Abstract
Previous studies have shown that a σ54–σS cascade regulates the expression of a few key lipoproteins in Borrelia burgdorferi, the agent of Lyme disease. Here, we demonstrate that these sigma factors, both together and independently, regulate a much more extensive number of genes and cellular processes. Microarray analyses of σ54 and σS mutant strains identified 305 genes regulated by σ54 and 145 regulated by σS, whereas the σ54–σS regulatory cascade appears to control 48 genes in B. burgdorferi. In silico analyses revealed that nearly 80% of genes with altered expression in the σ54 mutant were linked to potential σ54-dependent promoters. Many σ54-regulated genes are expressed in vivo, and through genetic complementation of the mutant, we demonstrated that σ54 was required by B. burgdorferi to infect mammals. Surprisingly, σ54 mutants were able to infect Ixodes scapularis ticks and be maintained for at least 24 wk after infection, suggesting the σ54–σS regulatory network was not involved in long-term survival in ticks. However, σ54 mutants did not enter the salivary glands during tick feeding, indicating that σ54-regulated genes were involved in the transmission process.
Keywords: infectivity, microarray, Lyme, transcription
The agent of Lyme disease, Borrelia burgdorferi, colonizes both a mammalian reservoir host and an arthropod vector during its infective cycle. This transmission cycle requires: (i) migration of spirochetes from tick midgut to salivary glands during a blood meal, (ii) entry into and colonization of mammalian tissues, (iii) establishment of a chronic/systemic infection, and (iv) uptake and colonization of an uninfected feeding tick. This process undoubtedly involves recognition by the bacterium of multiple environmental cues that modulates the expression of key genes required for a successful adaptation process. A remarkable characteristic of this bacterium is its ability to adapt to and thrive in these very different host environments. It is perhaps more remarkable that B. burgdorferi is able to complete the complex events required for transmission even with its relatively small genome consisting of a 910-kbp linear chromosome and 21 circular and linear plasmids totaling 610 kbp (1, 2).
A number of B. burgdorferi genes are known to be regulated within its hosts (3, 4) or under conditions that mimic host infection (5–7). Unfortunately, the genetic regulation of adaptation to different hosts is not well understood. It has been shown that both alternative sigma factors in B. burgdorferi (σ54 and σS, encoded by ntrA and rpoS) act in a cascade where σ54 controls σS production to regulate the expression of two lipoproteins, OspC and DbpA (8), that potentially play roles in B. burgdorferi's survival in mammals (9, 10). In this study, microarrays were used to identify many other genes both independently and coregulated by σ54 and σS, and the in vivo role of σ54 was evaluated in the murine and arthropod hosts.
Methods
Detailed protocols are provided as Supporting Methods, which is published as supporting information on the PNAS web site.
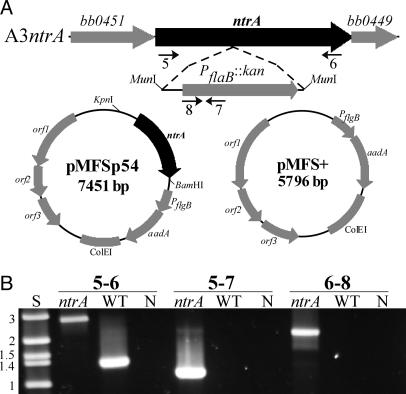
Bacterial Strains and Growth Conditions. B. burgdorferi WT clone B31-A3 (11) and all derivatives were grown at 34°C in BSK-II medium supplemented with 6% rabbit serum under either normal atmosphere or microaerobic conditions (3–5% O2, 5% CO2, 95% N2). Strain A3rpoS is a low-passage σS mutant that harbors all plasmids found in the parental strain B31-A3 (11). All plasmids were propagated in Escherichia coli DH5α or Top-10.
Construction of the σ54 Mutant and Complemented (Comp) Strains. The σ54 mutant strain was generated by allelic replacement of the WT copy of ntrA with a disrupted copy in the suicide vector pJSTJNK (12). This plasmid contains a 1.1-kb internal fragment of the ntrA gene (PCR-amplified with primers 1 and 2; all primers are shown in Table 2, which is published as supporting information on the PNAS web site) that is interrupted at the MunI site with the PflaB::kan cassette from pJLB12A (ref. 13; all plasmids are shown in Table 3, which is published as supporting information on the PNAS web site). Approximately 5 × 109 cells (B. burgdorferi B31-A3 or A3 derivatives; all strains are shown in Table 3) were electroporated with 10–50 μg of plasmid DNA, and transformants were plated in solid BSK (200 μg/ml kanamycin, 40 μg/ml gentamicin, or 50 μg/ml streptomycin) (11). Screening for disruption of ntrA was done by PCR with primers 1 and 2 and confirmed with primers 5–6, 5–7, and 6–8. Before complementation and animal infections, A3ntrA, which harbors all plasmids but lp25, and B31-A3 were transformed with plasmid DNA from B31-A3 lp25-Gm containing a PflaB::aacC1 marker on lp25 (14), yielding clones A3-Gm and A3ntrA-Gm. Strain A3ntrA-Gm was transformed with vector pMFS+ or pMFSp54 generating strains ntrA-VC (vector control) and ntrA-comp. Both strains harbored all plasmids but lp56. Details of plasmid and strain construction are found in Supporting Methods. All plasmid constructs were confirmed by DNA sequencing.
