Abstract
The number of people classified as obese, defined by the World Health Organization as having a body mass index ≥30, has been rising since the 1980s. Obesity is associated with comorbidities such as hypertension, diabetes mellitus, and nonalcoholic fatty liver disease. The current treatment paradigm emphasizes lifestyle modifications, including diet and exercise; however this approach produces only modest weight loss for many patients. When lifestyle modifications fail, the current “gold standard” therapy for obesity is bariatric surgery, including Roux-en-Y gastric bypass, sleeve gastrectomy, duodenal switch, and placement of an adjustable gastric band. Though effective, bariatric surgery can have severe short- and long-term complications. To fill the major gap in invasiveness between lifestyle modification and surgery, researchers have been developing pharmacotherapies and minimally invasive endoscopic techniques to treat obesity. Recently, interventional radiologists developed a percutaneous transarterial catheter-directed therapy targeting the hormonal function of the stomach. This review describes the current standard obesity treatments (including diet, exercise, and surgery), as well as newer endoscopic bariatric procedures and pharmacotherapies to help patients lose weight. We present data from two ongoing human trials of a new interventional radiology procedure for weight loss, bariatric embolization.
Keywords: bariatric surgery, BEAT Obesity, embolization, obesity, weight loss
1. Introduction
Obesity is a major medical problem worldwide. Radiologists have always played a role in the medical evaluation and treatment of obesity but as obesity rates continue to rise, it is important for the radiologist to be aware of emerging trends in obesity treatment. Obesity is defined as the presence of excess body fat; but because body fat is difficult to measure, body weight and body mass index are used as surrogates. Currently, obesity is defined by the World Health Organization (WHO) as a BMI of ≥30 for men and women. According to the United States Centers for Disease Control and Prevention (CDC) [1], a subcategory of obesity known as severe (morbid) obesity is defined as a BMI of ≥40.
There has been a marked increase in the rate of obesity among adults since the 1980s [2], and it is now formally recognized as an epidemic by the WHO [3, 4]. In fact, in 2013, it was estimated that more than 2 billion people worldwide were overweight or obese, of whom 671 million were obese [5].
Traditionally, radiologists have participated in the medical evaluation of obesity. Obesity increases the risk of noncommunicable diseases, including type-2 diabetes mellitus, hyperlipidemia, hypertension, stroke, metabolic syndrome, sleep apnea, gallstones, nonalcoholic fatty liver disease, depression, polycystic ovarian syndrome, and cancer. Familiarity with these associations is important when interpreting diagnostic examinations. Additionally, radiologists have played a key role in evaluating patients undergoing bariatric surgery by assessing anatomy before surgery and diagnosing complications afterward. Recently, there have been major advances in obesity treatment, with a trend toward minimally invasive procedures that pose less risk of the complications associated with invasive surgery. To update radiologists on the current and emerging trends in obesity therapy, this review provides an overview of current standard treatments for obesity and describes newer endoscopic bariatric procedures and a new interventional procedure—bariatric embolization.
2. Lifestyle modifications
The current paradigm in treating obesity is focused on lifestyle modifications (i.e., diet and exercise). Psychosocial and behavioral assessments should be performed when first evaluating a patient for obesity treatment to determine the patient’s readiness to lose weight and to identify any possible eating disorders (i.e., binge eating) [6]. Weight gain is always a result of higher energy intake than energy use, so attempts are made to alter this balance through lifestyle modification. Diet remains the cornerstone of weight loss, though compliance with a strict diet is often difficult because of low satiety and issues with palatability. Dietary modifications include moderate energy-deficit diets (i.e., decrease caloric intake typically by 500 kcal/day to achieve 0.45-kg/week weight loss), very low-calorie diets (total intake <800 kcal/day), low-carbohydrate/high-protein diets, low-fat diets, low–glycemic index diets, and meal-replacement diets [7].
Increasing energy expenditure is another approach to weight loss. The World Health Organization recommends adults aged 18–64 years engage in 150 minutes of moderate intensity aerobic physical activity per week [8]. With diet and exercise, most obese patients can lose up to 10% of their initial weight in 1 year [9]. In a study by Foster-Schubert et al. [10], postmenopausal women were randomized to 1 of 4 groups: reduced-calorie diet (n = 118), moderate-intensity aerobic exercise program (n = 117), combination of both (n = 117), or no lifestyle change (control) (n = 87). Women in the reduced-calorie group lost a mean of 7.2 kg (p < 0.001); those in the exercise group lost a mean of 2.0 kg (p = 0.034), and those in the combination group lost a mean of 8.9 kg (p < 0.0001) compared with a mean 0.7-kg decrease among controls. [10].
There are major limitations of relying on diet and exercise solely to control weight. Adhering to the prescribed regimen of a restricted diet and exercise can be extremely difficult. Maintaining weight loss requires long-term behavioral modifications that may not be feasible. Patients who regain weight after lifestyle modifications often consider further interventions, including bariatric surgery.
