Abstract
The fin-to-limb transition represents one of the major vertebrate morphological innovations associated with the transition from aquatic to terrestrial life and is an attractive model for gaining insights into the mechanisms of morphological diversity between species1. One of the characteristic features of limbs is the presence of digits at their extremities. Although most tetrapods have limbs with five digits (pentadactyl limbs), palaeontological data indicate that digits emerged in lobed fins of early tetrapods, which were polydactylous2. How the transition to pentadactyl limbs occurred remains unclear. Here we show that the mutually exclusive expression of the mouse genes Hoxa11 and Hoxa13, which were previously proposed to be involved in the origin of the tetrapod limb1–6, is required for the pentadactyl state. We further demonstrate that the exclusion of Hoxa11 from the Hoxa13 domain relies on an enhancer that drives antisense transcription at the Hoxa11 locus after activation by HOXA13 and HOXD13. Finally, we show that the enhancer that drives antisense transcription of the mouse Hoxa11 gene is absent in zebrafish, which, together with the largely overlapping expression of hoxa11 and hoxa13 genes reported in fish3–7, suggests that this enhancer emerged in the course of the fin-to-limb transition. On the basis of the polydactyly that we observed after expression of Hoxa11 in distal limbs, we propose that the evolution of Hoxa11 regulation contributed to the transition from polydactyl limbs in stem-group tetrapods to pentadactyl limbs in extant tetrapods.
Several studies provided evidence for the implication of Hox genes in the fin-to-limb transition8–13, notably Hoxa13 and Hoxd13 (Hox13 hereafter), which are required for digit morphogenesis10–14. Comparison of their expression pattern in fin and limb buds revealed a significant expansion of the Hox13 domain in distal limbs15 and engineered enlargement of the Hoxd13 domain in fish resulted in more chondrogenic tissue forming distally as well as fin fold reduction12—that is, morphological changes associated with the fin-to-limb transition. It was thus proposed that the evolution of Hox13 regulation has likely been instrumental to the emergence of the limb characteristic feature, that is, the digits10,12. In mice, this regulation relies on a series of remote transcriptional enhancers16,17, and although a subset of these enhancers exists in fish18, the expansion of the Hox13 domain in limb was probably associated with the emergence of tetrapod-specific enhancers during the fin-to-limb transition10–13. Another notable difference is the mutually exclusive expression of Hoxa11 and Hoxa13 in tetrapod limbs, contrasting with their largely overlapping expression in fins3–7. Two hypotheses have been put forward to explain how Hoxa11 gets proximally restricted in tetrapod limbs. One hypothesis suggested a Hoxa13-dependent repression of Hoxa11 in the presumptive autopod9,13,19, whereas the second proposed that antisense transcription at the Hoxa11 locus prevents expression of the gene distally20–22, but the functional importance of the mutually exclusive expression of Hoxa11 and Hoxa13 in tetrapod limbs is unknown.
Previous chromatin conformation analyses revealed that, in distal limbs, 5′ HoxA genes (that is, Hoxa9 to Hoxa13) are grouped within a chromatin sub-topological domain (sub-TAD) interacting with sub-TADs containing distal limb enhancers17. Yet, although Hoxa10 and Hoxa13 are both expressed distally, Hoxa11 expression is proximally restricted (Fig. 1a–c), suggesting that Hoxa11 is part of the distal limb regulatory landscape, but a specific, yet unknown, mechanism prevents its expression distally13,19. To test this possibility, we first took advantage of a mouse line in which the Hoxa11 gene is replaced by a PGK-neomycin resistance cassette23, which we used as a reporter transgene. We found neomycin expression in distal limbs (Fig. 1d), indicating that Hoxa11 proximal restriction is linked to specific feature(s) of the gene itself. We next analysed the putative implication of antisense long non-coding RNAs previously identified at the Hoxa11 locus20,21 and robustly expressed in the distal limb bud20 (Fig. 1e). Among the distinct Hoxa11 antisense transcripts (Hoxa11as, also known as Hoxa11os), two initiate upstream of the Hoxa11 gene and are thus non-overlapping with Hoxa11 (Hoxa11as-a; Fig. 1e) and the other two initiate within Hoxa11 exon 1 (Hoxa11as-b; Fig. 1f). Notably, only Hoxa11as-b expression pattern is mutually exclusive with Hoxa11 expression domain (Fig. 1f, compare with Fig. 1b). To test whether antisense transcription overlapping with Hoxa11 exon 1 prevents Hoxa11 expression distally, we took advantage of the Hoxa11eGFP mutant line, which lacks Hoxa11as-b start sites as the enhanced green fluorescent protein (eGFP) coding sequence replaces most of Hoxa11 exon 1 (ref. 24). This mutation disrupted antisense transcription normally initiating 3′ to Hoxa11 promoter (Extended Data Fig. 1a, b) while gfp expression driven by the Hoxa11 promoter was present both in the proximal and distal domains (Fig. 1g). By contrast, ectopic expression of Hoxa11as-b in the entire limb had no effect on Hoxa11 expression (Extended Data Fig. 2c–e), thereby excluding a trans-acting effect of Hoxa11as-b on Hoxa11 expression. Together, our data suggest that Hoxa11 distal repression is due to the antisense transcription event or the antisense Hoxa11as-b transcripts acting in cis.
Figure 1. The proximal restriction of Hoxa11 is linked to antisense transcription at the Hoxa11 locus.
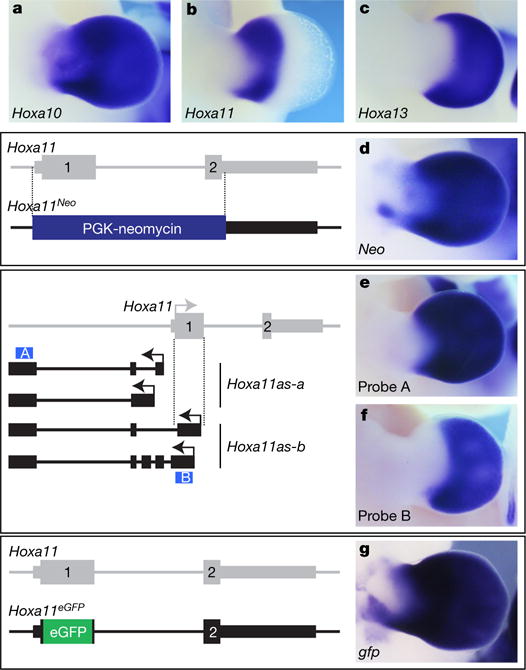
a–c, Expression of Hoxa10 (a), Hoxa11 (b) and Hoxa13 (c) in wild-type limb bud from embryonic day (E) 11.5 mouse. d, Replacement of the Hoxa11 gene with the PGK-neomycin cassette (Hoxa11Neo; scheme to the left), results in neomycin expression both in the proximal and distal domains. e, f, Expression of all antisense transcripts (e) and antisense transcripts overlapping with Hoxa11 exon 1 (f) in E11.5 wild-type limb. Schemes of the antisense transcripts and the probes used (blue boxes) are on the left. Note that the antisense transcripts overlapping with Hoxa11 exon 1 (Hoxa11as-b) are distally restricted (f), reminiscent of Hoxa13 expression (c) and mutually exclusive with the Hoxa11 pattern (b). g, Deletion of the antisense transcript start sites in Hoxa11 exon 1, via replacement of most of exon 1 with the eGFP coding sequence (Hoxa11eGFP; scheme to the left) and expression of gfp under the control of the Hoxa11 promoter (right). Original magnification, ×31.5 (for all images).
