Abstract
Cell replacement therapy utilizing mesenchymal stem cells as its main resource holds great promise for ultimate treatment of human neurological disorders. Parkinson's disease (PD) is a common, chronic neurodegenerative disorder hallmarked by localized degeneration of a specific set of dopaminergic neurons within a midbrain sub-region. The specific cell type and confined location of degenerating neurons make cell replacement therapy ideal for PD treatment since it mainly requires replenishment of lost dopaminergic neurons with fresh and functional ones. Endogenous as well as exogenous cell sources have been identified as candidate targets for cell replacement therapy in PD. In this review, umbilical cord mesenchymal stem cells (UCMSCs) are discussed as they provide an inexpensive unlimited reservoir differentiable towards functional dopaminergic neurons that potentially lead to long-lasting behavioral recovery in PD patients. We also present miRNAs-mediated neuronal differentiation of UCMSCs. The UCMSCs bear a number of outstanding characteristics including their non-tumorigenic, low-immunogenic properties that make them ideal for cell replacement therapy purposes. Nevertheless, more investigations as well as controlled clinical trials are required to thoroughly confirm the efficacy of UCMSCs for therapeutic medical-grade applications in PD.
Keywords: nerve regeneration, umbilical cord, mesenchymal stem cells, differentiation, neuronal, dopaminergic neurons, dopamine, substantia nigra, ventral mesencephalon, Parkinson's disease, cell replacement therapy, neural regeneration
Introduction
Cell replacement approaches for ultimate treatment of degenerative disorders by replenishing lost cells with fresh and functional ones largely rely on differentiation of potential cell sources. This is mainly because cell loss plays a central role in onset and progression of degenerative diseases (Fox et al., 2014).
Parkinson's disease (PD) is a typical neurodegenerative disorder characterized by progressive but largely localized degeneration of dopaminergic (DAergic) neurons chiefly within midbrain substantianigra pars compacta (SNpc) (Hirsch et al., 1997). Current pharmacotherapeutic approaches include administration of dopamine precursors such as L-dihydroxy phenyl alanine, dopamine agonists, monoamine oxidase B inhibitors, catechol-O-methyltransferase inhibitors and adenosine A2A antagonists (Stocchi, 2014), blockage of dopamine D3 receptors (Cortés et al., 2016), inhibition of neuronal nitric oxide synthase (Maccallini and Amoroso, 2016) and use of small-molecule epigenetic modifiers (Hegarty et al., 2016). Moreover, other alternatives such as surgical options, mainly deep brain stimulation, are being used in patients with advanced PD (Odekerken et al., 2013). Nevertheless, these treatments solely provide symptomatic relief and are not able to hinder disease progression. In this review, the potential therapeutic application of umbilical cord mesenchymal stem cells (UCMSCs) in PD will be discussed with an exclusive focus on generation of DAergic neurons.
In Search of Cell Sources for PD Cell Replacement: Criteria
The proof-of-principle for feasibility of cell replacement therapy (CRT) in PD is provided by the fact that clinical transplantation of human fetal tissues (in the open-label trials) obtained from ventral mesencephalon results in striatal re-innervation of DAergic neurons and symptomatic relief (Lindvall and Björklund, 2004). Cell replacement therapy (CRT) is aimed at constitution of a neuroprotective and/or neuroregenerative platform for human neurological disorders such as PD. However, prior to clinical translation of stem cells for PD, a number of requirements need to be taken to account in order to opt for a suitable cell type. They include 1) a standardized, medical grade differentiation protocol with minimum reliance on various growth factors, chemicals and any animal components; 2) phenotypical resemblance of cell sources upon differentiation. In the case of PD, stem cell-derived DAergic neurons have to display DAergic phenotype, express DAergic markers such as Pitx3, Nurr1, Engrailed-1, Lmx1a, tyrosine hydroxylase, aromatic acid decarboxylase and vesicular monoamine transporter and release dopamine in a controlled fashion. They must also display electrophysiological characteristics of substantia nigra (SN) neurons; 3) long-term survival of grafted DAergic neurons in the target tissue; 4) active integration into local neural network; target DAergic neurons should augment effective reconstitution of neural circuits and integrate into host striatum; 5) functionality of engrafted cells; functional DAergic neurons have to alleviate Parkinson's symptoms and improve behavioral motor conditions after transplantation in a rodent PD model without causing any sign of tumorigenicity and dyskinesias (Brundin and Hagell, 2001; Isacson et al., 2003; Braak and Del Tredici, 2008). It is noteworthy that transplantation of differentiated cells into various sub-regions in the striatum where A8, A9 and A10 dopaminergic cell groups extend their fibers to release dopamine would increase the chance of recovery and movement restoration in animal models of PD. On the other hand, impurities carrying non-DAergic neurons in our cell reservoir could offer therapeutic advantages to pure sources for cell therapy because not just dopaminergic but also neuronal types of serotonergic and others are damaged in the course of PD.
