Abstract
Oxidative stress and inflammation are part and parcel of cisplatin-induced nephrotoxicity. The purpose of this work is to study the role of soy isoflavone constituent, daidzein, in cisplatin-induced renal damage. Cisplatin-induced nephrotoxicity was evident by the histological damage in proximal tubular cells and by the increase in serum neutrophil gelatinase-associated lipocalin (NGAL), blood urea nitrogen (BUN), creatinine, and urinary kidney injury molecule-1 (KIM-1). Cisplatin-induced cell death was shown by TUNEL staining and caspase-3/7 activity. Daidzin treatment reduced all kidney injury markers (NGAL, BUN, creatinine, and KIM-1) and attenuated cell death (apoptotic markers). In cisplatin-induced kidney injury, renal oxidative/nitrative stress was manifested by the increase in lipid peroxidation and protein nitration. Cisplatin induced the reactive oxygen species-generating enzyme NOX-2 and impaired antioxidant defense enzyme activities such as glutathione peroxidase (GPX) and superoxide dismutase (SOD) activities. Cisplatin-induced oxidative/nitrative stress was attenuated by daidzein treatment. Cisplatin induced CD11b-positive macrophages in kidneys and daidzein attenuated CD11b-positive cells. Daidzein attenuated cisplatin-induced inflammatory cytokines tumor necrosis factor α (TNFα), interleukin 10 (IL-10), interleukin 18 (IL-18), and monocyte chemoattractant protein-1 (MCP-1). Daidzein attenuated cell death in vitro. Our data suggested that daidzein attenuated cisplatin-induced kidney injury through the downregulation of oxidative/nitrative stress, immune cells, inflammatory cytokines, and apoptotic cell death, thus improving kidney regeneration.
1. Introduction
Cisplatin is a commonly used anticancer drug for the treatment of solid tumors. The mechanism of cancer cell killing is through its DNA-binding properties by forming adducts and stopping replication of cancer cells. One of the major side effects of cisplatin is nephrotoxicity and is mediated by preferential absorption of cisplatin in proximal tubules through a specific transporter [1]. Hydration has been used to alleviate this issue with some success. However, the dose-dependent nephrotoxicity is thus a limiting factor during cisplatin chemotherapy. The mechanism of cisplatin-mediated nephrotoxicity is mediated by apoptotic cell death induced by oxidative stress and inflammation.
Cisplatin is mainly excreted by kidneys, and high concentration of cisplatin accumulated there due to the basolateral organic cation system [2]. Cisplatin also accumulates in mitochondria and modulates its bioenergetics [3]. However, substantial literatures indicate that oxidative stress plays a critical role in renal damage [4]. Cell death associated with oxidative stress leads to inflammatory response and is highly relevant to the pathogenesis of cisplatin-induced nephrotoxicity [5].
Various natural bioactive compounds, which have antioxidant and anti-inflammatory properties, exhibit renoprotective activity in an animal model of cisplatin nephrotoxicity. Daidzein is found in soybeans and is a constituent of Chinese traditional medicine Nao Mai Tong formula. Daidzein is an isoflavone and has antioxidant, anti-inflammatory, and phytoestrogenic properties. Daidzein demonstrates anti-inflammatory effects on endotoxin-induced RAW 264.7 macrophages [6]. In a clinical trial, both soy and purified daidzein improved renal function [7]. Daidzein also inhibits STAT-1 and NF-κB activations in an activated macrophage [8]. Daidzein has cardioprotective and antiarthritogenic effects on rheumatoid arthritis. A clinical trial (ClinicalTrials.gov NCT02075112) is currently ongoing with soy supplementation during cisplatin chemotherapy and radiation therapy for head and neck cancer to decrease side effects caused by treatments.
Here, we demonstrated that daidzein is protective against cisplatin-induced nephrotoxicity. The protective effect was mediated by its antioxidant and anti-inflammatory properties.
