Abstract
An approach for 3D bone tissue generation from embryonic stem (ES) cells was investigated. The ES cells were induced to differentiate into osteogenic precursors, capable of proliferating and subsequently differentiating into bone-forming cells. The differentiated cells and the seeded scaffolds were characterized using von Kossa and Alizarin Red staining, electron microscopy, and RT-PCR analysis. The results demonstrated that ES-derived bone-forming cells attached to and colonized the biocompatible and biodegradable scaffolds. Furthermore, these cells produced bone nodules when grown for 3–4 weeks in mineralization medium containing ascorbic acid and beta-glycerophosphate both in tissue culture plates and in scaffolds. The differentiated cells also expressed osteospecific markers when grown both in the culture plates and in 3D scaffolds. Osteogenic cells expressed alkaline phosphatase, osteocalcin, and osteopontin, but not an ES cell-specific marker, oct-4. These findings suggest that ES cell can be used for in vitro tissue engineering and cultivation of graftable skeletal structures.
INTRODUCTION
Although autogenous bone grafting remains the main choice for treatment of bone fractures, bone defects, and therapeutic arthrodesis, efficacy is limited by donor morbidity and the lack of tissue resource availability [1]. On the other hand, alloplastic materials lack desired properties and consequently result in a high rate of failure [2]. Therefore, there is a great need for renewable sources of materials based on the tissue engineering approach for bone regeneration, repair, and replacement.
Embryonic stem (ES) cells are pluripotent cells derived from the inner cell mass of preimplantation embryos and represent embryonic precursor cells that give rise to any cell type in the embryo [3]. When ES cells are allowed to differentiate in a suspension culture, they form spherical multicellular aggregates, called embryoid bodies (EBs), which have been shown to contain a variety of cell populations [4, 5]. Significant progress has been achieved in inducing murine ES (mES) cells to differentiate into particular types of cells, such as hematopoietic cells [6, 7], cardiomyocytes [8], smooth muscle cells [9, 10], neurons [11, 12, 13, 14], and even organs such as a functional gut-like unit [15] and pancreatic islet-like organization [16]. Specifically, it has been shown that ES cells can differentiate into osteogenic cells under selective culture conditions [17, 18, 19, 20]. The osteogenic cells are capable of in vitro producing extracellular organic matrix of bone collagen 1 and osteocalcin (OC) [17]. The ability of ES cells to in vitro differentiate into a variety of cell types provides novel opportunities to use them for new therapeutic strategies such as cell transplantation and tissue and organ regeneration, replacement, and repair.
The recent identification of osteogenesis-related transcription factors and osteoblast-specific markers has led to a rapid advancement in understanding the process of osteoblast differentiation [21]. Many growth factors and cytokines have been shown to promote the differentiation of osteoblasts, including insulin-like growth factor-1 [22], melatonin [23], and IL-6-related cytokines [24]. Bone morphogenic protein (BMP) family members have been shown to exert particularly strong osteoinductive effects. It was recently found that compounds that are able to upregulate the promoter of BMP 2 result in identification of statins, drugs of the HMG-CoA reductase inhibitor family [25, 26]. One of these drugs, compactin, has shown the potential of enhancing osteogenesis in mES cells [26, 27].
It is believed that most organ cells, including osteoblast or osteoblast-like cells, are anchorage-dependent and require specific environments for growth, which include the presence of a supporting scaffold [28]. This 3D scaffold will provide a supporting frame and act as a template for osteogenesis. In order to permit the ingress of bone cells and nutrients, a porous structure with interconnected channels for 3D penetration is crucial. The required characteristics of the material for the scaffold include biocompatibility and biodegradability. Various studies have been carried out to test the attachment and spreading of osteoblast or osteoblast-like cells on porous membranes or 3D scaffolds using collagen, calcium phosphate, poly lactic acid (PLA), and PLA's copolymers with glycolide [29, 30, 31, 32, 33, 34, 35]. Particularly, recent work by Wiedmann-Al-Ahmad et al [29] investigated the seeding and culture of human osteoblast-like cells on several different types of collagen. These previous studies indicated that the seeding and culture of osteoblast-like cells strongly depend on various factors of the scaffold material and its condition, such as topography, chemistry, and surface energy [36, 37].