In Search of Cell sources for PD Cell Replacement: Candidates
Cell sources are categorized based on their potentiality to undergo DAergic differentiation into endogenous and exogenous candidates. Endogenous candidates include primary cultures of ventral mesencephalon (VM) that consist of DAergic progenitor cells and neural stem cells from subventricular zone, striatum (ST) and SN (Fallon et al., 2000; Storch et al., 2004; Mohapel et al., 2005; Madhavan et al., 2009). Exogenous sources, on the other hand, include embryonic cells, induced pluripotent stem cells (iPSCs) and also mesenchymal stem cells derived from bone marrow, amniotic fluid, sertoli cells, retinal pigment epithelium (RPE), adipocytes, carotid body cells, adrenal medullary, cervical sympathetic ganglion neurons and olfactory mucosal cells (Bankiewicz et al., 1988; Espejo et al., 1998; Subramanian et al., 2002; Nakao et al., 2004; McLaughlin et al., 2006; Levy et al., 2008; Murrell et al., 2008; Glavaski-Joksimovic et al., 2009). These cell candidates have already been subjected to various treatments with inducing factors by adding these factors to cell medium and also by gene transfer to direct them towards DAergic fate. In addition, co-culture systems have been established by several helper cell lines including fibroblasts, pA6 stromal cell line, sertoli cells, RPE, as reviewed by Gardaneh (2010). They appear to secrete medium specific growth factors capable of inducing DAergic phenotype when interacting with the target cells. Furthermore, we previously proposed a combined, multi-factorial approach based on interaction of GPX-1-overexpressing DAergic neurons, GDNF secreting astrocytes and Nurr1-expressing microglia to potentiate survival and biological function of DAergic neurons against 6-OHDA toxicity (Gardaneh et al., 2010).
Mesenchymal Stem Cells (MSCs) versus Embryonic Stem Cells (ESCs)
Stem cells are regarded as undifferentiated cells that can undergo both proliferation and differentiation (Fuchs and Segre, 2000). ESCs are stem cells derived from the inner cell mass of the blastocysts (Thomson, 1998). MSCs are non-hematopoietic adult stem cells that possess the capacity to differentiate into various tissues including bone, cartilage and adipose tissue (Pountos and Giannoudis, 2005). MSCs can be isolated from bone marrow (Bianco et al., 2001), adipose tissue (Zuk et al., 2001), cord blood, amniotic fluid (In’t Anker, 2003) and placental tissue (Karahuseyinoglu et al., 2007).
MSCs have been described as plastic adherent multipotent cells represented by distinct terminologies such as “colony-forming fibroblastic cells” (Kuznetsov et al., 1997), “bone marrow stromal cells (BMSC)” (Peister, 2004), “multipotent adult progenitor cells” (Jiang et al., 2002) and “marrow isolated adult multi-lineage inducible cells” (D’Ippolito, 2004; Boroujeni et al., 2012). ESCs may appear as an appealing source for any cell-based therapy but their possible complications such as tumor formation, the need for immunosuppression, limited ESCs supply and above all, ethical concerns have substantially restricted their therapeutic use. Therefore, the employment of MSCs in the tissue regeneration has attracted great interest as therapeutic agents. Moreover, these cells are capable of treating a variety of maladies including spinal cord injury (Hofstetter et al., 2002) and stroke (Chen et al., 2001), although UCMSC-derived dopaminergic neurons have not be utilized in the clinic. This means that steps have to be taken to clarify both beneficial and deleterious consequences of such a therapy for human patients.
The plasticity and transdifferentiation capacity of MSCs have provided an effective platform as they differentiate into other lineages of ectodermal and endodermal cells. Mezey et al. (2000) initially described the in vivo differentiation of transplanted adult bone marrow cells into glial cells. To be utilized specifically for PD cell therapy, studies have reported the feasibility of neuronal differentiation of MSCs in which the paracrine effect of the cells has been taken into account (Kitada and Dezawa, 2012).
Umbilical Cord: a Reservoir of MSCs
The umbilical cord consists of two umbilical arteries and also one umbilical vein which delivers oxygenated, nutrient-rich blood to the fetus (Meyer et al., 1978). This vascular structure is buried within a jelly-like tissue called umbilical cord matrix or Wharton's jelly which is counted as the gelatinous connective tissue (Wang et al., 2004). These cells express MSC markers SH2 and SH3 but not CD35 and CD45 which are regarded as hematopoietic markers. In addition, they exhibit the capacity to differentiate into a wide range of lineages including adipocytes, osteocytes, chondrocytes, and neural lineages (Mitchell et al., 2003; Wei et al., 2012). UCMSCs have shown scores of advantages over other stem cell sources outlined below: 1) they exist in more primordial stages of differentiation than other mesenchymal cells including BMSCs (Hao et al., 1995). 2) They do not express many of immunological markers involved in tissue rejection as shown by successful transplantation of umbilical cord blood nucleated cells in a 23-month-old child suffering from hemophagocytic lymphohistiocytosis (Schwinger et al., 1998). 3) Isolation, expansion, and freezing of these cells are easier and less expensive compared to many other sources such as neural stem cells (Taghizadeh et al., 2011; Dalous et al., 2012). 4) They demonstrate high proliferation rate compared to BMSCs (Baksh et al., 2007; Boroujeni et al., 2012). 5) They can be genetically manipulated to express various factors and/or used as delivery vehicles for therapeutic applications (Kim et al., 2008; Li et al., 2013; Zhang et al., 2014).
