Abstract
The mutation rate of the mammalian mitochondrial genome is higher than that of the nuclear genome. Because mitochondrial and nuclear deoxyribonucleoside triphosphate (dNTP) pools are physically distinct and because dNTP concentrations influence replication fidelity, we asked whether mitochondrial dNTP pools are asymmetric with respect to each other. We report here that the concentrations of the four dNTPs are not equal in mitochondria isolated from several tissues of both young and old rats. In particular, in most tissues examined, mitochondrial dGTP concentrations are high relative to the other dNTPs. Moreover, in the presence of the biased dNTP concentrations measured in heart and skeletal muscle, the fidelity of DNA synthesis in vitro by normally highly accurate mtDNA polymerase γ is reduced, with error frequencies increased by as much as 3-fold, due to increased formation of template T·dGTP mismatches that are inefficiently corrected by proofreading. These data, plus some published data on specific mitochondrial mutations seen in human diseases, are consistent with the hypothesis that normal intramitochondrial dNTP pool asymmetries may contribute to spontaneous mutagenesis in the mammalian mitochondrial genome.
Mutations in mitochondrial DNA (mtDNA) are associated with a variety of human diseases, including neurodegenerative disorders, cardiomyopathies, and cancer (1–3). In addition, mutations in mtDNA accumulate with age (4–6) and cause premature aging in mice (7). These factors highlight the importance of understanding how genomic stability is maintained within the mitochondrion. The spontaneous mutation rate for mtDNA has been estimated to be as much as two orders of magnitude higher than that for the nuclear genome (8). Several factors could be responsible, such as excessive DNA damage generated by reactive oxygen species within the mitochondrion and/or the absence of protection against damage that may be afforded to nuclear genes by histones. Limited DNA repair could also be a significant factor in mtDNA mutagenesis because, although mammalian mitochondria possess a base excision repair system, other DNA repair systems have not been unequivocally demonstrated in mammalian mitochondria (9–11).
Analysis of human mitochondrial mutations suggests that another source of mitochondrial mutagenesis could be normal DNA replication errors (8, 12). Limiting this possibility is the fact that mtDNA polymerase (pol) γ can replicate DNA with very high fidelity, for example, with error rates for single-base substitutions of ≤10–5 (13–16). This accuracy results from the high nucleotide selectivity of the pol γ active site, combined with the ability of the intrinsic 3′ exonuclease activity of pol γ to excise nucleotide misinsertions during replication.
The contribution of nucleotide selectivity and proofreading to replication fidelity depends on the concentrations of the dNTP precursors available during replication. The probability of polymerase misinsertion opposite any template base will depend partly on the ratio of the correct dNTP to the three incorrect dNTPs. After a misinsertion has occurred, the efficiency with which it is excised before additional synthesis proceeds depends partly on the concentration of the next correct dNTP to be incorporated, which if high, will favor mismatch extension over proofreading. Thus, altering the relative and/or the absolute concentrations of the dNTP precursors can change DNA replication fidelity in a sequence-dependent manner (reviewed in ref. 17).
Although mitochondrial dNTP pools have been analyzed in cultured cells (18–20), no such data have been reported for mitochondria from animal tissues. Therefore, a major goal of this study was to measure mitochondrial dNTP pool sizes in several tissues of the rat, asking whether the pools are sufficiently asymmetric to affect replication fidelity within the organelle. This investigation was further motivated by recent evidence suggesting that imbalanced mitochondrial nucleotide pools may participate in the pathogenesis of several human diseases (21, 22). Also, because mitochondrial mutations accumulate with age (4–6), we wished to determine whether mitochondrial dNTP pools change significantly with age in a way that could influence replication fidelity and, possibly, the mutation rate of the mitochondrial genome. Related to this issue is that cardiac muscle contains two distinct populations of mitochondria that show significant differences in age-related changes in biological reductant status. Subsarcolemmal mitochondria (SSM) are located beneath the sarcolemma, whereas interfibrillary mitochondria (IFM) are located between the myofibrils (23). Previous studies showed a decline in oxidative phosphorylation with age in IFM (24) and age-related decreases of reduced glutathione levels and glutaredoxin reductase and glutathione reductase activities (25). Because glutaredoxin is a glutathione-dependent electron donor for ribonucleotide reductase, an enzyme found in mitochondria (26), an age-related decrease of glutaredoxin and glutaredoxin reductase and glutathione reductase activities in IFM could affect mitochondrial dNTP pools and mtDNA replication, with an eventual effect on IFM function. Therefore, this study was designed to allow independent measurements of dNTP pools in both SSM and IFM cardiac mitochondrial populations.
In this report, mitochondrial dNTP pools were measured in tissues from young and old rats, and this information was used to estimate molar dNTP concentration in mitochondrial fluid spaces. In surprising contrast to total cellular or nuclear dNTP pools, where dGTP is consistently observed to be the least abundant DNA precursor, dGTP appears to be the most abundant dNTP in mitochondrial pools. These results suggested fidelity assays designed to ask whether the mitochondrial dNTP pool asymmetries observed in tissues were sufficiently asymmetric to decrease the DNA replication fidelity of human DNA pol γ. This hypothesis was substantiated, thereby implicating pool imbalance as one possible contributor to the elevated mutation rate of the mitochondrial as compared with the nuclear genome.
