Abstract
The emergence and global spread of the 2009 pandemic H1N1 influenza virus reminds us that we are limited in the strategies available to control influenza infection. Vaccines are the best option for the prophylaxis and control of a pandemic; however the lag time between virus identification and vaccine distribution exceeds six months and concerns of vaccine safety are a growing issue leading to vaccination refusal. In the short term, antiviral therapy is vital to control the spread of influenza; however, we are currently limited to four licensed anti-influenza drugs: the neuraminidase (NA) inhibitors oseltamivir and zanamivir, and M2 ion-channel inhibitors amantadine and rimantadine. The value of NA inhibitors was clearly established during the initial phases of the 2009 pandemic when vaccines were not available, i.e. stockpiles of antivirals are valuable. Unfortunately, as drug-resistant variants continue to emerge naturally and through selective pressure applied by use of antiviral drugs, the efficacy of these drugs declines. Because we cannot predict the strain of influenza virus that will cause the next epidemic or pandemic, it is important that we develop novel anti-influenza drugs with broad reactivity against all strains and subtypes and consider moving to multiple drug therapy in the future. Here we review the experimental data on investigational antiviral agents undergoing clinical trials [parenteral zanamivir and peramivir; long acting NA inhibitors (LANI), and polymerase inhibitor T-705], experimental antiviral agents that target either the virus [hemagglutinin (HA) inhibitor Cyanovirin-N and Thiazolides] or the host [fusion protein inhibitor (DAS181), Cox-2 inhibitors and PPAR agonists].
1. Introduction
The influenza virus is a member of the Orthomyxoviridae family, which is divided into three types: A, B and C. Influenza A viruses are responsible for causing seasonal epidemics and caused the 3 pandemics that occurred in the twentieth century (1918, 1957, and 1968) as well as the 2009 pandemic H1N1, the first pandemic of the twenty-first century. Human influenza is predominantly an upper respiratory tract infection that is typically mild and self-limiting; however, in rare cases severe complications and death can occur. The onset of complications can arise as a result of viral characteristics or predisposition of the host. Severe complications attributed to the host response commonly occur in the very young or elderly after infection with seasonal influenza viruses,[1] whereas life threatening illness associated with infection by the 1918 pandemic virus or the highly-pathogenic (HP) H5N1 viruses is often found in healthy, immunocompetent adults.[2–4]
Annual immunization is our best option for prophylaxis and to control an influenza pandemic; however, limitations on vaccine efficacy, design, and delay in strain specific vaccine production have stimulated the search for antiviral drugs. Two classes of antiviral drugs are currently approved for both prophylaxis and therapeutic treatment of influenza virus infection: M2-ion channel inhibitors (amantadine and its derivative rimantadine) and NA inhibitors (zanamivir and oseltamivir). These drug classes differ significantly in their mechanisms of action, pharmacokinetics, tolerance profiles, and resistance patterns.[5–11] Amantadine and rimantadine inhibit viral replication by blocking the ion channel at the stage of virus entry into cells,[12] and prevent virus release by altering the conformation of the hemagglutinin (HA) protein.[13, 14] On the other hand, the NA inhibitors, zanamivir and oseltamivir, approved by the United States Food and Drug Administration (FDA) in 1999 block the activity of the neuraminidase (NA) enzyme and interrupt an established infection at a later stage of virus replication by inhibiting the release of virions from infected cells (Figure 1).[15, 16] NA inhibitors also cause aggregation of the released virions, thus inhibiting viral penetration into mucous secretions and ultimately spread to neighboring cells.[17]
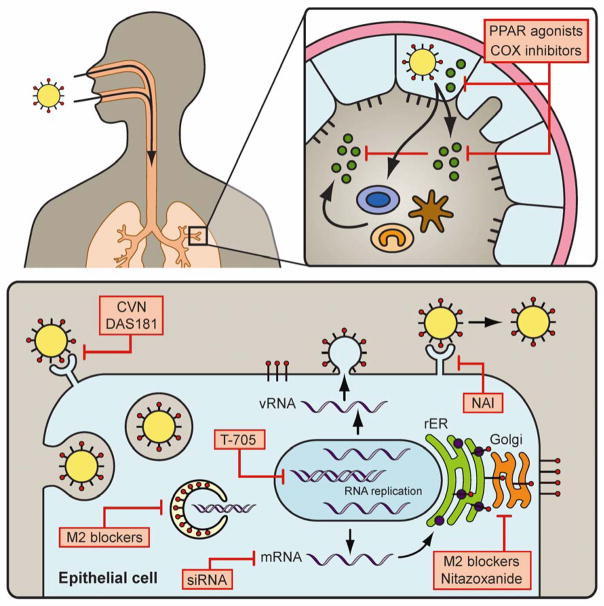
Figure 1. Sites of action of immunnomodulators and antiviral agents for the control influenza infection.
Influenza infects the epithelial cells of the respiratory tract eliciting an immune response characterized by the release of cytokines/chemokines and infiltration of leukocytes. Cox inhibitors and PPAR agonists reduce leukocyte infiltration, presumably by reducing cytokine/chemokine production. Infection is initiated when the virus attaches to sialic acid receptors on the cell surface. This attachment can be inhibited by sialidase inhibitor DAS-181 and hemagglutinin (HA) inhibitor Cyanovirin (CVN). After endocytosis, the pH of the endosome decreases resulting in HA-mediated fusion of the virus and endosomal membranes. Prior to membrane fusion, the M2 ion channel reduces the pH within the virion to allow for release of viral RNA. M2 blockers inhibit the decrease in pH within the virion, thus blocking the release of viral RNA into the cytoplasm. Viral RNA replication occurs in the nucleus and can be blocked by the polymerase inhibitor T-705. Newly made viral mRNA is transported to the cytoplasm where translation of viral proteins occurs. Silencing of specific viral genes can be achieved using small interfering RNAs (siRNA). The maturation of viral proteins such as the viral HA, which occurs in the Golgi, can be disrupted by M2 blockers and Nitazoxanide. Release of mature virions at the cell surface occurs through enzymatic cleavage of sialic acid receptors by the neuraminidase (NA) enzyme. NA inhibitors block NA activity thereby preventing the release of virions from the cell.
A major concern of using antiviral compounds to control influenza is the emergence of drug-resistant variants. The use of amantadine/rimantadine has driven the rapid emergence of viruses that are resistant to M2 blockers yet maintain full transmissibility.[11] The incidence of naturally occurring amantadine-resistant influenza A (H1N1 and H3N2) variants has increased significantly throughout the world. By 2003, these resistant viruses were found to have spread to all countries surveyed, including some where the use of M2 inhibitors was minimal.[18] In contrast, surveillance data from 1999–2007 revealed a low prevalence of resistance to the NA inhibitor oseltamivir (<1%).[19, 20] During 2007–2008, there was an increase in the number of H1N1 viruses carrying the H275Y NA mutation (molecular marker of oseltamivir resistance, N1 numbering) and data from the 2008–2009 influenza season show the prevalence of oseltamivir-resistant viruses exceeds 90%.[21–24] Of the pandemic 2009 H1N1 influenza viruses tested, all were resistant to adamantanes (amantadine and rimantadine).[25, 26] Although still rare, the emergence of the H275Y NA mutation in 2009 H1N1 viruses has been reported.[25, 27–29] Therefore, the US Centers for Disease Control and Prevention (CDC) has discouraged the use of M2 inhibitors and recommends the use of NA inhibitors to treat the 2009 H1N1 pandemic virus.
