Abstract
Purpose
Osteonecrosis of the jaw (ONJ) is emerging as one of the important complications in cancer patients treated with antiresorptive agents. This study explored the potential role of IL-17-mediated M1/M2 macrophage alterations in the pathogenesis of bisphosphonate-related osteonecrosis of the jaw (BRONJ).
Experimental Design
The expression of IL-17 and M1 and M2 macrophage markers at the local mucosal site of human BRONJ lesions was examined by immunofluorescence studies. BRONJ-like disease was induced in C57BL/6 mice and multiple myeloma (MM)-burdened mice by intravenous injection of zoledronate to evaluate the correlation of elevated IL-17 levels with changes in M1 and M2 macrophage phenotypes and the therapeutic effects of blocking IL-17 on pathogenesis of BRONJ-like disease.
Results
Increased Th17 cells and IL-17 cytokine correlate with an increase in M1/M2 macrophages ratio at the local mucosal site of both murine and human BRONJ lesion. Convincingly, in mice burdened with multiple myeloma, a combination of elevated suprabasal level and drug-induced IL-17 activity augmented the incidence of BRONJ; both systemic increase of IL-17 and disease severity could be reversed by adoptive transfer of ex vivo expanded M2 macrophages. Targeting IL-17 via specific neutralizing antibodies or a small inhibitory molecule, Laquinimod, significantly decreased M1/M2 ratio and concomitantly suppressed BRONJ-like condition in mice. Mechanistically, IL-17 enhanced IFN-γ-induced M1 polarization through augmenting STAT-1 phosphorylation while suppressed IL-4-mediated M2 conversion via inhibiting STAT-6 activation.
Conclusions
These findings have established a compelling linkage between activated IL-17-mediated polarization of M1 macrophages and the development of BRONJ-like conditions in both human disease and murine models.
Introduction
Nitrogen containing bisphosphonates (BPs), a widely used anti-bone resorptive agent, has been associated with osteonecrosis of the jaw (ONJ), a complication of significant impacts in both medical and dental communities (1, 2). Bisphosphonate related osteonecrosis of the jaw (BRONJ) is defined as exposed necrotic bone in the oral cavity that does not heal after appropriate intervention over 8 weeks in the absence of radiation therapy (2, 3). The incidence of BRONJ in cancer patients receiving high doses of intravenous BP such as zoledronic acid (ZA) and pamidronate (%) ranged from 0.8~12%, a rate much higher than that in patients with osteoporosis on oral BP treatment. Among the cancer group, highest prevalence of bisphosphonate-related osteonecrosis of the jaw (BRONJ) has been reported in patients with multiple myeloma, followed by breast and prostate cancer (4–7). To date, even though several risk factors, including invasive dental procedure, infection, mechanical trauma to the jaw bone, and concomitant use of immunosuppressive and chemotherapy drugs, have been implicated in the etiology of BRONJ (4, 5), its underlying mechanisms remains largely unknown.
Macrophages play important role in innate immune response and are an essential component of the wound healing cascade (8–10). Macrophages can be converted to M1 phenotype upon stimulation of Th1 cytokines such as interferon (IFN)-γ or lipopolysaccharide (LPS), while their M2 counterpart, also known as wound healing macrophages, are polarized in response to Th2 cytokines such as interleukin (IL)-4 or IL-13 (11). M1 macrophages are generally pro-inflammatory based on their production of nitric oxide (NO), reactive oxygen species (ROS), interleukin (IL)-12 and tumor necrosis factor (TNF)-α; whereas M2 macrophages more likely adapt an anti-inflammatory role, characterized by IL-10 and transforming growth factor (TGF)-β (12–14), contributing to resolution of inflammation and tissue modeling (15, 16). Given the importance of macrophages in wound healing and the fact that these cells share similar cell lineage as osteoclasts, it is plausible to hypothesize that macrophage function may potentially be altered by BP treatment and therefore, contributes to the defective healing in BRONJ (17). Most recent studies have suggested a potential effect of zoledronic acid (ZA) in the polarization of tumor associated macrophages (TAMs) in vitro (18, 19); it remains to be determined comprehensively whether and how the function and phenotypes (M1 vs M2) of macrophages participate in the pathogenesis of BRONJ.
Interleukin (IL)-17A, a signature cytokine of the T helper cell subset, Th17, has been indicated in the development of numerous inflammatory, autoimmune diseases, tumors, and in host defense against bacterial and fungal infection (20). Besides Th17 cells, γδ T cells and macrophages are capable to produce IL-17 (21–25). IL-17 can promote recruitment of macrophage and induce their cytokine/chemokine production, and thus capable of mediating a link between acquired and innate immunity, specifically, T cell and macrophage functions (26–30). Most recently, we have demonstrated that administration of zoledronic acid can induce BRONJ-like lesions in mice, in part by suppressing the adaptive regulatory T cells (Tregs) and activating the inflammatory Th17 cells (31); this immunomodulatory effect linking elevated IL-17 and the prolonged inflammatory state associated with delayed wound healing potentially serve the underlying mechanism of BRONJ-like lesions.
In the present study, we first demonstrated an elevated expression of IL-17 in the inflammatory socket wounds obtained from BRONJ-affected cancer patients with a distinct histological feature of increased M1 and decreased M2 macrophage infiltration. Using a BRONJ-like disease murine model, we observed a correlation between an elevated IL-17 level and a combination of increased M1 macrophages and decreased M2 macrophages (an increased M1/M2 ratio) in both long bone marrow and local necrotic lesions of the jaw bone. Interestingly, administration of IL-17 neutralizing antibodies or Laquinimod, a small molecule drug that exhibits inhibitory effects on IL-17 activity (32–34), led to a decrease in M1/M2 ratio and concomitantly, a reduction in the incidence of BRONJ-like lesion. Additionally, systemic infusion of ex vivo expanded M2 macrophages significantly decreased serum IL-17 levels and inhibited BRONJ formation. Mechanistically, we showed that IL-17 promoted IFN-γ-induced M1 macrophage polarization by enhancing the phosphorylation of STAT-1; on the other hand, this pro-inflammatory suppressed IL-4-induced M2 macrophage conversion through downregulation of STAT-6 phosphorylation. Therefore, we describe for the first time substantial evidence that IL-17-mediated alteration of M1/M2 macrophage polarization can contribute to the pathogenesis of BRONJ.
Materials and Methods
Animals
C57BL/6J mice (female, 8–10 week-old, Jackson Laboratory), beige nude/nude Xid (III) (female, 8–10 week-old, Harlan) were used in this study. All animal experiments were performed under an institutionally approved protocol for the use of animal research at University of Southern California (USC) (USC #10874, #10941 and #11327).
Patient sample collections
The mucosal tissues bordering the extraction sockets were collected from patients undergoing tooth extraction under different conditions (n=5): 1) BRONJ: patients manifested BRONJ lesion that were undergoing surgical debridement and tooth extraction for this condition; 2) Non-BRONJ: patients with history of BP treatment without clinical BRONJ who underwent tooth extraction for non-restorable indication; 3) Periodontitis: patients with diagnosis of inflammatory gum disease, specifically, active periodontitis with resultant tooth loss; and 4) Normal: healthy patients who underwent routine dental extraction for other non-inflammatory mucosa condition such as dental crowding indication. The BRONJ group includes two patients with multiple myeloma, two patients with breast cancer, and one patient with osteoporosis treated with IV or oral bisphosphonates, respectively. The study is approved by the Institutional Review Board (IRB#HS-08-00281) at University of Southern California.
Reagents and antibodies
Murine and human macrophage colony-stimulating factor (M-CSF), IFN-γ, IL-4, IL-13, and IL-17 were purchase from PeproTech. Rat PE-conjugated anti-mouse CD11b and FITC-conjugated anti-mouse F4/80, mouse PE-conjugated anti-human CD14 and CD68, FITC-conjugated anti-human CD206 and CD4 were purchased from BioLegend. Rabbit polyclonal antibodies for human and mouse iNOS and arginase-1 were from Santa Cruz. A neutralizing antibody against mouse IL-17A (clone MM17F3) was from eBiosciences. Lipopolysaccharide (LPS) from Escherichia coli 055:B5, and phorbol 12-myristate 13-acetate (PMA) were obtained from Sigma-Aldrich
Induction of BRONJ-like lesion in wild type mice and multiple myeloma mouse model
The BRONJ-like lesions in mice were induced according to the protocol we reported recently (31). Briefly, wild type C57BL/6 mice or beige nude/nude Xid (III) burdened with multiple myeloma cells (5TGM) were intravenously injected with zoledronate (Zometa®, 125μg/kg, Novartis Oncology) twice a week via the tail vein. One week after zoledronate injection, the first maxillary molar teeth were extracted under deep anesthesia by intraperitoneal injection of ketamine (Ketaject®, 100 mg/kg, Phoenix) and xylazine (AnaSed®, 20 mg/kg, LLOYD Laboratories Pharmaceutical, Inc.). A total of 6 doses of zoledronate were administered for 3 consecutive weeks. Untreated mice with tooth extraction were used as controls. Clinical evaluation of extracted tooth socket and determination of the incidence of BRONJ-like lesions were done according to the previous study (31).