3. Bariatric surgery
Bariatric surgery is an alternative method to treat obesity. Surgical approaches can be classified as malabsorptive (anatomic diversion leading to decreased absorption of food), restrictive (decreasing the amount that can be ingested), or a combination of both. Figure 1 depicts common bariatric procedures. In 1991, a consensus panel concluded that Roux-en-Y gastric bypass (RYGB) and vertical banded gastroplasty were safe and effective for patients with a BMI >40 or patients with a BMI >35 and serious medical complications [11]. Since then, the practice of bariatric surgery has greatly expanded and newer surgical approaches have been developed.
Fig. 1.
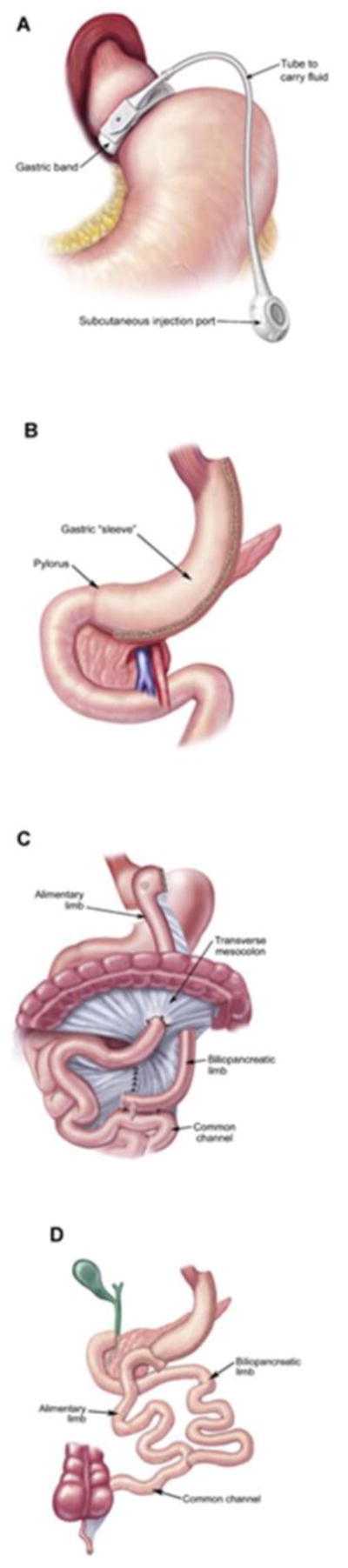
Most common bariatric surgical procedures. (a) Adjustable gastric band. (b) Sleeve gastrectomy. (c) Roux-en-Y-gastric bypass. (d) Biliopancreatic diversion with duodenal switch. (Reprinted with permission from Atlas of Metabolic and Weight Loss Surgery, Jones et al. Cine-Med, 2010. Fig. 1. Copyright of the book and illustrations are retained by Cine-Med.)
3.1. Malabsorptive procedures
The first bariatric surgical procedures were based primarily on malabsorption. An early procedure was the jejunoileal bypass, in which the proximal jejunum was bypassed to the distal ileum. This resulted in extreme weight loss because of excessive malabsorption and hypoproteinemia, sometimes creating life-threatening electrolyte abnormalities and other complications, including renal failure and acute liver failure; therefore, the procedure was discontinued [12]. A modified version of this procedure, the biliopancreatic diversion, creates a 200–250 mL horizontal pouch, distal gastrectomy, gastroenterostomy with a Roux limb, and anastomosis of the biliopancreatic limb to the Roux limb proximal to the ileocecal junction [13]. Traditionally, this procedure has been reserved for patients with a BMI >50. The biliopancreatic diversion with duodenal switch procedure is similar, but involves removal of the greater curvature and a long intestinal bypass.
3.2. Restrictive procedures
The next group of procedures developed were restrictive rather than malabsorptive. Restrictive horizontal and vertical gastroplasty procedures were developed when a suture line with staples was created to limit the capacity of the stomach. Though moderately effective, these procedures are not as favored by surgeons and patients as other bariatric surgical procedures [14]. Currently, most surgeons transect the stomach to prevent staple-line dehiscence. Gastric banding is another exclusively restrictive procedure in which a tight band is placed around the upper part of the stomach, creating a small gastric pouch. A reservoir port is then placed in the skin to allow for adjustments to the pouch size. Weight loss from the gastric band procedure is similar to that of gastroplasty procedures; however, the gastric band procedure is much simpler technically [13]. Though initially popular, there are many long-term band-related complications requiring removal in almost 50% of patients, including band slippage and pouch dilation [15].
Combined malabsorptive and restrictive operations include the stapled gastric bypass, transected gastric bypass, banded gastric bypass, and distal RYGB. Unlike the biliopancreatic diversion, in the distal RYGB procedure the distal stomach is not completely removed, but rather a small pouch is made. Though improved (with fewer malabsorption-related complications) compared with biliopancreatic diversion, this procedure is invasive and has severe complications, including diarrhea, vitamin deficiency, abdominal pain, and protein malnutrition [15]. Although RYGB can lead to vitamin and mineral deficiencies, it has been shown to be associated with endocrine changes, including a decrease in hunger-related hormones and changes in insulin sensitivity leading to an increased rate of remission of type-2 diabetes [16].
