TMCO1 undergoes reversible homotetramerization to form a Ca2+-selective ion channel in response to ER Ca2+ overloading or depletion (Wang et al., 2016).
Genetic sequence variants in TMCO1 are associated with POAG risk and IOP, an important glaucoma-related quantitative trait (Bailey et al., 2016; Burdon et al., 2011; Burdon et al., 2015; Chen et al., 2015b; Gibson et al., 2012; Hysi et al., 2014; Micheal et al., 2014; Ozel et al., 2014; Scheetz et al., 2016; van Koolwijk et al., 2012). It has been reported that POAG patients that are homozygous for the TMCO1 POAG-risk allele (rs4656461, allele G) develop glaucoma 4–5 years earlier than those without the risk allele (Sharma et al., 2012). Recently, a genetic study of subjects enrolled in the Ocular Hypertension Treatment Study (OHTS) found that non-Hispanic white subjects with TMCO1 risk alleles were at increased risk (12%) to develop POAG than subjects without these risk alleles after 13 years follow-up (Scheetz et al., 2016). In addition, the presence of glaucoma-risk associated TMCO1 alleles increased relative risk of glaucoma by 1.7 fold per allele, which is comparable to other glaucoma risk factors (i.e., elevated IOP and aging) (Scheetz et al., 2016). TMCO1 protein is expressed in most human ocular tissues, including TM and retina (Sharma et al., 2012). The mechanistic relationship between variants in the TMCO1 locus and IOP remains to be determined.
4.5 Actin filament associated protein 1 (AFAP1)
The AFAP1 gene is located on chromosome 4 and encodes actin filament associated protein 1. Variants within the AFAP1 gene locus have been associated with POAG in populations of European ancestry (Bailey et al., 2016; Gharahkhani et al., 2014). AFAP1 mRNA and protein are expressed in many human ocular tissues including retina, optic nerve, and cultured TM cells (Gharahkhani et al., 2014). POAG-associated SNPs within this region are correlated with the expression level of AFAP1 and AFAP1-AS1 in non-ocular human tissues including skin, fibroblast cells, brain, and whole blood (Consortium, 2015). The mechanistic role that AFAP1 variants play in POAG risk remains to be determined.
4.6 ATP binding cassette subfamily A member 1 (ABCA1)
The ABCA1 gene encodes a membrane-associated protein that is a member of the ATP-binding cassette (ABC) transporter family. This protein complex transports various molecules across extra- and intracellular membranes. ABCA1 acts as a cholesterol efflux pump in the cellular lipid removal pathway. Mutations in ABCA1 gene have been associated with Tangier’s disease and familial high-density lipoprotein deficiency. Variants located upstream of the ABCA1 gene are associated with POAG and IOP in multiple populations (Chen et al., 2014; Gharahkhani et al., 2014; Hysi et al., 2014; Luo et al., 2015). ABCA1 mRNA and protein are expressed in human TM, iris, ciliary body, cornea, optic nerve, and retina; especially in the ganglion cell layer (Chen et al., 2014; Gharahkhani et al., 2014; Tserentsoodol et al., 2006). The expression of ABCA1 protein is similar in glaucoma and non-glaucoma eyes (Gharahkhani et al., 2014). The IOP-related variant rs2472493 is associated with ABCA1 transcript levels in lymphoblastoid cell lines (Gharahkhani et al., 2014; Hysi et al., 2014). ABCA1 may regulate neuroinflammation and neurodegeneration in the mouse brain, which could potentially play a role in retinal and POAG pathogenesis.
4.7 Thioredoxin reductase 2 (TXNRD2)
The TXNRD2 gene, located on chromosome 22, encodes a mitochondrial protein that is required for redox homeostasis (Chen et al., 2006). Sequence variants in the TXNRD2 region have been associated with POAG (Bailey et al., 2016). TXNRD2 mRNA and protein are found in retina and optic nerve tissue (Bailey et al., 2016). Oxidative stress is known to cause RGC dysfunction in glaucoma models and clinical glaucoma samples (Chrysostomou et al., 2013). TXNRD2 regulates the cellular redox environment by scavenging reactive oxygen species in mitochondria. Overexpression of thioredoxin, produced by TXNRD2, is protective for; RGCs after optic nerve axotomy, pharmacologically induced oxidative stress in vitro, and in an animal model (rat) of glaucoma (Caprioli et al., 2009). Further, computational analysis indicates that POAG-associated SNPs may locate within enhancers to regulate the expression of TXNRD2 gene (Bailey et al., 2016). These data are consistent with the suspected role of oxidative damage in POAG pathogenesis and, more specifically, the contribution by pathways that include TXNRD2 that are related to mitochondrial biology.
4.8 FOXC1/GMDS
The gene Forkhead Box C1 (FOXC1) is physically located next to GDP-mannose 4,6-dehydratase (GMDS) on chromosome 6. Mutations in FOXC1 have been associated with primary congenital glaucoma and developmental glaucoma including Axenfeld-Rieger’s syndrome, Peter’s anomaly, and iridogoniodysgenesis (Gould et al., 2004b; Liu and Allingham, 2012; Nishimura et al., 2001; Sowden, 2007). GMDS encodes an enzyme that converts GDP-mannose to GDP-4-keto-deoxymannose and is ubiquitously expressed in human eye tissues (Gharahkhani et al., 2014). Sequence variants within the GMDS gene have been associated with advanced POAG (Gharahkhani et al., 2014) while variants located upstream of FOXC1 have also been associated with POAG (Bailey et al., 2016). Although these loci are near each other, a conditional analysis suggests that the associations are independent (Bailey et al., 2016). The most associated genetic variant, SNP rs2745572, near FOXC1 is associated with the expression of a long noncoding RNA – RP11-157J24.2 (ELF2P2, E74-like factor 2 pseudogene 2) (Consortium, 2015).
4.9 Ataxin 2 (ATXN2)
The gene Ataxin 2 (ATXN2), located on chromosome 12, encodes a membrane protein located in the ER and plasma membrane. This protein is involved in endocytosis and modulates mTOR signals that modify ribosomal translation and mitochondrial function. The association of genetic variants in the ATXN2 locus with POAG has been reported in subjects of European ancestry (Bailey et al., 2016). ATXN2 is expressed in normal human cornea, TM, ciliary body, retina, and optic nerve while the protein is expressed in mouse RGC and optic nerve (Bailey et al., 2016). Long expansions of CAG repeats within ATXN2 are associated with spinocerebellar ataxia 2 with optic atrophy while intermediate length expansions of this repeat are a strong risk factor for ALS (Lattante et al., 2014). Mutations in OPTN and TBK1, as previously discussed, also contribute to POAG and ALS. The relationship between these POAG-risk associated genes lends further support for the presence of a biological relationship between POAG and disorders of the central nervous system.
4.10 Other POAG-associated Loci
There are many other reported associations between various genetic loci and POAG that are supported by recent GWAS and summarized in Table 2 and are briefly described here. SRBD1 variants have been associated with POAG, especially NTG, in multiple studies (Gibson et al., 2012; Kanemaki et al., 2013; Mabuchi et al., 2011, 2015; Meguro et al., 2010). LRP12/ZFPM2 variants have been associated with NTG in multiple studies (Bailey et al., 2016; Wiggs et al., 2012). Variants in the FNDC3B, GAS7, and ARHGEF12 are consistently associated with POAG and IOP, a quantitative trait (Luo et al., 2015; Micheal et al., 2014; Ozel et al., 2014; Sharma et al., 2012; van Koolwijk et al., 2012). Variants in PMM2 and CDC-TGFBR3 are associated with POAG while variants in C12ORF23 have been associated with NTG (Bailey et al., 2016; Chen et al., 2014; Li et al., 2015). A variant in the MIR182 gene has been associated with POAG, especially in those with elevated IOP (Drewry et al., 2016; Liu et al., 2016). Interestingly, elevated expression of miR-182 has been reported to contribute to stress-induced premature senescence in human TM cells (Li et al., 2009).
4.11 Populations, GWAS and POAG
To date GWAS for POAG have primarily been limited to European and Asian populations. Currently, there are a growing number of GWAS in populations of African ancestry. Two African American studies are in progress or have been reported (Charlson et al., 2015; Hoffmann et al., 2014). Surprisingly, a recent GWAS of POAG in a west continental African (Ghana and Nigeria) and African American dataset found no association with either variants in CDKN2B-AS1 or TMCO1, genetic loci highly associated with POAG in Asian and European populations (Hauser et al., 2017). To date, no genome-wide associations for POAG have been reported in populations of African descent. However, because of the unique ancestral relationship between populations of Sub Saharan Africa and all other global populations, this information is likely to be highly informative and of great scientific interest.
4.12 Copy number variants (CNV)
DNA copy number variants (CNVs), i.e. genomic duplications and deletions, have been increasingly associated with a variety of human genetic disorders, including ocular disorders like hereditary benign epithelial dyskeratosis (HBID) (Allingham et al., 2001; White and Nelson, 1975), AMD (Cantsilieris et al., 2012; Grassmann et al., 2016; Park et al., 2012; Sawitzke et al., 2011; Schmid-Kubista et al., 2009; Sivakumaran et al., 2011), uveitis (Hou et al., 2015) and non-ocular conditions like autism and schizophrenia (Alkan et al., 2011). CNVs are defined as changes in DNA copy number of a specific genomic region when compared to a reference genome.
A number of CNVs have been reported in POAG cases. Davis and colleagues performed a study of 400 cases/500 non-glaucoma controls that suggested rare CNVs may play a role in the development of POAG (Davis et al., 2011). A study of POAG in subjects of Indian and European ethnicity reported rare large genomic deletions that were highly enriched in POAG patients (Kaurani et al., 2014). Deletions on chromosome 5q21.2 that contained the gene RAB9BP1, a gene that has been associated with IOP, was associated with higher IOP but was not associated with high tension POAG (Nag et al., 2013). In another study individuals heterozygous for a deletion of the GALC gene (galactosylceramidase) had 5 fold increased risk of POAG (Liu et al., 2011a). Interestingly, Krabbe disease, a rare generally fatal disorder of the myelin sheath that includes progressive optic neuropathy and vision loss occurs when both copies of GALC are nonfunctional (Wenger, 1993; Wenger et al., 1997; Wenger et al., 2000; Wenger et al., 1999). As discussed earlier, a CNV consisting of duplications in the TBK1 are associated with NTG (Awadalla et al., 2015; Fingert et al., 2017; Fingert et al., 2016; Fingert et al., 2011; Kawase et al., 2012; Ritch et al., 2014). A study of DNA copy number variations in approximately 1,600 POAG cases and 1,500 controls identified the presence of rare genomic deletions/duplications in a large number of POAG-associated genes (Liu et al., 2014b). These data further support the role of CNVs as another contributor to POAG pathogenesis.
4.13 POAG-associated Molecular Pathways
POAG is a complex genetic disorder, which in most cases has contributions from multiple genetic factors that participate in a wide variety of cellular signaling pathways (Abu-Amero et al., 2015; Cooke Bailey et al., 2013; Fan and Wiggs, 2010; Janssen et al., 2013; Libby et al., 2005; Wang and Wiggs, 2014; Weinreb et al., 2014). MYOC mutations reportedly affect the ER stress response, which has been implicated in the pathogenesis of glaucoma (Chrysostomou et al., 2013; Zode et al., 2012; Zode et al., 2011). ER stress pathways also appear to play a role in the pathogenesis of POAG in the retina based on mouse models of optic nerve injury or transgenic mutated myocilin (Carbone et al., 2011; Hu et al., 2012; Yang et al., 2016a; Zode et al., 2012; Zode et al., 2011). Mitochondrial-related pathways have also been implicated in POAG pathogenesis. A genetic pathway analysis of mitochondrial genetic variations suggests a relationship between lipid and carbohydrate metabolism and POAG risk (Khawaja et al., 2016). Targeted pathway analyses have suggest the contribution of genes involved in pathways related to vascular tone and estrogen metabolism in women (Dewundara et al., 2016; Kang et al., 2014; Pasquale et al., 2013b).
A genome-wide hypothesis-independent pathway analysis implicated gamma-aminobutyric acid (GABA) and acetyl-CoA metabolism in glaucoma pathogenesis (Bailey et al., 2014). Multiple genetic and molecular studies indicate the important contribution of autophagy to the pathogenesis of POAG (Frost et al., 2014; Hirt and Liton, 2017; Liton, 2016; Minegishi et al., 2016; Porter et al., 2015; Porter et al., 2013; Porter et al., 2014; Slowicka et al., 2016; Tucker et al., 2014; Wong and Holzbaur, 2014). As discussed earlier, a number of genetic factors are involved in or associated with the pathogenesis of POAG. Since a single genetic factor can play a role in multiple molecular pathways, several have been discussed here, any of which may play an important role in POAG pathogenesis (Janssen et al., 2013).
In addition to molecular pathways, interaction between glaucoma-associated genes may also contribute to pathogenesis of POAG. For example, interactions between MYOC and CYP1B1, OPTN and TBK1, and SIX6 with CDKN2B have been reported (Kaur et al., 2005; Minegishi et al., 2013; Minegishi et al., 2016; Mookherjee et al., 2012; Morton et al., 2008; Richter et al., 2016; Seo et al., 2013; Shen et al., 2015; Skowronska-Krawczyk et al., 2015; Vincent et al., 2002). A gene-based SNP-SNP interaction analysis identified several gene-gene interactions with POAG. These include interactions between ZNF385B and ELMO1, ALX4 and RBFOX1, OPCML and RYR3, ROBO1 and HTR2A, and CTNND2 with NRG3 (Verma et al., 2016). Current and future genetic will help unravel POAG’s complex inheritance.
5. Genetic Analyses of POAG-associated Traits (Endophenotypes)
Endophenotypes, a term coined by Bernard John and Kenneth Lewis in studies of grasshopper distribution, are defined as heritable, stable traits that coexist in families and are not state dependent, so often exist whether illness is present or not (John and Lewis, 1966). In other words, though endophenotypes may be related to a disease, they are not disease dependent. Endophenotypes are typically quantitative traits. Quantitative traits, by their very nature, are frequently more amenable to analysis. Association analysis of endophenotypes can be a useful approach to dissect an inherited disease, for example psychiatric disorders, where a disease phenotype may be vague or difficult to define. Well-known and studied endophenotypes for POAG include IOP, CCT, VCDR, and RNFL thickness. Heritability of ocular traits, the variation in a phenotypic trait that is attributable to genetic factors, can be high. For example, estimates for the heritability of IOP is 0.29–0.50, VCDR is 0.48–0.80, CCT is 0.71–0.95 and RNFL thickness is 0.48 (Chang et al., 2005; Charlesworth et al., 2010; Klein et al., 2004; Levene et al., 1970; Schwartz et al., 1975; Toh et al., 2005; van Koolwijk et al., 2007; Vitart et al., 2010a; Zheng et al., 2008). These traits, used to study and dissect a variety of ocular disorders, are individually addressed below.
5.1 IOP
IOP reduction is currently the only clinically validated treatment for glaucoma. Family-based genome-wide linkage analyses have identified several genomic loci (chr5q22, 14q22, and 10q22) that contribute to variation in IOP (Axenovich et al., 2011; Charlesworth et al., 2005; Duggal et al., 2005; Lee et al., 2010; Rotimi et al., 2006). GWAS have identified a large number of IOP-associated genetic variants near or within genetic loci. These loci include TMCO1, GAS7, CAV1/CAV2, GLCCI1/ICA1, MVB12B (aka FAM125B), ARHGEF12, FNDC3B, ABCA1, ABO, ADAMTS8, and chr11p11.2 (Blue Mountains Eye and Wellcome Trust Case Control, 2013; Chen et al., 2015a; Hysi et al., 2014; Nag et al., 2014; Ozel et al., 2014; Springelkamp et al., 2015a; Springelkamp et al., 2017; van Koolwijk et al., 2012). Not surprisingly most IOP-associated variants are associated with POAG, the only exceptions in this list are ABO (blood group) and ADAMTS8 loci. Most IOP-associated genes are expressed in adult human ocular tissues. Interestingly, when combined these loci accounts for less than 2% of the phenotypic variability observed in IOP, which leaves the vast majority of IOP heritability unexplained (Hysi et al., 2014). In addition to finding new IOP-associated loci, identifying rare variants that that lie in IOP-associated loci and contribute a large effect on IOP may account for some of the “missing” inheritance. IOP reduction is currently the only proven, effective therapy for glaucoma. Determining the mechanisms that underlie this quantitative trait will improve our understanding its contribution to glaucoma pathogenesis and will likely provide novel therapeutic targets.
5.2 CCT
CCT is a highly heritable trait that is estimated between 0.71–0.95 and is influenced by ethnicity (Aghaian et al., 2004; Charlesworth et al., 2010; Toh et al., 2005; Zheng et al., 2008). CCT is reportedly thinner in people of African ancestry (Chua et al., 2014; Sample et al., 2009). Although the mechanism is unclear, thinner CCT has been reported as a strong predictor of POAG risk (Deol et al., 2015; Gordon et al., 2002; Sng et al., 2017). Thicker CCT is associated with ocular hypertension and glaucoma suspect status in adults and children (Gordon et al., 2002; Muir et al., 2004). GWAS have identified a large number of CCT-associated sequence variants near or within many genes. These include ZNF469-BANP, FOXO1, COL5A1, RXRA-COL5A1, AVGR8, AKAP13, COL8A2, WNT7B, WNT10A-USP37, NR3C2, GPR15, TIPARP, LCN12-PTGDS, CWC27-ADAMTS6, GLT8D2, SMAD3, VKORC1L1, COL4A3, FAM46A-IBTK, LPAR1, ARID5B, TBL1XR1-KCNMB2, ARHGAP20-POU2AF1, C7ORF42, MPDZ-NF1B, FGF9-SGCG, TJP1, LRRK1, CHSY1, HS3ST3B1-PMP22, and FNDC3B (Cuellar-Partida et al., 2015; Gao et al., 2013; Hoehn et al., 2012; Lu et al., 2010; Lu et al., 2013; Ulmer et al., 2012; Vitart et al., 2010b; Vithana et al., 2011). A pathway analysis of CCT in a population of European ancestry found that genes involved in collagen and extracellular matrix metabolism, collagen fibril organization, and myosin binding were enriched (Lu et al., 2013). However, similar to IOP, the combined genetic effect of these loci accounted for only a small portion (10%) of heritability (Charlesworth et al., 2010; Lu et al., 2013; Toh et al., 2005; Zheng et al., 2008). The relationship between CCT and POAG risk has been a subject of considerable discussion. Although CCT is considered a risk factor for POAG, the only genetic locus that is associated with CCT and POAG risk is FNDC3B (Lu et al., 2013). CCT has been inversely associated with oxygen levels in the human anterior chamber angle (Siegfried et al., 2015). This is consistent with the long-held belief that damage to components of the aqueous outflow pathways by reactive oxygen species (ROS) contributes to elevated IOP, a common feature observed in POAG (Siegfried et al., 2015). In other studies a correlation between CCT, optic nerve head topography, and visual field damage was found (Cankaya et al., 2008; Coman et al., 2014; Iester et al., 2012; Insull et al., 2010; Kaushik et al., 2006; Mokbel and Ghanem, 2010; Pakravan et al., 2007; Papadia et al., 2007; Saenz-Frances et al., 2015; Sullivan-Mee et al., 2005). The relationship between POAG risk and CCT is complex and poorly understood.
5.3 VCDR and RNFL
VCDR is a continuous variable that is used clinically as a measure of optic nerve head cupping to diagnose and follow disease status in glaucoma cases. It is a relatively simple and moderately robust method to approximate loss of neuroretinal rim in glaucoma patients (Foster et al., 2002). Optic nerve head and retinal parameters measured by high resolution imaging technologies like OCT, scanning laser polarimetry, and scanning laser topography are far more accurate and robust (Fallon et al., 2017). A number of genetic loci have been associated with optic nerve head and retinal parameters. These VCDR-associated variants are located near or within many genetic loci, including CDKN2B-AS1, SIX6, ATOH7-PBLD, FRMD8-SCYL1, CDC7-TGFBR3, DCLK1, CHEK2, COL8A1, DUSP1, EXOC2, HSF2, PLCE1, SSSCA1, ADAMTS8, RPAP3, TMTC2, SALL1, BMP2, CHEK2, RERE, RPE65, F5, PDZD2, RREB1, DGKB, VCAN, PSCA, ENO4, RBM23, and CARD10 (Nannini et al., 2017; Ramdas et al., 2010; Springelkamp et al., 2014; Springelkamp et al., 2017). Variants in or near CDC42BPA, CRISPLD1, FAM169B, CDKN1A, COL8A1, ATOH7, PLCE1, TMTC2, ASB7, CHEK2, SIX6, CDKN2B-AS1, SSSCA1, ADAMTS8, DCLK1, BMP2, FLNB, FAM101A, RERE, TRIB2, DUSP1, DDHD1, SALL1, BCAS3, EFEMP1/PNPT, TRIOBP, and HSF2 are associated with cup area (Springelkamp et al., 2014; Springelkamp et al., 2017; Springelkamp et al., 2015b). Variants in or near ATOH7, TMTC2, CHEK2, CDC7-TGFBR3, SALL1, ELP4-PAX6, CARD10, CDC42BPA, F5, DIRC3, RARB, ABI3BP, COL8A1, DCAF4L2, TMTC2, PRDM16, UGT8, CTNNA3, GADD45A, VGLL4, ASB7, and HORMAD2, have been associated with optic disc area, which is the area of the oval scleral opening where the retinal ganglion cell axons exit the eye (Gasten et al., 2012; Ramdas et al., 2010; Springelkamp et al., 2015b). Variants in or near CDKN2B-AS1, SIX6, ATOH7, CDC7-TGFBR3, and CDKN1A are associated with POAG (Carnes et al., 2014; Iglesias et al., 2014; Li et al., 2015; Liu et al., 2013b; Ramdas et al., 2011b; Skowronska-Krawczyk et al., 2015; Springelkamp et al., 2017; Wiggs et al., 2012). It is interesting to note that there are two well-known long noncoding RNAs (lncRNAs), NEAT1 (nuclear paraspeckle assembly transcript 1) and MALAT1 (metastasis associated lung adenocarcinoma transcript 1), are located in the genomic region between FRMD8 and SCYL1 genes. lncRNA MALAT1 has been associated with retinal neurodegeneration and diabetic retinopathy (Liu et al., 2014a; Yan et al., 2014; Yang et al., 2016b; Yao et al., 2016; Zhou et al., 2015). It is not known if these lncRNAs contribute to the development of POAG.