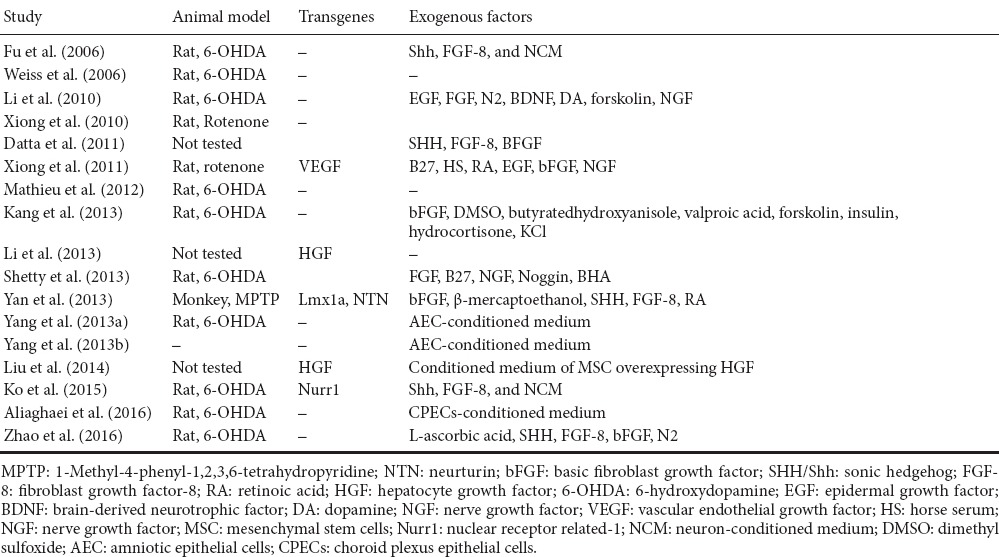
Dopaminergic Differentiation of UCMSCs
Production of functional DAergic neurons relies fundamentally on signaling factors such as Shh, FGF8 and Wnt1 that initiate DAergic neurogenesis. Subsequently, the gene expression of LIM homeodomain family members (Lmx1a, Lmx1b) and FoxA2 facilitates specification of DAergic progenitors, which paves the way for terminal differentiation, promoted by cooperative function of Nurr1 and Pitx3 (Chakrabarty et al., 2012; Hegarty et al., 2013). In order to demystify the precise mechanisms of DAergic differentiation in MSCs, early events parallel with late events need to be examined. Such studies will clarify the innate preparedness and potential of MSCs for neuronal/DAergic differentiation. Reports indicate that UCMSCs are capable of displaying neuronal phenotype by expressing neuron-specific enolase (Mitchell et al., 2003), astrocytic marker GFAP and oligodendrocytic marker CNPase (Ha et al., 2004; Tracy et al., 2008). The UCMSCs can be induced to differentiate into DAergic neurons comparatively to a high or low degree of success as shown in Table 1. These studies mainly applied transcription factors and/or growth factors as transgenes from within or as supplements from outside cells as inducing forces for neurogenesis and neuroprotection. Inclusion of exogenous genes in target cells that are destined to be part of patients’ live tissue can be a source of biological and ethical concerns. It is not possible to readily predict the physiological consequences of exogenous gene expression, neither are scientists prepared to fully satisfy the society of unforeseeable complications inherent with transgene transfer into human body. Further, undifferentiated UCMSCs were used for transplantation and mostly resulted in behavioral recovery in animal models of PD based on parkinsonian toxins 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) or rotenone. For instance, Weiss et al. (2006) and Xiong et al. (2010) merely used undifferentiated UCMSCs without applying any growth factors for transplantation and made promising observations for decreased rotations by 50% and 67% respectively. Other investigators employed several inducing factors including epidermal growth factor (EGF), sonic hedgehog (SHH), nerve growth factor (NGF) and/or specific conditioned media (e.g., from amniotic epithelial cells, AEC) to facilitate DAergic neuron production and, upon PD brain transplantation, observed alleviation of motor symptoms (Fu et al., 2006; Li et al., 2010; Kang et al., 2013; Shetty et al., 2013; Yang et al., 2013a; Zhao et al., 2016). Our laboratory has recently tested the inducing effect of murine cerebrospinal fluid on DAergic differentiation of UCMSCs which were then transplanted to rat striatum (Aliaghaei et al., 2016). The cells survived and resulted in rotational recovery and reduced rate of neuronal apoptosis in animal's injured brain. Besides, we successfully generated MSCs-derived DAergic secreting neurons using a cocktail of choroid plexus epithelial cells-conditioned medium and knockout serum replacement (Boroujeni et al., 2017). A combinatorial approach to convert UCMSCs to DAergic neurons employed both transgenes (such as Lmx1a and neurturin, NTN) and external inducing factors (basic fibroblast growth factor, β-mercaptoethanol, SHH, fibroblast growth factor-8, retinoic acid) that upon cell transplantation demonstrated improvements in behavioral deficits against MPTP (Yan et al., 2013). Measures outlined above could pave the ground for survival as well as regeneration of host neurons and ultimately for augmented efficacy of repairing target neural tissues. In accordance with this notion, MSCs transplanted into the brain stimulate endogenous neuronal growth and synaptogenesis and enhance functional recovery of damaged neurons (Lin et al., 2011; Olson et al., 2012). These trophic effects are implemented by MSC-mediated secretion of a variety of cytokines and neurotrophic factors (Joyce et al., 2010). Besides, the analysis of UCMSCs conditioned medium secretome revealed it contains neuro-regulatory components [such as Cyr61 protein and colony-stimulating factor 1 (CSF-1)] essential for the maintenance and maturation of neural stem cells, respectively (Pires et al., 2016). Hence, it is important in these studies to distinguish between neurogenic and paracrine protective effects of the administered UCMSCs so their fate as well as their ultimate therapeutic effects can be clearly determined.