Comparing the effectiveness of these surgical procedures has been difficult because of a lack of randomized clinical trials. A comprehensive systematic review of 136 studies reported weight loss outcomes for various procedures and found that RYGB was more effective than gastric banding [17]. This systematic review [17] also showed weight loss outcomes for various procedures, with excess weight loss (EWL) (calculated as weight loss / excess weight) of 50% for adjustable gastric banding, 68% for RYGB, 69% for vertical banded gastroplasty, and 72% for biliopancreatic diversion with duodenal switch [14, 15, 18]. Advantages and disadvantages for common bariatric procedures are summarized in Table 1.
Table 1.
Advantages and disadvantages of common bariatric surgical procedures [17].
| Bariatric surgery | Advantages | Disadvantages | Excess weight loss (%) |
|---|---|---|---|
| Roux-en-Y gastric bypass | Long-term weight loss; alters level of hormones, decreasing appetite; alters insulin sensitivity, increasing rate of remission of type-2 diabetes | Complex surgical procedure; malabsorption of vitamins and minerals because duodenum is bypassed; major short- and long-term surgical complications possible | 62 |
| Sleeve gastrectomy | Few vitamin and mineral deficiencies because duodenum is not bypassed; alters level of hormones, decreasing appetite | Possible early and late surgical complications, including leak and obstruction; remnant sleeve can dilate over time | 68 |
| Adjustable gastric banding | Short operative time; reversible; adjustable; few mineral and vitamin deficiencies because duodenum is not bypassed | High rate of repeat intervention because of obstruction or band malfunction; instrumentation associated with the band can break | 48 |
| Biliopancreatic diversion with duodenal switch | Greatest weight loss; highest rate of remission of type-2 diabetes | Vitamin and mineral deficiencies can be more severe and harder to treat compared with Roux-en-Y gastric bypass | 70 |
Despite its effectiveness, bariatric surgery carries risks. Serious complications such as anastomotic leaks, sepsis, bleeding, and venous thromboembolism have been reported [19], and the procedure is associated with severe morbidity and mortality. Considering the success rate of nonoperative treatment is modest compared with bariatric surgery, a major gap in invasiveness of treatments exists for patients who do not want or are not candidates for bariatric surgery. As part of a therapeutic continuum from lifestyle modifications to surgery, alternative therapies are being developed to help treat obesity, including medications and novel endoscopic and percutaneous procedures.
4. Pharmacotherapy
Because of the substantial complications and costs associated with bariatric surgery, managing weight with medications has long been a goal of the medical community. Pharmacotherapy may be a viable option for patients with obesity refractory to lifestyle modifications, those who do not wish to undergo bariatric surgery, or those who are not surgical candidates.
There are currently 4 U.S. Food and Drug Administration (FDA)–approved noradrenergic medications for short-term use: phentermine, diethylpropion, phendimetrazine, and benzphetamine. Phentermine, a sympathomimetic agent related to amphetamine that acts as an appetite suppressant, is the most commonly prescribed. A meta-analysis of randomized controlled trials of patients taking phentermine for 2–24 weeks reported a mean 3.6-kg greater weight loss compared with those who received a placebo [20]. Common adverse effects include dry mouth and insomnia, as well as cardiovascular effects like palpitations and tachycardia [21].
Medications for long-term weight control have been developed to address the chronic nature of obesity and its associated morbidities. The FDA has recently approved orlistat, lorcaserin, phentermine/topiramate, liraglutide, and naltrexone/bupropion (Table 2).
Table 2.
Weight loss medications.
| Medication | Mechanism of action | Adverse effects | Weight loss | ||
|---|---|---|---|---|---|
| With drug, % | With placebo, % | Time period, years | |||
| Orlistat [23] | Pancreatic lipase inhibitor | Decreased absorption of fat-soluble vitamins; oily stools; diarrheal abdominal pain | 5.8* | 3.0* | 4 |
| Lorcaserin [25] | Selective serotonin 2C receptor agonist | Headache, dizziness, fatigue, dry mouth, constipation | 5.0 | 1.5 | 1 |
| Phentermine/topiramate [26] | Sympathomimetic, raises concentration of norepinephrine | Paresthesias, constipation, and dry mouth | 9.3–10 | 1.8 | 2 |
| Liraglutide [28] | Glucagon-like peptide-1 agonist | Nausea, diarrhea, constipation, vomiting, dyspepsia, upper abdominal pain, nasopharyngitis, upper respiratory tract infection, sinusitis, influenza, headache, dizziness, decreased appetite, back pain, arthralgias, fatigue, injection site hematomas | 8.0 | 2.6 | 1.1 |
| Naltrexone/bupropion [30] | Bupropion, dopamine/norepinephrine reuptake inhibitor; naltrexone, opioid receptor agonist | Nausea, constipation, headache, and psychiatric and sleep disturbances | 9.3 | 5.1 | 1.1 |
Expressed in kg.
4.1. Orlistat
Orlistat (Xenical, Genentech, San Francisco, CA, USA), a pancreatic lipase inhibitor, prevents absorption of triglycerides [22]. Orlistat has been shown to achieve statistically significant weight loss (5.8 kg) compared with a placebo (3.0 kg) during a 4-year period and to decrease the risk of developing type-2 diabetes by improving insulin sensitivity and decreasing serum glucose [23]. Except for gastrointestinal adverse effects such as oily stools, diarrhea, and abdominal pain, orlistat is generally well tolerated, although the fat absorption–blocking effects also may decrease absorption of fat-soluble vitamins [20].