The high incidence of influenza viruses developing resistance to both M2 and NA inhibitors, the only drugs currently licensed for treatment of influenza virus infection, underscores the need for the development of novel antiviral drugs (Table 1). In this article, we will discuss: 1) antiviral drugs that are at the early stages of clinical trials, 2) new antiviral agents in development that target other virus genes and/or host pathways, and 3) immunomodulators that show potential in limiting the immune-induced pathology associated with HP influenza infections.
Table I.
Influenza therapeutics and interventions
| Agent | Investigational Phasea | Target | References |
|---|---|---|---|
| Peramivir IV | Phase II-III | Influenza neuraminidase | 30–49 |
| Zanamivir IV | Phase II | Influenza neuraminidase | 8,17,28, 50–53 |
| CS-8958 | Phase III | Influenza neuraminidase | 54–60 |
| T-705 | Phase III | Influenza polymerase | 61–64, 67–69 |
| Cyanovirin-N | Experimental | Influenza hemagglutinin | 70–73 |
| DAS181 | Phase I | Host sialic acid residues | 75–80 |
| Nitazoxanide | Experimental | Influenza hemagglutinin | 83 |
| siRNA | Experimental | Host or viral genes | 88–94 |
| Combination therapy | Phase I & II | Influenza neuraminidase and polymerase | 114–117 |
| Cox inhibitors | Experimental | Cox-2 enzyme | 109–111 |
| PPAR agonists | Experimental | Peroxisome proliferator-activated receptor | 114–117 |
Phase of drug evaluation for use against influenza.
2. Investigational antiviral agents for influenza in clinical trails
2.1. Intravenous (IV) peramivir
Peramivir (BCX-1812, RWJ-270201), generated using a structure-based drug design, is an NA inhibitor that is currently being developed. Unlike existing NA inhibitors, peramivir is a cyclopentane derivative with a negatively charged carboxylate group, a positively charged guanidino group, and lipophilic side chains.[30] Peramivir has proven efficacious in reducing replication of influenza A and B viruses in vitro and when used as an oral formulation in experimental animal models.[30–35] Although peramivir has been well tolerated in humans when given at an oral dose of 800 mg/day, findings from a phase III clinical trial demonstrated no statistically significant difference in the time required for resolution of symptoms between treatment and placebo groups.[36] These findings are likely due to the low (<3%) bioavailability of peramivir when administered orally in humans.
To address the limited bioavailability of orally administered peramivir, parenteral formulations are currently being evaluated in preclinical studies and clinical trials for the treatment of seasonal influenza A infection. In human volunteers, parenterally administered peramivir was well tolerated and resulted in high levels of the drug in the blood.[37, 38] Peramivir has a half-life of 22 hours within human plasma and remains bound to the influenza NA for an extended period of time.[39] One benefit of the protracted interaction between peramivir and the influenza NA is that the required frequency of dosing is diminished. Pre-clinical studies in mice showed that intramuscularly (IM) injected peramivir is effective in treating infection with the contemporary H1N1 and H3N2 influenza A viruses,[39] as well as treating both sub-lethal and lethal H5N1 infections.[40, 41] Recently, studies were conducted in Asia to measure the efficacy of intravenous (IV) administered peramivir in humans. In a phase II clinical study, IV administration of peramivir (200 or 400 mg) to treat patients hospitalized within 72 hours of the onset of symptoms proved to be clinically beneficial.[42] In phase III clinical studies a single IV administration of peramivir with 300 or 600 mg of peramivir was found to reduce the time required for alleviation of symptoms and fever.[43] Moreover, treatment with 600 mg of peramivir resulted in a significant reduction in viral titers. In a second phase III study, conducted by Shionogi & Co., Ltd., peramivir administered IV over multiple days at 300 mg or 600 mg per day alleviated symptoms (median time 68.6 hours) in all treated patients.[44]
The overall safety of peramivir and the need for a parenterally administered antiviral drug prompted the FDA to issue the emergency use authorization (EUA) of peramivir IV as treatment for the 2009 H1N1 influenza in patients not responding to the currently approved antiviral therapies.[45] As directed by the EUA, peramivir IV may be administered at 600 mg once daily for 5–10 days in patients infected with the 2009 H1N1 influenza. Although the use of peramivir IV is approved for pandemic 2009 H1N1-infected patients, its success in clinical trials provides support for the potential of parenterally administered antiviral drugs. The decreased susceptibility of oseltamivir resistant viruses that have the H275Y NA mutation to peramivir has prevented its use in patients infected with a virus that is highly suspected or documented to be oseltamivir resistant.[46, 47] In a phase II clinical trial during 2007–2008 season, H1N1 influenza A viruses isolated from 79% of study subjects carried the H275Y NA mutation which may have contributed to the unfavorable results of the study.[48] Clinical resistance to peramivir IV treatment was recently described in an immunocompromised patient infected with 2009 H1N1 influenza virus carrying the H275Y NA mutation.[49]
2.2. Intravenous (IV) zanamivir
Intravenous (IV) zanamivir is being evaluated as a potential therapy for severe influenza infections. Zanamivir is a potent inhibitor of NAs of influenza A and B viruses, including the oseltamivir-resistant viruses possessing the H275Y mutation.[8, 17] The high incidence of the H275Y mutation makes zanamivir an excellent alternative for treatment of infection with oseltamivir-resistant viruses. The combined prophylactic and therapeutic treatment with IV zanamivir (10 mg/kg) has been shown to reduce viral load and lung pathology in macaques infected with HP H5N1 influenza virus.[50] In a clinical study wherein participants received IV zanamivir (600 mg) twice daily beginning 4 hours prior to inoculation, the drug was well tolerated, decreased viral shedding, and alleviated clinical symptoms.[51] IV zanamivir is available for compassionate use from GlaxoSmithKline via an emergency Investigational New Drug (IND) application to the FDA. There have been 2 reports of the emergency use of IV zanamivir to treat pandemic 2009 H1N1 influenza virus. In the first case, an immunocompromised child infected with oseltamivir-resistant 2009 H1N1 influenza was successfully treated with IV zanamivir. The child failed to respond to oseltamivir treatment and the presence of H275Y NA mutation was confirmed by PCR.