Table 1.
Umbilical cord mesenchymal stem cells-based therapy for in vitro generation of dopaminergic neurons
MiRNAs-Mediated Neuronal Differentiation of UCMSCs
In order to develop UCMSCs-based therapeutic strategies, a comprehensive understanding of signaling pathways involved in proliferation and differentiation of UCMSCs is needed. These biological processes are considerably modulated by genetic and epigenetic mechanisms. Recently, microRNAs (miRNAs) as small non-coding RNA have been demonstrated to play essential roles in a plethora of biological functions including neural differentiation and neurodegeneration (Christensen and Schratt, 2009). Extensive studies have been carried out and shed light on the gene expression profile of miRNAs involved particularly in neurogenesis. For instance, miR-124a and miR-9 have been regarded as most specific and well-studied brain miRNAs which promote neural differentiation (Liu and Zhao, 2009). Four miRNAs (miR-1290, miR-26b, miR-194, and miR-124a) have been recently shown to be up-regulated during neuronal differentiation of UCMSCs. This study also disclosed two down-regulated miRNAs (miR-4521 and miR-543) with unknown functions in neurogenesis (Zhuang et al., 2015). Moreover, Meng et al. (2016) compared the gene expression profile of miRNAs in umbilical cord and cord blood-derived MSCs and interestingly demonstrated that the expression of genes related to neurogenesis was increased in UCMSCs, which is contrary to the finding performed by Secco et al. (2009). This dissimilarity demands a detailed investigation into miRNA signature of tissue-specific MSCs prior to any clinical application.
As mentioned above, miRNAs have critical roles in cellular processes. Autophagy is a self-degradation process of cytosolic components, in which defective cellular constituents are degraded through delivery to lysosomes. A recent study has suggested the significant implication of autophagic flux in neural differentiation (Vessoni et al., 2011). Indeed, genetic ablation of Atg5 and Atg7, both involved in autophagic machinery, in the mouse brain has been shown to lead to neurodegenerative disorders (Nikoletopoulou et al., 2015). Further, emerging evidence indicates a strong link between miRNA regulatory networks and autophagy pathways (Frankel and Lund, 2012). Thus, miRNA-autophagy interactions during neuronal differentiation of UCMSCs should be well elucidated to improve the therapeutic potential of UCMSCs.
Conclusion
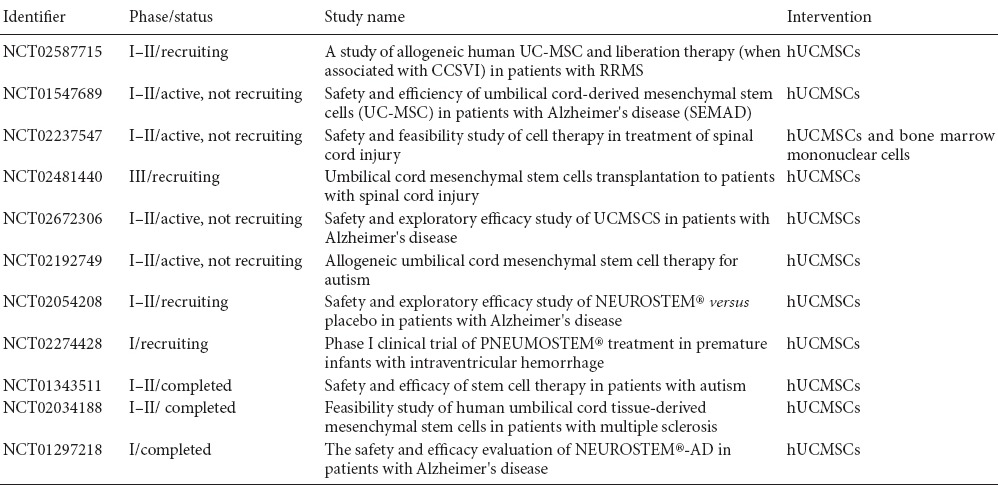
Pharmacotherapeutic options (including Mitoquinone, PYM50028 and Rasagiline) hitherto failed to improve PD patients in recent clinical trials, despite their great promise in experimental researches (Athauda and Foltynie, 2015). Likewise, Levodopa administration to restore dopamine levels causes dyskinesia few years after treatment initiates (Prashanth et al., 2011). Moreover, the new drug development process is very costly and time-consuming (Sherer et al., 2012). MSCs-based approaches have opened a promising avenue into treatment of patients with neurological disorders such as PD. In this review, the potential therapeutic application of UCMSCs in PD patients was outlined. As discussed above, due mainly to their non-tumorigenic as well as low immunogenic capacity, UCMSCs could be considered as suitable cell candidates for neuro-regeneration and neuro-repair in treating neurodegenerative diseases with specific focus on PD. Above and beyond, the release of dopamine by UCMSCs has to be under control by measures such as use of regulating genetic elements. However, clinical trials using UCMSCs for PD have not been registered in ClinicalTrials.gov (in April 2017) whereas for other brain diseases, studies are in progress as shown in Table 2. The completed studies are basically indicating that UCMSCs are stable and safe. Considering collected data from clinical trial failures related to neuroprotective drugs, enriched comprehension of mechanisms underlying PD is highly demanded if CRT is being employed. Additionally, the detailed outcomes of these trials will surely assist in setting up improved methods of cell preparation, purification and DAergic neuron enrichment, pre-injection cell manipulation, transplantation procedures, cell integration to neural network and their paracrine effects beside long-term potential recovery from PD symptoms.