Microarray, RT-PCR, and Quantitative RT-PCR Analyses. B. burgdorferi strains B31-A3, A3ntrA, and A3rpoS were grown in triplicate to 4 × 107 cells per ml under microaerobic conditions, and growth rates were determined by dark-field microscopy. Bacteria were harvested by centrifugation at 25,000 × g for 1 min at 4°C, and RNA was extracted by TRI-reagent. RNA was treated with DNase-I, followed by DNA-Free DNase removal reagent, and purity was confirmed by PCR using primers 2 and 12.
RNA for microarray analysis was labeled by reverse transcription with Alexa Fluor 546 or Alexa Fluor 647 by using the ARES system (Molecular Probes). Labeled cDNAs were mixed (WT plus mutant) and hybridized to microarrays at 50°C for 16 h. Microarrays consisted of 1,742 custom 70-mer oligonucleotides (Tm-matched and screened to reduce cross-hybridization) covering essentially all ORFs in the B31 genome, printed in duplicate on Ultra-GAPS slides. Because of the large number of paralogs in the B31 genome (1), it was impossible to produce entirely unique probes for some genes. Those with identity >80% of the length of another probe are indicated in Tables 4–6, which are published as supporting information on the PNAS web site.
Fluorescence intensities from six hybridizations per strain (two hybridizations of three biological replicates) were quantified by a Scanarray 5000 with quantarray 3.0 software (PerkinElmer). Data were normalized by using the standard lowess algorithm in genespring 5.1 (Silicon Genetics, Redwood City, CA). Genes significantly up- or down-regulated vs. WT were identified by using significance analysis of microarrays (sam) version 1.21 (15). For a detailed description of the microarray analysis, see Supporting Methods.
For RT-PCR, total RNA (2 μg) from WT or mutant B. burgdorferi cells was converted to cDNA with 400 units of Superscript II reverse transcriptase with 40 units of RNase inhibitor and 3.8 μM arbitrary decamers (Supporting Methods) to prime cDNA synthesis. Reactions without reverse transcriptase confirmed the lack of DNA in RNA samples. The cDNA was used as template for PCR using primers 14 and 15 for ntrA expression and primers 17 and 18 for rpoS expression.
Quantitative RT-PCR (TaqMan) analysis (16) was used to confirm microarray data for 27 genes as described in detail in Supporting Methods. Log fold-change values from both microarray and TaqMan assays for selected genes were compared by linear regression with Microsoft excel.
Bioinformatic Analyses. B. burgdorferi chromosomal and plasmid DNA sequences (1) were analyzed with seqscan (www.bmb.psu.edu/seqscan) by using a scoring matrix derived from 186 known σ54-dependent promoters (17). The resulting promoter candidates were screened for validity in excel and sas (SAS Institute, Cary, NC) by using criteria based on seqscan score, orientation, and spacing of promoters relative to downstream genes, then compiled and merged with microarray data (Supporting Methods).
Experimental Infections. Naïve Swiss–Webster mice were anesthetized and bled before inoculation. Animals were infected with 4 × 103 spirochetes i.p. plus 1 × 103 s.c. or by tick bite as described (11). At 3 wk after inoculation, serum samples were collected, and preinoculation or postinoculation sera were screened by immunoblotting for P39 reactivity (Supporting Methods). Seroconversion, or development of antibodies, to P39 in mice is an indicator of active B. burgdorferi infection (18). At 5 wk after inoculation, spirochetes were cultured under aerobic conditions from tissues (ear, bladder, and joint) as described (11). Correct ntrA loci of tissue isolates were confirmed by PCR (primer pairs 1–2 and 13–16).
Tick Infection and Immunofluorescence Assays (IFAs). Ixodes scapularis larvae were infected by immersion in logarithmic growth phase cultures of B. burgdorferi strains A3-Gm, A3ntrA-Gm, ntrA-comp, and ntrA-VC as described (19). Infected larvae were allowed to feed to repletion on mice and molt to nymphs. The resulting nymphs were maintained for 18–24 wk at 98% humidity before being fed on mice and removed at 64 and 89 h after attachment. Midguts and salivary glands were isolated and examined by IFA (20) with rabbit anti-B. burgdorferi serum (1:100) as the primary antibody, and Alexa Fluor 488-labeled anti-rabbit IgG (1:100) as secondary antibody. Tissues were counterstained with DRAQ5 (1:1,000, Biostatus, Shepshed, U.K.). Midguts were analyzed by epifluorescent microscopy (Nikon Eclipse E800), and complete salivary glands were examined through their entire thickness by confocal microscopy (Zeiss LSM-510). 3D surface generating and surface cutaway projections of confocal image Z-stacks were generated by imaris surpass software (Bitplane, St. Paul) as described (21).