[28] The patient received 600 mg of zanamivir IV every 12 hours for 15 days, and there was an observed decrease in virus load during therapy. In the second case, a woman suffering from neutropenia following chemotherapy did not respond to oseltamivir treatment and required mechanical ventilation.[52] IV zanamivir (600 mg) twice daily was initiated and her condition improved within 48 hours. Oseltamivir resistance was not confirmed but was assumed because of the failure of oseltamivir treatment to alleviate symptoms. These 2 cases provide promise for the use of IV zanamivir. A phase II clinical trial is planned to evaluate the safety and tolerability of IV zanamivir (600 mg) twice daily for 5 days in hospitalized patients with laboratory confirmed influenza infection.[53]
2.3. Long-acting NA inhibitors (LANI)
For optimal efficacy, the NA inhibitors described thus far must be administered twice daily. Because of this, the amount of drug needed for pandemic stockpiling is tremendous, thus the development of high-potency drugs requiring less frequent administration is desired. To this end, Biota Holdings of Australia and Sankyo Pharmaceuticals of Japan are working in conjunction to develop a multimeric compound of zanamivir, CS-8958 (also known as R-118958) that maintains longer acting NA inhibition to allow for once weekly administration. These multimeric compounds have proven more potent than zanamivir and have provided the desired long acting NA inhibition when tested in an influenza-infected murine model.[54, 55] The active metabolite R-125489 of the esterified prodrug CS-8958 (R-118958) has broad reactivity and has proven effective in inhibiting the NA activities of influenza A subtypes N1 to N9 in vitro, including oseltamivir-resistant viruses.[56] In a lethal mouse model, survival was comparable between animals given a single dose of R-125489 or zanamivir; however, CS-8958 significantly increased survival when treatment was given prophylactically as early as 10 days before infection.[56] The increased survival correlated with a significant reduction in virus titers within the infected lung when CS-8958 was given 7 days prior to infection. In ferrets, administration of CS-8958 4 hours after infection caused a reduction in viral titers in the nasal wash, thus confirming the findings from the murine model.[57] In a clinical trial using healthy male subjects, when administered by inhalation R-118958 was found to be well tolerated at doses of 1, 2, 5, or 10 mg with a long retention of the active metabolite.[58] In a phase III trial using adult subjects, inhaled CS-8958 administered once daily was found to be as effective as a 75 mg dose of oseltamivir administered twice daily for 5 consecutive days.[59] Phase III clinical trials are currently underway in Japan to evaluate the safety of CS-8958 and to measure influenza transmission in participants receiving CS-8958 or a placebo [60]
2.4. Polymerase inhibitors
Presently, ribavirin is the only polymerase inhibitor available to treat influenza infection; however, it’s approved use is limited to a few countries. In 2002, Furuta et al. reported that a novel pyrazine molecule, T-705 (favipiravir), inhibited influenza virus infections in cell culture and in mice.[61] T-705 inhibits replication of both influenza A and B viruses. [61–63] Similar to ribavirin, T-705 is an inhibitor of the influenza RNA polymerase (Figure 1); however, unlike ribavirin, T-705 does not interfere with host DNA or RNA synthesis and is only weakly inhibitory to the host inosine monophosphate dehydrogenase, thus rendering T-705 less cytotoxic.[62, 64] These properties make T-705 an attractive candidate for the treatment of influenza virus infections in humans. High doses of T-705 resulted in little or no cytotoxicity, and repeated passage of influenza virus in cell culture in the presence of the drug did not result in the development of resistance.[61] Influenza viruses resistant to ribavirin have yet to be described; however the generation in cell culture of ribavirin resistant polio virus and resistant cell lines hepatitis C virus replicon containing has been described.[65, 66] Ribavirin resistance has not been identified in the clinical setting; therefore, it is possible to hypothesize that the possibility of emergence of influenza viruses resistant to T-705 is low.
Although T-705 was found to reduce influenza virus replication less than that of oseltamivir when measured in vitro, it was more protective than oseltamivir in vivo. Mice given 200 mg/kg/day orally beginning either 1 or 13 hours after challenge with a lethal H1N1 virus displayed increased survival and reduced virus titers from the infected lungs as compared to control animals.[61, 67] Phase I/II studies of T-705 are currently underway in the US and Japan, and T-705 is entering phase III trials in Japan.[68, 69]
3. Experimental antiviral agents for influenza
3.1. Cyanovirin-N (CVN)
Cyanovirin-N (CVN) is a carbohydrate-binding protein that inhibits the entry of viruses into cells by specifically binding to high-mannose oligosaccharides on the surface glycoproteins of enveloped viruses (Figure 1). Initially developed as an antiviral drug to treat human immunodeficiency virus (HIV) infection, CVN was found to also have potent antiviral activity against most strains of influenza A and B viruses.[70] The binding of CVN to influenza viruses and the resulting antiviral activity was limited by the degree of glycosylation of the HA. In the mouse and ferret models, prophylaxis and early initiation of treatment with CVN caused a significant, dose-dependent reduction of viral titers in the lungs and nasal washes.[71] Resistance to CVN has been reported for influenza viruses isolated in the 1930s, as well as after mouse adaption in the absence of drug or after passage in cell culture in the presence of drug.[72] Resistance to CVN may be explained by the lack or loss of glycosylation on the HA. Glycosylation patterns of human influenza viruses suggest that CVN may be effective against seasonal influenza viruses; however, the pandemic 2009 H1N1 influenza viruses lack glycosylation on the HA similar to influenza viruses isolated from the 1930s.[73] The effectiveness of CVN across all strains and HA subtypes of influenza may therefore be limited.