2. Materials and Methods
2.1. Mouse Experiments
All protocols were approved by the Committee on the Ethics of Animal Experiments of the First Affiliated Hospital, College of Medicine, Zhejiang University, under the guidance of the Chinese Academy of Sciences. The mouse strain C57BL/6 was used as described before [9]. Male mice of ~8 weeks of age with weights of 18–22 g were used in all experiments. Mice were sacrificed under deep anesthesia with 5% isoflurane followed by cervical dislocation on the third day (72 hours) after a single injection of cisplatin (cis-diammineplatinum (II) dichloride, Sigma) at dose 25 mg/kg i.p. in 5% DMSO/saline vehicle. High-quality daidzein (>98% pure) was purchased from Nanjing Zelang Medical Technology Co. Ltd. Daidzein was dissolved in DMSO/saline and administered at 200 mg/kg, i.p., for two days, starting 1 h after the cisplatin administration. Daidzein and vehicle were also administered alone (without cisplatin treatment) as a separate group.
2.2. Kidney Function
Serum levels of blood urea nitrogen (BUN) and creatinine were measured as described earlier [10]. Serum NGAL and urinary KIM-1 were measured from serum using Mouse NGAL Quantikine ELISA Kit and Mouse KIM-1 Quantikine ELISA Kit (R&D Systems China Co. Ltd., Changning, China) according to the manufacturer's instruction.
2.3. Histology
Periodic acid-Schiff (PAS) staining for histological examination was performed as described earlier [10]. Slides with PAS staining were examined based on the following four histological criteria and scored. Tubular damage in PAS-stained sections was examined under the microscope and scored based on the percentage of cortical tubules showing epithelial necrosis: 0—normal, 1—<10%, 2—10 to 25%, 3—26 to 75%, and 4—>75%. Tubular necrosis was defined as the loss of the proximal tubular brush border, blebbing of apical membranes, tubular epithelial cell detachment from the basement membrane, or intraluminal aggregation of cells and proteins. The morphometric examinations were performed in a blinded manner.
Protein nitrotyrosine staining using monoclonal anti-nitrotyrosine antibody (Cayman Chemical, NeoBioscience Technology, Shenzhen, China) was performed as described earlier [10].
2.4. Fluorescence Microscopy
Kidneys were sectioned with a microtome, deparaffinized, and stained as provided below with a fluorescence microscope. Apoptosis was detected in the kidneys by the TUNEL assay (Roche Diagnostics, Indianapolis, IN, USA) along with nuclear staining using Hoechst 33342 (Solarbio, China) as described earlier [10]. CD11b-conjugated FITC (BD Biosciences, USA) for neutrophils/monocytes (leukocytes) and nuclear stain Hoechst 33342 (Solarbio, China) were used in kidney sections.
2.5. Renal Apoptosis
Caspase-3/7 activity of the lysate was measured using Apo-ONE Homogenous Caspase-3/7 Assay Kit (Promega Corp., Madison, WI, USA) as described earlier [9]. Caspase-3/7 activity was presented as caspase-3 activity in the figure and text. The activity was expressed as fold change.
2.6. Renal HNE Protein Adducts and Protein Nitration
Nitrotyrosine content was evaluated by ELISA as described [9] HNE adducts were determined using OxiSelect™ HNE Adduct ELISA Kit (Cell Biolabs, Genetimes Technology Inc., Shanghai, China) as described earlier [9].
2.7. Quantitative Determination of SOD Activity
SOD activity was determined from tissue lysates using an SOD activity kit (Enzo Life Sciences International Inc., Plymouth Meeting, PA, USA) as described before [9].
2.8. Glutathione Peroxidase Assay
Glutathione peroxidase was measured using a Glutathione Peroxidase (GPX) Assay Kit (Abcam Trading Company Ltd., Shanghai, China) according to the manufacturer's instruction.
2.9. Glutathione Content
Glutathione (GSH) was determined by using a colorimetric kit (Jiancheng Bioengineering Institute, China) according to the manufacturer's instructions.
2.10. Real-Time PCR
Isolation of RNA and real-time PCR were carried out as described earlier [9, 10]. The primer sets for TNFα (PPM03113G), IL-18 (PPM03112B), IL-10 (PPM03017C), MCP-1 (PPM03151G), NOX2 (PPM32951A), and β-actin (PPM02945B) were purchased from Qiagen (Pudong, Shanghai, China).
2.11. Renal Western Blot
Western blot was performed as described previously [11].
2.12. Cell Culture and Flow Cytometry Analyses
HK-2 cells were grown and processed as described earlier [9]. Cisplatin was added at 50 μM and vehicle or daidzein at 30 μM after a 30 min delay to cisplatin addition for 24 hours. Flow cytometry experiments were performed as described earlier [9].