Materials and Methods
Isolation of Mitochondria. Young male Wistar and Fischer 344 rats (3–7 months of age) were purchased from Simonsen Laboratories (Gilroy, CA). Old male Fischer 344 rats (26–28 months of age) were purchased from the National Institute of Aging (Bethesda). All animal procedures were approved by the Institutional Animal Care and Use Committee of Oregon State University. Animals were anesthetized with diethyl ether and killed by decapitation, one animal per analysis. Tissues (1 g or more) of heart, liver, forebrain, and skeletal muscle were immediately removed, washed and minced in cold isolation buffer containing 220 mM mannitol, 70 mM sucrose, 5 mM Mops (pH 7.4), 2 mM EGTA, and 0.2 mg/ml BSA. Liver and brain were homogenized in cold isolation buffer with a glass-Teflon motorized homogenizer. The mitochondrial fraction was isolated by differential centrifugation and subsequently washed twice and resuspended in 2 ml of isolation buffer. Immediately after that, most of the mitochondrial suspension was centrifuged to pellet mitochondria, and the remaining suspension was saved for determination of mitochondrial respiratory control ratio and protein concentration. The skeletal muscle mitochondria isolation procedure was the same as described above except for using more vigorous homogenization and a different isolation buffer consisting of 0.1 M KCl, 50 mM Tris·HCl (pH 7.4), 5 mM MgCl2, and 1 mM EGTA. Two subpopulations of cardiac mitochondria were isolated as described by Suh et al. (25). Lactate dehydrogenase and respiratory control ratio were determined as described in refs. 27 and 28. Mitochondrial protein was estimated by the Bradford method. The purity of the mitochondrial preparation was monitored by assaying for contaminating lactate dehydrogenase activity. The preparations routinely showed lactate dehydrogenase specific activity <1% the specific activity of corresponding cytosolic extracts. Functional integrity of the preparations was routinely monitored by determining the respiratory control ratio and the P/O ratio for succinate. All P/O ratios measured were found to be >1.7, close to the theoretical value of 2. The respiratory control ratios varied with the tissue analyzed, but all values determined were in the range expected for functionally intact mitochondria (28).
Mitochondrial dNTP Pool Extraction and Analysis. Immediately after their preparation, mitochondria were resuspended in 1 ml of ice-cold 60% methanol and incubated at –20°C for 1 h to extract nucleotide pools. The suspension was heated for 3 min in a boiling water bath, followed by centrifugation for 20 min at 17,000 × g. The supernatant was transferred to a fresh tube and dried under a vacuum. The residue was dissolved in sterile water and stored at –20°C for later analysis. Analysis of the dNTP pools in each extract was carried out by the pol-based method as described in ref. 19 with some modifications. Reaction mixtures (25 μl) contained 100 mM Hepes buffer (pH 7.5), 10 mM MgCl2, 0.1 units of Escherichia coli pol I Klenow fragment (United States Biochemical), 0.25 μM oligonucleotide template, 5 μg of BSA (New England Biolabs), and 1.25 μCi (1 Ci = 37 GBq) of [3H]dATP (Amersham Pharmacia Biosciences) or [3H]dTTP (Perkin-Elmer Life Sciences). Incubation was carried out for 45 min at 37°C.
Fidelity Assays. His-6 affinity-tagged recombinant human pol γ catalytic (p140) subunit (exonuclease-proficient and -deficient forms) and accessory subunits were purified separately to homogeneity and reconstituted as described in refs. 29 and 30. Human pol γ fidelity was measured as described in ref. 13. Briefly, pol γ was used to copy a single-stranded region of the M13 lacZ α-complementation gene. Gap-filling reaction mixtures (25 μl) contained 25 mM Hepes·KOH (pH 7.6), 2 mM DTT, 2 mM MgCl2, 50 μg/ml BSA, 0.1 M NaCl, ≈150 ng of gapped M13mp2 DNA, 40 ng of Exo+ or Exo– p140 pol γ, a 1.3-fold molar excess of the p55 accessory subunit, and dNTPs at the indicated concentrations. Gap-filling reactions were run to completion as monitored by agarose gel electrophoresis. Products containing completely filled gaps were introduced by electroporation into the host strain, and replication errors were scored by plating as described in ref. 31. M13 DNA from independent mutant M13 plaques was isolated and sequenced to determine the types of polymerization errors. Error rates were calculated from these data as described in ref. 31.