Culture of bone marrow-derived macrophages (BMDMs) and adoptive transfer of M1 or M2 macrophages
Bone marrow cells were flashed out from mice bone marrow cavity of femurs and tibias with PBS containing heat-inactivated 3% fetal bovine serum (FBS; Equitech-Bio) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin; Biofluids). All nuclear cells were seeded at 15 x 106 onto 100 mm culture dishes (Corning) and initially incubated for 3h under 37°C at 5% CO2 condition. Non-adherent cells were removed by washing the dishes twice with PBS. The attached cells were continuously cultured in complete DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin, and 10μg/mL murine M-CSF (10ng/mL) for 6 days. Macrophages were incubated for 48h in the presence of either 20ng/mL IFN-γ or 10ng/mL IL-4 to polarize macrophages into M1 and M2 macrophages, respectively. M1 macrophages were characterized by the expression of inducible nitric oxide synthase (iNOS) and secretion of TNF-α in response to LPS (100ng/mL) stimulation for 24h, while M2 macrophages were characterized by the expression of arginase-1 and the secretion of IL-10 after stimulation with LPS (100ng/mL) for 24h (13). One day after tooth extraction, cells were collected and intraperitoneally (i. p.) administered into mice (1.0 × 106/ mice).
Treatment of mice with murine IL-17 neutralizing antibody, or Laquinimod during induction of BRONJ-like lesions
One day after tooth extraction, 0.1mg of mouse monoclonal anti-murine IL-17 neutralizing antibody (1mg/ml) was intravenously (i. v.) injected into mice twice a week for 2 weeks, while mice received subclass-matched control antibody (1mg/mouse) served as control. In addition, one day after tooth extraction, Laquinimod (TEVA Pharmaceuticals Industries, Ltd), a quinoline-3-Carboxamide, was dissolved in purified water and administered daily (25 mg/kg) by oral gavage for 2 weeks (32–34). Mice without any treatment (control) and only with tooth extraction served as controls.
Histological and immunohistochemical studies
Paraffin-embedded and frozen sections of socket soft tissues from BRONJ patients were prepared and standard hematoxylin and eosin (H & E) staining and dual-color immunofluorescence studies using specific primary antibodies for human CD68, iNOS and arginase-1 were performed. Frozen sections of mice tissues including BRONJ-like lesions, spleens and long-bone marrows were immunostained with specific antibodies for mice F4/80 and iNOS or arginase-1 to recognize M1 and M2 macrophages as described before (35). Isotype-matched control antibodies (eBiosciences) were used as negative controls. For semi-quantification, positive signals in at least 6 random high-power fields (HPF) were visualized, counted and expressed as percentage of total DAPI-positive cells (mean ± SD).
Western blot analysis
Cell lysates or homogenate tissue lysates (50~100 μg of total protein) were separated on polyacrylamide-SDS gel and electroblotted onto nitrocellulose membrane (BioRad, Hercules, CA). After blocking with TBS/5% nonfat dry milk, the membrane was incubated with antibodies against mouse p-STAT-1 (Tyr701) (Cell Signal), pSTAT-6 (Tyr641) (Millipore) or arginase-1(Santa Cruz Biotech, Inc.) followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody, and the signals were visualized by enhanced chemiluminescence detection (ECL) (PIERCE, Rockford, IL). The blots were also re-probed with a specific antibody against β-actin (Sigma).
ELISA
The concentration of IL-10, TNF-α and IL-12 (p70) in supernatants of cultured cells was detected using ELISA kits (BioLegend) according to the manufacturer’s procedures.
Statistical analysis
All data are expressed as mean ± SEM from at least three independent experiments. Differences between experimental and control groups were analyzed by two-tailed unpaired Student’s t-test using SPSS. P-values less than 0.05 were considered statistically significant.
Results
Elevated Th17 cell/IL-17 activity and polarized M1 macrophages at the nonhealing extraction socket of BRONJ patients
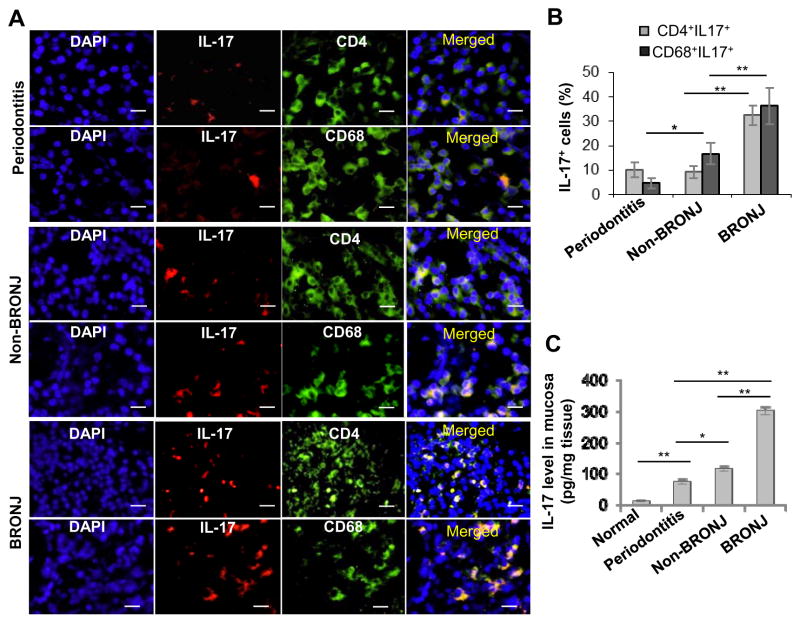
We have recently reported that intravenous administration of zoledronate, a potent nitrogen-bisphosphonate, induces BRONJ-like condition in mice undergoing tooth extraction; this adverse event is correlated with a decrease in regulatory T cells (Tregs) and a concomitant increase in Th17 cells and IL-17 production (31). Here, using immunofluorescence staining of the mucosal tissues bordering the non-healing extraction socket obtained from BRONJ patients, we confirmed that IL-17 positive cells were dramatically increased at the local extraction sockets of patients manifested BRONJ lesion that were undergoing surgical debridement for this condition, as compared to that from control patients without clinical BRONJ (Fig. 1A and B). The control group included: 1) patients with diagnosis of inflammatory gum disease, specifically, active periodontitis with resultant tooth loss; and 2) patients with history of bisphosphonate treatment who did not present with clinical BRONJ at the time of tooth extraction for non-restorable indication. These IL-17 positive cells were positive for CD4+ and CD68+, indicating T cell, specifically Th17 subset, and macrophage lineage, respectively. The elevated IL-17 expression was also confirmed by ELISA using tissue lysates obtained from the above patient groups (Fig. 1C).
Figure 1.
Increased expression of IL-17 in oral mucosal tissues bordering the non-healing extraction socket of BRONJ patients. A and B, Immunofluorescence studies showed increased expression of IL-17 in both CD4+ T cells and CD68+ macrophages in mucosal tissues bordering the extraction sockets of patients manifested BRONJ lesions (BRONJ) or patients with history of zoledronate treatment without clinical BRONJ (Non-BRONJ) (n=5), whereby oral mucosal tissues from healthy patients who underwent routine dental extraction for other non-inflammatory mucosa conditions (Normal) or healthy patients with diagnosis of inflammatory gum disease, specifically, active periodontitis with resultant tooth loss (Periodontitis) were used as controls (n=5). Scale bars, 50μm. C, IL-17 levels in the tissue lysates were examined by ELISA. Data are mean ± SEM of multiple fields in n=5 per group. *P < 0.05; **P<0.01.