2.13. Statistical Analysis
All data were presented as the means ± SEMs. Multiple comparisons (Tukey) were performed using one-way ANOVA. The analyses were performed with GraphPad Prism software (GraphPad Software Inc., CA, USA). A p value <0.05 was considered statistically significant.
3. Results and Discussion
3.1. Effect of Daidzein on Cisplatin-Induced Tubular Damage, Kidney Injury, and Cell Death
Cisplatin administration to C57BL6 mice led to significant tubular damage at 72 hours as observed in PAS staining (Figure 1). Histological examination and quantification revealed vacuolation, protein cast formation, and desquamation of epithelial cells in the renal tubules. The damage was significantly attenuated by daidzein treatment in mice. Cisplatin also induced renal dysfunction as found by the kidney injury parameters such as NGAL, BUN, creatinine, and urinary KIM-1 (Figure 2). Cisplatin administration resulted in a 2.46-, 6.92-, 9.93-, and 20-fold increase in NGAL, BUN, creatinine, and KIM-1, respectively. Daidzein attenuated all kidney injury markers. Daidzein at 200 mg/kg reduced cisplatin-induced kidney injury as shown by a decrease in serum levels of NGAL (2.93 to 1.60), BUN (153.5 to 103.6), creatinine (1.65 to 1.11), and urinary KIM-1 (3.7 to 1.98). Cisplatin is known to cause apoptotic cell death in the kidney. We have observed a significant increase in TUNEL staining in cisplatin kidney, and the number reduced after daidzein treatment (Figure 3(a)). Quantitative determination of caspase-3/7 activity demonstrated that cisplatin induced 3.86-fold increases and daidzein treatment reduced 43.9% of caspase-3/7 activity (Figure 3(b)).
Figure 1.
Effect of daidzein on cisplatin-induced kidney tubular damage in mice. (a) Cisplatin induced tubular damage as shown by PAS staining. The damage was attenuated by daidzein (daidz) treatment at dose 200 mg/kg. (b) Quantification of the tubular damage score from PAS-stained slide. Results are mean ± SEM (n = 6/group). ∗p < 0.05 versus vehicle and #p < 0.05 versus cisplatin.
Figure 2.
Effect of daidzein on cisplatin-induced renal dysfunction in mice. Cisplatin caused significant renal dysfunction as determined by the levels of NGAL (a), BUN (b), creatinine (c), and urinary KIM-1 at 72 hours (d). Cisplatin induced kidney injury which was attenuated by daidzein treatment. Results are mean ± SEM (n = 6/group). ∗p < 0.05 versus vehicle and #p < 0.05 versus cisplatin.
Figure 3.
Effects of daidzein on cisplatin-induced cell death. Histological examination (a) demonstrated cisplatin-induced TUNEL staining (green) in the kidney and TUNEL staining was significantly attenuated with daidzein administration. Nuclei were stained with Hoechst 33342 (blue). (b) Caspase-3 activities were determined and daidzein attenuated cisplatin-induced caspase-3 activity. Results are mean ± SEM (n = 6/group). ∗p < 0.05 versus vehicle and #p < 0.05 versus cisplatin.
Nephrotoxic drug-related acute kidney injury in hospital was approximately 20% and increased to 66% for the elderly [5]. Cisplatin accumulated at high concentration in the kidneys by the renal transport system, and its toxicity is dose dependent [12]. Whole soy and its constituent daidzein have a positive effect on renal function in a clinical trial [7]. Pharmacokinetics of daidzein in human, mouse, and rat demonstrates its presence as glucuronides [13, 14]. Comparative pharmacokinetics of traditional Chinese medicine Nao Mai Tong in a rat study also demonstrates the presence of daidzein [15]. In our study, daidzein significantly reduced cisplatin-induced acute kidney injuries by improving kidney function and prevented tubular cell death.
3.2. Effect of Daidzein on Cisplatin-Induced Oxidative Stress and Impaired Antioxidant Defense
Cisplatin-induced nephrotoxicity is mediated by oxidative stress [16]. We evaluated the effect of daidzein on cisplatin-induced oxidative footprints such as HNE protein adducts and protein nitration. Both oxidative stress markers HNE protein adducts and protein nitration were increased 2.6- and 2.9-fold in cisplatin-treated mice (Figure 4(a)). Treatment with daidzein reduced 39.9% and 48.7% of HNE protein adducts and protein nitration, respectively. We also examined protein nitration by histological staining, and daidzein significantly reduced cisplatin-induced protein nitration (Figure 4(b)). In all above experiments, daidzein did not change any oxidative stress marker when administered alone.