Previous mapping of active enhancers in distal limbs17 (referred to as ‘digit’ enhancers hereafter) uncovered a putative ‘digit’ enhancer embedded in Hoxa11 intron. We thus proposed that this enhancer might control Hoxa11as-b expression. We first tested the transcriptional enhancer activity of this DNA region in transgenic embryos and confirmed its ability to act as a transcriptional enhancer in distal limbs (Fig. 2a). Next, we generated mutant mice lacking this enhancer (Hoxa11ΔInt/ΔInt; Extended Data Fig. 2) to examine its potential implication in Hoxa11as-b expression. Analysis of antisense transcription in Hoxa11ΔInt/ΔInt limbs showed no detectable expression of Hoxa11as-b in the most distal cells (Fig. 2b, c), indicating that in these cells, the identified enhancer is required for antisense transcription overlapping with Hoxa11 exon 1. Some Hoxa11as-b expression remained in proximal cells of the presumptive handplate (presumptive carpal region; Fig. 2c), which suggests that additional cis-regulatory element(s) trigger antisense transcription in these cells. Notably, the deletion of the enhancer abrogating Hoxa11as-b expression in the most distal cells also resulted in ectopic expression of Hoxa11 in the presumptive digits (Fig. 2d, e). The gain-of-sense transcription in Hoxa11eGFP/eGFP distal limbs (Fig. 1g) indicates that it is not the intronic regulatory region per se but Hoxa11as-b expression or the antisense transcription event that represses Hoxa11 expression distally.
Figure 2. Deletion of the distal enhancer in Hoxa11 intron results in impaired antisense transcription and gain of sense transcription in distal cells.
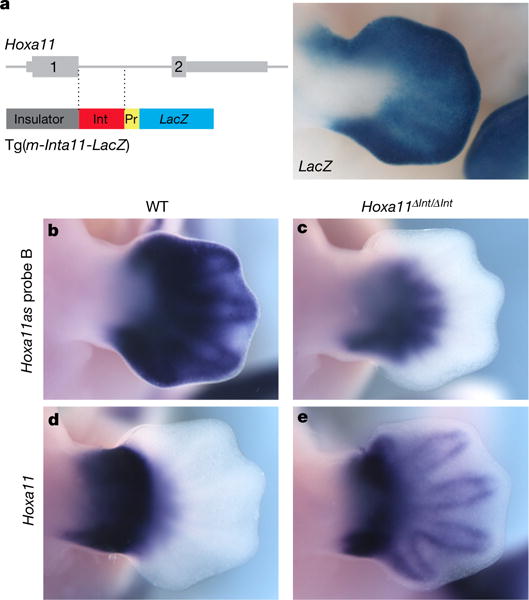
a, Left, scheme of the Tg(m-Inta11-LacZ) transgene carrying the predicted distal enhancer (Int, red box). Right, X-gal staining of E12.5 transgenic embryos (n = 5). B–e, Expression of Hoxa11as-b (b, c) and Hoxa11 (d, e) in wild-type (WT; b, d) and Hoxa11ΔInt/ΔInt (c, e) mouse limbs at E12.5. Note that based on the observed gain of Hoxa11 expression, other regulatory input(s) could be implicated in Hoxa11 regulation in distal cells. Pr, minimal promoter. Original magnification, × 31.5 (for all images).
Analysis of the enhancer sequence revealed several putative binding sites for HOXA13, the expression of which occurs in digit progenitor cells25 and is required in conjunction with HOXD13 for digit morphogenesis14. Chromatin immunoprecipitation followed by high-throughput sequencing (ChIP-seq) indicated that, in distal limb cells, HOXA13 as well as HOXD13 bind to the identified enhancer (Extended Data Fig. 3a). Moreover, transcription assay in 293T cells shows that HOXA13 has a positive effect on the enhancer activity (Extended Data Fig. 3b). Together, these results raised the possibility that distal Hoxa11 antisense transcription relies on HOX13. We thus analysed Hoxa11 antisense transcription in the Hoxa13;Hoxd13 allelic series. We used the probe recognizing all antisense transcripts such that expression in the proximal limb, where Hox13 genes are not expressed, served as internal control. We found that although antisense transcription is barely modified in single mutants (Extended Data Fig. 4), it markedly decreases in the Hoxa13−/− Hoxd13+/− mutant (Fig. 3c, compare to Fig. 3a), and is completely abrogated in Hoxa13−/−Hoxd13−/− distal limbs (Fig. 3e). Analysis of the distal-specific antisense transcripts (Hoxa11as-b) confirmed that distal antisense transcription requires HOX13 function (Extended Data Fig. 5). Importantly, concomitant with the abrogation of antisense transcription, Hoxa11 expression was gained distally (Fig. 3d–f, compare with Fig. 3b) consistent with the requirement of antisense transcription for Hoxa11 proximal restriction.
Figure 3. Hox13 inactivation disrupts Hoxa11 antisense transcription in distal cells and distal Hoxa11 expression results in the formation of supernumerary digits.
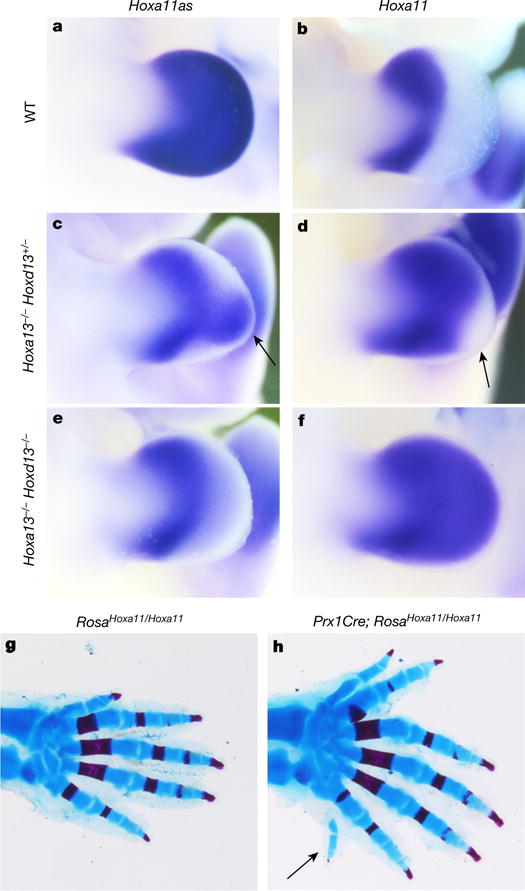
a–f, Hoxa11as (probe A) (a, c, e) and Hoxa11 (b, d, f) expression in E11.5 limb buds from wild-type (a, b), Hoxa13−/− Hoxd13+/− (c, d) and Hoxa13−/− Hoxd13−/− (e, f) mouse embryos. Arrows in c and d show the group of cells still expressing Hoxa11as in Hoxa13−/−Hoxd13+/− limbs (c), which corresponds to distal cells in which Hoxa11 expression is not gained (d). g, h, Skeleton of RosaHoxa11/Hoxa11 (g) and Prx1Cre; RosaHoxa11/Hoxa11 (h) distal forelimb at postnatal day 0 (P0). Anterior is up. Original magnification, ×31.5 (a–f) and ×20 (g, h).