Results
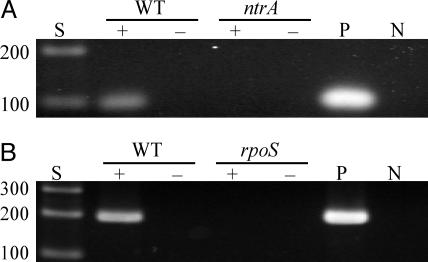
Inactivation of ntrA. Because the σ54–σS cascade regulates the potential virulence factors ospC and dbpA (8–10), we used two genetic approaches to characterize mutants in this regulatory network. Microarray analysis was used to identify other genes regulated by these global regulators, and a σ54 mutant was evaluated for its role in the mouse-tick infectious cycle. A clone, A3ntrA, was confirmed to have ntrA insertionally inactivated (Fig. 1). RT-PCR was used to examine the expression of ntrA and rpoS in the respective mutants before microarray analysis. As expected, the σ54 mutant did not express ntrA (Fig. 2A), and the σS mutant failed to express rpoS (Fig. 2B), confirming inactivation of these genes. All strains exhibited growth characteristics very similar to WT when cultivated in BSK-II medium at 34°C.
Fig. 1.
Disruption of ntrA and complementation of the σ54 mutant. (A) Flanking ORFs and insertion point of the PflaB::kan cassette in strain A3ntrA are shown above the complementation (pMFSp54) and VC (pMFS+) plasmids. Numbered arrows indicate the relative positions of primers used for PCR confirmation of the mutant. (B) PCR analyses to confirm ntrA disruption in A3ntrA. Size standards (S) in kbp and negative controls (N) are indicated.
Fig. 2.
RT-PCR analysis of ntrA and rpoS expression. RNA isolated from each strain was converted to cDNA and PCR-amplified with primers specific for ntrA or rpoS. (A) Expression of ntrA in B31-A3 (WT) and A3ntrA (ntrA, σ54 mutant). (B) Expression of rpoS in B31-A3 (WT) and A3rpoS (rpoS, σS mutant). Size standards (S) are in bp, and positive (P, B31-A3 DNA) and negative (N) controls are indicated. The presence or absence of reverse transcriptase is indicated by + or –.
Microarray-Based Identification of the σ54 and σS Regulons. A custom oligonucleotide array was used to identify genes differentially transcribed in the σ54 and σS mutants relative to WT. In the σ54 mutant, which lacked plasmid lp25, 305 genes were differentially expressed at statistically significant levels. Of these, 146 were underexpressed in A3ntrA compared with WT (Fig. 3 and Tables 4 and 6). In the σS mutant, 145 genes showed significantly altered expression. Of these, 81 were underexpressed in A3rpoS relative to WT (Fig. 3 and Tables 5 and 6). Both mutants shared a common set of 51 significantly changed genes. Of these, 48 were coordinately expressed in the mutants, thus they appeared to be regulated by the σ54–σS cascade (Fig. 3 and Table 6). Therefore, three distinct regulatory groups were identified: group 1, 254 genes regulated by σ54 alone (Table 4); group 2, 94 genes regulated by σS alone (Table 5); and group 3, 51 genes regulated by both σ54 and σS (Table 6).
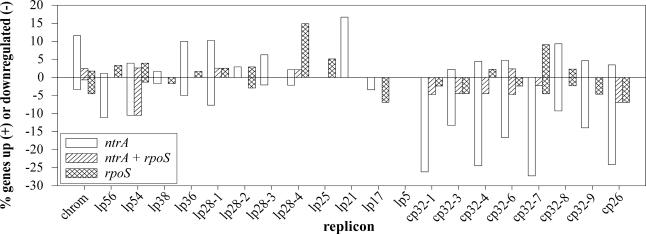
Fig. 3.
Distribution of regulated genes by replicon. Microarray data were categorized by replicon and sigma factor regulatory group and are shown as percent of ORFs significantly changed on each replicon. ntrA, genes regulated only by σ54; ntrA + rpoS, genes regulated by both σ54 and σS; rpoS, genes regulated only by σS.
Cp26 and several cp32 plasmids had the largest percentage of their ORFs significantly regulated (25–30%) by σ54 (group 1), whereas lp28-4 had the largest percentage of genes (15%) differentially expressed by σS (group 2). As seen in other B. burgdorferi array experiments (5, 6), a large percentage of lp54 ORFs were also changed in both mutants (group 3, Fig. 3). The genes regulated by σ54 and σS fell into several major categories (e.g., cell envelope, cellular processes) as annotated in the B. burgdorferi genome (1). Role categories containing three or more regulated genes are shown in Fig. 6, which is published as supporting information on the PNAS web site. TaqMan assays (using primers and probes listed in Table 7, which is published as supporting information on the PNAS web site) were used to confirm differential expression of 27 genes identified in the microarray analyses (Tables 4–6). There was a strong, significant linear correlation between the log fold-change of the TaqMan data and that of the array data for both the σ54 and σS mutant comparisons (r = 0.73, P = 3.8 × 10–6), validating the microarray results.