In the present study, we investigated a new approach for 3D bone tissue generation from stem cells. First, the ES cells were induced to differentiate into osteogenic precursors, capable of proliferating and subsequently differentiating into osteoblasts, bone forming cells. The osteogenic cells were then seeded into a three dimensional scaffold for in vitro bone tissue generation. The mES cells were used to verify the feasibility of this approach. The experimental result showed that bone-forming cells derived from mES cells can be seeded into a PLA scaffold and subsequently grow and form bone nodules under selective cultivation conditions.
MATERIALS AND METHODS
Scaffold
OPLA scaffolds [38] from BD Biosciences, Billerica, Mass, were used in the study. The BD OPLA scaffold is a 3D synthetic polymer scaffold that is synthesized from D,DL,L polylactic acid. This material has an open-cell facetted architecture, which is effective for culturing high density cell suspensions [38]. It is of cylindrical shape, with a diameter of 4.2–5.2 mm and a height of 3.9–4.5 mm. The ratio of dry weight to wet weight is 5.2 mg/34 mg. Average pore size ranges from 100–200 μm. The hydration capacity of the scaffold is 30 μL. The modulus of elasticity at 25% strain is 0.92 ± 0.03 Mega Pascal. During in vivo biodegradation, the scaffold is structurally functional for 13 weeks and completely resorbed at the end of 12 months.
Stem cell culture
ES cells (D3 [39], obtained from Dr K. Sue O'Shea, University of Michigan, Mich) were maintained on gelatin-coated dishes without feeder cells in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 0.1 mM 2-mercaptoethanol (Sigma, St Lois, Mo), 0.1 mM nonessential amino acids (Invitrogen, Carisbad, Calif), 1 mM sodium pyruvate (Sigma), and 1,000 U/mL of leukemia inhibitory factor (LIF; Chemicon International Inc, Temecula, Calif).
Differentiation of ES cells to embryoid bodies
ES cells were cultured as described above. EBs were prepared using the “hanging drop” method [17]. Approximately 1000 cells per 20 μL were incubated on the inverted lid of petri plates for three days. The resulting EBs were then transferred to fresh petri plates and cultured with 10−7 M cis-retinoic acid for three days with a change of medium each day.
Differentiation of EBs to osteogenic cells
To differentiate the EBs to osteogenic cells, the EBs were transferred to gelatin-coated tissue culture plates in ES medium supplemented with ascorbic acid phosphate (50 μg/mL) and β-glycerol phosphate (10 mM) (mineralization medium). The resulting cells were incubated under the same condition for three weeks with a medium change every 2 days.
Seeding of scaffold
The EBs were subjected to selective differentiation of osteogenic cells in the mineralization medium as described above and shown to produce bone nodules (see Results). The differentiated cells were treated with trypsin and the scaffolds were seeded as follows.
The scaffolds were placed in six-well tissue culture plates (Fisher Scientific, Hanover, Ill) and seeded with the osteogenic cells derived from EBs (a concentration of 200 μL of 106 cells per mL). The cells were incubated for 0, 2, 4, and 8 hours at 37°C in 5% CO2 incubator before supplementing with 2.5 mL of the culture medium, and then incubated for 4 weeks with a medium change every 2–3 days. Seeded and unseeded controls included scaffolds seeded with and without ES cells, respectively, and were incubated in the same way in the mineralization medium. The scaffolds were periodically checked under a light microscope and one set for each of the scaffolds including controls (unseeded scaffolds) was sacrificed at intervals of 0, 1, 2, 3, and 4 weeks, and analyzed by scanning electron microscope for the purpose of monitoring cell growth and production of bone nodules as well as expression of specific genetic markers. Simultaneously, six-well culture plates with 100 μL of 106 cells per mL were incubated as above. One set of the wells was sacrificed at intervals of 0, 1, 2, 3, and 4 weeks and analyzed as above. All experiments were run in triplicates.