Table 2.
Current trials using human umbilical cord mesenchymal stem cells (UCMSCs) for neurological disorders (retrieved from ClinicalTrials.gov, April 2017)
Acknowledgments
The authors would like to thank all colleagues and staff in National Institute of Genetic Engineering and Biotechnology where they assisted us in this review.
Footnotes
Conflicts of interest: None declared.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Copyedited by Li CH, Song LP, Zhao M
References
- 1.Aliaghaei A, Gardaneh M, Maghsoudi N, Salehinejad P, Gharib E. Dopaminergic induction of umbilical cord mesenchymal stem cells by conditioned medium of choroid plexus epithelial cells reduces apomorphine-induced rotation in Parkinsonian rats. Arch Iran Med. 2016;19:561–570. [PubMed] [Google Scholar]
- 2.Athauda D, Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol. 2015;11:25–40. doi: 10.1038/nrneurol.2014.226. [DOI] [PubMed] [Google Scholar]
- 3.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 4.Bankiewicz KS, Plunkett RJ, Kophin IJ, Jacobowitz DM, London WT, Oldfield EH. Transient behavioral recovery in hemiparkinsonian primates after adrenal medullary allografts. Prog Brain Res. 1988;78:543–549. doi: 10.1016/s0079-6123(08)60329-5. [DOI] [PubMed] [Google Scholar]
- 5.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 6.Boroujeni ME, Gowda P, Johnson J, Rao J, Saremy S. The proliferation and differentiation capacity of bone marrow derived human mesenchymal stem cells in early and late doubling. Asian J Biochem. 2012;7:27–36. [Google Scholar]
- 7.Boroujeni ME, Gardaneh M, Shahriari MH, Aliaghaei A, Hasani S. Synergy between choroid plexus epithelial cells-conditioned medium and knockout serum replacement converts human adipose-derived stem cells to dopamine-secreting neurons. Rejuvenation Res. 2017 doi: 10.1089/rej.2016.1887. doi: 10.1089/rej.2016.1887. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Del Tredici K. Assessing fetal nerve cell grafts in Parkinson's disease. Nat Med. 2008;14:483–485. doi: 10.1038/nm0508-483. [DOI] [PubMed] [Google Scholar]
- 9.Brundin P, Hagell P. The neurobiology of cell transplantation in Parkinson's disease. Clin Neurosci Res. 2001;1:507–520. [Google Scholar]
- 10.Chakrabarty K, Von Oerthel L, Hellemons A, Clotman F, Espana A, Koerkamp MG, Holstege FC, Pasterkamp RJ, Smidt MP. Genome wide expression profiling of the mesodiencephalic region identifies novel factors involved in early and late dopaminergic development. Biol Open. 2012;1:693–704. doi: 10.1242/bio.20121230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 12.Christensen M, Schratt GM. MicroRNA involvement in developmental and functional aspects of the nervous system and in neurological diseases. Neurosci Lett. 2009;466:55–62. doi: 10.1016/j.neulet.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 13.Cortés A, Moreno E, Rodríguez-Ruiz M, Canela EI, Casadó V. Targeting the dopamine D3 receptor: an overview of drug design strategies. Expert Opin Drug Discov. 2016;11:641–664. doi: 10.1080/17460441.2016.1185413. [DOI] [PubMed] [Google Scholar]
- 14.D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 15.Dalous J, Larghero J, Baud O. Transplantation of umbilical cord-derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives. Pediatr Res. 2012;71:482–490. doi: 10.1038/pr.2011.67. [DOI] [PubMed] [Google Scholar]
- 16.Datta I, Mishra S, Mohanty L, Pulikkot S, Joshi PG. Neuronal plasticity of human Wharton's jelly mesenchymal stromal cells to the dopaminergic cell type compared with human bone marrow mesenchymal stromal cells. Cytotherapy. 2011;13:918–932. doi: 10.3109/14653249.2011.579957. [DOI] [PubMed] [Google Scholar]
- 17.Espejo EF, Montoro RJ, Armengol JA, López-Barneo J. Cellular and functional recovery of Parkinsonian rats after intrastriatal transplantation of carotid body cell aggregates. Neuron. 1998;20:197–206. doi: 10.1016/s0896-6273(00)80449-3. [DOI] [PubMed] [Google Scholar]
- 18.Fallon J, Reid S, Kinyamu R, Opole I, Opole R, Baratta J, Korc M, Endo TL, Duong A, Nguyen G, Karkehabadhi M, Twardzik D, Patel S, Loughlin S. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci U S A. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox IJ, Daley GQ, Goldman SA, Huard J, Kamp TJ, Trucco M. Use of differentiated pluripotent stem cells in replacement therapy for treating disease. Science. 2014:345. doi: 10.1126/science.1247391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankel LB, Lund AH. MicroRNA regulation of autophagy. Carcinogenesis. 2012;33:2018–2025. doi: 10.1093/carcin/bgs266. [DOI] [PubMed] [Google Scholar]
- 21.Fu YS, Cheng YC, Lin MYA, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs E, Segre JA. Stem Cells. Cell. 2000;100:143–155. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- 23.Gardaneh M. Dopamine-synthesizing neurons: An overview of their development and application for cell therapy. Iran J Biotechnol. 2010;8:8. [Google Scholar]