4.2. Lorcaserin
Lorcaserin (BELVIQ, Eisai, Woodcliff Lake, NJ, USA) is another chronic weight management medication that regulates energy balance and satiety. It is a selective serotonin 2C receptor agonist leading to weight loss [24]. The Behavioral Modification and Lorcaserin for Obesity and Overweight Management in Diabetes Mellitus study reported that obese patients with type-2 diabetes showed significantly more weight loss in the group taking lorcaserin compared with a placebo group (5.0% vs 1.5% total body weight, respectively), as well as improvements in mean ± SD HbA1c level (decrease of 1.0% ± 0.09% vs 0.4% ± 0.06%, respectively; p<0.001) [25]. Adverse effects of lorcaserin include headache, dizziness, fatigue, dry mouth, and constipation.
4.3. Phentermine/topiramate
Phentermine/topiramate (Qsymia, VIVUS, Mountain View, CA, USA) is a dual agent, extended-release therapy for chronic weight loss. Phentermine is a sympathomimetic agent that helps reduce appetite by increasing concentrations of norepinephrine in the central nervous system. The mechanism of action of topiramate related to weight loss is not well understood [20]. The SEQUEL study showed a mean 10.5% loss of baseline body weight after 2 years in 295 patients taking 15-mg phentermine/92-mg controlled-release topiramate [26]. Adverse effects reported in this study included paresthesias, constipation, and dry mouth, which appeared to be dose-dependent.
4.4. Liraglutide
Liraglutide (Saxenda, Novo Nordisk, Plainsboro, NJ, USA) is an injectable glucagon-like peptide-1 agonist that is also used as a treatment for type-2 diabetes at lower doses [27]. A randomized study of 3731 patients comparing liraglutide plus lifestyle intervention vs placebo and lifestyle intervention reported mean (± standard deviation) weight loss of 8.0 ± 6.7%vs 2.6 ± 5.7%, respectively [28]. Common adverse effects include nausea, hypoglycemia, and diarrhea.
4.5. Naltrexone/bupropion
Naltrexone/bupropion (Contrave, Orexigen Therapeutics, La Jolla, CA, USA) is another combination therapy. Naltrexone is an opioid antagonist that has anorexigenic effects, and bupropion is a dopamine/norepinephrine reuptake inhibitor, which has antidepressant and anorexigenic effects. The clinical efficacy of naltrexone/bupropion has been evaluated in 4 phase-3 clinical trials. The Contrave Obesity Research-BMOD (intensive behavior modification) trial showed up to 9.3% loss of initial body weight at 56 weeks for those treated with naltrexone/bupropion and behavior modification [20, 29, 30]. The most common adverse effects reported included nausea, constipation headache, and psychiatric and sleep disturbances.
5. Endoscopic bariatric techniques
The development of endoscopic bariatric techniques is a new trend in obesity treatment. Endoscopic techniques are less invasive and usually reversible, in contrast to bariatric surgery. These therapies can be classified into 6 overlapping categories: space-occupying devices, restrictive procedures, bypass liners, aspiration therapy, gastric stimulation, and transpyloric shuttle (Fig. 2, Table 3).
Fig. 2.
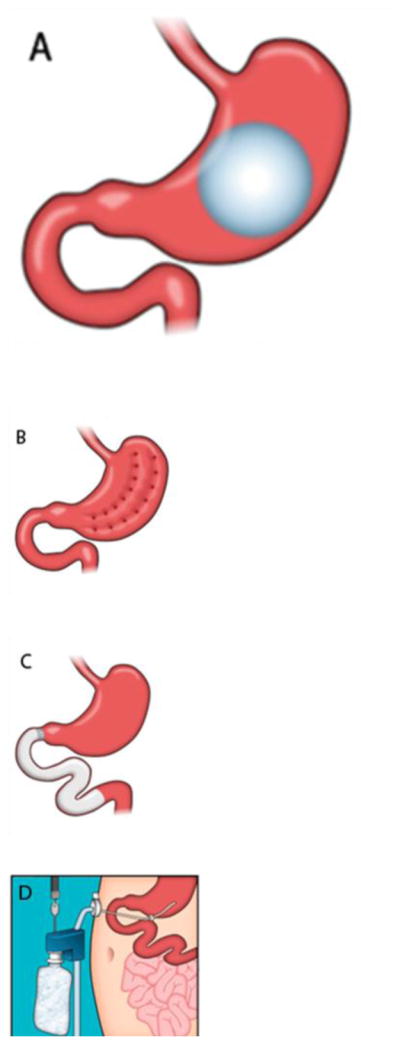
Illustrations of endoscopic bariatric procedures. (a) Space-occupying device. (b) Restrictive procedure. (c) Bypass liner. (d) Aspiration therapy. (e) Gastric stimulation. (f) Transpyloric shuttle. (Reprinted with permission from Neylan, D.T. Dempsey, C.M. Tewksbury, N.N. Williams, K.R. Dumon, Endoscopic treatments of obesity: a comprehensive review, Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery 12(5) (2016) 1108–15. Fig. 1.)
Table 3.