Family-based linkage analysis in a large Dutch pedigree identified two loci for RNFL thickness at chr3p22.2 (DCLK3 locus) and 14q22–q23 (SIX6 locus) (Axenovich et al., 2011). A quantitative analysis of rs33912345, a SIX6 coding variant and a variant in strong linkage disequilibrium with this variant, rs10483727, have been associated with RNFL thickness (Cheng et al., 2015; Kuo et al., 2015). Both variants have been associated with POAG risk. As discussed earlier, there is strong evidence that the SIX6 coding variant rs33912345 has a functional effect that influences RNFL thickness.
6. Differential Expression Tissue Studies in POAG
Microarray or next generation sequencing –based gene expression studies are used to identify disease-related genes and pathways (Jakobs, 2014). Expression profiling in normal and glaucoma-affected ocular tissues offers valuable new information that has advanced our genetic and molecular understanding of POAG. Due to the size of the field the focus of this review will be limited to studies that have used normal and/or glaucomatous human ocular tissues. There are excellent reviews of expression analyses in cell culture and animal models for glaucoma (Jakobs, 2014; Libby et al., 2005; Tezel, 2014; Yang and Zack, 2011). Expression profiling of normal human eye tissues has been performed for TM, ciliary body, iris, cornea, optic nerve head, RGC layer, and retina (Carnes et al., 2016; Diehn et al., 2005; Drewry et al., 2016; Farkas et al., 2013; Janssen et al., 2012; Karali et al., 2016; Kim et al., 2006; Liu et al., 2011b; Miao et al., 2008; O’Brien et al., 2014; Pinelli et al., 2016; Wagner et al., 2013; Whitmore et al., 2014). These studies have generated extensive, tissue specific information for baseline gene expression. These datasets and related databases are excellent tools for use by glaucoma researchers. These include The Ocular Tissue Database (genome.uiowa.edu/otdb), sSyTE (bioinformatics.udel.edu/research/isyte), NEIBank (neibank.nei.nih.gov), and EyeBrowse (https://hpcwebapps.cit.nih.gov/eyebrowse) (Lachke et al., 2012; Wagner et al., 2013; Wistow et al., 2008). Many of these datasets can be found and downloaded from NCBI Gene Expression Omnibus (GEO) repository (www.ncbi.nlm.nih.gov/gds).
TM tissues obtained from glaucoma cases, post-mortem or post-surgical, have been used for differential gene/protein expression studies to identify pathways that contribute to POAG. These pathways include those related to inflammation, cell adhesion, extracellular matrix, and exosome-related pathways in POAG (Borras, 2003; Diskin et al., 2006; Liton et al., 2006; Liu et al., 2013a; Micera et al., 2016; Wang et al., 2008). Expression analysis with glaucoma-affected human Schlemm canal endothelial cells suggests the contribution of cell adhesion, heparin binding, glycosaminoglycan binding, and extracellular matrix remodeling pathways in the pathogenesis of POAG (Cai et al., 2015; Overby et al., 2014). Microarray analyses in astrocytes obtained from optic nerve head tissue have demonstrated the involvement of a variety of biological processes in glaucoma, which include cell adhesion and proliferation, cell motility, extracellular matrix synthesis and degradation, neurosteroids, and signal transduction (Agapova et al., 2003; Hernandez et al., 2002; Lukas et al., 2008; Ricard et al., 1999). Expression analysis of primary lamina cribrosa cells has implicated involvement of TGF-β, extracellular matrix, and fibrosis in POAG (Kirwan et al., 2009). Proteomics analysis using human retina has identified the differential regulation of complement activation related to oxidative stress in glaucomatous retina (Tezel et al., 2010) as well as pathways related to cellular development, stress, and cell death (Funke et al., 2016).
Proteomic studies of tears from patients with POAG provide additional support for the role of inflammation in POAG (Pieragostino et al., 2013; Pieragostino et al., 2012). Elevated levels of zymosterol and glucopyranosyl cholesterol and decreased levels of galactosylceramide and glucosylceramide in glaucomatous aqueous humor suggest the potential contribution of glycoceramide in glaucoma (Aljohani et al., 2013; Aribindi et al., 2013), which is consistent with involvement of GALC in glaucoma (Liu et al., 2011a). Proteomic studies of aqueous humor from POAG patients in comparison to non-glaucoma controls have found elevated concentrations of transthyretin, TGF-β1, TGF-β2, IL-6, IL-8, IL-10, IL-12, TNF-α;, IFN-γ, and HGF (Balaiya et al., 2011; Chua et al., 2012; Cvenkel et al., 2010; Duan et al., 2010; Freedman and Iserovich, 2013; Grus et al., 2008; Hu and Ritch, 2001; Inatani et al., 2001; Janciauskiene et al., 2011; Kuchtey et al., 2010; Min et al., 2006; Ochiai and Ochiai, 2002; Ozcan et al., 2004; Picht et al., 2001; Sawada et al., 2010; Takai et al., 2012; Trivedi et al., 2011; Yamamoto et al., 2005). It is interesting that transthyretin, an abundant protein found in cerebrospinal fluid (CSF), has been shown to form amyloid deposits (Saraiva, 2001). The elevated level of transthyretin in glaucomatous aqueous humor may promote the formation of amyloid deposit in the eye while elevated levels of serum amyloid A has been identified in glaucomatous TM and aqueous humor (Takai et al., 2012; Wang et al., 2008). In summary, differential gene/protein expression analyses support evidence that processes involved in inflammation, extracellular matrix metabolism, cell adhesion, TGF-β, and cell motility in POAG pathology.
7. Conclusion
The rapid development in genome technology has accelerated the identification of POAG-related genetic factors using genome-wide association studies in glaucoma patients. Striking progress has been made toward our understanding of Mendelian and complex inherited forms of POAG. The identification of associations for disease- or endophenotype- related genetic factors has generated new opportunities to investigate the cellular and molecular mechanisms that contribute to factors related to glaucoma. For example, the discovery of common and functional genetic variants for POAG (SIX6) will enable targeted investigations into POAG-associated pathways.
During the last few years, significant genomic resources including the ENCODE project (Encyclopedia of DNA Elements, www.encodeproject.org) (Kellis et al., 2014) and the GTEx project (Genotype-Tissue Expression project, www.gtexportal.org) (Consortium, 2015) have dramatically promoted “post-GWAS” research for a broad array of human disorders, such as cardiovascular disease, diabetes, cancer, and many neurodegenerative disorders. This has highlighted the need to expand the availability of human ocular tissues in health and disease that has slowed progress in the study of many human eye disorders including glaucoma. Specifically, it is of central importance to have access to surgical and postmortem human ocular samples with detailed clinical information for future research on functional genomics and epigenetics. The Duke-Miracles in Sight Program located in Durham, NC, and others have implemented mechanisms to obtain clinically documented ocular tissues that are necessary for high level ophthalmic research (Williams et al., 2016a; Williams et al., 2016b). Ultimately our advanced understanding of glaucoma development provided by these efforts will lead to more effective preventive, diagnostic and therapeutic options for patients with this common leading cause of irreversible blindness.
Acknowledgments
This work was supported by the Glaucoma Research Foundation (San Francisco, CA, USA) (YL), the Glaucoma Foundation (New York, NY, USA) (YL), BrightFocus Foundation (Formerly called American Health Assistance Foundation) (Clarksburg, MD, USA) (YL), Research to Prevent Blindness, Inc. (New York, NY, USA), National Eye Institute (NEI, Bethesda, MD, USA) Grant R01EY023242 (YL), R03EY014939 (RRA), R01EY015543 (RRA), and R01EY023646 (RRA)
Abbreviations
- POAG
primary open-angle glaucoma
- IOP
intraocular pressure
- CCT
central cornea thickness
- GWAS
genome-wide association studies
- TM
trabecular meshwork
- RGC
retinal ganglion cells
- VCDR
vertical cup-to-disc ratio
- RNFL
retinal nerve fiber layer
- HTG
high tension glaucoma
- NTG
normal tension glaucoma
- CNV
copy number variants
- ER
endoplasmic reticulum
- SNP
single nucleotide polymorphism
- ALS
amyotrophic lateral sclerosis
- lncRNA
long non-coding RNA
References
- Abu-Amero K, Kondkar AA, Chalam KV. An Updated Review on the Genetics of Primary Open Angle Glaucoma. Int J Mol Sci. 2015;16:28886–28911. doi: 10.3390/ijms161226135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aga M, Bradley JM, Wanchu R, Yang YF, Acott TS, Keller KE. Differential effects of caveolin-1 and -2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells. Investigative ophthalmology & visual science. 2014;55:5497–5509. doi: 10.1167/iovs.14-14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agapova OA, Yang P, Wang WH, Lane DA, Clark AF, Weinstein BI, Hernandez MR. Altered expression of 3 alpha-hydroxysteroid dehydrogenases in human glaucomatous optic nerve head astrocytes. Neurobiology of disease. 2003;14:63–73. doi: 10.1016/s0969-9961(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Aghaian E, Choe JE, Lin S, Stamper RL. Central corneal thickness of Caucasians, Chinese, Hispanics, Filipinos, African Americans, and Japanese in a glaucoma clinic. Ophthalmology. 2004;111:2211–2219. doi: 10.1016/j.ophtha.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Yatsu K, Ota M, Katsuyama Y, Kashiwagi K, Mabuchi F, Iijima H, Kawase K, Yamamoto T, Nakamura M, Negi A, Sagara T, Kumagai N, Nishida T, Inatani M, Tanihara H, Ohno S, Inoko H, Mizuki N. Microsatellite analysis of the GLC1B locus on chromosome 2 points to NCK2 as a new candidate gene for normal tension glaucoma. The British journal of ophthalmology. 2008;92:1293–1296. doi: 10.1136/bjo.2008.139980. [DOI] [PubMed] [Google Scholar]
- Aljohani AJ, Munguba GC, Guerra Y, Lee RK, Bhattacharya SK. Sphingolipids and ceramides in human aqueous humor. Molecular vision. 2013;19:1966–1984. [PMC free article] [PubMed] [Google Scholar]
- Alkan C, Coe BP, Eichler EE. Genome structural variation discovery and genotyping. Nature reviews. Genetics. 2011;12:363–376. doi: 10.1038/nrg2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Experimental eye research. 2009;88:837–844. doi: 10.1016/j.exer.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham RR, Seo B, Rampersaud E, Bembe M, Challa P, Liu N, Parrish T, Karolak L, Gilbert J, Pericak-Vance MA, Klintworth GK, Vance JM. A duplication in chromosome 4q35 is associated with hereditary benign intraepithelial dyskeratosis. American journal of human genetics. 2001;68:491–494. doi: 10.1086/318194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham RR, Shields MB. Shields’ textbook of glaucoma. 6th. Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia: 2011. [Google Scholar]
- Allingham RR, Wiggs JL, De La Paz MA, Vollrath D, Tallett DA, Broomer B, Jones KH, Del Bono EA, Kern J, Patterson K, Haines JL, Pericak-Vance MA. Gln368STOP myocilin mutation in families with late-onset primary open-angle glaucoma. Investigative ophthalmology & visual science. 1998;39:2288–2295. [PubMed] [Google Scholar]
- Allingham RR, Wiggs JL, Hauser ER, Larocque-Abramson KR, Santiago-Turla C, Broomer B, Del Bono EA, Graham FL, Haines JL, Pericak-Vance MA, Hauser MA. Early Adult-Onset POAG Linked to 15q11-13 Using Ordered Subset Analysis. Invest Ophthalmol Vis Sci. 2005;46:2002–2005. doi: 10.1167/iovs.04-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A) The New England journal of medicine. 1998;338:1022–1027. doi: 10.1056/NEJM199804093381503. [DOI] [PubMed] [Google Scholar]
- Ariani F, Longo I, Frezzotti P, Pescucci C, Mari F, Caporossi A, Frezzotti R, Renieri A. Optineurin gene is not involved in the common high-tension form of primary open-angle glaucoma. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2006;244:1077–1082. doi: 10.1007/s00417-005-0079-3. [DOI] [PubMed] [Google Scholar]
- Aribindi K, Guerra Y, Piqueras Mdel C, Banta JT, Lee RK, Bhattacharya SK. Cholesterol and glycosphingolipids of human trabecular meshwork and aqueous humor: comparative profiles from control and glaucomatous donors. Current eye research. 2013;38:1017–1026. doi: 10.3109/02713683.2013.803123. [DOI] [PubMed] [Google Scholar]
- Aung T, Rezaie T, Okada K, Viswanathan AC, Child AH, Brice G, Bhattacharya SS, Lehmann OJ, Sarfarazi M, Hitchings RA. Clinical features and course of patients with glaucoma with the E50K mutation in the optineurin gene. Investigative ophthalmology & visual science. 2005;46:2816–2822. doi: 10.1167/iovs.04-1133. [DOI] [PubMed] [Google Scholar]
- Awadalla MS, Fingert JH, Roos BE, Chen S, Holmes R, Graham SL, Chehade M, Galanopolous A, Ridge B, Souzeau E, Zhou T, Siggs OM, Hewitt AW, Mackey DA, Burdon KP, Craig JE. Copy number variations of TBK1 in Australian patients with primary open-angle glaucoma. American journal of ophthalmology. 2015;159:124–130 e121. doi: 10.1016/j.ajo.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axenovich T, Zorkoltseva I, Belonogova N, van Koolwijk LM, Borodin P, Kirichenko A, Babenko V, Ramdas WD, Amin N, Despriet DD, Vingerling JR, Lemij HG, Oostra BA, Klaver CC, Aulchenko Y, van Duijn CM. Linkage and association analyses of glaucoma related traits in a large pedigree from a Dutch genetically isolated population. Journal of medical genetics. 2011;48:802–809. doi: 10.1136/jmedgenet-2011-100436. [DOI] [PubMed] [Google Scholar]
- Bailey JN, Loomis SJ, Kang JH, Allingham RR, Gharahkhani P, Khor CC, Burdon KP, Aschard H, Chasman DI, Igo RP, Jr, Hysi PG, Glastonbury CA, Ashley-Koch A, Brilliant M, Brown AA, Budenz DL, Buil A, Cheng CY, Choi H, Christen WG, Curhan G, De Vivo I, Fingert JH, Foster PJ, Fuchs C, Gaasterland D, Gaasterland T, Hewitt AW, Hu F, Hunter DJ, Khawaja AP, Lee RK, Li Z, Lichter PR, Mackey DA, McGuffin P, Mitchell P, Moroi SE, Perera SA, Pepper KW, Qi Q, Realini T, Richards JE, Ridker PM, Rimm E, Ritch R, Ritchie M, Schuman JS, Scott WK, Singh K, Sit AJ, Song YE, Tamimi RM, Topouzis F, Viswanathan AC, Verma SS, Vollrath D, Wang JJ, Weisschuh N, Wissinger B, Wollstein G, Wong TY, Yaspan BL, Zack DJ, Zhang K, Study, E.N., Consortium, A. Weinreb RN, Pericak-Vance MA, Small K, Hammond CJ, Aung T, Liu Y, Vithana EN, MacGregor S, Craig JE, Kraft P, Howell G, Hauser MA, Pasquale LR, Haines JL, Wiggs JL. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nature genetics. 2016;48:189–194. doi: 10.1038/ng.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JN, Yaspan BL, Pasquale LR, Hauser MA, Kang JH, Loomis SJ, Brilliant M, Budenz DL, Christen WG, Fingert J, Gaasterland D, Gaasterland T, Kraft P, Lee RK, Lichter PR, Liu Y, McCarty CA, Moroi SE, Richards JE, Realini T, Schuman JS, Scott WK, Singh K, Sit AJ, Vollrath D, Wollstein G, Zack DJ, Zhang K, Pericak-Vance MA, Allingham RR, Weinreb RN, Haines JL, Wiggs JL. Hypothesis-independent pathway analysis implicates GABA and Acetyl-CoA metabolism in primary open-angle glaucoma and normal-pressure glaucoma. Human genetics. 2014;133:1319–1330. doi: 10.1007/s00439-014-1468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird PN, Foote SJ, Mackey DA, Craig J, Speed TP, Bureau A. Evidence for a novel glaucoma locus at chromosome 3p21-22. Human genetics. 2005;117:249–257. doi: 10.1007/s00439-005-1296-x. [DOI] [PubMed] [Google Scholar]
- Balaiya S, Edwards J, Tillis T, Khetpal V, Chalam KV. Tumor necrosis factor-alpha (TNF-alpha) levels in aqueous humor of primary open angle glaucoma. Clinical ophthalmology. 2011;5:553–556. doi: 10.2147/OPTH.S19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SR, Alward WL, Folberg R. An autosomal dominant form of low-tension glaucoma. American journal of ophthalmology. 1989;108:238–244. doi: 10.1016/0002-9394(89)90112-8. [DOI] [PubMed] [Google Scholar]
- Bettencourt C, Houlden H. Exome sequencing uncovers hidden pathways in familial and sporadic ALS. Nature neuroscience. 2015;18:611–613. doi: 10.1038/nn.4012. [DOI] [PubMed] [Google Scholar]
- Blanchette M, Kent WJ, Riemer C, Elnitski L, Smit AF, Roskin KM, Baertsch R, Rosenbloom K, Clawson H, Green ED, Haussler D, Miller W. Aligning multiple genomic sequences with the threaded blockset aligner. Genome research. 2004;14:708–715. doi: 10.1101/gr.1933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Marchite C, Sanchez-Sanchez F, Lopez-Garrido MP, Inigez-de-Onzono M, Lopez-Martinez F, Lopez-Sanchez E, Alvarez L, Rodriguez-Calvo PP, Mendez-Hernandez C, Fernandez-Vega L, Garcia-Sanchez J, Coca-Prados M, Garcia-Feijoo J, Escribano J. WDR36 and P53 gene variants and susceptibility to primary open-angle glaucoma: analysis of gene-gene interactions. Investigative ophthalmology & visual science. 2011;52:8467–8478. doi: 10.1167/iovs.11-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue Mountains Eye, S., Wellcome Trust Case Control, C. Genome-wide association study of intraocular pressure identifies the GLCCI1/ICA1 region as a glaucoma susceptibility locus. Human molecular genetics. 2013;22:4653–4660. doi: 10.1093/hmg/ddt293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borras T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Progress in retinal and eye research. 2003;22:435–463. doi: 10.1016/s1350-9462(03)00018-1. [DOI] [PubMed] [Google Scholar]
- Budenz DL, Bandi JR, Barton K, Nolan W, Herndon L, Whiteside-de Vos J, Hay-Smith G, Kim H, Tielsch J. Blindness and visual impairment in an urban West African population: the Tema Eye Survey. Ophthalmology. 2012a;119:1744–1753. doi: 10.1016/j.ophtha.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budenz DL, Barton K, Whiteside-de Vos J, Schiffman J, Bandi J, Nolan W, Herndon L, Kim H, Hay-Smith G, Tielsch JM. Prevalence of glaucoma in an urban west African population: the Tema Eye Survey. Arch Ophthalmol. 2012b doi: 10.1001/jamaophthalmol.2013.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon KP, Crawford A, Casson RJ, Hewitt AW, Landers J, Danoy P, Mackey DA, Mitchell P, Healey PR, Craig JE. Glaucoma Risk Alleles at CDKN2B-AS1 Are Associated with Lower Intraocular Pressure, Normal-Tension Glaucoma, and Advanced Glaucoma. Ophthalmology. 2012 doi: 10.1016/j.ophtha.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Burdon KP, Macgregor S, Hewitt AW, Sharma S, Chidlow G, Mills RA, Danoy P, Casson R, Viswanathan AC, Liu JZ, Landers J, Henders AK, Wood J, Souzeau E, Crawford A, Leo P, Wang JJ, Rochtchina E, Nyholt DR, Martin NG, Montgomery GW, Mitchell P, Brown MA, Mackey DA, Craig JE. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nature genetics. 2011 doi: 10.1038/ng.824. [DOI] [PubMed] [Google Scholar]
- Burdon KP, Mitchell P, Lee A, Healey PR, White AJ, Rochtchina E, Thomas PB, Wang JJ, Craig JE. Association of open-angle glaucoma loci with incident glaucoma in the Blue Mountains Eye Study. American journal of ophthalmology. 2015;159:31–36 e31. doi: 10.1016/j.ajo.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Perkumas KM, Qin X, Hauser MA, Stamer WD, Liu Y. Expression Profiling of Human Schlemm’s Canal Endothelial Cells From Eyes With and Without GlaucomaIdentification of Glaucoma Genes in Primary Cultured SC Cells. Investigative ophthalmology & visual science. 2015;56:6747–6753. doi: 10.1167/iovs.15-17720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cankaya AB, Elgin U, Batman A, Acaroglu G. Relationship between central corneal thickness and parameters of optic nerve head topography in healthy subjects. European journal of ophthalmology. 2008;18:32–38. doi: 10.1177/112067210801800106. [DOI] [PubMed] [Google Scholar]