Results
Mitochondrial dNTP Pool Analysis. We measured mitochondrial dNTP pools from heart, brain, liver, and skeletal muscle from both young and old rats as described in Materials and Methods. Values recorded as pmol/mg mitochondrial protein were converted to molar concentrations by using the estimated aqueous volume of rat tissue mitochondria. For this purpose, a value of 0.82 μl/mg for rat heart mitochondrial protein was used (32). This value is close to 0.84, which we calculated as an average of values reported for rat heart and liver mitochondria in three earlier reports (33–35); these earlier values range from 0.67 to 1.04 μl/mg mitochondrial protein. The calculated molar concentrations of mitochondrial dNTPs (Table 1) revealed no significant difference in mitochondrial dNTP levels between young and old rats or between heart subsarcolemmal mitochondria and interfibrillary mitochondria. This result suggests that the age-related decrease of glutaredoxin reductase and oxidized glutathione reductase activities in IFM does not affect mitochondrial dNTP levels. The results further show that mitochondrial dNTP pools in all tissues analyzed are highly asymmetric. Surprisingly, dGTP was the most abundant dNTP present in most tissues. In heart and skeletal muscle mitochondria, dGTP comprises between 85% and 91% of total dNTPs. By contrast, dTTP accounts for only 0.5% of total dNTPs. In brain mitochondria, dGTP comprises ≈62% of total dNTPs, whereas dTTP accounts for only ≈4% of total dNTPs. In liver mitochondria, dGTP, dCTP, dATP, and dTTP account for 37%, 51%, 9%, and 3% of total dNTPs, respectively. These values contrast sharply with whole-cell measurements, which primarily reflect cytosolic dNTP pools that supply nuclear DNA replication. As measured in cultured cells, dGTP usually comprises just 5–10% of the total dNTP pool (36). The differences in relative dGTP abundance in total versus mitochondrial dNTP pools is not simply due to the use of rat tissues versus cultured cells, because we have found that even in HeLa cells, mitochondrial dGTP accounts for 33% of total dNTPs, whereas the corresponding value is just 7.6% for total HeLa cell extracts (19) and just 10% in extracts of whole rat embryos (37). For comparison, Table 1 lists estimated dNTP concentrations in HeLa cell mitochondria, as determined in our earlier study (19).
Table 1. Estimated mitochondrial dNTP concentrations in rat tissues.
| dNTP concentration, μM
|
|||||
|---|---|---|---|---|---|
| Source of mitochondria | n | dATP | dTTP | dCTP | dGTP |
| Young rats | |||||
| Heart, subsarcolemmal | 3 | 3.6 ± 1.0 | 0.7 ± 0.21 | 13 ± 4.4 | 110 ± 48 |
| Heart, interfibrillary | 3 | 4.0 ± 0.43 | 0.8 ± 0.46 | 12 ± 6.6 | 140 ± 62 |
| Liver | 2 | 3.8 ± 0.23 | 1.3 ± 0.87 | 23 ± 3.9 | 16 ± 4.4 |
| Brain | 3 | 11 ± 5.4 | 3.4 ± 2.1 | 16 ± 9.1 | 49 ± 7.4 |
| Skeletal muscle | 2 | 2.8 ± 1.4 | 0.27 ± 0.22 | 5.3 ± 1.8 | 82 ± 13 |
| Old rats | |||||
| Heart, subsarcolemmal | 2 | 4.9 ± 0.63 | 0.9 ± 0.23 | 14 ± 1.0 | 87 ± 4.1 |
| Heart, interfibrillary | 2 | 6.1 ± 2.0 | 1.2 ± 0.43 | 15 ± 6.4 | 130 ± 18 |
| Liver | 2 | 3.3 ± 1.0 | 1.9 ± 1.6 | 19 ± 1.1 | 14 ± 6.3 |
| Brain | 1 | 7.7 | 2.5 | 21 | 50 |
| HeLa cells | 13.4 | 35.4 | 7.9 | 26.8 | |
dNTP pools were determined as described in Materials and Methods. Values are mean ± SD. HeLa cell data are from ref. 19. n, number of animals analyzed.
Pol γ Fidelity with Biased dNTP Pools. The high dGTP concentration in mitochondria and the fact that dGTP is generally more abundant than the other three dNTPs, particularly in heart and skeletal muscle mitochondria, suggests that dNTP asymmetries could be a contributor to the high mutation rate for the mitochondrial genome. To test this idea, we measured the fidelity of human pol γ during synthesis in vitro to fill a 407-nt gap containing the 275-nt lacZ α-complementation gene sequence in M13mp2. We compared results for reaction mixtures containing equimolar dNTPs at 1, 10, 100, and 1,000 μM versus those containing intramitochondrial dNTP concentrations measured in rat tissues. A forward mutation assay for loss of α-complementation function (blue to light blue and colorless M13 plaques on indicator plates) was used to score substitution, deletion, and addition errors in a variety of sequence contexts. To determine the extent to which the dNTP pools asymmetries affected the nucleotide selectivity of the polymerase and the proofreading efficiency of the intrinsic 3′ exonuclease, fidelity results were obtained for both wild-type (i.e., exonuclease-proficient) and exonuclease-deficient forms of pol γ. Note that Km values for pol γ range from 0.5 to 5 μM, depending on the dNTP, the DNA substrate, and the presence of the accessory subunit (30, 38–40).