3.2. Conjugated sialidase (DAS181)
Influenza A and B viruses bind to saccharides containing terminal sialic acids (SA)-α2,3-galactose (SAα2,3Gal) or (SA)-α2,6-galactose (SAα2,6Gal) on the surface of host cells. Both forms of sialic acids are expressed on the surface of the human respiratory epithelial cells;[74] therefore, enzymatic removal of these sialic acids via a sialidase delivered as an inhalant may reduce or eliminate influenza infection (Figure 1). DAS181 is a sialidase fusion construct, consisting of a sialidase from Actinomyces viscosus fused with a respiratory epithelium-anchoring domain to increase its retention within the respiratory tract.[75] DAS181 has exhibited potent antiviral activity against a range of seasonal influenza A and B viruses when grown in Madin-Darby canine kidney (MDCK) cells, with EC50 values ranging from 0.1 to 13.7 nM.[75] This efficacy in vitro has been confirmed in vivo through a reduction in virus titers from the lungs of mice and in nasal washes from ferrets subsequent to influenza infection. When tested for efficacy against the potentially pandemic H5N1 influenza A virus and the pandemic 2009 H1N1 influenza A virus, DAS181 inhibited virus replication in human airway epithelial (HAE) cultures and enhanced the survival of infected mice. [76, 77] This protection was found to be elicited through the desialylation of the epithelial cell surface within the respiratory tract. When DAS181 was applied to HAE cultures and ex vivo bronchi tissues, desialylation occurred immediately, resulting in an inhibitory effect lasting for at least 2 days.[78] One concern when considering new antiviral drugs is whether existing mutations would confer cross resistance. However, DAS181 displayed antiviral activity against known oseltamivir resistant viruses.[79] The demonstrated broad antiviral activity against various influenza subtypes, including oseltamivir-resistant viruses, suggests that DAS181 may represent a future therapeutic and prophylactic treatment for influenza. Thus far, DAS181 was shown to be well tolerated with no serious adverse events in phase I trials, and phase II trials have been initiated. [80]
3.3. Thiazolides
Thiazolides are a class of novel, broad-spectrum drugs initially designed for the treatment of parasitic infections. Nitazoxanide is a licensed thiazolide for enteritis and has been found to have antiviral activity against DNA and RNA viruses, including hepatitis B and C viruses.[81, 82] The mechanism of action of nitazoxanide remains unclear for hepatitis B and C viruses; however, studies using influenza viruses suggest thiazolides block the maturation of influenza glycoproteins (Figure 1). When tested against influenza infection in vitro, nitazoxanide blocked terminal glycosylation of the HA. This impaired the trafficking of the HA glycoprotein between the endoplasmic reticulum and the Golgi complex, thereby preventing its transport to the cell surface.[83] Nitazoxanide has been found to be safe and effective and is currently in clinical trails for the treatment of rotavirus, gastroenteritis, and chronic hepatitis B and C infection.[82, 84, 85] Thus far, the emergence of drug resistant variants has not been reported.[86] Data obtained from influenza infection in vitro data support the potential of thiazolides as anti-influenza therapeutics; however, their efficacy in vivo remains to be studied.
3.4. siRNA
Small interfering RNAs (siRNAs) are nucleic acid-based molecules designed to inhibit gene expression. These short (21–26 nt) sequences are double stranded RNAs that selectively reduce specific RNA molecules thus inhibiting the expression of a target gene (Figure 1).[87] siRNAs specific for influenza mRNA have effectively inhibited replication of multiple influenza subtypes in culture and provide protection against lethal challenge in mice.[88–91] Protection in vivo was elicited through the delivery of siRNA via inhalation and IV administration. The high mutation rate of influenza could result in resistance to selected siRNAs; therefore, the addition of host targets could minimize the likelihood of antiviral resistance. siRNA has been used to identify host genes required for the replication of influenza viruses through genome wide RNAi screening.[92–94] Silencing the host genes that were identified in these studies effectively reduced viral replication in vitro. Although siRNA antiviral therapy for influenza has yet to enter clinical trials, RNAi is currently in phase II clinical trials for Respiratory Syncytial Virus (RSV). The siRNAs delivered via inhalation are safe and well tolerated, and have demonstrated significant anti-viral efficacy.[95, 96] siRNAs show promising antiviral potential to provide a potent therapy for the control of influenza by targeting viral or host genes.
4. Combination chemotherapy
Each of the previously described antivirals is effective in inhibiting the influenza virus replication alone; however, when used in combination an additive or synergistic effect can be observed. Under investigation are the combinations of oseltamivir, adamantanes, and ribavirin against influenza infection. Initial studies of combination chemotherapy included evaluating combination of oseltamivir and adamantanes. This therapy was found to exert an additive and synergistic antiviral effect against multiple subtypes of influenza in vitro and in vivo thus providing an advantage over single drug treatment.[97–100] An oseltamivir and ribavirin combination therapy was tested against H5N1 influenza virus in a mouse model and shown to be beneficial; however the results were dependent on the H5N1 influenza strain used.[101] More recent studies have shown a triple combination of amantadine, oseltamivir and ribavirin to be efficacious against multiple influenza virus strains in vitro including drug resistant strains.[102, 103] This triple combination was effective against amantadine- and oseltamivir- resistant viruses with synergy greater than any double combination tested. An additional advantage to the use of combination chemotherapy may be the potential for reducing the emergence of drug-resistant influenza variants; however this requires further investigation.[104] As novel therapeutics are identified, they may prove to be most effective when used in combination.
5. Immunomodulators
Until recently, most efforts have been focused on development of therapeutic strategies that target the virus.[105] However, the pathogenic effects of an exuberant immune response, otherwise known as a cytokine storm, following infection with HP influenza viruses are undeniable.[2, 4, 106, 107] This, along with the growing number of reports that demonstrate virus replication and host survival are not always correlated,[108] suggest that new strategies centered around modulating influenza-induced inflammation are sorely needed. Therapeutic agents known to dampen inflammation initiated by pathogens other than influenza or autoimmune disorders, which may also positively influence the outcome of a HP influenza infection, were recently reviewed in detail.[108] Here, we will focus on therapies that are beginning to be evaluated for efficacy during an experimental influenza infection.
5.1. Cyclooxygenase (COX) inhibitors
The cyclooxygenase (COX) pathway, and in particular COX-2, is known for modulating inflammation (Figure 1). Indeed COX-2 has been found to be elevated in H5N1-infected macrophages in vitro and in lung tissue obtained from patients who died from H5N1 infection, leading to speculation that targeting COX-2 signaling may improve the outcome of a HP influenza infection.[109] Further supporting this idea, a study by Carey et. al demonstrated that a COX-1 deficiency was detrimental while a COX-2 deficiency proved beneficial for the murine host response to influenza infection.[110] While lung virus titers were elevated in COX-2−/− animals, both mortality and inflammation was markedly reduced as compared to COX-1−/− or wild-type animals following infection with H3N2 influenza.
A more recent study tested the hypothesis that combination therapy using the NA inhibitor zanamivir along with the COX-2 inhibitors celecoxib and mesalazine would decrease H5N1virus-induced mortality.[111] Administration of zanamivir 4 hours after infection provided complete protection following H5N1 virus infection in mice. However, in real clinical situations, H5N1virus-infected patients often fail to seek medical advice until 2–4 days after the onset of symptoms. Consistent with previous findings, when zanamivir treatment was delayed for 48 hours the survival rate decreased from 100% to 13%. However, when celecoxib and mesalazine were administered 48 hours after infection in addition to zanamivir, the survival rate increased to 53%. Concomitant with increased survival was a decrease in proinflammatory cytokine production and ultimately decreased pulmonary damage. In this study, the improved survival realized by the combination therapy was attributed to inhibition of COX-2, but it is likely that there were other contributing factors. In particular, the anti-inflammatory effects of mesalazine are known to be dependent on activation of the peroxisome proliferator-activated receptor (PPAR)-γ,[112] which leads to the next potential therapeutic target to dampen the excessive influenza-induced inflammation, the PPAR axis.