Figure 4.
Effect of daidzein on cisplatin-induced oxidative/nitrative stress. (a) Quantitative measurement of HNE adducts and protein nitration by ELISA demonstrated cisplatin-induced lipid peroxidation and protein nitration. Daidzein attenuated both cisplatin-induced oxidative/nitrative stress markers. (b) Histological staining of protein nitration. A trend similar to quantitative protein nitration was observed. Results are mean ± SEM (n = 6/group). ∗p < 0.05 versus vehicle and #p < 0.05 versus cisplatin.
The balance of reactive oxygen species- (ROS-) generating enzymes and antioxidant defense enzymes is critical for cisplatin nephrotoxicity [10, 17]. We found that cisplatin-induced gene expression of the ROS-generating enzyme NOX2 is significantly attenuated by daidzein (3.8- to 2.3-fold, Figure 5(a)). Glutathione plays a critical role in cisplatin-induced kidney injury [18]. Daidzein also improved up to 66% of cisplatin-mediated depletion of reduced glutathione in mouse kidney (Figure 5(b)). In addition to that, daidzein enhanced a cisplatin-mediated reduction in glutathione peroxidase activity and total SOD activity up to 49% and 55%, respectively (Figures 5(c) and 5(d)).
Figure 5.
Effect of daidzein on cisplatin-induced changes in the ROS-generating enzyme NOX2 and antioxidant defense in mice. (a) Cisplatin induced the ROS-generating enzyme NOX2 mRNA as determined by real-time PCR, and daidzein attenuated cisplatin-reduced reduced glutathione reserve, glutathione peroxidase activity, and SOD activity. Daidzein administration restored those antioxidant defenses close to the control group. Results are mean ± SEM (n = 6/group). ∗p < 0.05 versus vehicle and #p < 0.05 versus cisplatin.
Oxidative stress plays a critical role in cisplatin-induced acute kidney injury [16, 19, 20]. Previous studies show that cisplatin mediated an increase in lipid peroxidation (one oxidative stress marker) and is attenuated by flavonoids and antioxidants [21–23]. Protein nitration is mediated by peroxynitrite and another hallmark of cisplatin-induced oxidative stress in the kidney [9]. Here, we demonstrated that daidzein attenuated cisplatin-induced protein nitration and lipid peroxidation (HNE adducts). NOX2 is one among several sources for oxidative stress [24, 25]. Daidzein attenuated cisplatin-induced NOX2 expression. Antioxidant defense is also critical in cisplatin-induced nephropathy [9, 26]. Cisplatin reduces the reserve of reduced glutathione, SOD activity, and glutathione peroxidase activity in kidneys [27–29]. Consistent with earlier studies, cisplatin impaired reduced glutathione reserve and decreased glutathione peroxidase activity and SOD activity. Daidzein improved all three antioxidant defense mechanisms and thus ameliorated cisplatin-induced oxidative stress.
3.3. Effect of Daidzein on Cisplatin-Induced Leukocyte Infiltration and Inflammatory Cytokines in the Kidney
Cisplatin-induced inflammatory response followed by infiltration of neutrophils and macrophages has been reported earlier [10, 19, 24]. Consistent with earlier findings, we observed CD11b-positive cells in cisplatin-induced kidney injury and daidzein significantly reduced CD11b-positive cells (Figure 6). Cisplatin induces several cytokines such as TNFα, IL-10, IL-18, and MCP-1 in kidney injury [30–32]. Cisplatin induced TNFα, IL-10, IL-18, and MCP-1 mRNA expression to 4.3-, 3.5-, 3.4-, and 2.98-fold, respectively (Figure 7(a)). Daidzein treatment attenuated 39.9%, 46.2%, 47%, and 43.2% of TNFα, IL-10, IL-18, and MCP-1 mRNA expression, respectively. We further verified one of the cytokines TNFα by Western blot analyses (Figure 7(b)), and the result was consistent with mRNA level. GAPDH was used as a loading control.
Figure 6.