Histology
The osteogenic cells, after growing in the cell culture plates, were analyzed for bone mineral deposition by the von Kossa staining method [40]. Briefly, the cells were rinsed twice with 10 mM phosphate-buffered saline (PBS), and then fixed in 0.2% glutaraldehyde for 1 hour. After rinsing in water, cells were dehydrated in ascending ethanol series (70%, 90%, 100% ethanol) and then air dried. The cells were rehydrated in a descending ethanol series (100%, 90%, 70% ethanol) and water. The plates were then stained with 2% silver nitrate for 1 hour, exposed to a 70 W lamp, rinsed in water, and counterstained in safranin for 2–3 minutes. Finally, the plates were rinsed in 70% ethanol and observed. Alternatively, cell culture was fixed in 10% formalin-buffered saline for 20 minutes, washed with PBS, and stained for 10 minutes with 1% Alizarin Red [41]. Plates were washed in running tap water and then left to air dry. Bone mineral nodules stained bright red.
Electron microscopic studies
The cell colonization of the scaffold was analyzed by scanning electron microscopy (SEM). The samples were fixed in 4% paraformaldehyde for 2 hours at room temperature and incubated in 8% formaldehyde for 2 days at 4°C. The samples were dehydrated in ascending ethanol series (30%, 50%, 70%, 80%, 90%, one time each and twice in 100%). After critical point drying, according to standard procedure using liquid carbon dioxide, the samples were sputtered with gold-palladium and scanned using SEM (ISI model DS130).
For transmission electron microscopy (TEM), the cells were fixed in 3% glutaraldehyde in PBS for 3 hours at room temperature. They were then washed with PBS for 30 minutes, post-fixed with 1% osmium oxide in PBS for 45 minutes and washed overnight with PBS. The samples were dehydrated in ascending ethanol series (50%, 60%, 70%, 80%, 90%, 100%), treated with Epon 812 at a ratio of 1 : 3, 1 : 1, and 3 : 1 for 4 hours each and 100% overnight. The samples were analyzed using TEM (Model 410, Philips FEI, Bruckmanrig, Germany).
Extraction of RNA and RT-PCR
Cells were detached from the cell culture plates and collected by centrifugation. The RNA from the cells was extracted using Rneasy Kit (Qiagen Inc, Valencia, Calif) following the manufacturer's instructions. Analysis of the RNA samples was performed using the one-step RT-PCR kit (Qiagen GmbH). PCR conditions used were as follows: reverse transcription, 50°C, 30 minutes; Taq polymerase activation, 95°C, 15 minutes; then thermal cycling, 94°C, 30 seconds, 55°C, 30 seconds, 72°C, 30 seconds, for 35 cycles; followed by a single elongation step at 72°C, 10 minutes. The primer sequences and expected product sizes are listed in Table 1. RT-PCR products were analyzed by 1.5% agarose gel electrophoresis.
Table 1.
RT-PCR primers.
| Gene | Primer sequence | Product size | |
|---|---|---|---|
| octv-4 | Sense | GCAACTCAGAGGGAACCTCCT | 62 bp |
| Antisense | TCTCCAACTTCACGGCATTG | ||
| Alkaline phosphate | Sense | AGGCAGGATTGACCACGG | 138 bp |
| Antisense | TGTAGTTCTGCTCATGGA | ||
| Osteocalcin | Sense | CTTGGGTTCTGACTGGGTGT | 212 bp |
| Antisense | GCCCTCTGCAGGTCATAGAG | ||
| Osteopontin | Sense | TCACCATTCGGATGAGTCTG | 436 bp |
| Antisense | ACTTGTGGCTCTGATGTTCC | ||
RESULTS
Differentiation of ES cells
The ES cells formed EBs after a period of 3 days in suspension in the ES cell medium lacking Leukemia Inhibitory Factor (LIF). The EBs were then plated in the same medium and treated with retinoic acid for 3 days before being transferred to the mineralization medium containing beta-glycerophosphate and ascorbic acid. Retinoic acid has been reported to be an important factor in osteogenesis of ES cells [17]. As shown in Figure 1, the EBs are being differentiated into progenitor osteogenic cells (differentiated cells are seen in the periphery of the EB). The isolated cells displayed morphological features similar to osteogenic cells (ie, osteoblasts and osteocytes) as shown in Figure 2. Figure 2b shows the characteristic cytoplasmic extensions contacting the adjacent osteocytes when grown for 4 weeks in the mineralization medium.
Figure 1.