5.2. Peroxisome proliferator-activated receptor (PPAR) agonists
PPARs are a family of lipid-activated transcription factors that are key regulators of lipid and glucose metabolism as well as inflammation.[113] There are three distinct members of the PPAR family: PPAR-alpha, PPAR-delta (sometimes referred to as PPAR-beta), and PPAR-gamma. It has been speculated that the use of fibrates (PPAR-alpha agonists) and/or glitazones (PPAR-gamma agonists) may be sufficient to decrease immune-mediated pulmonary damage during H5N1 virus infection[114]. To determine if PPAR-alpha activation was a viable method to moderate morbidity and decrease mortality during influenza infection, Budd et. al treated mice with the PPAR-alpha agonist gemfibrozil on days 4–10 after infection with an H2N2 virus [115]. The authors found that treatment with gemfibrozil (60 mg/kg) increased survival from 26% to 52%. Unfortunately, inflammation was not measured in this study, nor were virus titers, making it difficult to conclude the mechanism of protection. Considering the known anti-inflammatory effects of PPAR-alpha agonists, it is reasonable to hypothesize that protection was conferred through reduced inflammation.
Recently, a specific subset of CCR2+ inflammatory monocytes were found to be significantly elevated during HP influenza infections.[116, 117] Although elimination of these inflammatory cells was an appealing approach to combat lethal infection, it was discovered that they are required for the full realization of antigen-specific CD8+ T cell-mediated immunity.[116] The blunted CD8+ T cell response delayed virus clearance, thus there was no therapeutic benefit realized. It was hypothesized that reducing, without eliminating the accumulation of these monocytes may prove beneficial to the host. Chemokine (C-C motif) ligand 2 (CCL2) is the preferred ligand for CCR2 and is known to be suppressed by the PPAR-gamma agonist pioglitazone.[118] Aldridge et al., demonstrated that treatment with pioglitazone (60 mg/kg) results in increased survival of mice from 8% to 50% after a HP influenza virus challenge, effectively reducing the accumulation of CCR2 responsive monocytes without compromising virus clearance.[116] The effects of pioglitazone are therefore believed to be directly related to decreased inflammation (Figure 1), as there was no decrease in virus replication when tested either in vivo or in vitro. Because the study was designed to determine if moderating the numbers of CCR2 responsive monocytes would prove beneficial during a HP influenza infection, pioglitazone was given as a prophylactic. Therefore, further studies are necessary to determine if it is an effective therapy for an established infection. Of the immunomodulators that have been tested during influenza infection thus far, the potential for PPAR agonists appear to be the most exciting, the potential benefits are also supported by the limited side effects of pioglitazone (diarrhea, nausea, and upset stomach) reported in clinical trials.
6. Conclusion
Novel antiviral drugs that are currently being developed for the prophylaxis and treatment of influenza hold great promise. Future management strategies for influenza infection include developing therapies targeting viral genes other than NA, using new delivery methods to improve the efficacy of previously approved drugs, identifying drugs for other pathogens that have antiviral activity against influenza and finding approaches to regulate host immunity. It is likely that the influenza virus will become resistant to monotherapy using antiviral drugs currently in development, as it has for those already licensed for use. The rapid emergence and spread of oseltamivir resistance among seasonal H1N1 influenza viruses in 2007–2008 demonstrates that we cannot be complacent about the ability of influenza viruses to circumvent any devised control strategy. One of the attractive options is combination chemotherapy with the drugs affecting different virus proteins or different factors of viral pathogenicity. As more information about the pathogenesis of the influenza infection becomes available, novel antiviral drugs and immunomodulators will be discovered to provide additional treatment options.
Acknowledgments
We thank Betsy Williford for the figure design and Vani Shanker, PhD, ELS for expert editorial review of the article. The authors laboratories are funded in part by the National Institute Allergy and Infectious Diseases, National Institutes of Health, under contract number HHSN 266200700005C and by the American Lebanese Syrian Associated Charities. JRA is funded by grant 1F32AI078667 by the National Institute Allergy and Infectious Diseases, National Institutes of Health. RGW and EAG receive research funding from Hoffmann-La Roche and BioCryst Pharmaceuticals.
References
- 1.Glezen WP, Greenberg SB, Atmar RL, et al. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283(4):499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 2.Chan MC, Cheung CY, Chui WH, et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kash JC, Tumpey TM, Proll SC, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443(7111):578–81. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loo YM, Gale M., Jr Influenza: fatal immunity and the 1918 virus. Nature. 2007;445(7125):267–8. doi: 10.1038/445267a. [DOI] [PubMed] [Google Scholar]
- 5.Monto AS. The role of antivirals in the control of influenza. Vaccine. 2003;21(16):1796–800. doi: 10.1016/s0264-410x(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 6.Sidwell RW, Huffman JH, Barnard DL, et al. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antiviral Res. 1998;37(2):107–20. doi: 10.1016/s0166-3542(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 7.Monto AS, Fleming DM, Henry D, et al. Efficacy and safety of the neuraminidase inhibitor zanamivirin the treatment of influenza A and B virus infections. J Infect Dis. 1999;180(2):254–61. doi: 10.1086/314904. [DOI] [PubMed] [Google Scholar]
- 8.McKimm-Breschkin JL. Management of influenza virus infections with neuraminidase inhibitors: detection, incidence, and implications of drug resistance. Treat Respir Med. 2005;4(2):107–16. doi: 10.2165/00151829-200504020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay AJ, Wolstenholme AJ, Skehel JJ, et al. The molecular basis of the specific anti-influenza action of amantadine. The EMBO journal. 1985;4(11):3021–4. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69(3):517–28. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 11.Hayden FG. Amantadine and rimantadine-clinical aspects. In: Richman DD, editor. Antiviral Drug Resistance. New York: John Wiley & Sons, Inc; 1996. pp. 59–77. [Google Scholar]
- 12.Wang C, Takeuchi K, Pinto LH, et al. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993;67(9):5585–94. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grambas S, Hay AJ. Maturation of influenza A virus hemagglutinin--estimates of the pH encountered during transport and its regulation by the M2 protein. Virology. 1992;190(1):11–8. doi: 10.1016/0042-6822(92)91187-y. [DOI] [PubMed] [Google Scholar]
- 14.Betakova T, Ciampor F, Hay AJ. Influence of residue 44 on the activity of the M2 proton channel of influenza A virus. J Gen Virol. 2005;86(Pt 1):181–4. doi: 10.1099/vir.0.80358-0. [DOI] [PubMed] [Google Scholar]