To assess the functional significance of the HOXA13/D13-mediated repression of Hoxa11, we investigated the phenotypic outcome of distal Hoxa11 expression. Although the deletion of the enhancer driving antisense transcription results in Hoxa11 expression in distal limbs, the deletion extends up to the exon 1-intron boundary, thereby precluding the use of this mutant line to assess the phenotype resulting from distal Hoxa11 expression. We thus generated a Hoxa11 conditional gain-of-function allele (Rosa26Hoxa11; Extended Data Fig. 6) to express Hoxa11 ectopically and distally. We found that embryos carrying the Rosa26Hoxa11 allele and either Hoxa13:Cre (ref. 25) or Prx1:Cre (ref. 26) have limbs with extra digits (Fig. 3g, h), including postaxial extra digits (arrow in Fig. 3h and Extended Data Fig. 7). While some variations in the digit phenotype were observed among individuals, all homozygous mutants analysed were polydactylous (Extended Data Fig. 7c–e). Increased expression of Hoxd11 in the presumptive autopod in the absence of Hoxd13 also resulted in polydactyly, whereas a similar gain of Hoxd10 or Hoxd12 had no effect on digit number27. These data raise the possibility that the formation of extra digits upon ectopic expression of Hoxa11 or Hoxd11 distally reflects the divergence between Hoxa11/Hoxd11 targets and those of the other 5′ HoxA/D genes. Notably, the evidence that Hoxa11 expression in the distal limb results in the formation of extra digits indicates that the proximal restriction of Hoxa11 expression is required for the pentadactyl state.
In contrast to the mutually exclusive Hoxa11 and Hoxa13 pattern in tetrapod limbs, Hoxa11 and Hoxa13 gene expression is largely overlapping in zebrafish fins3–7 (Extended Data Fig. 8) as well as in other teleosts28 (the medaka Oryzias latipes) and in fish models of both chondrichthyans5 (Scyliorhinus canicula) and basal actinopterygians3 (Polyodon spathula). The HOXA13/D13-mediated repression of Hoxa11 identified in distal limb cells was thus probably implemented after the separation of actinopterygians and chondrichthyans, during the evolution of vertebrates towards tetrapod species. Consistent with this hypothesis, no Hoxa11 antisense transcription has been reported in fish22,29 (Extended Data Fig. 9). Moreover, sequence comparison of the mouse Hoxa11 intron showed robust conservation among tetrapods, whereas considerably weaker sequence conservation was observed with fish Hoxa11 orthologues (Fig. 4a). To examine whether the lack of Hoxa11 antisense transcription in fish could be due to the absence of a distal enhancer within Hoxa11 intron, we tested the zebrafish Hoxa11a and Hoxa11b intronic sequences for potential enhancer activity using transgenic reporter assays in both zebrafish and mice. Neither the Hoxa11a nor Hoxa11b intron was capable of triggering expression of a reporter gene in fin nor in mouse limb buds (Extended Data Table 1), indicating that there is no distal enhancer in Hoxa11a nor Hoxa11b intron. By contrast, when we tested the transcriptional activity of the mouse Hoxa11 intron in zebrafish, the analysis of four stable transgenic lines revealed that the mouse Hoxa11 intron was able to drive reporter gene expression in the pectoral fin mesenchyme (Fig. 4b, c). At 60 hours post-fertilization (hpf), eGFP-positive cells were present at the distal rim of the endoskeletal disc and migrating into the fin fold (Fig. 4b) and by 72 hpf most eGFP-positive cells were found in the fin fold mesenchyme (Fig. 4c). The expression of the reporter transgene was reminiscent of Hoxa13a expression at 60 hpf (Fig. 4d) and 72 hpf (Fig. 4e), indicating that the mouse enhancer in Hoxa11 intron was active in the Hoxa13 domain also in zebrafish. Together, our data indicate that all the transcription factors required for the activity of the mouse enhancer are present in zebrafish fins, and that the enhancer driving Hoxa11 antisense transcription does not exist in the intron of the zebrafish Hoxa11a and Hoxa11b genes. We therefore propose that the emergence of the enhancer triggering Hoxa11 antisense transcription, and thus distal repression of Hoxa11, occurred in the course of evolution towards tetrapod species.
Figure 4. The mouse Hoxa11 antisense enhancer is functional in distal fins.
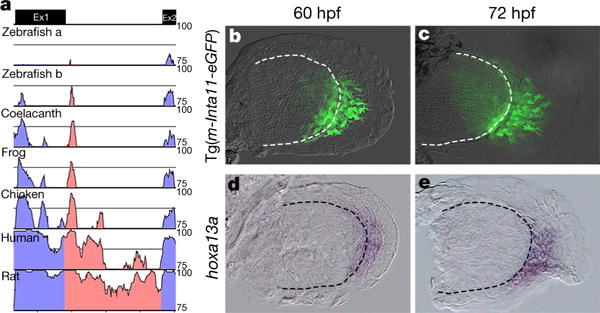
a, mVISTA sequence conservation plot of the mouse Hoxa11 intron (red) with tetrapod (rat, human, chicken and frog) and fish representatives (coelacanth and zebrafish). Ex1, exon 1; Ex2, exon 2. Note that zebrafish has two Hoxa11 genes expressed in developing fins, Hoxa11a and Hoxa11b. b, c, GFP expression in fin buds of Tg(m-Inta11-eGFP) transgenic zebrafish embryos at 60 hpf (b) and 72 hpf (c), revealing the enhancer activity of the mouse Hoxa11 intron in fish. Note the filopodia-like protrusions in GFP+ mesenchymal cells suggestive of a migration towards the fin fold. d, e, Hoxa13a expression in developing fins at 60 hpf (d) and 72 hpf (e). Original magnification, ×400.
In summary, our work reveals that the mutually exclusive expression of Hoxa11 and Hoxa13 in tetrapods is associated with the emergence of a transcriptional enhancer in Hoxa11 intron, which upon HOXA13/ D13-dependent activation, triggers antisense transcription and thereby prevents Hoxa11 expression distally. On the basis of the evidence that this HOX13-mediated regulation of Hoxa11 probably emerged during the fin-to-limb transition and the polydactyly resulting from distal expression of Hoxa11 in mice, we propose that the evolution of Hoxa11 regulation has contributed to the transition from polydactyly in stem-group (extinct) tetrapods to pentadactyly in extant tetrapods.
METHODS
No statistical methods were used to predetermine sample size.
Mouse lines
Hoxa11Neo, Hoxa11eGFP, Hoxa13null (Hoxa13Str) and Hoxd13null (Hoxd13lacZ) mouse lines were previously described14,23,24,30.
RosaHoxa11 knock-in allele was constructed as followed: PacI-AscI fragment from pBTG (Addgene plasmid 15037)31 was inserted into the previously described Rosa26 targeting vector32 pROSA26Am1 (Addgene plasmid 15036)31. The mouse Hoxa11 cDNA was inserted at the Smal site within the MCS. The vector was linearized by SwaI digest prior electroporation into embryonic stem (ES) cells. After double selection using G418 and DTA negative selection, 96 ES cell clones were analysed by Southern blot for homologous recombination. Two independent clones were injected into blastocysts obtained from C57BL/6J mice, subsequently implanted into pseudo-pregnant females. After germline transmission of the RosaHoxa11 allele, mice and embryos were genotyped by Southern blot (a scheme with restriction sites and probes used is presented in Extended Data Fig. 6) and PCR. The following PCR primers were used: fw_wt: 5′-GCAATACCTTTCTGGGAGTTCT-3′, rev_wt : 5′-TCGGGTGAGCATGTCTTTTAATC-3′, rev_flox : 5′-TTCAATGGCCGATCCCATATT-3′, rev_del : 5′-AGGTTGGAGGAGTAGGAGTATG-3′. Wildtype band: 384 bp, flox band: 881 bp, del band: 583 bp. The moderate transcription resulting from the Rosa26 promoter allowed for Rosa26Hoxa11 expression at a level comparable to the Hoxa11 gain observed in our series of mutants.