Advantages and disadvantages of endoscopic bariatric procedures.
| Procedures | Advantages | Disadvantages | Excess weight loss |
|---|---|---|---|
| Space-occupying devices | Easily placed endoscopically; restrict food consumption; well tolerated; effective; can be reversed/removed | Balloon may deflate over long term; can migrate, leading to perforation; FDA-required removal at 6 months with poor long-term weight loss | 39% at 1 year after removal [32] |
| Restrictive procedures | Permanently reduces stomach capacity; effective; well tolerated | Not easily reversible; plication durability varies according to device | Up to 54% ± 40% at 12 months (n = 10) [31] |
| Bypass liners | Pancreaticobiliary secretions can still travel along the sleeve, as opposed to surgical bypass | High risk of hepatic abscesses; not currently available in the U.S. because of complications | Up to 36% at 1 year [31] |
| Aspiration therapy | Patients can continue to eat normal diet | Easily abused and can promote poor eating habits and eating disorders | 41% after 6 months [31] |
FDA, U.S. Food and Drug Administration
5.1. Space-occupying devices (Fig. 2A)
Intragastric balloons, made of silicone and filled with air or fluid, occupy space normally occupied by food. They can be single balloons or consist of 2 connected spheres. Currently, the balloons are required, per FDA guidance, to be removed after 6 months because of concern that they slowly deflate and migrate, causing complications such as obstruction. At the time of removal, there has been up to 30%–50% mean EWL [31]. One such balloon, the BioEnterics Intragastric Balloon (Inamed Corp., Santa Barbara, CA, USA), now known as the Orbera balloon (Obalon Therapeutics, Carlsbad, CA, USA), showed a 58% ± 19% EWL at the time of removal (6 months), 39% ± 14% at 1 year, 25% ± 8% at 2 years, and 17% ± 8% at 5 years after removal [32] in a study of 122 patients. The ReShape device (ReShape Medical, San Clemente, CA, USA) is another form of intragrastric balloon consisting of 2 attached balloons. It is designed to prevent migration if 1 of the balloons deflates. The transpyloric shuttle is another space-occupying device, in which the smaller of 2 spheres enters the duodenal bulb and pulls a larger sphere into the pylorus, occasionally occluding the pylorus during peristalsis. It showed a 6-month total weight loss of 14% ± 5.8% in 20 patients[33]. Although the balloons have been shown to cause weight loss, one study found that the results were temporary, with patients regaining weight after balloon removal [34].
5.2. Restrictive procedures (Fig. 2B)
These procedures reduce gastric volume via suturing, stapling, or tissue anchors. The Incisionless Operating Platform (USGI Medical, San Clemente, CA, USA) has the ability to perform full-thickness plication. This device is used to perform the primary obesity surgery, endoluminal (POSE) procedure, which creates parallel plications in the gastric fundus to decrease gastric volume [35]. This procedure is currently being evaluated in the sham-controlled ESSENTIAL trial [36]. The OverStitch device (Apollo Endosurgery, Austin, TX, USA) is another tool used endoscopically that allows for full-thickness sutures and creates a full-thickness vertical gastroplasty similar to sleeve gastrectomy. One study of 50 patients showed a 19% ± 11% total body weight loss at 1 year [37]. The EndoCinch Suturing System (Davol, Warwick, RI, USA), now known as the RESTORe suturing system (Davol, Murray Hill, NJ, USA), creates a superficial suture to perform vertical gastroplasty and at 1 year has shown a mean EWL of 28% ± 22% in the 18 patients in the TRIM trial [38].
5.3. Bypass liner (Fig. 2C)
The duodenal-jejunal bypass liner (EndoBarrier, GI Dynamics, Lexing, MA, USA) is a Teflon (Chemours, Wilmington, DE, USA) sleeve that is implanted into the duodenal bulb and extends into the small bowel, which allows food to bypass the duodenum and proximal jejunum. It is left in place for 6 months. Although patients treated with the device showed a mean 32% EWL, a significant decrease in HbA1c, and a significant decrease in antidiabetic drug requirement, the trial for this procedure was prematurely terminated because of the incidence of hepatic abscesses [31, 39]. The EndoBarrier has CE mark approval in Europe and is indicated for patients with type-2 diabetes and obesity, but is not approved for sale in the U.S. [40].
5.4. Aspiration therapy (Fig. 2D)
The AspireAssist (Aspire Bariatrics, King of Prussia, PA, USA) is an FDA-approved device that involves inserting a gastrostomy tube into an obese patient’s stomach. The patient uses this device to manually aspirate approximately 30% of the meal after consumption. One study of 22 patients treated with this device showed a mean total weight loss of 15% ± 6.3% at 6 months [41]. Physicians must assess patients for eating disorders before tube placement because this device can be easily abused.
5.5. Gastric stimulation (Fig. 2E)
Currently, implantable gastric stimulator devices are placed laparoscopically. Retrograde gastric electrical stimulation is now being attempted to place 2 electrode pairs in the pylorus endoscopically [31]. Gastric stimulation was associated with decrease caloric intake and gastric accommodation, but weight loss was not measured in this study because a temporary pacing device was used [42]. Of note, there is a surgically placed device of this kind in use (vBloc, EnteroMedics, St. Paul, MN, USA), which has shown 21% EWL (8% total weight loss) after 2 years [43].