- 15.von Itzstein M, Wu WY, Kok GB, et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363(6428):418–23. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 16.Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet. 2000;355(9206):827–35. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- 17.Roberts NA, Govorkova EA. The Activity of Neuraminidase Inhibitor Oseltamivir Against All Subtypes of Influenza Viruses. In: Mitrasinovic PM, editor. Global View of the Fight against Influenza. Nova Science Publishers; 2009. pp. 93–118. [Google Scholar]
- 18.Bright RA, Medina MJ, Xu X, et al. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 2005;366(9492):1175–81. doi: 10.1016/S0140-6736(05)67338-2. [DOI] [PubMed] [Google Scholar]
- 19.Monto AS, McKimm-Breschkin JL, Macken C, et al. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Chemother. 2006;50(7):2395–402. doi: 10.1128/AAC.01339-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheu TG, Deyde VM, Okomo-Adhiambo M, et al. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother. 2008;52(9):3284–92. doi: 10.1128/AAC.00555-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijer A, Lackenby A, Hungnes O, et al. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 Season. Emerg Infect Dis. 2009;15(4):552–60. doi: 10.3201/eid1504.081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dharan NJ, Gubareva LV, Meyer JJ, et al. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301(10):1034–41. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- 23.Hurt AC, Ernest J, Deng YM, et al. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antiviral Res. 2009;83(1):90–3. doi: 10.1016/j.antiviral.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med. 2009;360(10):953–6. doi: 10.1056/NEJMp0900648. [DOI] [PubMed] [Google Scholar]
- 25.Deyde VM, Sheu TG, Trujillo AA, et al. Detection of molecular markers of drug resistance in the 2009 pandemic influenza A (H1N1) viruses using pyrosequencing. Antimicrob Agents Chemother. 2009 doi: 10.1128/AAC.01417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) Update: influenza activity - United States, August 30–October 31, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(44):1236–41. [PubMed] [Google Scholar]
- 27.Le QM, Wertheim HF, Tran ND, et al. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med. 2010;362(1):86–7. doi: 10.1056/NEJMc0910448. [DOI] [PubMed] [Google Scholar]
- 28.Gaur AH, Bagga B, Barman S, et al. Intravenous zanamivir for oseltamivir-resistant 2009 H1N1 influenza. N Engl J Med. 2010;362(1):88–9. doi: 10.1056/NEJMc0910893. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Cheung CL, Tai H, et al. Oseltamivir-resistant influenza A pandemic (H1N1) 2009 virus, Hong Kong, China. Emerg Infect Dis. 2009;15(12):1970–2. doi: 10.3201/eid1512.091057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babu YS, Chand P, Bantia S, et al. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43(19):3482–6. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- 31.Bantia S, Parker CD, Ananth SL, et al. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob Agents Chemother. 2001;45(4):1162–7. doi: 10.1128/AAC.45.4.1162-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drusano GL, Preston SL, Smee D, et al. Pharmacodynamic evaluation of RWJ-270201, a novel neuraminidase inhibitor, in a lethal murine model of influenza predicts efficacy for once-daily dosing. Antimicrob Agents Chemother. 2001;45(7):2115–8. doi: 10.1128/AAC.45.7.2115-2118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smee DF, Huffman JH, Morrison AC, et al. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activities. Antimicrob Agents Chemother. 2001;45(3):743–8. doi: 10.1128/AAC.45.3.743-748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sidwell RW, Smee DF, Huffman JH, et al. In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RJW-270201. Antimicrob Agents Chemother. 2001;45(3):749–57. doi: 10.1128/AAC.45.3.749-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Govorkova EA, Leneva IA, Goloubeva OG, et al. Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob Agents Chemother. 2001;45(10):2723–32. doi: 10.1128/AAC.45.10.2723-2732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barroso L, Treanor J, Gubareva L, et al. Efficacy and tolerability of the oral neuraminidase inhibitor peramivir in experimental human influenza: randomized, controlled trials for prophylaxis and treatment. Antivir Ther. 2005;10(8):901–10. [PubMed] [Google Scholar]
- 37.Kilpatrick JM, Harman LA, Collis PJ, Aitee G, Mead E, Alexander WJ. Pharmacokinetics and safety of peramivir by intramuscular administration. Proceedings of the Options for the Control of Influenza VI; 2007; Toronto, Canada. p. 916. [Google Scholar]
- 38.Beigel J, Harman LA, Collis PJ, et al. Pharmacokinetic and safety evaluations of escalating doses of peramivir administered intravenously in healthy volunteers. Proceedings of the Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007; Chicago, IL. [Google Scholar]
- 39.Bantia S, Arnold CS, Parker CD, et al. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antiviral Res. 2006;69(1):39–45. doi: 10.1016/j.antiviral.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Boltz DA, Ilyushina NA, Arnold CS, et al. Intramuscularly administered neuraminidase inhibitor peramivir is effective against lethal H5N1 influenza virus in mice. Antiviral Res. 2008;80(2):150–7. doi: 10.1016/j.antiviral.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yun NE, Linde NS, Zacks MA, et al. Injectable peramivir mitigates disease and promotes survival in ferrets and mice infected with the highly virulent influenza virus, A/Vietnam/1203/04 (H5N1) Virology. 2008;374(1):198–209. doi: 10.1016/j.virol.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ison MG, McGeer AJ, Hui DS, et al. Safety and efficacy of multiple-day treatment with intravenous peramivir or oral oseltamivir in hospitalized adults with acute influenza. Proceedings of the XI International Symposium on Respiratory Viral Infections; 2009; Bangkok, Thailand. [Google Scholar]
- 43.Kohno S, Yen MY, Cheong HJ, et al. Single-intravenous peramivir vs. oral oseltamivir to treat acute, uncomplicated influenza in the outpatient setting: a phase III randomized, double-blind trial. Proceedings of the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009 September 12–15; San Francisco, CA. [Google Scholar]
- 44.BioCryst Pharmaceuticals. [Accessed January 20 2010];BioCryst pharmaceuticals reports positive results of Shionogi & Co. sponsored phase 3 studies of i.v. peramivir for influenza [online] Available from URL: http://investor.shareholder.com/biocryst/ReleaseDetail.cfm?ReleaseID=397310.
- 45.U.S. Food and Drug Administration. [Accessed January 21 2010];Emergency use authorization of peramivir iv fact sheet for health care providers [online] Available from URL: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM187811.pdf.
- 46.Baz M, Abed Y, Boivin G. Characterization of drug-resistant recombinant influenza A/H1N1 viruses selected in vitro with peramivir and zanamivir. Antiviral Res. 2007;74(2):159–62. doi: 10.1016/j.antiviral.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Hurt AC, Holien JK, Barr IG. In vitro generation of neuraminidase inhibitor resistance in A(H5N1) influenza viruses. Antimicrob Agents Chemother. 2009;53(10):4433–40. doi: 10.1128/AAC.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.BioCryst Pharmaceuticals. [Accessed January 20 2010];Biocryst provides peramivir update and reports first quarter 2009 financial results [online] Available from URL: http://investor.shareholder.com/biocryst/releasedetail.cfm?ReleaseID=382718.