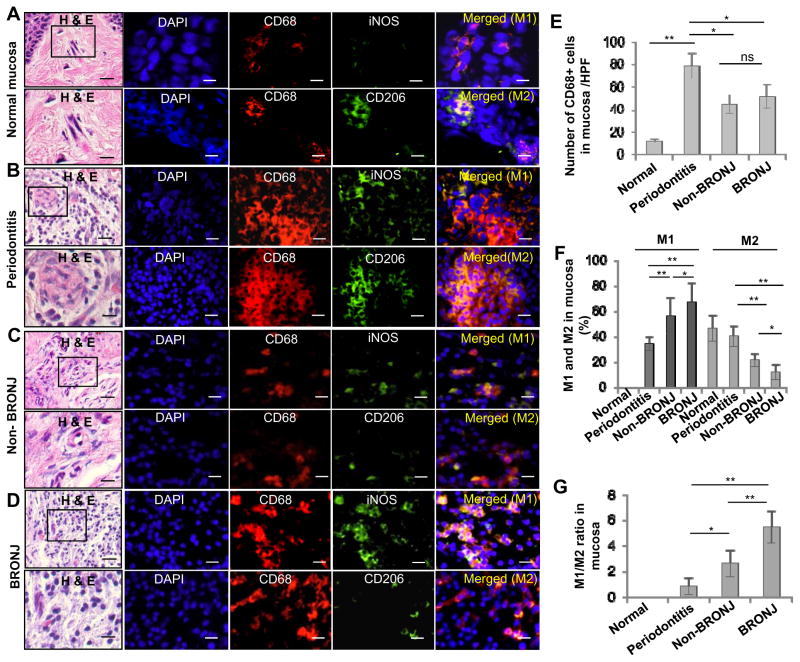
To investigate the phenotype of macrophage, specifically in the context of wound healing, we examine the distribution of M1 and M2 macrophages at the BRONJ socket site. A remarkable infiltration of CD68+ macrophages was noticed in mucosal tissues adjacent to the extraction sockets of periodontitis affected tooth as compared to clinically healthy tissues (P<0.01) of patients exposed or not exposed to BP treatment (Fig. 2A–E); likewise, abundant CD68+ macrophages were also observed in mucosal tissues of BRONJ extraction sockets relative to those of non-BRONJ (P<0.05) (Fig. 2E). Among the general surge in CD68+ macrophages activity, we noticed a remarkable shift in iNOS-positive (M1) over CD206+ (M2), resulting in an increased M1/M2 ratio, at the non-healing sockets of patients with history of zoledronate treatment that were diagnosed with or without BRONJ, as compared to those of periodontitis affected patients that were naïve of zoledronate exposure (P<0.01) (Fig. 2A–D; Fig. 2F and G). Of note, polarization of M1/M2 ratio was more pronounced at the mucosal tissue bordering the extraction sockets of BRONJ relative to non-BRONJ patients (Fig. 2F and G). The abundant activity of M1 macrophages was also confirmed using a quick cell smear screening approach obtained from the socket of BRONJ patients (Supplementary Fig. S1). These results suggest that a combination of enhanced IL-17 and M1/M2 activity may situate the wound environment more of a prolonged inflammatory state, rendering a generalized delay in the healing of the open extraction socket and subsequently, osteonecrosis of the jaw.
Figure 2.
Altered M1 and M2 macrophage infiltrations in oral mucosal tissues bordering the non-healing extraction socket of BRONJ patients. A–D, Immunofluorescence studies showed an increased infiltration of CD68+ iNOS+ M1 macrophages and a decreased infiltration of CD206+ M2 macrophages in mucosal tissues bordering the extraction sockets of patients manifested BRONJ lesions (BRONJ) or patients with history of zoledronate treatment without clinical BRONJ (Non-BRONJ) (n=5), whereby oral mucosal tissues from healthy patients who underwent routine dental extraction for other non-inflammatory mucosa conditions (Normal) or patients with diagnosis of inflammatory gum disease, specifically, active periodontitis with resultant tooth loss (Periodontitis) were used as controls (n=5). Scale bars, 50μm. E, Quantification of total CD68+ macrophages in 6 randomly selected high-power fields (HPFs). F and G, Quantification of M1 and M2 macrophages in 6 randomly selected high-power fields (HPFs). Data are mean ± SEM of multiple fields in n=5 per group. *P < 0.05; **P<0.01; ns, no significant statistical differences.
Systemic IL-17 elevation and a shift to M1 activity render mice vulnerable to osteonecrosis of the jaw bone, not skeletal bone
To determine whether the enhanced Th17 and IL-17 activity is limited to the local mucosal tissue bordering the non-healing extraction socket of BRONJ mice, we tested the effect of elevated serum IL-17 on the development of BRONJ. Here, we performed a series of human serum transfusion in our murine model using samples collected from BRONJ patients undergoing surgical debridement versus non-BRONJ patients with history of BP treatment or healthy controls. We measured serum IL-17 level in BRONJ affected patients, range 100–200pg/mL (182.5±12.4 pg/mL), in non-BRONJ patients, range ~50pg/mL (58.2±9.8 pg/mL), as well as in healthy controls, less than 30pg/mL (25.4±6.3pg/mL) (n=5/group) (Supplementary Fig. S2A). During the course of BP-induction of BRONJ, serum sample from each patient group was intravenously infused into C57BL/6 mice one day after tooth extraction and manifestation of BRONJ-like condition was assessed as previously described (31). Interestingly, we observed development of exposed bone (ONJ) or BRONJ-like condition in mice that underwent systemic infusion of serum from patients who manifested active BRONJ, and these ONJ mice showed relatively high serum levels of murine IL-17 and increased percentage of M1 macrophage or M1/M2 ratio as detected in the long bone marrow (Supplementary Fig. S2B–E). Despite the seemingly correlation between infused human serum IL-17 and ONJ incidence in our murine model, we cannot rule out other factors that potentially contribute to this effect. Further studies are ongoing to address this finding.
Since it is well-established that BRONJ occurred predominantly in the jaw bone, we examined whether there is any difference in IL-17 level in bone marrow derived from jaw versus long bone. Interestingly, we found a remarkably increase in Th17 cells and IL-17 expression at both mRNA and protein levels in jaw bone marrow as compared to long bone marrow of C57BL/6 mice, while Treg cells and IL-10 activities were relatively suppressed (Supplementary Fig. S3A–F); similar trends of increased Th17 and decreased Treg cells were consistently observed in bone marrow compartment of jaw versus long bone in other species, C3H mice (Supplementary Fig. S3G–H), and rats (Supplementary Fig. S3I and J). We next ask whether the differential IL-17 activity affect bone healing at different skeletal sites. Consistent with previous findings, we showed that IL-17 elevation correlated with high incidence of exposed bone or ONJ in zoledronate treated wild type C57BL/6 or Bg ZID (III) nu/nu mice (31) following tooth extraction. However, when subjected nu/nu mice to surgically induced bone cavitation in the appendicular bone, specifically the tibia, there was apparently no difference in bone healing between zoledronate treated and non-treated groups (Supplementary Fig. S4). When evaluating jaw bone healing, residual necrotic alveolar bone was evident with absolutely no signs of bone filling in the extraction socket of the zoledronate treated mice (Supplementary Fig. S4A); whereas, in the long bone cavitation defect, similar callus formation and bone filling was observed in mice that manifested clinical BRONJ in the jaw bone as compared to nontreated control (Supplementary Fig. S4B). Taken together, the inherent increase in Th17 activity unique to the jaw bone, the open tooth extraction wound exposed to the oral microbial environment versus the closed and sterile wound of the long bone cavitation, and specifically its oral mucosa immunology may explain the limited manifestation of BRONJ at this bone type.