- Cantsilieris S, White SJ, Richardson AJ, Guymer RH, Baird PN. Comprehensive analysis of Copy Number Variation of genes at chromosome 1 and 10 loci associated with late age related macular degeneration. PloS one. 2012;7:e35255. doi: 10.1371/journal.pone.0035255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Jiao X, Liu X, Hennis A, Leske MC, Nemesure B, Hejtmancik JF. CDKN2B Polymorphism Is Associated with Primary Open-Angle Glaucoma (POAG) in the Afro-Caribbean Population of Barbados, West Indies. PloS one. 2012;7:e39278. doi: 10.1371/journal.pone.0039278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli J, Munemasa Y, Kwong JM, Piri N. Overexpression of thioredoxins 1 and 2 increases retinal ganglion cell survival after pharmacologically induced oxidative stress, optic nerve transection, and in experimental glaucoma. Transactions of the American Ophthalmological Society. 2009;107:161–165. [PMC free article] [PubMed] [Google Scholar]
- Carbone MA, Chen Y, Hughes GA, Weinreb RN, Zabriskie NA, Zhang K, Anholt RR. Genes of the unfolded protein response pathway harbor risk alleles for primary open angle glaucoma. PloS one. 2011;6:e20649. doi: 10.1371/journal.pone.0020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnes MU, Allingham RR, Ashley-Koch A, Hauser MA. Transcriptome analysis of adult and fetal trabecular meshwork, cornea, and ciliary body tissues by RNA sequencing. Experimental eye research. 2016 doi: 10.1016/j.exer.2016.11.021. [DOI] [PubMed] [Google Scholar]
- Carnes MU, Liu YP, Allingham RR, Whigham BT, Havens S, Garrett ME, Qiao C, Investigators, N.C. Katsanis N, Wiggs JL, Pasquale LR, Ashley-Koch A, Oh EC, Hauser MA. Discovery and functional annotation of SIX6 variants in primary open-angle glaucoma. PLoS genetics. 2014;10:e1004372. doi: 10.1371/journal.pgen.1004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Kaur K, Komatireddy S, Acharya M, Devi KR, Mukhopadhyay A, Mandal AK, Hasnain SE, Chandrasekhar G, Thomas R, Ray K. Gln48His is the prevalent myocilin mutation in primary open angle and primary congenital glaucoma phenotypes in India. Molecular vision. 2005;11:111–113. [PubMed] [Google Scholar]
- Chang TC, Congdon NG, Wojciechowski R, Munoz B, Gilbert D, Chen P, Friedman DS, West SK. Determinants and heritability of intraocular pressure and cup-to-disc ratio in a defined older population. Ophthalmology. 2005;112:1186–1191. doi: 10.1016/j.ophtha.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth J, Kramer PL, Dyer T, Diego V, Samples JR, Craig JE, Mackey DA, Hewitt AW, Blangero J, Wirtz MK. The path to open-angle glaucoma gene discovery: endophenotypic status of intraocular pressure, cup-to-disc ratio, and central corneal thickness. Investigative ophthalmology & visual science. 2010;51:3509–3514. doi: 10.1167/iovs.09-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth JC, Dyer TD, Stankovich JM, Blangero J, Mackey DA, Craig JE, Green CM, Foote SJ, Baird PN, Sale MM. Linkage to 10q22 for maximum intraocular pressure and 1p32 for maximum cup-to-disc ratio in an extended primary open-angle glaucoma pedigree. Investigative ophthalmology & visual science. 2005;46:3723–3729. doi: 10.1167/iovs.05-0312. [DOI] [PubMed] [Google Scholar]
- Charlesworth JC, Stankovich JM, Mackey DA, Craig JE, Haybittel M, Westmore RN, Sale MM. Confirmation of the adult-onset primary open angle glaucoma locus GLC1B at 2cen-q13 in an Australian family. Ophthalmologica. 2006;220:23–30. doi: 10.1159/000089271. [DOI] [PubMed] [Google Scholar]
- Charlson ES, Sankar PS, Miller-Ellis E, Regina M, Fertig R, Salinas J, Pistilli M, Salowe RJ, Rhodes AL, Merritt WT, 3rd, Chua M, Trachtman BT, Gudiseva HV, Collins DW, Chavali VR, Nichols C, Henderer J, Ying GS, Varma R, Jorgenson E, O’Brien JM. The primary open-angle african american glaucoma genetics study: baseline demographics. Ophthalmology. 2015;122:711–720. doi: 10.1016/j.ophtha.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Klein AP, Klein BE, Lee KE, Truitt B, Klein R, Iyengar SK, Duggal P. Exome array analysis identifies CAV1/CAV2 as a susceptibility locus for intraocular pressure. Investigative ophthalmology & visual science. 2015a;56:544–551. doi: 10.1167/iovs.14-15204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LJ, Ng TK, Fan AH, Leung DY, Zhang M, Wang N, Zheng Y, Liang XY, Chiang SW, Tam PO, Pang CP. Evaluation of NTF4 as a causative gene for primary open-angle glaucoma. Molecular vision. 2012;18:1763–1772. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cai J, Jones DP. Mitochondrial thioredoxin in regulation of oxidant-induced cell death. FEBS letters. 2006;580:6596–6602. doi: 10.1016/j.febslet.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hughes G, Chen X, Qian S, Cao W, Wang L, Wang M, Sun X. Genetic Variants Associated With Different Risks for High Tension Glaucoma and Normal Tension Glaucoma in a Chinese Population. Investigative ophthalmology & visual science. 2015b;56:2595–2600. doi: 10.1167/iovs.14-16269. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lin Y, Vithana EN, Jia L, Zuo X, Wong TY, Chen LJ, Zhu X, Tam PO, Gong B, Qian S, Li Z, Liu X, Mani B, Luo Q, Guzman C, Leung CK, Li X, Cao W, Yang Q, Tham CC, Cheng Y, Zhang X, Wang N, Aung T, Khor CC, Pang CP, Sun X, Yang Z. Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nature genetics. 2014;46:1115–1119. doi: 10.1038/ng.3078. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Allingham RR, Aung T, Tham YC, Hauser MA, Vithana EN, Khor CC, Wong TY. Association of common SIX6 polymorphisms with peripapillary retinal nerve fiber layer thickness: the Singapore Chinese Eye Study. Investigative ophthalmology & visual science. 2015;56:478–483. doi: 10.1167/iovs.14-15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi ZL, Akahori M, Obazawa M, Minami M, Noda T, Nakaya N, Tomarev S, Kawase K, Yamamoto T, Noda S, Sasaoka M, Shimazaki A, Takada Y, Iwata T. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Human molecular genetics. 2010a;19:2606–2615. doi: 10.1093/hmg/ddq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi ZL, Yasumoto F, Sergeev Y, Minami M, Obazawa M, Kimura I, Takada Y, Iwata T. Mutant WDR36 directly affects axon growth of retinal ganglion cells leading to progressive retinal degeneration in mice. Human molecular genetics. 2010b;19:3806–3815. doi: 10.1093/hmg/ddq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow G, Wood JP, Sharma S, Dimasi DP, Burdon KP, Casson RJ, Craig JE. Ocular expression and distribution of products of the POAG-associated chromosome 9p21 gene region. PloS one. 2013;8:e75067. doi: 10.1371/journal.pone.0075067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP, Los Angeles Latino Eye Study, G. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:227–232 e221. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TH, Tomarev S, Porciatti V. Transgenic mice expressing mutated Tyr437His human myocilin develop progressive loss of retinal ganglion cell electrical responsiveness and axonopathy with normal iop. Investigative ophthalmology & visual science. 2014;55:5602–5609. doi: 10.1167/iovs.14-14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou V, Rezania F, Trounce IA, Crowston JG. Oxidative stress and mitochondrial dysfunction in glaucoma. Current opinion in pharmacology. 2013;13:12–15. doi: 10.1016/j.coph.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Chua J, Tham YC, Liao J, Zheng Y, Aung T, Wong TY, Cheng CY. Ethnic differences of intraocular pressure and central corneal thickness: the Singapore Epidemiology of Eye Diseases study. Ophthalmology. 2014;121:2013–2022. doi: 10.1016/j.ophtha.2014.04.041. [DOI] [PubMed] [Google Scholar]
- Chua J, Vania M, Cheung CM, Ang M, Chee SP, Yang H, Li J, Wong TT. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Molecular vision. 2012;18:431–438. [PMC free article] [PubMed] [Google Scholar]
- Cirulli ET, Lasseigne BN, Petrovski S, Sapp PC, Dion PA, Leblond CS, Couthouis J, Lu YF, Wang Q, Krueger BJ, Ren Z, Keebler J, Han Y, Levy SE, Boone BE, Wimbish JR, Waite LL, Jones AL, Carulli JP, Day-Williams AG, Staropoli JF, Xin WW, Chesi A, Raphael AR, McKenna-Yasek D, Cady J, Vianney de Jong JM, Kenna KP, Smith BN, Topp S, Miller J, Gkazi A, Consortium F.S. Al-Chalabi A, van den Berg LH, Veldink J, Silani V, Ticozzi N, Shaw CE, Baloh RH, Appel S, Simpson E, Lagier-Tourenne C, Pulst SM, Gibson S, Trojanowski JQ, Elman L, McCluskey L, Grossman M, Shneider NA, Chung WK, Ravits JM, Glass JD, Sims KB, Van Deerlin VM, Maniatis T, Hayes SD, Ordureau A, Swarup S, Landers J, Baas F, Allen AS, Bedlack RS, Harper JW, Gitler AD, Rouleau GA, Brown R, Harms MB, Cooper GM, Harris T, Myers RM, Goldstein DB. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347:1436–1441. doi: 10.1126/science.aaa3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman L, Costescu M, Alecu M, Coman OA. Correlation between corneal thickness and optic disc morphology in normal tension glaucoma using modern technical analysis. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2014;55:857–862. [PubMed] [Google Scholar]
- Consortium, G.T. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte I, Morcillo J, Bovolenta P. Comparative analysis of Six 3 and Six 6 distribution in the developing and adult mouse brain. Dev Dyn. 2005;234:718–725. doi: 10.1002/dvdy.20463. [DOI] [PubMed] [Google Scholar]
- Cooke Bailey JN, Sobrin L, Pericak-Vance MA, Haines JL, Hammond CJ, Wiggs JL. Advances in the genomics of common eye diseases. Human molecular genetics. 2013;22:R59–65. doi: 10.1093/hmg/ddt396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks KR, Allingham RR, Qin X, Liu Y, Gibson JR, Santiago-Turla C, Larocque-Abramson KR, Del Bono E, Challa P, Herndon LW, Akafo S, Wiggs JL, Schmidt S, Hauser MA. Genome-wide linkage scan for primary open angle glaucoma: influences of ancestry and age at diagnosis. PloS one. 2011;6:e21967. doi: 10.1371/journal.pone.0021967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar-Partida G, Springelkamp H, Lucas SE, Yazar S, Hewitt AW, Iglesias AI, Montgomery GW, Martin NG, Pennell CE, van Leeuwen EM, Verhoeven VJ, Hofman A, Uitterlinden AG, Ramdas WD, Wolfs RC, Vingerling JR, Brown MA, Mills RA, Craig JE, Klaver CC, van Duijn CM, Burdon KP, MacGregor S, Mackey DA. WNT10A exonic variant increases the risk of keratoconus by decreasing corneal thickness. Human molecular genetics. 2015;24:5060–5068. doi: 10.1093/hmg/ddv211. [DOI] [PubMed] [Google Scholar]
- Cvenkel B, Kopitar AN, Ihan A. Inflammatory molecules in aqueous humour and on ocular surface and glaucoma surgery outcome. Mediators of inflammation. 2010;2010:939602. doi: 10.1155/2010/939602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LK, Meyer KJ, Schindler EI, Beck JS, Rudd DS, Grundstad AJ, Scheetz TE, Braun TA, Fingert JH, Alward WL, Kwon YH, Folk JC, Russell SR, Wassink TH, Sheffield VC, Stone EM. Copy number variations and primary open-angle glaucoma. Investigative ophthalmology & visual science. 2011;52:7122–7133. doi: 10.1167/iovs.10-5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deol M, Taylor DA, Radcliffe NM. Corneal hysteresis and its relevance to glaucoma. Curr Opin Ophthalmol. 2015;26:96–102. doi: 10.1097/ICU.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewundara SS, Wiggs JL, Sullivan DA, Pasquale LR. Is Estrogen a Therapeutic Target for Glaucoma? Seminars in ophthalmology. 2016;31:140–146. doi: 10.3109/08820538.2015.1114845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehn JJ, Diehn M, Marmor MF, Brown PO. Differential gene expression in anatomical compartments of the human eye. Genome Biol. 2005;6:R74. doi: 10.1186/gb-2005-6-9-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin S, Kumar J, Cao Z, Schuman JS, Gilmartin T, Head SR, Panjwani N. Detection of differentially expressed glycogenes in trabecular meshwork of eyes with primary open-angle glaucoma. Investigative ophthalmology & visual science. 2006;47:1491–1499. doi: 10.1167/iovs.05-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismuke WM, Challa P, Navarro I, Stamer WD, Liu Y. Human aqueous humor exosomes. Experimental eye research. 2015;132:73–77. doi: 10.1016/j.exer.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan RK, Hill SE, Freeman DM, Nguyen E, Orwig SD, Turnage KC, Lieberman RL. Structural basis for misfolding in myocilin-associated glaucoma. Human molecular genetics. 2015;24:2111–2124. doi: 10.1093/hmg/ddu730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan RK, Hill SE, Turnage KC, Orwig SD, Lieberman RL. The glaucoma-associated olfactomedin domain of myocilin is a novel calcium binding protein. The Journal of biological chemistry. 2012;287:43370–43377. doi: 10.1074/jbc.M112.408906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry M, Helwa I, Allingham RR, Hauser MA, Liu Y. miRNA Profile in Three Different Normal Human Ocular Tissues by miRNA-Seq. Investigative ophthalmology & visual science. 2016;57:3731–3739. doi: 10.1167/iovs.16-19155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Xue P, Wang N, Dong Z, Lu Q, Yang F. Proteomic analysis of aqueous humor from patients with primary open angle glaucoma. Molecular vision. 2010;16:2839–2846. [PMC free article] [PubMed] [Google Scholar]
- Duggal P, Klein AP, Lee KE, Iyengar SK, Klein R, Bailey-Wilson JE, Klein BE. A genetic contribution to intraocular pressure: the beaver dam eye study. Investigative ophthalmology & visual science. 2005;46:555–560. doi: 10.1167/iovs.04-0729. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Elliott MH, Ashpole NE, Gu X, Herrnberger L, McClellan ME, Griffith GL, Reagan AM, Boyce TM, Tanito M, Tamm ER, Stamer WD. Caveolin-1 modulates intraocular pressure: implications for caveolae mechanoprotection in glaucoma. Sci Rep. 2016;6:37127. doi: 10.1038/srep37127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelli A, Izquierdo NJ, Townsend W. Eye diseases in Puerto Rico. Puerto Rico health sciences journal. 2005;24:287–290. [PubMed] [Google Scholar]
- Fallon M, Valero O, Pazos M, Anton A. Diagnostic accuracy of imaging devices in glaucoma: A meta-analysis. Survey of ophthalmology. 2017 doi: 10.1016/j.survophthal.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Fan BJ, Ko WC, Wang DY, Canlas O, Ritch R, Lam DS, Pang CP. Fine mapping of new glaucoma locus GLC1M and exclusion of neuregulin 2 as the causative gene. Molecular vision. 2007;13:779–784. [PMC free article] [PubMed] [Google Scholar]
- Fan BJ, Wang DY, Cheng CY, Ko WC, Lam SC, Pang CP. Different WDR36 mutation pattern in Chinese patients with primary open-angle glaucoma. Molecular vision. 2009;15:646–653. [PMC free article] [PubMed] [Google Scholar]
- Fan BJ, Wang DY, Pasquale LR, Haines JL, Wiggs JL. Genetic variants associated with optic nerve vertical cup-to-disc ratio are risk factors for primary open angle glaucoma in a US Caucasian population. Investigative ophthalmology & visual science. 2011;52:1788–1792. doi: 10.1167/iovs.10-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan BJ, Wiggs JL. Glaucoma: genes, phenotypes, and new directions for therapy. The Journal of clinical investigation. 2010;120:3064–3072. doi: 10.1172/JCI43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas MH, Grant GR, White JA, Sousa ME, Consugar MB, Pierce EA. Transcriptome analyses of the human retina identify unprecedented transcript diversity and 3.5 Mb of novel transcribed sequence via significant alternative splicing and novel genes. BMC genomics. 2013;14:486. doi: 10.1186/1471-2164-14-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH. Primary open-angle glaucoma genes. Eye (Lond) 2011;25:587–595. doi: 10.1038/eye.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Alward WL, Kwon YH, Shankar SP, Andorf JL, Mackey DA, Sheffield VC, Stone EM. No association between variations in the WDR36 gene and primary open-angle glaucoma. Arch Ophthalmol. 2007;125:434–436. doi: 10.1001/archopht.125.3.434-b. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Darbro BW, Qian Q, Van Rheeden R, Miller K, Riker M, Solivan-Timpe F, Roos BR, Robin AL, Mullins RF. TBK1 and flanking genes in human retina. Ophthalmic genetics. 2014;35:35–40. doi: 10.3109/13816810.2013.768674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Human molecular genetics. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Miller K, Hedberg-Buenz A, Roos BR, Lewis CJ, Mullins RF, Anderson MG. Transgenic TBK1 mice have features of normal tension glaucoma. Human molecular genetics. 2017;26:124–132. doi: 10.1093/hmg/ddw372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Robin AL, Scheetz TE, Kwon YH, Liebmann JM, Ritch R, Alward WL. Tank-Binding Kinase 1 (TBK1) Gene and Open-Angle Glaucomas (An American Ophthalmological Society Thesis) Transactions of the American Ophthalmological Society. 2016;114:T6. [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Robin AL, Stone JL, Roos BR, Davis LK, Scheetz TE, Bennett SR, Wassink TH, Kwon YH, Alward WL, Mullins RF, Sheffield VC, Stone EM. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Human molecular genetics. 2011;20:2482–2494. doi: 10.1093/hmg/ddr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Roos BR, Solivan-Timpe F, Miller KA, Oetting TA, Wang K, Kwon YH, Scheetz TE, Stone EM, Alward WL. Analysis of ASB10 variants in open angle glaucoma. Human molecular genetics. 2012;21:4543–4548. doi: 10.1093/hmg/dds288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Survey of ophthalmology. 2002;47:547–561. doi: 10.1016/s0039-6257(02)00353-3. [DOI] [PubMed] [Google Scholar]
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. The British journal of ophthalmology. 2002;86:238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J, Iserovich P. Pro-inflammatory cytokines in glaucomatous aqueous and encysted Molteno implant blebs and their relationship to pressure. Investigative ophthalmology & visual science. 2013;54:4851–4855. doi: 10.1167/iovs.13-12274. [DOI] [PubMed] [Google Scholar]
- Freischmidt A, Wieland T, Richter B, Ruf W, Schaeffer V, Muller K, Marroquin N, Nordin F, Hubers A, Weydt P, Pinto S, Press R, Millecamps S, Molko N, Bernard E, Desnuelle C, Soriani MH, Dorst J, Graf E, Nordstrom U, Feiler MS, Putz S, Boeckers TM, Meyer T, Winkler AS, Winkelman J, de Carvalho M, Thal DR, Otto M, Brannstrom T, Volk AE, Kursula P, Danzer KM, Lichtner P, Dikic I, Meitinger T, Ludolph AC, Strom TM, Andersen PM, Weishaupt JH. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nature neuroscience. 2015;18:631–636. doi: 10.1038/nn.4000. [DOI] [PubMed] [Google Scholar]
- Frezzotti P, Pescucci C, Papa FT, Iester M, Mittica V, Motolese I, Peruzzi S, Artuso R, Longo I, Mencarelli MA, Mittica P, Motolese E, Renieri A. Association between primary open-angle glaucoma (POAG) and WDR36 sequence variance in Italian families affected by POAG. The British journal of ophthalmology. 2011;95:624–626. doi: 10.1136/bjo.2009.167494. [DOI] [PubMed] [Google Scholar]
