Human gait adaptation implies that the nervous system senses energetic cost, yet this signal is unknown. We tested the hypothesis that the blood gas receptors sense cost for gait optimization by controlling blood O2 and CO2 with step frequency as people walked. At the simulated energetic minimum, ventilation and perceived exertion were lowest, yet subjects preferred walking at their original frequency. This suggests that blood gas receptors are not critical for sensing cost during gait.
Keywords: energetic cost, locomotion, motor control, O2 and CO2 chemoreceptors, optimal control
Abstract
People can adapt their gait to minimize energetic cost, indicating that walking’s neural control has access to ongoing measurements of the body’s energy use. In this study we tested the hypothesis that an important source of energetic cost measurements arises from blood gas receptors that are sensitive to O2 and CO2 concentrations. These receptors are known to play a role in regulating other physiological processes related to energy consumption, such as ventilation rate. Given the role of O2 and CO2 in oxidative metabolism, sensing their levels can provide an accurate estimate of the body’s total energy use. To test our hypothesis, we simulated an added energetic cost for blood gas receptors that depended on a subject’s step frequency and determined if subjects changed their behavior in response to this simulated cost. These energetic costs were simulated by controlling inspired gas concentrations to decrease the circulating levels of O2 and increase CO2. We found this blood gas control to be effective at shifting the step frequency that minimized the ventilation rate and perceived exertion away from the normally preferred frequency, indicating that these receptors provide the nervous system with strong physiological and psychological signals. However, rather than adapt their preferred step frequency toward these lower simulated costs, subjects persevered at their normally preferred frequency even after extensive experience with the new simulated costs. These results suggest that blood gas receptors play a negligible role in sensing energetic cost for the purpose of optimizing gait.
NEW & NOTEWORTHY Human gait adaptation implies that the nervous system senses energetic cost, yet this signal is unknown. We tested the hypothesis that the blood gas receptors sense cost for gait optimization by controlling blood O2 and CO2 with step frequency as people walked. At the simulated energetic minimum, ventilation and perceived exertion were lowest, yet subjects preferred walking at their original frequency. This suggests that blood gas receptors are not critical for sensing cost during gait.
people prefer to walk in ways that minimize their energy use. For example, people’s preferred speed minimizes the metabolic energetic cost per unit distance traveled (Ralston 1958). At a fixed speed, preferred step frequencies, step widths, and patterns of arm swing all minimize cost (Bertram and Ruina 2001; Collins et al. 2009; Dean and Kuo 2009; Donelan et al. 2001). Models that minimize the energetic cost of gait also predict gait kinematics and ground reaction forces, providing further evidence to support this idea (Anderson and Pandy 2001; Miller et al. 2012). The mechanisms of energy optimization are not yet fully understood but likely combine several processes that operate over different timescales. On the longest timescale, much theorizing has emphasized energetically beneficial adaptations that are established through natural selection (Alexander 1996, 2001; Middleton et al. 2008; Rodman and McHenry 1980). Optima may also be established over a lifetime, both because the nervous system can learn optimal control strategies through the course of development and because muscle and other physiological properties can remodel to fit control strategies that are constantly repeated (Berger and Adolph 2007). On the shortest timescale, the nervous system may compute new control strategies in real time that minimize the energetic cost of movement.
Our recent work has focused on understanding optimization on this shortest timescale and has shown that the nervous system can indeed compute motor commands that minimize energetic cost. In these experiments, subjects walked while wearing exoskeletons to change the normal relationship between step frequency and the actual energetic cost of gait (Selinger et al. 2015). By controlling the amount of energetic penalty added by the braces as a function of step frequency, we systematically reshaped the energetic cost landscape so that the minimum energetic cost occurred at a different step frequency from subjects’ normal preferred gait. We found that subjects adapted their step frequency to be energetically optimal with no more than 30 min of walking experience with the new landscape. Remarkably, our subjects readily adapted their preferred step frequency for energetic savings as small as 4%. Thus the nervous system can act rapidly and frugally when adapting the characteristics of gait to reduce energetic cost.
For the nervous system to optimize cost, it must be able to sense it. Sensory systems that directly affect respiratory physiology are among the logical candidates for cost sensing because ventilation rate rapidly responds to changing energetic demands of the body, rising proportionally with energetic cost during exercise (Davis et al. 1980). This increase allows muscle cells to consume more energy because oxygen (O2) and carbon dioxide (CO2), together with stored fat, carbohydrates, proteins, and water, comprise the substrates and byproducts of cellular oxidative metabolism. One of the main sensory systems hypothesized to underlie the control of ventilation involves blood gas receptors that are sensitive to O2 and CO2 (Casaburi 2012; Whipp and Ward 1998). These receptors can be found both centrally in the medulla oblongata, located in the brain stem, and peripherally in the carotid bodies, located in the carotid artery (Heymans and Heymans 1927; Miller and Tenney 1975). One reason blood gas receptors are thought to contribute to ventilation control is that changes in their afferent feedback precede changes in ventilation; CO2 and O2 concentrations increase in the arteries during exercise before an increase in ventilation brings these values toward homeostatic levels (Forster et al. 1993; Whipp and Ward 1998). Similarly, elevated CO2 in expired air at the beginning of exercise also happens before ventilation changes (Casaburi et al. 1977), and on surgical removal of these sensors, the ventilatory response to exercise is slower and smaller (Lugliani et al. 1971; Wasserman et al. 1975). These findings suggest that blood gas receptors contribute to ventilation control, which also makes them logical candidates for sensing energetic cost.
In the present study we test the hypothesis that the nervous system uses blood gas receptors that are sensitive to O2 and CO2 to sense energetic cost and optimize our movements. To test our hypothesis, we used a custom-built gas control system to stimulate blood gas receptors as subjects walked on a treadmill. We systematically controlled circulating gas concentrations to alter the signals generated by these receptors from those produced during normal walking, while leaving essentially unchanged the actual energetic cost of walking. To shift the simulated energetic optimum to lower step frequencies, we used a “penalize-high” control function that lowered blood O2 and raised CO2 at higher step frequencies, thereby simulating an added energetic cost at higher step frequencies. To shift the simulated energetic optimum to higher step frequencies, we used a “penalize-low” control function that did the opposite. Subjects experienced these simulated energetic cost landscapes using the same protocol that was sufficient for subjects to minimize the actual energetic cost in a novel energetic landscape (Selinger et al. 2015). If subjects adapted their gait toward the new minimum in the simulated energetic cost, it would suggest that blood gas receptors are an important contributor to the sensation of energetic cost during gait optimization. Alternatively, no adaptation of gait to the simulated cost would suggest that the blood gas sensory system plays no role, or at least a small role, in the nervous system’s estimate of energetic cost for optimum movement. Importantly, there are several other candidate mechanisms for sensing energetic cost (c.f. discussion). We have elected to test the role of the blood gas sensory system first because it seems reasonable that the nervous system may use measures of blood O2 and CO2 to estimate global energetic cost and because we can readily manipulate blood O2 and CO2, allowing for experiments that rigorously test the importance of this sensory system in energy optimization.