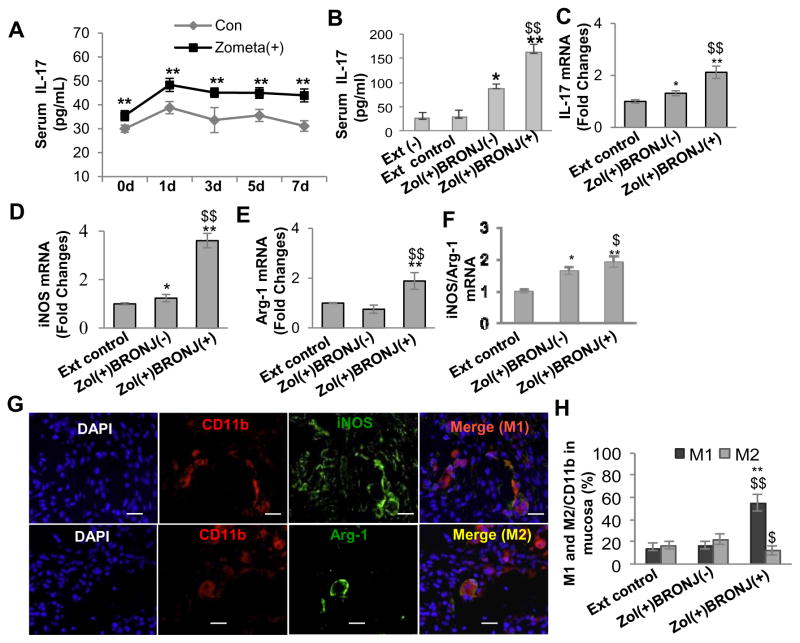
We further ask whether zoledronate directly affect systemic IL-17 level using our established model of BRONJ-like disease that showed a high correlation between IL-17 level and incidence of BRONJ in wild type C57BL/6 mice (31). Consistent with previous findings, zoledronate treatment augmented serum level of IL-17 (Fig. 3A) over the entire course of treatment; more importantly, mice that manifested BRONJ-like condition displayed a much higher level of serum IL-17 than those without BRONJ (P<0.01) (Fig. 3B). At the local mucosa of the non-healing socket, elevated expression of IL-17 mRNA was detected as confirmed by real-time PCR (Fig. 3C). These results further support the notion that an elevated IL-17 expression at both systemic and local levels contributes to the development of BRONJ-like condition.
Figure 3.
An elevated IL-17 level was correlated with altered M1 and M2 macrophage phenotypes in mucosal tissues bordering extraction socket of C57BL/6 mice with BRONJ-like lesions. Mice were intravenously given one dose of zoledronate (Zol) (125μg/kg) one week before tooth extraction followed by intravenous injection of Zol twice a week for two weeks. A, Dynamic changes in serum IL-17 levels of C57BL/6 mice within one week after tooth extraction. Data represent mean ± SEM (n=5 per group). **P<0.01. B, An elevated serum IL17 level was correlated with BRONJ-like lesion development. Ext (−), normal mice without tooth extraction; Ext control, mice with tooth extraction but without Zol treatment; Zol(+)BRONJ(−), mice with tooth extraction and Zol treatment but without BRONJ-like lesion development; Zol(+)BRONJ(+), mice with tooth extraction and Zol treatment developed BRONJ-like lesions. Data represent mean ± SEM (n=5 per group). *P<0.05, **P<0.01 as compared with Ext control. $$P < 0.01 as compared with Zol (+) BRONJ (−). C–F, Real-time PCR analysis showed increased expression of IL-17, iNOS mRNA but decreased expression of arginase-1 mRNA in soft socket tissues of Zol(+)BRONJ(+) mice as compared with those of Zol(+)BRONJ(−) mice. Data represent mean ± SEM (n=5 per group). *P<0.05, **P<0.01 as compared with Ext (+) control. $P < 0.05, $$P < 0.01 as compared with Zol (+) BRONJ (−). G and H, Immunofluorescence studies showed an increased number of CD11b+ iNOS+ M1 macrophages and a decreased number of CD11b+ Arginase-1+ (Arg-1) M2 macrophages (an increased M1/M2 ratio) in mucosal tissues bordering socket tissues of Zol(+)BRONJ(+) mice as compared with those of Zol(+)BRONJ(−) mice. Scale bars, 100 μm. Data represent mean ± SEM quantification of M1/M2 macrophages (multiple images, n=3 per group). **P<0.01 as compared with Ext control. $P < 0.05, $$P < 0.01 as compared with Zol (+) BRONJ (−).
It has been suggested that the impaired wound healing characteristic of BRONJ may be attributed to macrophage dysfunction (17); however, up to date, evidence remains lacking. Here, using our established BRONJ-like murine model we explore the potential involvement of macrophages in IL-17-mediated inflammation as a mechanism of impaired healing in osteonecrosis of the jaw bone. To this purpose, we initially analyzed mRNA expression of iNOS and arginase-1, a marker for M1 and M2 macrophages, respectively, in mucosal tissues bordering extraction socket of mice following treatment with zoledronate. As shown in Fig. 3D–F, an overall elevation of both iNOS and arginase-1 mRNA expression was detected, with a more remarkable increase in iNOS than arginase-1 (P<0.05), suggesting a dominance of M1 macrophages in the non-healing extraction socket of BRONJ. To further confirm this, we examined the local and systemic distribution of M1 and M2 macrophages in C57BL/6 mice after zoledronate treatment. Using dual-color immunofluorescence staining of mucosal tissue sections obtained from ONJ sockets we showed a significantly increased percentage of CD11b+ cells expressing iNOS (M1 macrophages) and a decreased percentage of CD11b+ cells expressing arginase-1 (M2 macrophages) (P<0.01) (Fig. 3G and H). To determine whether zoledronate has direct effect on macrophage phenotype switching and function, we exposed bone marrow-derived macrophages (BMDMs) from BRONJ and non-BRONJ mice under M1 and M2 macrophage induction conditions, respectively. Our results indicated that a higher percentage of M1 macrophages and a lower percentage of M2 macrophages were induced from BMDMs of zoledronate-treated mice that manifested BRONJ-like conditions, as compared to those of non-BRONJ mice (P<0.05), or non-treated mice (P<0.01) (Supplementary Fig. S5A–D). Taken together, these findings support for the first time, an immune underlying mechanism, linking the elevated IL-17 activity and a shift in M1/M2 macrophage ratio to the development of BRONJ-like condition in the murine model.
Blocking IL-17 activity negates M1/M2 macrophage polarization and the incidence of BRONJ-like condition in mice burdened with multiple myeloma (MM)
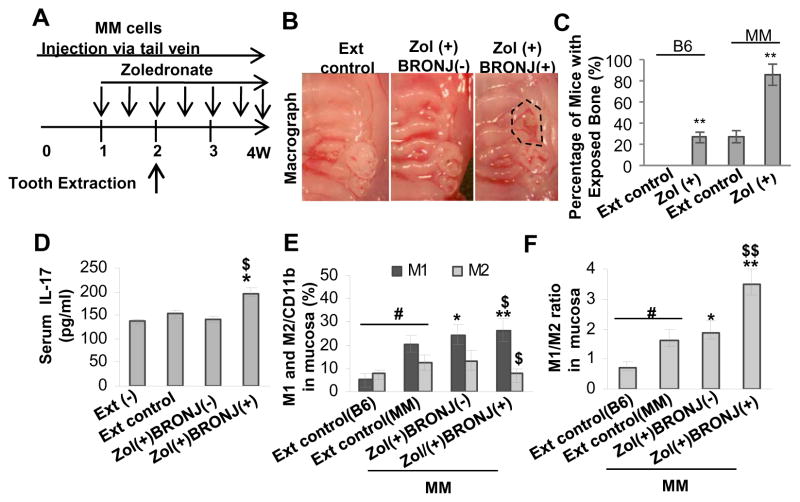
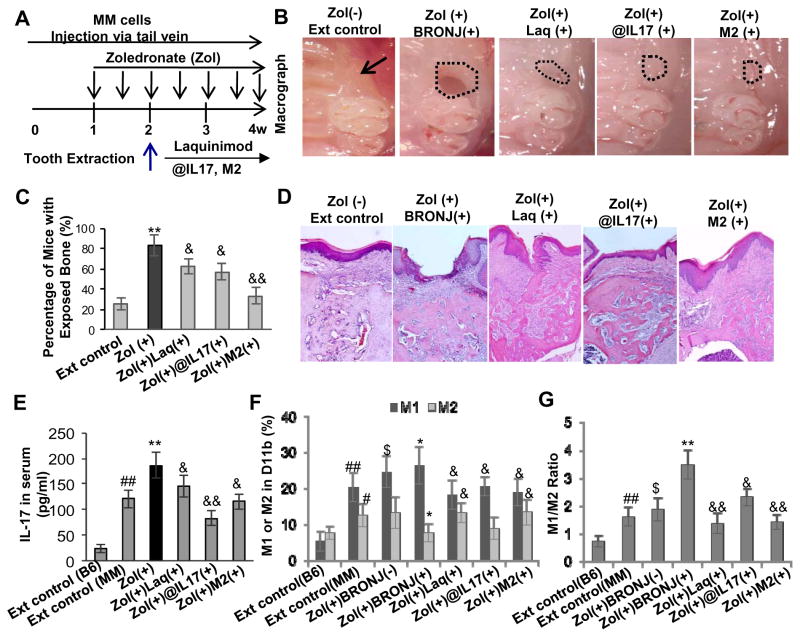
Recent studies have reported a significantly elevated baseline and induced frequency of Th17 cells in peripheral blood mononuclear cells (PBMCs) and an increased serum IL-17 levels in multiple myeloma (MM) patients (36), implying a critical role of Th17 cells in the genesis of MM bone lytic diseases (37). Additionally, it has been well-documented that the highest prevalence of BRONJ occurs in MM patients receiving zoledronate treatment (4, 5). To test the potential contribution of IL-17 in the pathogenesis of BRONJ in MM, we induced BRONJ-like condition in a well-established MM murine model (Fig. 4A–C) adapting the protocol used in wild type C57BL/6 mice as previously published (31). Manifestation of BRONJ-like phenotype followed tooth extraction was confirmed by clinical examination (Fig. 4B). Overall, MM mice displayed a significantly elevated basal level of serum IL-17 as compared to wild type C57BL/6 mice (Fig. 4D vs Fig. 3B), and zoledronate treatment further augmented serum IL-17 levels above the baseline (P<0.05). Consistent with the incidence of BRONJ in zoledronate induced wild type C57BL/6 (31), in MM mice we observed a baseline disease of 20% (Fig. 4C), which correlated with similar serum IL-17 level in both disease models (Fig. 3B; Fig. 4D). Intravenous administration of zoledronate into MM mice caused a remarkably higher incidence of BRONJ (~80%) as compared to wild type C57BL/6 mice (Fig. 4B and C). The 2–3 fold increase in ONJ incidence in drug-induced MM mice corresponded to the augmented serum IL-17 level (Fig. 4D); furthermore, consistent with previous findings in wild type mice, we observed an increased M1 and a decreased M2 macrophage activity, specifically a significant shift in M1/M2 ratio, in mucosa of MM mice sustained BRONJ-like condition (P<0.05) (Fig. 4E and F).