- Frost LS, Mitchell CH, Boesze-Battaglia K. Autophagy in the eye: implications for ocular cell health. Experimental eye research. 2014;124:56–66. doi: 10.1016/j.exer.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchshofer R, Stephan DA, Russell P, Tamm ER. Gene expression profiling of TGFbeta2- and/or BMP7-treated trabecular meshwork cells: Identification of Smad7 as a critical inhibitor of TGF-beta2 signaling. Experimental eye research. 2009;88:1020–1032. doi: 10.1016/j.exer.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke S, Perumal N, Beck S, Gabel-Scheurich S, Schmelter C, Teister J, Gerbig C, Gramlich OW, Pfeiffer N, Grus FH. Glaucoma related Proteomic Alterations in Human Retina Samples. Sci Rep. 2016;6:29759. doi: 10.1038/srep29759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo ME, Lopez-Rios J, Fernaud-Espinosa I, Granadino B, Sanz R, Ramos C, Ayuso C, Seller MJ, Brunner HG, Bovolenta P, Rodriguez de Cordoba S. Genomic cloning and characterization of the human homeobox gene SIX6 reveals a cluster of SIX genes in chromosome 14 and associates SIX6 hemizygosity with bilateral anophthalmia and pituitary anomalies. Genomics. 1999;61:82–91. doi: 10.1006/geno.1999.5916. [DOI] [PubMed] [Google Scholar]
- Gallenberger M, Kroeber M, Marz L, Koch M, Fuchshofer R, Braunger BM, Iwata T, Tamm ER. Heterozygote Wdr36-deficient mice do not develop glaucoma. Experimental eye research. 2014;128:83–91. doi: 10.1016/j.exer.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Gallenberger M, Meinel DM, Kroeber M, Wegner M, Milkereit P, Bosl MR, Tamm ER. Lack of WDR36 leads to preimplantation embryonic lethality in mice and delays the formation of small subunit ribosomal RNA in human cells in vitro. Human molecular genetics. 2011;20:422–435. doi: 10.1093/hmg/ddq478. [DOI] [PubMed] [Google Scholar]
- Gao J, Ohtsubo M, Hotta Y, Minoshima S. Oligomerization of optineurin and its oxidative stress- or E50K mutation-driven covalent cross-linking: possible relationship with glaucoma pathology. PloS one. 2014;9:e101206. doi: 10.1371/journal.pone.0101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Jakobs TC. Mice Homozygous for a Deletion in the Glaucoma Susceptibility Locus INK4 Show Increased Vulnerability of Retinal Ganglion Cells to Elevated Intraocular Pressure. Am J Pathol. 2016;186:985–1005. doi: 10.1016/j.ajpath.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Gauderman WJ, Liu Y, Marjoram P, Torres M, Haritunians T, Kuo JZ, Chen YD, Allingham RR, Hauser MA, Taylor KD, Rotter JI, Varma R. A genome-wide association study of central corneal thickness in Latinos. Investigative ophthalmology & visual science. 2013;54:2435–2443. doi: 10.1167/iovs.13-11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasten AC, Ramdas WD, Broer L, van Koolwijk LM, Ikram MK, de Jong PT, Aulchenko YS, Wolfs RC, Hofman A, Rivadeneira F, Uitterlinden AG, Oostra BA, Lemij HG, Klaver CC, Jansonius NM, Vingerling JR, van Duijn CM. A genetic epidemiologic study of candidate genes involved in the optic nerve head morphology. Investigative ophthalmology & visual science. 2012;53:1485–1491. doi: 10.1167/iovs.11-7384. [DOI] [PubMed] [Google Scholar]
- Gharahkhani P, Burdon KP, Fogarty R, Sharma S, Hewitt AW, Martin S, Law MH, Cremin K, Bailey JN, Loomis SJ, Pasquale LR, Haines JL, Hauser MA, Viswanathan AC, McGuffin P, Topouzis F, Foster PJ, Graham SL, Casson RJ, Chehade M, White AJ, Zhou T, Souzeau E, Landers J, Fitzgerald JT, Klebe S, Ruddle JB, Goldberg I, Healey PR, Wellcome Trust Case Control, C., Consortium, N. Mills RA, Wang JJ, Montgomery GW, Martin NG, Radford-Smith G, Whiteman DC, Brown MA, Wiggs JL, Mackey DA, Mitchell P, MacGregor S, Craig JE. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nature genetics. 2014;46:1120–1125. doi: 10.1038/ng.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharahkhani P, Burdon KP, Hewitt AW, Law MH, Souzeau E, Montgomery GW, Radford-Smith G, Mackey DA, Craig JE, MacGregor S. Accurate Imputation-Based Screening of Gln368Ter Myocilin Variant in Primary Open-Angle Glaucoma. Investigative ophthalmology & visual science. 2015;56:5087–5093. doi: 10.1167/iovs.15-17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J, Griffiths H, De Salvo G, Cole M, Jacob A, Macleod A, Yang Y, Menon G, Cree A, Ennis S, Lotery A. Genome-wide association study of primary open angle glaucoma risk and quantitative traits. Molecular vision. 2012;18:1083–1092. [PMC free article] [PubMed] [Google Scholar]
- Gijselinck I, Van Mossevelde S, van der Zee J, Sieben A, Philtjens S, Heeman B, Engelborghs S, Vandenbulcke M, De Baets G, Baumer V, Cuijt I, Van den Broeck M, Peeters K, Mattheijssens M, Rousseau F, Vandenberghe R, De Jonghe P, Cras P, De Deyn PP, Martin JJ, Cruts M, Van Broeckhoven C, Consortium B. Loss of TBK1 is a frequent cause of frontotemporal dementia in a Belgian cohort. Neurology. 2015;85:2116–2125. doi: 10.1212/WNL.0000000000002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girkin CA. Differences in optic nerve structure between individuals of predominantly African and European ancestry: Implications for disease detection and pathogenesis. Clinical ophthalmology. 2008;2:65–69. doi: 10.2147/opth.s1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girkin CA, Sample PA, Liebmann JM, Jain S, Bowd C, Becerra LM, Medeiros FA, Racette L, Dirkes KA, Weinreb RN, Zangwill LM, Group, A African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol. 2010;128:541–550. doi: 10.1001/archophthalmol.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. discussion 829-730. [DOI] [PubMed] [Google Scholar]
- Gould DB, Miceli-Libby L, Savinova OV, Torrado M, Tomarev SI, Smith RS, John SW. Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol Cell Biol. 2004a;24:9019–9025. doi: 10.1128/MCB.24.20.9019-9025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould DB, Reedy M, Wilson LA, Smith RS, Johnson RL, John SW. Mutant myocilin nonsecretion in vivo is not sufficient to cause glaucoma. Mol Cell Biol. 2006;26:8427–8436. doi: 10.1128/MCB.01127-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould DB, Smith RS, John SW. Anterior segment development relevant to glaucoma. Int J Dev Biol. 2004b;48:1015–1029. doi: 10.1387/ijdb.041865dg. [DOI] [PubMed] [Google Scholar]
- Grassmann F, Cantsilieris S, Schulz-Kuhnt AS, White SJ, Richardson AJ, Hewitt AW, Vote BJ, Schmied D, Guymer RH, Weber BH, Baird PN. Multiallelic copy number variation in the complement component 4A (C4A) gene is associated with late-stage age-related macular degeneration (AMD) J Neuroinflammation. 2016;13:81. doi: 10.1186/s12974-016-0548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grus FH, Joachim SC, Sandmann S, Thiel U, Bruns K, Lackner KJ, Pfeiffer N. Transthyretin and complex protein pattern in aqueous humor of patients with primary open-angle glaucoma. Molecular vision. 2008;14:1437–1445. [PMC free article] [PubMed] [Google Scholar]
- Gu X, Reagan A, Yen A, Bhatti F, Cohen AW, Elliott MH. Spatial and temporal localization of caveolin-1 protein in the developing retina. Advances in experimental medicine and biology. 2014;801:15–21. doi: 10.1007/978-1-4614-3209-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Reagan AM, McClellan ME, Elliott MH. Caveolins and caveolae in ocular physiology and pathophysiology. Progress in retinal and eye research. 2017;56:84–106. doi: 10.1016/j.preteyeres.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hardy KM, Hoffman EA, Gonzalez P, McKay BS, Stamer WD. Extracellular trafficking of myocilin in human trabecular meshwork cells. The Journal of biological chemistry. 2005;280:28917–28926. doi: 10.1074/jbc.M504803200. [DOI] [PubMed] [Google Scholar]
- Hauser MA, Allingham RR, Linkroum K, Wang J, LaRocque-Abramson K, Figueiredo D, Santiago-Turla C, del Bono EA, Haines JL, Pericak-Vance MA, Wiggs JL. Distribution of WDR36 DNA sequence variants in patients with primary open-angle glaucoma. Investigative ophthalmology & visual science. 2006a;47:2542–2546. doi: 10.1167/iovs.05-1476. [DOI] [PubMed] [Google Scholar]
- Hauser MA, Khor CC, Aung T, Akafo SK, Ashaye A, Olawoye S, Jorgenson E, Hoffmann T, Risch N, Allingham RR. Genetic risk factors for primary open-angle glaucoma in populations of African ancestry. The Annual Meeting of ARVO; Baltimore, MD. 2017. [Google Scholar]
- Hauser MA, Sena DF, Flor J, Walter J, Auguste J, Larocque-Abramson K, Graham F, Delbono E, Haines JL, Pericak-Vance MA, Rand Allingham R, Wiggs JL. Distribution of optineurin sequence variations in an ethnically diverse population of low-tension glaucoma patients from the United States. Journal of glaucoma. 2006b;15:358–363. doi: 10.1097/01.ijg.0000212255.17950.42. [DOI] [PubMed] [Google Scholar]
- Heo JM, Ordureau A, Paulo JA, Rinehart J, Harper JW. The PINK1-PARKIN Mitochondrial Ubiquitylation Pathway Drives a Program of OPTN/NDP52 Recruitment and TBK1 Activation to Promote Mitophagy. Mol Cell. 2015;60:7–20. doi: 10.1016/j.molcel.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MR, Agapova OA, Yang P, Salvador-Silva M, Ricard CS, Aoi S. Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia. 2002;38:45–64. doi: 10.1002/glia.10051. [DOI] [PubMed] [Google Scholar]
- Hewitt AW, Dimasi DP, Mackey DA, Craig JE. A Glaucoma Case-control Study of the WDR36 Gene D658G sequence variant. American journal of ophthalmology. 2006;142:324–325. doi: 10.1016/j.ajo.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Hewitt AW, Mackey DA, Craig JE. Myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008;29:207–211. doi: 10.1002/humu.20634. [DOI] [PubMed] [Google Scholar]
- Hirt J, Liton PB. Autophagy and mechanotransduction in outflow pathway cells. Experimental eye research. 2017;158:146–153. doi: 10.1016/j.exer.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn R, Zeller T, Verhoeven VJ, Grus F, Adler M, Wolfs RC, Uitterlinden AG, Castagne R, Schillert A, Klaver CC, Pfeiffer N, Mirshahi A. Population-based meta-analysis in Caucasians confirms association with COL5A1 and ZNF469 but not COL8A2 with central corneal thickness. Human genetics. 2012;131:1783–1793. doi: 10.1007/s00439-012-1201-3. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Perkumas KM, Highstrom LM, Stamer WD. Regulation of myocilin-associated exosome release from human trabecular meshwork cells. Investigative ophthalmology & visual science. 2009;50:1313–1318. doi: 10.1167/iovs.08-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann TJ, Tang H, Thornton TA, Caan B, Haan M, Millen AE, Thomas F, Risch N. Genome-wide association and admixture analysis of glaucoma in the Women’s Health Initiative. Human molecular genetics. 2014;23:6634–6643. doi: 10.1093/hmg/ddu364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Kijlstra A, Yang P. Molecular Genetic Advances in Uveitis. Prog Mol Biol Transl Sci. 2015;134:283–298. doi: 10.1016/bs.pmbts.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Howell GR, Walton DO, King BL, Libby RT, John SW. Datgan, a reusable software system for facile interrogation and visualization of complex transcription profiling data. BMC genomics. 2011;12:429. doi: 10.1186/1471-2164-12-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DN, Ritch R. Hepatocyte growth factor is increased in the aqueous humor of glaucomatous eyes. Journal of glaucoma. 2001;10:152–157. doi: 10.1097/00061198-200106000-00002. [DOI] [PubMed] [Google Scholar]
- Hu Y, Park KK, Yang L, Wei X, Yang Q, Cho KS, Thielen P, Lee AH, Cartoni R, Glimcher LH, Chen DF, He Z. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012;73:445–452. doi: 10.1016/j.neuron.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Li M, Guo X, Li S, Xiao X, Jia X, Liu X, Zhang Q. Mutation analysis of seven known glaucoma-associated genes in Chinese patients with glaucoma. Investigative ophthalmology & visual science. 2014;55:3594–3602. doi: 10.1167/iovs.14-13927. [DOI] [PubMed] [Google Scholar]
- Hulleman JD. Malattia Leventinese/Doyne Honeycomb Retinal Dystrophy: Similarities to Age-Related Macular Degeneration and Potential Therapies. Advances in experimental medicine and biology. 2016;854:153–158. doi: 10.1007/978-3-319-17121-0_21. [DOI] [PubMed] [Google Scholar]
- Hysi PG, Cheng CY, Springelkamp H, Macgregor S, Bailey JN, Wojciechowski R, Vitart V, Nag A, Hewitt AW, Hohn R, Venturini C, Mirshahi A, Ramdas WD, Thorleifsson G, Vithana E, Khor CC, Stefansson AB, Liao J, Haines JL, Amin N, Wang YX, Wild PS, Ozel AB, Li JZ, Fleck BW, Zeller T, Staffieri SE, Teo YY, Cuellar-Partida G, Luo X, Allingham RR, Richards JE, Senft A, Karssen LC, Zheng Y, Bellenguez C, Xu L, Iglesias AI, Wilson JF, Kang JH, van Leeuwen EM, Jonsson V, Thorsteinsdottir U, Despriet DD, Ennis S, Moroi SE, Martin NG, Jansonius NM, Yazar S, Tai ES, Amouyel P, Kirwan J, van Koolwijk LM, Hauser MA, Jonasson F, Leo P, Loomis SJ, Fogarty R, Rivadeneira F, Kearns L, Lackner KJ, de Jong PT, Simpson CL, Pennell CE, Oostra BA, Uitterlinden AG, Saw SM, Lotery AJ, Bailey-Wilson JE, Hofman A, Vingerling JR, Maubaret C, Pfeiffer N, Wolfs RC, Lemij HG, Young TL, Pasquale LR, Delcourt C, Spector TD, Klaver CC, Small KS, Burdon KP, Stefansson K, Wong TY, Consortium, N., Wellcome Trust Case Control, C. Viswanathan A, Mackey DA, Craig JE, Wiggs JL, van Duijn CM, Hammond CJ, Aung T. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nature genetics. 2014;46:1126–1130. doi: 10.1038/ng.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iester M, Telani S, Frezzotti P, Manni G, Uva M, Figus M, Perdicchi A, Italian Glaucoma R. Differences in central corneal thickness between the paired eyes and the severity of the glaucomatous damage. Eye (Lond) 2012;26:1424–1430. doi: 10.1038/eye.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias AI, Springelkamp H, van der Linde H, Severijnen LA, Amin N, Oostra B, Kockx CE, van den Hout MC, van Ijcken WF, Hofman A, Uitterlinden AG, Verdijk RM, Klaver CC, Willemsen R, van Duijn CM. Exome sequencing and functional analyses suggest that SIX6 is a gene involved in an altered proliferation-differentiation balance early in life and optic nerve degeneration at old age. Human molecular genetics. 2014;23:1320–1332. doi: 10.1093/hmg/ddt522. [DOI] [PubMed] [Google Scholar]
- Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- Insull E, Nicholas S, Ang GS, Poostchi A, Chan K, Wells A. Optic disc area and correlation with central corneal thickness, corneal hysteresis and ocular pulse amplitude in glaucoma patients and controls. Clin Exp Ophthalmol. 2010;38:839–844. doi: 10.1111/j.1442-9071.2010.02373.x. [DOI] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Human molecular genetics. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- Jakobs TC. Differential gene expression in glaucoma. Cold Spring Harb Perspect Med. 2014;4:a020636. doi: 10.1101/cshperspect.a020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janciauskiene S, Westin K, Grip O, Krakau T. Detection of Alzheimer peptides and chemokines in the aqueous humor. European journal of ophthalmology. 2011;21:104–111. doi: 10.5301/ejo.2010.2108. [DOI] [PubMed] [Google Scholar]
- Janssen SF, Gorgels TG, Bossers K, Ten Brink JB, Essing AH, Nagtegaal M, van der Spek PJ, Jansonius NM, Bergen AA. Gene expression and functional annotation of the human ciliary body epithelia. PloS one. 2012;7:e44973. doi: 10.1371/journal.pone.0044973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen SF, Gorgels TG, Ramdas WD, Klaver CC, van Duijn CM, Jansonius NM, Bergen AA. The vast complexity of primary open angle glaucoma: disease genes, risks, molecular mechanisms and pathobiology. Progress in retinal and eye research. 2013;37:31–67. doi: 10.1016/j.preteyeres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- John B, Lewis KR. Chromosome variability and geographic distribution in insects. Science. 1966;152:711–721. doi: 10.1126/science.152.3723.711. [DOI] [PubMed] [Google Scholar]
- Kachaner D, Genin P, Laplantine E, Weil R. Toward an integrative view of Optineurin functions. Cell cycle. 2012;11:2808–2818. doi: 10.4161/cc.20946. [DOI] [PubMed] [Google Scholar]
- Kanemaki N, Tchedre KT, Imayasu M, Kawarai S, Sakaguchi M, Yoshino A, Itoh N, Meguro A, Mizuki N. Dogs and humans share a common susceptibility gene SRBD1 for glaucoma risk. PloS one. 2013;8:e74372. doi: 10.1371/journal.pone.0074372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Loomis SJ, Yaspan BL, Bailey JC, Weinreb RN, Lee RK, Lichter PR, Budenz DL, Liu Y, Realini T, Gaasterland D, Gaasterland T, Friedman DS, McCarty CA, Moroi SE, Olson L, Schuman JS, Singh K, Vollrath D, Wollstein G, Zack DJ, Brilliant M, Sit AJ, Christen WG, Fingert J, Forman JP, Buys ES, Kraft P, Zhang K, Allingham RR, Pericak-Vance MA, Richards JE, Hauser MA, Haines JL, Wiggs JL, Pasquale LR. Vascular tone pathway polymorphisms in relation to primary open-angle glaucoma. Eye (Lond) 2014;28:662–671. doi: 10.1038/eye.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karali M, Persico M, Mutarelli M, Carissimo A, Pizzo M, Singh Marwah V, Ambrosio C, Pinelli M, Carrella D, Ferrari S, Ponzin D, Nigro V, di Bernardo D, Banfi S. High-resolution analysis of the human retina miRNome reveals isomiR variations and novel microRNAs. Nucleic acids research. 2016;44:1525–1540. doi: 10.1093/nar/gkw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC, Siscovick DS, O’Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Melander O, Altshuler D, Merlini PA, Berzuini C, Bernardinelli L, Peyvandi F, Tubaro M, Celli P, Ferrario M, Fetiveau R, Marziliano N, Casari G, Galli M, Ribichini F, Rossi M, Bernardi F, Zonzin P, Piazza A, Yee J, Friedlander Y, Marrugat J, Lucas G, Subirana I, Sala J, Ramos R, Meigs JB, Williams G, Nathan DM, MacRae CA, Havulinna AS, Berglund G, Hirschhorn JN, Asselta R, Duga S, Spreafico M, Daly MJ, Nemesh J, Korn JM, McCarroll SA, Surti A, Guiducci C, Gianniny L, Mirel D, Parkin M, Burtt N, Gabriel SB, Thompson JR, Braund PS, Wright BJ, Balmforth AJ, Ball SG, Hall AS, Linsel-Nitschke P, Lieb W, Ziegler A, Konig I, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Ouwehand W, Deloukas P, Scholz M, Cambien F, Li M, Chen Z, Wilensky R, Matthai W, Qasim A, Hakonarson HH, Devaney J, Burnett MS, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Knouff CW, Waterworth DM, Walker MC, Mooser V, Epstein SE, Scheffold T, Berger K, Huge A, Martinelli N, Olivieri O, Corrocher R, McKeown P, Erdmann E, Konig IR, Holm H, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Do R, Xie C, Siscovick D. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nature genetics. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Meguro A, Nomura E, Uemoto R, Nomura N, Ota M, Kashiwagi K, Mabuchi F, Iijima H, Kawase K, Yamamoto T, Nakamura M, Negi A, Sagara T, Nishida T, Inatani M, Tanihara H, Aihara M, Araie M, Fukuchi T, Abe H, Higashide T, Sugiyama K, Kanamoto T, Kiuchi Y, Iwase A, Chin S, Ohno S, Inoko H, Mizuki N. Association study of genetic variants on chromosome 7q31 with susceptibility to normal tension glaucoma in a Japanese population. Eye (Lond) 2013;27:979–983. doi: 10.1038/eye.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K, Mandal AK, Chakrabarti S. Primary Congenital Glaucoma and the Involvement of CYP1B1. Middle East Afr J Ophthalmol. 2011;18:7–16. doi: 10.4103/0974-9233.75878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K, Reddy AB, Mukhopadhyay A, Mandal AK, Hasnain SE, Ray K, Thomas R, Balasubramanian D, Chakrabarti S. Myocilin gene implicated in primary congenital glaucoma. Clin Genet. 2005;67:335–340. doi: 10.1111/j.1399-0004.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- Kaurani L, Vishal M, Kumar D, Sharma A, Mehani B, Sharma C, Chakraborty S, Jha P, Ray J, Sen A, Dash D, Ray K, Mukhopadhyay A. Gene-rich large deletions are overrepresented in POAG patients of Indian and Caucasian origins. Investigative ophthalmology & visual science. 2014;55:3258–3264. doi: 10.1167/iovs.14-14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Gyatsho J, Jain R, Pandav SS, Gupta A. Correlation between retinal nerve fiber layer thickness and central corneal thickness in patients with ocular hypertension: an optical coherence tomography study. American journal of ophthalmology. 2006;141:884–890. doi: 10.1016/j.ajo.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Kawase K, Allingham RR, Meguro A, Mizuki N, Roos B, Solivan-Timpe FM, Robin AL, Ritch R, Fingert JH. Confirmation of TBK1 duplication in normal tension glaucoma. Experimental eye research. 2012;96:178–180. doi: 10.1016/j.exer.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Wirtz MK. Working your SOCS off: The role of ASB10 and protein degradation pathways in glaucoma. Experimental eye research. 2017;158:154–160. doi: 10.1016/j.exer.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Yang YF, Sun YY, Sykes R, Acott TS, Wirtz MK. Ankyrin repeat and suppressor of cytokine signaling box containing protein-10 is associated with ubiquitin-mediated degradation pathways in trabecular meshwork cells. Molecular vision. 2013;19:1639–1655. [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Yang YF, Sun YY, Sykes R, Gaudette ND, Samples JR, Acott TS, Wirtz MK. Interleukin-20 receptor expression in the trabecular meshwork and its implication in glaucoma. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2014;30:267–276. doi: 10.1089/jop.2013.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellis M, Wold B, Snyder MP, Bernstein BE, Kundaje A, Marinov GK, Ward LD, Birney E, Crawford GE, Dekker J, Dunham I, Elnitski LL, Farnham PJ, Feingold EA, Gerstein M, Giddings MC, Gilbert DM, Gingeras TR, Green ED, Guigo R, Hubbard T, Kent J, Lieb JD, Myers RM, Pazin MJ, Ren B, Stamatoyannopoulos JA, Weng Z, White KP, Hardison RC. Defining functional DNA elements in the human genome. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6131–6138. doi: 10.1073/pnas.1318948111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja AP, Cooke Bailey JN, Kang JH, Allingham RR, Hauser MA, Brilliant M, Budenz DL, Christen WG, Fingert J, Gaasterland D, Gaasterland T, Kraft P, Lee RK, Lichter PR, Liu Y, Medeiros F, Moroi SE, Richards JE, Realini T, Ritch R, Schuman JS, Scott WK, Singh K, Sit AJ, Vollrath D, Wollstein G, Zack DJ, Zhang K, Pericak-Vance M, Weinreb RN, Haines JL, Pasquale LR, Wiggs JL. Assessing the Association of Mitochondrial Genetic Variation With Primary Open-Angle Glaucoma Using Gene-Set Analyses. Investigative ophthalmology & visual science. 2016;57:5046–5052. doi: 10.1167/iovs.16-20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, Johnson RL. Targeted Disruption of the Myocilin Gene (Myoc) Suggests that Human Glaucoma-Causing Mutations Are Gain of Function. Mol Cell Biol. 2001;21:7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CY, Kuehn MH, Clark AF, Kwon YH. Gene expression profile of the adult human retinal ganglion cell layer. Molecular vision. 2006;12:1640–1648. [PubMed] [Google Scholar]