Bioinformatic Analysis of Potential σ54-Dependent Promoters. Because genes encoding potential regulatory proteins were differentially expressed in the σ54 mutant, a bioinformatic approach was used to identify putative σ54-regulated promoters upstream of significantly regulated genes. This approach should identify genes that may be directly regulated by σ54. When criteria were set to allow genes in potential operons to be recognized, 78% of the regulated ORFs could be linked to a potential σ54-dependent promoter (data not shown). Even by limiting criteria to exclude all but a single gene within 500 bp of a promoter, 42% of the genes regulated in the σ54 mutant possessed potential σ54-dependent promoters (Supporting Methods and Tables 4 and 6).
The σ54 Mutant Is Not Infectious in Mice. To determine whether σ54 is required by B. burgdorferi to infect mammals, we inoculated mice by i.p./s.c. injection (5 × 103 cells) or tick bite with strains A3-Gm (WT), A3ntrA-Gm (σ54 mutant), ntrA-comp, or ntrA-VC. All strains harbored a selectable copy of lp25. Three of four mice injected with WT and 4/4 with ntrA-comp cells seroconverted to P39 (18), and spirochetes were cultured from tissue samples (Table 1). In contrast, no mice injected with A3ntrA-Gm or ntrA-VC seroconverted and no spirochetes were recovered from any tissues. Likewise, all mice challenged by ticks infected with WT or ntrA-comp seroconverted and yielded spirochetes from tissues, whereas none challenged with A3ntrA-Gm or ntrA-VC seroconverted and no bacteria were recovered from tissues (Table 1). PCR confirmed that all strains isolated from tissue contained the correct ntrA loci. These data show that σ54 is required for B. burgdorferi to infect mammals regardless of the infection route.
Table 1. Summary of experimental mouse infections with B. burgdorferi A3-Gm, A3ntrA-Gm, ntrA-comp, and ntraA-VC strains.
| Strain | Route* | Seroconversion† | Culture‡ |
|---|---|---|---|
| A3-Gm | Needle | 3/4 | 3/4 |
| Tick | 3/3 | 3/3 | |
| A3ntrA-Gm | Needle | 0/4 | 0/4 |
| Tick | 0/3 | 0/3 | |
| ntrA-comp | Needle | 4/4 | 4/4 |
| Tick | 3/3 | 3/3 | |
| ntrA-VC | Needle | 0/4 | 0/4 |
| Tick | 0/3 | 0/3 |
Needle inoculation with 5 × 103 bacteria i.p./s.c. or by tick bite with larvae (one mouse) or nymphs (two mice).
No. of P39 or culture positive/total no. of mice tested.
All tissues (skin, bladder, and joint) gave the same culture results.
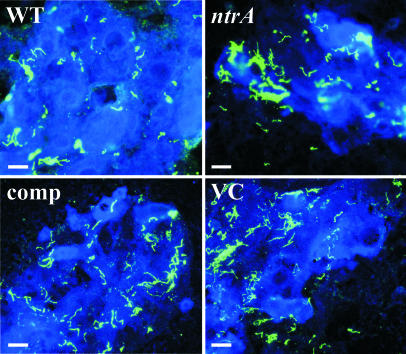
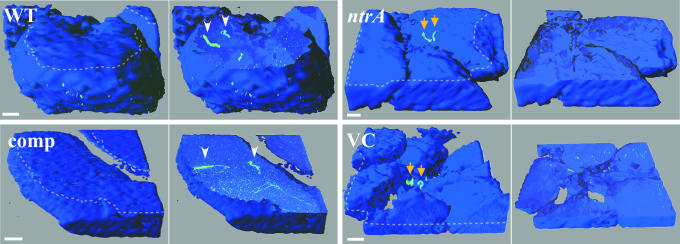
The σ54 Mutant Fails to Enter Tick Salivary Glands. I. scapularis ticks infected with A3-Gm, A3ntrA-Gm, ntrA-comp, and ntrA-VC strains were monitored by IFA for 24 wk. There was no apparent defect in the mutant's ability to survive in the midgut (Fig. 4). However, when salivary glands from feeding ticks were examined by confocal microscopy, a striking difference was observed. Both WT (13/19 ticks) and ntrA-comp (13/17) strains were able to enter the ascini of salivary glands, whereas neither the A3ntrA-Gm (0/19) nor the ntrA-VC (0/13) strain was observed inside salivary gland ascini (Fig. 5). These data suggest that σ54-regulated genes are necessary for transmission of B. burgdorferi.
Fig. 4.
IFA of midguts from fed ticks 24 wk after infection. Midguts isolated from ticks infected with A3-Gm (WT), A3ntrA-Gm (ntrA, σ54 mutant), ntrA-comp, and ntrA-VC strains were examined by IFA. Spirochetes (green) were detected by fluorescent microscopy with rabbit anti-B. burgdorferi primary and Alexa Fluor 488-labeled anti-rabbit secondary antisera. (Scale bars, 20 μm.)
Fig. 5.
3D surface and cutaway projections of salivary gland ascini. Salivary glands were isolated from partially fed ticks infected with A3-Gm (WT), A3ntrA-Gm (ntrA, σ54 mutant), ntrA-comp, and ntrA-VC strains. The left image in each case shows the outer surface of intact ascini, and the right image shows a computer-generated internal section of the corresponding structure. The cutting plane used in the right image is denoted by the dotted line in the left image. Arrowheads indicate spirochetes (green) located within ascini, and arrows denote those on the exterior of the structure. Spirochetes were detected by confocal microscopy using rabbit anti-B. burgdorferi primary and Alexa Fluor 488-labeled anti-rabbit secondary antisera, and salivary glands were stained with DRAQ5. (Scale bars, 10 μm.)