Effect of daidzein on cisplatin-induced CD11b-positive monocyte/macrophage in mice. Immunofluorescence examination revealed significant CD11b-positive cells (yellow) of the cisplatin-treated group. Nuclear staining (blue) was carried out using Hoechst 33342. In the cisplatin group, a zoom image of single cells was provided as an inset to demonstrate that staining covers surface staining and a larger area than nuclear staining. Daidzein treatment reduced the number of CD11b-positive cells. Either vehicle (Veh) or daidzein (daidz) control group does not have any CD11b-positive cells.
Figure 7.
Effect of daidzein on cisplatin-induced proinflammatory cytokines in mice. (a) Real-time PCR-based analyses of proinflammatory cytokines TNFα, IL-10, IL-18, and MCP-1 indicated a profound increase in cisplatin-treated mice. Daidzein treatment attenuated cisplatin-induced cytokine MRNA expression. Results are mean ± SEM (n = 6/group). ∗p < 0.05 versus vehicle and #p < 0.05 versus cisplatin. (b) Western blot analyses of TNFα and control GAPDH.
In the pathogenesis of cisplatin-induced nephrotoxicity, inflammation plays another major role [12, 33]. Different immune cells, namely, neutrophils, macrophages, T cells, and dendritic cells, play their role during the inflammatory response [12]. Neutrophils and monocyte-derived macrophages are myeloid cells which pursue common goals to neutralize danger in cisplatin-mediated nephropathy [32, 34]. CD11b is a αM integrin, a member of the integrin family, primarily expressed on monocytes/macrophages. Consistent with earlier data, we also observed a significant increase in a CD11b-positive macrophage in cisplatin-induced kidney injury [35]. Daidzein reduced cisplatin-induced macrophage accumulation in the kidneys. Daidzein inhibits production of nitric oxide and IL-6 in a lipopolysaccharide-induced macrophage [36].
Cisplatin activates the NF-κB pathway, thus facilitating inflammatory cytokines such as TNFα. Daidzein attenuated cisplatin-induced TNFα, and consistent with previous findings, berberine, curcumin, and chlorogenic acid mediated a renoprotective effect [21, 37, 38]. IL-18 is also crucial in cisplatin-mediated toxicity [39]. Genetic deletion of caspase-1, which cleaved IL-18 to make it active, reduced cisplatin kidney injury and neutrophil infiltration [40]. IL-18 was induced by cisplatin and attenuated with daidzein treatment in our study. IL-10 and MCP-1 also play a role in cisplatin-induced kidney injury [31, 41, 42]. We also observed that cisplatin induced both IL-10 and MCP-1 mRNA and those were attenuated by daidzein. Dendritic cells produce IL-10, and the modulation of dendritic cells by daidzein in cisplatin nephropathy cannot be excluded.
3.4. Effect of Daidzein on Cisplatin-Induced Cell Death of Proximal Tubular Cell Line In Vitro
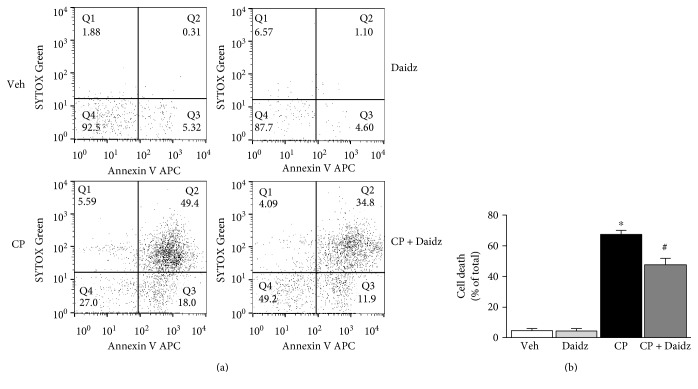
Daidzein reduced both oxidative stress and inflammation. We also examined its effect on cell death under in vitro condition using HK-2 proximal tubular cell line. Cisplatin induced both apoptotic and necrotic cell death at 50 μM for 24 hours (Figure 8(a)). Daidzein addition at 30 μM attenuated cell death by 28.29% (Figure 8(b)). There was no effect of daidzein on HK-2 cell death when added without cisplatin.
Figure 8.