Figure 4.
An elevated IL-17 level was correlated with altered M1 and M2 macrophage phenotypes in mucosal tissues bordering extraction sockets of multiple myeloma (MM)-burdened mice with BRONJ-like lesions. A, Treatment regimen. Mice were intravenously given one dose of zoledronate (Zol) (125μg/kg) one week before tooth extraction followed by intravenous injection of Zol twice a week for two weeks. B and C, Induction of BRONJ-like lesions in MM mice. Data represent mean ± SD (n=10 per group). **P<0.01 as compared with Ext control (C). D, An elevated serum IL-17 level in Zol(+) BRONJ(+) mice as compared with that of Zol(+)BRONJ(−) mice. Data represent mean ± SEM (n=5 per group). *P<0.05 as compared with Ext (+) control; $P < 0.05 as compared with Zol (+) BRONJ (−). E and F, An increased number of CD11b+ iNOS+ M1 macrophages and a decreased number of CD11b+ Arginase-1+ M2 macrophages in mucosal tissues bordering extract sockets of Zol(+) BRONJ(+) MM mice as compared with that of Zol(+) BRONJ(−) MM mice as determined by immunofluorescence studies. Data represent mean ± SEM quantification of M1/M2 macrophages in mucosal tissues of BRONJ-like lesions of mice (multiple images n=3 per group). #P<0.01 as compared with C57BL/6 mice (B6); *P<0.05, **P<0.01 as compared with Ext control; $P < 0.05, $P < 0.01 as compared with Zol (+) BRONJ (−). Ext (−), normal mice without tooth extraction; Ext control, mice with tooth extraction but without Zol treatment; Zol(+)BRONJ(−), mice with tooth extraction and Zol treatment but without manifestation of BRONJ-like lesions; Zol(+)BRONJ(+), mice with tooth extraction and Zol treatment developed BRONJ-like lesions.
To further explore the critical role of IL-17 and altered M1/M2 macrophage balance in the development of BRONJ-like condition, we tested several therapeutic approaches modifying macrophage function or targeting IL-17 using: 1) adoptive transfer of ex vivo expanded M1 and M2 macrophages; 2) treatment with a popular small molecule inhibitor of IL-17 activity (Laquinimod); and 3) treatment with a specific IL-17 neutralizing antibody (Fig. 5A). We showed that adoptive transfer of M1 macrophages had no obvious effect on serum IL-17 levels and BRONJ incidence (data not shown); on the contrary, infusion of M2 macrophages significantly suppressed the incidence of BRONJ in MM mice (Fig. 5B–D) and serum levels of IL-17 (Fig. 5E). In addition, we demonstrated that oral administration of Laquinimod reduced the incidence of BRONJ (Fig. 5B–D), and simultaneously led to a decrease in serum IL-17 levels (Fig. 5E) and M1/M2 macrophage ratio in long bone marrow of MM mice (Fig. 5F and G). Convincingly, administration of IL-17 neutralizing antibody led to a remarkable decrease in M1/M2 ratio in long bone marrow (Fig. 5F and G), and concomitantly, a significant reduction in the incidence of BRONJ in MM mice (Fig. 5B–D). Taken together, these results have provided substantial evidence that an elevated IL-17 level and a shift in M1/M2 macrophage polarization contribute to the delayed healing in zoledronate induced osteonecrosis of the extracted socket in murine jaw bone.
Figure 5.
Systemic infusion of M2 macrophages and blocking IL-17 activity suppress BRONJ-like lesion formation in MM mice. A, Treatment regimen. Mice were intravenously given one dose of zoledronate (Zol) (125μg/kg) one week before tooth extraction followed by intravenous injection of Zol twice a week for two weeks. One day after tooth extraction, mice were adoptively transferred with ex vivo expanded M2 macrophages (1x106) or administered with laquinimod (Laq; 5mg/kg) or IL-17 neutralizing antibody (@IL-17, 0.3mg/mice). B–D, Amelioration of BRONJ-like lesion formation by adoptive transfer of ex vivo expanded M2 macrophages, daily oral administration of Laq or intraperitoneal (i.p.) administration of murine neutralizing @IL-17 one day after tooth extraction. Data represent mean ± SEM (n=10 per group). **P<0.01 as compared with Ext control; &P < 0.05 and &&P<0.01 as compared with Zol (+). E, A reduction in serum IL-17 level after adoptive transfer of ex vivo expanded M2 macrophages, daily oral administration of Laq or intraperitoneal (i.p.) administration of murine neutralizing @IL-17 one day after tooth extraction. Data represent mean ± SEM (n=5 per group). ##P<0.01 as compared with C57BL/6 mice (B6); **P<0.01 as compared with Ext control; &P < 0.05 and &&P<0.01 as compared with Zol (+). F and G, A decrease in M1/M2 ratio in mucosal tissues bordering extraction sockets of mice after adoptive transfer of ex vivo expanded M2 macrophages, daily oral administration of Laq or intraperitoneal (i. p.) administration of murine neutralizing @IL-17 one day after tooth extraction. Data represent mean ± SEM of quantification of M1/M2 macrophages in BRONJ-like lesions of mice (multiple images, n=3 per group). #P<0.05 and ##P<0.01 as compared with C57BL/6 mice (B6); $P<0.05 as compared with Ext control; *P<0.05 and **P<0.01 as compared with Zol (+) BRONJ (−); &P < 0.05 and &&P<0.01 as compared with Zol(+)BRONJ(+).