Hoxa11AInt mouse line was generated through pronuclei injection of single-guide RNAs (sgRNAs). We used the CRISPR (http://crispr.mit.edu/) platform to design sgRNAs flanking the region to delete. Complementary strands were annealed, phos-phorylated and cloned into the BbsI site of pX330 CRISPR/Cas9 vector (Addgene plasmid 42230)33. SgInt1_fw : 5′-CACCGACT CCCCTTT CATAAAGCCC-3′; SgInt1_rev : 5′-AAACGCGCTTTATGAAAGGGGAGTC-3′; SgInt2_fw : 5′-CACCGAGCAACAGGCGAGTTTGCGC-3′; SgInt2_rev : 5′-AAACGCGCAAACTCGCCTGTTGCTC-3′. Mice and embryos were genotyped by Southern blot (a scheme with restriction sites and probe used is presented in Extended Data Fig. 2) as well as PCR. The Southern blot probe corresponds to the ScaI-HpaI fragment in the 3′ untranslated region (UTR) of the Hoxa11 gene. Primers used for PCR genotyping, fw: 5′-GGCCACCTAAGGAAGGAGAG-3′; rev: 5′-GGCTCCGGTGCGTATAAAG-3′
Three Prx1-Hoxa11as transgenic lines were derived from three distinct founders obtained from pronuclear injection of the Prx1-Hoxa11as transgene. The Prx1-Hoxa11as transgene carries the Prx1 promoter upstream of the mouse Hoxa11as (GenBank: U20367.1 and U20366.1) and the SV40 polyadenylation sequence was inserted downstream Hoxa11as. Embryos were genotyped by PCR using DNA from the amniotic membrane and the following pair of primers: fw: 5′-CTTTCTCTCTGGCTCTGATG-3′ and rev: 5′-GACAAGAACGCCGAGAA-3′ (for U20367.1) or fw: 5′-GTCCGAGGAAAAGGAGGTAG-3′ and rev: 5′-GCTCCTCTAACATGTATTTG-3′ (for U20366.1).
All mice were of mixed background (C57BL/6 X 129).
The Tg(m-Inta11-LacZ) transgene was generated by subcloning the mouse Hoxa11 intron upstream of the Hbb (β-globin) minimal promoter and a LacZΔCpG NLS reporter. The H19 insulator was inserted upstream of the Hoxa11 intron. Tg(m-Inta11-LacZ) embryos were produced by pronuclear injection.
Whole-mount in situ hybridization, X-gal staining, skeletal preparations and imaging
For skeletal preparation, newborn mice were processed using the standard alcian blue alizarin red staining protocol34 (n = 10 for each genotype).
Whole-mount in situ hybridizations were performed using previously described protocol35 and probes35 (gfp36, Neo, Hoxa11, Hoxa13). Embryos were genotyped prior in situ hybridization (no blinding). Hoxa11as probes were generated using limb cDNA and the following primers: fw 5′-AGAGGCGCTGAGGAGCCTTCTC-3′ and rev 5′-GGCCGCTGTGGACACTAGCATATACC-3′ (probe A); fw 5′-CCTTCTCGGCGTTCTTGTC-3′ and rev ′-GGCATACTCCTACTCCTCCAACCTW (probe B).
X-gal staining was performed using standard protocol35. Embryos were geno-typed after X-gal staining (which results in blinding test).
All mouse specimens were imaged using the Leica DFC450C camera. For each experiment, a minimum of three embryos per genotype was used as we considered that reproducible staining/expression patterns with three distinct embryos of the same genotype are significant. The experiments shown were repeated at least twice. We did not use the randomization method.
Subcloning of zebrafish hoxa11a/b intron and microinjections in zebrafish embryos
The zebrafish hoxa11a (713 bp; gene ID 58061, NCBI) and hoxa11b (747bp; gene ID 30382, NCBI) introns were amplified from zebrafish genomic DNA using the following primers: hoxa11a intron: fw 5′-GAATTCAACAGTAAGTACGAGCTCAAC-3′; rev 5′-GGTACCACCTAAATGTAAATACACGT-3′; hoxa11b intron: fw 5′-GAATTCCAGCGGCAGCAGCAGTACGT-3′; rev 5′-GGTACCCCGTGTCTTTTGTCCATCTAA-3′.
The zebrafish hoxa11a and hoxa11b and the mouse Hoxa11 introns were subcloned into the pEGFP-N1 vector (CLONTECH Laboratories, Inc.) in which the CMV promoter upstream of eGFP was replaced with the human HBB minimal promoter using the following primers: fw 5′-GGATCCCTGGGCATAAAAGTCAG-3′, rev 5′-ACCGGTTCTGCTTCTGGAAGGCT-3′. This vector also contains the Tol2 arms to increase transgenesis efficiency. For screening purposes, a heart marker (cmlc2:mCherry37) was added to zebrafish Tg(z-Inta11a-eGFP) and Tg(z-Inta11b-eGFP) constructs. All constructs were microinjected in one-cell stage wild-type zebrafish embryos at a concentration of 100 ng μl1 together with 50 ng μl-1 transposase mRNA.
Generation of zebrafish transgenic lines
Primary injected zebrafish (P1) are raised until 3 months of age, and then are screened for transgenic progeny (F1). P1 fish are crossed with wild-type fish and the embryos are screened at 2 days post-fertilization (dpf). Owing to lack of fin fold eGFP expression in the Tg(z-Inta11a-eGFP; cmlc2:mCherry), Tg(z-Inta11b-eGFP; cmlc2:mCherry) injected fish, embryos were screened for the presence of the cmlc2:mCherry heart marker and genotyped to confirm the presence of the hoxa11a/b intron:eGFP elements. The following primers were used for genotyping: hoxa11a: fw 5′-GGTACCACCTAAATGTAAATACACGT-3′, rev (eGFP) 5′-GTCCTCCTTGAAGTCGATGC-3′; hoxa11b: fw 5′-GGTACCCC GTGTCTTTTGTCCATCTAA-3′, rev (eGFP) 5′-GTCCTCCTTGAAGTC GATGC-3′.
Three transgenic lines for Tg(m-Inta11-eGFP) were obtained to confirm the expression pattern. A fourth line containing the cmlc2:mCherry heart marker was also created. To confirm the Hbb minimal promoter does not drive tissue-specific expression alone, a transgenic line Tg(HBB:eGFP; cmlc2:mCherry) was also created and genotyped using the following primers: Hbb: fw 5′-GGATCCCTGGGCATAAAAGTCAG-3′, rev (eGFP) 5′-GTCCTCCTTGAAGTCGATGC-3′.