- Kim S, Kim K, Heo DW, Kim JS, Park CK, Kim CS, Kang C. Expression-associated polymorphisms of CAV1-CAV2 affect intraocular pressure and high-tension glaucoma risk. Molecular vision. 2015;21:548–554. [PMC free article] [PubMed] [Google Scholar]
- Kirwan RP, Wordinger RJ, Clark AF, O’Brien CJ. Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Molecular vision. 2009;15:76–88. [PMC free article] [PubMed] [Google Scholar]
- Kitsos G, Eiberg H, Economou-Petersen E, Wirtz MK, Kramer PL, Aspiotis M, Tommerup N, Petersen MB, Psilas K. Genetic linkage of autosomal dominant primary open angle glaucoma to chromosome 3q in a Greek pedigree. European journal of human genetics : EJHG. 2001;9:452–457. doi: 10.1038/sj.ejhg.5200645. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Lee KE. Heritability of risk factors for primary open-angle glaucoma: the Beaver Dam Eye Study. Investigative ophthalmology & visual science. 2004;45:59–62. doi: 10.1167/iovs.03-0516. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight OJ, Girkin CA, Budenz DL, Durbin MK, Feuer WJ, Cirrus, O.C.T.N.D.S.G. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by Cirrus HD-OCT. Arch Ophthalmol. 2012;130:312–318. doi: 10.1001/archopthalmol.2011.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Rosenhammer B, Koschade SE, Braunger BM, Volz C, Jagle H, Tamm ER. Myocilin modulates programmed cell death during retinal development. Experimental eye research. 2014;125:41–52. doi: 10.1016/j.exer.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Kramer PL, Samples JR, Monemi S, Sykes R, Sarfarazi M, Wirtz MK. The role of the WDR36 gene on chromosome 5q22.1 in a large family with primary open-angle glaucoma mapped to this region. Arch Ophthalmol. 2006;124:1328–1331. doi: 10.1001/archopht.124.9.1328. [DOI] [PubMed] [Google Scholar]
- Kryndushkin D, Ihrke G, Piermartiri TC, Shewmaker F. A yeast model of optineurin proteinopathy reveals a unique aggregation pattern associated with cellular toxicity. Mol Microbiol. 2012;86:1531–1547. doi: 10.1111/mmi.12075. [DOI] [PubMed] [Google Scholar]
- Kuchtey J, Rezaei KA, Jaru-Ampornpan P, Sternberg P, Jr, Kuchtey RW. Multiplex cytokine analysis reveals elevated concentration of interleukin-8 in glaucomatous aqueous humor. Investigative ophthalmology & visual science. 2010;51:6441–6447. doi: 10.1167/iovs.10-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MH, Wang K, Roos B, Stone EM, Kwon YH, Alward WL, Mullins RF, Fingert JH. Chromosome 7q31 POAG locus: ocular expression of caveolins and lack of association with POAG in a US cohort. Molecular vision. 2011;17:430–435. [PMC free article] [PubMed] [Google Scholar]
- Kuo JZ, Zangwill LM, Medeiros FA, Liebmann JM, Girkin CA, Hammel N, Rotter JI, Weinreb RN. Quantitative Trait Locus Analysis of SIX1-SIX6 With Retinal Nerve Fiber Layer Thickness in Individuals of European Descent. American journal of ophthalmology. 2015;160:123–130 e121. doi: 10.1016/j.ajo.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HS, Johnson TV, Joe MK, Abu-Asab M, Zhang J, Chan CC, Tomarev SI. Myocilin mediates myelination in the peripheral nervous system through ErbB2/3 signaling. The Journal of biological chemistry. 2013;288:26357–26371. doi: 10.1074/jbc.M112.446138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HS, Nakaya N, Abu-Asab M, Kim HS, Tomarev SI. Myocilin is involved in NgR1/Lingo-1-mediated oligodendrocyte differentiation and myelination of the optic nerve. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:5539–5551. doi: 10.1523/JNEUROSCI.4731-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. The New England journal of medicine. 2009;360:1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Ho JW, Kryukov GV, O’Connell DJ, Aboukhalil A, Bulyk ML, Park PJ, Maas RL. iSyTE: integrated Systems Tool for Eye gene discovery. Investigative ophthalmology & visual science. 2012;53:1617–1627. doi: 10.1167/iovs.11-8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattante S, Millecamps S, Stevanin G, Rivaud-Pechoux S, Moigneu C, Camuzat A, Da Barroca S, Mundwiller E, Couarch P, Salachas F, Hannequin D, Meininger V, Pasquier F, Seilhean D, Couratier P, Danel-Brunaud V, Bonnet AM, Tranchant C, LeGuern E, Brice A, Le Ber I, Kabashi E, French Research Network on, F.T.D., Ftd, A.L.S. Contribution of ATXN2 intermediary polyQ expansions in a spectrum of neurodegenerative disorders. Neurology. 2014;83:990–995. doi: 10.1212/WNL.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Woo SJ, Kim JI, Cho SI, Kim H, Sung J, Seo JS, Kim DM. Replication of a glaucoma candidate gene on 5q22.1 for intraocular pressure in mongolian populations: the GENDISCAN Project. Investigative ophthalmology & visual science. 2010;51:1335–1340. doi: 10.1167/iovs.09-3979. [DOI] [PubMed] [Google Scholar]
- Lei Y, Song M, Wu J, Xing C, Sun X. eNOS Activity in CAV1 Knockout Mouse Eyes. Investigative ophthalmology & visual science. 2016;57:2805–2813. doi: 10.1167/iovs.15-18841. [DOI] [PubMed] [Google Scholar]
- Levene RZ, Workman PL, Broder SW, Hirschhorn K. Heritability of ocular pressure in normal and suspect ranges. Arch Ophthalmol. 1970;84:730–734. doi: 10.1001/archopht.1970.00990040732006. [DOI] [PubMed] [Google Scholar]
- Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Alterations in microRNA expression in stress-induced cellular senescence. Mech Ageing Dev. 2009;130:731–741. doi: 10.1016/j.mad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, McClellan ME, Tanito M, Garteiser P, Towner R, Bissig D, Berkowitz BA, Fliesler SJ, Woodruff ML, Fain GL, Birch DG, Khan MS, Ash JD, Elliott MH. Loss of caveolin-1 impairs retinal function due to disturbance of subretinal microenvironment. The Journal of biological chemistry. 2012;287:16424–16434. doi: 10.1074/jbc.M112.353763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Perissi V, Liu F, Rose DW, Rosenfeld MG. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297:1180–1183. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- Li Z, Allingham RR, Nakano M, Jia L, Chen Y, Ikeda Y, Mani B, Chen LJ, Kee C, Garway-Heath DF, Sripriya S, Fuse N, Abu-Amero KK, Huang C, Namburi P, Burdon K, Perera SA, Gharahkhani P, Lin Y, Ueno M, Ozaki M, Mizoguchi T, Krishnadas SR, Osman EA, Lee MC, Chan AS, Tajudin LS, Do T, Goncalves A, Reynier P, Zhang H, Bourne R, Goh D, Broadway D, Husain R, Negi AK, Su DH, Ho CL, Blanco AA, Leung CK, Wong TT, Yakub A, Liu Y, Nongpiur ME, Han JC, Hon DN, Shantha B, Zhao B, Sang J, Zhang N, Sato R, Yoshii K, Panda-Jonas S, Ashley Koch AE, Herndon LW, Moroi SE, Challa P, Foo JN, Bei JX, Zeng YX, Simmons CP, Bich Chau TN, Sharmila PF, Chew M, Lim B, Tam PO, Chua E, Ng XY, Yong VH, Chong YF, Meah WY, Vijayan S, Seongsoo S, Xu W, Teo YY, Cooke Bailey JN, Kang JH, Haines JL, Cheng CY, Saw SM, Tai ES, Consortium I.C.-G., Consortium N. Richards JE, Ritch R, Gaasterland DE, Pasquale LR, Liu J, Jonas JB, Milea D, George R, Al-Obeidan SA, Mori K, Macgregor S, Hewitt AW, Girkin CA, Zhang M, Sundaresan P, Vijaya L, Mackey DA, Wong TY, Craig JE, Sun X, Kinoshita S, Wiggs JL, Khor CC, Yang Z, Pang CP, Wang N, Hauser MA, Tashiro K, Aung T, Vithana EN. A common variant near TGFBR3 is associated with primary open angle glaucoma. Human molecular genetics. 2015;24:3880–3892. doi: 10.1093/hmg/ddv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Gould DB, Anderson MG, John SW. Complex genetics of glaucoma susceptibility. Annual review of genomics and human genetics. 2005;6:15–44. doi: 10.1146/annurev.genom.6.080604.162209. [DOI] [PubMed] [Google Scholar]
- Lin Y, Liu T, Li J, Yang J, Du Q, Wang J, Yang Y, Liu X, Fan Y, Lu F, Chen Y, Pu Y, Zhang K, He X, Yang Z. A genome-wide scan maps a novel autosomal dominant juvenile-onset open-angle glaucoma locus to 2p15-16. Molecular vision. 2008;14:739–744. [PMC free article] [PubMed] [Google Scholar]
- Liton PB. The autophagic lysosomal system in outflow pathway physiology and pathophysiology. Experimental eye research. 2016;144:29–37. doi: 10.1016/j.exer.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Molecular vision. 2006;12:774–790. [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Yao J, Li XM, Song YC, Wang XQ, Li YJ, Yan B, Jiang Q. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell death & disease. 2014a;5:e1506. doi: 10.1038/cddis.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Allingham RR. Molecular genetics in glaucoma. Experimental eye research. 2011;93:331–339. doi: 10.1016/j.exer.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Allingham RR. Glaucoma. In: Ginsburg GS, Willard HF, editors. Genomic and Personalized Medicine. 2nd. Academic Press; Oxford: 2012. [Google Scholar]
- Liu Y, Allingham RR, Qin X, Layfield D, Dellinger AE, Gibson J, Wheeler J, Ashley-Koch AE, Stamer WD, Hauser MA. Gene expression profile in human trabecular meshwork from patients with primary open-angle glaucoma. Investigative ophthalmology & visual science. 2013a;54:6382–6389. doi: 10.1167/iovs.13-12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bailey JC, Helwa I, Dismuke WM, Cai J, Drewry M, Brilliant MH, Budenz DL, Christen WG, Chasman DI, Fingert JH, Gaasterland D, Gaasterland T, Gordon MO, Igo RP, Jr, Kang JH, Kass MA, Kraft P, Lee RK, Lichter P, Moroi SE, Realini A, Richards JE, Ritch R, Schuman JS, Scott WK, Singh K, Sit AJ, Song YE, Vollrath D, Weinreb R, Medeiros F, Wollstein G, Zack DJ, Zhang K, Pericak-Vance MA, Gonzalez P, Stamer WD, Kuchtey J, Kuchtey RW, Allingham RR, Hauser MA, Pasquale LR, Haines JL, Wiggs JL. A Common Variant in MIR182 Is Associated With Primary Open-Angle Glaucoma in the NEIGHBORHOOD Consortium. Investigative ophthalmology & visual science. 2016;57:3974–3981. doi: 10.1167/iovs.16-19688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Garrett ME, Yaspan BL, Bailey JC, Loomis SJ, Brilliant M, Budenz DL, Christen WG, Fingert JH, Gaasterland D, Gaasterland T, Kang JH, Lee RK, Lichter P, Moroi SE, Realini A, Richards JE, Schuman JS, Scott WK, Singh K, Sit AJ, Vollrath D, Weinreb R, Wollstein G, Zack DJ, Zhang K, Pericak-Vance MA, Haines JL, Pasquale LR, Wiggs JL, Allingham RR, Ashley-Koch AE, Hauser MA. DNA copy number variants of known glaucoma genes in relation to primary open-angle glaucoma. Investigative ophthalmology & visual science. 2014b;55:8251–8258. doi: 10.1167/iovs.14-15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gibson J, Wheeler J, Kwee LC, Santiago-Turla CM, Akafo SK, Lichter PR, Gaasterland DE, Moroi SE, Challa P, Herndon LW, Girkin CA, Budenz DL, Richards JE, Allingham RR, Hauser MA. GALC deletions increase the risk of primary open-angle glaucoma: the role of Mendelian variants in complex disease. PloS one. 2011a;6:e27134. doi: 10.1371/journal.pone.0027134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hauser MA, Akafo SK, Qin X, Miura S, Gibson JR, Wheeler J, Gaasterland DE, Challa P, Herndon LW, Ritch R, Moroi SE, Pasquale LR, Girkin CA, Budenz DL, Wiggs JL, Richards JE, Ashley-Koch AE, Allingham RR. Investigation of known genetic risk factors for primary open angle glaucoma in two populations of african ancestry. Investigative ophthalmology & visual science. 2013b;54:6248–6254. doi: 10.1167/iovs.13-12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu W, Crooks K, Schmidt S, Allingham RR, Hauser MA. No evidence of association of heterozygous NTF4 mutations in patients with primary open-angle glaucoma. American journal of human genetics. 2010;86:498–499. doi: 10.1016/j.ajhg.2009.11.018. author reply 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Munro D, Layfield D, Dellinger A, Walter J, Peterson K, Rickman CB, Allingham RR, Hauser MA. Serial analysis of gene expression (SAGE) in normal human trabecular meshwork. Molecular vision. 2011b;17:885–893. [PMC free article] [PubMed] [Google Scholar]
- Loomis SJ, Kang JH, Weinreb RN, Yaspan BL, Cooke Bailey JN, Gaasterland D, Gaasterland T, Lee RK, Lichter PR, Budenz DL, Liu Y, Realini T, Friedman DS, McCarty CA, Moroi SE, Olson L, Schuman JS, Singh K, Vollrath D, Wollstein G, Zack DJ, Brilliant M, Sit AJ, Christen WG, Fingert J, Kraft P, Zhang K, Allingham RR, Pericak-Vance MA, Richards JE, Hauser MA, Haines JL, Pasquale LR, Wiggs JL. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. Ophthalmology. 2014;121:508–516. doi: 10.1016/j.ophtha.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Dimasi DP, Hysi PG, Hewitt AW, Burdon KP, Toh T, Ruddle JB, Li YJ, Mitchell P, Healey PR, Montgomery GW, Hansell N, Spector TD, Martin NG, Young TL, Hammond CJ, Macgregor S, Craig JE, Mackey DA. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 influence the blinding disease risk factor central corneal thickness. PLoS genetics. 2010;6:e1000947. doi: 10.1371/journal.pgen.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Vitart V, Burdon KP, Khor CC, Bykhovskaya Y, Mirshahi A, Hewitt AW, Koehn D, Hysi PG, Ramdas WD, Zeller T, Vithana EN, Cornes BK, Tay WT, Tai ES, Cheng CY, Liu J, Foo JN, Saw SM, Thorleifsson G, Stefansson K, Dimasi DP, Mills RA, Mountain J, Ang W, Hoehn R, Verhoeven VJ, Grus F, Wolfs R, Castagne R, Lackner KJ, Springelkamp H, Yang J, Jonasson F, Leung DY, Chen LJ, Tham CC, Rudan I, Vatavuk Z, Hayward C, Gibson J, Cree AJ, MacLeod A, Ennis S, Polasek O, Campbell H, Wilson JF, Viswanathan AC, Fleck B, Li X, Siscovick D, Taylor KD, Rotter JI, Yazar S, Ulmer M, Li J, Yaspan BL, Ozel AB, Richards JE, Moroi SE, Haines JL, Kang JH, Pasquale LR, Allingham RR, Ashley-Koch A, Mitchell P, Wang JJ, Wright AF, Pennell C, Spector TD, Young TL, Klaver CC, Martin NG, Montgomery GW, Anderson MG, Aung T, Willoughby CE, Wiggs JL, Pang CP, Thorsteinsdottir U, Lotery AJ, Hammond CJ, van Duijn CM, Hauser MA, Rabinowitz YS, Pfeiffer N, Mackey DA, Craig JE, Macgregor S, Wong TY. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nature genetics. 2013;45:155–163. doi: 10.1038/ng.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas TJ, Miao H, Chen L, Riordan SM, Li W, Crabb AM, Wise A, Du P, Lin SM, Hernandez MR. Susceptibility to glaucoma: differential comparison of the astrocyte transcriptome from glaucomatous African American and Caucasian American donors. Genome Biol. 2008;9:R111. doi: 10.1186/gb-2008-9-7-r111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Chen Y, Ye Z, Sun X, Shi Y, Luo Q, Gong B, Shuai P, Yang J, Zhou Y, Liu X, Zhang K, Tan C, Li Y, Lin Y, Yang Z. Evaluation of the Association Between Common Genetic Variants Near the ABCA1 Gene and Primary Angle Closure Glaucoma in a Han Chinese Population. Investigative ophthalmology & visual science. 2015;56:6248–6254. doi: 10.1167/iovs.15-16741. [DOI] [PubMed] [Google Scholar]
- Mabuchi F, Sakurada Y, Kashiwagi K, Yamagata Z, Iijima H, Tsukahara S. Association between SRBD1 and ELOVL5 gene polymorphisms and primary open-angle glaucoma. Investigative ophthalmology & visual science. 2011;52:4626–4629. doi: 10.1167/iovs.11-7382. [DOI] [PubMed] [Google Scholar]
- Mabuchi F, Sakurada Y, Kashiwagi K, Yamagata Z, Iijima H, Tsukahara S. Association between genetic variants associated with vertical cup-to-disc ratio and phenotypic features of primary open-angle glaucoma. Ophthalmology. 2012;119:1819–1825. doi: 10.1016/j.ophtha.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Mabuchi F, Sakurada Y, Kashiwagi K, Yamagata Z, Iijima H, Tsukahara S. Involvement of genetic variants associated with primary open-angle glaucoma in pathogenic mechanisms and family history of glaucoma. American journal of ophthalmology. 2015;159:437–444 e432. doi: 10.1016/j.ajo.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Mackay DS, Bennett TM, Shiels A. Exome Sequencing Identifies a Missense Variant in EFEMP1 Co-Segregating in a Family with Autosomal Dominant Primary Open-Angle Glaucoma. PloS one. 2015;10:e0132529. doi: 10.1371/journal.pone.0132529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein L. Association of EFEMP1 with malattia leventinese and age-related macular degeneration: a mini-review. Ophthalmic genetics. 2004;25:219–226. doi: 10.1080/13816810490498305. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, Suzuki H, Komure O, Matsuura S, Kobatake K, Morimoto N, Abe K, Suzuki N, Aoki M, Kawata A, Hirai T, Kato T, Ogasawara K, Hirano A, Takumi T, Kusaka H, Hagiwara K, Kaji R, Kawakami H. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Shimogori T, Hattori N, Nukina N. TBK1 controls autophagosomal engulfment of polyubiquitinated mitochondria through p62/SQSTM1 phosphorylation. Human molecular genetics. 2015;24:4429–4442. doi: 10.1093/hmg/ddv179. [DOI] [PubMed] [Google Scholar]
