Abstract
Drought, rising temperatures, and expanding human populations are increasing water demands. Many countries are extending potable water supplies by irrigating crops with wastewater. Unfortunately, wastewater contains biologically active, long-lived pharmaceuticals, even after treatment. Run-off from farms and wastewater treatment plant overflows contribute high concentrations of pharmaceuticals to the environment. This study assessed the effects of common pharmaceuticals on a cosmopolitan saprophagous insect, Megaselia scalaris (Diptera: Phoridae). Larvae were reared on artificial diets spiked with contaminants of emerging concern (CECs) at environmentally relevant concentrations. Female flies showed no oviposition preference for treated or untreated diets. Larvae exposed to caffeine in diets showed increased mortality, and larvae fed antibiotics and hormones showed signs of slowed development, especially in females. The normal sex ratio observed in M. scalaris from control diets was affected by exposure to caffeine and pharmaceutical mixture treatments. There was an overall effect of treatment on the flies’ microbial communities; notably, caffeine fed insects displayed higher microbial variability. Eight bacterial families accounted for approximately 95% of the total microbes in diet and insects. Our results suggest that CECs at environmentally relevant concentrations can affect the biology and microbial communities of an insect of ecological and medical importance.
Introduction
Pharmaceuticals have been increasingly prescribed for the past 30 years, and prescription rates have almost tripled in the past 14 years1, 2. In food-producing animals alone, there were 9.1 million kg of medically important antibiotics (antibiotics used in both humans and animals) used in 2013. Of those 9.1 million kg used, 73.6% was used for the purpose of increasing production of the animals, and this use continues to increase3. Many antibiotics and other common Contaminants of Emerging Concern (CECs) (acetaminophen, mental stimulants, heartburn medications, allergy, and bacterial infection treatments), are excreted by both humans and animals with little change in their chemical structure4. It is no surprise pharmaceuticals have been appearing in wastewater, and in some cases tap water, over the past few years5, 6.
Standard wastewater treatment facilities are ill equipped to remove pharmaceuticals7, 8. Many pharmaceuticals are released during heavy storms in the untreated wastewater, due to overflow, which then flows directly to the environment9. These pharmaceuticals are now found at biologically active concentrations in surface waters around the world10–14. In addition to runoff, there is an increasing effort to use reclaimed wastewater in drought affected areas, such as Southern California15, 16. In agriculture/livestock operations, pharmaceuticals are also found in manure that is then used as fertilizer, effectively compounding the pharmaceutical concentrations10, 17, 18. Current research shows these chemicals tend to be both long lived in soil and detrimental to soil microbes11, 19–22.
Recent studies on the effects of pharmaceuticals on aquatic insects show that at environmentally relevant concentrations they can alter development of the mosquito Culex quinquefaciatus, its susceptibility to a common larvicide, and its larval microbial communities23, 24. Watts et al.25 showed alterations and deformities in the midge Chironomus riparius after treatment with a common birth control agent, 17α-ethinylestradiol, and a common plasticizer, Bisphenol-A. Interestingly, many chemicals used by humans, which are not intended for use on microbial communities, have been shown to affect microbes. For example, caffeine, a common mental stimulant, alters biofilm respiration, and an antihistamine, diphenhydramine, has been demonstrated to modify the microbial community and respiration of lake biofilms26. Because of unexpected pharmaceutical effects, it is relatively difficult to predict what will occur in model organisms. This problem is exacerbated by a lack of information regarding pharmaceuticals’ effects on terrestrial insects: no available publications report the effects on any terrestrial insects’ microbial community.
Arthropods, such as insects and crustaceans, rely on hormones to grow, develop, mate and even produce pigmentation27–29. However, many pharmaceuticals, especially hormones, resemble chemicals that these organisms rely on for growth and development. These pharmaceuticals then bind to receptors and either over-express or suppress their counterparts’ natural function. This has been reported in birds, reptiles, and arthropods where endocrine disruption occurs, primary and secondary sexual characteristics are modified, and courtship behaviors change27, 30–34. While most arthropod hormones do not closely match those of mammals, their molting hormone (ecdysone), is very similar to 17β-estradiol (the mammalian female sex hormone). In crustaceans, mammalian hormones have been known to cause both increased molting events and inhibition of chitobiase, the enzyme responsible for digestion of the cuticle during insect molting35, 36. In insects, 17α-ethinylestradiol, a common synthetic birth control hormone, has been shown to alter molting and lead to deformities of C. riparius. Also, Bisphenol-A, a common plasticizer, can bind and activate estrogen receptors in humans, and the ecdysone-binding protein in insects25, 37. In addition to these effects, pharmaceuticals have been shown to cause effects to insects over multiple generations38.
Megaselia scalaris (Lowe, Diptera: Phoridae) is a common saprophagous pest. They are known to infect living humans (myiasis), provide important ecological roles as detritivores, and because they often feed on human corpses are commonly used in forensic entomology to determine time of death39, 40. This species will generally feed on a variety of decomposing plant and animal tissues, and acts as a vector of pathogens39, 41. These insects are both fecund and hardy because females can lay over 650 eggs in 16 days and are tolerant of heavy metals42, 43. The white, roughly football-shaped eggs, hatch after approximately 24 hours into white translucent larvae. When they have matured to third instar (life-stage) they pupariate39, 40. Their detritivorous larval life history exposes them to a wide diversity of microorganisms that may act as pathogens, commensals, and symbionts. There is currently no record of how M. scalaris acquires their microbiota or if any symbionts are required. However, it stands to reason that they, like so many other insects, would rely on microbial symbionts44, 45. There are many ways insects acquire symbionts: from their diet, the environment, their social network, or vertical transmittance (maternally inherited)46–49.
Currently there is little to no information regarding pharmaceutical effects at the concentrations found in reclaimed water on the growth or microbial community composition of any terrestrial detritivore. These detritvores become exposed to contaminants after the CECs enter surface waters, soil, and plants from overflow and wastewater reuse. There are studies involving antibiotics at high doses to determine necessity of microbiota in several insects, but these have not tested relevant concentrations found in reclaimed water or joint effects of other pharmaceuticals, which often coexist with antibiotics45, 50. To assess potential effects of common pharmaceuticals, we used a series of bioassays to determine the possibility of individual and joint contamination on development, mortality and population sex ratios of M. scalaris. Any effects would have potentially important implications from medical, ecological, and forensic perspectives. Also, as there is currently no information on M. scalaris’ microbial community, information generated from this study could serve as novel information into the role possible symbionts play in M. scalaris development.
Results
Oviposition Choice, Mortality, Days to Pupariation, and Sex Ratio Differences
These insects did not respond to these contaminants because pharmaceuticals in reclaimed water did not affect oviposition preferences (χ2 = 3.66, df = 5, p = 0.60). Mortality was increased (χ2 = 23.21, df = 5, p < 0.001) when M. scalaris were fed diets containing caffeine (p < 0.01) (Fig. 1). Increased mortality was evident both in the larval life-stage (χ2 = 22.81, df = 5, p < 0.001) and the pupal stage (χ2 = 17.41, df = 5, p < 0.01). The largest increase in mortality was in larval-stage caffeine treatments (p < 0.05).
Figure 1.