Effect of daidzein on cisplatin-induced cell death in vitro. (a) Representative dot plot of flow cytometric data of an HK-2 cell treated with either saline or daidzein in the presence or absence of cisplatin. x-axis represented the apoptotic cell death marker Annexin V whereas y-axis represented the dead cell marker SYTOX Green. (b) Quantitative determination of cell death (combined Q2 and Q3) among different groups. Results are mean ± SEM (n = 3/group). ∗p < 0.05 versus vehicle and #p < 0.05 versus cisplatin.
Daidzein-mediated protection of cisplatin-induced proximal tubular cell death demonstrated its direct role. Cisplatin-induced nephrotoxicity is a far more complex situation where interplay of oxidative stress, inflammation, and cell death is partially correlated. Because anti-inflammatory properties of daidzein is reported earlier and our data demonstrated its direct role in inflammatory cytokines, we concluded that daidzein had anti-inflammatory properties in addition to its function to prevent cell death. It is also important to note that daidzein has antiestrogenic properties [43]. However, these specific properties of daidzein had no effect on our study as we have used male mice.
4. Conclusions
Cisplatin-induced tubular cell damage, cell death, and associated kidney injury were significantly attenuated with the administration of daidzein in mice. The molecular mechanism of protection is mediated through the interplay of oxidative stress and inflammation (Figure 9). Daidzein modulated cisplatin-induced lipid peroxidation, protein nitration, and NOX2 mRNA. Daidzein also improved cisplatin-impaired antioxidant defense such as reduced glutathione reserve, GPX activity, and SOD activity. Cisplatin-induced macrophage accumulation and proinflammatory cytokine production were attenuated by daidzein.
Figure 9.
Schematic diagram of the protection mechanism of daidzein in cisplatin-induced kidney injury. EGCG inhibit cisplatin-induced ROS by attenuating ROS-generating enzymes and improving cisplatin-impaired antioxidant defense mechanisms in the renal tubular cells which caused cell death. Cell death also leads to proinflammatory response with cytokines and infiltrating leukocytes with the additional release of ROS. Daidzein attenuates cell death directly. Daidzein also neutralize cytokines and infiltrating leukocytes. Both antioxidant and anti-inflammatory effects leads to reduced cell death, thus protecting against cisplatin-induced kidney injury.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (no. 81000302), Science and Technology Department of Zhejiang Province (no. 2015C33214), and Zhejiang Medical and Health Science and Technology Plan Project (2014KYA070).
Disclosure
The funding agency has no role in the study design or conclusion.
Conflicts of Interest
All authors hereby declare that no conflict of interest existed in this study.
Authors' Contributions
Hongzhou Meng, Guanghou Fu, and Jie Shen contributed equally to this work.
References
- 1.Hanigan M. H., Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Therapy. 2003;1:47–61. [PMC free article] [PubMed] [Google Scholar]
- 2.Ciarimboli G., Deuster D., Knief A., et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. The American Journal of Pathology. 2010;176:1169–1180. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos N. A., Bezerra C. S., Martins N. M., Curti C., Bianchi M. L., Santos A. C. Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemotherapy and Pharmacology. 2008;61:145–155. doi: 10.1007/s00280-007-0459-y. [DOI] [PubMed] [Google Scholar]
- 4.Hosohata K. Role of oxidative stress in drug-induced kidney injury. International Journal of Molecular Sciences. 2016;17 doi: 10.3390/ijms17111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peres L. A., da Cunha A. D., Jr. Acute nephrotoxicity of cisplatin: molecular mechanisms. Jornal Brasileiro de Nefrologia. 2013;35:332–340. doi: 10.5935/0101-2800.20130052. [DOI] [PubMed] [Google Scholar]
- 6.Chinta S. J., Ganesan A., Reis-Rodrigues P., Lithgow G. J., Andersen J. K. Anti-inflammatory role of the isoflavone diadzein in lipopolysaccharide-stimulated microglia: implications for Parkinson’s disease. Neurotoxicity Research. 2013;23:145–153. doi: 10.1007/s12640-012-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z. M., Ho S. C., Chen Y. M., Tang N., Woo J. Effect of whole soy and purified isoflavone daidzein on renal function--a 6-month randomized controlled trial in equol-producing postmenopausal women with prehypertension. Clinical Biochemistry. 2014;47:1250–1256. doi: 10.1016/j.clinbiochem.2014.05.054. [DOI] [PubMed] [Google Scholar]