Zebrafish in situ hybridization
In situ hybridization on whole-mount embryos was performed as previously described38. Digoxigenin-labelled antisense RNA probes were generated using the following cDNAs: hoxa13a (500 bp; Addgene 36463), hoxa13b (700 bp; Addgene 36568), hoxa11b (probe 1 (Extended Data Fig. 8c, d); 800 bp; Addgene 36466). For hoxa11a/b antisense/sense RNA probes (Extended Data Fig. 9a, b), hoxa11a (713 bp; Gene ID 58061, NCBI) and hoxa11b (747 bp; gene ID 30382, NCBI) partial cDNAs (exon 1) were obtained by PCR with reverse transcription from total RNA of 24–48 hpf embryos using the following primers: hoxa11a exon 1: fw 5′-ATGATGGATTTTGACGAAAGGGTT-3′, rev 5′-TGTTCCCACCGCTAGTTTTT TCCT-3′; hoxa11b exon 1: fw 5′-ATGATGGATTTTGATGAGCGGGTA-3′, rev 5′-TGCTGCTGCCGCTGAATTTATCTT-3′.
For accurate comparison, hoxa11a and hoxa11b sense and antisense probes, respectively, are identical in length and were transcribed using the same RNA polymerase. In situ hybridizations were also performed in parallel with identical staining times.
Transfection and gene expression analysis
293T cells (ATCC) were transfected using lipofactamine. Cells (800,000) were plated in 6-well plates. Cells were checked for mycoplasma contamination using Venor GeM Mycoplasma Detection Kit (MP0025 SIGMA). A total of 2 μg of DNA (250 ng reporter plasmid, 250 ng effector plasmid or empty expression vector), 25ng of mCherry expression vector as internal control and 1.45 μg carrier pBSK plasmid was used for each transfection. All transfections were performed in duplicates. Then, 24 h after transfection, the medium was changed and 48 h after transfection, cells were processed for RNA extraction. Reporter gene expression was normalized to internal control mCherry (n = 3). Gene expression (Hoxa11) was measured in dissected E11.5 forelimb buds of the RosaHoxa11 knock-in embryos that were stored in RNA later before RNA extraction (n = 4).
RNA extraction was done using RNAeasy Plus mini kit (Qiagen 74134). cDNA synthesis was performed using M-MuLV reverse transcriptase (NEB) and a mix of random primers and oligo-dT on 1ug of total RNA. Quantitative real-time-PCR was performed with cDNA and the SYBR Green kit (applied biosystems) using the following primers: fw 5′-AGGAGAAGGAGCGACGG-3′ and rev 5′-GGTATTTGGTATAAGGGCAGCG-3′ (Hoxa11); fw 5′-CTTTGTCAAGCTCATTTCCTGG-3′ and rev 5′-TCTTGCTCAGTGTCCTTGC-3′ (Gapdh); fw 5′-TTGACCTAAAGACCATTGCACTTC-3′ and rev 5′-TTCTCA TGATGACTGCAGCAAA-3′ (Tbp); fw 5′-GCCTACAACGTCAACATCAAG-3′ and rev 5′-GCGTTCGTACTGTTCCAC-3′ (mCherry); fw 5′-GACCCTGA AGTTCATCTGCA-3′ and rev 5′-CCGTCGTCCTTGAAGAAGA-3′ (gfp).
Study approval
All mice experiments described in this article were approved by the Animal Care Commitee of the Institut de Recherches Cliniques de Montréal (protocols 2011-39 and 2014-14) and zebrafish experiments were approved by uOttawa Animal Care Committee (protocol BL-2317-R1).
Extended Data
Extended Data Figure 1. Absence of antisense transcription 3′ to the Hoxa11 promoter in the Hoxa11eGFP/eGFP limb and evidence that Hoxa11as-b transcripts produced in trans have no effect on Hoxa11 expression.
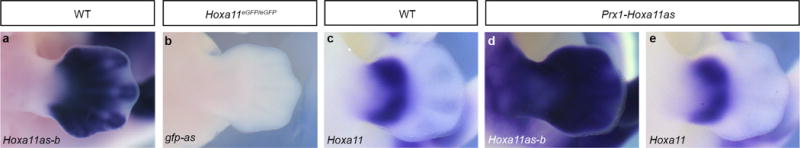
a, b, Detection of Hoxa11as-b transcripts in wild-type limb buds at E12.5 (a), and whole-mount in situ hybridization to detect gfp antisense transcripts in Hoxa11eGFP/eGFP limb buds at E12.5 (b). c–e, Hoxa11 expression in wild-type limb buds (c), and Hoxa11as-b (d) and Hoxa11 (e) expression in Prx1-Hoxa11as limb buds. Original magnification, ×31.5.
Extended Data Figure 2. Deletion of the distal enhancer in Hoxa11 intron using CRISPR-Cas9.
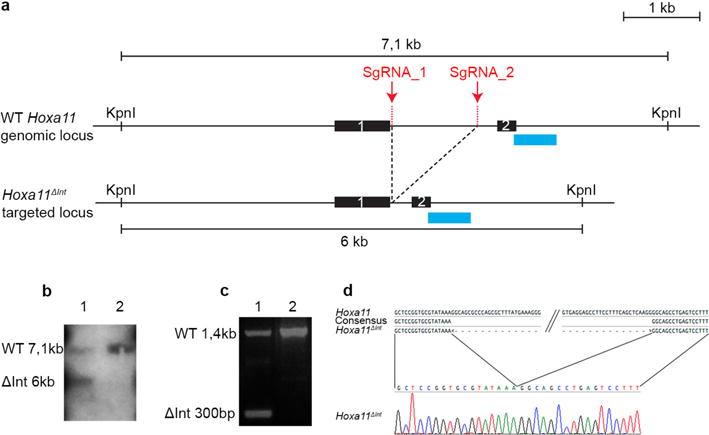
a, Scheme of the wild-type and targeted (Hoxa11ΔInt) loci. Sites targeted by the single-guide RNAS (sgRNA_1 and sgRNA_2) for the CRISPR-Cas9-mediated deletion of the distal enhancer. The blue rectangles indicate the position of the DNA probe used to confirm the deletion by Southern blot in b. b, Lane 1 shows the 6-kb KpnI band resulting from the CRISPR-Cas9-mediated deletion. Lane 2 was loaded with wild-type DNA. c, PCR reaction using a forward primer located upstream of sgRNA_1 and a reverse primer located downstream sgRNA_2 shows the presence of a 300 bp (ΔInt 300 bp) fragment expected for the Hoxa11ΔInt allele. d, The sequence of the 300-bp PCR fragment confirms the CRISPR-Cas9-mediated deletion of the Hoxa11 intronic region containing the distal enhancer (only the sequence encompassing the deletion breakpoints is shown).
Extended Data Figure 3. The distal enhancer located in the Hoxa11 intron is bound by HOXA13 and HOXD13 in distal limb cells and its activity is increased by HOXA13 in 293T cells.