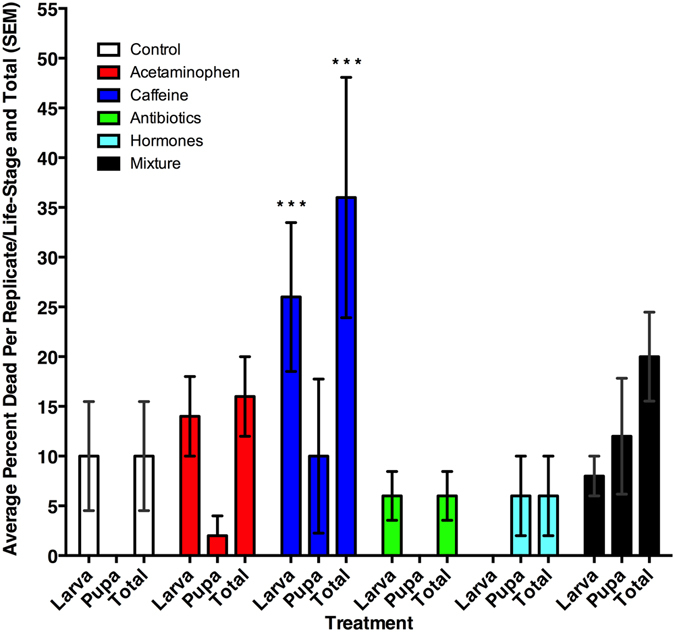
Average (SEM) mortality of larvae, pupae, and total insects for each treatment group. ***Denotes significant difference (α = 0.05) relative to the control.
There was an increased time to pupariation (χ2 = 24.71, df = 5, p < 0.001) for M. scalaris fed antibiotic (p < 0.01) or hormone (p < 0.001) containing diets (Fig. 2). While there were no overall differences of sex ratio (χ2 = 4.54, df = 5, p = 0.48), sex did have an effect on pupariation time (χ2 = 52.59, df = 1, p < 0.001), and there was an interaction of treatment and sex (χ2 = 26.88, df = 5, p < 0.001), which was most evident in the mixture treatment (p < 0.01). Within individual treatments, however, there were sex ratio differences (Fig. 3) for M. scalaris exposed to diets in the control (p < 0.05), acetaminophen (p < 0.05), antibiotics (p < 0.001), and hormone (p < 0.001) treatments. Interestingly, there were no sex ratio differences in the caffeine (p = 0.15) and mixture (p = 0.88) treatments. Comparing the time to pupariation of opposite sexes (χ2 = 44.25, df = 5, p < 0.001), male times to pupariation in treatments were not different (χ2 = 7.34, df = 5, p = 0.20) than the controls. However, female (χ2 = 44.25, df = 5, p < 0.001) development in the antibiotic (p < 0.01) and hormone (p < 0.001) treated diets took significantly longer to pupariate than the control females (Fig. 2).
Figure 2.
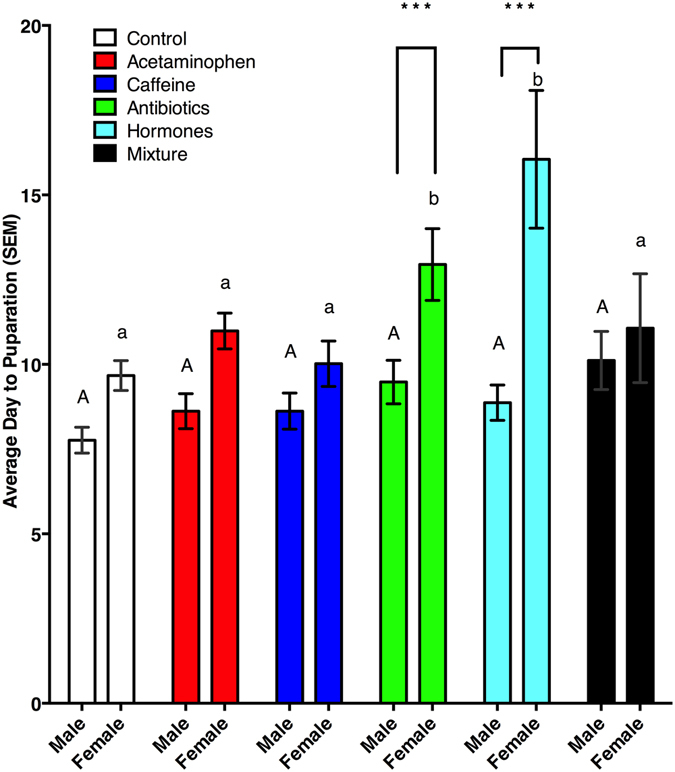
Average day to pupariation of male and female Megaselia scalaris by treatment. Upper case letters denote significant differences in days to pupariation (DTP) from male control. Lower case letters denote significant differences in DTP from female control. ***Denotes an overall day to pupariation difference from controls.
Figure 3.
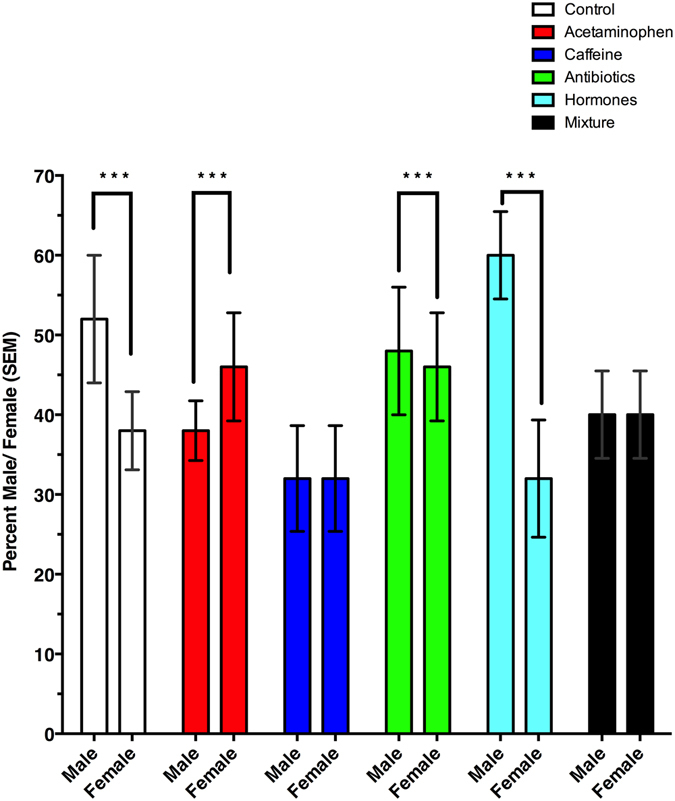
Male: female ratios of Megaselia scalaris fed diets contaminated with common pharmaceuticals found in reclaimed water. ***Denotes a significant difference in sex ratio with respect to treatment.
Bacterial Community Analysis
There were 752,855 total raw reads, with an average of 10,456 reads per sample, and a total of 772 distinct operational taxonomic units (OTUs), DNA sequences which are at least 97% identical, after removing OTUs identified as mitochondria, chloroplast, and obvious contaminant DNA through BLAST analysis. Overall, there was an effect of treatment (Adonis PERMANOVA: F = 1.92; df = 5, 44; p < 0.05) on the bacterial community of M. scalaris. Based on adjusted p-values (BH False Discovery Rate), a majority of differences in the treatments occurred between third instars and adults (Table 1). There were 30 different OTUs responsible for these differences and of those 28 were different in the third instar/adult stage comparisons. There were eight bacterial families that accounted for at least 2.5% (by proportional abundance of OTUs) of the total bacterial families found in at least one sample (Fig. 4 and Table 2) and, collectively, they account for at least 94% of the total microbial community found in all life-stages. Six of the eight families in Fig. 4 showed differences between third instars and adults (Table 1). Only Burkholderiaceae did not show a difference between third instar and pupal stages. Xanthomonadaceae and Sphingomonadaceae were the only families on Fig. 4 not also in Table 1. According to a non-metric multidimensional scaling (NMDS) plot (Supp. Fig. 2), the least dissimilarities were observed among (1) pupae and diets from hormone treatments, (2) the adults, pupae, and diets, from mixture treatments and (3) the individuals exposed to antibiotics.