Discussion
Very little is known about gene regulatory systems in B. burgdorferi. We have begun to address this problem by identifying genes under the control of two global regulators, the alternative sigma factors σ54 and σS. In other bacteria, σ54 regulates such processes as nitrogen homeostasis and motility, and σS often regulates general stress responses (reviewed in refs. 22 and 23). Because these sigma factors are also required for virulence in other organisms (24, 25), we examined the ability of a B. burgdorferi σ54 mutant to infect a mammalian host. Through complementation analysis, we demonstrated that a functional ntrA gene is required for infectivity in mice (Table 1). Further, we showed that σ54 is required by B. burgdorferi to enter tick salivary glands, a critical step in vector transmission.
B. burgdorferi strain B31-A3 was chosen for ntrA mutagenesis because it is a clonal, infectious derivative of strain B31 used for genomic sequencing (1) and the parent strain of the σS mutant (11). Because both mutants were generated in the same background, differential gene expression in the mutants relative to WT could be directly compared. The σ54 and σS mutants were shown by RT-PCR to lack expression of ntrA and rpoS, respectively (Fig. 2), indicating that these mutants were appropriate for microarray analysis. Because we sought to identify all genes regulated by these alternative sigma factors, no arbitrary fold-change restriction was imposed on the microarray data. Rather, conservative statistical criteria were used to provide the most robust, yet complete data set possible. It is important to note that because sigma factors are primarily activators of transcription, genes up-regulated in the mutants are probably regulated indirectly (e.g., nonactivation of a repressor) or pleiotropically (e.g., nonactivation of an RNase slowing message turnover). However, σ54 can act directly as a negative regulator by a mechanism termed sigma factor antagonism (26). Therefore, some genes overexpressed in the σ54 mutant may in fact be directly repressed by σ54. Down-regulated genes could be directly or indirectly (e.g., σ54–σS regulation of ospC) regulated by the respective sigma factor.
By comparing both the σ54 and the σS mutants to WT, we identified sets of genes regulated by σ54 alone (group 1, Table 4), σS alone (group 2, Table 5), and both σ54 and σS (group 3, Table 6). Microarray comparison of the σ54 mutant with WT revealed 305 genes with significantly altered expression (Tables 4 and 6). Although the σ54 mutant analyzed by microarray lacked lp25, it is unlikely that this affected overall gene expression profiles because no transcriptional regulators or ribonucleases have been identified on this plasmid (1). In the σS mutant, only 145 genes showed significant changes (Tables 5 and 6). The expression of 254 genes was significantly altered in the σ54, but not the σS mutant (Table 4), whereas 94 genes showed significant differential expression only in the σS mutant (Table 5).
Both mutants showed altered expression of 51 shared genes (Table 6). The vast majority (48/51) were expressed in the same direction in both mutants, suggesting they may be coregulated by the σ54–σS cascade. Based on previous work (8), we would predict ospC (bbb19), dbpA (bba24), and dbpB (bba25, which is cotranscribed with dbpA) (27) to be in regulatory group 3. As expected, both ospC and dbpB were found in group 3 but dbpA was significantly changed only in the σS mutant (Tables 5 and 6). However, closer inspection revealed that dbpA was down-regulated in 5/6 microarray hybridizations (–2.1- to –1.3-fold) and up-regulated in one (1.3-fold) in the σ54 mutant. Without the latter data point, the average fold-change was –1.54, which was comparable to the –1.62 value observed in the σS mutant. This finding confirmed previous data (8) and suggested that our statistical criteria for identifying significantly regulated genes were too conservative, thus there are potentially more genes that are regulated by these sigma factors.
Previous work suggests rpoS (bb0771) should also be in regulatory group 3 (8) but the rpoS signal was below the detection threshold in all strains and all microarray hybridizations. This finding suggested that there was a problem with the rpoS oligo target or the levels of rpoS mRNA were below the detection limit. No rpoS mRNA was detected by RT-PCR in the σS mutant, but it was easily detectable in WT (Fig. 2B) and the σ54 mutant (TaqMan rpoS levels of –2.63-fold vs. WT), implicating a faulty array target. Together, these observations confirmed that rpoS was expressed under the conditions used for the arrays and was regulated by σ54.
Because the σ54 mutant cannot infect mice, one would expect some genes regulated by σ54 to be involved in infectivity. Moreover, genes up-regulated in the host may be necessary for in vivo growth or survival. Thus, we hypothesized that a search for genes up-regulated in rat dialysis membrane chambers (5, 7) but down-regulated in the σ54 mutant should identify B. burgdorferi genes involved in infectivity. Not surprisingly, this comparison yielded 12 genes that may be required for infectivity and are likely under the control of σ54. A similar analysis to identify genes expressed in mammalian tissues (3, 28) but down-regulated in the σ54 mutant yielded 25 additional genes, thus totaling 37 potentially involved in the virulence of B. burgdorferi (Table 8, which is published as supporting information on the PNAS web site). Several of these genes have been either shown (ntrA and ospC) or suggested (dbpB, bba64, bbo39, bbs41, and vlsE) to be required for the infectivity or pathogenesis of B. burgdorferi (this study and refs. 9 and 29–31).