- 8.Hamalainen M., Nieminen R., Vuorela P., Heinonen M., Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators of Inflammation. 2007;2007:10. doi: 10.1155/2007/45673.45673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan H., Chen J., Shen K., et al. Mitochondrial modulation by epigallocatechin 3-gallate ameliorates cisplatin induced renal injury through decreasing oxidative/nitrative stress, inflammation and NF-kB in mice. PLoS One. 2015;10(4, article e0124775) doi: 10.1371/journal.pone.0124775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan H., Shen K., Wang X., Meng H., Wang C., Jin B. Protective effect of metalloporphyrins against cisplatin-induced kidney injury in mice. PLoS One. 2014;9, article e86057 doi: 10.1371/journal.pone.0086057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan H., Shen Z., Mukhopadhyay P., et al. Anaphylatoxin C5a contributes to the pathogenesis of cisplatin-induced nephrotoxicity. American Journal of Physiology-Renal Physiology. 2009;296:F496–F504. doi: 10.1152/ajprenal.90443.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller R. P., Tadagavadi R. K., Ramesh G., Reeves W. B. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010;2:2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Morato J., Farre M., Perez-Mana C., et al. Pharmacokinetic comparison of soy isoflavone extracts in human plasma. Journal of Agricultural and Food Chemistry. 2015;63:6946–6953. doi: 10.1021/acs.jafc.5b02891. [DOI] [PubMed] [Google Scholar]
- 14.Soukup S. T., Helppi J., Muller D. R., et al. Phase II metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: a cross-species and sex comparison. Archives of Toxicology. 2016;90:1335–1347. doi: 10.1007/s00204-016-1663-5. [DOI] [PubMed] [Google Scholar]
- 15.Wu C., Zhao L., Rong Y., Zhu G., Liang S., Wang S. The pharmacokinetic screening of multiple components of the Nao Mai Tong formula in rat plasma by liquid chromatography tandem mass spectrometry combined with pattern recognition method and its application to comparative pharmacokinetics. Journal of Pharmaceutical and Biomedical Analysis. 2016;131:345–354. doi: 10.1016/j.jpba.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Ojha S., Venkataraman B., Kurdi A., Mahgoub E., Sadek B., Rajesh M. Plant-derived agents for counteracting cisplatin-induced nephrotoxicity. Oxidative Medicine and Cellular Longevity. 2016;2016:27. doi: 10.1155/2016/4320374.4320374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez R., Romay C., Borrego A., et al. Lipid peroxides and antioxidant enzymes in cisplatin-induced chronic nephrotoxicity in rats. Mediators of Inflammation. 2005;2005:139–143. doi: 10.1155/MI.2005.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appenroth D., Winnefeld K. Role of glutathione for cisplatin nephrotoxicity in young and adult rats. Renal Failure. 1993;15:135–139. doi: 10.3109/08860229309046144. [DOI] [PubMed] [Google Scholar]
- 19.Ozkok A., Edelstein C. L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Research International. 2014;2014:17. doi: 10.1155/2014/967826.967826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chirino Y. I., Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Experimental and Toxicologic Pathology. 2009;61:223–242. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Domitrovic R., Cvijanovic O., Susnic V., Katalinic N. Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury. Toxicology. 2014;324:98–107. doi: 10.1016/j.tox.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Luo X., Pan H., et al. Pharmacological inhibition of NADPH oxidase protects against cisplatin induced nephrotoxicity in mice by two step mechanism. Food and Chemical Toxicology. 2015;83:251–260. doi: 10.1016/j.fct.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Sahu B. D., Mahesh Kumar J., Sistla R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-kappaB pathways. PLoS One. 2015;10, article e0134139 doi: 10.1371/journal.pone.0134139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahu B. D., Kalvala A. K., Koneru M., et al. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-kappaB activation and antioxidant defence. PLoS One. 2014;9, article e105070 doi: 10.1371/journal.pone.0105070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhopadhyay P., Pan H., Rajesh M., et al. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. British Journal of Pharmacology. 2010;160:657–668. doi: 10.1111/j.1476-5381.2010.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues M. A., Rodrigues J. L., Martins N. M., et al. Carvedilol protects against cisplatin-induced oxidative stress, redox state unbalance and apoptosis in rat kidney mitochondria. Chemico-Biological Interactions. 2011;189:45–51. doi: 10.1016/j.cbi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Darwish M. A., Abo-Youssef A. M., Khalaf M. M., Abo-Saif A. A., Saleh I. G., Abdelghany T. M. Vitamin E mitigates cisplatin-induced nephrotoxicity due to reversal of oxidative/nitrosative stress, suppression of inflammation and reduction of total renal platinum accumulation. Journal of Biochemical and Molecular Toxicology. 2017;31:1–9. doi: 10.1002/jbt.21833. [DOI] [PubMed] [Google Scholar]