METHODS
Ethical approval.
Twenty subjects (age: 20.4 ± 3.6 yr, mean ± SD) participated in the study, and we randomly assigned them to one of 3 groups: 8 in experiment 1 (2-day sessions, 1 group) and 12 in experiment 2 (3-day sessions, 2 groups). All were healthy adults with no known history of cardiovascular, musculoskeletal, neurological, or respiratory impairments. Written informed consent was obtained from subjects before participation. The Simon Fraser University Research Ethics Board approved all procedures.
Gas control system.
The gas control system regulated the levels of O2 and CO2 within the blood on a breath-by-breath basis (described in detail in O’Connor et al. 2016). Briefly, subjects breathed from a 1.5-liter reservoir bag connected to compressed sources of O2, CO2, and nitrogen (N2) via electronically controlled valves. Subjects breathed from the bag through a respiratory mask that measured flow rate and sampled inspired and expired air concentrations (Vmax Encore metabolic cart; ViaSys). A custom real-time control system calculated the partial pressure of expired O2 and CO2 at the end of each breath (Simulink; The MathWorks). At a given atmospheric pressure, partial pressures are proportional to the volume concentrations of O2 (and CO2). The partial pressure at the end of each breath is referred to as end-tidal partial pressure, which we denote and . and values are a close proxy for the levels of O2 and CO2 circulating within the arterial blood (Jones et al. 1979; Robbins et al. 1990). The blood that eventually reaches the blood gas receptors has approximately the same concentrations of gases as the blood that leaves the lungs because there are no major energy-consuming tissues encountered in transit (Jones et al. 1979). After each expiration, the control system calculated the difference between these measured partial pressures and target partial pressures (which depend on step frequency). It minimized these error signals using a combination of feedforward and feedback control to adjust the partial pressures of inspiratory O2, CO2, and N2 contained within the reservoir bag. As described in O’Connor et al. 2016, this system accurately controls end-tidal blood gases with low latency, including effectively tracking complicated and rapid changes in end-tidal values.
We used the gas control system to simulate novel relationships between gait characteristics and energetic cost. We accomplished this by shifting the step frequency at which subjects maximized the O2 in their blood, and minimized the CO2, away from their normally preferred and energetically optimal step frequency. In principle, we could have used any aspect of gait to control blood gases. We chose to manipulate the relationship between step frequency and energetic cost because step frequency is a fundamental characteristic of gait, people have strong preferences for particular step frequencies, and these preferred frequencies are energetically optimal (Bertram and Ruina 2001; Selinger et al. 2015; Umberger and Martin 2007). Furthermore, we have previously shown that people can minimize the actual energetic cost of walking by adapting their step frequency (Selinger et al. 2015). We computed step frequency on a step-by-step basis from treadmill foot contact events (Pagliara et al. 2014).
Our goal was to simulate a cost for blood gas sensors that was large enough to be physiologically relevant. We used the known effect that blood gas receptors have on ventilation control as an estimate of the magnitude of our gas perturbation’s simulated cost. At steady-state and moderate levels of exercise, ventilation rate correlates directly with metabolic rate (Davis et al. 1980; Wasserman et al. 1973). To simulate an energetic penalty in the range of 20%, we therefore controlled blood gas values such that ventilation rate increased by ~20% when subjects walked at their initial step frequency, compared with when they walked at the new simulated energetic minimum. Circulating CO2 gas has a much stronger effect on ventilation than O2 (Khoo et al. 1982; Rebuck et al. 1977; Severinghaus and Crawford 1978), and in pilot experiments we found that controlling to +5 mmHg of steady state achieved this magnitude of shift in ventilation. We simultaneously shifted in the opposite direction, because O2 is consumed when CO2 is produced in the normal physiological system. We used a respiratory exchange rate (RER) coefficient of 0.85 to dictate the ratio of CO2 to O2, thereby simulating the steady-state metabolism of a typical combination of carbohydrate and fat foodstuffs (Schmidt-Nielsen 1997; Simonson and DeFronzo 1990).
Our controller adjusted blood gases as a function of step frequency in a manner that matched the normal dynamic relationship between changes in energetic cost and circulating blood gases. Whereas the energetic demands on muscle change instantaneously with changes to step frequency, the resulting changes to the circulating O2 and CO2 in the proximity of the blood gas receptors occur much more slowly (Davis et al. 1980; Selinger and Donelan 2014; Wasserman and Whipp 1983; Whipp and Davis 1979). To estimate the dynamics of these circulation and inspiration processes, we simulated blood gases dynamics using a two-compartment model of CO2 and O2 concentrations in the lung and body, similar to Khoo et al. (1982). This model allowed us to determine how changes in circulating CO2 and O2 depended on both changes in the body’s metabolism and changes to inspired gases. Inspired gases had a much faster effect on circulating gases, indicating that we need to slow the dynamics between measured step frequency and inspired gases. Specifically, our simulations determined that inserting a 0.01-Hz 1st-order forward-direction low-pass Butterworth filter between measured step frequency and desired blood gases would well approximate the dynamics between muscle metabolism and blood gas concentrations.