Potential mechanisms underlying IL-17-mediated alterations in the phenotype and function of M1/M2 macrophages
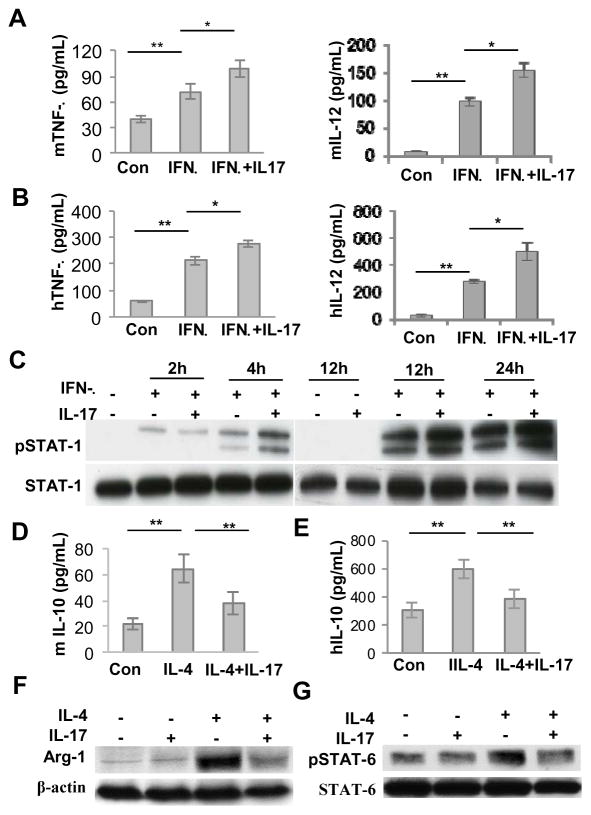
We next explored the effects of IL-17 on polarization of M1/M2 macrophages in vitro and their potential mechanisms. To this end, BMDMs from C57BL/6 mice or human macrophages (THP-1) were cultured under M2 macrophage induction condition in the presence or absence of IL-17 for 48 hours. Exposure to exogenous IL-17 enhanced murine and human M1 macrophage polarization as represented by an increased secretion of pro-inflammatory cytokines TNF-α and IL-12 (P<0.05) (Fig. 6A and B). Since INF-γ-mediated STAT-1 signaling pathway plays a central role in polarization of M1 macrophages (38), we then examined whether exogenous IL-17 has any effect on INF-γ-mediated activation of STAT-1. We showed that IFNγ induced phosphorylation of STAT-1 (Tyr701) at 2h and 4h time intervals, and even a more pronounced induction of p-STAT1 was noticed at 12h and 24h after IFNγ stimulation (Fig. 6C). Semi-quantitative analysis showed that addition of IL-17 enhanced IFNγ-induced phosphorylation of STAT-1 by about 2-fold at 4h, and by about 1.5-fold at 12 and 24h, respectively (Supplementary Fig. S6). These results suggest that IL-17 promotes M1 macrophage polarization possibly by enhancing INF-γ-mediated activation of STAT-1 signaling pathway.
Figure 6.
Mechanisms underlying IL-17-mediated alterations of M1 and M2 macrophage phenotypes. Mice bone marrow-derived monocytes were cultured in the presence of M-CSF (10ng/mL) for 6 days to induce differentiation of mice bone marrow-derived macrophages (BMDMs), while human leukemic monocytes (THP-1) were differentiated into macrophages in the presence of PMA (200 nmol/L) for 24 hours. A and B, Mice BMDMs or human macrophages were continuously cultured under M1 macrophage polarization condition (IFN-γ, 20ng/mL) in the presence or absence of murine or human IL-17 (10ng/mL) for another 24 hours. The production of murine or human TNF-α in the supernatants was determined by ELISA. C, Mice BMDMs were continuously cultured under M1 macrophage polarization condition (IFN-γ, 20ng/mL) in the presence or absence of murine IL-17 (10ng/mL) for different time periods, and the phosphorylated levels of STAT-1 were determined by Western blot. D and E, Mice BMDMs or human macrophages were continuously cultured under M2 macrophage polarization condition (IL-4 10ng/mL) in the presence or absence of murine or human IL-17 (10ng/mL) for another 24 hours, and the production of murine or human IL-10 in the supernatants were determined by ELISA. F, Mice BMDMs were continuously cultured under M2 macrophage polarization condition (IL-4 10ng/mL) in the presence or absence of murine IL-17 (10ng/mL) for another 24 hours, and the expression of arginase-1 (Arg-1) was determined by Western blot analysis. G, Mice BMDMs were continuously cultured under M2 macrophage polarization condition (IL-4 10ng/mL) in the presence or absence of murine IL-17 (10ng/mL) for another 2 hours, and the phosphorylated levels of STAT-6 were determined by Western blot analysis. Data represent mean ± SEM of three independent experiments. *P < 0.05; **P<0.01.
We then examined the effects of exogenous IL-17 on M2 macrophage polarization and its underlying mechanism. Using a series of studies with BMDMs from C57BL/6 mice or human macrophages (THP-1), we applied M2 macrophage polarization condition in the absence or presence of IL-17. Our results showed that pretreatment with IL-17 dramatically inhibited murine and human M2 macrophage polarization as represented by a reduced secretion of the anti-inflammatory cytokine IL-10 (Fig. 6D and E) and a decreased expression of arginase-1in mice BMDMs (Fig. 6F), a specific marker for M2 macrophages. We next examined the effects of exogenous IL-17 on IL-4-mediated STAT-6 signaling pathway in the polarization of M2 macrophages. The data indicated that pretreatment with IL-17 significantly suppressed the phosphorylation of STAT-6 in mice BMDMs stimulated by IL-4 (Fig. 6G), suggesting that IL-17 inhibited M2 macrophage polarization by directly suppressing IL-4-mediated activation of STAT-6 signaling pathway. Further in-depth studies are underway to delineate the underlying mechanism of IL-17-mediated activation of STAT-1/STAT-6 balance and how they contribute to M1 and M2 switching in delayed healing in drug-induced osteonecrosis of the jaw bone.
Discussion
Over the last decade, osteonecrosis of the jaw (ONJ) has emerged as a devastating and debilitating condition of oncological patients receiving treatment with high doses of anti-resorptive agents (bisphosphonates and denosumab) (39). Epidemiological data from the latest cohort studies indicate that 88% of the 2408 ONJ cases were associated with intravenous therapy primarily with zoledronate alone, or zoledronate sequentially with pamidronate, while only 261 cases (11%) had ever received oral BP treatment. Among these ONJ cases, the majority of them (89%) were oncological patients, with multiple myeloma accounting for 43%, breast cancer for 32%, prostate cancer for 9% and other cancers for 5% (40). To date, several thousands of ONJ cases have been reported in the literature; however, the pathophysiology or etiology of this condition remains largely unknown. Even though several potential mechanisms have been implicated in the pathophysiology of bisphosphonate-related osteonecrosis of jaw (BRONJ), including over-suppressed alveolar bone turnover, toxicity to oral mucosal tissues, altered angiogenesis and deregulated immune functions that are directly or indirectly caused by BP therapy (17, 39), none of them can delineate the full spectrum of the pathophysiology of BRONJ. Conceivably, both epidemiological/retrospective and clinical prospective studies have identified several major risk factors for BRONJ including the type, the route and the cumulative dose of BPs, preexisting infection, oral trauma/injury such as dentures and tooth extraction, the presence of metastases and obesity, and other medical morbidity conditions (17, 39). In spite of these factors identified so far that are related to BRONJ prevalence, no direct causal effects have been established due to the lack of evidence-based studies and a well-accepted BRONJ-like animal model that can recapitulate the major pathophysiological characteristics of BRONJ in patients.
Most recently, we have established a BRONJ-like model in mice by intravenous administration of zoledronate in conjunction with the addition of immunosuppressive agent dexamethasone preceding tooth extraction, which displayed similar hallmarks of human diseases including the persistent presence of BRONJ-like clinical, radiographic and histologic features at the extraction site (31). More importantly, we showed that the BRONJ-like condition was closely associated with an altered immune homeostasis, specifically a deficiency in Tregs and a significant increase in Th17 cells and IL-17 level in peripheral blood, which might contribute to the prolonged inflammation at the local extraction sites (31). In the present study, an elevated IL-17 expression was also observed in the serum and local mucosal tissues bordering the non-healing extraction socket of BRONJ patients (Fig.1; Supplementary Fig. 2A). We further showed that an elevated serum level of IL-17 was tightly correlated to an increased incidence of BRONJ-like conditions in wild type C57BL/6 mice (Fig. 3) and MM mice (Fig. 4). Moreover, blocking IL-17 activity using specific neutralizing antibody significantly reduced the incidence rate of BRONJ in MM mice (Fig. 5), thus further confirmed the essential role of IL-17 in the pathogenesis of this condition. These findings further support the notion that an elevated IL-17 level may serve as a major risk factor in patients sustaining BRONJ. Consistent with our findings in both murine model and human BRONJ disease, a recent study reported that MM patients experienced an elevated serum level of IL-17 and other related pro-inflammatory cytokines, including IL-21, IL-22 and IL-23 (36). However, an earlier short letter described decreased serum IL-17 levels in MM patients following administration of bisphosphonates and those presented with ONJ as compared to non-treated MM or MM patients without ONJ (41). This seemingly controversial observation needs to be addressed in light of the well-recognized immune-stimulatory effect of the aminophosphates and related compounds (42, 43). Clinical studies by our group using increased sample size for ONJ and non-ONJ patients in oncologic patients with MM or other type of cancers are in progress to further confirm the changes in IL-17 levels both locally and systemically, and to determine whether these levels correlate with ONJ phenotypes.