- McKay BS, Congrove NR, Johnson AA, Dismuke WM, Bowen TJ, Stamer WD. A role for myocilin in receptor-mediated endocytosis. PloS one. 2013;8:e82301. doi: 10.1371/journal.pone.0082301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro A, Inoko H, Ota M, Mizuki N, Bahram S. Genome-wide association study of normal tension glaucoma: common variants in SRBD1 and ELOVL5 contribute to disease susceptibility. Ophthalmology. 2010;117:1331–1338 e1335. doi: 10.1016/j.ophtha.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Memarzadeh F, Ying-Lai M, Chung J, Azen SP, Varma R, Los Angeles Latino Eye Study G. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Investigative ophthalmology & visual science. 2010;51:2872–2877. doi: 10.1167/iovs.08-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Chen L, Riordan SM, Li W, Juarez S, Crabb AM, Lukas TJ, Du P, Lin SM, Wise A, Agapova OA, Yang P, Gu CC, Hernandez MR. Gene expression and functional studies of the optic nerve head astrocyte transcriptome from normal African Americans and Caucasian Americans donors. PloS one. 2008;3:e2847. doi: 10.1371/journal.pone.0002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micera A, Quaranta L, Esposito G, Floriani I, Pocobelli A, Sacca SC, Riva I, Manni G, Oddone F. Differential Protein Expression Profiles in Glaucomatous Trabecular Meshwork: An Evaluation Study on a Small Primary Open Angle Glaucoma Population. Adv Ther. 2016;33:252–267. doi: 10.1007/s12325-016-0285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheal S, Ayub H, Islam F, Siddiqui SN, Khan WA, Akhtar F, Qamar R, Khan MI, den Hollander AI. Variants in the ASB10 Gene Are Associated with Primary Open Angle Glaucoma. PloS one. 2015;10:e0145005. doi: 10.1371/journal.pone.0145005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheal S, Ayub H, Khan MI, Bakker B, Schoenmaker-Koller FE, Ali M, Akhtar F, Khan WA, Qamar R, den Hollander AI. Association of known common genetic variants with primary open angle, primary angle closure, and pseudoexfoliation glaucoma in Pakistani cohorts. Molecular vision. 2014;20:1471–1479. [PMC free article] [PubMed] [Google Scholar]
- Min SH, Lee TI, Chung YS, Kim HK. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean journal of ophthalmology : KJO. 2006;20:162–165. doi: 10.3341/kjo.2006.20.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Iejima D, Kobayashi H, Chi ZL, Kawase K, Yamamoto T, Seki T, Yuasa S, Fukuda K, Iwata T. Enhanced optineurin E50K-TBK1 interaction evokes protein insolubility and initiates familial primary open-angle glaucoma. Human molecular genetics. 2013;22:3559–3567. doi: 10.1093/hmg/ddt210. [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Nakayama M, Iejima D, Kawase K, Iwata T. Significance of optineurin mutations in glaucoma and other diseases. Progress in retinal and eye research. 2016;55:149–181. doi: 10.1016/j.preteyeres.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fuse N, Mengkegale M, Ryu M, Seimiya M, Wada Y, Nishida K. Association between primary open-angle glaucoma and WDR36 DNA sequence variants in Japanese. Molecular vision. 2007;13:1912–1919. [PubMed] [Google Scholar]
- Mokbel TH, Ghanem AA. Correlation of central corneal thickness and optic nerve head topography in patients with primary open-angle glaucoma. Oman journal of ophthalmology. 2010;3:75–80. doi: 10.4103/0974-620X.64231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Heon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Human molecular genetics. 2005;14:725–733. doi: 10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- Mookherjee S, Acharya M, Banerjee D, Bhattacharjee A, Ray K. Molecular basis for involvement of CYP1B1 in MYOC upregulation and its potential implication in glaucoma pathogenesis. PloS one. 2012;7:e45077. doi: 10.1371/journal.pone.0045077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookherjee S, Chakraborty S, Vishal M, Banerjee D, Sen A, Ray K. WDR36 variants in East Indian primary open-angle glaucoma patients. Molecular vision. 2011;17:2618–2627. [PMC free article] [PubMed] [Google Scholar]
- Moore AS, Holzbaur EL. Dynamic recruitment and activation of ALS-associated TBK1 with its target optineurin are required for efficient mitophagy. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E3349–3358. doi: 10.1073/pnas.1523810113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton S, Hesson L, Peggie M, Cohen P. Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS letters. 2008;582:997–1002. doi: 10.1016/j.febslet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Muir KW, Jin J, Freedman SF. Central corneal thickness and its relationship to intraocular pressure in children. Ophthalmology. 2004;111:2220–2223. doi: 10.1016/j.ophtha.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Murakami K, Meguro A, Ota M, Shiota T, Nomura N, Kashiwagi K, Mabuchi F, Iijima H, Kawase K, Yamamoto T, Nakamura M, Negi A, Sagara T, Nishida T, Inatani M, Tanihara H, Aihara M, Araie M, Fukuchi T, Abe H, Higashide T, Sugiyama K, Kanamoto T, Kiuchi Y, Iwase A, Ohno S, Inoko H, Mizuki N. Analysis of microsatellite polymorphisms within the GLC1F locus in Japanese patients with normal tension glaucoma. Molecular vision. 2010;16:462–466. [PMC free article] [PubMed] [Google Scholar]
- Nag A, Venturini C, Hysi PG, Arno M, Aldecoa-Otalora Astarloa E, Macgregor S, Hewitt AW, Young TL, Mitchell P, Viswanathan AC, Mackey DA, Hammond CJ. Copy number variation at chromosome 5q21.2 is associated with intraocular pressure. Investigative ophthalmology & visual science. 2013;54:3607–3612. doi: 10.1167/iovs.13-11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag A, Venturini C, Small KS, International Glaucoma Genetics C. Young TL, Viswanathan AC, Mackey DA, Hysi PG, Hammond C. A genome-wide association study of intra-ocular pressure suggests a novel association in the gene FAM125B in the TwinsUK cohort. Human molecular genetics. 2014;23:3343–3348. doi: 10.1093/hmg/ddu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushana A, Chalasani ML, Jain N, Radha V, Rangaraj N, Balasubramanian D, Swarup G. Regulation of endocytic trafficking of transferrin receptor by optineurin and its impairment by a glaucoma-associated mutant. BMC Cell Biol. 2010;11:4. doi: 10.1186/1471-2121-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Ikeda Y, Taniguchi T, Yagi T, Fuwa M, Omi N, Tokuda Y, Tanaka M, Yoshii K, Kageyama M, Naruse S, Matsuda A, Mori K, Kinoshita S, Tashiro K. Three susceptible loci associated with primary open-angle glaucoma identified by genome-wide association study in a Japanese population. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12838–12842. doi: 10.1073/pnas.0906397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannini DR, Torres M, Chen YI, Taylor KD, Rotter JI, Varma R, Gao X. A Genome-Wide Association Study of Vertical Cup-Disc Ratio in a Latino Population. Investigative ophthalmology & visual science. 2017;58:87–95. doi: 10.1167/iovs.16-19891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SK, Burdon KP, Fitzgerald JT, Zhou T, Fogarty R, Souzeau E, Landers J, Mills RA, Casson RJ, Ridge B, Graham SL, Hewitt AW, Mackey DA, Healey PR, Wang JJ, Mitchell P, MacGregor S, Craig JE. Genetic Association at the 9p21 Glaucoma Locus Contributes to Sex Bias in Normal-Tension Glaucoma. Investigative ophthalmology & visual science. 2016;57:3416–3421. doi: 10.1167/iovs.16-19401. [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Searby CC, Alward WL, Walton D, Craig JE, Mackey DA, Kawase K, Kanis AB, Patil SR, Stone EM, Sheffield VC. A spectrum of FOXC1 mutations suggests gene dosage as a mechanism for developmental defects of the anterior chamber of the eye. American journal of human genetics. 2001;68:364–372. doi: 10.1086/318183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien ET, Wang Y, Ying H, Yue BY. Differential expression of genes in cells cultured from juxtacanalicular trabecular meshwork and Schlemm’s canal. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2014;30:291–299. doi: 10.1089/jop.2013.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Japanese journal of ophthalmology. 2002;46:249–253. doi: 10.1016/s0021-5155(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Osman W, Low SK, Takahashi A, Kubo M, Nakamura Y. A genome-wide association study in the Japanese population confirms 9p21 and 14q23 as susceptibility loci for primary open angle glaucoma. Human molecular genetics. 2012;21:2836–2842. doi: 10.1093/hmg/dds103. [DOI] [PubMed] [Google Scholar]
- Overby DR, Zhou EH, Vargas-Pinto R, Pedrigi RM, Fuchshofer R, Braakman ST, Gupta R, Perkumas KM, Sherwood JM, Vahabikashi A, Dang Q, Kim JH, Ethier CR, Stamer WD, Fredberg JJ, Johnson M. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13876–13881. doi: 10.1073/pnas.1410602111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan AA, Ozdemir N, Canataroglu A. The aqueous levels of TGF-beta2 in patients with glaucoma. International ophthalmology. 2004;25:19–22. doi: 10.1023/b:inte.0000018524.48581.79. [DOI] [PubMed] [Google Scholar]
- Ozel AB, Moroi SE, Reed DM, Nika M, Schmidt CM, Akbari S, Scott K, Rozsa F, Pawar H, Musch DC, Lichter PR, Gaasterland D, Branham K, Gilbert J, Garnai SJ, Chen W, Othman M, Heckenlively J, Swaroop A, Abecasis G, Friedman DS, Zack D, Ashley-Koch A, Ulmer M, Kang JH, Consortium N. Liu Y, Yaspan BL, Haines J, Allingham RR, Hauser MA, Pasquale L, Wiggs J, Richards JE, Li JZ. Genome-wide association study and meta-analysis of intraocular pressure. Human genetics. 2014;133:41–57. doi: 10.1007/s00439-013-1349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakravan M, Parsa A, Sanagou M, Parsa CF. Central corneal thickness and correlation to optic disc size: a potential link for susceptibility to glaucoma. The British journal of ophthalmology. 2007;91:26–28. doi: 10.1136/bjo.2006.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang CP, Fan BJ, Canlas O, Wang DY, Dubois S, Tam PO, Lam DS, Raymond V, Ritch R. A genome-wide scan maps a novel juvenile-onset primary open angle glaucoma locus to chromosome 5q. Molecular vision. 2006;12:85–92. [PubMed] [Google Scholar]
- Papadia M, Sofianos C, Iester M, Bricola G, Mete M, Traverso CE. Corneal thickness and visual field damage in glaucoma patients. Eye (Lond) 2007;21:943–947. doi: 10.1038/sj.eye.6702350. [DOI] [PubMed] [Google Scholar]
- Park B, Ying H, Shen X, Park JS, Qiu Y, Shyam R, Yue BY. Impairment of protein trafficking upon overexpression and mutation of optineurin. PloS one. 2010;5:e11547. doi: 10.1371/journal.pone.0011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Lee S, Yu HG, Kim JI, Seo JS. Copy number variation of age-related macular degeneration relevant genes in the Korean population. PloS one. 2012;7:e31243. doi: 10.1371/journal.pone.0031243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale LR, Loomis SJ, Kang JH, Yaspan BL, Abdrabou W, Budenz DL, Chen TC, Delbono E, Friedman DS, Gaasterland D, Gaasterland T, Grosskreutz CL, Lee RK, Lichter PR, Liu Y, McCarty CA, Moroi SE, Olson LM, Realini T, Rhee DJ, Schuman JS, Singh K, Vollrath D, Wollstein G, Zack DJ, Allingham RR, Pericak-Vance MA, Weinreb RN, Zhang K, Hauser MA, Richards JE, Haines JL, Wiggs JL. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. American journal of ophthalmology. 2013a;155:342–353 e345. doi: 10.1016/j.ajo.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale LR, Loomis SJ, Weinreb RN, Kang JH, Yaspan BL, Bailey JC, Gaasterland D, Gaasterland T, Lee RK, Scott WK, Lichter PR, Budenz DL, Liu Y, Realini T, Friedman DS, McCarty CA, Moroi SE, Olson L, Schuman JS, Singh K, Vollrath D, Wollstein G, Zack DJ, Brilliant M, Sit AJ, Christen WG, Fingert J, Kraft P, Zhang K, Allingham RR, Pericak-Vance MA, Richards JE, Hauser MA, Haines JL, Wiggs JL. Estrogen pathway polymorphisms in relation to primary open angle glaucoma: an analysis accounting for gender from the United States. Molecular vision. 2013b;19:1471–1481. [PMC free article] [PubMed] [Google Scholar]
- Pasutto F, Keller KE, Weisschuh N, Sticht H, Samples JR, Yang YF, Zenkel M, Schlotzer-Schrehardt U, Mardin CY, Frezzotti P, Edmunds B, Kramer PL, Gramer E, Reis A, Acott TS, Wirtz MK. Variants in ASB10 are associated with open-angle glaucoma. Human molecular genetics. 2012;21:1336–1349. doi: 10.1093/hmg/ddr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasutto F, Mardin CY, Michels-Rautenstrauss K, Weber BH, Sticht H, Chavarria-Soley G, Rautenstrauss B, Kruse F, Reis A. Profiling of WDR36 missense variants in German patients with glaucoma. Investigative ophthalmology & visual science. 2008;49:270–274. doi: 10.1167/iovs.07-0500. [DOI] [PubMed] [Google Scholar]
- Pasutto F, Matsumoto T, Mardin CY, Sticht H, Brandstatter JH, Michels-Rautenstrauss K, Weisschuh N, Gramer E, Ramdas WD, van Koolwijk LM, Klaver CC, Vingerling JR, Weber BH, Kruse FE, Rautenstrauss B, Barde YA, Reis A. Heterozygous NTF4 mutations impairing neurotrophin-4 signaling in patients with primary open-angle glaucoma. American journal of human genetics. 2009;85:447–456. doi: 10.1016/j.ajhg.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkumas KM, Hoffman EA, McKay BS, Allingham RR, Stamer WD. Myocilin-associated exosomes in human ocular samples. Experimental eye research. 2007;84:209–212. doi: 10.1016/j.exer.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philomenadin FS, Asokan R, N V, George R, Lingam V, Sarangapani S. Genetic association of SNPs near ATOH7, CARD10, CDKN2B, CDC7 and SIX1/SIX6 with the endophenotypes of primary open angle glaucoma in Indian population. PloS one. 2015;10:e0119703. doi: 10.1371/journal.pone.0119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picht G, Welge-Luessen U, Grehn F, Lutjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- Pieragostino D, Agnifili L, Fasanella V, D’Aguanno S, Mastropasqua R, Di Ilio C, Sacchetta P, Urbani A, Del Boccio P. Shotgun proteomics reveals specific modulated protein patterns in tears of patients with primary open angle glaucoma naive to therapy. Mol Biosyst. 2013;9:1108–1116. doi: 10.1039/c3mb25463a. [DOI] [PubMed] [Google Scholar]
- Pieragostino D, Bucci S, Agnifili L, Fasanella V, D’Aguanno S, Mastropasqua A, Ciancaglini M, Mastropasqua L, Di Ilio C, Sacchetta P, Urbani A, Del Boccio P. Differential protein expression in tears of patients with primary open angle and pseudoexfoliative glaucoma. Mol Biosyst. 2012;8:1017–1028. doi: 10.1039/c1mb05357d. [DOI] [PubMed] [Google Scholar]
- Pilli M, Arko-Mensah J, Ponpuak M, Roberts E, Master S, Mandell MA, Dupont N, Ornatowski W, Jiang S, Bradfute SB, Bruun JA, Hansen TE, Johansen T, Deretic V. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity. 2012;37:223–234. doi: 10.1016/j.immuni.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinelli M, Carissimo A, Cutillo L, Lai CH, Mutarelli M, Moretti MN, Singh MV, Karali M, Carrella D, Pizzo M, Russo F, Ferrari S, Ponzin D, Angelini C, Banfi S, di Bernardo D. An atlas of gene expression and gene co-regulation in the human retina. Nucleic acids research. 2016;44:5773–5784. doi: 10.1093/nar/gkw486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Hirt J, Stamer WD, Liton PB. Autophagic dysregulation in glaucomatous trabecular meshwork cells. Biochimica et biophysica acta. 2015;1852:379–385. doi: 10.1016/j.bbadis.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Nallathambi J, Lin Y, Liton PB. Lysosomal basification and decreased autophagic flux in oxidatively stressed trabecular meshwork cells: implications for glaucoma pathogenesis. Autophagy. 2013;9:581–594. doi: 10.4161/auto.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KM, Jeyabalan N, Liton PB. MTOR-independent induction of autophagy in trabecular meshwork cells subjected to biaxial stretch. Biochimica et biophysica acta. 2014;1843:1054–1062. doi: 10.1016/j.bbamcr.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. The British journal of ophthalmology. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, West SK, Rodriguez J, Munoz B, Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819–1826. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- Ramdas WD, van Koolwijk LM, Cree AJ, Janssens AC, Amin N, de Jong PT, Wolfs RC, Gibson J, Kirwan JF, Hofman A, Rivadeneira F, Oostra BA, Uitterlinden AG, Ennis S, Lotery AJ, Lemij HG, Klaver CC, Vingerling JR, Jansonius NM, van Duijn CM. Clinical implications of old and new genes for open-angle glaucoma. Ophthalmology. 2011a;118:2389–2397. doi: 10.1016/j.ophtha.2011.05.040. [DOI] [PubMed] [Google Scholar]
- Ramdas WD, van Koolwijk LM, Ikram MK, Jansonius NM, de Jong PT, Bergen AA, Isaacs A, Amin N, Aulchenko YS, Wolfs RC, Hofman A, Rivadeneira F, Oostra BA, Uitterlinden AG, Hysi P, Hammond CJ, Lemij HG, Vingerling JR, Klaver CC, van Duijn CM. A genome-wide association study of optic disc parameters. PLoS genetics. 2010;6:e1000978. doi: 10.1371/journal.pgen.1000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdas WD, van Koolwijk LM, Lemij HG, Pasutto F, Cree AJ, Thorleifsson G, Janssen SF, Jacoline TB, Amin N, Rivadeneira F, Wolfs RC, Walters GB, Jonasson F, Weisschuh N, Mardin CY, Gibson J, Zegers RH, Hofman A, de Jong PT, Uitterlinden AG, Oostra BA, Thorsteinsdottir U, Gramer E, Welgen-Lussen UC, Kirwan JF, Bergen AA, Reis A, Stefansson K, Lotery AJ, Vingerling JR, Jansonius NM, Klaver CC, van Duijn CM. Common genetic variants associated with open-angle glaucoma. Human molecular genetics. 2011b;20:2464–2471. doi: 10.1093/hmg/ddr120. [DOI] [PubMed] [Google Scholar]
- Rao KN, Kaur I, Parikh RS, Mandal AK, Chandrasekhar G, Thomas R, Chakrabarti S. Variations in NTF4, VAV2, and VAV3 genes are not involved with primary open-angle and primary angle-closure glaucomas in an indian population. Investigative ophthalmology & visual science. 2010;51:4937–4941. doi: 10.1167/iovs.10-5553. [DOI] [PubMed] [Google Scholar]
- Resch ZT, Fautsch MP. Glaucoma-associated myocilin: a better understanding but much more to learn. Experimental eye research. 2009;88:704–712. doi: 10.1016/j.exer.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch ZT, Hann CR, Cook KA, Fautsch MP. Aqueous humor rapidly stimulates myocilin secretion from human trabecular meshwork cells. Experimental eye research. 2010;91:901–908. doi: 10.1016/j.exer.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Ricard CS, Pena JD, Hernandez MR. Differential expression of neural cell adhesion molecule isoforms in normal and glaucomatous human optic nerve heads. Brain research. Molecular brain research. 1999;74:69–82. doi: 10.1016/s0169-328x(99)00264-8. [DOI] [PubMed] [Google Scholar]
- Richter B, Sliter DA, Herhaus L, Stolz A, Wang C, Beli P, Zaffagnini G, Wild P, Martens S, Wagner SA, Youle RJ, Dikic I. Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:4039–4044. doi: 10.1073/pnas.1523926113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritch R, Darbro B, Menon G, Khanna CL, Solivan-Timpe F, Roos BR, Sarfarzi M, Kawase K, Yamamoto T, Robin AL, Lotery AJ, Fingert JH. TBK1 gene duplication and normal-tension glaucoma. JAMA ophthalmology. 2014;132:544–548. doi: 10.1001/jamaophthalmol.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, Sanchez R, Munoz B, West SK, Broman A, Snyder RW, Klein R, Quigley H. Causes of blindness and visual impairment in a population-based sample of U.S. Hispanics. Ophthalmology. 2002;109:737–743. doi: 10.1016/s0161-6420(01)01008-9. [DOI] [PubMed] [Google Scholar]