For reaction mixtures containing equimolar dNTPs at concentrations of 1 and 1,000 μM, which largely span the range of concentrations in tissues reported in Table 1, the products of gap-filling reactions by wild-type pol γ yielded lacZ mutant frequencies of 7.8 × 10–4 and 11 × 10–4, respectively (Table 2). These values are near the background mutant frequency for uncopied DNA (historically between 5 × 10–4 and 10 × 10–4) and are consistent with previous studies (13–16), indicating that wild-type pol γ is an accurate polymerase. The products of gap-filling reaction by exonuclease-deficient pol γ yielded lacZ mutant frequencies that were higher, with similar frequencies obtained at equimolar dNTPs concentrations ranging from 1 to 1,000 μM (values from 45 × 10–4 to 62 × 10–4). These values are all greater than for the wild-type polymerase because of loss of proofreading activity, thereby reflecting the nucleotide selectivity of the polymerase alone. Importantly, in comparison with results with equimolar dNTP concentrations, the lacZ mutant frequencies are 2- to 6-fold higher (Table 2) for reactions performed in the presence of dNTP concentrations estimated in heart (SSM and IFM) and skeletal muscle mitochondria. These two tissues have the largest dNTP pool asymmetries and the highest dGTP concentrations. The lacZ mutant frequencies for reactions performed in the presence of dNTP concentrations estimated in liver and brain mitochondria, which are less asymmetric, are similar to those observed at equimolar dNTPs. Collectively, the mutant frequency data in Table 2 thus provide an initial indication that the dNTP pool asymmetries in mitochondria of rat heart and skeletal muscle tissues increase nucleotide misinsertion and decrease proofreading by pol γ.
Table 2. lacZ mutant frequencies for products of pol γ gap filling.
| Wild-type pol γ
|
Exonuclease-deficient pol γ
|
|||
|---|---|---|---|---|
| dNTP pool used | Mutant frequency × 10-4 | Relative frequency | Mutant frequency × 10-4 | Relative frequency |
| Equimolar dNTP pools, μM | ||||
| 1,000 | 11 | 1.4 | 62 | 1.4 |
| 100 | ND | — | 56 | 1.2 |
| 10 | ND | — | 48 | 1.1 |
| 1 | 7.8 | 1.0 | 45 | 1.0 |
| Intramitochondrial dNTP pools | ||||
| Heart, SSM | 23 | 2.9 | 160 | 3.6 |
| Heart, IFM | 21 | 2.7 | 170 | 3.8 |
| Liver | ≤22 | — | 42 | 0.9 |
| Brain | ND | — | 60 | 1.3 |
| Skeletal muscle | 13 | 1.7 | 270 | 6.0 |
ND, not determined. Relative frequencies were normalized to 1 μM equimolar dNTP pools for each enzyme. The data for the 1,000 μM reaction mixture are from ref. 13.
A priori, the intramitochondrial pool asymmetries in Table 1 would predict increased fidelity for some mismatches. As one example, a high proportion of dGTP should lower error rates opposite template C. Conversely, fidelity should be decreased for other mismatches. For example, the most common base substitution error made by pol γ is misincorporation of incoming dGTP opposite template T (13), an error that should be strongly promoted by the high ratio of dGTP to dATP observed in rat muscle mitochondrial dNTP pools (Table 1). The use of the M13mp2 forward mutation assay provides a test of such predictions because it monitors all 12 possible base–base mismatches in many different sequence contexts. Thus, to determine which nucleotide changes led to the pool asymmetry-dependent increases in lacZ mutation frequencies (i.e., lower fidelity), we sequenced DNA from independent lacZ mutant plaques obtained from reactions performed with equimolar (1 μM) dNTPs and with the three dNTP pool asymmetries observed in mitochondria of rat heart and skeletal muscle tissues.
Base substitution errors comprise the majority of errors made by wild-type and exonuclease-deficient pol γ for all conditions examined (Table 3). Among these, T to C substitutions resulting from stable misincorporation of incoming dGTP opposite template T were the most common. With equimolar dNTPs, among 45 and 15 total substitutions recovered from wild-type and exonuclease-deficient pol γ reactions, respectively, 5 (11%) and 2 (13%) substitutions were T to C. In contrast, in reactions containing the biased dNTP pools, about one-half of the base substitutions by exonuclease-deficient pol γ were T to C. These errors are predicted by the excess dGTP present in these reactions. Moreover, in reactions mimicking intramitochondrial dNTP pools in heart mitochondria (both SSM and IFM), 25% of the base substitutions made by wild-type pol γ were T to C. This finding implies that some dGMP misinsertions opposite template T promoted by the excess dGTP escaped proofreading by this highly accurate polymerase. This interpretation is supported by the observation that among 13 T to C substitutions by the wild-type polymerase (five with SSM conditions, eight with IFM conditions) (Table 3), 10 had a template C 5′ to the T (Table 3, column labeled T(C) to C). This sequence context bias in base substitution specificity is predicted by the high dGTP concentrations (110 and 140 μM, respectively, for SSM and IFM, Table 1), because extension of T-dG mismatches would be promoted by more frequent correct incorporation of dG opposite the next template C, thus reducing proofreading efficiency.
Table 3. Sequence analysis of lacZ mutants.
| No. of mutants observed
|
||||
|---|---|---|---|---|
| dNTP pool | T to C | T(C) to C* | Substitution | Total |
| Exonuclease-deficient pol γ | ||||
| Equimolar (1 μM) | 5 | 2 | 45 | 70 |
| Heart (SSM) | 20 | 10 | 27 | 38 |
| Heart (IFM) | 28 | 16 | 44 | 57 |
| Skeletal muscle | 27 | 14 | 58 | 65 |
| Wild-type pol γ | ||||
| Equimolar (1 μM) | 2 | 1 | 15 | 15 |
| Heart (SSM) | 5 | 4 | 21 | 23 |
| Heart (IFM) | 8 | 6 | 32 | 35 |
| Skeletal muscle | 1 | 0 | 16 | 18 |
T to C substitution with a C 5′ to the T.