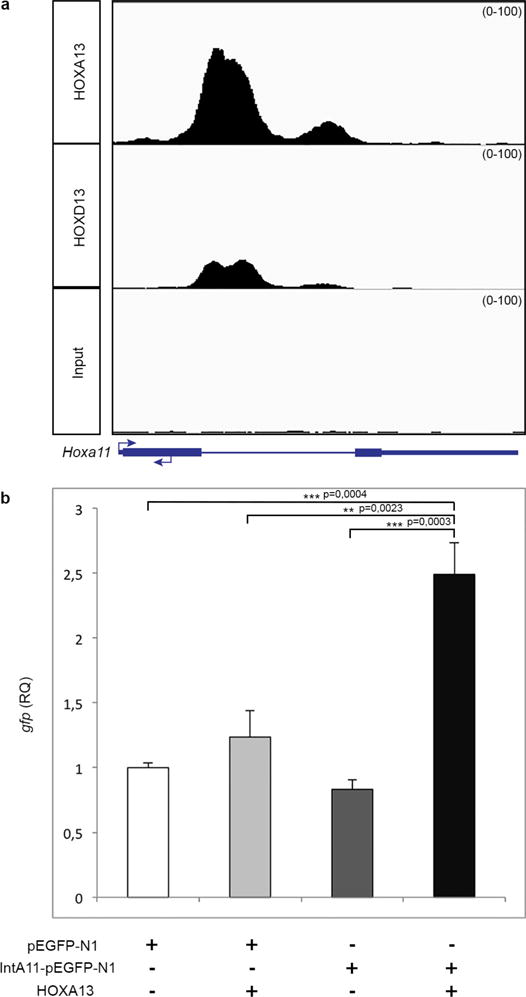
a, Integrative genomics viewer (IGV) screenshot showing HOXA13 and HOXD13 ChIP-seq data at the Hoxa11 locus. These ChIP-seq data were obtained using chromatin from distal forelimb buds of wild-type E11.5 mouse embryos (R. Sheth et al., manuscript submitted). b, Transfection assay shows HOXA13 dependent activation of Hoxa11 intron driving reporter gene expression. Two-tailed Tukey’s multiple comparisons test was performed. Error bars indicate s.d (n = 3). RQ, relative quantification.
Extended Data Figure 4. Individual inactivation of Hoxa13 or Hoxd13 is not sufficient to fully abrogate antisense transcription in distal limbs.
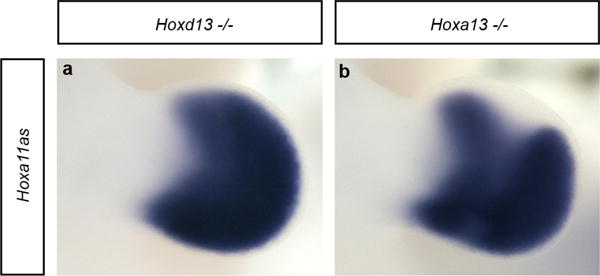
a, b, Whole-mount in situ hybridization, using probe A (see Fig. 1) to detect all antisense transcripts, on Hoxd13−/− (a) and Hoxa13−/− (b) mouse limb buds at E11.5. Antisense transcription in distal limbs remains robust in both mutants but a clear reduction is seen in the distal Hoxa13−/− limbs. Original magnification, ×31.5.
Extended Data Figure 5. Inactivation of both Hoxa13 and Hoxd13 disrupts antisense transcription overlapping with the Hoxa11 exon 1.
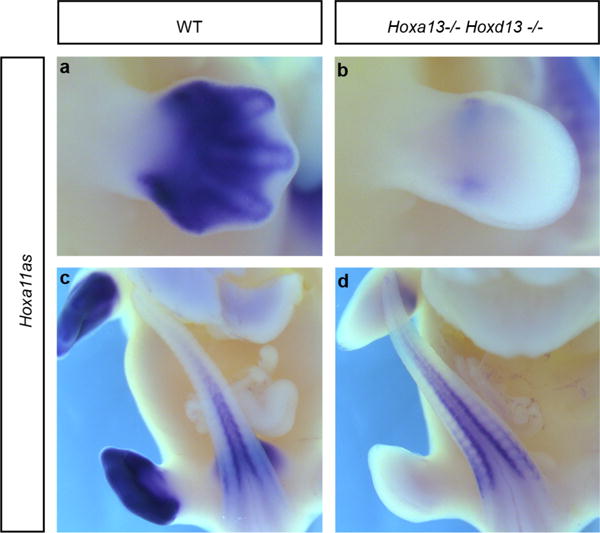
a–d, Hoxa11as-b expression (probe B in Fig. 1) in limb buds (a, b) and tail buds (c, d) from wild-type (a, c) and Hoxa13−/− Hoxd13−/− (b, d) E12.5 mouse embryos. Whole-mount in situ hybridization shows that Hoxa11as-b expression in tail buds (internal control) is similar in both the wild-type (c) and double-mutant (d) embryos, whereas there is almost no expression remaining in Hoxa13−/− Hoxd13−/− limb buds (b). Original magnification, ×31.5.
Extended Data Figure 6. Generation of the RosaHoxa11 knock-in mouse line.
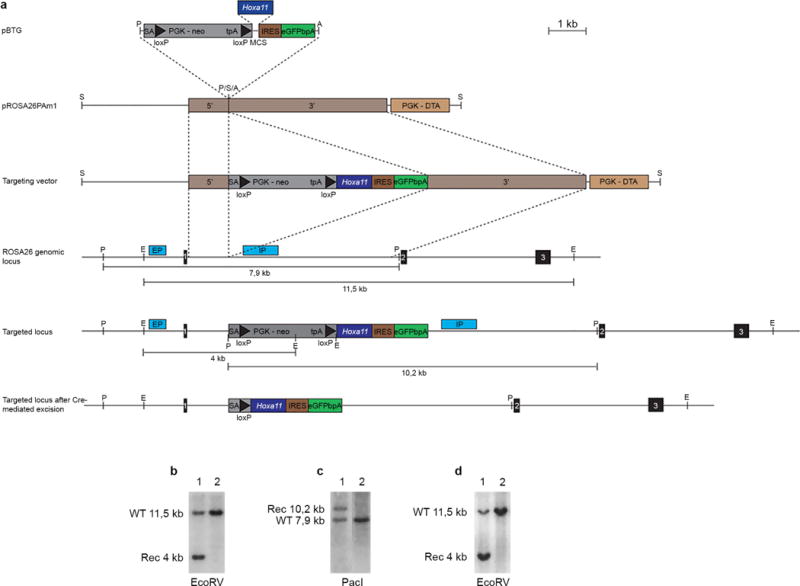
a, Targeting of the endogenous Rosa26 locus (top three lines). The wild-type Rosa26 locus is shown below (middle). Regions used as homologous arms for the recombination in ES cells are indicated by brown rectangles labelled 5′ and 3′, respectively. Scheme of the targeted locus after homologous recombination in ES cells and after Cre-mediated recombination is shown at the bottom. The position of the internal (IP) and external (EP) probes and restriction sites used for Southern blot analysis are indicated on both the wild-type and targeted locus. b, c, Southern blots of ES cells clones using the internal probe (b) and external probe (c) to detect the targeted allele (lane 1). d, Southern blot of wild-type (lane 2) and heterozygous (lane 1) mice. A, AscI; E, EcoRV; P, PacI; S, SwaI.
Extended Data Figure 7. The conditional gain of Hoxa11 using the Hoxa13Cre allele results in the formation of supernumerary digits.