- 24.Gardaneh M, Panahi Y, Shojaei S, Mazaheri-Tehrani E, Maghsudi N. Neuroprotection in Parkinson's disease: a multi-directional genetic strategy for maximum protection of dopaminergic neurons against Parkinsonian toxicity. J Med Hyp Ideas. 2010;4:7. [Google Scholar]
- 25.Glavaski-Joksimovic A, Virag T, Chang QA, West NC, Mangatu TA, McGrogan MP, Dugich-Djordjevic M, Bohn MC. Reversal of dopaminergic degeneration in a Parkinsonian rat following micrografting of human bone marrow-derived neural progenitors. Cell Transplant. 2009;18:801–814. doi: 10.3727/096368909X470801. [DOI] [PubMed] [Google Scholar]
- 26.Ha Y, Yoon SH, Park HC, Kim KN, Yoon DH, Cho YE. Transplantation of human umbilical cord blood improves neurological outcomes in the rats after traumatic spinal cord injury. J Korean Neurosurg Soc. 2004;35:302–308. [Google Scholar]
- 27.Hao QL, Shah AJ, Thiemann FT, Smogorzewska EM, Crooks G. A functional comparison of CD34+ CD38– cells in cord blood and bone marrow. Blood. 1995;86:3745–3753. [PubMed] [Google Scholar]
- 28.Hegarty SV, Sullivan AM, O’Keeffe GW. Midbrain dopaminergic neurons: a review of the molecular circuitry that regulates their development. Dev Biol. 2013;379:123–138. doi: 10.1016/j.ydbio.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Hegarty SV, Sullivan AM, O’Keeffe GW. The Epigenome as a therapeutic target for Parkinson's disease. Neural Regen Res. 2016;11:1735. doi: 10.4103/1673-5374.194803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch EC, Faucheux B, Damier P, Mouatt-Prigent A, Agid Y. Advances in Research on Neurodegeneration. Springer Science + Business Media; 1997. Neuronal vulnerability in Parkinson’s disease; pp. 79–88. [DOI] [PubMed] [Google Scholar]
- 31.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FHJ, Willemze R, Fibbe WE, Kanhai HHH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 33.Isacson O, Bjorklund LM, Schumacher JM. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson's disease by stem cells. Ann Neurol. 2003;53:S135–S148. doi: 10.1002/ana.10482. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 35.Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang EJ, Lee YH, Kim MJ, Lee YM, Mohana Kumar B, Jeon BG, Ock SA, Kim HJ, Rho GJ. Transplantation of porcine umbilical cord matrix mesenchymal stem cells in a mouse model of Parkinson's disease. J Tissue Eng Regen Med. 2013;7:169–182. doi: 10.1002/term.504. [DOI] [PubMed] [Google Scholar]
- 37.Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DÖ, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319–331. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- 38.Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, Oh W, Park SH, Sung YC, Jeun SS. Gene therapy using TRAIL-secreting human umbilical cord blood–derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 39.Kitada M, Dezawa M. Parkinson's disease and mesenchymal stem cells: potential for cell-based therapy. Parkinsons Dis. 2012;2012:1–9. doi: 10.1155/2012/873706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko TL, Fu YY, Shih YH, Lin YH, Ko MH, Fu TW, Lin TY, Hsiao HS, Chu PM, Fu YS. A high efficiency induction of dopaminergic cells from human umbilical mesenchymal stem cells for the treatment of hemiparkinsonian rats. Cell Transplant. 2015;24:2251–2262. doi: 10.3727/096368914X685078. [DOI] [PubMed] [Google Scholar]
- 41.Kuznetsov SA, Friedenstein AJ, Gehron Robey P. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997;97:561–570. doi: 10.1046/j.1365-2141.1997.902904.x. [DOI] [PubMed] [Google Scholar]
- 42.Levy YS, Bahat-Stroomza M, Barzilay R, Burshtein A, Bulvik S, Barhum Y, Panet H, Melamed E, Offen D. Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson's disease. Cytotherapy. 2008;10:340–352. doi: 10.1080/14653240802021330. [DOI] [PubMed] [Google Scholar]
- 43.Li JF, Yin HL, Shuboy A, Duan HF, Lou JY, Li J, Wang HW, Wang YL. Differentiation of hUC-MSC into dopaminergic-like cells after transduction with hepatocyte growth factor. Mol Cell Biochem. 2013;381:183–190. doi: 10.1007/s11010-013-1701-z. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Zhang SZ, Guo YW, Cai YQ, Yan ZJ, Zou Z, Jiang XD, Ke YQ, He XY, Jin ZL. Human umbilical vein-derived dopaminergic-like cell transplantation with nerve growth factor ameliorates motor dysfunction in a rat model of Parkinson's disease. Neurochem Res. 2010;35:1522–1529. doi: 10.1007/s11064-010-0211-6. [DOI] [PubMed] [Google Scholar]