Table 1.
Bacterial families and genera in each treatment that are significantly different in at least one life-stage pairing.
| Treatment | Phylum | Family | Genus | Species | Third Instar-Pupa | Third Instar- Adult | Pupa-Adult |
|---|---|---|---|---|---|---|---|
| Control | Actinobacteria | Corynebacteriaceae | Corynebacterium | sp. | * | ||
| Bacteroidetes | Chitinophagaceae | Sediminibacterium | sp. | * | * | ||
| Proteobacteria | Alcaligenaceae | Achromobacter | sp. | * | * | ||
| Caulobacteraceae | Caulobacter | sp. | * | * | |||
| Enterobacteriaceae | Klebsiella | sp. | * | * | |||
| Enterobacter | sp. | * | |||||
| Erwiniaceae | Erwinia | sp. | * | * | |||
| Hyphomicrobiaceae | Pedomicrobium | sp. | * | ||||
| Methylobacteriaceae | Methylobacterium | sp. | * | * | |||
| NA | NA | sp. | * | * | |||
| Pseudomonadaceae | Pseudomonas | veronii | * | * | |||
| sp. | * | * | |||||
| sp. | * | * | |||||
| Sinobacteraceae | Steroidobacter | sp. | * | ||||
| Acetaminophen | Proteobacteria | Pseudomonadaceae | Pseudomonas | sp. | * | ||
| Caffeine | Actinobacteria | Cornebacteriacea | Corynebacterium | sp. | * | ||
| Proteobacteria | Bradyrhizobiaceae | Afipia | sp. | * | * | ||
| Burkholderiaceae | Burkholderia | sp. | * | * | |||
| Enterobacteriaceae | Enterobacter | sp. | * | * | |||
| Erythrobacteraceae | Altererythrobacter | sp. | * | * | |||
| Methylobacteriaceae | Methylobacterium | sp. | * | * | |||
| sp. | * | * | |||||
| Pseudomonadaceae | Pseudomonas | sp. | * | * | |||
| sp. | * | * | |||||
| Antibiotics | Actinobacteria | Microbacteriaceae | Microbacterium | sp. | * | ||
| Micrococcaceae | Micrococcus | sp. | * | ||||
| Firmicutes | Lactobacillaceae | Lactobacillus | brevis | * | * | ||
| Proteobacteria | Burkholderiaceae | Burkholderia | sp. | * | * | ||
| Hormones | Actinobacteria | Mycobacteriaceae | Mycobacterium | sp. | * | * | |
| Nocardioidaceae | Nocardioides | sp. | * | ||||
| Bacteroidetes | Sphingobacteriaceae | Sphingobacterium | multivorum | * | * | ||
| Proteobacteria | Alcaligenaceae | Achromobacter | sp. | * | * | ||
| Enterobacteriaceae | Klebsiella | sp. | * | ||||
| Enterobacter | sp. | * | * | ||||
| Erwiniaceae | Erwinia | sp. | * | * | |||
| Methylobacteriaceae | Methylobacterium | sp. | * | * | |||
| Moraxellaceae | Acinetobacter | lwoffii | * | * | |||
| NA | NA | sp. | * | * | |||
| Pseudomonadaceae | Pseudomonas | veronii | * | * | |||
| sp. | * | * | |||||
| sp. | * | * | |||||
| Rickettsiaceae | Rickettsia | sp. | * | * | |||
| Mixture | Proteobacteria | Burkholderiaceae | Burkholderia | sp. | * | * | |
| Erwiniaceae | Erwinia | sp. | * | * | |||
| Orbaceae | Orbus | sp. | * |
*Denotes adjusted p value of <0.05 in those genera for each life-stage pairing in a treatment.
Figure 4.
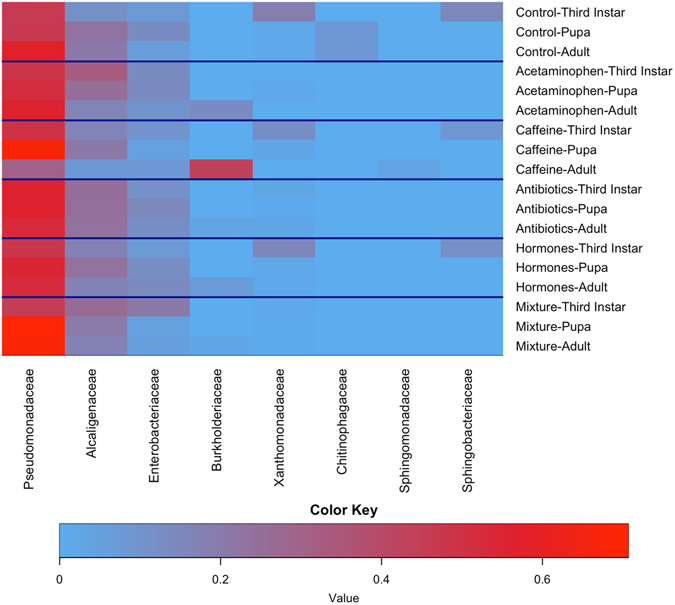
Heatmap of the most abundant bacterial families (each accounting for at least 2.5% of the total OTUs) by average OTUs of treatment life-stage pairing Increased red coloration is an increase in indicative of increased proportional abundance.
Table 2.
Average percentage of bacterial families by insect life-stage.
| Bacterial Phylum | Bacterial Family | Avg. Percentage Third Instar | Avg. Percentage Pupa | Avg. Percentage Adult |
|---|---|---|---|---|
| Proteobacteria | Alcaligenaceae | 26.41 | 22.90 | 17.23 |
| Enterobacteriaceae | 13.31 | 10.64 | 9.42 | |
| Pseudomonadaceae | 50.61 | 58.02 | 52.26 | |
| Burkholderiaceae | 0.00 | 0.02 | 12.25 | |
| Xanthomonadaceae | 3.57 | 1.63 | 1.21 | |
| Sphingomonadaceae | 0.16 | 0.19 | 0.67 | |
| Bacteroidetes | Chitinophagaceae | >0.01 | 1.40 | 1.49 |
| Sphingobacteriaceae | 1.42 | 0.00 | 0.02 | |
| Sum Percentages | 95.48% | 94.80% | 94.55% |
Discussion
Megaselia scalaris, a common detritivore, has been known to develop on substances as diverse as human wounds and corpses51, 52, modeling clay, and emulsion paint53, 54. Their ability to grow and mature on these diets, with minimal effect on their survival, and their tolerance to heavy metals42 makes any effect of pharmaceuticals at very low doses found in reclaimed water even more surprising. In our study, the females had no preference for untreated diets versus any treated diets. This poses a problem for the insect population, as there was higher larval mortality when developing on a caffeine-contaminated food source. Because females require an additional 24 hours39 after emergence in order to be receptive to males, populations exposed to hormones or antibiotics would be adversely affected. If females require an extra six days to emerge and become receptive, there is a reasonable possibility the males would leave the area or perish before mating. In addition, the suitability of decaying food sources tends to be temporary55. Collectively, these factors could likely negatively influence population growth. Also, these changes in population growth rate could hinder forensic scientists from determining an accurate time of death if there were long lasting or even moderate concentrations of these pharmaceuticals in the body at death.