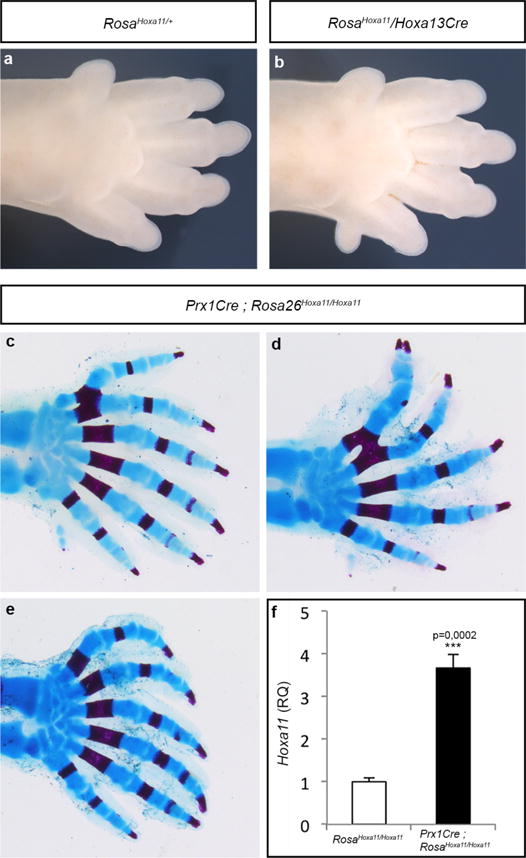
a, b, Autopod of RosaHoxa11/+ (a) and RosaHoxa11 Hoxa13Cre (b) at E15.5. Anterior is up. The Rosa26 locus and Hoxa13Cre allele being on the same chromosome (Chr6), the gain-of-function phenotype was assessed with only one copy of the RosaHoxa11 allele. c–e, Autopod skeletons of Prx1Cre; RosaHoxa11/Hoxa11 mice at P0 from four distinct mutants (anterior is up). The number of digits varies from 6 to 7, with often a small post-axial extra-digit (posterior). The extra-digit phenotype is fully penetrant upon Cre-activation of two copies of the RosaHoxa11 allele (n = 10). Original magnification, × 20. d, Quantification of Hoxa11 expression level by quantitative reverse transcriptase PCR (RT-qPCR) on RNA extracted from E11.5 forelimb, relative to both Gapdh and Tbp mRNA of Prx1Cre; RosaHoxa11/Hoxa11 embryos. Two-tailed t-test was performed. Error bars indicate s.d (n = 4).
Extended Data Figure 8. Hoxa11 and hoxa13 are expressed in overlapping domains in zebrafish fins.
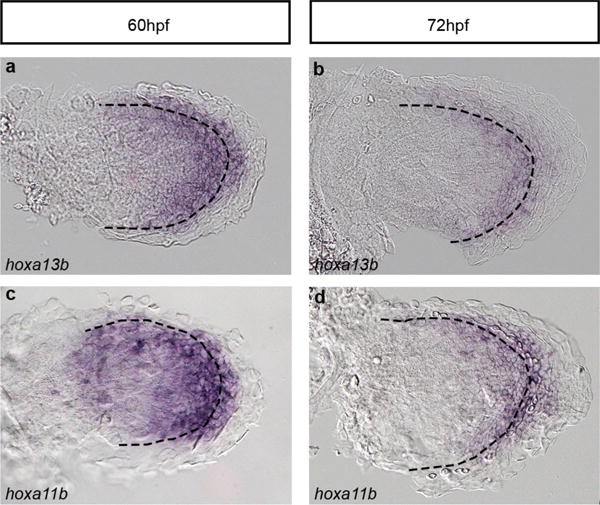
a–d, Expression of hoxa13b (a, b) and Hoxa11b (c, d) in zebrafish fins at 60 hpf (a, c) and 72 hpf (b, d). Dotted lines indicate the boundary between the endochondral disc and the fin fold. Original magnification, ×400.
Extended Data Figure 9. Absence of antisense transcription at the Hoxa11a and Hoxa11b loci in zebrafish fins.
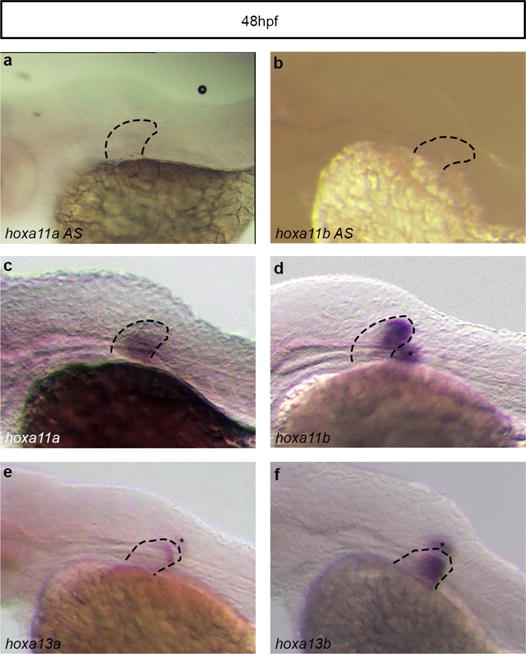
a, b, Whole-mount in situ hybridization with probes designed to detect putative antisense transcription at Hoxa11a (a) and Hoxa11b (b). c–f, No antisense transcription is detected, whereas expression of Hoxa11a (c), Hoxa11b (d), hoxa13a (e) and Hoxa13b (f) is observed in zebrafish fins at the same stage. Asterisks correspond to the staining from the fin on the other side of the embryo. Original magnification, ×63.
Extended Data Table 1.
Summary of transient transgenic embryos analysed
| Zebrafish Transient Transgenics
| |
|---|---|
| Construct | % of eGFP positive fish |
| Tg(HBB:eGFP) | 0% (n=74) |
| Tg(z-Inta11a-eGFP) | 0% (n=105) |
| Tg(z-Inta11b-eGFP) | 1.19% (n=84) |
| Tg(m-Inta11-eGFP) | 91.9% (n=123) |
| Tg(HBB:eGFP; cmlc2:mCherry) | 1.25% (n=94) |
| Tg(z-Inta11a-eGFP; cmlc2:mCherry) | 0% (n = 200) |
| Tg(z-Inta11b-eGFP; cmlc2:mCherry) | 0% (n = 300) |
| Tg(m-Inta11-eGFP; cmlc2:mCherry) | 88.9% (n=53) |
|
| |
| Mouse Transient Transgenics | |
|
| |
| Construct | % of eGFP positive embryos (# eGFP positive / # genotyped positive) |
|
| |
| Tg(z-Inta11a-eGFP) | 0% (n=0/10) |
| Tg(z-Inta11b-eGFP) | 0% (n=0/7) |
Zebrafish stable lines for Tg(z-Inta11a-eGFP; cmlc2:mCherry); Tg(z-Inta11b-eGFP; cmlc2:mCherry) were also generated and three genotyped Fi embryos per line were analysed and confirmed for the absence of gfp expression. For Tg(m-Inta11-eGFP; cmlc2:mCherry), four distinct transgenic lines were also generated and analysed.
Acknowledgments
We thank Q. Zhu and L. Lian from the IRCM transgenic core facility for the ES cell work and production of transgenic mouse lines. We are particularly grateful to A. Kania for critical reading of the manuscript as well as laboratory members for insightful discussions and sharing reagents. This work was supported by the Canadian Institute for Health Research (MOP-115127) and the Canada Research Chair program to M.K. (RCHS0192), the Natural Sciences and Engineering Research Council of Canada (155817-2012) to M.-A.A. and Shriners Hospital Research grant 85400 to H.S.S. Y.K. was supported by a fellowship from the Molecular Biology program of the Université de Montréal and the IRCM fellowship Michel-Bélanger. R.S. was supported by a post-doctoral fellowship from the Canadian Institute for Health Research. D.M.W. and K.M.P. were supported by NIH NIAMS AR061402, with K.M.P. additionally supported by NIH T32 DE007057.
Footnotes
Author Contributions Y.K. and M.K. conceived the study and analysed the data. Y.K. designed and conducted all mouse experiments with the help of R.S. for the generation of the mouse lines. All fish experiments were performed by R.L.L. under the supervision of M.-A.A. R.S. performed the ChIP-seq experiments. A.D. provided technical help for the mouse experiments. G.M. performed preliminary experiments related to Figs 2a and 3c, e. D.M.W. and K.M.P provided Hoxa11eGFP/eGFP embryos. H.S.S. provided the HOXA13 and HOXD13 antibodies. M.K. wrote the paper. All authors commented on the manuscript.