- Rong SS, Chen LJ, Leung CK, Matsushita K, Jia L, Miki A, Chiang SW, Tam PO, Hashida N, Young AL, Tsujikawa M, Zhang M, Wang N, Nishida K, Pang CP. Ethnic specific association of the CAV1/CAV2 locus with primary open-angle glaucoma. Sci Rep. 2016;6:27837. doi: 10.1038/srep27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotimi CN, Chen G, Adeyemo AA, Jones LS, Agyenim-Boateng K, Eghan BA, Jr, Zhou J, Doumatey A, Lashley K, Huang H, Fasanmade O, Akinsola FB, Ezepue F, Amoah A, Akafo S, Chen Y, Oli J, Johnson T. Genomewide scan and fine mapping of quantitative trait loci for intraocular pressure on 5q and 14q in West Africans. Investigative ophthalmology & visual science. 2006;47:3262–3267. doi: 10.1167/iovs.05-1537. [DOI] [PubMed] [Google Scholar]
- Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM, Jr, Brophy PD, Berkman J, Gattas M, Hyland V, Ruf EM, Schwartz C, Chang EH, Smith RJ, Stratakis CA, Weil D, Petit C, Hildebrandt F. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8090–8095. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz-Frances F, Janez L, Borrego-Sanz L, Berrozpe-Villabona C, Martinez-de-la-Casa JM, Morales-Fernandez L, Garcia-Sanchez J, Santos-Bueso E, Garcia-Feijoo J. Correlations between corneal and optic nerve head variables in healthy subjects and patients with primary open angle glaucoma. International journal of ophthalmology. 2015;8:1156–1161. doi: 10.3980/j.issn.2222-3959.2015.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlender DA, Roberts RC, Arden SD, Spudich G, Taylor MJ, Luzio JP, Kendrick-Jones J, Buss F. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J Cell Biol. 2005;169:285–295. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample PA, Girkin CA, Zangwill LM, Jain S, Racette L, Becerra LM, Weinreb RN, Medeiros FA, Wilson MR, De Leon-Ortega J, Tello C, Bowd C, Liebmann JM. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples JR, Kitsos G, Economou-Petersen E, Steinkamp P, Sykes R, Rust K, Patzer C, Grigoriadou M, Aperis G, Psilas K, Petersen MB, Wirtz MK. Refining the primary open-angle glaucoma GLC1C region on chromosome 3 by haplotype analysis. Clin Genet. 2004;65:40–44. doi: 10.1111/j..2004.00182.x. [DOI] [PubMed] [Google Scholar]
- Sang J, Jia L, Zhao B, Wang H, Zhang N, Wang N. Association of three single nucleotide polymorphisms at the SIX1-SIX6 locus with primary open angle glaucoma in the Chinese population. Sci China Life Sci. 2016;59:694–699. doi: 10.1007/s11427-016-5073-y. [DOI] [PubMed] [Google Scholar]
- Saraiva MJ. Transthyretin mutations in hyperthyroxinemia and amyloid diseases. Hum Mutat. 2001;17:493–503. doi: 10.1002/humu.1132. [DOI] [PubMed] [Google Scholar]
- Sarfarazi M, Child A, Stoilova D, Brice G, Desai T, Trifan OC, Poinoosawmy D, Crick RP. Localization of the fourth locus (GLC1E) for adult-onset primary open-angle glaucoma to the 10p15-p14 region. American journal of human genetics. 1998;62:641–652. doi: 10.1086/301767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Fukuchi T, Tanaka T, Abe H. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Investigative ophthalmology & visual science. 2010;51:903–906. doi: 10.1167/iovs.09-4247. [DOI] [PubMed] [Google Scholar]
- Sawitzke J, Im KM, Kostiha B, Dean M, Gold B. Association assessment of copy number polymorphism and risk of age-related macular degeneration. Ophthalmology. 2011;118:2442–2446. doi: 10.1016/j.ophtha.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz TE, Faga B, Ortega L, Roos BR, Gordon MO, Kass MA, Wang K, Fingert JH. Glaucoma Risk Alleles in the Ocular Hypertension Treatment Study. Ophthalmology. 2016;123:2527–2536. doi: 10.1016/j.ophtha.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Kubista KE, Tosakulwong N, Wu Y, Ryu E, Hecker LA, Baratz KH, Brown WL, Edwards AO. Contribution of copy number variation in the regulation of complement activation locus to development of age-related macular degeneration. Investigative ophthalmology & visual science. 2009;50:5070–5079. doi: 10.1167/iovs.09-3975. [DOI] [PubMed] [Google Scholar]
- Schwartz JT, Reuling FH, Feinleib M. Size of the physiologic cup of the optic nerve head. hereditary and environmental factors. Arch Ophthalmol. 1975;93:776–778. doi: 10.1001/archopht.1975.01010020670002. [DOI] [PubMed] [Google Scholar]
- Senatorov V, Malyukova I, Fariss R, Wawrousek EF, Swaminathan S, Sharan SK, Tomarev S. Expression of mutated mouse myocilin induces open-angle glaucoma in transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:11903–11914. doi: 10.1523/JNEUROSCI.3020-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Solivan-Timpe F, Roos BR, Robin AL, Stone EM, Kwon YH, Alward WL, Fingert JH. Identification of proteins that interact with TANK binding kinase 1 and testing for mutations associated with glaucoma. Current eye research. 2013;38:310–315. doi: 10.3109/02713683.2012.754047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Burdon KP, Chidlow G, Klebe S, Crawford A, Dimasi DP, Dave A, Martin S, Javadiyan S, Wood JP, Casson R, Danoy P, Griggs K, Hewitt AW, Landers J, Mitchell P, Mackey DA, Craig JE. Association of genetic variants in the TMCO1 gene with clinical parameters related to glaucoma and characterization of the protein in the eye. Investigative ophthalmology & visual science. 2012;53:4917–4925. doi: 10.1167/iovs.11-9047. [DOI] [PubMed] [Google Scholar]
- Sheffield VC, Stone EM, Alward WL, Drack AV, Johnson AT, Streb LM, Nichols BE. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nature genetics. 1993;4:47–50. doi: 10.1038/ng0593-47. [DOI] [PubMed] [Google Scholar]
- Shen WC, Li HY, Chen GC, Chern Y, Tu PH. Mutations in the ubiquitin-binding domain of OPTN/optineurin interfere with autophagy-mediated degradation of misfolded proteins by a dominant-negative mechanism. Autophagy. 2015;11:685–700. doi: 10.4161/auto.36098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shete S, Hosking FJ, Robertson LB, Dobbins SE, Sanson M, Malmer B, Simon M, Marie Y, Boisselier B, Delattre JY, Hoang-Xuan K, El Hallani S, Idbaih A, Zelenika D, Andersson U, Henriksson R, Bergenheim AT, Feychting M, Lonn S, Ahlbom A, Schramm J, Linnebank M, Hemminki K, Kumar R, Hepworth SJ, Price A, Armstrong G, Liu Y, Gu X, Yu R, Lau C, Schoemaker M, Muir K, Swerdlow A, Lathrop M, Bondy M, Houlston RS. Genome-wide association study identifies five susceptibility loci for glioma. Nature genetics. 2009;41:899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim MS, Takihara Y, Kim KY, Iwata T, Yue BY, Inatani M, Weinreb RN, Perkins GA, Ju WK. Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration. Sci Rep. 2016;6:33830. doi: 10.1038/srep33830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried CJ, Shui YB, Bai F, Beebe DC. Central corneal thickness correlates with oxygen levels in the human anterior chamber angle. American journal of ophthalmology. 2015;159:457–462 e451. doi: 10.1016/j.ajo.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumaran TA, Igo RP, Jr, Kidd JM, Itsara A, Kopplin LJ, Chen W, Hagstrom SA, Peachey NS, Francis PJ, Klein ML, Chew EY, Ramprasad VL, Tay WT, Mitchell P, Seielstad M, Stambolian DE, Edwards AO, Lee KE, Leontiev DV, Jun G, Wang Y, Tian L, Qiu F, Henning AK, LaFramboise T, Sen P, Aarthi M, George R, Raman R, Das MK, Vijaya L, Kumaramanickavel G, Wong TY, Swaroop A, Abecasis GR, Klein R, Klein BE, Nickerson DA, Eichler EE, Iyengar SK. A 32 kb critical region excluding Y402H in CFH mediates risk for age-related macular degeneration. PloS one. 2011;6:e25598. doi: 10.1371/journal.pone.0025598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarie JM, Link BA. The primary open-angle glaucoma gene WDR36 functions in ribosomal RNA processing and interacts with the p53 stress-response pathway. Human molecular genetics. 2008;17:2474–2485. doi: 10.1093/hmg/ddn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronska-Krawczyk D, Zhao L, Zhu J, Weinreb RN, Cao G, Luo J, Flagg K, Patel S, Wen C, Krupa M, Luo H, Ouyang H, Lin D, Wang W, Li G, Xu Y, Li O, Chung C, Yeh E, Jafari M, Ai M, Zhong Z, Shi W, Zheng L, Krawczyk M, Chen D, Shi C, Zin C, Zhu J, Mellon PL, Gao W, Abagyan R, Zhang L, Sun X, Zhong S, Zhuo Y, Rosenfeld MG, Liu Y, Zhang K. P16INK4a Upregulation Mediated by SIX6 Defines Retinal Ganglion Cell Pathogenesis in Glaucoma. Mol Cell. 2015;59:931–940. doi: 10.1016/j.molcel.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowicka K, Vereecke L, van Loo G. Cellular Functions of Optineurin in Health and Disease. Trends Immunol. 2016;37:621–633. doi: 10.1016/j.it.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Sng CC, Ang M, Barton K. Central corneal thickness in glaucoma. Curr Opin Ophthalmol. 2017;28:120–126. doi: 10.1097/ICU.0000000000000335. [DOI] [PubMed] [Google Scholar]
- Souzeau E, Burdon KP, Dubowsky A, Grist S, Usher B, Fitzgerald JT, Crawford A, Hewitt AW, Goldberg I, Mills RA, Ruddle JB, Landers J, Mackey DA, Craig JE. Higher prevalence of myocilin mutations in advanced glaucoma in comparison with less advanced disease in an Australasian disease registry. Ophthalmology. 2013;120:1135–1143. doi: 10.1016/j.ophtha.2012.11.029. [DOI] [PubMed] [Google Scholar]
- Souzeau E, Glading J, Keane M, Ridge B, Zhou T, Burdon KP, Craig JE. Predictive genetic testing experience for myocilin primary open-angle glaucoma using the Australian and New Zealand Registry of Advanced Glaucoma. Genetics in medicine : official journal of the American College of Medical Genetics. 2014;16:558–563. doi: 10.1038/gim.2013.196. [DOI] [PubMed] [Google Scholar]
- Souzeau E, Glading J, Ridge B, Wechsler D, Chehade M, Dubowsky A, Burdon KP, Craig JE. Predictive genetic testing in minors for Myocilin juvenile onset open angle glaucoma. Clin Genet. 2015;88:584–588. doi: 10.1111/cge.12558. [DOI] [PubMed] [Google Scholar]
- Sowden JC. Molecular and developmental mechanisms of anterior segment dysgenesis. Eye (Lond) 2007;21:1310–1318. doi: 10.1038/sj.eye.6702852. [DOI] [PubMed] [Google Scholar]
- Springelkamp H, Hohn R, Mishra A, Hysi PG, Khor CC, Loomis SJ, Bailey JN, Gibson J, Thorleifsson G, Janssen SF, Luo X, Ramdas WD, Vithana E, Nongpiur ME, Montgomery GW, Xu L, Mountain JE, Gharahkhani P, Lu Y, Amin N, Karssen LC, Sim KS, van Leeuwen EM, Iglesias AI, Verhoeven VJ, Hauser MA, Loon SC, Despriet DD, Nag A, Venturini C, Sanfilippo PG, Schillert A, Kang JH, Landers J, Jonasson F, Cree AJ, van Koolwijk LM, Rivadeneira F, Souzeau E, Jonsson V, Menon G, Blue Mountains Eye Study G.g. Weinreb RN, de Jong PT, Oostra BA, Uitterlinden AG, Hofman A, Ennis S, Thorsteinsdottir U, Burdon KP, Consortium N., Wellcome Trust Case Control C. Spector TD, Mirshahi A, Saw SM, Vingerling JR, Teo YY, Haines JL, Wolfs RC, Lemij HG, Tai ES, Jansonius NM, Jonas JB, Cheng CY, Aung T, Viswanathan AC, Klaver CC, Craig JE, Macgregor S, Mackey DA, Lotery AJ, Stefansson K, Bergen AA, Young TL, Wiggs JL, Pfeiffer N, Wong TY, Pasquale LR, Hewitt AW, van Duijn CM, Hammond CJ. Meta-analysis of genome-wide association studies identifies novel loci that influence cupping and the glaucomatous process. Nature communications. 2014;5:4883. doi: 10.1038/ncomms5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springelkamp H, Iglesias AI, Cuellar-Partida G, Amin N, Burdon KP, van Leeuwen EM, Gharahkhani P, Mishra A, van der Lee SJ, Hewitt AW, Rivadeneira F, Viswanathan AC, Wolfs RC, Martin NG, Ramdas WD, van Koolwijk LM, Pennell CE, Vingerling JR, Mountain JE, Uitterlinden AG, Hofman A, Mitchell P, Lemij HG, Wang JJ, Klaver CC, Mackey DA, Craig JE, van Duijn CM, MacGregor S. ARHGEF12 influences the risk of glaucoma by increasing intraocular pressure. Human molecular genetics. 2015a;24:2689–2699. doi: 10.1093/hmg/ddv027. [DOI] [PubMed] [Google Scholar]
- Springelkamp H, Iglesias AI, Mishra A, Hohn R, Wojciechowski R, Khawaja AP, Nag A, Wang YX, Wang JJ, Cuellar-Partida G, Gibson J, Bailey JN, Vithana EN, Gharahkhani P, Boutin T, Ramdas WD, Zeller T, Luben RN, Yonova-Doing E, Viswanathan AC, Yazar S, Cree AJ, Haines JL, Koh JY, Souzeau E, Wilson JF, Amin N, Muller C, Venturini C, Kearns LS, Kang JH, Consortium N. Tham YC, Zhou T, van Leeuwen EM, Nickels S, Sanfilippo P, Liao J, van der Linde H, Zhao W, van Koolwijk LM, Zheng L, Rivadeneira F, Baskaran M, van der Lee SJ, Perera S, de Jong PT, Oostra BA, Uitterlinden AG, Fan Q, Hofman A, Tai ES, Vingerling JR, Sim X, Wolfs RC, Teo YY, Lemij HG, Khor CC, Willemsen R, Lackner KJ, Aung T, Jansonius NM, Montgomery G, Wild PS, Young TL, Burdon KP, Hysi PG, Pasquale LR, Wong TY, Klaver CC, Hewitt AW, Jonas JB, Mitchell P, Lotery AJ, Foster PJ, Vitart V, Pfeiffer N, Craig JE, Mackey DA, Hammond CJ, Wiggs JL, Cheng CY, van Duijn CM, MacGregor S. New insights into the genetics of primary open-angle glaucoma based on meta-analyses of intraocular pressure and optic disc characteristics. Human molecular genetics. 2017;26:438–453. doi: 10.1093/hmg/ddw399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springelkamp H, Mishra A, Hysi PG, Gharahkhani P, Hohn R, Khor CC, Cooke Bailey JN, Luo X, Ramdas WD, Vithana E, Koh V, Yazar S, Xu L, Forward H, Kearns LS, Amin N, Iglesias AI, Sim KS, van Leeuwen EM, Demirkan A, van der Lee S, Loon SC, Rivadeneira F, Nag A, Sanfilippo PG, Schillert A, de Jong PT, Oostra BA, Uitterlinden AG, Hofman A, Consortium N. Zhou T, Burdon KP, Spector TD, Lackner KJ, Saw SM, Vingerling JR, Teo YY, Pasquale LR, Wolfs RC, Lemij HG, Tai ES, Jonas JB, Cheng CY, Aung T, Jansonius NM, Klaver CC, Craig JE, Young TL, Haines JL, MacGregor S, Mackey DA, Pfeiffer N, Wong TY, Wiggs JL, Hewitt AW, van Duijn CM, Hammond CJ. Meta-analysis of Genome-Wide Association Studies Identifies Novel Loci Associated With Optic Disc Morphology. Genetic epidemiology. 2015b;39:207–216. doi: 10.1002/gepi.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Hoffman EA, Luther JM, Hachey DL, Schey KL. Protein profile of exosomes from trabecular meshwork cells. Journal of proteomics. 2011;74:796–804. doi: 10.1016/j.jprot.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoilova D, Child A, Trifan OC, Crick RP, Coakes RL, Sarfarazi M. Localization of a locus (GLC1B) for adult-onset primary open angle glaucoma to the 2cen-q13 region. Genomics. 1996;36:142–150. doi: 10.1006/geno.1996.0434. [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Stone EM, Lotery AJ, Munier FL, Heon E, Piguet B, Guymer RH, Vandenburgh K, Cousin P, Nishimura D, Swiderski RE, Silvestri G, Mackey DA, Hageman GS, Bird AC, Sheffield VC, Schorderet DF. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nature genetics. 1999;22:199–202. doi: 10.1038/9722. [DOI] [PubMed] [Google Scholar]
- Sullivan-Mee M, Halverson KD, Saxon GB, Saxon MC, Shafer KM, Sterling JA, Sterling MJ, Qualls C. The relationship between central corneal thickness-adjusted intraocular pressure and glaucomatous visual-field loss. Optometry. 2005;76:228–238. doi: 10.1016/s1529-1839(05)70298-0. [DOI] [PubMed] [Google Scholar]
- Surgucheva I, Surguchov A. Expression of caveolin in trabecular meshwork cells and its possible implication in pathogenesis of primary open angle glaucoma. Molecular vision. 2011;17:2878–2888. [PMC free article] [PubMed] [Google Scholar]
- Suriyapperuma SP, Child A, Desai T, Brice G, Kerr A, Crick RP, Sarfarazi M. A new locus (GLC1H) for adult-onset primary open-angle glaucoma maps to the 2p15-p16 region. Arch Ophthalmol. 2007;125:86–92. doi: 10.1001/archopht.125.1.86. [DOI] [PubMed] [Google Scholar]
- Takai Y, Tanito M, Ohira A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Investigative ophthalmology & visual science. 2012;53:241–247. doi: 10.1167/iovs.11-8434. [DOI] [PubMed] [Google Scholar]
- Takamoto M, Kaburaki T, Mabuchi A, Araie M, Amano S, Aihara M, Tomidokoro A, Iwase A, Mabuchi F, Kashiwagi K, Shirato S, Yasuda N, Kawashima H, Nakajima F, Numaga J, Kawamura Y, Sasaki T, Tokunaga K. Common variants on chromosome 9p21 are associated with normal tension glaucoma. PloS one. 2012;7:e40107. doi: 10.1371/journal.pone.0040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Hayashi T, Bedell M, Zhang K, Yamada H, Tsuneoka H. A novel haplotype with the R345W mutation in the EFEMP1 gene associated with autosomal dominant drusen in a Japanese family. Investigative ophthalmology & visual science. 2010;51:1643–1650. doi: 10.1167/iovs.09-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm ER. Myocilin and glaucoma: facts and ideas. Progress in retinal and eye research. 2002;21:395–428. doi: 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Templeton JP, Freeman NE, Nickerson JM, Jablonski MM, Rex TS, Williams RW, Geisert EE. Innate immune network in the retina activated by optic nerve crush. Investigative ophthalmology & visual science. 2013;54:2599–2606. doi: 10.1167/iovs.12-11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G. A decade of proteomics studies of glaucomatous neurodegeneration. Proteomics Clin Appl. 2014;8:154–167. doi: 10.1002/prca.201300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Kain AD, Powell DW, Kuehn MH, Kaplan HJ. Oxidative stress and the regulation of complement activation in human glaucoma. Investigative ophthalmology & visual science. 2010;51:5071–5082. doi: 10.1167/iovs.10-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DS, Tam PO, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JC, Karwatowski WS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U, Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nature genetics. 2010;42:906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA : the journal of the American Medical Association. 1991;266:369–374. [PubMed] [Google Scholar]
- Toh T, Liew SH, MacKinnon JR, Hewitt AW, Poulsen JL, Spector TD, Gilbert CE, Craig JE, Hammond CJ, Mackey DA. Central corneal thickness is highly heritable: the twin eye studies. Investigative ophthalmology & visual science. 2005;46:3718–3722. doi: 10.1167/iovs.04-1497. [DOI] [PubMed] [Google Scholar]
- Trifan OC, Traboulsi EI, Stoilova D, Alozie I, Nguyen R, Raja S, Sarfarazi M. A third locus (GLC1D) for adult-onset primary open-angle glaucoma maps to the 8q23 region. American journal of ophthalmology. 1998;126:17–28. doi: 10.1016/s0002-9394(98)00073-7. [DOI] [PubMed] [Google Scholar]
- Trivedi RH, Nutaitis M, Vroman D, Crosson CE. Influence of race and age on aqueous humor levels of transforming growth factor-beta 2 in glaucomatous and nonglaucomatous eyes. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2011;27:477–480. doi: 10.1089/jop.2010.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng HC, Riday TT, McKee C, Braine CE, Bomze H, Barak I, Marean-Reardon C, John SW, Philpot BD, Ehlers MD. Visual impairment in an optineurin mouse model of primary open-angle glaucoma. Neurobiol Aging. 2015;36:2201–2212. doi: 10.1016/j.neurobiolaging.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Molecular vision. 2006;12:1319–1333. [PubMed] [Google Scholar]
- Tucker BA, Solivan-Timpe F, Roos BR, Anfinson KR, Robin AL, Wiley LA, Mullins RF, Fingert JH. Duplication of TBK1 Stimulates Autophagy in iPSC-derived Retinal Cells from a Patient with Normal Tension Glaucoma. J Stem Cell Res Ther. 2014;3:161. doi: 10.4172/2157-7633.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello DA, Waxse BJ, Arden SD, Bright NA, Kendrick-Jones J, Buss F. Autophagy receptors link myosin VI to autophagosomes to mediate Tom1-dependent autophagosome maturation and fusion with the lysosome. Nature cell biology. 2012;14:1024–1035. doi: 10.1038/ncb2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull C, Ahmed S, Morrison J, Pernet D, Renwick A, Maranian M, Seal S, Ghoussaini M, Hines S, Healey CS, Hughes D, Warren-Perry M, Tapper W, Eccles D, Evans DG, Hooning M, Schutte M, van den Ouweland A, Houlston R, Ross G, Langford C, Pharoah PD, Stratton MR, Dunning AM, Rahman N, Easton DF. Genome-wide association study identifies five new breast cancer susceptibility loci. Nature genetics. 2010;42:504–507. doi: 10.1038/ng.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer M, Li J, Yaspan BL, Ozel AB, Richards JE, Moroi SE, Hawthorne F, Budenz DL, Friedman DS, Gaasterland D, Haines J, Kang JH, Lee R, Lichter P, Liu Y, Pasquale LR, Pericak-Vance M, Realini A, Schuman JS, Singh K, Vollrath D, Weinreb R, Wollstein G, Zack DJ, Zhang K, Young T, Allingham RR, Wiggs JL, Ashley-Koch A, Hauser MA. Genome-Wide Analysis of Central Corneal Thickness in Primary Open-Angle Glaucoma Cases in the NEIGHBOR and GLAUGEN Consortia. Investigative ophthalmology & visual science. 2012;53:4468–4474. doi: 10.1167/iovs.12-9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno S, Zembutsu H, Hirasawa A, Takahashi A, Kubo M, Akahane T, Aoki D, Kamatani N, Hirata K, Nakamura Y. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nature genetics. 2010;42:707–710. doi: 10.1038/ng.612. [DOI] [PubMed] [Google Scholar]