Differentiation of mouse EBs in mineralization medium.
Figure 2.

Differentiation of ES cells to osteogenic cells.
Electron micrographs of the ES and differentiated osteogenic cells are shown in Figure 3. The differentiated cells exhibit characteristics of differentiated cells including cellular organelles (Golgi apparatus and rough endoplasmic reticulum), which are not present in the ES cells. Additional details of the differentiated cells are shown in Figure 5.
Figure 3.


Electron micrographs of (a) ES cell and (b) osteogenic cell.
Figure 5.

TEM micrograph of osteogenic cells producing bone nodules. B: bone nodules; M: mitochondria; N: nucleus.
Production of bone nodules
The osteogenic cells grown for 3 to 4 weeks and subjected to von Kossa staining showed spots of dark brown coloration (Figure 4). Alizarin Red was also used to stain the osteogenic cells (results not shown). ES cells, EBs, or EBs differentiated into cells other than osteogenic cells did not stain under the same conditions. The results shown in Figure 4 suggest that the differentiated osteogenic cells produced bone nodules. Similarly, Phillips et al [27] have reported that osteogenic cells produced extracellular matrix material that was stained by von Kossa staining.
Figure 4.

The von Kossa stain.
Simultaneously, the differentiated cells derived from EBs were analyzed by TEM. A comparison of ES cells (Figure 3a) and the differentiated cells (Figure 5) shows that the differentiated cell structure has an array of bone nodules in the vicinity of well-developed and extensive amount of mitochondria. Presence of a large number of mitochondria supports the fact that the production of bone nodules is an energy-demanding process.
Growth of osteogenic cells in scaffolds
Osteogenic cell growth, attachment, and invasion of scaffold were investigated by the scanning electron microscope. The results in Figure 6 show an extensive growth of cellular biomass completely covering the scaffold as compared to the control-unseeded scaffold. Growth and invasion of the scaffolds by the osteogenic cells could be seen after 1 week of incubation in mineralization medium. No nodules were detected during the first 2 and 3 weeks in cell plates and scaffolds, respectively. After 4 weeks of incubation of seeded scaffolds, small nodular patches of excreted extracellular matrix (bone nodule structures) could be seen. Production of bone nodules by the osteogenic cells seeded onto scaffolds and cultured on the plate was judged by the von Kossa staining, as well as the expression of osteospecific surface markers as determined by the RT-PCR (see below). No nodular structures were found in control scaffolds.
Figure 6.


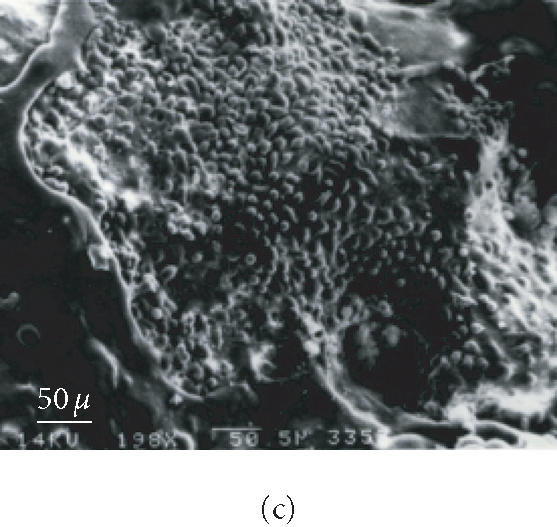
Scanning electron micrographs of scaffold seeded and unseeded with osteogenic cells and incubated in mineralization medium. (a) Unseeded scaffold. (b) Seeded scaffold after 2 weeks of incubation. (c) Seeded scaffold after 4 weeks of incubation.
Molecular characterization of the osteoprogenitors
The ES cells exhibit expression of specific molecular markers, such as oct-4 and several cell surface antigens. The differentiated osteoprogenitors also express specific markers, such as alkaline phosphatase (ALP), OC, and osteopontin (OP) [27]. We investigated the expression of selected markers in both the ES cells and differentiated cells producing bone nodules. The results of the experiments are shown in Figure 7. The oct-4 was expressed only in the ES cells but not in the EB cells. The expression of osteospecific markers, ALP, OP, and OC, was evident in the differentiated cells that produced bone nodules. In some cases, low levels of expression of OC was also detected in messenger RNA that was derived from ES cell (results not shown). The reason for this observation is an unusual splicing event where one to several introns are retained along with the exons. This result is the presence of higher molecular weight transcript that will be expressed in many nonosseous tissues [42].