- 49.Memoli MJ, Hrabal RJ, Hassantoufighi A, et al. Rapid selection of oseltamivir- and peramivir-resistant pandemic H1N1 virus during therapy in 2 immunocompromised hosts. Clin Infect Dis. 2010;50(9):1252–5. doi: 10.1086/651605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stittelaar KJ, Tisdale M, van Amerongen G, et al. Evaluation of intravenous zanamivir against experimental influenza A (H5N1) virus infection in cynomolgus macaques. Antiviral Res. 2008;80(2):225–8. doi: 10.1016/j.antiviral.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Calfee DP, Peng AW, Cass LM, et al. Safety and efficacy of intravenous zanamivir in preventing experimental human influenza A virus infection. Antimicrob Agents Chemother. 1999;43(7):1616–20. doi: 10.1128/aac.43.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kidd IM, Down J, Nastouli E, et al. H1N1 pneumonitis treated with intravenous zanamivir. Lancet. 2009;374(9694):1036. doi: 10.1016/S0140-6736(09)61528-2. [DOI] [PubMed] [Google Scholar]
- 53.GlaxoSmithKline. [Accessed January 10 2010];An open-label, multi-center, single arm study to evaluate the safety and tolerability of intravenous zanamivir in the treatment of hospitalized adult, adolescent and pediatric subjects with confirmed influenza infection [online] Available from URL: http://www.gsk-clinicalstudyregister.com/protocol_detail.jsp?protocolId=113678&studyId=29102&compound=Zanamivir.
- 54.Honda T, Kubo S, Masuda T, et al. Synthesis and in vivo influenza virus-inhibitory effect of ester prodrug of 4-guanidino-7-O-methyl-Neu5Ac2en. Bioorganic & medicinal chemistry letters. 2009;19(11):2938–40. doi: 10.1016/j.bmcl.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 55.Koyama K, Takahashi M, Oitate M, et al. CS-8958, a prodrug of the novel neuraminidase inhibitor R-125489, demonstrates a favorable long-retention profile in the mouse respiratory tract. Antimicrob Agents Chemother. 2009;53(11):4845–51. doi: 10.1128/AAC.00731-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamashita M, Tomozawa T, Kakuta M, et al. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob Agents Chemother. 2009;53(1):186–92. doi: 10.1128/AAC.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kubo S, Tomozawa T, Kakuta M, et al. CS-8958 (laninamivir prodrug), a long-acting neuraminidase inhibitor, shows superior anti-influenza virus activity after a single administration. Antimicrob Agents Chemother. 2010 doi: 10.1128/AAC.01311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rennecke J, Hirota T, Puchler K, et al. R-118958, a unique anti-influenza agent: safety, tolerability and pharmacokinetics (PK) in healthy male volunteers. Proceedings of the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2003; Washington, DC. American Society for Microbiology; p. F-1834. [Google Scholar]
- 59.Biota. [Accessed December 27 2009];LANI Phase III clinical trials in Asia prove successful [online] Available from URL: http://www.biota.com.au/uploaded/154/1021542_25laniphaseiiiclinicaltri.pdf.
- 60.Biota. [Accessed December 27 2009];Phase III trial with CS-8958 for flu prevention underway [online] Available from URL: http://www.biota.com.au/uploaded/154/1021585_54cs8958phaseiiipreventio.pdf.
- 61.Furuta Y, Takahashi K, Fukuda Y, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46(4):977–81. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furuta Y, Takahashi K, Kuno-Maekawa M, et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005;49(3):981–6. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gowen BB, Wong MH, Jung KH, et al. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007;51(9):3168–76. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Streeter DG, Witkowski JT, Khare GP, et al. Mechanism of action of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci U S A. 1973;70(4):1174–8. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pfeiffer JK, Kirkegaard K. A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc Natl Acad Sci U S A. 2003;100(12):7289–94. doi: 10.1073/pnas.1232294100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfeiffer JK, Kirkegaard K. Ribavirin resistance in hepatitis C virus replicon-containing cell lines conferred by changes in the cell line or mutations in the replicon RNA. J Virol. 2005;79(4):2346–55. doi: 10.1128/JVI.79.4.2346-2355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi K, Furuta Y, Fukuda Y, et al. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir Chem Chemother. 2003;14(5):235–41. doi: 10.1177/095632020301400502. [DOI] [PubMed] [Google Scholar]
- 68.Fujifilm. [Accessed December 18 2009];notice of start of phase III clinical trials of T-705 as a treatment for influenza infections in Japan [online] Available from URL: http://www.fujifilmholdings.com/en/pdf/investors/library/ff_announcement_20091029_001.pdf.
- 69.Furuta Y, Takahashi K, Shiraki K, et al. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82(3):95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Keefe BR, Smee DF, Turpin JA, et al. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother. 2003;47(8):2518–25. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smee DF, Bailey KW, Wong MH, et al. Treatment of influenza A (H1N1) virus infections in mice and ferrets with cyanovirin-N. Antiviral Res. 2008;80(3):266–71. doi: 10.1016/j.antiviral.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smee DF, Wandersee MK, Checketts MB, et al. Influenza A (H1N1) virus resistance to cyanovirin-N arises naturally during adaptation to mice and by passage in cell culture in the presence of the inhibitor. Antivir Chem Chemother. 2007;18(6):317–27. doi: 10.1177/095632020701800604. [DOI] [PubMed] [Google Scholar]
- 73.Igarashi M, Ito K, Yoshida R, et al. Predicting the antigenic structure of the pandemic (H1N1) 2009 influenza virus hemagglutinin. PLoS One. 2010;5(1):e8553. doi: 10.1371/journal.pone.0008553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shinya K, Ebina M, Yamada S, et al. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440(7083):435–6. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 75.Malakhov MP, Aschenbrenner LM, Smee DF, et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother. 2006;50(4):1470–9. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan RW, Chan MC, Wong AC, et al. DAS181 inhibits H5N1 influenza virus infection of human lung tissues. Antimicrob Agents Chemother. 2009;53(9):3935–41. doi: 10.1128/AAC.00389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Triana-Baltzer GB, Gubareva LV, Nicholls JM, et al. Novel pandemic influenza A(H1N1) viruses are potently inhibited by DAS181, a sialidase fusion protein. PLoS One. 2009;4(11):e7788. doi: 10.1371/journal.pone.0007788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Triana–Baltzer GB, Babizki M, Chan MC, et al. DAS181, a sialidase fusion protein, protects human airway epithelium against influenza virus infection: an in vitro pharmacodynamic analysis. J Antimicrob Chemother. 2009 doi: 10.1093/jac/dkp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Triana-Baltzer GB, Gubareva LV, Klimov AI, et al. Inhibition of neuraminidase inhibitor-resistant influenza virus by DAS181, a novel sialidase fusion protein. PLoS One. 2009;4(11):e7838. doi: 10.1371/journal.pone.0007838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.NexBio. [Accessed January 24 2010];NexBio Initiates Phase II Trial of DAS181 (Fludase®*) for Treatment of Influenza, Including Pandemic Influenza A(H1N1) [online] Available from URL: http://www.nexbio.com/docs/DAS181_Phase_2_Press_Release_010710.pdf.