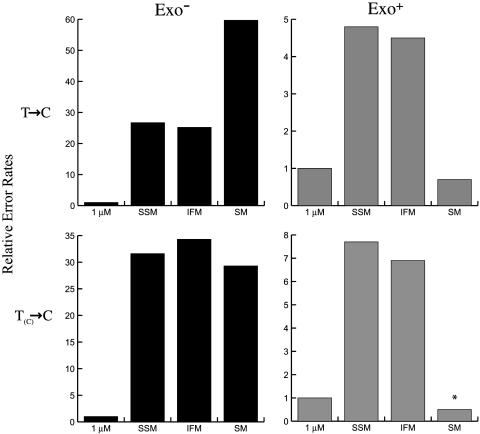
To quantify the effects of the pool asymmetries on pol γ nucleotide selectivity and proofreading, we used the mutant frequency (Table 2) and sequencing data (Table 3) to calculate (as described in ref. 31) the error rates for T to C substitutions for the four dNTP pool conditions. When dNTP pools are balanced and low, the error rates of both wild-type and exonuclease-deficient pol γ are low (Fig. 1). This conclusion is so when considering T to C substitutions at all 27 different detectable template T positions in the lacZ template or only those 8 template Ts flanked by a 5′ template C. In reaction mixtures containing dNTPs at concentrations intended to mimic the ≈30:1 ratio of dGTP:dATP seen in mitochondria of young rats (Table 1), the T to C error rate for exonuclease-deficient pol γ is ≈30-fold higher (Fig. 1 Left). This increase in error rate is a remarkably close match between the predicted and observed effect on error rate for this specific mismatch when made by a polymerase whose fidelity depends only on nucleotide selectivity. Of potentially greater biological relevance, the dNTP pool imbalances found in heart mitochondria even reduce the fidelity of wild-type pol γ (Fig. 1 Right). When all sequence contexts are considered, fidelity is reduced by 4- to 5-fold (Fig. 1 Upper Right), indicating that proofreading is still operational but that some dGMP misinsertions opposite T are not edited. When the T(C) sequence context alone is considered, fidelity is reduced even more by the pool imbalances (7- to 8-fold, Fig. 1 Lower Right), consistent with even less efficient proofreading driven by mismatch extension due to the high concentration of the immediate next correct nucleotide. Curiously, the fidelity of wild-type pol γ remained high when using the pool imbalance found in skeletal muscle. This high fidelity of incorporation may possibly reflect the slightly lower concentration of each of the four dNTPs for skeletal muscle as opposed to heart mitochondrial pools (Table 1), which would less effectively promote mismatch extension at the expense of proofreading.
Fig. 1.
Relative error rates for T to C substitutions by pol γ with varying dNTP pools. Wild-type and exonuclease-deficient pol γ were used to fill M13mp2-gapped DNA as described in Materials and Methods by using the dNTP concentrations indicated in Table 1. Error rates were calculated from the data in Tables 2 and 3 (as described in ref. 31) for all T to C changes and for T(C) to C changes for T having template C as a 5′neighbor. The values plotted are rates relative to error rates observed by using 1 μM of each of the four dNTPs, which were assigned a value of 1. For exonuclease-deficient pol γ, the actual rates are 2.1 × 10–5 and 3.0 × 10–5 for T to C and T(C) to C errors, respectively. For wild-type pol γ, the actual rates are 0.64 × 10–5 and 1.1 × 10–5 for T to C and T(C) to C errors, respectively. *, theoretical value if at least one mutant plaque had been detected. SM, skeletal muscle.
Additional analysis of pol γ error specificity indicates that next nucleotide effect of dNTP pool asymmetries is not limited to base substitutions. In studies of the large Klenow fragment of E. coli pol I (a family A homolog of pol γ), dNTP pool imbalances were found to initiate single-base deletions that were suggested to result from nucleotide misinsertion, followed by strand realignment, to create correct termini for extension (41). This model can explain single-base deletion errors observed in the current study, where biased dNTP pools elevated the rate of single-nucleotide deletions by exonuclease-deficient pol γ by 14- to 30-fold more than equimolar dNTPs (Table 4). These errors include loss of template bases whose 5′ neighbor was a C (as observed for substitution errors), consistent with the high dGTP concentration. As for Klenow polymerase, proofreading by pol γ can apparently edit most of these errors, but a few of these frameshift intermediates appear to have escaped proofreading. In Table 4, note that one frameshift mutant was detected in each of the two “heart” reactions with exonuclease-plus polymerase, whereas none were seen in the equimolar (1 μM) reaction mixture.