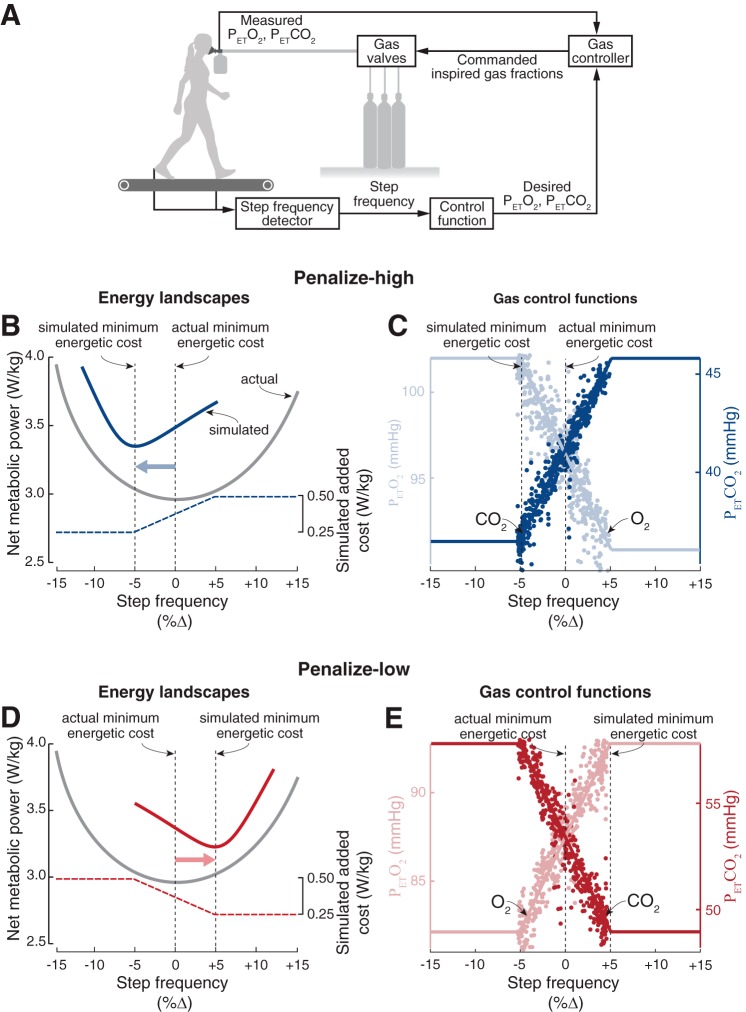
The resulting steady-state relationships between step frequency and end-tidal gases, which we refer to as control functions, shift the simulated energetic minimum in opposite directions. The gas control system made end-tidal gases a linear function of step frequency over the range ±5% of preferred step frequency (Fig. 1, C and E). The penalize-low control function (Fig. 1, B and C) simulated energetic penalties at lower step frequencies by decreasing blood O2 and increasing blood CO2, shifting the simulated energetic minimum to high step frequencies. The penalize-high control function (Fig. 1, D and E) had the opposite relationship, thereby simulating higher energetic penalties at higher step frequencies and shifting the simulated energetic minimum to low step frequencies.
Fig. 1.
Gas control system design. A: apparatus. Subjects walked on an instrumented treadmill that detected foot contact events for computing step frequency. Expired gas values were measured at the mouth, and subjects breathed air from a reservoir bag that was filled from compressed gas source tanks. The control system compared measured partial pressures of expired gas to desired values, computed the partial pressures of gases necessary to be delivered to the breathing bag to achieve desired values, and electronically operated solenoid valves that regulated the flow of compressed gas to the reservoir bag. To simulate a shift in the energetic minimum to a step frequency that differed from a subject’s preferred gait, we created a simulated energetic penalty that varied with step frequency, which we measure as the percent change from baseline preferred. B shows the predictions of the penalize-high control function experiments, and D shows those for the penalize-low control function experiments. In both, the gray line is the actual energetic cost landscape as a function of step frequency, the colored dashed line is the added cost that we simulate with the gas-control system, and the colored solid line is the sum of the actual cost and the added cost, which we refer to as the simulated energetic cost landscape. Importantly, the penalize-high control function shifts the simulated minimum energetic cost to step frequencies lower than normally preferred, whereas the penalize-low control functions shifts the new minimum to higher step frequencies. C shows the control of end-tidal gases for the penalize-high control function experiments, and E shows that for the penalize-low control function experiments, for a single representative subject. In both, the solid lines denote the commanded end-tidal gases as a function of step frequency, and the filled circles denote actual values of O2 and CO2 achieved by the gas control system on a breath-by-breath basis.
Protocol.
At the beginning of testing, subjects received 5 min of familiarization with walking on the treadmill. Next, we measured each subject’s preferred step frequency during a 20-min initial walking period while subjects breathed room air through the gas control system. We established each subject’s baseline step frequency as the average step frequency during the final 3 min of this walking period. Next, become familiarized with breathing different gas concentrations, subjects stood quietly for 5 min while we presented gas concentrations with elevated CO2 and depressed levels of O2. Because there is effectively no CO2 in room air, circulating CO2 cannot be decreased by reducing inspired CO2 levels below atmospheric values. Thus we chose to control CO2 around a nominal value of +2 SD above the median baseline end-tidal CO2 value. O2 was controlled around the median value measured during baseline walking. After this familiarization period, subjects returned to the treadmill, and we then engaged a single control function (i.e., penalize-high or penalize-low) for the remainder of the experiment. While the control function was on, we administered a protocol that gave subjects experience with the new simulated energetic landscape and tested for gait adaptation.
The adaptation protocol (Fig. 2) was designed specifically to determine if subjects alter their step frequency to minimize simulated cost. We previously used the same protocol to measure gait adaptation in response to changes in actual energetic cost and determined that it is sufficient for subjects to shift their self-selected step frequencies to frequencies that minimize actual energetic cost (Selinger et al. 2015). In brief, the protocol presented subjects with the novel simulated energetic cost landscape and gradually increased the subject’s experience with this landscape across four periods. In the first period (Initial), we sought to determine if subjects would adapt their step frequency spontaneously when they experienced the novel simulated cost landscape. Thus we engaged the control function at 5 min into a 10-min walking period. In the second period (Exploration) we gave our subjects experience with the novel energetic landscape across a wide range of step frequencies and then once again looked for gait adaptations toward the novel energetic minima. We accomplished this by instructing subjects to explore walking with high and low step frequencies (3 min). We then used a metronome to prescribe consecutive steady-state frequencies (3 min each, at +5 and −5% of preferred, in random order). The metronome then prescribed sinusoidally varying step frequencies (3 min). Subjects were then asked to again explore walking with high and low step frequencies (3 min). This period of experience was followed by self-selected walking (5 min). In the third period (Hold-release), we determined if long steady-state holds at new step frequencies were sufficient for subjects to make changes to preferred step frequency. We had subjects match steady-state metronome tempos for 5 min that held them at higher simulated energetic costs (penalize-high, +5 penalize-low, −5%) and lower simulated energetic costs (penalize-high, −5%; penalize-low, +5%), the order of which was randomized. After each steady-state metronome tempo, subjects were presented with white noise and released to self-select their step frequency for another 10 min. In the fourth period (Continuous) we gave subjects additional continuous experience with the new relationship between the simulated energetic cost and step frequency by prescribing step frequencies that changed sinusoidally over time with a period of 6 min, at an amplitude of 5% step frequency, beginning at baseline preferred and terminating at 7.5 min to release subjects at high and low frequencies. Each subject repeated the entire protocol twice, once for each of the two control functions. These took place on separate days, and we randomized the order of the control functions. We assessed adaptation by measuring each subject’s preferred step frequency during each of the self-selected periods: Initial, Explore, Hold 1, Hold 2, and Final. Preferred step frequency was quantified as the mean frequency over the final 3 min.