Although we did not test the σS mutant in mice, 19 genes that are expressed in mammals were down-regulated in the σS mutant (Table 8), and thus may be another subset of genes involved in virulence (3, 5, 7, 28). In support of this idea, bbl40 and another σS-regulated homolog (bbo40), both members of the erp gene family, have been implicated in survival in mammals by allowing evasion of the complement system (30). Another potential virulence gene down-regulated in the σS mutant is bb0810, which encodes a MviN homolog. Salmonella typhimurium mutants lacking MviN, a protein of unknown function, are attenuated in a mouse model of infection (32). Recently, Caimano et al. (33) demonstrated that a B. burgdorferi σS mutant was avirulent in mice. Our data could provide insight into which σS-regulated genes are required for virulence.
The genes regulated by both sigma factors fall into several major functional categories (1), including cell envelope, cellular processes, and transport/binding proteins (Fig. 6 and Tables 4–6). In the σ54 mutant, the expression of 18 cell envelope genes was affected, and 9 are potentially involved in virulence as described above (Table 8). Of the 22 σS-regulated genes involved in the cell envelope, 16 encode putative membrane proteins. This result is not surprising because B. burgdorferi devotes ≈8% of its genome to lipoproteins (1). Most of the envelope genes are down-regulated in the mutants, indicating direct regulation by σ54 and/or σS. Because it has been shown that B. burgdorferi lipoproteins are down-regulated during long-term colonization of mice (28), σ54 and/or σS-dependent regulation of these proteins may be important for chronic infections.
The largest group of σ54- and σS-regulated genes in the cellular processes category is involved in chemotaxis and motility. In some bacteria (e.g., Vibrio fischeri and Campylobacter jejuni), motility genes are known to be regulated by σ54 and may play a role in colonization and pathogenesis (34, 35). The role of chemotaxis and motility systems in the pathogenesis of Lyme disease is not well characterized, but lack of flagella has been correlated with attenuation in cell culture and mouse infection models (36, 37). Although several chemotaxis and motility genes showed altered expression in both sigma factor mutants (17 genes, Tables 4–6), both remained motile. This finding is not surprising because most of the underexpressed chemotaxis genes are involved in regulation and B. burgdorferi has a redundant system for regulating motility (1). Additionally, most of the nonregulatory motility genes underexpressed in the σS mutant were related to flagellar protein secretion (e.g., fliQ and fliR) rather than flagellar structural proteins (e.g., FlaB). The down-regulation of these secretion genes in the σS mutant may have a more subtle effect on the function or number of flagella. Interestingly, Sellek et al. (37) demonstrated that a decrease in the number of flagella in B. garinii was correlated with reduced invasiveness. One could speculate, because of the different environments encountered during the complex infectious cycle, that chemotaxis would be very important at key stages of the process. For example, functional chemotaxis could be required for dissemination in the mammal and/or migration from the midgut to the salivary glands.
Microarray analysis of a Listeria monocytogenes σ54 mutant showed altered transcription of many genes involved in carbohydrate utilization and transport (38). Our data also show that 11 carbohydrate metabolism and transport genes are regulated by σ54 (Tables 4 and 6). Five of these (bbb05-6 and bb002-4) have putative roles in transport and metabolism of chitobiose (39). Because chitobiose is an amino sugar, this finding may indicate a role for nitrogen in σ54-dependent regulation as is commonly seen in other bacteria (22). Alternatively, these gene products could simply be used, but not required (39), for acquiring precursors for peptidoglycan biosynthesis and energy because chitobiose is likely present in the arthropod vector.
Recently, Tokarz et al. (40) reported the transcriptional response of B. burgdorferi to blood. They demonstrated that rpoS was among 75 genes induced in blood. Presumably with increased transcription of rpoS, some σS-regulated genes would also be up-regulated. In fact, comparison of the blood-induced genes with our data shows that 17 genes underexpressed in the σS mutant (bb0418, bb0548, bb0565, bb0567, bb0680-1, bb0728, bb0844, bba24, bba64-6, bba71-2, bbb19, bbj25, and bbn28) were also induced in blood, further confirming our microarray data.
Neither K-means nor QT clustering analyses, methods to subset microarray data by expression patterns, provided insights into a unifying theme of sigma factor regulation in B. burgdorferi. Although many bacteria use alternative sigma factors to regulate distinct sets of genes for certain metabolic or physical processes, it appears that B. burgdorferi, perhaps because of its limited repertoire, may instead use alternative sigma factors as general regulators of many simultaneous biological processes.