Figure 7.

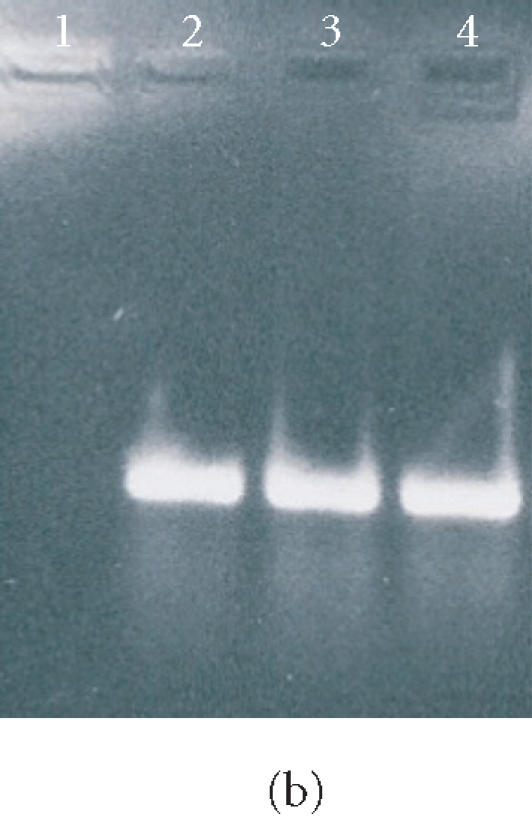
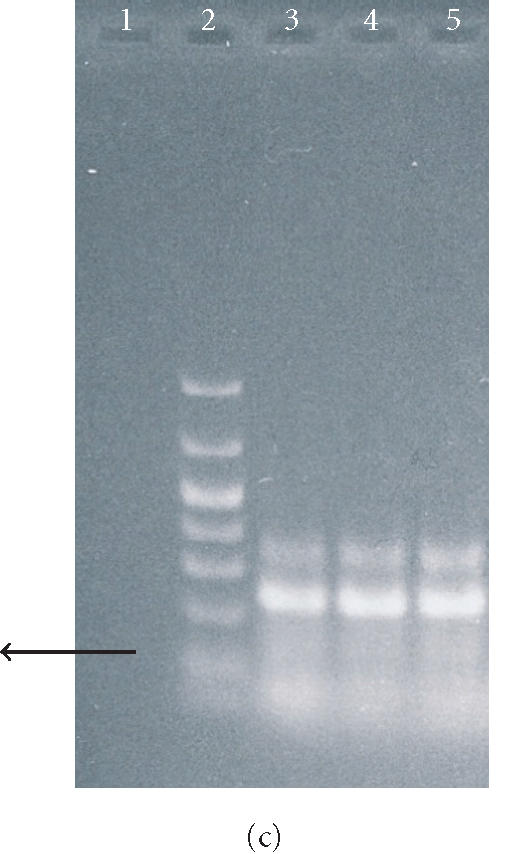
Agarose gel electrophoresis of RT-PCR products of mRNA isolated from ES cells, EBs, and osteogenic cells. In (a), lanes 1 and 2 show the RT-PCR product of oct4, and lane 3 shows molecular weight markers (1000, 700, 500, 400, 300, 200, 100, and 50 bp). In (b), lanes 1 and 2 represent the RT-PCR product of OP from ES cells and EBs, respectively. Lanes 3 and 4 represent the RT-PCR product of OP from osteogenic cells grown in the tissue culture plate and 3D scaffold, respectively. The arrow indicates the molecular size of 400 bp. In (c), lanes 1, 3, 4, and 5 represent the RT-PCR product of OC from the cells as described in (b) above. Lane 2 represents the molecular weight markers as described in (a).