Fig. 2.

Adaptation protocol. Step frequency (%change from baseline preferred) is shown (top) as a function of time for a representative subject during the penalize-high control function. In the first period, we determined if subjects adapted their step frequency spontaneously (Initial). We then provided subjects with further experience with the control function. We gave subjects an exploration period (Exploration), followed by 2 periods of 6-min hold and release (Hold-release), and finally a period of continuous experience with the control function (Continuous). We used a metronome to prescribe step frequencies (red lines). At the end of each of these periods, we asked subjects to self-select the way they walked (absence of red lines). The 3-min window over which we averaged preferred step frequency during each self-selected bout of walking is indicated by the gray vertical bars. The end-tidal partial pressures of oxygen (; middle) and carbon dioxide (; bottom) were measured during the protocol.
Following the adaptation protocol, we evaluated each subject’s perceived difficulty of walking at the different step frequencies. During these trials, subjects walked with the control function on while step frequencies were prescribed with the metronome at −5%, 0%, and +5% relative to their initial step frequency. Participants were asked to report their level of rated perceived exertion (RPE; Borg 1982) after 3 min of steady-state walking at each of these three frequencies. We performed these measurements in six of our eight subjects; our two first subjects in experiment 1 completed the experiment before we designed and implemented our RPE protocol. Because we wanted subjects to remain blind to the purpose of the study, we performed these measurements at the end of the second day of testing to avoid inadvertently affecting how subjects would consciously perceive our perturbations during the subsequent periods of self-selected walking. As a result, we only collected RPE values for a single control function for a given subject, therefore totaling three sets of RPE responses for each control function.
We used a second experiment to test whether subjects require more experience with the simulated cost landscape to adapt toward the simulated cost minimum. This experiment 2 protocol design was similar to that for experiment 1, but rather than only having a single experimental day of experience with a particular simulated cost landscape, subjects completed 3 consecutive days of ~50 min of treadmill walking exclusively under a single control function. On each day, subjects received four bouts of metronome-prescribed step frequencies, with two bouts at +5% and two bouts at −5% initial step frequency, each of which was followed by 5 min of self-selected walking. Subjects did not receive additional familiarization after the first day, and they did not complete the protocol where we evaluated their perceived exertion.
Statistics.
For experiment 1, we used a within-subjects ANOVA to test for reliable differences in subjects’ preferred step frequency during each of five self-selected walking periods presented to the subjects throughout the protocol. To compare changes in ventilation by subjects as they walked with the simulated energetic penalty, we measured ventilation during the metronome prescribed steady-state step frequencies and during the self-selected walking periods. We used a within-subjects ANOVA to test for differences in ventilation that occurred for subjects as they walked at simulated high energetic costs, simulated low energetic costs, and their preferred step frequency (averaged over the final 3 min). We used a mixed-ANOVA with two within-subjects effects (prescribed step frequency: levels −5, 0, and +5; and control function direction: levels high and low) to test for differences in the performance of the gas control system. Paired t-tests were used to test for differences between RPE values. Paired t-tests were used to test for differences in experiment 2 subjects’ preferred step frequency. A P value of 0.05 was considered to be significant in all cases. Bonferroni post hoc corrections were made to control for type I error in post hoc tests.
RESULTS
The gas control system was effective at controlling blood gases as a function of step frequency (Fig. 1, C and E). We quantified its accuracy as the average error between the actual end-tidal partial pressure of oxygen () and carbon dioxide () values and the desired and values during the metronome-prescribed steady-state phases of the protocol. We quantified its precision as the variability in this error, calculated as the root-mean-square error (RMS error) on a breath-by-breath basis during these same periods. The average and RMS error of measured were −0.02 ± 0.17 and 1.80 ± 0.63 mmHg (means ± SD), respectively. For , they were 0.02 ± 0.13 and 1.12 ± 0.35 mmHg, respectively. As a percentage of the desired pressures, these steady-state and RMS errors were 0.00 ± 0.23% and 1.71 ± 0.76% for and 0.00 ± 0.31% and 1.89 ± 0.94% for , respectively. Controller performance did not vary with the direction of the control function condition or the level of prescribed step frequency (F2,28 < 1.5, P > 0.05 in all cases). These results collectively indicate that our gas control system created the desired relationship between end-tidal gas and step frequency under both control functions.
Our simulated energetic cost landscapes were effective at shifting the step frequency that minimized ventilation rate away from the preferred frequency (Fig. 3B). During walking under the penalize-high control function, where the control function paired lower step frequencies with a simulated minimum energetic cost, ventilation rate decreased by 20 ± 9% at low step frequencies compared with initial frequency (P = 0.0011). The penalize-low control function produced the opposite response: ventilation rate increased at low step frequencies and decreased by 15 ± 13% at high step frequencies (P = 0.024). Under normal conditions, changes in ventilation rate are approximately proportional to changes in energetic cost (Davis et al. 1980; Wasserman et al. 1973). We can use this 1:1 relationship to roughly calibrate the magnitude of energetic costs simulated by our gas control system. A 15–20% reduction in energetic cost at this walking speed is thus ~0.5 W/kg, or about what the average walker experiences after removing a backpack weighing 20–25% of their body weight (Pandolf et al. 1977). People will adapt step frequency to gain reductions in actual energetic cost as small as 4% (Selinger et al. 2015), suggesting that our simulated changes to energetic cost are of sufficient magnitude to test the role of blood gas receptors in this process.
Fig. 3.