The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper. Correspondence and requests for materials should be addressed to M.K. (marie.kmita@ircm.qc.ca).
Reviewer Information Nature thanks R. Freitas, J. L. Gomez-Skarmeta and S. Mackem for their contribution to the peer review of this work.
Online Content Methods, along with any additional Extended Data display items and Source Data, are available in the online version of the paper; references unique to these sections appear only in the online paper.
References
- 1.Shubin N, Tabin C, Carroll S. Deep homology and the origins of evolutionary novelty. Nature. 2009;457:818–823. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
- 2.Coates MI, Jeffery JE, Rut M. Fins to limbs: what the fossils say. Evol Dev. 2002;4:390–401. doi: 10.1046/j.1525-142x.2002.02026.x. [DOI] [PubMed] [Google Scholar]
- 3.Davis MC, Dahn RD, Shubin NH. An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature. 2007;447:473–476. doi: 10.1038/nature05838. [DOI] [PubMed] [Google Scholar]
- 4.Metscher BD, et al. Expression of Hoxa-11 and Hoxa-13 in the pectoral fin of a basal ray-finned fish, Polyodon spathula: implications for the origin of tetrapod limbs. Evol Dev. 2005;7:186–195. doi: 10.1111/j.1525-142X.2005.05021.x. [DOI] [PubMed] [Google Scholar]
- 5.Sakamoto K, et al. Heterochronic shift in Hox-mediated activation of sonic hedgehog leads to morphological changes during fin development. PLoS One. 2009;4:e5121. doi: 10.1371/journal.pone.0005121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sordino P, Duboule D, Kondo T. Zebrafish Hoxa and Evx-2 genes: cloning, developmental expression and implications for the functional evolution of posterior Hox genes. Mech Dev. 1996;59:165–175. doi: 10.1016/0925-4773(96)00587-4. [DOI] [PubMed] [Google Scholar]
- 7.Ahn D, Ho RK. Tri-phasic expression of posterior Hox genes during development of pectoral fins in zebrafish: implications for the evolution of vertebrate paired appendages. Dev Biol. 2008;322:220–233. doi: 10.1016/j.ydbio.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Nature. 1997;388:639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- 9.Wagner GP, Chiu CH. The tetrapod limb: a hypothesis on its origin. J Exp Zool. 2001;291:226–240. doi: 10.1002/jez.1100. [DOI] [PubMed] [Google Scholar]
- 10.Sordino P, van der Hoeven F, Duboule D. Hox gene expression in teleost fins and the origin of vertebrate digits. Nature. 1995;375:678–681. doi: 10.1038/375678a0. [DOI] [PubMed] [Google Scholar]
- 11.Freitas R, Zhang G, Cohn MJ. Biphasic Hoxd gene expression in shark paired fins reveals an ancient origin of the distal limb domain. PLoS One. 2007;2:e754. doi: 10.1371/journal.pone.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freitas R, Gómez-Marin C, Wilson JM, Casares F, Gómez-Skarmeta JL. Hoxd13 contribution to the evolution of vertebrate appendages. Dev Cell. 2012;23:1219–1229. doi: 10.1016/j.devcel.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Woltering JM, Noordermeer D, Leleu M, Duboule D. Conservation and divergence of regulatory strategies at Hox loci and the origin of tetrapod digits. PLoS Biol. 2014;12:e1001773. doi: 10.1371/journal.pbio.1001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fromental-Ramain C, et al. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development. 1996;122:2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- 15.Schneider I, Shubin NH. The origin of the tetrapod limb: from expeditions to enhancers. Trends Genet. 2013;29:419–426. doi: 10.1016/j.tig.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Montavon T, et al. A regulatory archipelago controls Hoix genes transcription in digits. Cell. 2011;147:1132–1145. doi: 10.1016/j.cell.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Berlivet S, et al. Clustering of tissue-specific sub-TADs accompanies the regulation of HoxA genes in developing limbs. PLoS Genet. 2013;9:e1004018. doi: 10.1371/journal.pgen.1004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehrke AR, et al. Deep conservation of wrist and digit enhancers in fish. Proc Natl Acad Sci USA. 2015;112:803–808. doi: 10.1073/pnas.1420208112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheth R, Bastida MF, Kmita M, Ros M. “Self-regulation,” a new facet of Hox genes’ function. Dev Dyn. 2014;243:182–191. doi: 10.1002/dvdy.24019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh-Li HM, et al. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995;121:1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- 21.Potter SS, Branford WW. Evolutionary conservation and tissue-specific processing of Hoxa 11 antisense transcripts. Mamm Genome. 1998;9:799–806. doi: 10.1007/s003359900870. [DOI] [PubMed] [Google Scholar]
- 22.Leite-Castro J, Beviano V, Rodrigues PN, Freitas R, Hox A. Genes and the fin-to-limb transition in vertebrates. J Dev Biol. 2016;4:10. doi: 10.3390/jdb4010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Small KM, Potter SS. Homeotic transformations and limb defects in Hox A11 mutant mice. Genes Dev. 1993;7:2318–2328. doi: 10.1101/gad.7.12a.2318. [DOI] [PubMed] [Google Scholar]
- 24.Nelson LT, Rakshit S, Sun H, Wellik DM. Generation and expression of a Hoxa11eGFP targeted allele in mice. Dev Dyn. 2008;237:3410–3416. doi: 10.1002/dvdy.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scotti M, Kherdjemil Y, Roux M, Kmita M. A Hoxa13:Cre mouse strain for conditional gene manipulation in developing limb, hindgut, and urogenital system. Genesis. 2015;53:366–376. doi: 10.1002/dvg.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan M, et al. Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 27.Kmita M, Fraudeau N, Hérault Y, Duboule D. Serial deletions and duplications suggest a mechanism for the collinearity of Hoxd genes in limbs. Nature. 2002;420:145–150. doi: 10.1038/nature01189. [DOI] [PubMed] [Google Scholar]
- 28.Takamatsu N, et al. Duplicated Abd-B class genes in medaka hoxAa and hoxAb clusters exhibit differential expression patterns in pectoral fin buds. Dev Genes Evol. 2007;217:263–273. doi: 10.1007/s00427-007-0137-4. [DOI] [PubMed] [Google Scholar]
- 29.Ulitsky I, Shkumatava A, Jan CH, Sive H, Bartel D. P Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell. 2011;147:1537–1550. doi: 10.1016/j.cell.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kmita M, Kondo T, Duboule D. Targeted inversion of a polar silencer within the HoxD complex re-allocates domains of enhancer sharing. Nat Genet. 2000;26:451–454. doi: 10.1038/82593. [DOI] [PubMed] [Google Scholar]
- 31.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 33.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheth R, et al. Hox genes regulate digit patterning by controlling the wavelength of a Turing-type mechanism. Science. 2012;338:1476–1480. doi: 10.1126/science.1226804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scotti M, Kmita M. Recruitment of 5′ Hoxa genes in the allantois is essential for proper extra-embryonic function in placental mammals. Development. 2012;139:731–739. doi: 10.1242/dev.075408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattar P, et al. Basic helix-loop-helix transcription factors cooperate to specify a cortical projection neuron identity. Mol Cell Biol. 2008;28:1456–1469. doi: 10.1128/MCB.01510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yelon D, Horne SA, Stainier D. Y Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- 38.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protocols. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]