- 45.Lin YT, Chern Y, Shen CK, Wen HL, Chang YC, Li H, Cheng TH, Hsieh-Li HM. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington's disease mouse models. PLoS One. 2011;6:e22924. doi: 10.1371/journal.pone.0022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindvall O, Björklund A. Cell therapy in Parkinson's disease. Neurotherapeutics. 2004;1:382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C, Zhao X. MicroRNAs in adult and embryonic neurogenesis. Neuromolecular Med. 2009;11:141–152. doi: 10.1007/s12017-009-8077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu XS, Li JF, Wang SS, Wang YT, Zhang YZ, Yin HL, Geng S, Gong HC, Han B, Wang YL. Human umbilical cord mesenchymal stem cells infected with adenovirus expressing HGF promote regeneration of damaged neuron cells in a Parkinson's disease model. Biomed Res Int. 2014;2014:909657. doi: 10.1155/2014/909657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maccallini C, Amoroso R. Targeting neuronal nitric oxide synthase as a valuable strategy for the therapy of neurological disorders. Neural Regen Res. 2016;11:1731–1734. doi: 10.4103/1673-5374.194707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madhavan L, Daley BF, Paumier KL, Collier TJ. Transplantation of subventricular zone neural precursors induces an endogenous precursor cell response in a rat model of Parkinson's disease. J Comp Neurol. 2009;515:102–115. doi: 10.1002/cne.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathieu P, Roca V, Gamba C, del Pozo A, Pitossi F. Neuroprotective effects of human umbilical cord mesenchymal stromal cells in an immunocompetent animal model of Parkinson's disease. J Neuroimmunol. 2012;246:43–50. doi: 10.1016/j.jneuroim.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 52.McLaughlin D, Tsirimonaki E, Vallianatos G, Sakellaridis N, Chatzistamatiou T, Stavropoulos-Gioka C, Tsezou A, Messinis I, Mangoura D. Stable expression of a neuronal dopaminergic progenitor phenotype in cell lines derived from human amniotic fluid cells. J Neurosci Res. 2006;83:1190–1200. doi: 10.1002/jnr.20828. [DOI] [PubMed] [Google Scholar]
- 53.Meng X, Sun B, Xue M, Xu P, Hu F, Xiao Z. Comparative analysis of microRNA expression in human mesenchymal stem cells from umbilical cord and cord blood. Genomics. 2016;107:124–131. doi: 10.1016/j.ygeno.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 54.Meyer W, Rumpelt H, Yao A, Lind J. Structure and closure mechanism of the human umbilical artery. Eur J Pediatr. 1978;128:247–259. doi: 10.1007/BF00445610. [DOI] [PubMed] [Google Scholar]
- 55.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T. Matrix cells from Wharton's jelly form neurons and glia. Stem cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 57.Mohapel P, Frielingsdorf H, Häggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132:767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 58.Murrell W, Wetzig A, Donnellan M, Féron F, Burne T, Meedeniya A, Kesby J, Bianco J, Perry C, Silburn P. Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson's disease. Stem Cells. 2008;26:2183–2192. doi: 10.1634/stemcells.2008-0074. [DOI] [PubMed] [Google Scholar]
- 59.Nakao N, Shintani-Mizushima A, Kakishita K, Itakura T. The ability of grafted human sympathetic neurons to synthesize and store dopamine: a potential mechanism for the clinical effect of sympathetic neuron autografts in patients with Parkinson's disease. Exp Neurol. 2004;188:65–73. doi: 10.1016/j.expneurol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Nikoletopoulou V, Papandreou M, Tavernarakis N. Autophagy in the physiology and pathology of the central nervous system. Cell Death Differ. 2015;22:398–407. doi: 10.1038/cdd.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, Beute GN, van Vugt JP, Lenders MW, Contarino MF. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12:37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- 62.Olson SD, Pollock K, Kambal A, Cary W, Mitchell GM, Tempkin J, Stewart H, McGee J, Bauer G, Kim HS. Genetically engineered mesenchymal stem cells as a proposed therapeutic for Huntington's disease. Mol Neurobiol. 2012;45:87–98. doi: 10.1007/s12035-011-8219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peister A. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 64.Pires AO, Mendes-Pinheiro B, Teixeira FG, Anjo SI, Ribeiro-Samy S, Gomes ED1, Serra SC, Silva NA, Manadas B, Sousa N, Salgado AJ. Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: a proteomic analysis. Stem Cells Dev. 2016;25:1073–1083. doi: 10.1089/scd.2016.0048. [DOI] [PubMed] [Google Scholar]
- 65.Pountos I, Giannoudis PV. Biology of mesenchymal stem cells. Injury. 2005;36:S8–12. doi: 10.1016/j.injury.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 66.Prashanth L, Fox S, Meissner WG. l-Dopa-induced dyskinesia-clinical presentation, genetics, and treatment. Int Rev Neurobiol. 2011;98:31–54. doi: 10.1016/B978-0-12-381328-2.00002-X. [DOI] [PubMed] [Google Scholar]
- 67.Schwinger W, Urban C, Lackner H, Benesch M, Kerbl R, Dornbusch H, Sovinz P, Kogler G. CASE REPORTS-Unrelated 5/6-locus matched umbilical cord blood transplantation in a 23-month-old child with hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 1998;22:393–396. doi: 10.1038/sj.bmt.1701338. [DOI] [PubMed] [Google Scholar]