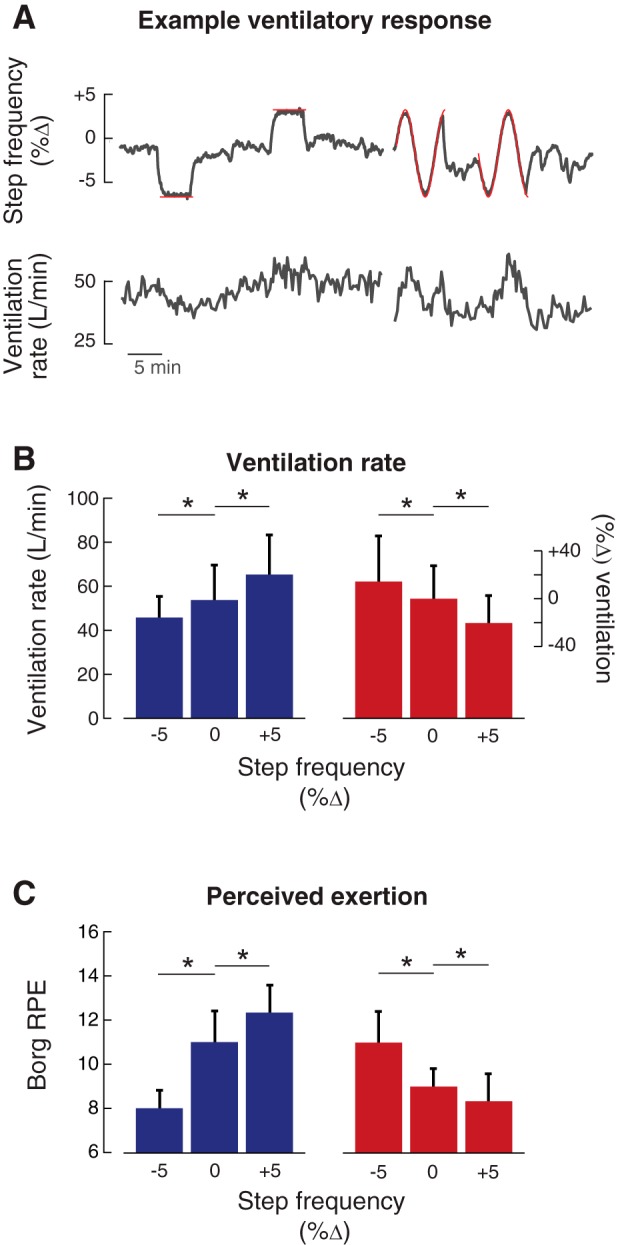
Ventilation rate and perceived exertion. A: ventilation rate as a function of time for a representative subject, showing how the gas control function created a relationship between step frequency (gray line, top; %change from baseline preferred) and ventilation rate (gray lines, bottom). Depicted are the Hold 1, Hold 2, and Continuous periods of the experiment where the protocol alternated between prescribing step frequencies for the subject using a metronome (red lines) and self-selected walking (absence of red lines). These representative data were recorded during adaptation to a penalize-high control function, which results in increases in ventilation rate with increases in step frequency. B: group-averaged ventilation (liters/min) at −5%, 0%, and +5% from baseline preferred step frequency during walking under both the penalize-high control function (blue) and the penalize-low control function (red). C: group-averaged rate of perceived exertion (RPE) measures at −5%, 0%, and +5% of initial step frequency under the two control functions penalize-high (blue) and penalize-low control functions were also effective at shifting the minimum perceived effort toward the simulated minimum energetic cost. *P < 0.05, statistically significant difference.
Subjects perceived that their level of exertion was higher at their initially preferred frequency than the frequency for which we simulated an energetic minimum (Fig. 3C). During walking under the penalize-high control function, the perceived exertion was 8.0 ± 1.0 (out of 20) for low step frequencies compared with 11.0 ± 1.7 for the initial preferred step frequency, with each of the 3 subjects in this group ranking preferred step frequency as more effortful. As with the ventilatory response, the penalize-low control function produced the opposite pattern: perceived exertion was lower for high step frequencies (8.3 ± 1.5) compared with the initial step frequency (9.0 ± 1.0), again with all subjects in this group ranking preferred step frequency as more effortful. Our measured differences in perceived effort are approximately equal to those reported by subjects walking at similar speeds when carrying between 7.5% and 15% of their body weight (Robertson et al. 1982). These results suggest that our gas control system was effective at making subjects consciously feel that the step frequency that minimized their effort occurred at a step frequency that was different from what they normally prefer.
Subjects did not show any preference to walk at steady-state step frequencies that lowered the simulated energetic cost (Fig. 4A). In our earlier experiments, where we manipulated actual energetic cost, the largest shifts in step frequency occurred at the end of the protocol, after subjects had the greatest experience with the new cost landscape (Selinger et al. 2015). In contrast, our subjects preferred to use the same step frequency at the end of protocol, after considerable experience with the simulated cost landscapes, as they preferred when they were breathing room air. Specifically, the changes in step frequency were −0.5 ± 0.8% (P = 0.34) and −0.3 ± 1.4% (P = 0.63) under the penalize-high and penalize-low control functions, respectively. Earlier phases of the protocol also showed no evidence of step frequency adaptation (Fig. 4A). One reason subjects may not have adapted their gait to lower the simulated cost is that the nervous system requires more experience with the novel relationship between simulated energetic cost and gait. However, subjects who experienced second and third days each having 50 min of treadmill walking under a single control function also exhibited no shift in preferred step frequency to lower the simulated energetic cost (Fig. 4B; P > 0.05).
Fig. 4.
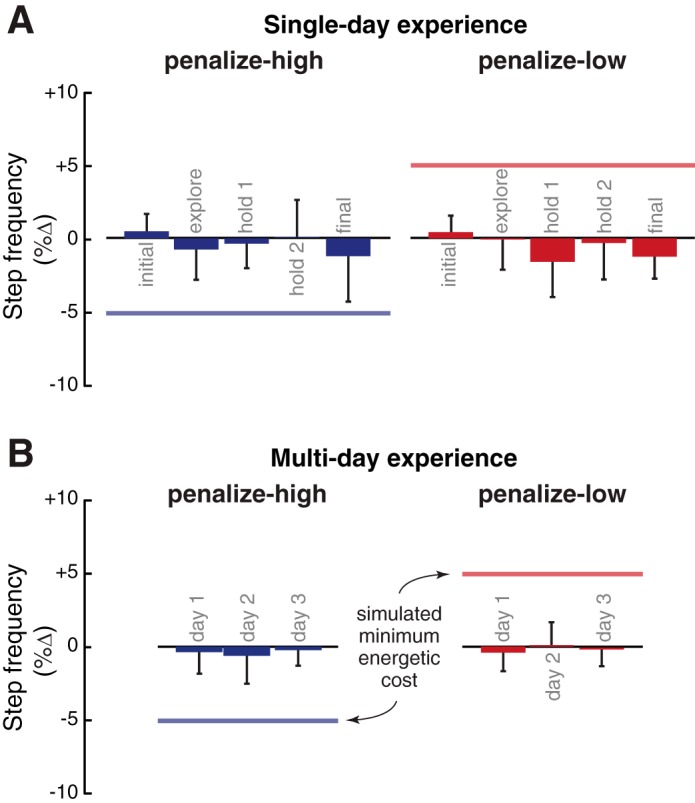
Adaptation results. A: preferred step frequency (%change from baseline preferred) across subjects at the 5 self-selected points throughout the adaptation protocol for experiment 1. These subjects each experienced both penalize-high (blue) and penalize-low (red) control functions on separate days. The colored horizontal line denotes the step frequency at which the simulated energetic cost was minimal. We observed no changes to preferred step frequency. B: group-averaged changes to preferred step frequency for experiment 2 subjects, who experienced a single control function over 3 days of repeated exposure. These subjects also did not demonstrate changes to preferred step frequency.