Our in vivo analyses of the σ54 mutant demonstrated that ntrA is required by B. burgdorferi to infect mice and to enter tick salivary glands, an essential step in the transmission process. Because midgut contents (including spirochetes) often contaminate other tissues during dissection of fed ticks, it is unclear whether the mutant's defect in entry is caused by an inability to escape the midgut, penetrate the salivary glands, or both. Grimm et al. (9) reported that a B31-A3 ospC mutant was not infectious in mice but was able to enter tick salivary glands. Although the σ54 mutant did not express OspC in vitro and behaved like an ospC mutant in mice, it had a different phenotype in the tick; it could not enter the salivary glands. Recently, Pal et al. (41) showed that a different ospC mutant was unable to enter tick salivary glands. Their study used nymphs infected by microinjection, which differs considerably from the immersion-infection method used in this study and by Grimm et al. Microinjected B. burgdorferi cells were assayed for transmission 3 days after infection and were not tested after long-term survival or passage through the molting process. In contrast, immersion-infected larvae were assayed after molting to nymphs, which may account for the different salivary gland entry results seen in the two ospC mutants. Bacteria residing longer in the midgut may undergo OspC-independent adaptations, whereas short-term residence may not allow B. burgdorferi to fully adapt. Together, these observations suggest that OspC facilitates, but is not required for salivary gland entry.
An intriguing finding was that the σ54 mutant was able to survive long term in the tick. In an unfed tick, one might expect spirochetes to require stationary phase-regulated proteins. Because σ54 regulates the expression of σS, the stationary-phase sigma factor, we predicted the mutant would not persist in the tick. Its survival suggests a level of rpoS regulation independent of σ54.
The search for sigma factor regulons by microarray analysis has provided a wealth of knowledge about the regulatory networks present in bacteria (42, 43). In this work, we used microarray technology to begin to identify the alternative sigma factor regulons of B. burgdorferi and demonstrated that they regulate distinct and overlapping sets of genes. Many of these are either required for infectivity or expressed in vivo, implicating their involvement in growth or survival in animals. In support of these observations, we have established, by means of targeted mutagenesis and genetic complementation, that the ntrA gene is a virulence determinant required by B. burgdorferi for transmission to, and infection of, its mammalian host.
Supplementary Material
Acknowledgments
We thank T. Schwan and P. Policastro for tick/IFA help, D. Sturdevant for array support, J. Treglown for pJSTJNK, D. Dorward and F. DeLeo for help with confocal microscopy, R. Larson for help with mice, G. Hettrick for graphics, and G. Sylva for sequencing.
Author contributions: M.A.F. and F.C.G. designed research; M.A.F. performed research; M.A.F., D.G., A.F.E., and P.E.S. contributed new reagents/analytic tools; M.A.F. and A.K.H. analyzed data; and M.A.F. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: IFA, immunofluorescence assay; VC, vector control; comp, complemented.
See Commentary on page 4933.
References
- 1.Fraser, C. M., Casjens, S., Huang, W. M., Sutton, G. G., Clayton, R., Lathigra, R., White, O., Ketchum, K. A., Dodson, R., Hickey, E. K., et al. (1997) Nature 390, 580–586. [DOI] [PubMed] [Google Scholar]
- 2.Casjens, S., Palmer, N., van Vugt, R., Huwang, W. M., Stevenson, B., Rosa, P., Lathigra, R., Sutton, G. G., Peterson, J., Dodson, R., et al. (2000) Mol. Microbiol. 35, 490–516. [DOI] [PubMed] [Google Scholar]
- 3.Narasimhan, S., Camaino, M. J., Liang, F. T., Santiago, F., Laskowski, M., Philipp, M. T., Pachner, A. R., Radolf, J. D. & Fikrig, E. (2003) Proc. Natl. Acad. Sci. USA 100, 15953–15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narasimhan, S., Santiago, F., Koski, R. A., Brei, B., Anderson, J. F., Fish, D. & Fikrig, E. (2002) J. Bacteriol. 184, 3122–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks, C. S., Hefty, P. S., Jolliff, S. E. & Akins, D. R. (2003) Infect. Immun. 71, 3371–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojaimi, C., Brooks, C., Casjens, S., Rosa, P., Elias, A., Barbour, A., Jasinskas, A., Benach, J., Katona, L., Radolf, J., et al. (2003) Infect. Immun. 71, 1689–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Revel, A. T., Talaat, A. M. & Norgard, M. V. (2002) Proc. Natl. Acad. Sci. USA 99, 1562–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hübner, A., Yang, X., Nolen, D. M., Popova, T. G., Cabello, F. C. & Norgard, M. V. (2001) Proc. Natl. Acad. Sci. USA 98, 12724–12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm, D., Tilly, K., Byram, R., Stewart, P. E., Krum, J. G., Bueschel, D. M., Schwan, T. G., Policastro, P. F., Elias, A. F. & Rosa, P. A. (2004) Proc. Natl. Acad. Sci. USA 101, 3142–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo, B. P., Brown, E. L., Dorward, D. W., Rosenberg, L. C. & Hook, M. (1998) Mol. Microbiol. 30, 711–723. [DOI] [PubMed] [Google Scholar]
- 11.Elias, A. F., Stewart, P. E., Grimm, D., Caimano, M. J., Eggers, C. H., Tilly, K., Bono, J. L., Akins, D. R., Radolf, J. D., Schwan, T. G., et al. (2002) Infect. Immun. 70, 2139–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treglown, J. S. (2004) Ph.D. thesis (University of Georgia, Athens).