Sex ratios of emergent adults were also affected in the caffeine and mixture treatments. The sex ratios found in control treatments in our study are similar to those reported in Benner & Ostermeyer56 of a male: female sex ratio at 25 °C of 1.18:1. However, sex ratios from the acetaminophen, caffeine, and mixture treatments differed significantly from the controls. A major difference in sex ratio would change the reproductive capacity of a population. It is unclear why acetaminophen and caffeine would alter sex ratios, however acetaminophen as been recorded to hinder the production of arachadonic acid in mosquitoes, another Dipteran, and it could be playing a similar role here57. Ibuprofen, another analgesic and antipyretic has been shown to alter the sex ratio in another mosquito58.
Many insects rely on their microbial communities and endosymbionts to grow and develop59. However, Adonis, the statistical method used to analyze these data, does not have a post hoc test available that would allow direct pairwise comparisons between treatments. Nonetheless, there are changes in the bacterial community (Fig. 4 and Table 1) based on adjusted p-values evaluating differential abundance. We found significant shifts in the microbial community in the various life stages examined within the control treatments. A similar result has been reported for mosquitoes24 and other insects60, 61. Not surprisingly, insects that undergo complete metamorphosis and also rely on a different food source as adults would require a different bacterial community; however there is one family, Pseudomonadaceae, which appears in all treatments and life-stages. Species in this family are gram-negative Proteobacteria that cannot survive in acidic environments62. They are fairly common in insects63, and can be responsible for 90 + % of the bacterial community64. They are resistant to antibiotics62, which potentially explains why they are so prevalent in many of our treatments. Pseudomonadaceae is responsible for ~ 50% of the bacteria in all life-stages, followed by Alcaligenaceae, Enterobacteriaceae, and Xanthomonadaceae. Pseudomonadaceae and Enterobacteriaceae families contain known symbionts in insects48, 65–67 and could be filling the same role in M. scalaris.
When Pseudomonadaceae is removed from the heatmap (Supp. Fig. 1), it becomes clear how the next three highly proportional families change with life-stage. Alcaligenaceae tends to become more proportionally abundant in pupae and adults than in larvae. Species in the family Alcaligenaceae are oxidase- and catalase-positive and utilize a variety of organic and amino acids as carbon sources68. Enterobacteriaceae has higher proportions in larvae than in adults. Species of Enterobacteriaceae are likely to be either symbionts or a pathogen to their hosts62. Enterobacteriaceae includes Buchnera, an important endosymbiont of aphids69, and other species that inhabit various insects to provide facultative benefits48, 70. Xanthomonadaceae, like Enterobacteriaceae, is more prominent in larvae than in other life-stages except in acetaminophen, antibiotic, and mixture containing diets, where Enterobacteriaceae dominate. Most species in Xanthomonadaceae are plant pathogens62, and have been known to make use of chitin as a carbon source and utilize insects as vectors71, 72. It is possible that some of the bacteria in this family may act as symbionts with insects as they have been found in a variety of insect orders73–75.
In the NMDS plot (Supp. Fig. 2) there is distinct clustering in the microbiota by treatment. In individuals exposed to antibiotics in their diets, there is a lack of dissimilarity among their microbial communities. Insects reared on diets containing hormones had less microbial diversity in pupae. Unfortunately, we do not know if this is due to similarity in the microbial communities of third instar individuals as the statistical process of rarefication removed that particular life-stage in larvae exposed to hormones, controls, and caffeine treatments. However, the insects feeding on the mixture treatments show a distinct clustering of microbial groups in the pupal and adult stages, whereas the larval stage contains individuals with more variable microbiota. This is likely due to the early instars being exposed directly to the microbe-laden diet, while the later life-stages are only exposed to a subset of bacteria left after the gut contents were expelled at time of pupariation. The greatest dissimilarity was found in the caffeine-treated adults (Fig. 5 and Supp. Fig. 2). The adult stage, regardless of treatment, also seems to be where the majority of variation in microbiota occurs (Fig. 5).
Figure 5.
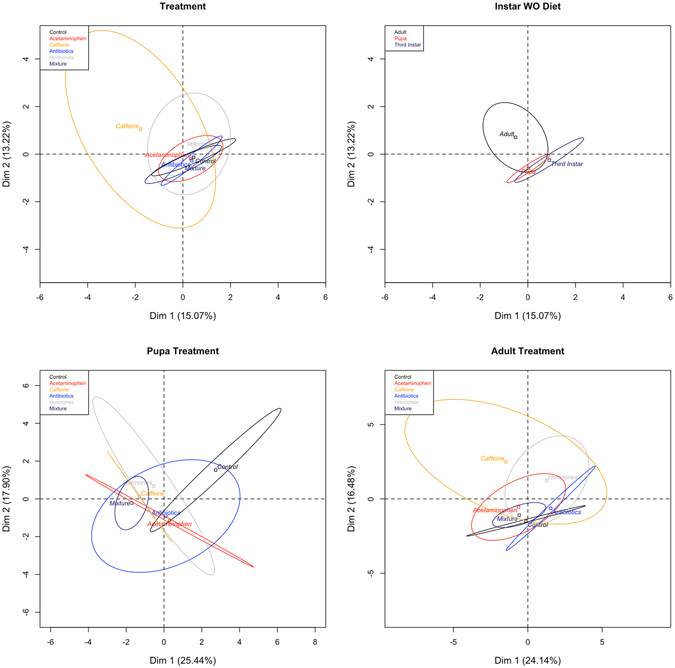
Principal Component Analysis of treatments, life-stage, pupa by treatment, and adult by treatment. Ellipses denote range of individuals around a centroid barycentre.
Megaselia scalaris has been suggested as a model organism for bioassays for drugs and pollutants39, and our findings support this claim. However, our results also suggest that the presence of even very low concentrations of some pharmaceuticals could affect the forensic estimation of time of death based on emergence patterns of adult M. scalaris. We also caution that the pharmaceuticals used in this trial were at low concentrations found in wastewater and could be much higher in cadavers, as pharmaceuticals in humans tend to be higher than what is found in the environment76–79. Also, due to increases in concentrations caused by water loss (on a weight/weight basis), pharmaceuticals could have higher toxicity in decaying matter. Perhaps most importantly, pharmaceuticals in reclaimed water are having unintended effects on the microbial community of these flies, which could lead to decreased viability of these ecologically useful detritivores.
Methods and Materials
Chemicals
Test compounds included: acetaminophen, caffeine, three antibiotics, and four estrogenic steroidal hormones. Six treatments were examined: acetaminophen, caffeine, an antibiotic mixture (lincomycin, oxytetracycline, and ciprofloxacin), a hormone mixture (estrone, 19-norethindrone, 17β- estradiol, and 17α- ethynylestradiol), a mixture of all chemicals (as would be found in overflow or wastewater effluent), and a control, consisting of only distilled water. Distilled water was tested for CECs and found to not contain any. Treatment groups were chosen as representative compounds for pain relievers, mental stimulants, antibiotics commonly used on humans and livestock, hormones normally either produced or prescribed to humans, and as a mixture that would be simple, yet representative of wastewater effluent or reclaimed wastewater. Artificial diets were prepared at room temperature to negate any decomposition of the CECs. Acetaminophen (10 μg/L), caffeine (6 μg/L), estrone (0.112 μg/L), 19-norethindrone (0.872 μg/L), 17β- estradiol (0.2 μg/L), 17α- ethynylestradiol (0.831 μg/L), lincomycin (0.73 μg/L), and oxytetracycline (72.9 μg/L) concentrations were chosen based on the maximum concentrations measured by Kolpin et al.13. Ciprofloxacin (6,500 μg/L) concentration was chosen from the maximum lake water concentration reported by Mutiyar and Mittal14.