DISCUSSION
To test the role of blood gas receptors in optimizing the energetic cost of movement, we regulated inspired gases according to each subject’s step frequency, successfully shifting the frequency that maximized blood O2 and minimized blood CO2 away from the subject’s preferred value. This blood gas control was also effective at shifting the frequency that minimized ventilation rate and perceived exertion away from the preferred frequency, indicating that the targeted receptors provide the nervous system with strong physiological and psychological signals. However, our subjects did not adapt their gait toward the simulated energetic minima when given the freedom to self-select their step frequency. Instead, they persevered at their normally preferred step frequency while enduring the substantially higher ventilation rates and perceived exertion. These results strongly suggest that blood gas receptors do not play a principal role in sensing energetic cost for the purpose of optimizing movements.
Importantly, we cannot fully rule out a role for these receptors, because it is likely that the nervous system estimates energetic cost by integrating sensory signals from many sources. Our experiment does not affect actual energetic cost, resulting in a sensory conflict between the receptors that we are manipulating (blood gas chemoreceptors) and those that we are not (perhaps muscle afferents). To overcome this conflict between multiple sensors and shift the sensed energetic minimum away from the actual energetic minimum, the nervous system may require larger perturbations to blood gas sensed cost than was simulated in our protocol. However, in our other experiments, we have shown that people adapt their gait to reduce actual energetic costs by as little as 4% (Selinger et al. 2015). This is evidence that the nervous system can be sensitive to very small changes in cost when the various sensory mechanisms measuring energetic cost are simultaneously perturbed. Under normal conditions, a 4% change in cost produces a proportional 4% increase in ventilation, yet in the present study we provided relatively large changes in blood gas signals that produced a 20% change in ventilation and which elicited no effect on the nervous system’s control of movement. Therefore, if there is a contribution of blood gas receptors to any real-time minimization of energetic cost, its contribution appears to be small.
As mentioned in the Introduction, there are other candidate sensory systems for sensing energetic cost. A second candidate sensory system involves group III and IV afferents from muscles. Some of these group III/IV afferent receptors respond to the byproducts of muscle metabolism at the level of the individual muscle (Amann et al. 2011; Iwamoto et al. 1985; Mitchell et al. 1983; Smith et al. 2006). When stimulated directly, these afferents produce increases in ventilation (Coote et al. 1971). When these sensors are chemically disabled, reduced ventilatory response to exercise is observed (Amann 2012; Amann et al. 2010). Another interesting possibility is that the nervous system could combine signals from proprioceptors to form a proxy estimate of energetic cost. A muscle’s energy use is related to the force it generates, the mechanical work it performs, and its efficiency (Hill 1938; Wilkie 1949), all of which can be deduced from the muscle length, velocity, and force signals contained with the muscle spindle and Golgi tendon organ pathways (Prochazka 1999). Many decades of research have demonstrated that proprioceptive feedback makes important contributions to the timing and magnitude of muscle activity (Duysens and Pearson 1980; Goodwin et al. 1972; Hall and McCloskey 1983; Oscarsson and Rosen 1963; Pearson 1995). The role of proprioception in energy optimization is unclear, but research is ongoing (Dean 2013; Hubbuch et al. 2015). In principle, any or all of these systems could contribute to sensing energetic cost. It is also possible that the nervous system dynamically changes its use of energetic cost signals, increasing or decreasing its reliance of particular sensory signals depending on the task. We chose to first focus our work on a candidate sensory system that seemed likely to contribute to sensing cost while also being experimentally tractable.
Although we have attempted to design and implement experiments that provide a strong test of our hypothesis, the experiments are not without limitations. One particular challenge that exists when performing closed-loop control of a sensory system is to make a compelling simulated environment. This is because the nervous system may ignore sensory perturbations that it classifies as nonphysiological, perhaps because the timing, magnitude, or covariation with other sensory signals is outside the normal range. Research on virtual reality systems that generate artificial visual scenes emphasizes the difficulty in creating true presence, defined as a person’s subjective sense of being within the scene being visually simulated (Barfield 1995). In our case, simulated costs may have failed to produce believable illusions, and instead are treated as sensory failure by the nervous system, or as generated by an environmental source entirely beyond the nervous system’s control. Although we cannot rule out this possibility entirely, our simulated costs were effective at manipulating both ventilation rates and perceived effort, “fooling” the nervous system in at least these two respects. Another possible reason for a false-negative result is that the simulated relationship between behavior and cost could not overcome the relationship between behavior and cost that the nervous system has learned from decades of walking (Pagliara et al. 2014; Snaterse et al. 2011; Snyder et al. 2012). Indeed, our earlier exoskeleton study showed that this predictive process is both strong and present when adapting to gait perturbations (Selinger et al. 2015). Those subjects, however, learned to reprogram their prediction to the new optimal gait following experience with the new actual cost landscape, whereas our present subjects did not, even after our subjects had much greater experience over the course of multiple experimental sessions. Because subjects optimized their gait in our previous study after less than one-fifth of the time of adaptation in this study, it seems unlikely that the amount of time we used for adaptation limits our conclusions about the role of blood gas sensors for gait optimization. A third possibility is that this sensory system does play a major role in optimizing energetic cost, but only after specific sensory cues categorize the distinct context that initiates the nervous system to adapt gait. For example, proprioceptive cues associated with wearing the exoskeleton in our earlier study may have provided sufficient cues to initiate optimization, whereas similar physical cues were absent in our current project. It would be interesting to follow up on this possibility by testing whether blood gas control could accentuate or interfere with ongoing gait adaptation to actual energetic cost changes due to exoskeleton resistance (Selinger et al. 2015), different treadmill belt speeds (Finley et al. 2013), or other similar motor learning paradigms.