- 81.Korba BE, Montero AB, Farrar K, et al. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res. 2008;77(1):56–63. doi: 10.1016/j.antiviral.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 82.Rossignol JF, Keeffe EB. Thiazolides: a new class of drugs for the treatment of chronic hepatitis B and C. Future Microbiol. 2008;3:539–45. doi: 10.2217/17460913.3.5.539. [DOI] [PubMed] [Google Scholar]
- 83.Rossignol JF, La Frazia S, Chiappa L, et al. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem. 2009;284(43):29798–808. doi: 10.1074/jbc.M109.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rossignol JF, Abu-Zekry M, Hussein A, et al. Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet. 2006;368(9530):124–9. doi: 10.1016/S0140-6736(06)68852-1. [DOI] [PubMed] [Google Scholar]
- 85.Rossignol JF, Elfert A, El-Gohary Y, et al. Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin. Gastroenterology. 2009;136(3):856–62. doi: 10.1053/j.gastro.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 86.Korba BE, Elazar M, Lui P, et al. Potential for hepatitis C virus resistance to nitazoxanide or tizoxanide. Antimicrob Agents Chemother. 2008;52(11):4069–71. doi: 10.1128/AAC.00078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hannon GJ. RNA interference. Nature. 2002;418(6894):244–51. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 88.Ge Q, McManus MT, Nguyen T, et al. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc Natl Acad Sci U S A. 2003;100(5):2718–23. doi: 10.1073/pnas.0437841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tompkins SM, Lo CY, Tumpey TM, et al. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci U S A. 2004;101(23):8682–6. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou H, Jin M, Yu Z, et al. Effective small interfering RNAs targeting matrix and nucleocapsid protein gene inhibit influenza A virus replication in cells and mice. Antiviral Res. 2007;76(2):186–93. doi: 10.1016/j.antiviral.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 91.Zhang W, Wang CY, Yang ST, et al. Inhibition of highly pathogenic avian influenza virus H5N1 replication by the small interfering RNA targeting polymerase A gene. Biochem Biophys Res Commun. 2009;390(3):421–6. doi: 10.1016/j.bbrc.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 92.Karlas A, Machuy N, Shin Y, et al. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- 93.Konig R, Stertz S, Zhou Y, et al. Human host factors required for influenza virus replication. Nature. 2009 doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hao L, Sakurai A, Watanabe T, et al. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature. 2008;454(7206):890–3. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DeVincenzo J, Cehelsky JE, Alvarez R, et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV) Antiviral Res. 2008;77(3):225–31. doi: 10.1016/j.antiviral.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 96.Alnylam. [Accessed January 28 2010];ALN-RSV: Respiratory Syncytial Virus [online] Available from URL: http://www.alnylam.com/Programs-and-Pipeline/Programs/index.php.
- 97.Galabov AS, Simeonova L, Gegova G. Rimantadine and oseltamivir demonstrate synergistic combination effect in an experimental infection with type A (H3N2) influenza virus in mice. Antivir Chem Chemother. 2006;17(5):251–8. doi: 10.1177/095632020601700502. [DOI] [PubMed] [Google Scholar]
- 98.Govorkova EA, Fang HB, Tan M, et al. Neuraminidase inhibitor-rimantadine combinations exert additive and synergistic anti-influenza virus effects in MDCK cells. Antimicrob Agents Chemother. 2004;48(12):4855–63. doi: 10.1128/AAC.48.12.4855-4863.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ilyushina NA, Hoffmann E, Salomon R, et al. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir Ther. 2007;12(3):363–70. [PubMed] [Google Scholar]
- 100.Masihi KN, Schweiger B, Finsterbusch T, et al. Low dose oral combination chemoprophylaxis with oseltamivir and amantadine for influenza A virus infections in mice. J Chemother (Florence, Italy) 2007;19(3):295–303. doi: 10.1179/joc.2007.19.3.295. [DOI] [PubMed] [Google Scholar]
- 101.Ilyushina NA, Hay A, Yilmaz N, et al. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob Agents Chemother. 2008;52(11):3889–97. doi: 10.1128/AAC.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen JT, Hoopes JD, Le MH, et al. Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS One. 2010;5(2):e9332. doi: 10.1371/journal.pone.0009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen JT, Hoopes JD, Smee DF, et al. Triple combination of oseltamivir, amantadine, and ribavirin displays synergistic activity against multiple influenza virus strains in vitro. Antimicrob Agents Chemother. 2009;53(10):4115–26. doi: 10.1128/AAC.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ilyushina NA, Bovin NV, Webster RG, et al. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antiviral Res. 2006;70(3):121–31. doi: 10.1016/j.antiviral.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 105.Basler CF. Influenza viruses: basic biology and potential drug targets. Infect Disord Drug Targets. 2007;7(4):282–93. doi: 10.2174/187152607783018745. [DOI] [PubMed] [Google Scholar]
- 106.Cheung CY, Poon LL, Lau AS, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360(9348):1831–7. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 107.Kobasa D, Jones SM, Shinya K, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445(7125):319–23. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 108.Fedson DS. Confronting the next influenza pandemic with anti-inflammatory and immunomodulatory agents: why they are needed and how they might work. Influenza Other Respi Viruses. 2009;3(4):129–42. doi: 10.1111/j.1750-2659.2009.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee SM, Cheung CY, Nicholls JM, et al. Hyperinduction of cyclooxygenase-2-mediated proinflammatory cascade: a mechanism for the pathogenesis of avian influenza H5N1 infection. J Infect Dis. 2008;198(4):525–35. doi: 10.1086/590499. [DOI] [PubMed] [Google Scholar]
- 110.Carey MA, Bradbury JA, Seubert JM, et al. Contrasting effects of cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza A viral infection. J Immunol. 2005;175(10):6878–84. doi: 10.4049/jimmunol.175.10.6878. [DOI] [PubMed] [Google Scholar]
- 111.Zheng BJ, Chan KW, Lin YP, et al. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci U S A. 2008;105(23):8091–6. doi: 10.1073/pnas.0711942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rousseaux C, Lefebvre B, Dubuquoy L, et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med. 2005;201(8):1205–15. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–7. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 114.Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis. 2008;8(9):571–6. doi: 10.1016/S1473-3099(08)70070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Budd A, Alleva L, Alsharifi M, et al. Increased survival after gemfibrozil treatment of severe mouse influenza. Antimicrob Agents Chemother. 2007;51(8):2965–8. doi: 10.1128/AAC.00219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aldridge JR, Jr, Moseley CE, Boltz DA, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A. 2009;106(13):5306–11. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin KL, Suzuki Y, Nakano H, et al. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180(4):2562–72. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 118.Di Gregorio GB, Yao-Borengasser A, Rasouli N, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54(8):2305–13. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]