The chemicals used were purchased as follows: acetaminophen with a purity of ≥90%; (MP Biomedicals, LLC, Santa Ana, CA); caffeine at laboratory grade purity (Fisher Scientific, Hanover Park, IL); lincomycin, oxytetracycline, and ciprofloxacin with purities of ≥98% (Alfa Aesar, Ward Hill, MA); estrone, 19-norethindrone, 17β- estradiol, and 17α- ethynylestradiol at ≥98% purity (Sigma-Aldrich, St. Louis, MO). Blue formula 4–24® instant Drosophila medium, hereafter known as ‘blue diet’, was purchased from Carolina Biological Supply Company (Burlington, NC). Hydrochloric acid (12.1 M) was obtained from Fisher Scientific. Sodium hydroxide was acquired from Sigma-Aldrich (St. Louis, MO) as anhydrous pellets. Stock solutions were prepared by adding powdered chemicals to deionized water. Approximately 5 mL 80% ethanol was added to 250 mL steroidal hormone solutions to facilitate dissolution. Hydrochloric acid (1 M) was added to antibiotic chemical solutions to facilitate dissolution and pH was adjusted using NaOH (1 M) to pH 4.00. Compounds were added to distilled water to the desired concentrations for each treatment and then an equal amount of blue diet flakes was added as described by the manufacturer. In all experiments, preparations and concentrations of treatment groups were prepared as stated previously.
Insects
A M. scalaris colony was established in 2015, and the species verified at the Los Angeles Natural History Museum by Ms. Emily Hartop. The colony was maintained on an alfalfa diet as in Mandeville et al.80. For all experiments, eggs were collected from adults and were stored in an incubator (model 818: Precision Scientific Inc., Buffalo, NY) at 26 °C, approximately 70% RH, and a light: dark cycle of 16:8. In order to standardize the age of larvae in all of the following experiments, eggs were collected by placing 9 cm Petri dishes, containing blue diet, inside the colony for ~12 hr. Petri dishes were wrapped in aluminum foil in a funnel shape to exclude colony larvae. Eggs were then transferred, by microspatula, to bifurcated 9 cm Petri dishes or individual 50 mL centrifuge tubes each containing 25 mL or 2 mL, respectively, of treated or control blue diet. Nine centimeter Petri dishes contained blue diet only on one side of the bifurcation to allow larvae to migrate to the empty side for pupariation. Centrifuge tubes allowed for monitoring of individual larvae, while the Petri dishes were used to rear multiple insects for microbial community analyses.
Oviposition choice assay
Following blue diet preparation, size 12 cork-borer plugs were taken from each Petri dish. Ten individual 9 cm Petri dish arenas were prepared by placing one plug of each treatment and the control in a circle (6 plugs per dish), with plugs placed equidistantly. Ten male/female pairings were added to each arena and allowed to choose and oviposit for 24 h. Eggs on each plug in each replicate were then counted and recorded.
Mortality, Days to Pupariation, and Sex differences
Individual eggs were transferred to 50 mL centrifuge tubes by microspatula. There were 10 centrifuge tubes per replicate, and 5 replicates for each treatment (n = 50/n = 300 across all treatments). Inside each centrifuge tube, a strip of filter paper was placed inside to reduce excess moisture and provide a pupariation surface. Individuals were monitored daily for pupariation and adult emergence until all individuals had emerged or died. Adults were then sacrificed at −60 °C, and their sexes were determined based on the structure of genitalia.
Insect rearing for bacterial analysis
Eggs were transferred, by microspatula, to the blue diet of each 9 cm Petri dish. There were 3 replicates per life stage for each of the 6 treatments (n = 54). Lids contained a size 7 cork-borer hole affixed with 2 layers of fine organza mesh to allow for moisture and gas exchange. Following egg placement, Petri dish lids and bottoms were aligned and secured with parafilm. Petri dishes were monitored daily for development. Six individuals were collected at third instar, pupa, and adult life-stages, triple washed with 200 proof ethanol, and stored in clean 200 proof ethanol at −60 °C until DNA extraction. During the collection of each treatment group and life-stage, blanks in triplicate of DDI H2O were used to monitor contamination. Before extraction, triplicate blanks were pooled.
DNA extractions and Illumina sequencing of whole body Megaselia scalaris bacteria
All DNA extractions and Illumina preparations were performed as in McFrederick and Rehan46. Briefly, DNA extractions were performed using a DNeasy Blood and Tissue kit (Qiagen, Valencia, CA). An individual from each life-stage (n = 3), each treatment group (n = 6), and replicate group (n = 3), along with triplicates of the pooled blank (DDI H2O), for each treatment group (n = 18), were acquired (n = 72) and placed in individual wells of a 96-well plate provided in the kit. Into each well, we added 180 μL of buffer ATL, a sterile 3.2 mm chrome-steel bead and 100 μL of 0.1 mm glass (Biospec, Bartlesville, OK). A Qiagen tissuelyzer was then used to bead-beat each sample for 6 min at 30 Hz. We then added 20 μL of Proteinase K to each sample and incubated at 57 °C overnight. The standard DNeasy extraction protocol was then followed.
Following extraction, dual-index inline barcoding was used to prepare libraries for sequencing on the Illumina MiSeq. We used primers that included either the forward or reverse Illumina sequencing primer, a unique eight-nucleotide long barcode, and the forward or reverse genomic oligonucleotide as in Kembel et al.81. For the bacterial 16S rDNA sequences we used the primers 799F-mod3 CMGGATTAGATACCCKGG82 and 1115 R AGGGTTGCGCTCGTTG81, which have been shown to minimize contamination from plastids.
We used these primers to generate 16S rRNA gene amplicons for Illumina sequencing using PCR. PCRs were performed using 10 μL ultrapure water, 10 μL of 2× Pfusion High-Fidelity DNA polymerase (New England Biolabs, Ipswich, MA), 0.5 μL of each 10 μM primer stock, and 4 μL of DNA. We used a 52 °C annealing temperature, 35 cycles, and negative controls for each reaction. To remove unincorporated primers and dNTPs, we used the Ultraclean PCR clean up kit (MoBio, Carlsbad, CA). We used 1 μL of the clean PCR product as a template for another PCR, using HPLC purified primers to complete the Illumina sequencing construct as in Kembel et al.81: CAAGCAGAAGACGGCATACGAGATCGGTCTCGGCATTCCTGC, and AATGATACG GCGACCACCGAGATCTACACTCTTTCCCTACACGACG. For the reactions, we used a 58 °C annealing temperature, 35 cycles and negative controls. Once the PCR cycles were finished, we used 18 μL of the PCR product and SequalPrep Normilization plates (ThermoFisher Scientific, Waltham, MA) to normalize the amount of DNA in each sample. We pooled 5 μL of each normalized sample, performed another cleanup, and then used a 2100 Bioanalyzer (Agilent, Santa Clara, CA) to assess our library quality. After quality control, we sequenced the libraries using the MiSeq Reagent kit v3 with 2 × 300 cycles. Raw data are available on the NCBI Sequence Read Archive (SRA) accession number SRP099221.