- 28.Hassan S. M., Khalaf M. M., Sadek S. A., Abo-Youssef A. M. Protective effects of apigenin and myricetin against cisplatin-induced nephrotoxicity in mice. Pharmaceutical Biology. 2017;55:766–774. doi: 10.1080/13880209.2016.1275704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He X., Li C., Wei Z., et al. Protective role of apigenin in cisplatin-induced renal injury. European Journal of Pharmacology. 2016;789:215–221. doi: 10.1016/j.ejphar.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Ramesh G., Reeves W. B. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. The Journal of Clinical Investigation. 2002;110:835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tadagavadi R. K., Reeves W. B. Endogenous IL-10 attenuates cisplatin nephrotoxicity: role of dendritic cells. Journal of Immunology. 2010;185:4904–4911. doi: 10.4049/jimmunol.1000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faubel S., Lewis E. C., Reznikov L., et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. The Journal of Pharmacology and Experimental Therapeutics. 2007;322:8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 33.Ali B. H., Al Moundhri M. S. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food and Chemical Toxicology. 2006;44:1173–1183. doi: 10.1016/j.fct.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Chauhan P., Sodhi A., Shrivastava A. Cisplatin primes murine peritoneal macrophages for enhanced expression of nitric oxide, proinflammatory cytokines, TLRs, transcription factors and activation of MAP kinases upon co-incubation with L929 cells. Immunobiology. 2009;214:197–209. doi: 10.1016/j.imbio.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Lu L. H., Oh D. J., Dursun B., et al. Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. The Journal of Pharmacology and Experimental Therapeutics. 2008;324:111–117. doi: 10.1124/jpet.107.130161. [DOI] [PubMed] [Google Scholar]
- 36.Choi E. Y., Jin J. Y., Lee J. Y., Choi J. I., Choi I. S., Kim S. J. Anti-inflammatory effects and the underlying mechanisms of action of daidzein in murine macrophages stimulated with Prevotella intermedia lipopolysaccharide. Journal of Periodontal Research. 2012;47:204–211. doi: 10.1111/j.1600-0765.2011.01422.x. [DOI] [PubMed] [Google Scholar]
- 37.Domitrovic R., Cvijanovic O., Pernjak-Pugel E., Skoda M., Mikelic L., Crncevic-Orlic Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food and Chemical Toxicology. 2013;62:397–406. doi: 10.1016/j.fct.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Ueki M., Ueno M., Morishita J., Maekawa N. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. Journal of Bioscience and Bioengineering. 2013;115:547–551. doi: 10.1016/j.jbiosc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Nozaki Y., Kinoshita K., Yano T., et al. Signaling through the interleukin-18 receptor alpha attenuates inflammation in cisplatin-induced acute kidney injury. Kidney International. 2012;82:892–902. doi: 10.1038/ki.2012.226. [DOI] [PubMed] [Google Scholar]
- 40.Faubel S., Ljubanovic D., Reznikov L., Somerset H., Dinarello C. A., Edelstein C. L. Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney International. 2004;66:2202–2213. doi: 10.1111/j.1523-1755.2004.66010.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim M. G., Yang H. N., Kim H. W., Jo S. K., Cho W. Y., Kim H. K. IL-10 mediates rosiglitazone-induced kidney protection in cisplatin nephrotoxicity. Journal of Korean Medical Science. 2010;25:557–563. doi: 10.3346/jkms.2010.25.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramesh G., Reeves W. B. Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney International. 2004;65:490–499. doi: 10.1111/j.1523-1755.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 43.Guo J. M., Xiao B. X., Dai D. J., Liu Q., Ma H. H. Effects of daidzein on estrogen-receptor-positive and negative pancreatic cancer cells in vitro. World Journal of Gastroenterology. 2004;10:860–863. doi: 10.3748/wjg.v10.i6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]