Table 4. Single-base deletions generated by pol γ.
| No. of mutants
|
Error rate × 10-4
|
Fold difference
|
||
|---|---|---|---|---|
| dNTP pool | -1total | -1(C) | ||
| Exonuclease-deficient pol γ | ||||
| Equimolar (1 μM) | 10 | 1 | 0.19 | 1.0 |
| Heart (SSM) | 5 | 2 | 2.6 | 14 |
| Heart (IFM) | 10 | 5 | 3.5 | 18 |
| Skeletal muscle | 4 | 2 | 5.7 | 30 |
| Wild-type pol γ | ||||
| Equimolar (1 μM) | 0 | 0 | ≤2.5 | 1.0 |
| Heart (SSM) | 1 | 1 | 9.6 | ≥3.8 |
| Heart (IFM) | 1 | 1 | 8.8 | ≥3.5 |
| Skeletal muscle | 0 | 0 | — | — |
-1total no. of mutants, number of single-base deletions observed in the total number of mutants observed described in Table 3. The error rate of 2.5 × 10-4 for wild-type pol γ with equimolar dNTPs is the error rate calculated if one mutant had been observed.
Discussion
The extent of the asymmetries in mitochondrial dNTP pools from rat heart and muscle tissues reported here was unexpected. The abundance of dGTP was particularly surprising given extensive literature indicating that dGTP is the least abundant dNTP in studies of whole-cell and nuclear extracts (36). The estimated dGTP concentrations in heart mitochondria are ≈5-fold higher than our reported value for HeLa cell mitochondria (19). Equally surprising, perhaps, are the low dTTP concentrations determined in most of the tissues examined, 1 μM or less. Given that the Km values for pol γ are in that same range, these data suggest that the dTTP pool may be rate-limiting for mtDNA replication, an unexpected scenario for a continuous replication process.
The observed dNTP asymmetries provide conditions that should strongly increase misinsertion during mtDNA replication. For example, the 30:1 dGTP:dATP ratio and the 19:1 dCTP:dTTP ratio in heart muscle (SSM) of young rats (Table 1) predicts increased formation of T·dGMP and A·dCMP mismatches that, if not corrected by proofreading or DNA repair, would result in A·T to G·C transition mutations. This prediction is confirmed by our in vitro studies showing an ≈30-fold increase in the T to C error rate for exonuclease-deficient pol γ (Fig. 1). This conclusion raises the biologically relevant question of whether these mitochondrial dNTP pool asymmetries detectably affect the fidelity of wild-type mitochondrial pol γ, whose accuracy is normally strongly enhanced by proofreading. Our in vitro data suggest a positive answer to this question as well because the dNTP pool asymmetries observed in heart mitochondria reduce the fidelity of wild-type pol γ forTtoC substitutions by 4- to 8-fold (Fig. 1). The in vitro data suggest that A·TtoG·C transitions should represent a significant proportion of spontaneous mitochondrial mutations in vivo. Three sets of observations support this prediction. A summary of published data on mtDNA point mutations in cardiomyopathy showed 62% A·T to G·C transitions (42). A survey of several studies of mtDNA point mutations in mice reported 55% A·TtoG·C transitions (5). Finally, in mtDNA from patients with progressive external ophthalmoplegia, a heritable mitochondrial disorder characterized by the accumulation of multiple point mutations and large deletions in mtDNA, 8 of 14 cases (57%) listed in the MitoMap database were A·T to G·C transitions (43). As investigations on this subject continue, it is important to keep in mind that tissue-specific variations in dNTP pool asymmetries, such as those already seen here (Table 1), may preferentially promote different mutational pathways in different tissues.
The mutagenic effects of dNTP pool imbalances have been extensively documented in the literature (17). However, all studies reported to date involve the effect of imbalances artificially induced, for example, by metabolic inhibitors, thymidine limitation, or mutational alteration of allosteric control sites. The data recorded here suggest that natural precursor pool asymmetries influence the spontaneous mutation rate and the spectrum of spontaneous mutations.
As noted earlier, mtDNA point mutations have been shown to accumulate with age (4–6), and it was of interest to determine whether mitochondrial dNTP pools change with age in a manner that could account for any age-related differences in mitochondrial mutation rate. Our initial data presented here reveal no significant aging-related changes in mitochondrial dNTP pools. Whether the mutation rate actually does increase with age is still an open question, but it is also possible that the accumulation of mitochondrial mutations with age is simply the consequence of a spontaneous mutation rate that is high at birth and remains so throughout life.
The dGTP excess over the other dNTPs in these tissues is so high as to suggest the possibility of metabolic roles other than its function as a DNA precursor. The literature on non-DNA-related functions of dNTPs is sparse. There is one report (44) of dGTP accumulation in the microtubule cytoskeleton of neuronal cells cultured with nerve growth factor, but no biological function for this dGTP was proposed. Also, dATP and cytochrome c released from mitochondria have been proposed to activate a chain of caspase reactions in an apoptotic cascade (45). More recently, dATP was reported to be much more effective than ATP in stimulating contraction of rat heart muscle (46). These results and the present study suggest possible metabolic functions of dNTPs other than as DNA precursors. To our knowledge, the only known non-DNA-related metabolic function of deoxyribonucleotides is the use of thymidine diphosphate sugars as carriers in the biosynthesis of bacterial cell wall lipopolysaccharides. These nucleotide-linked sugars are not known to exist in mammalian mitochondria.