6. Bariatric embolization
Bariatric arterial embolization is a new percutaneous, transcatheter procedure performed by an interventional radiologist. It is designed to induce weight loss by embolizing the blood supply to the gastric fundus. The rationale for this procedure is based on the hormonal role played by the stomach in hunger and satiety. Ghrelin is an orexigenic hormone that is produced primarily (90%) by the gastric fundus. In addition to inducing hunger, the balance of ghrelin and other hormones (glucagon-like peptide-1, leptin, and neuropeptide Y) is important in satiety (i.e., the sensation of being full). Plasma ghrelin levels have been shown to rise before meals and decrease after ingestion [44, 45]. In postprocedural weight loss patients, ghrelin has been implicated in the mechanism for weight loss [46]. The premise of bariatric embolization is to create ischemia to ghrelin-producing cells in the gastric fundus.
Arepally et al. [47] first described left gastric artery embolization using a swine model. Multiple animal studies using distally penetrating embolic agents showed up to a 60% decrease in serum ghrelin levels, with decreased weight gain in growing swine compared with control groups [48–50]. Bawudun et al. [51] also showed suppressed ghrelin and weight gain after gastric artery embolization in a canine model. Using a porcine model, Diana et al. [52] showed significant ghrelin reduction in the pigs that underwent gastric artery embolization with 100–300-μm beads followed by coil embolization, compared with the group treated with 500–700-μm beads alone. A retrospective analysis of 19 patients who underwent left gastric artery embolization for gastrointestinal bleeding showed a 7.3% reduction in weight compared with patients who had a different blood vessel embolized for upper gastrointestinal bleeding [53]. However, a causal relationship between fundal ischemia and weight loss could not be established because malignancy could be a confounding variable in this study.
Kipshidze et al. [54] performed the first prospective human trial in which embolization of the left gastric artery via a femoral approach was performed for 5 patients using 300–500-μm particles (BeadBlock, Biocompatibles UK Ltd., Surrey, UK). The study showed a mean weight loss of 20 kg at 6 months after the procedure with no adverse events. Mean weight was reduced by 10%, 13%, 16%, 17%, and 17% at 1, 3, 6, 12, and 20–24 months of follow-up, respectively. Serum ghrelin levels in these patients showed an initial decrease of 36% at 3 months but began trending upward at 6 months. Although this study showed promising results, there were substantial limitations, including the lack of a control group or specific dietary and lifestyle modifications [54].
Two FDA-approved clinical trials are ongoing: the Gastric Artery Embolization Trial for Lessening of Appetite Nonsurgically (GET LEAN) at Dayton Interventional Radiology and Ohio State University; and Bariatric Embolization of Arteries for the Treatment of Obesity (BEAT Obesity) at The Johns Hopkins University and Mount Sinai Hospital. In the GET LEAN trial, 4 patients (3 women; mean age, 41 years; mean BMI, 42.4) underwent embolization using 300–500-μm particles (BeadBlock, BTG International, West Conshohocken, PA, USA) via a right common femoral or left radial approach. No serious adverse events were reported. A minor complication of superficial gastric ulcers that resolved in 30 days occurred in 3 of 4 patients. Mean body weight change at 6 months was 9.2 kg (range, −2.7 to −9.5 kg), which corresponded to a 17% EWL [55].
BEAT Obesity [56] is a multicenter trial approved for 20 severely obese patients (BMI ≥40), who are otherwise healthy. Before embolization, patients are referred to a multidisciplinary weight management center with a 4-week run-in period, similar to bariatric trials. The patients are given proton-pump inhibitors and sucralfate both before and 4 weeks after embolization. The screening process for the procedure includes a computed tomography angiogram to assess anatomy, a preprocedure endoscopy to assess gastric health, and a gastric emptying study. The embolization is then performed via a transfemoral or transradial approach. The left gastric artery and/or the gastroepiploic artery are embolized using 300–500-μm particles (Embosphere, Merit Medical Systems, Jordan, UT, USA). Figure 3 shows representative images from a bariatric embolization procedure.
Fig. 3.