The present results suggest that the nervous system uses sensory signals other than blood gas receptors for optimizing energetic cost. Group III and IV afferents and proprioceptive signals from spindles and Golgi tendon organs are other possible candidates. Rather than signaling a global measure of ongoing energetic cost, these receptors reflect local costs at the level of individual muscles, and perhaps even individual motor units. Use of these local signals presents the nervous system with a computational challenge: it must appropriately weight and sum these signals to accurately estimate the body’s total energetic cost. However, local signals also have a major advantage over global cost measures: the nervous system can readily attribute changes in global energetic cost to the energetic demands of individual muscles or motor units, overcoming the credit assignment problem (Berniker and Kording 2008; Sutton 1984). The nervous system is not always concerned with only minimizing energetic cost. In such cases the afferent signals from group III/IV may inform the nervous system about other important states of the body, such as individual muscle fatigue or sense of effort (Hreljac 1993; Prilutsky and Gregor 2001). For all motor tasks, credit assignment is necessary to appropriately adjust muscle coordination to optimize for any cost function. Use of delayed and global signals produced by blood gas receptors may well be possible, but it is potentially easier to use rapid and local signals.
GRANTS
This work was supported by U.S. Army Research Office Grant W911NF-13-1-0268 (to J. M. Donelan), a Michael Smith Foundation for Health Research Fellowship (to J. D. Wong), and a Vanier Canadian Graduate Scholarship (to J. C. Selinger).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.W., S.M.O., and J.M.D. conceived and designed research; J.D.W. performed experiments; J.D.W., S.M.O., J.C.S., and J.M.D. analyzed data; J.D.W., S.M.O., J.C.S., and J.M.D. interpreted results of experiments; J.D.W. prepared figures; J.D.W. drafted manuscript; J.D.W., S.M.O., J.C.S., and J.M.D. edited and revised manuscript; J.D.W., J.C.S., and J.M.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Andy Ruina, Art Kuo, and Dinant Kistemaker for helpful discussions.
REFERENCES
- Alexander RM. Optima for Animals. Princeton, NJ: Princeton University Press, 1996. [Google Scholar]
- Alexander RM. Design by numbers. Nature 412: 591, 2001. doi: 10.1038/35088155. [DOI] [PubMed] [Google Scholar]
- Amann M. Significance of group III and IV muscle afferents for the endurance exercising human. Clin Exp Pharmacol Physiol 39: 831–835, 2012. doi: 10.1111/j.1440-1681.2012.05681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 589: 5299–5309, 2011. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson FC, Pandy MG. Dynamic optimization of human walking. J Biomech Eng 123: 381–390, 2001. doi: 10.1115/1.1392310. [DOI] [PubMed] [Google Scholar]
- Barfield W. Presence and performance within virtual environments. In: Virtual Environments and Advanced Interface Design, edited by Barfield W and Furness TA. New York: Oxford University Press, 1995, p. 473–513. [Google Scholar]
- Berger SE, Adolph KE. Learning and development in infant locomotion. Prog Brain Res 164: 237–255, 2007. doi: 10.1016/S0079-6123(07)64013-8. [DOI] [PubMed] [Google Scholar]
- Berniker M, Kording K. Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci 11: 1454–1461, 2008. doi: 10.1038/nn.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram JE, Ruina A. Multiple walking speed-frequency relations are predicted by constrained optimization. J Theor Biol 209: 445–453, 2001. doi: 10.1006/jtbi.2001.2279. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- Casaburi R. The mechanism of the exercise hyperpnea: the ultrasecret revisited. Am J Respir Crit Care Med 186: 578–579, 2012. doi: 10.1164/rccm.201207-1278ED. [DOI] [PubMed] [Google Scholar]
- Casaburi R, Whipp BJ, Wasserman K, Beaver WL, Koyal SN. Ventilatory and gas exchange dynamics in response to sinusoidal work. J Appl Physiol Respir Environ Exerc Physiol 42: 300–301, 1977. [DOI] [PubMed] [Google Scholar]
- Collins SH, Adamczyk PG, Kuo AD. Dynamic arm swinging in human walking. Proc Biol Sci 276: 3679–3688, 2009. doi: 10.1098/rspb.2009.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Whipp BJ, Wasserman K. The relation of ventilation to metabolic rate during moderate exercise in man. Eur J Appl Physiol Occup Physiol 44: 97–108, 1980. doi: 10.1007/BF00421087. [DOI] [PubMed] [Google Scholar]
- Dean JC. Proprioceptive feedback and preferred patterns of human movement. Exerc Sport Sci Rev 41: 36–43, 2013. doi: 10.1097/JES.0b013e3182724bb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JC, Kuo AD. Elastic coupling of limb joints enables faster bipedal walking. J R Soc Interface 6: 561–573, 2009. doi: 10.1098/rsif.2008.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. Mechanical and metabolic determinants of the preferred step width in human walking. Proc Biol Sci 268: 1985–1992, 2001. doi: 10.1098/rspb.2001.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187: 321–332, 1980. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Finley JM, Bastian AJ, Gottschall JS. Learning to be economical: the energy cost of walking tracks motor adaptation. J Physiol 591: 1081–1095, 2013. doi: 10.1113/jphysiol.2012.245506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV, Dunning MB, Lowry TF, Erickson BK, Forster MA, Pan LG, Brice AG, Effros RM. Effect of asthma and ventilatory loading on arterial Pco2 of humans during submaximal exercise. J Appl Physiol (1985) 75: 1385–1394, 1993. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science 175: 1382–1384, 1972. doi: 10.1126/science.175.4028.1382. [DOI] [PubMed] [Google Scholar]
- Hall LA, McCloskey DI. Detections of movements imposed on finger, elbow and shoulder joints. J Physiol 335: 519–533, 1983. doi: 10.1113/jphysiol.1983.sp014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymans JF, Heymans CJ. Sur les modifications directes et sur la régulation réflexe de l'activité du centre respiratoire de la tête isolé du chien. Arch Int Pharmacodyn 33: 273–370, 1927. [Google Scholar]
- Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci 126: 136–195, 1938. doi: 10.1098/rspb.1938.0050. [DOI] [PubMed] [Google Scholar]
- Hreljac A. Preferred and energetically optimal gait transition speeds in human locomotion. Med Sci Sports Exerc 25: 1158–1162, 1993. doi: 10.1249/00005768-199310000-00012. [DOI] [PubMed] [Google Scholar]