- 68.Secco M, Moreira YB, Zucconi E, Vieira NM, Jazedje T, Muotri AR, Okamoto OK, Verjovski-Almeida S, Zatz M. Gene expression profile of mesenchymal stem cells from paired umbilical cord units: cord is different from blood. Stem Cell Rev. 2009;5:387–401. doi: 10.1007/s12015-009-9098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sherer TB, Chowdhury S, Peabody K, Brooks DW. Overcoming obstacles in Parkinson's disease. Mov Disord. 2012;27:1606–1611. doi: 10.1002/mds.25260. [DOI] [PubMed] [Google Scholar]
- 70.Shetty P, Thakur AM, Viswanathan C. Dopaminergic cells, derived from a high efficiency differentiation protocol from umbilical cord derived mesenchymal stem cells, alleviate symptoms in a Parkinson's disease rodent model. Cell Biol Int. 2013;37:167–180. doi: 10.1002/cbin.10029. [DOI] [PubMed] [Google Scholar]
- 71.Stocchi F. Therapy for Parkinson's disease: what is in the pipeline? Neurotherapeutics. 2014;11:24–33. doi: 10.1007/s13311-013-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Storch A, Sabolek M, Milosevic J, Schwarz SC, Schwarz J. Midbrain-derived neural stem cells: from basic science to therapeutic approaches. Cell Tissue Res. 2004;318:15–22. doi: 10.1007/s00441-004-0923-5. [DOI] [PubMed] [Google Scholar]
- 73.Subramanian T, Marchionini D, Potter EM, Cornfeldt ML. Striatal xenotransplantation of human retinal pigment epithelial cells attached to microcarriers in hemiparkinsonian rats ameliorates behavioral deficits without provoking a host immune response. Cell Transplant. 2002;11:207–214. [PubMed] [Google Scholar]
- 74.Taghizadeh R, Cetrulo K, Cetrulo C. Wharton's Jelly stem cells: future clinical applications. Placenta. 2011;32:S311–315. doi: 10.1016/j.placenta.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 75.Thomson JA. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 76.Tracy E, Aldrink J, Panosian J, Beam D, Thacker J, Reese M, Kurtzberg J. Isolation of oligodendrocyte-like cells from human umbilical cord blood. Cytotherapy. 2008;10:518–525. doi: 10.1080/14653240802154586. [DOI] [PubMed] [Google Scholar]
- 77.Vessoni AT, Muotri AR, Okamoto OK. Autophagy in stem cell maintenance and differentiation. Stem Cells Dev. 2011;21:513–520. doi: 10.1089/scd.2011.0526. [DOI] [PubMed] [Google Scholar]
- 78.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 79.Wei X, Peng G, Zheng S, Wu X. Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Prolif. 2012;45:101–110. doi: 10.1111/j.1365-2184.2012.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem cells. 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 81.Xiong N, Cao X, Zhang Z, Huang J, Chen C, Zhang Z, Jia M, Xiong J, Liang Z, Sun S. Long-term efficacy and safety of human umbilical cord mesenchymal stromal cells in rotenone-induced hemiparkinsonian rats. Biol Blood Marrow Transplant. 2010;16:1519–1529. doi: 10.1016/j.bbmt.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Xiong N, Zhang Z, Huang J, Chen C, Jia M, Xiong J, Liu X, Wang F, Cao X, Liang Z. VEGF-expressing human umbilical cord mesenchymal stem cells, an improved therapy strategy for Parkinson's disease. Gene Ther. 2011;18:394–402. doi: 10.1038/gt.2010.152. [DOI] [PubMed] [Google Scholar]
- 83.Yan M, Sun M, Zhou Y, Wang W, He Z, Tang D, Lu S, Wang X, Li S, Wang W. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopamine neurons mediated by the Lmx1a and neurturin in vitro: potential therapeutic application for Parkinson's disease in a rhesus monkey model. PLoS One. 2013;8:e64000. doi: 10.1371/journal.pone.0064000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Yang S, Sun HM, Yan JH, Xue H, Wu B, Dong F, Li WS, Ji FQ, Zhou DS. Conditioned medium from human amniotic epithelial cells may induce the differentiation of human umbilical cord blood mesenchymal stem cells into dopaminergic neuron-like cells. J Neurosci Res. 2013a;91:978–986. doi: 10.1002/jnr.23225. [DOI] [PubMed] [Google Scholar]
- 85.Yang S, Xue DD, Wu B, Sun HM, Li XS, Dong F, Li WS, Ji FQ, Zhou DS. Pleiotrophin is involved in the amniotic epithelial cell-induced differentiation of human umbilical cord blood-derived mesenchymal stem cells into dopaminergic neuron-like cells. Neurosci Lett. 2013b;539:86–91. doi: 10.1016/j.neulet.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Wang J, Ren M, Li M, Chen D, Chen J, Shi F, Wang X, Dou J. Gene therapy of ovarian cancer using IL-21-secreting human umbilical cord mesenchymal stem cells in nude mice. J Ovarian Res. 2014;7:8. doi: 10.1186/1757-2215-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao C, Li H, Zhao XJ, Liu ZX, Zhou P, Liu Y, Feng MJ. Heat shock protein 60 affects behavioral improvement in a rat model of Parkinson's disease grafted with human umbilical cord mesenchymal stem cell-derived dopaminergic-like neurons. Neurochem Res. 2016;41:1238–1249. doi: 10.1007/s11064-015-1816-6. [DOI] [PubMed] [Google Scholar]
- 88.Zhuang H, Zhang R, Zhang S, Shu Q, Zhang D, Xu G. Altered expression of microRNAs in the neuronal differentiation of human Wharton's Jelly mesenchymal stem cells. Neurosci Lett. 2015;600:69–74. doi: 10.1016/j.neulet.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 89.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]