Bioinformatics
All genomic information was processed using macQIIME ver. 1.9.1–2015060483. We used USEARCH v6.184 to identify and remove chimeric sequences, and SUMACLUST85 to cluster OTUs and remove any with less than two reads per sample. We used 97% sequence identity to bin OTUs and choose representative OTUs. We then performed standard alpha and beta diversity analyses in QIIME. To assign taxonomy to OTUs, Greengenes taxonomy86 and the RDP Naïve Bayesian Classifier87 were utilized, and we also performed BLASTN searches against NCBI’s online Nucleotide Collection (nr/nt) and 16 S ribosomal RNA sequences (Bacteria and Archea) databases (accessed January 17, 2017). Taxonomy was then used to identify any mitochondria or chloroplast OTUs, which were removed from the dataset as in McFrederick & Rehan46. We aligned the quality-filtered dataset using the pynast aligner88 and the Greengenes database86. We then reconstructed the phylogeny of the bacterial OTUs using FASTTREE version 2.1.389. Next we performed weighted and unweighted UniFrac analyses90 using the generated phylogeny and OTU tables. Using the generated distance matrices, we performed Adonis91 and created PCA92 graphs in R version 3.3.193. For alpha diversity, we plotted rarefaction curves in GraphPad Prism version 6.00 software (La Jolla, California), and used gplots94 to create a heatmap of the most abundant bacterial families; a 0.025 proportional abundance in at least one sample was used as the cutoff.
Statistics
All statistical analyses were performed using R (version 3.3.1). Normality was determined using Shapiro-Wilk normality tests. Mortality was determined using a generalized linear model with a binomial family. Differences in days to pupariation were determined using the ‘survival’ and the ‘OIsurv’ packages95, 96. In all cases, when data were not considered normal, either a Poisson distribution or a negative binomial generalized linear model was used and the best fitting model was determined from Akaike’s ‘An Information Criterion’. Adonis within the R package “vegan”91 was used for all PERMANOVA analyses. As there is no post-hoc97 test for Adonis, we used adjusted p values obtained from metagenomeHIT_zig in R through QIIME83, 98 to determine differentially abundant OTUs in treatments between life stages.
Electronic supplementary material
Acknowledgements
The authors would like to thank G. Kund for his knowledge and guidance in experimental design and methodology and D. De la Riva, T. Dang, and S. Truong for edits on this manuscript. The authors would also like to thank the UCR Genomics Core facility staff for sequencing expertise. This research was supported by the Environmental Protection Agency (Award No: R835829) and through a fellowship awarded to Jason A. Rothman by the National Aeronautics and Space Administration MIRO Fellowships in Extremely Large Data Sets (Award No: NNX15AP99A). This work was also supported in part by the USDA National Institute of Food and Agriculture, Hatch Project (Project No.: 1011669).
Author Contributions
Pennington, Rothman, and Trumble designed the experiments. Pennington, Rothman, and Trumble wrote the manuscript. Pennington and Rothman conducted the analyses and generated all figures and tables. The Trumble, Gan and McFrederick laboratories funded the project. All authors reviewed and edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08683-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Center for Health Statistics. Health, United States, 2013: with special feature on prescription drugs (2014). [PubMed]
- 2.Schumock GT, et al. National trends in prescription drug expenditures and projections for 2014. Am. J. Health. Syst. Pharm. 2014;71:482–99. doi: 10.2146/ajhp130767. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services. Antimicrobials sold or distributed for use in food-producing animals (2013).
- 4.Wright L, et al. Fate and transport of thirteen pharmaceutical and personal pare products in a controlled irrigated turfgrass system. Agron. J. 2012;104 doi: 10.2134/agronj2011.0394. [DOI] [Google Scholar]
- 5.Monteiro, S. & Boxall, A. B. A. In Reviews of Environmental Contamination and Toxicology225, 159–195 (2013).
- 6.Sklerov, F. & Saucier, M. Follow-up study confirms no risk from pharmaceuticals and personal care products in NYC drinking water (2011).
- 7.Hedgespeth ML, et al. Pharmaceuticals and personal care products (PPCPs) in treated wastewater discharges into Charleston Harbor, South Carolina. Sci. Total Environ. 2012;437:1–9. doi: 10.1016/j.scitotenv.2012.07.076. [DOI] [PubMed] [Google Scholar]
- 8.Gros M, Petrović M, Ginebreda A, Barceló D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010;36:15–26. doi: 10.1016/j.envint.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Phillips PJ, et al. Combined sewer overflows: an environmental source of hormones and wastewater micropollutants. Environ. Sci. Technol. 2012;46:5336–43. doi: 10.1021/es3001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei R, Ge F, Huang S, Chen M, Wang R. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere. 2011;82:1408–14. doi: 10.1016/j.chemosphere.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez DA, et al. Bioassay of estrogenicity and chemical analyses of estrogens in streams across the United States associated with livestock operations. Water Res. 2013;47:3347–63. doi: 10.1016/j.watres.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Huang B, et al. Occurrence, removal and bioaccumulation of steroid estrogens in Dianchi Lake catchment, China. Environ. Int. 2013;59:262–73. doi: 10.1016/j.envint.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Kolpin DW, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002;36:1202–11. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 14.Mutiyar PK, Mittal AK. Risk assessment of antibiotic residues in different water matrices in India: key issues and challenges. Environ. Sci. Pollut. Res. Int. 2014;21:7723–36. doi: 10.1007/s11356-014-2702-5. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Conkle JL, Gan J. Multi-residue determination of pharmaceutical and personal care products in vegetables. J. Chromatogr. A. 2012;1254:78–86. doi: 10.1016/j.chroma.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Brown, E. et al. Policy for water quality control for recycled water (Recycled Water Policy). California State Water Resources Control Board (2013).