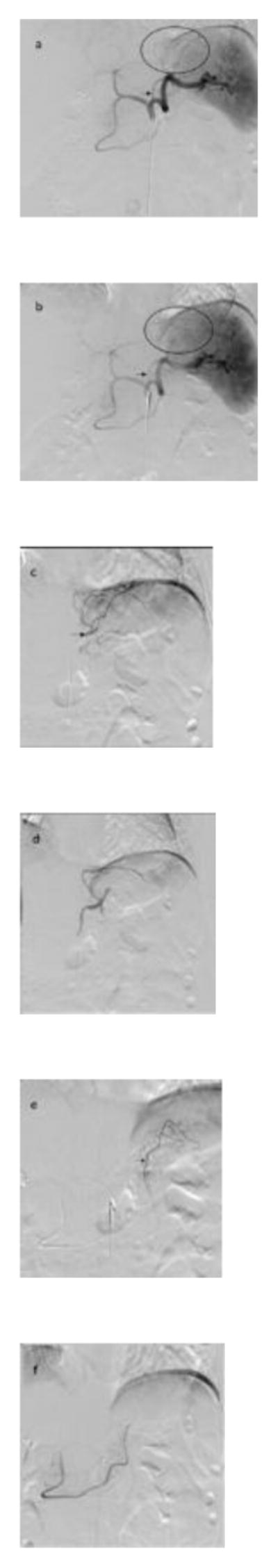
Bariatric embolization angiograms from the BEAT Obesity trial. (a) Celiac axis before embolization. Note the normal vascular distribution in the region of the gastric fundus (circle) from the left gastric artery (arrow). (b) Celiac axis angiogram postembolization showing decreased opacification of the distal branches in the region of the gastric fundus (circle) and decreased flow in the left gastric artery (arrow). (c) Selective left gastric artery angiogram. The arrow shows the location of the microcatheter within the distal left gastric artery at the time of embolization. (d) Selective left gastric artery angiogram postembolization showing decreased markedly truncated vessels flow. (e) Selective gastroepiploic artery angiogram showing flow to the gastric fundus. The arrow shows the location of the microcatheter within the distal gastroepiploic artery at the time of embolization. (f) Selective gastroepiploic artery angiogram postembolization showing markedly truncated vessel flow.
To date, no major adverse events have been reported in the BEAT Obesity trial. Minor adverse events, including superficial gastric ulcerations, were seen in 8 of 20 patients, which healed by the 3-month follow-up endoscopy. The superficial gastric ulcerations were considered minor because they were asymptomatic and were already healed or healing at the time of endoscopy. One patient had subclinical pancreatitis, evidenced by increased serum pancreatic enzymes. The current data from the BEAT Obesity trial have shown mean EWL of 8.4%, 12%, and 18% at 1-, 3-, and 6-month follow-up, respectively. Continued weight loss will be monitored for the entire cohort for 12 months. It will be essential to determine the durability of the embolization as evidenced by serum hormone levels and weight changes. These results will require continued follow-up to determine the durability and efficacy of bariatric embolization. However, it is likely that the weight and hormone level changes will need to be studied in a randomized clinical trial to determine the role of bariatric embolization for the treatment of obesity.
7. Conclusion
Obesity is a worsening global epidemic that presents a major burden for health care systems. The current approach to treating obesity is modification of diet and exercise, followed by referral for bariatric surgery. Though bariatric surgery has shown positive outcomes regarding weight loss and reversing obesity-related comorbidities, the high cost, invasiveness, and potential morbidities have led to development of less invasive therapies.
These new procedures manipulate the physical capacity of the gastrointestinal system and/or the hormonal function of the stomach through minimally invasive approaches. The endoscopic procedures, which include restrictive balloons, bypass sleeves, and aspiration devices have shown promising results at 6 months. Similarly, early data on endovascular bariatric embolization have shown safety and efficacy in the short and intermediate terms. It is hoped that as long-term safety and efficacy data become available, these new interventions will be integrated into the multidisciplinary treatment paradigm for obesity.
Supplementary Material
Highlights.
Review of the current obesity problem.
Review of lifestyle modifications and bariatric surgery.
Discussion of emerging/novel obesity therapies.
Introduction to bariatric embolization.
Acknowledgments
Role of the Funding Source: This work was supported by Merit Medical, Siemens Healthcare, the National Institute of Biomedical Imaging and Bioengineering, (grant R01EB017615), and a National Institutes of Health T32 training grant (T32EB006351).
Abbreviations
- BEAT Obesity
Bariatric Embolization of Arteries for the Treatment of Obesity
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- EWL
excess weight loss
- FDA
Food and Drug Administration
- GET LEAN
Gastric Artery Embolization Trial for Lessening of Appetite Nonsurgically
- RYGB
Roux-en-Y gastric bypass
- WHO
World Health Organization
Footnotes
Conflicts of Interest: The authors do not have any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. [Accessed on March 29, 2017];Defining adult overweight and obesity. Available at https://www.cdc.gov/obesity/adult/defining.html.
- 2.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 4.Dent KM, Harper C, Kearney L, Lieber C, Finucane B. Embracing the unique role of genetic counselors: response to the commentary by Madeo et al. American Journal of Medical Genetics Part A. 2011;155A(8):1791–3. doi: 10.1002/ajmg.a.34111. [DOI] [PubMed] [Google Scholar]
- 5.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheskin LJ, Poddar K. Obesity management. In: Jordan WH, editor. Principles of Human Nutrition. HardPress Publishing; Miami, FL: 2012. pp. 788–801. [Google Scholar]
- 7.United States Department of Health and Human Services, National Institutes of Health, National Heart Lung and Blood Institute. Managing overweight and obesity in adults. [Accessed on March 22, 2017];Systematic evidence review from the obesity expert panel. 2013 Available at https://www.nhlbi.nih.gov/sites/www.nhlbi.nih.gov/files/obesity-evidence-review.pdf.