- Hubbuch JE, Bennett BW, Dean JC. Proprioceptive feedback contributes to the adaptation toward an economical gait pattern. J Biomech 48: 2925–2931, 2015. doi: 10.1016/j.jbiomech.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto GA, Waldrop TG, Kaufman MP, Botterman BR, Rybicki KJ, Mitchell JH. Pressor reflex evoked by muscular contraction: contributions by neuraxis levels. J Appl Physiol (1985) 59: 459–467, 1985. [DOI] [PubMed] [Google Scholar]
- Jones NL, Robertson DG, Kane JW. Difference between end-tidal and arterial Pco2 in exercise. J Appl Physiol Respir Environ Exerc Physiol 47: 954–960, 1979. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol Respir Environ Exerc Physiol 53: 644–659, 1982. [DOI] [PubMed] [Google Scholar]
- Lugliani R, Whipp BJ, Seard C, Wasserman K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N Engl J Med 285: 1105–1111, 1971. doi: 10.1056/NEJM197111112852002. [DOI] [PubMed] [Google Scholar]
- Middleton KM, Kelly SA, Garland T Jr. Selective breeding as a tool to probe skeletal response to high voluntary locomotor activity in mice. Integr Comp Biol 48: 394–410, 2008. doi: 10.1093/icb/icn057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Tenney SM. Hypoxia-induced tachypnea in carotid-deafferented cats. Respir Physiol 23: 31–39, 1975. [DOI] [PubMed] [Google Scholar]
- Miller RH, Umberger BR, Hamill J, Caldwell GE. Evaluation of the minimum energy hypothesis and other potential optimality criteria for human running. Proc Biol Sci 279: 1498–1505, 2012. doi: 10.1098/rspb.2011.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- O’Connor SM, Wong JD, Donelan JM. A generalized method for controlling end-tidal respiratory gases during nonsteady physiological conditions. J Appl Physiol (1985) 121: 1363–1378, 2016. doi: 10.1152/japplphysiol.00274.2016. [DOI] [PubMed] [Google Scholar]
- Oscarsson O, Rosen I. Projection to cerebral cortex of large muscle-spindle afferents in forelimb nerves of the cat. J Physiol 169: 924–945, 1963. doi: 10.1113/jphysiol.1963.sp007305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara R, Snaterse M, Donelan JM. Fast and slow processes underlie the selection of both step frequency and walking speed. J Exp Biol 217: 2939–2946, 2014. doi: 10.1242/jeb.105270. [DOI] [PubMed] [Google Scholar]
- Pandolf KB, Givoni B, Goldman RF. Predicting energy expenditure with loads while standing or walking very slowly. J Appl Physiol Respir Environ Exerc Physiol 43: 577–581, 1977. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Proprioceptive regulation of locomotion. Curr Opin Neurobiol 5: 786–791, 1995. doi: 10.1016/0959-4388(95)80107-3. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Gregor RJ. Swing- and support-related muscle actions differentially trigger human walk-run and run-walk transitions. J Exp Biol 204: 2277–2287, 2001. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Quantifying proprioception. Prog Brain Res 123: 133–142, 1999. doi: 10.1016/S0079-6123(08)62850-2. [DOI] [PubMed] [Google Scholar]
- Ralston HJ. Energy-speed relation and optimal speed during level walking. Int Z Angew Physiol 17: 277–283, 1958. [DOI] [PubMed] [Google Scholar]
- Rebuck AS, Slutsky AS, Mahutte CK. A mathematical expression to describe the ventilatory response to hypoxia and hypercapnia. Respir Physiol 31: 107–116, 1977. doi: 10.1016/0034-5687(77)90069-X. [DOI] [PubMed] [Google Scholar]
- Robbins PA, Conway J, Cunningham DA, Khamnei S, Paterson DJ. A comparison of indirect methods for continuous estimation of arterial Pco2 in men. J Appl Physiol (1985) 68: 1727–1731, 1990. [DOI] [PubMed] [Google Scholar]
- Robertson RJ, Caspersen CJ, Allison TG, Skrinar GS, Abbott RA, Metz KF. Differentiated perceptions of exertion and energy cost of young women while carrying loads. Eur J Appl Physiol Occup Physiol 49: 69–78, 1982. doi: 10.1007/BF00428965. [DOI] [PubMed] [Google Scholar]
- Rodman PS, McHenry HM. Bioenergetics and the origin of hominid bipedalism. Am J Phys Anthropol 52: 103–106, 1980. doi: 10.1002/ajpa.1330520113. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Animal Physiology: Adaptation and Environment. Cambridge: Cambridge University Press, 1997. [Google Scholar]
- Selinger JC, Donelan JM. Estimating instantaneous energetic cost during non-steady-state gait. J Appl Physiol (1985) 117: 1406–1415, 2014. doi: 10.1152/japplphysiol.00445.2014. [DOI] [PubMed] [Google Scholar]
- Selinger JC, O’Connor SM, Wong JD, Donelan JM. Humans can continuously optimize energetic cost during walking. Curr Biol 25: 2452–2456, 2015. doi: 10.1016/j.cub.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW, Crawford RD. Regulation of respiration. Acta Anaesthesiol Scand Suppl 70: 188–191, 1978. [PubMed] [Google Scholar]
- Simonson DC, DeFronzo RA. Indirect calorimetry: methodological and interpretative problems. Am J Physiol 258: E399–E412, 1990. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- Snaterse M, Ton R, Kuo AD, Donelan JM. Distinct fast and slow processes contribute to the selection of preferred step frequency during human walking. J Appl Physiol (1985) 110: 1682–1690, 2011. doi: 10.1152/japplphysiol.00536.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder KL, Snaterse M, Donelan JM. Running perturbations reveal general strategies for step frequency selection. J Appl Physiol (1985) 112: 1239–1247, 2012. doi: 10.1152/japplphysiol.01156.2011. [DOI] [PubMed] [Google Scholar]
- Sutton R. Temporal Credit Assignment in Reinforcement Learning University of Massachusetts (PhD thesis). Amherst, MA: University of Massachusetts, 1984. [Google Scholar]
- Umberger BR, Martin PE. Mechanical power and efficiency of level walking with different stride rates. J Exp Biol 210: 3255–3265, 2007. doi: 10.1242/jeb.000950. [DOI] [PubMed] [Google Scholar]
- Wasserman DH, Whipp BJ. Coupling of ventilation to pulmonary gas exchange during nonsteady-state work in men. J Appl Physiol Respir Environ Exerc Physiol 54: 587–593, 1983. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Whipp BJ, Koyal SN, Cleary MG. Effect of carotid body resection on ventilatory and acid-base control during exercise. J Appl Physiol 39: 354–358, 1975. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Whipp BJ, Koyl SN, Beaver WL. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol 35: 236–243, 1973. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Davis JA. Peripheral chemoreceptors and exercise hyperpnea. Med Sci Sports 11: 204–212, 1979. [PubMed] [Google Scholar]
- Whipp BJ, Ward SA. Determinants and control of breathing during muscular exercise. Br J Sports Med 32: 199–211, 1998. doi: 10.1136/bjsm.32.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie DR. The relation between force and velocity in human muscle. J Physiol 110: 249–280, 1949. doi: 10.1113/jphysiol.1949.sp004437. [DOI] [PMC free article] [PubMed] [Google Scholar]