The ES-cell derived differentiated cells not only colonized the scaffolds but also produced bone nodules as judged by the scanning electron micrography (Figure 6) and von Kossa staining. The RT-PCR analysis of the transcripts of the cells colonizing the scaffold showed expression of osteospecific markers. The mRNA from these cells yielded RT-PCR amplified products of ALP, OC, and OP.
DISCUSSION
Totipotent ES cells have been induced to differentiate into a variety of cell types in vitro [6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16]. We present in this study that ES-cell-derived osteoprogenitor cells attached to and colonized the scaffolds. These results show that ES cells can be used for tissue engineering of bone structures/grafts. In vitro grown bone grafts could facilitate skeletal reconstructions in cases involving defects created by tumor resection, injury, and skeletal abnormalities. The grafts developed from the differentiated cells of well-characterized ES cells may provide an alternative to allografts and nonbiodegradable material such as bone cements and metals, especially titanium and ceramics. The use of allografts is disadvantageous due to donor morbidity [43, 44]. The nonbiodegradable materials do not promote bone ingrowth [45].
Therefore, tissue engineering of bone structures and their subsequent use as skeletal reconstruction biomaterials, which are bioresorbable, biocompatible, not toxic and which allow the bone ingrowth, are desirable. Various studies have been carried out to test the attachment and spreading of osteoblast or osteoblast-like cells on porous membranes or 3D scaffolds using collagen, calcium phosphate, PLA, and PLGA [29, 30, 31, 32, 33, 34, 35]. Particularly, recent work by Wiedmann-Al-Ahmad et al [29] investigated the seeding and culture of human osteoblast-like cells on several different types of collagen. All previous studies indicated that the seeding and culture of osteoblast-like cells strongly depend on various factors of the scaffold material and its condition, such as topography, chemistry, and surface energy [36, 37]. We found that the time interval between seeding of the osteogenic cells into the scaffold and adding growth medium had little or no effect on colonization, production of bone nodules, and expression of the osteospecific markers. These results are contrary to the previous study [29] reporting that the time interval between seeding osteoblasts and adding culture medium significantly affected the osteoblast proliferation. However, osteoblasts used in this study were derived from patients and were not derived from ES cells as is the case in our study.
The osteogenic cells used for the scaffold seeding study could be characterized as predominantly osteoblast-like or osteoprogenitor since they produced bone nodules and expressed osteospecific markers, ALP, OC, and OP [27]. ALP is an indicator for osteoblast-type cells and OC, an extracellular noncollagenous matrix protein, is exclusively expressed by osteoblasts. However, in our study, no attempt was made to isolate, grow, or determine types of cells in the EB-derived cells. It is likely that the osteogenic cells derived from EBs had multiple types of cell lineage. Therefore, further investigation to enrich and prolong the growth of osteoblast-type cells should be helpful for in vitro tissue engineering and cultivation of graftable skeletal structures.
The use of ES cells as the source for generating graftable skeletal tissues is advantageous compared with existing sources (such as bone marrows) for bone tissue engineering. The supply of autologous tissues and compatible bone-marrow-derived grafts is limited, while ES cells can provide a renewable source for the production of engineered grafts. ES cells can potentially yield compatible grafts on sustained bases or they may be produced on demand.
In conclusion, our study demonstrates that osteogenic cells can be derived from ES cells which can colonize and grow when seeded into the biocompatible and biodegradable scaffolds. Furthermore, these cells expressed osteospecific markers and produced bone nodules when grown both in the culture plates and in 3D scaffolds. Additional improvements in the protocol could allow cultivation of graftable tissues. Further studies with other biomaterials used as scaffolds should be helpful for identifying optimal conditions for in vitro cultivation of graftable osseous structures.
ACKNOWLEDGMENT
This study was supported by the College of Arts and Sciences, Oakland University. The authors wish to thank Dr K. S. Oshea for the mouse embryonic stem cells and Todd Miller and L. T. N. Dang for the TEM and SEM work.