- 17.Shappell NW, et al. Estrogenic activity and steroid hormones in swine wastewater through a lagoon constructed-wetland system. Environ. Sci. Technol. 2007;41:444–50. doi: 10.1021/es061268e. [DOI] [PubMed] [Google Scholar]
- 18.Kumar K, Gupta SC, Baidoo SK, Chander Y, Rosen CJ. Antibiotic uptake by plants from soil fertilized with animal manure. J Env. Qual. 2005;34:2082–2085. doi: 10.2134/jeq2005.0026. [DOI] [PubMed] [Google Scholar]
- 19.Gan J, Bondarenko S, Oki L, Haver D, Li JX. Occurrence of fipronil and its biologically active derivatives in urban residential runoff. Environ. Sci. Technol. 2012;46:1489–95. doi: 10.1021/es202904x. [DOI] [PubMed] [Google Scholar]
- 20.Kinney CA, Furlong ET, Werner SL, Cahill JD. Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ. Toxicol. Chem. 2006;25:317–26. doi: 10.1897/05-187R.1. [DOI] [PubMed] [Google Scholar]
- 21.Thiele-Bruhn S. Pharmaceutical antibiotic compounds in soils—a review. J Plant Nutr Soil Sci. 2003;166:145–67. doi: 10.1002/jpln.200390023. [DOI] [Google Scholar]
- 22.Chefetz B, Mualem T, Ben-Ari J. Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere. 2008;73:1335–43. doi: 10.1016/j.chemosphere.2008.06.070. [DOI] [PubMed] [Google Scholar]
- 23.Pennington MJ, Rivas NG, Prager SM, Walton WE, Trumble JT. Pharmaceuticals and personal care products alter the holobiome and development of a medically important mosquito. Environ. Pollut. 2015;203:199–207. doi: 10.1016/j.envpol.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Pennington MJ, Prager SM, Walton WE, Trumble JT. Culex quinquefasciatus larval microbiomes vary with instar and exposure to common wastewater contaminants. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watts MM, Pascoe D, Carroll K. Exposure to 17 alpha-ethinylestradiol and bisphenol A-effects on larval moulting and mouthpart structure of Chironomus riparius. Ecotoxicol. Environ. Saf. 2003;54:207–15. doi: 10.1016/S0147-6513(02)00029-5. [DOI] [PubMed] [Google Scholar]
- 26.Rosi-Marshall EJ, et al. Pharmaceuticals suppress algal growth and microbial respiration and alter bacterial communities in stream biofilms. Ecol. Appl. 2013;23:583–93. doi: 10.1890/12-0491.1. [DOI] [PubMed] [Google Scholar]
- 27.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 28.Knowles SFGW, Carlisle DB. Endocrine control in the Crustacea. Biol. Rev. 1956;31:396–467. doi: 10.1111/j.1469-185X.1956.tb01556.x. [DOI] [Google Scholar]
- 29.Martín D, Wang SF, Raikhel AS. The vitellogenin gene of the mosquito Aedes aegypti is a direct target of ecdysteroid receptor. Mol. Cell. Endocrinol. 2001;173:75–86. doi: 10.1016/S0303-7207(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez G, Sorci G, Smith LC. Testosterone and sexual signalling in male house sparrows (Passer domesticus) Behav. Ecol. Sociobiol. 2001;50:557–562. [Google Scholar]
- 31.Hoffmann F, Kloas W. Estrogens can disrupt amphibian mating behavior. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tompsett AR, Wiseman S, Higley E, Giesy JP, Hecker M. Effects of exposure to 17α-ethynylestradiol during larval development on growth, sexual differentiation, and abundances of transcripts in the liver of the wood frog (Lithobates sylvaticus) Aquat. Toxicol. 2013;126:42–51. doi: 10.1016/j.aquatox.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Tompsett AR, et al. Effects of 17α-ethynylestradiol on sexual differentiation and development of the African clawed frog (Xenopus laevis) Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2012;156:202–10. doi: 10.1016/j.cbpc.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Segner H, et al. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: report from the European IDEA project. Ecotoxicol. Environ. Saf. 2003;54:302–314. doi: 10.1016/S0147-6513(02)00039-8. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez EM, Medesani DA, Fingerman M. Endocrine disruption in crustaceans due to pollutants: a review. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2007;146:661–71. doi: 10.1016/j.cbpa.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Zou E, Fingerman M. Effects of estrogenic xenobiotics on molting of the water flea, Daphnia magna. Ecotoxicol. Environ. Saf. 1997;38:281–5. doi: 10.1006/eesa.1997.1589. [DOI] [PubMed] [Google Scholar]
- 37.Planelló R, Martínez-Guitarte JL, Morcillo G. The endocrine disruptor bisphenol A increases the expression of HSP70 and ecdysone receptor genes in the aquatic larvae of Chironomus riparius. Chemosphere. 2008;71:1870–6. doi: 10.1016/j.chemosphere.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 38.Watts MM, Pascoe D, Carroll K. Chronic exposure to 17 alpha-ethinylestradiol and bisphenol A-effects on development and reproduction in the freshwater invertebrate Chironomus riparius (Diptera: Chironomidae) Aquat. Toxicol. 2001;55:113–24. doi: 10.1016/S0166-445X(01)00148-5. [DOI] [PubMed] [Google Scholar]
- 39.Disney RHL. Natural history of the scuttle fly, Megaselia scalaris. Annu. Rev. Entomol. 2008;53:39–60. doi: 10.1146/annurev.ento.53.103106.093415. [DOI] [PubMed] [Google Scholar]
- 40.Disney, R. H. L. Scuttle Flies: the Phoridae. (Chapman & Hall, 1994).
- 41.Benner DB. Oocyte development and fecundity in Megaselia scalaris (Phoridae: Diptera) Int. J. Entomol. 1985;27:280–288. [Google Scholar]
- 42.Sorensen MA, Chase-Dunn CM, Trumble JT. Chronic exposure to elevated levels of manganese and nickel is not harmful to a cosmopolitan detritivore, Megaselia scalaris (Diptera: Phoridae) Insect Sci. 2009;16:73–79. doi: 10.1111/j.1744-7917.2009.00256.x. [DOI] [Google Scholar]
- 43.Prawirodisastro M, Benjamin DM. Laboratory study on the biology and ecology of Megaselia scalaris (Diptera: Phoridae) J. Med. Entomol. 2014;16:317–320. doi: 10.1093/jmedent/16.4.317. [DOI] [PubMed] [Google Scholar]
- 44.Engel P, Moran NA. The gut microbiota of insects - diversity in structure and function. FEMS Microbiol. Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 45.Chouaia, B. et al. Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiol. 12 Suppl 1 (2012). [DOI] [PMC free article] [PubMed]
- 46.McFrederick QS, Rehan SM. Characterization of pollen and bacterial community composition in brood provisions of a small carpenter bee. Mol. Ecol. 2016;25:2302–2311. doi: 10.1111/mec.13608. [DOI] [PubMed] [Google Scholar]
- 47.Estes AM, et al. Brood ball-mediated transmission of microbiome members in the dung beetle, Onthophagus taurus (Coleoptera: Scarabaeidae) PLoS One. 2013;8 doi: 10.1371/journal.pone.0079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moran NA, Russell JA, Koga R, Fukatsu T. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 2005;71:3302–3310. doi: 10.1128/AEM.71.6.3302-3310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minard G, Mavingui P, Moro CV. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit. Vectors. 2013;6:1–12. doi: 10.1186/1756-3305-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kafil M, Bandani AR, Kaltenpoth M, Goldansaz SH, Alavi SM. Role of symbiotic bacteria in the growth and development of the Sunn pest, Eurygaster integriceps. J. Insect Sci. 2013;13:1–12. doi: 10.1673/031.013.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hira PR, et al. Myiasis in Kuwait: Nosocomial infections caused by Lucilia sericata and Megaselia scalaris. Am. J. Trop. Med. Hyg. 2004;70:386–389. [PubMed] [Google Scholar]
- 52.Greenberg B, Wells JD. Forensic use of Megaselia abdita and M. scalaris (Phoridae: Diptera): Case studies, development rates, and egg structure. J. Med. Entomol. 1998;35:205–209. doi: 10.1093/jmedent/35.3.205. [DOI] [PubMed] [Google Scholar]
- 53.Alcaine-Colet A, Wotton KR, Jimenez-Guri E. Rearing the scuttle fly Megaselia scalaris (Diptera: Phoridae) on industrial compounds: implications on size and lifespan. PeerJ. 2015;3 doi: 10.7717/peerj.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCrae A. Infestation of emulsion paint by the fly Megaselia scalaris (Loew)(Dipt., Phoridae) Entomol. Mon. Mag. 1967;102:241–243. [Google Scholar]
- 55.Jensen PD, Rivas MD, Trumble JT. Developmental responses of a terrestrial insect detritivore, Megaselia scalaris (Loew) to four selenium species. Ecotoxicology. 2005;14:313–22. doi: 10.1007/s10646-003-6368-x. [DOI] [PubMed] [Google Scholar]
- 56.Benner DB, Ostermeyer EC. Some observations on the life-history of the fly Megaselia scalaris Loew (Phoridae) with special reference to the eclosion pattern. J. Tennessee Acad. Sci. 1980;55:103–105. [Google Scholar]
- 57.Dadd RH. Prostaglandin synthetase inhibitors modulate the effect of essential dietary arachadonic acid in the mosquito. Culex pipiens. Pergamon Press Ltd. 1984;30:721–728. [Google Scholar]
- 58.Prud’homme SM, Chaumot A, Cassar E, David JP, Reynaud S. Impact of micropollutants on the life-history traits of the mosquito Aedes aegypti: On the relevance of transgenerational studies. Environ. Pollut. 2017;220:242–254. doi: 10.1016/j.envpol.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 59.Dillon RJ, Dillon VM. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 60.Hail D, Dowd SE, Bextine B. Identification and location of symbionts associated with potato psyllid (Bactericera cockerelli) lifestages. Environ. Entomol. 2012;41:98–107. doi: 10.1603/EN11198. [DOI] [PubMed] [Google Scholar]
- 61.Vasanthakumar A, Handelsman J, Schloss PD, Bauer LS, Raffa KF. Gut microbiota of an invasive subcortical beetle, Agrilus planipennis Fairmaire, across various life stages. Environ. Entomol. 2008;37:1344–1353. doi: 10.1093/ee/37.5.1344. [DOI] [PubMed] [Google Scholar]
- 62.Brenner, D. J., Krieg, N. R. & Staley, J. T. Bergey’s Manual of Systematic Bacteriology Volume 2 Part B. (Springer Science + Buisness Media Inc., 2005).