As a potent pro-inflammatory cytokine, IL-17 is principally produced by a subset of CD4+ T helper (Th17) cells, but recent evidence has shown that it can also be produced by innate immune cells, including γδ T cells, macrophages and mast cells under certain pathophysiological settings (21–25). Accumulating evidence has shown that IL-17-mediated inflammation is crucial to the pathogenesis of several inflammatory and autoimmune diseases including rheumatoid arthritis (RA), multiple sclerosis (MS), systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD) and psoriasis (20). Most recently, several lines of evidence have demonstrated the important role of IL-17-mediated inflammation in bone loss or destruction-related diseases (37, 44–46). IL-17 drives irreversible bone loss or destruction by directly promoting osteoclastogenesis (37, 47) and/or by interplaying with other pro-inflammatory cytokines like TNF-α to enhance the expression of several important inflammatory mediators (48). In addition, the interplay between IL-17 and macrophages may exaggerate IL-17-mediated inflammatory responses. On one aspect, classically activated or M1 macrophages can produce cytokines such as IL-6 and IL-23 that are essential for Th17 differentiation (11, 20); on the other aspect, IL-17 can promote the recruitment and pro-inflammatory cytokine/chemokine production of macrophages (26–30), suggesting a potential role of IL-17 in the induction of M1 macrophages. Under certain conditions, secretion of IL-17 by macrophages in response to pro-inflammatory or allergic stimuli can play a major role in the inflammatory process (22, 23). In this study, we have provided the first line of evidence that an elevated IL-17 expression was closely correlated with an increased M1/M2 macrophage ratio at the local mucosal tissue of non-healing extraction socket of BRONJ patients, whereby the elevated IL-17 level at the local sites was shown to be attributed to both Th17 cells and macrophages (Fig. 1; Fig. 2). Using mice BRONJ-like disease model, we have shown that an elevated IL-17 production in mice with BRONJ is closely associated with a significant increase in the percentage of M1 macrophages and a decrease in M2 macrophages, which is represented as an increased M1/M2 ratio (Fig. 3; Supplementary Fig. S5); moreover, blocking of IL-17 activity by using a neutralizing antibody or small immunosuppressive molecule such as Laquinimod reversed the alteration in M1/M2 macrophages ratio and concomitantly reduced the incidence of BRONJ in mice (Fig. 5). Convincingly, adoptive transfer of M2 macrophages led to a remarkable decrease in serum levels of IL-17 and a corresponding reduction in the incidence of BRONJ in MM mice (Fig. 5). In vitro studies further showed that exogenous IL-17 promoted M1 macrophage polarization via augmenting IFN-γ-mediated activation of STAT-1 signaling pathway, and inhibited M2 macrophage polarization by interfering IL-4-mediated activation of STAT-6 signaling pathway (Fig. 6). These findings have provided compelling evidence that IL-17-mediated alterations in M1/M2 macrophages contribute a crucial role in the pathophysiology of BRONJ-like disease.
Zoledronic acid and other BPs are capable of suppressing bone loss via inhibition of osteoclast activity (1–3). Several evidences describe the close interplay between Th17 cells and M1 macrophages (11, 20; 26–30); it remains largely unknown whether these interactions participate in zoledronate mediated IL-17 increase. Recent studies have demonstrated that suppressor of cytokine signaling-3 (SOCS3) can repress the proinflammatory M1 macrophage phenotype, whereas SOCS3 deficiency promotes M1 macrophage polarization and inflammation via an increased production of proinflammatory cytokines IL-1β, IL-6, IL-12 and IL23, rendering a microenvironment conditioned for the differentiation of Th1 and Th17 cells (49, 50). Interestingly, zoledronic acid has been shown to induce M2-like tumor associated macrophages (TAMs) toward an M1 phenotype (51) and enhances proinflammatory cytokine production through inhibition of SOCS3 expression in macrophages (19). These findings could explain the mechanisms whereby zoledronic acid drives Th17 differentiation, at least in part, through promoting M1-macrophage polarization and activation (18). Based on these studies and our current findings, we postulate a positive feedback loop between zoledronate-induced IL-17 elevation and M1/ M2 macrophages switch, regulated in part via STAT-1/STAT-6 activation, as an underlying mechanism in BRONJ pathogenesis (Supplementary Fig. S7). However, it is noteworthy that microbial flora and its biofilm, a common feature of ONJ (52), can also affect differentiation and function of both macrophages and Th17 cells. Further in-depth studies are required to delineate how zoledronic acid and the oral microbial flora can dependently and/or independently modulate the complex network among Th17, M1/M2 macrophages and other types of immune cells in ONJ. In addition to zoledronate, recent clinical trials have reported that patients treated with denosumab, a monoclonal antibody against human receptor activator of NFκB ligand (RANKL) popularly prescribed as an anti-resorptive drug in osteoporosis and certain cancer patients, experienced similar incidence of ONJ (39, 53). The fundamental question whether denosumab induced ONJ shares similar pathways as that of BP-related ONJ, particularly their effects on the interplays between Th17 cells and M1/M2 macrophages, remains to be determined.
A growing body of evidence has shown that activation M1 macrophage within tumor microenvironment can boost inflammatory reaction as a mechanism for immune surveillance of cancer cells. This same mechanism is contributory to the prolonged inflammatory state associated with delayed wound healing, as observed in the refractory soft tissue collected from BRONJ site in human studies (Fig. 2). The tumor associated macrophages (TAMs) with similar characteristics of M2 macrophages can suppress immune response and promote angiogenesis and metastasis of tumor cells, thus facilitating cancer progression, or attenuating inflammation and enhancing wound healing (54). Recently, both in vitro and in vivo studies have shown that tumor macrophages are potential targets of BPs (18, 19, 51), possibly via the manipulation of macrophage phenotypes in tumor microenvironment, and by similar mechanisms, sustain prolonged inflammation leading to an increased susceptibility to BRONJ. Epidemiologic study has identified several risk factors associated with ONJ; in cancer patients receiving high-dose intravenous BPs for the prevention and treatment of skeletal-related complications, treatment with other anti-tumor drugs including glucocorticoid (dexamethasone), chemotherapeutic or/and anti-angiogenic drugs incur additional risks; as such, it should be further explored whether BPs and these anti-tumor drugs have any cooperative effects on the immune system, specifically on the interplay between Th17 cells and M1/M2 macrophages, as these immune cells potentially function as a “double-edge” sword within the tumor microenvironment to enhance both anti-tumor immunity and inflammatory responses (18, 39). Such mechanisms inadvertently result in prolonged inflammation, and thus delayed healing or bone necrosis manifested as BRONJ.
In summary, we have demonstrated for the first time to our knowledge that a combination of elevated IL-17 activity and a shift in M1/M2 macrophages ratio, contributes to the pathophysiology of BRONJ-like condition in both human and murine models. Blocking IL-17/M1 macrophage axis using small molecules or neutralizing antibodies can offer novel therapeutic modality for preventing and treating BRONJ condition in the vulnerable cancer patients.
Supplementary Material
Translational Relevance.
Osteonecrosis of the jaw (ONJ) is emerging as one of the important complications in cancer patients with metastatic bone disease treated with antiresorptive agents. This work reports that increased Th17 cells and IL-17 cytokine levels correlate with an increase in M1/M2 macrophages ratio at the local mucosal site of both BRONJ patients and zoledronate-induced ONJ-like lesions in mice; adoptive transfer of ex vivo expanded M2 macrophages could reverse systemic increase of IL-17 and ONJ severity and blocking IL-17 activity significantly decreased M1/M2 ratio and concomitantly suppressed BRONJ-like condition in mice. IL-17-mediated inflammation related to M1/M2 macrophage alterations induced by zoledronate may be beneficial for cancer therapy, but also contributes to an increased susceptibility to BRONJ. These results suggest that it would be critical to establish optimal approaches for zoledronate administration that can balance its dual effects on cancer therapy and BRONJ development.
Acknowledgments
Grant Support
This work was supported by National Institute of Health Research Grant, R01DE 019932, Oral and Maxillofacial Surgery Foundation (OMSF) Research Grant, USC Institutional funding (CTSI and Zumberge awards), and the Schoenleber funding support.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: Q-Z. Zhang, S-T. Shi, A. Le; Development of methodology: Q-Z. Zhang, I. Atsuta, S-Y. Liu, C. Chen, S-H. Shi; Acquisition of data: Q-Z. Zhang, I. Atsuta, S-Y. Liu, C. Chen, S-H. Shi; Analysis and interpretation of data: Q-Z. Zhang, I. Atsuta, S-Y. Liu, C. Chen; Writing, review, and/or revision of the manuscript: Q-Z. Zhang, S-T. Shi, A. Le; Administrative, technical, or material support (i. e., reporting or organizing data, constructing data base): Q-Z. Zhang, I. Atsuta, S-Y. Liu, C. Chen; Study supervision: S-T. Shi, A. Le.