In summary, we find that normal intramitochondrial dNTP pools in rat tissues are highly asymmetric, and in vitro fidelity studies show that these imbalanced pools can stimulate base substitution and frameshift mutations with a substitution pattern that correlates with mitochondrial substitution mutations in vivo. These findings suggest that normal intramitochondrial dNTP pool asymmetries could contribute to mitochondrial mutagenesis and possibly to the origins of mitochondrial diseases.
Acknowledgments
We thank Dr. Tory M. Hagen of the Linus Pauling Institute, Oregon State University, for advice on mitochondrial isolation and characterization and Drs. Samuel Bennett (Oregon State University) and Roel Schaaper (National Institute of Environmental Health Sciences) for thoughtful suggestions regarding improvement of the manuscript. S.S. and C.K.M. thank Ms. Linda Wheeler for technical assistance, particularly with optimization of the nucleotide analytical procedures. Z.F.P. and T.A.K. thank Dinh Nguyen for technical assistance. The work at Oregon State University was supported by Grant LS-45039 of the U.S. Army Research Office. Additional support came from Center Grant ES00040 of the National Institute of Environmental Health Sciences.
Author contributions: S.S., Z.F.P., T.A.K., and C.K.M. designed research; S.S. and Z.F.P. performed research; S.S., W.C.C., and M.J.L. contributed new reagents/analytic tools; S.S., Z.F.P., W.C.C., T.A.K., and C.K.M. analyzed data; and S.S., Z.F.P., and C.K.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: pol, DNA polymerase; SSM, subsarcolemmal mitochondria; IFM, interfibrillary mitochondria; SM, skeletal muscle.
References
- 1.Linnane, A. W., Marzuki, S., Ozawa, T. & Tanaka, M. (1989) Lancet 1, 642–645. [DOI] [PubMed] [Google Scholar]
- 2.Wallace, D. C. (1999) Science 283, 1482–1488. [DOI] [PubMed] [Google Scholar]
- 3.Penta, J. S., Johnson, F. M., Wachsman, J. T. & Copeland, W. C. (2001) Mut. Res. 488, 119–133. [DOI] [PubMed] [Google Scholar]
- 4.Wang, Y., Michikawa, Y., Mallidis, C., Bai, Y., Woodhouse, L., Yarasheski, K. E., Miller, C. A., Askanas, V., Engel, W. K., Bhasin, S. & Attardi, G. (2001) Proc. Natl. Acad. Sci. USA 98, 4022–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khaidakov, M., Heflich, R. H., Manjanatha, M. G., Myers, M. B. & Aidoo, A. (2003) Mutat. Res. 526, 1–7. [DOI] [PubMed] [Google Scholar]
- 6.Michikawa, Y., Mazzucchelli, F., Bresolin, N., Scarlato, G. & Attardi, G. (1999) Science 286, 774–779. [DOI] [PubMed] [Google Scholar]
- 7.Trifunovic, A., Wredenberg, A., Falkenberg, M., Spelbrink, J. N., Rovio, A. T., Bruder, C. E., Bohlooly, Y. M., Gidlof, S., Oldfors, A., Wibom, R., et al. (2004) Nature 429, 417–423. [DOI] [PubMed] [Google Scholar]
- 8.Marcelino, L. & Thilly, W. G. (1999) Mutat. Res. 434, 177–203. [DOI] [PubMed] [Google Scholar]
- 9.Bogenhagen, D. F. (1999) Am. J. Hum. Genet. 64, 1276–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dianov, G. L., Souza-Pinto, N., Nyaga, S. G., Thybo, T., Stevnsner, T. & Bohr, V. A. (2001) Prog. Nucleic Acid Res. Mol. Biol. 68, 285–297. [DOI] [PubMed] [Google Scholar]
- 11.Mason, P. A. & Lightowlers, R. N. (2003) FEBS Lett. 554, 6–9. [DOI] [PubMed] [Google Scholar]
- 12.Khrapko, K., Coller, H. A., André, P. C., Li, X.-C., Hanekamp, J. S. & Thilly, W. G. (1997) Proc. Natl. Acad. Sci. USA 94, 13798–13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longley, M. J., Nguyen, D., Kunkel, T. A. & Copeland, W. C. (2001) J. Biol. Chem. 276, 38555–38562. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, A. A. & Johnson, K. A. (2001) J. Biol. Chem. 276, 38090–38096. [DOI] [PubMed] [Google Scholar]
- 15.Kunkel, T. A. & Soni, A. (1988) J. Biol. Chem. 263, 4450–4459. [PubMed] [Google Scholar]
- 16.Kunkel, T. A. & Mosbaugh, D. W. (1989) Biochemistry 28, 988–995. [DOI] [PubMed] [Google Scholar]