- Vaibhava V, Nagabhushana A, Chalasani ML, Sudhakar C, Kumari A, Swarup G. Optineurin mediates a negative regulation of Rab8 by the GTPase-activating protein TBC1D17. J Cell Sci. 2012;125:5026–5039. doi: 10.1242/jcs.102327. [DOI] [PubMed] [Google Scholar]
- van Koolwijk LM, Despriet DD, van Duijn CM, Pardo Cortes LM, Vingerling JR, Aulchenko YS, Oostra BA, Klaver CC, Lemij HG. Genetic contributions to glaucoma: heritability of intraocular pressure, retinal nerve fiber layer thickness, and optic disc morphology. Investigative ophthalmology & visual science. 2007;48:3669–3676. doi: 10.1167/iovs.06-1519. [DOI] [PubMed] [Google Scholar]
- van Koolwijk LM, Ramdas WD, Ikram MK, Jansonius NM, Pasutto F, Hysi PG, Macgregor S, Janssen SF, Hewitt AW, Viswanathan AC, ten Brink JB, Hosseini SM, Amin N, Despriet DD, Willemse-Assink JJ, Kramer R, Rivadeneira F, Struchalin M, Aulchenko YS, Weisschuh N, Zenkel M, Mardin CY, Gramer E, Welge-Lussen U, Montgomery GW, Carbonaro F, Young TL, Group, DER. Bellenguez C, McGuffin P, Foster PJ, Topouzis F, Mitchell P, Wang JJ, Wong TY, Czudowska MA, Hofman A, Uitterlinden AG, Wolfs RC, de Jong PT, Oostra BA, Paterson AD, Wellcome Trust Case Control C. Mackey DA, Bergen AA, Reis A, Hammond CJ, Vingerling JR, Lemij HG, Klaver CC, van Duijn CM. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS genetics. 2012;8:e1002611. doi: 10.1371/journal.pgen.1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven VJ, Hysi PG, Wojciechowski R, Fan Q, Guggenheim JA, Hohn R, MacGregor S, Hewitt AW, Nag A, Cheng CY, Yonova-Doing E, Zhou X, Ikram MK, Buitendijk GH, McMahon G, Kemp JP, Pourcain BS, Simpson CL, Makela KM, Lehtimaki T, Kahonen M, Paterson AD, Hosseini SM, Wong HS, Xu L, Jonas JB, Parssinen O, Wedenoja J, Yip SP, Ho DW, Pang CP, Chen LJ, Burdon KP, Craig JE, Klein BE, Klein R, Haller T, Metspalu A, Khor CC, Tai ES, Aung T, Vithana E, Tay WT, Barathi VA, Consortium for Refractive E. Myopia. Chen P, Li R, Liao J, Zheng Y, Ong RT, Doring A, Diabetes C, Complications Trial/Epidemiology of Diabetes, I., Complications Research, G. Evans DM, Timpson NJ, Verkerk AJ, Meitinger T, Raitakari O, Hawthorne F, Spector TD, Karssen LC, Pirastu M, Murgia F, Ang W, Wellcome Trust Case Control, C. Mishra A, Montgomery GW, Pennell CE, Cumberland PM, Cotlarciuc I, Mitchell P, Wang JJ, Schache M, Janmahasatian S, Igo RP, Jr, Lass JH, Chew E, Iyengar SK, Fuchs’ Genetics Multi-Center Study, G. Gorgels TG, Rudan I, Hayward C, Wright AF, Polasek O, Vatavuk Z, Wilson JF, Fleck B, Zeller T, Mirshahi A, Muller C, Uitterlinden AG, Rivadeneira F, Vingerling JR, Hofman A, Oostra BA, Amin N, Bergen AA, Teo YY, Rahi JS, Vitart V, Williams C, Baird PN, Wong TY, Oexle K, Pfeiffer N, Mackey DA, Young TL, van Duijn CM, Saw SM, Bailey-Wilson JE, Stambolian D, Klaver CC, Hammond CJ. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nature genetics. 2013;45:314–318. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SS, Cooke Bailey JN, Lucas A, Bradford Y, Linneman JG, Hauser MA, Pasquale LR, Peissig PL, Brilliant MH, McCarty CA, Haines JL, Wiggs JL, Vrabec TR, Tromp G, Ritchie MD, e, M.N., Consortium N. Epistatic Gene-Based Interaction Analyses for Glaucoma in eMERGE and NEIGHBOR Consortium. PLoS genetics. 2016;12:e1006186. doi: 10.1371/journal.pgen.1006186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, Williams-Lyn D, Heon E. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. American journal of human genetics. 2002;70:448–460. doi: 10.1086/338709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A, Zhu Y, May D, Afzal V, Gong E, Attanasio C, Blow MJ, Cohen JC, Rubin EM, Pennacchio LA. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature. 2010;464:409–412. doi: 10.1038/nature08801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishal M, Sharma A, Kaurani L, Chakraborty S, Ray J, Sen A, Mukhopadhyay A, Ray K. Evaluation of genetic association of the INK4 locus with primary open angle glaucoma in East Indian population. Sci Rep. 2014;4:5115. doi: 10.1038/srep05115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitart V, Bencic G, Hayward C, Herman JS, Huffman J, Campbell S, Bucan K, Zgaga L, Kolcic I, Polasek O, Campbell H, Wright A, Vatavuk Z, Rudan I. Heritabilities of ocular biometrical traits in two croatian isolates with extended pedigrees. Investigative ophthalmology & visual science. 2010a;51:737–743. doi: 10.1167/iovs.09-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitart V, Bencic G, Hayward C, Skunca Herman J, Huffman J, Campbell S, Bucan K, Navarro P, Gunjaca G, Marin J, Zgaga L, Kolcic I, Polasek O, Kirin M, Hastie ND, Wilson JF, Rudan I, Campbell H, Vatavuk Z, Fleck B, Wright A. New loci associated with central cornea thickness include COL5A1, AKAP13 and AVGR8. Human molecular genetics. 2010b;19:4304–4311. doi: 10.1093/hmg/ddq349. [DOI] [PubMed] [Google Scholar]
- Vithana EN, Aung T, Khor CC, Cornes BK, Tay WT, Sim X, Lavanya R, Wu R, Zheng Y, Hibberd ML, Chia KS, Seielstad M, Goh LK, Saw SM, Tai ES, Wong TY. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Human molecular genetics. 2011;20:649–658. doi: 10.1093/hmg/ddq511. [DOI] [PubMed] [Google Scholar]
- Vithana EN, Nongpiur ME, Venkataraman D, Chan SH, Mavinahalli J, Aung T. Identification of a novel mutation in the NTF4 gene that causes primary open-angle glaucoma in a Chinese population. Molecular vision. 2010;16:1640–1645. [PMC free article] [PubMed] [Google Scholar]
- Wagner AH, Anand VN, Wang WH, Chatterton JE, Sun D, Shepard AR, Jacobson N, Pang IH, Deluca AP, Casavant TL, Scheetz TE, Mullins RF, Braun TA, Clark AF. Exon-level expression profiling of ocular tissues. Experimental eye research. 2013;111:105–111. doi: 10.1016/j.exer.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DY, Fan BJ, Chua JK, Tam PO, Leung CK, Lam DS, Pang CP. A genome-wide scan maps a novel juvenile-onset primary open-angle glaucoma locus to 15q. Investigative ophthalmology & visual science. 2006;47:5315–5321. doi: 10.1167/iovs.06-0179. [DOI] [PubMed] [Google Scholar]
- Wang QC, Zheng Q, Tan H, Zhang B, Li X, Yang Y, Yu J, Liu Y, Chai H, Wang X, Sun Z, Wang JQ, Zhu S, Wang F, Yang M, Guo C, Wang H, Zheng Q, Li Y, Chen Q, Zhou A, Tang TS. TMCO1 Is an ER Ca(2+) Load-Activated Ca(2+) Channel. Cell. 2016;165:1454–1466. doi: 10.1016/j.cell.2016.04.051. [DOI] [PubMed] [Google Scholar]
- Wang R, Wiggs JL. Common and rare genetic risk factors for glaucoma. Cold Spring Harb Perspect Med. 2014;4:a017244. doi: 10.1101/cshperspect.a017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, McNatt LG, Pang IH, Hellberg PE, Fingert JH, McCartney MD, Clark AF. Increased expression of serum amyloid A in glaucoma and its effect on intraocular pressure. Investigative ophthalmology & visual science. 2008;49:1916–1923. doi: 10.1167/iovs.07-1104. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA : the journal of the American Medical Association. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisschuh N, Wolf C, Wissinger B, Gramer E. Variations in the WDR36 gene in German patients with normal tension glaucoma. Molecular vision. 2007;13:724–729. [PMC free article] [PubMed] [Google Scholar]
- Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff L, Parkinson H. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic acids research. 2014;42:D1001–1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger DA. In: Krabbe Disease. Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews(R); Seattle (WA): 1993. [Google Scholar]
- Wenger DA, Rafi MA, Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Hum Mutat. 1997;10:268–279. doi: 10.1002/(SICI)1098-1004(1997)10:4<268::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wenger DA, Rafi MA, Luzi P, Datto J, Costantino-Ceccarini E. Krabbe disease: genetic aspects and progress toward therapy. Molecular genetics and metabolism. 2000;70:1–9. doi: 10.1006/mgme.2000.2990. [DOI] [PubMed] [Google Scholar]
- Wenger DA, Victoria T, Rafi MA, Luzi P, Vanier MT, Vite C, Patterson DF, Haskins MH. Globoid cell leukodystrophy in cairn and West Highland white terriers. The Journal of heredity. 1999;90:138–142. doi: 10.1093/jhered/90.1.138. [DOI] [PubMed] [Google Scholar]
- White RC, Nelson TE. Analytical gel chromatography of rabbit muscle amylo-1,6-glucosidase/4-alpha-glucanotransferase under denaturing and non-denaturing conditions. Biochimica et biophysica acta. 1975;400:154–161. doi: 10.1016/0005-2795(75)90136-1. [DOI] [PubMed] [Google Scholar]
- Whitmore SS, Wagner AH, DeLuca AP, Drack AV, Stone EM, Tucker BA, Zeng S, Braun TA, Mullins RF, Scheetz TE. Transcriptomic analysis across nasal, temporal, and macular regions of human neural retina and RPE/choroid by RNA-Seq. Experimental eye research. 2014;129:93–106. doi: 10.1016/j.exer.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Allingham RR, Hossain A, Kern J, Auguste J, DelBono EA, Broomer B, Graham FL, Hauser M, Pericak-Vance M, Haines JL. Genome-wide scan for adult onset primary open angle glaucoma. Human molecular genetics. 2000;9:1109–1117. doi: 10.1093/hmg/9.7.1109. [DOI] [PubMed] [Google Scholar]
- Wiggs JL, Kang JH, Yaspan BL, Mirel DB, Laurie C, Crenshaw A, Brodeur W, Gogarten S, Olson LM, Abdrabou W, DelBono E, Loomis S, Haines JL, Pasquale LR. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Human molecular genetics. 2011;20:4707–4713. doi: 10.1093/hmg/ddr382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Lynch S, Ynagi G, Maselli M, Auguste J, Del Bono EA, Olson LM, Haines JL. A genomewide scan identifies novel early-onset primary open-angle glaucoma loci on 9q22 and 20p12. American journal of human genetics. 2004;74:1314–1320. doi: 10.1086/421533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Yaspan BL, Hauser MA, Kang JH, Allingham RR, Olson LM, Abdrabou W, Fan BJ, Wang DY, Brodeur W, Budenz DL, Caprioli J, Crenshaw A, Crooks K, Delbono E, Doheny KF, Friedman DS, Gaasterland D, Gaasterland T, Laurie C, Lee RK, Lichter PR, Loomis S, Liu Y, Medeiros FA, McCarty C, Mirel D, Moroi SE, Musch DC, Realini A, Rozsa FW, Schuman JS, Scott K, Singh K, Stein JD, Trager EH, Vanveldhuisen P, Vollrath D, Wollstein G, Yoneyama S, Zhang K, Weinreb RN, Ernst J, Kellis M, Masuda T, Zack D, Richards JE, Pericak-Vance M, Pasquale LR, Haines JL. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS genetics. 2012;8:e1002654. doi: 10.1371/journal.pgen.1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, Dotsch V, Bumann D, Dikic I. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Perkumas KM, Perry I, Wen JC, Keeling J, Tramber M, Liton PB, Stamer WD. Successful Implementation of a Program for Increasing Donor Eyes for Research: The Duke-Miracles In Sight Program. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2016a;32:145–149. doi: 10.1089/jop.2015.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Stamer WD, Allingham RR. Increasing the Availability and Quality of Donor Eyes for Research. JAMA ophthalmology. 2016b;134:351–352. doi: 10.1001/jamaophthalmol.2015.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Carmichael TR, Allingham RR, Hauser M, Ramsay M. The genetics of POAG in black South Africans: a candidate gene association study. Sci Rep. 2015;5:8378. doi: 10.1038/srep08378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz MK, Keller KE. The Role of the IL-20 Subfamily in Glaucoma. Mediators of inflammation. 2016;2016:4083735. doi: 10.1155/2016/4083735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz MK, Samples JR, Kramer PL, Rust K, Topinka JR, Yount J, Koler RD, Acott TS. Mapping a gene for adult-onset primary open-angle glaucoma to chromosome 3q. American journal of human genetics. 1997;60:296–304. [PMC free article] [PubMed] [Google Scholar]
- Wirtz MK, Samples JR, Rust K, Lie J, Nordling L, Schilling K, Acott TS, Kramer PL. GLC1F, a new primary open-angle glaucoma locus, maps to 7q35-q36. Arch Ophthalmol. 1999;117:237–241. doi: 10.1001/archopht.117.2.237. [DOI] [PubMed] [Google Scholar]
- Wistow G, Peterson K, Gao J, Buchoff P, Jaworski C, Bowes-Rickman C, Ebright JN, Hauser MA, Hoover D. NEIBank: genomics and bioinformatics resources for vision research. Molecular vision. 2008;14:1327–1337. [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Holzbaur EL. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4439–4448. doi: 10.1073/pnas.1405752111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodroffe A, Krafchak CM, Fuse N, Lichter PR, Moroi SE, Schertzer R, Downs CA, Duren WL, Boehnke M, Richards JE. Ordered subset analysis supports a glaucoma locus at GLC1I on chromosome 15 in families with earlier adult age at diagnosis. Experimental eye research. 2006;82:1068–1074. doi: 10.1016/j.exer.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Wrensch M, Jenkins RB, Chang JS, Yeh RF, Xiao Y, Decker PA, Ballman KV, Berger M, Buckner JC, Chang S, Giannini C, Halder C, Kollmeyer TM, Kosel ML, LaChance DH, McCoy L, O’Neill BP, Patoka J, Pico AR, Prados M, Quesenberry C, Rice T, Rynearson AL, Smirnov I, Tihan T, Wiemels J, Yang P, Wiencke JK. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nature genetics. 2009;41:905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Itonaga K, Marunouchi T, Majima K. Concentration of transforming growth factor beta2 in aqueous humor. Ophthalmic research. 2005;37:29–33. doi: 10.1159/000083019. [DOI] [PubMed] [Google Scholar]
- Yan B, Tao ZF, Li XM, Zhang H, Yao J, Jiang Q. Aberrant expression of long noncoding RNAs in early diabetic retinopathy. Investigative ophthalmology & visual science. 2014;55:941–951. doi: 10.1167/iovs.13-13221. [DOI] [PubMed] [Google Scholar]
- Yang L, Li S, Miao L, Huang H, Liang F, Teng X, Xu L, Wang Q, Xiao W, Ridder WH, 3rd, Ferguson TA, Chen DF, Kaufman RJ, Hu Y. Rescue of Glaucomatous Neurodegeneration by Differentially Modulating Neuronal Endoplasmic Reticulum Stress Molecules. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016a;36:5891–5903. doi: 10.1523/JNEUROSCI.3709-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yao H, Li M, Li H, Wang F. Long Non-Coding RNA MALAT1 Mediates Transforming Growth Factor Beta1-Induced Epithelial-Mesenchymal Transition of Retinal Pigment Epithelial Cells. PloS one. 2016b;11:e0152687. doi: 10.1371/journal.pone.0152687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zack DJ. What has gene expression profiling taught us about glaucoma? Experimental eye research. 2011;93:191–195. doi: 10.1016/j.exer.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Yao J, Wang XQ, Li YJ, Shan K, Yang H, Wang YN, Yao MD, Liu C, Li XM, Shen Y, Liu JY, Cheng H, Yuan J, Zhang YY, Jiang Q, Yan B. Long non-coding RNA MALAT1 regulates retinal neurodegeneration through CREB signaling. EMBO molecular medicine. 2016;8:346–362. doi: 10.15252/emmm.201505725. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Mo D, Cong P, He Z, Ling F, Li A, Niu Y, Zhao X, Zhou C, Chen Y. Molecular cloning, expression patterns and subcellular localization of porcine TMCO1 gene. Mol Biol Rep. 2010;37:1611–1618. doi: 10.1007/s11033-009-9573-8. [DOI] [PubMed] [Google Scholar]
- Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A, Leo PJ, Dahia CL, Park-Min KH, Tobias JH, Kooperberg C, Kleinman A, Styrkarsdottir U, Liu CT, Uggla C, Evans DS, Nielson CM, Walter K, Pettersson-Kymmer U, McCarthy S, Eriksson J, Kwan T, Jhamai M, Trajanoska K, Memari Y, Min J, Huang J, Danecek P, Wilmot B, Li R, Chou WC, Mokry LE, Moayyeri A, Claussnitzer M, Cheng CH, Cheung W, Medina-Gomez C, Ge B, Chen SH, Choi K, Oei L, Fraser J, Kraaij R, Hibbs MA, Gregson CL, Paquette D, Hofman A, Wibom C, Tranah GJ, Marshall M, Gardiner BB, Cremin K, Auer P, Hsu L, Ring S, Tung JY, Thorleifsson G, Enneman AW, van Schoor NM, de Groot LC, van der Velde N, Melin B, Kemp JP, Christiansen C, Sayers A, Zhou Y, Calderari S, van Rooij J, Carlson C, Peters U, Berlivet S, Dostie J, Uitterlinden AG, Williams SR, Farber C, Grinberg D, LaCroix AZ, Haessler J, Chasman DI, Giulianini F, Rose LM, Ridker PM, Eisman JA, Nguyen TV, Center JR, Nogues X, Garcia-Giralt N, Launer LL, Gudnason V, Mellstrom D, Vandenput L, Amin N, van Duijn CM, Karlsson MK, Ljunggren O, Svensson O, Hallmans G, Rousseau F, Giroux S, Bussiere J, Arp PP, Koromani F, Prince RL, Lewis JR, Langdahl BL, Hermann AP, Jensen JE, Kaptoge S, Khaw KT, Reeve J, Formosa MM, Xuereb-Anastasi A, Akesson K, McGuigan FE, Garg G, Olmos JM, Zarrabeitia MT, Riancho JA, Ralston SH, Alonso N, Jiang X, Goltzman D, Pastinen T, Grundberg E, Gauguier D, Orwoll ES, Karasik D, Davey-Smith G, Consortium A. Smith AV, Siggeirsdottir K, Harris TB, Zillikens MC, van Meurs JB, Thorsteinsdottir U, Maurano MT, Timpson NJ, Soranzo N, Durbin R, Wilson SG, Ntzani EE, Brown MA, Stefansson K, Hinds DA, Spector T, Cupples LA, Ohlsson C, Greenwood CM, Consortium U.K. Jackson RD, Rowe DW, Loomis CA, Evans DM, Ackert-Bicknell CL, Joyner AL, Duncan EL, Kiel DP, Rivadeneira F, Richards JB. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526:112–117. doi: 10.1038/nature14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Ge J, Huang G, Zhang J, Liu B, Hur YM, He M. Heritability of central corneal thickness in Chinese: the Guangzhou Twin Eye Study. Investigative ophthalmology & visual science. 2008;49:4303–4307. doi: 10.1167/iovs.08-1934. [DOI] [PubMed] [Google Scholar]
- Zhou RM, Wang XQ, Yao J, Shen Y, Chen SN, Yang H, Jiang Q, Yan B. Identification and characterization of proliferative retinopathy-related long noncoding RNAs. Biochemical and biophysical research communications. 2015;465:324–330. doi: 10.1016/j.bbrc.2015.07.120. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Grinchuk O, Tomarev SI. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Investigative ophthalmology & visual science. 2008;49:1932–1939. doi: 10.1167/iovs.07-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Wu CJ, Zhao Y, Ashwell JD. Optineurin negatively regulates TNFalpha- induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Curr Biol. 2007;17:1438–1443. doi: 10.1016/j.cub.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Zillig M, Wurm A, Grehn FJ, Russell P, Tamm ER. Overexpression and properties of wild-type and Tyr437His mutated myocilin in the eyes of transgenic mice. Investigative ophthalmology & visual science. 2005;46:223–234. doi: 10.1167/iovs.04-0988. [DOI] [PubMed] [Google Scholar]
- Zode GS, Bugge KE, Mohan K, Grozdanic SD, Peters JC, Koehn DR, Anderson MG, Kardon RH, Stone EM, Sheffield VC. Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma. Investigative ophthalmology & visual science. 2012;53:1557–1565. doi: 10.1167/iovs.11-8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. The Journal of clinical investigation. 2011;121:3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]