References
- 1.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–7. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 2.Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65:369–77. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Ruggiero SL, Drew SJ. Osteonecrosis of the jaws and bisphosphonate therapy. J Dent Res. 2007;86:1013–21. doi: 10.1177/154405910708601101. [DOI] [PubMed] [Google Scholar]
- 4.Silverman SL, Landesberg R. Osteonecrosis of the jaw and the role of bisphosphonates: a critical review. Am J Med. 2009;122(2 suppl):S33–S45. doi: 10.1016/j.amjmed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Bagan J, Scully C, Sabater V, Jimenez Y. Osteonecrosis of the jaws in patients treated with intravenous bisphosphonates (BRONJ): A concise update. Oral Oncol. 2009;45:551–4. doi: 10.1016/j.oraloncology.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–45. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cetiner S, Sucak GT, Kahraman SA, Aki SZ, Kocakahyaoglu B, Gultekin SE, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with zoledronic acid. J Bone Miner Metab. 2009;27:435–43. doi: 10.1007/s00774-009-0047-9. [DOI] [PubMed] [Google Scholar]
- 8.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 9.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–9. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 10.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175:2454–62. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 12.Aki K, Shimizu A, Masuda Y, Kuwahara N, Arai T, Ishikawa A, et al. ANG II receptor blockade enhances anti-inflammatory macrophages in anti-glomerular basement membrane glomerulonephritis. Am J Physiol Renal Physiol. 2010;298:F870–F882. doi: 10.1152/ajprenal.00374.2009. [DOI] [PubMed] [Google Scholar]
- 13.Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, Beck PL, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–9. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 15.Menzies FM, Henriquez FL, Alexander J, Roberts CW. Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clin Exp Immunol. 2010;160:369–79. doi: 10.1111/j.1365-2249.2009.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pazianas M. Osteonecrosis of the jaw and the role of macrophages. J Natl Cancer Inst. 2011;103:232–40. doi: 10.1093/jnci/djq516. [DOI] [PubMed] [Google Scholar]
- 18.Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheller EL, Hankenson KD, Reuben JS, Krebsbach PH. Zoledronic acid inhibits macrophage SOCS3 expression and enhances cytokine production. J Cell Biochem. 2011;112:3364–72. doi: 10.1002/jcb.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 Cells: Biology, Pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 21.Vykhovanets EV, Maclennan GT, Vykhovanets OV, Gupta S. IL-17 Expression by macrophages is associated with proliferative inflammatory atrophy lesions in prostate cancer patients. Int J Clin Exp Pathol. 2011;4:552–65. [PMC free article] [PubMed] [Google Scholar]
- 22.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–24. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 23.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181:4279–86. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336–40. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 25.Korn T, Petermann F. Development and function of interleukin 17-producing γδ T cells. Ann N Y Acad Sci. 2012;1247:34–45. doi: 10.1111/j.1749-6632.2011.06355.x. [DOI] [PubMed] [Google Scholar]
- 26.Barin JG, Baldeviano GC, Talor MV, Wu L, Ong S, Quader F, et al. Macrophages participate in IL-17-mediated inflammation. Eur J Immunol. 2012;42:726–36. doi: 10.1002/eji.201141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang JW, Li JC, Au KY, Yim HC, Lau AS. Interleukin-17A differentially modulates BCG induction of cytokine production in human blood macrophages. J Leukoc Biol. 2011;90:333–41. doi: 10.1189/jlb.0510311. [DOI] [PubMed] [Google Scholar]
- 28.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–21. [PubMed] [Google Scholar]
- 29.von Vietinghoff S, Koltsova EK, Mestas J, Diehl CJ, Witztum JL, Ley K. Mycophenolate mofetil decreases atherosclerotic lesion size by depression of aortic T-lymphocyte and interleukin-17-mediated macrophage accumulation. J Am Coll Cardiol. 2011;57:2194–204. doi: 10.1016/j.jacc.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q, et al. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol. 2012;7:1091–100. doi: 10.1097/JTO.0b013e3182542752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuiri T, Kim I, Yamaza T, Akiyama K, Zhang Q, Li Y, et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res. 2010;25:1668–79. doi: 10.1002/jbmr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wegner C, Stadelmann C, Pförtner R, Raymond E, Feigelson S, Alon R, et al. Laquinimod interferes with migratory capacity of T cells and reduces IL-17 levels, inflammatory demyelination and acute axonal damage in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;227:133–143. doi: 10.1016/j.jneuroim.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Thöne J, Ellrichmann G, Seubert S, Peruga I, Lee DH, Conrad R, et al. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am J Pathol. 2012;180:267–74. doi: 10.1016/j.ajpath.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 34.Comi G, Jeffery D, Kappos L, Montalban X, Boyko A, Rocca MA, et al. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med. 2012;366:1000–9. doi: 10.1056/NEJMoa1104318. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–98. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115:5385–92. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noonan K, Marchionni L, Anderson J, Pardoll D, Roodman GD, Borrello I. A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood. 2010;116:3554–63. doi: 10.1182/blood-2010-05-283895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid IR, Cornish J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat Rev Rheumatol. 2012;8:90–6. doi: 10.1038/nrrheum.2011.181. [DOI] [PubMed] [Google Scholar]
- 40.Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010;136:1117–24. doi: 10.1007/s00432-010-0907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oteri G, Allegra A, Bellomo G, Alonci A, Nastro E, Penna G, et al. Reduced serum levels of Interleukin 17 in patients with osteonecrosis of the jaw and in multiple myeloma subjects after bisphosphonates administration. Cytokine. 2008;43:103–4. doi: 10.1016/j.cyto.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Benzaïd I, Mönkkönen H, Bonnelye E, Mönkkönen J, Clézardin P. In vivo phosphoantigen levels in bisphosphonate-treated human breast tumors trigger Vγ9Vδ2 T-cell antitumor cytotoxicity through ICAM-1 engagement. Clin Cancer Res. 2012;18:6249–59. doi: 10.1158/1078-0432.CCR-12-0918. [DOI] [PubMed] [Google Scholar]
- 43.Nussbaumer O, Gruenbacher G, Gander H, Thurnher M. DC-like cell-dependent activation of human natural killer cells by the bisphosphonate zoledronic acid is regulated by γδ T lymphocytes. Blood. 2011;118:2743–51. doi: 10.1182/blood-2011-01-328526. [DOI] [PubMed] [Google Scholar]
- 44.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–73. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zwerina K, Koenders M, Hueber A, Marijnissen RJ, Baum W, Heiland GR, et al. Anti-IL-17A therapy inhibits bone loss in TNF-α-mediated murine arthritis by modulation of the T-cell balance. Eur J Immunol. 2012;42:413–23. doi: 10.1002/eji.201141871. [DOI] [PubMed] [Google Scholar]
- 46.Won HY, Lee JA, Park ZS, Song JS, Kim HY, Jang SM, et al. Prominent bone loss mediated by RANKL and IL-17 produced by CD4+ T cells in TallyHo/JngJ mice. PLoS One. 2011;6:e18168. doi: 10.1371/journal.pone.0018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pöllinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C, et al. Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011;186:2602–12. doi: 10.4049/jimmunol.1003370. [DOI] [PubMed] [Google Scholar]
- 48.Koenders MI, Marijnissen RJ, Devesa I, Lubberts E, Joosten LA, Roth J, et al. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1β, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum. 2011;63:2329–39. doi: 10.1002/art.30418. [DOI] [PubMed] [Google Scholar]
- 49.Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189:3439–48. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin H, Yeh WI, De Sarno P, Holdbrooks AT, Liu Y, Muldowney MT, et al. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci U S A. 2012;109:5004–9. doi: 10.1073/pnas.1117218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, et al. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010;14:2803–15. doi: 10.1111/j.1582-4934.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sedghizadeh PP, Kumar SK, Gorur A, Schaudinn C, Shuler CF, Costerton JW. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J Am Dent Assoc. 2009;140:1259–65. doi: 10.14219/jada.archive.2009.0049. [DOI] [PubMed] [Google Scholar]
- 53.Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–19. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 54.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–26. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.