- 63.Steinhaus EA. A study of the bacteria associated with thirty species of insects. J. Bacteriol. 1941;42:757–790. doi: 10.1128/jb.42.6.757-790.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bansal R, et al. Pyrosequencing reveals the rredominance of Pseudomonadaceae in gut microbiome of a Gall Midge. Pathogens. 2014;3:459–472. doi: 10.3390/pathogens3020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feldhaar H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011;36:533–543. doi: 10.1111/j.1365-2311.2011.01318.x. [DOI] [Google Scholar]
- 66.Kellner RLL, Dettner K. Differential efficacy of toxic pederin in deterring potential arthropod predators of Paederus (Coleoptera: Staphylinidae) offspring. Oecologia. 1996;107:293–300. doi: 10.1007/BF00328445. [DOI] [PubMed] [Google Scholar]
- 67.Piel J, Höfer I, Hui D. Evidence for a symbiosis island involved in horizontal acquisition of pederin biosynthestic capabilities by the bacterial symbiont of Paederus fuscipes beetles. J. Bacteriol. 2004;186:1280–1286. doi: 10.1128/JB.186.5.1280-1286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brenner, D. J., Krieg, N. R. & Staley, J. T. Bergey’s Manual of Systematic Bacteriology Volume 2 Part C. (Springer Science + Buisness Media Inc., 2005).
- 69.Douglas, A. E. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 17–37 (1998). [DOI] [PubMed]
- 70.Husník, F., Chrudimský, T. & Hypša, V. Multiple origins of endosymbiosis within the Enterobacteriaceae (γ-Proteobacteria): convergence of complex phylogenetic approaches. BMC Biol. 9 (2011). [DOI] [PMC free article] [PubMed]
- 71.Killiny N, Prado SS, Almeida RPP. Chitin utilization by the insect-transmitted bacterium Xylella fastidiosa. Appl. Environ. Microbiol. 2010;76:6134–6140. doi: 10.1128/AEM.01036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Retchless, A. C. et al. In Genomics of Plant-Associated Bacteria (eds Gross, D. C., Lichens-Park, A. & Kole, C.) 177–202, doi:10.1007/978-3-642-55378-3 (Springer-Verlag Berlin Heidelberg, 2014).
- 73.Valiente Moro C, Tran FH, Raharimalala FN, Ravelonandro P, Mavingui P. Diversity of culturable bacteria including Pantoea in wild mosquito Aedes albopictus. BMC Microbiol. 2013;13:1–11. doi: 10.1186/1471-2180-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reid NM, Addison SL, Macdonald LJ, Lloyd-Jones G. Biodiversity of active and inactive bacteria in the gut flora of wood-feeding huhu beetle larvae (Prionoplus reticularis) Appl. Environ. Microbiol. 2011;77:7000–6. doi: 10.1128/AEM.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bansal R, et al. Pyrosequencing reveals the redominance of Pseudomonadaceae in gut microbiome of a Gall Midge. Pathogens. 2014;3:459–472. doi: 10.3390/pathogens3020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peter Guengerich F. Metabolism of 17 alpha-ethynylestradiol in humans. Life Sci. 1990;47:1981–1988. doi: 10.1016/0024-3205(90)90431-P. [DOI] [PubMed] [Google Scholar]
- 77.Goldzieher JW, et al. Plasma levels and pharmacokinetics of ethynyl estrogens in various populations. Contraception. 1980;21:1–16. doi: 10.1016/0010-7824(80)90134-1. [DOI] [PubMed] [Google Scholar]
- 78.Morlet N, et al. Pharmacokinetics of ciprofloxacin in the human eye: a clinical study and population pharmacokinetic analysis. Antimicrob Agents Chemother. 2000;44:1674–1679. doi: 10.1128/AAC.44.6.1674-1679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamoto T, et al. Autopsy report for a caffeine intoxication case and review of the current literature. J Toxicol Pathol. 2015;28:33–36. doi: 10.1293/tox.2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mandeville JD, Mullens BA, Meyer JA. Rearing and host age suitability of Fannia canzcularzs (L.) (Diptera: Muscidae) for parasitization by Muscidifurax zaraptor Kogan and Legner (Hymenoptera: Pteromalidae) Can. Entomol. 1988;120:153–159. doi: 10.4039/Ent120153-2. [DOI] [Google Scholar]
- 81.Kembel SW, et al. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc. Natl. Acad. Sci. USA. 2014;111:13715–20. doi: 10.1073/pnas.1216057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanshew AS, Mason CJ, Raffa KF, Currie CR. Minimization of chloroplast contamination in 16S rRNA gene pyrosequencing of insect herbivore bacterial communities. J. Microbiol. Methods. 2013;95:149–155. doi: 10.1016/j.mimet.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caporaso JG, et al. QIIME allows analysis of high- throughput community sequencing data. Nat. Publ. Gr. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–61. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 85.Mercier, C., Boyer, F., Bonin, A. & Coissac, E. SUMATRA and SUMACLUST: fast and exact comparison and clustering of sequences. in SeqBio 2013 Workshop (2013).
- 86.McDonald D, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–8. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caporaso JG, et al. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2 - Approximately maximum-likelihood trees for large alignments. PLoS One5 (2010). [DOI] [PMC free article] [PubMed]
- 90.Hamady M, Lozupone C, Knight R. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J. 2010;4:17–27. doi: 10.1038/ismej.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oksanen, J. et al. The Vegan Package. 1–190 (2008).
- 92.Husson, F., Josse, J., Le, S. & Mazet, J. FactoMineR: Multivariate exploratory data analysis and data mining with R. R Packag. version 1.14 102–23 (2010).
- 93.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/ (2016).
- 94.Warnes, G. R. et al. gplots: Various R programming tools for plotting data. R Foundation for Statistical Computing, Vienna, Austria. https://cran.r-project.org/package=gplots (2016).
- 95.Fox J. Cox proportional-hazards regression for survival data. Most. 2002;2008:1–18. [Google Scholar]
- 96.Therneau, T. M. & Lumley, T. Package ‘ survival’. CRAN 1–136, doi:10.1016/j.jhydrol.2011.07.022 (2014).
- 97.r-sig-ecology & Oksanen, J. post-hoc test for adonis PERMANOVA. r-sig-ecology (2012). Available at: http://r-sig-ecology.471788.n2.nabble.com/post-hoc-test-for-adonis-PERMANOVA-td7577679.html (Accessed: 1st January 2017).
- 98.Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods. 2013;10:1200–2. doi: 10.1038/nmeth.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