References
- 1.Marx R.E, Morales M.J. Morbidity from bone harvest in major jaw reconstruction: a randomized trial comparing the lateral anterior and posterior approaches to the ilium. J Oral Maxillofac Surg. 1988;46(3):196–203. doi: 10.1016/0278-2391(88)90083-3. [DOI] [PubMed] [Google Scholar]
- 2.Closer Look-Growing Scandal over TMJ and Jaw Implants. Medical Materials Update. 1994 Jun [Google Scholar]
- 3.Evans M.J, Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 4.Robertson E.J. Embryo-derived stem cell lines. In: Robertson E.J, editor. Teratocarcinomas and Embryonic Stem Cells: a Practical Approach. 1st ed. Washington, DC: IRL Press; 1987. pp. 71–112. [Google Scholar]
- 5.Keller G.M. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7(6):862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 6.Potocnik A.J, Kohler H, Eichmann K. Hemato-lymphoid in vivo reconstitution potential of subpopulations derived from in vitro differentiated embryonic stem cells. Proc Natl Acad Sci USA. 1997;94(19):10295–10300. doi: 10.1073/pnas.94.19.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling V, Neben S. In vitro differentiation of embryonic stem cells: immunophenotypic analysis of cultured embryoid bodies. J Cell Physiol. 1997;171(1):104–115. doi: 10.1002/(SICI)1097-4652(199704)171:1<104::AID-JCP12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Klug M.G, Soonpaa M.H, Koh G.Y, Field L.J. Genetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98(1):216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng W.A, Doetschman T, Robbins J, Lessard J.L. Muscle isoactin expression during in vitro differentiation of murine embryonic stem cells. Pediatr Res. 1997;41(2):285–292. doi: 10.1203/00006450-199702000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Drab M, Haller H, Bychkov R, et al. From totipotent embryonic stem cells to spontaneously contracting smooth muscle cells: a retinoic acid and db-cAMP in vitro differentiation model. FASEB J. 1997;11(11):905–915. doi: 10.1096/fasebj.11.11.9285489. [DOI] [PubMed] [Google Scholar]
- 11.Bain G, Kitchens D, Yao M, Huettner J.E, Gottlieb D.I. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168(2):342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- 12.Brustle O, Jones K.N, Learish R.D, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285(5428):754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 13.McDonald J.W, Liu X.Z, Qu Y, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5(12):1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.H, Lumelsky N, Studer L, Auerbach J.M, McKay R.D. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18(6):675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 15.Yamada T, Yoshikawa M, Takaki M, et al. In vitro functional gut-like organ formation from mouse embryonic stem cells. Stem Cells. 2002;20(20):41–49. doi: 10.1634/stemcells.20-1-41. [DOI] [PubMed] [Google Scholar]
- 16.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292(5520):1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 17.Buttery L.D, Bourne S, Xynos J.D, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7(1):89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 18.Peng H, Wright V, Usas A, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110(6):751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda S, Nobukuni T, Shimo-Onoda K, et al. Sortilin is upregulated during osteoblastic differentiation of mesenchymal stem cells and promotes extracellular matrix mineralization. J Cell Physiol. 2002;193(1):73–79. doi: 10.1002/jcp.10151. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto T, Aoyama T, Nakayama T, et al. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun. 2002;295(2):354–361. doi: 10.1016/s0006-291x(02)00661-7. [DOI] [PubMed] [Google Scholar]
- 21.Karsenty G. The genetic transformation of bone biology. Genes Dev. 1999;13(23):3037–3051. doi: 10.1101/gad.13.23.3037. [DOI] [PubMed] [Google Scholar]
- 22.Jia D, Heersche J.N. Insulin-like growth factor-1 and -2 stimulate osteoprogenitor proliferation and differentiation and adipocyte formation in cell populations derived from adult rat bone. Bone. 2000;27(6):785–794. doi: 10.1016/s8756-3282(00)00400-2. [DOI] [PubMed] [Google Scholar]