- 17.Kunz, B. A., Kohalmi, S. E., Kunkel, T. A., Mathews, C. K., McIntosh, E. M. & Reidy, J. A. (1994) Mutat. Res. 318, 1–64. [DOI] [PubMed] [Google Scholar]
- 18.Bestwick, R. K., Moffett, G. L. & Mathews, C. K. (1982) J. Biol. Chem. 257, 9300–9304. [PubMed] [Google Scholar]
- 19.Song, S., Wheeler, L. J. & Mathews, C. K. (2003) J. Biol. Chem. 278, 43893–43896. [DOI] [PubMed] [Google Scholar]
- 20.Rampazzo, C., Ferraro, P., Pontarin, G., Fabris, S., Reichard, P. & Bianchi, V. (2004) J. Biol. Chem. 279, 17019–17026. [DOI] [PubMed] [Google Scholar]
- 21.Nishino, I., Spinazzola, A. & Hirano, M. (1999) Science 283, 689–692. [DOI] [PubMed] [Google Scholar]
- 22.Kaukonen, J., Juselius, J., Tiranti, V., Kyttala, A., Zeviani, M., Comi, G., Keranen, S., Peltonen, L. & Suomalainen, A. (2000) Science 289, 782–785. [DOI] [PubMed] [Google Scholar]
- 23.Palmer, J. W., Tandler, B. & Hoppel, C. L. (1977) J. Biol. Chem. 252, 8731–8739. [PubMed] [Google Scholar]
- 24.Fannin, S. W., Lesnefsky, E. J., Slabe, T. J., Hassan, M. O. & Hoppel, C. L. (1999) Arch Biochem. Biophys. 372, 399–407. [DOI] [PubMed] [Google Scholar]
- 25.Suh, J. H., Heath, S. H. & Hagen, T. M. (2003) Free Radical Biol. Med. 35, 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young, P., Leeds, J. M., Slabaugh, M. B. & Mathews, C. K. (1994) Biochem. Biophys. Res. Commun. 203, 46–52. [DOI] [PubMed] [Google Scholar]
- 27.Wroblewski, F. & LaDue, J. S. (1955) Proc. Soc. Exp. Bio. Med. 9, 210–213. [DOI] [PubMed] [Google Scholar]
- 28.Rickwood, D. (1987) in Practical Approach Series, eds. Darley-Usmar, V. M., Rickwood, D. & Wilson, M. T., (IRL, Washington, D. C.), pp. 12–14.
- 29.Longley, M. J., Ropp, P. A., Lim, S. E. & Copeland, W. C. (1998) Biochemistry 37, 10529–10539. [DOI] [PubMed] [Google Scholar]
- 30.Lim, S. E., Longley, M. J. & Copeland, W. C. (1999) J. Biol. Chem. 274, 38197–38203. [DOI] [PubMed] [Google Scholar]
- 31.Bebenek, K. & Kunkel, T. A. (1995) Methods Enzymol. 262, 217–232. [DOI] [PubMed] [Google Scholar]
- 32.Vinnakota, K. C. & Bassingthwaighte, J. B. (2004) Am J. Physiol. 286, H1742–H1749. [DOI] [PubMed] [Google Scholar]
- 33.Whipps, D. E. & Halestrap, A. E. (1984) Biochem. J. 221, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim, K. H. H., Javadov, S. J., Das, M., Clarke, S. J., Suleiman, S.-M. & Halestrap, A. P. (2002) J. Physiol. 545, 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das, M., Parker, J. E. & Halestrap, A. P. (2003) J. Physiol. 547, 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathews, C. K. & Ji, J. (1992) Bioessays 14, 295–301. [DOI] [PubMed] [Google Scholar]
- 37.Mole, M. L., Hunter, D. L., Gao, P. & Lau, C. (1998) Anal. Biochem. 259, 245–252. [DOI] [PubMed] [Google Scholar]
- 38.Longley, M. J., Prasad, R., Srivastava, D. K., Wilson, S. H. & Copeland, W. C. (1998) Proc. Natl. Acad. Sci. USA 95, 12244–12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graziewicz, M. A., Longley, M. J., Bienstock, R. J., Zeviani, M. & Copeland, W. C. (2004) Nat. Struct. Mol. Biol. 11, 770–776. [DOI] [PubMed] [Google Scholar]
- 40.Johnson, A. A., Tsai, Y., Graves, S. W. & Johnson, K. A. (2000) Biochemistry 39, 1702–1708. [DOI] [PubMed] [Google Scholar]
- 41.Bebenek, K. & Kunkel, T. A. (1990) Proc. Natl. Acad. Sci. USA 87, 4946–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marin-Garcia, J. Goldenthal, M. J. & Moe, G. W. (2001) Cardiovasc. Res. 49, 17–26. [DOI] [PubMed] [Google Scholar]
- 43.Ponamarev, M. V., Longley, M. J., Nguyen, D., Kunkel, T. A. & Copeland, W. C. (2002) J. Biol. Chem. 277, 15225–15228. [DOI] [PubMed] [Google Scholar]
- 44.Angelastro, J. M. & Purich, D. L. (1992) J. Biol. Chem. 267, 25685–25689. [PubMed] [Google Scholar]
- 45.Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S., Ahmad, M., Alnemri, E. S. & Wang, X. (1997) Cell 91, 479–489. [DOI] [PubMed] [Google Scholar]
- 46.Regnier, M., Rivera, A. J., Chen, Y. & Chase, P. B. (2000) Circ. Res. 86, 1211–1217. [DOI] [PubMed] [Google Scholar]