- 13.Bono, J. L., Elias, A. F., Kupko, J. J., 3rd, Stevenson, B., Tilly, K. & Rosa, P. (2000) J. Bacteriol. 182, 2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm, D., Eggers, C. H., Caimano, M. J., Tilly, K., Stewart, P. E., Elias, A. F., Radolf, J. D. & Rosa, P. A. (2004) Infect. Immun. 72, 5938–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tusher, V. G., Tibshirani, R. & Chu, G. (2001) Proc. Natl. Acad. Sci. USA 98, 5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heid, C. A., Stevens, J., Livak, K. J. & Williams, P. M. (1996) Genome Res. 6, 986–994. [DOI] [PubMed] [Google Scholar]
- 17.Barrios, H., Valderrama, B. & Morett, E. (1999) Nucleic Acids Res. 27, 4305–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson, W. J., Burgdorfer, W., Schrumpf, M. E., Karstens, R. H. & Schwan, T. G. (1991) J. Clin. Microbiol. 29, 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Policastro, P. F. & Schwan, T. G. (2003) J. Med. Entomol. 40, 364–370. [DOI] [PubMed] [Google Scholar]
- 20.Schwan, T. G. & Piesman, J. (2000) J. Clin. Microbiol. 38, 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaus, A. V., Kulasekera, V. L. & Schawaroch, V. (2003) J. Microsc. 212, 107–121. [DOI] [PubMed] [Google Scholar]
- 22.Kustu, S., Santero, E., Keener, J., Plpham, D. & Weiss, D. (1989) Microbiol. Rev. 53, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hengge-Aronis, R. (1999) Curr. Opin. Microbiol. 2, 148–152. [DOI] [PubMed] [Google Scholar]
- 24.Fang, F. C., Libby, S. J., Buchmeier, N. A., Loewen, P. C., Switala, J., Harwood, J. & Guiney, D. G. (1992) Proc. Natl. Acad. Sci. USA 89, 11978–11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendrickson, E. L., Plotnikova, J., Mahajan-Miklos, S., Rahme, L. G. & Ausubel, F. M. (2001) J. Bacteriol. 183, 7126–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boucher, J. C., Schurr, M. J. & Deretic, V. (2000) Mol. Microbiol. 36, 341–351. [DOI] [PubMed] [Google Scholar]
- 27.Hagman, K. E., Lahdenne, P., Popova, T. G., Porcella, S. F., Akins, D. R., Radolf, J. D. & Norgard, M. V. (1998) Infect. Immun. 66, 2674–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang, F. T., Nelson, F. K. & Fikrig, E. (2002) J. Exp. Med. 196, 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anguita, J., Samanta, S., Revilla, B., Suk, K., Das, S., Barthold, S. W. & Fikrig, E. (2000) Infect. Immun. 68, 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson, B., El-Hage, N., Hines, M. A., Miller, J. C. & Babb, K. (2002) Infect. Immun. 70, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, J. R. & Norris, S. J. (1998) Infect. Immun. 66, 3689–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carsiotis, M., Stocker, B. A., Weinstein, D. L. & O`Brien, A. D. (1989) Infect. Immun. 57, 3276–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caimano, M. J., Eggers, C. H., Hazlett, K. R. & Radolf, J. D. (2004) Infect. Immun. 72, 6433–6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfe, A. J., Millikan, D. S., Campbell, J. M. & Visick, K. L. (2004) Appl. Environ. Microbiol. 70, 2520–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helmann, J. D. (1991) Mol. Microbiol. 5, 2875–2882. [DOI] [PubMed] [Google Scholar]
- 36.Sadziene, A., Thomas, D. D., Bundoc, V. G., Holt, S. C. & Barbour, A. G. (1991) J. Clin. Invest. 88, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sellek, R. E., Escudero, R., Gil, H., Rodriguez, I., Chaparro, E., Perez-Pastrana, E., Vivo, A. & Anda, P. (2002) Infect. Immun. 70, 4851–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arous, S., Buchrieser, C., Folio, P., Glaser, P., Namane, A., Hebraud, M. & Hechard, Y. (2004) Microbiology 150, 1581–1590. [DOI] [PubMed] [Google Scholar]
- 39.Tilly, K., Grimm, D., Bueschel, D. M., Krum, J. G. & Rosa, P. (2004) Vector Borne Zoonotic Dis. 4, 159–168. [DOI] [PubMed] [Google Scholar]
- 40.Tokarz, R., Anderton, J. M., Katona, L. I. & Benach, J. L. (2004) Infect. Immun. 72, 5419–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal, U., Yang, X., Chen, M., Bockenstedt, L. K., Anderson, J. F., Flavell, R. A., Norgard, M. V. & Fikrig, E. (2004) J. Clin. Invest. 113, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Britton, R. A., Eichenberger, P., Gonzalez-Pastor, J. E., Fawcett, P., Monson, R., Losick, R. & Grossman, A. D. (2002) J. Bacteriol. 184, 4881–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun, R., Converse, P. J., Ko, C., Tyagi, S., Morrison, N. E. & Bishai, W. R. (2004) Mol. Microbiol. 52, 25–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.