- 23.Roth J.A, Kim B.G, Lin W.L, Cho M.I. Melatonin promotes osteoblast differentiation and bone formation. J Biol Chem. 1999;274(31):22041–22047. doi: 10.1074/jbc.274.31.22041. [DOI] [PubMed] [Google Scholar]
- 24.Bellido T, Borba V.Z.C, Roberson P, Manolagas S.C. Activation of the Janus kinase/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology. 1997;138(9):3666–3676. doi: 10.1210/endo.138.9.5364. [DOI] [PubMed] [Google Scholar]
- 25.Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama M, Kodama T, Konishi K, Abe K, Asami S, Oikama S. Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human osteosarcoma cells. Biochem Biophys Res Commun. 2000;271(3):688–692. doi: 10.1006/bbrc.2000.2697. [DOI] [PubMed] [Google Scholar]
- 27.Phillips B.W, Belmonte N, Vernochet C, Ailhaud G, Dani C. Compactin enhances osteogenesis in murine embrymonic stem cells. Biochem Biophys Res Commun. 2001;284(2):478–484. doi: 10.1006/bbrc.2001.4987. [DOI] [PubMed] [Google Scholar]
- 28.Yang S, Leong K.F, Du Z.H, Chua C.K. Review: The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Engineering. 2001;7(6):679–690. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 29.Wiedmann-Al-Ahmad M, Gutwald R, Lauer G, Hubner U, Schmelzeisen R. How to optimize seeding and culturing of human osteoblast-like cells on various biomaterials. Biomaterials. 2002;23(16):3319–3328. doi: 10.1016/s0142-9612(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 30.Jansen J.A, de Wijn J.R, Wolters-Lutgerhorst J.M.L, van Mullem P.J. Ultrastructural study of epithelial cell attachment to implant materials. J Dent Res. 1985;64(6):891–896. doi: 10.1177/00220345850640060601. [DOI] [PubMed] [Google Scholar]
- 31.Brunette D.M. The effects of implant surface topography on the behavior of cells. Int J Oral Maxillofac Implants. 1988;3(4):231–246. [PubMed] [Google Scholar]
- 32.Hormia M, Kononen M, Kivilahti J, Virtanen I. Immunolocalization of proteins specific for adhaerens junctions in human gingival epithelial cells grown on differently processed titanium surfaces. J Periodont Res. 1991;26(6):491–497. doi: 10.1111/j.1600-0765.1991.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 33.Kononen M, Hormia M, Kivilahti J, Hautaniemi J, Thesleff I. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. J Biomed Mater Res. 1992;26(10):1325–1341. doi: 10.1002/jbm.820261006. [DOI] [PubMed] [Google Scholar]
- 34.Glantz P.O. The choice of alloplastic materials for oral implants: does it really matter? Int J Prosthodont. 1998;11(5):402–407. [PubMed] [Google Scholar]
- 35.Zeng H, Chittur K.K, Lacefield W.R. Analysis of bovine serum albumin adsorption on calcium phosphate and titanium surfaces. Biomaterials. 1999;20(4):377–384. doi: 10.1016/s0142-9612(98)00184-7. [DOI] [PubMed] [Google Scholar]
- 36.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21(7):667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 37.Boyan B.D, Hummert T.W, Dean D.D, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 38.BD™ three dimensional OPLA® Scffold, guideline for use. Catalog from BD Biosciences.
- 39.Galli S.J, Zsebo K.M, Geissler E.N. The kit ligand, stem cell factor. Adv Immunol. 1994;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- 40.Humason G.L. London: Freeman; 1979. Animal Tissue Techniques. [Google Scholar]
- 41.Cooper M.S, Hewison M, Stewart P.M. Glucocorticoid activity, inactivity and the osteoblast. J Endocrinol. 1999;163:159–164. doi: 10.1677/joe.0.1630159. [DOI] [PubMed] [Google Scholar]
- 42.Jung C, Ou Y.C, Yeung F, Frierson H.F. Jr, Kao C. Osteocalcin is incompletely spliced in non-osseous tissues. Gene. 2001;271:143–150. doi: 10.1016/s0378-1119(01)00513-3. [DOI] [PubMed] [Google Scholar]
- 43.Ishaug S.L, Crane G.M, Miller M.J, Yasko A.W, Yaszemski M.J, Mikos A.G. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36(1):17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 44.Crane G.M, Ishaug S.L, Mikos A.G. Bone tissue engineering. Nat Med. 1995;1(12):1322–1324. doi: 10.1038/nm1295-1322. [DOI] [PubMed] [Google Scholar]
- 45.Yaszemski M.J, Payne R.G, Hayes W.C, Langer R, Mikos A.G. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17(2):175–185. doi: 10.1016/0142-9612(96)85762-0. [DOI] [PubMed] [Google Scholar]