- 8.World Health Organization. [Accessed on March 29, (2017)];Physical activity and adults. Available at http://www.who.int/dietphysicalactivity/factsheet_adults/en/
- 9.Carvajal R, Wadden TA, Tsai AG, Peck K, Moran CH. Managing obesity in primary care practice: a narrative review. Ann N Y Acad Sci. 2013;1281:191–206. doi: 10.1111/nyas.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, Bain CE, Wang CY, Blackburn GL, McTiernan A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring, Md) 2012;20(8):1628–38. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115(12):956–61. [PubMed] [Google Scholar]
- 12.Singh D, Laya AS, Clarkston WK, Allen MJ. Jejunoileal bypass: a surgery of the past and a review of its complications. World Journal of Gastroenterology. 2009;15(18):2277–9. doi: 10.3748/wjg.15.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fobi MA. Surgical treatment of obesity: a review. J Natl Med Assoc. 2004;96(1):61–75. [PMC free article] [PubMed] [Google Scholar]
- 14.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ (Clinical Research ed) 2014;349:g3961. doi: 10.1136/bmj.g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neylan CJ, Kannan U, Dempsey DT, Williams NN, Dumon KR. The Surgical Management of Obesity. Gastroenterol Clin North Am. 2016;45(4):689–703. doi: 10.1016/j.gtc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Cohen RV, Rubino F, Schiavon C, Cummings DE. Diabetes remission without weight loss after duodenal bypass surgery. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2012;8(5):e66–8. doi: 10.1016/j.soard.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA: The Journal of the American Medical Association. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 18.Buchwald H. Gastric stimulation: a new paradigm for management of morbid obesity. Obes Surg. 2004;14(Suppl 1):S2. doi: 10.1007/BF03342130. [DOI] [PubMed] [Google Scholar]
- 19.Bult MJ, van Dalen T, Muller AF. Surgical treatment of obesity. European Journal of Endocrinology. 2008;158(2):135–45. doi: 10.1530/EJE-07-0145. [DOI] [PubMed] [Google Scholar]
- 20.Patel D. Pharmacotherapy for the management of obesity. Metabolism. 2015;64(11):1376–85. doi: 10.1016/j.metabol.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Cosentino G, Conrad AO, Uwaifo GI. Phentermine and topiramate for the management of obesity: a review. Drug Design, Development and Therapy. 2013;7:267–78. doi: 10.2147/DDDT.S31443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bray GA. Medical treatment of obesity: the past, the present and the future. Best Practice & Research Clinical Gastroenterology. 2014;28(4):665–84. doi: 10.1016/j.bpg.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–61. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 24.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. Multicenter, placebo-controlled trial of lorcaserin for weight management. The New England Journal of Medicine. 2010;363(3):245–56. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 25.O'Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, Raether B, Anderson CM, Shanahan WR. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring, Md) 2012;20(7):1426–36. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 26.Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. The American Journal of Clinical Nutrition. 2012;95(2):297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saunders KH, Shukla AP, Igel LI, Kumar RB, Aronne LJ. Pharmacotherapy for Obesity. Endocrinol Metab Clin North Am. 2016;45(3):521–38. doi: 10.1016/j.ecl.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP, Randomized A. Controlled Trial of 3.0 mg of Liraglutide in Weight Management. The New England Journal of Medicine. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 29.Wadden TA, Wilson GT, Stunkard AJ, Berkowitz RI. Preface. Obesity and associated eating disorders: a guide for mental health professionals. The Psychiatric Clinics of North America. 2011;34(4):xiii–xvi. doi: 10.1016/j.psc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O'Neil PM, Perri MG, Pi-Sunyer FX, Rock CL, Erickson JS, Maier HN, Kim DD, Dunayevich E. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring, Md) 2011;19(1):110–20. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neylan CJ, Dempsey DT, Tewksbury CM, Williams NN, Dumon KR. Endoscopic treatments of obesity: a comprehensive review. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2016;12(5):1108–15. doi: 10.1016/j.soard.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E. 500 intragastric balloons: what happens 5 years thereafter? Obes Surg. 2012;22(6):896–903. doi: 10.1007/s11695-012-0607-2. [DOI] [PubMed] [Google Scholar]
- 33.Marinos G, Eliades C, Raman Muthusamy V, Greenway F. Weight loss and improved quality of life with a nonsurgical endoscopic treatment for obesity: clinical results from a 3- and 6-month study. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2014;10(5):929–34. doi: 10.1016/j.soard.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Dumonceau JM. Evidence-based review of the Bioenterics intragastric balloon for weight loss. Obes Surg. 2008;18(12):1611–7. doi: 10.1007/s11695-008-9593-9. [DOI] [PubMed] [Google Scholar]
- 35.Kumar N. Endoscopic therapy for weight loss: Gastroplasty, duodenal sleeves, intragastric balloons, and aspiration. World Journal of Gastrointestinal Endoscopy. 2015;7(9):847–59. doi: 10.4253/wjge.v7.i9.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. National Institutes of Health. [Accessed on March 16, 2017];The USGI Medical ESSENTIAL Study for Weight Loss. Available at https://clinicaltrials.gov/ct2/show/NCT01958385.
- 37.Lopez-Nava G, Galvao MP, Bautista-Castano I, Jimenez-Banos A, Fernandez-Corbelle JP. Endoscopic Sleeve Gastroplasty: How I Do It? Obes Surg. 2015;25(8):1534–8. doi: 10.1007/s11695-015-1714-7. [DOI] [PubMed] [Google Scholar]
- 38.Brethauer SA, Chand B, Schauer PR, Thompson CC. Transoral gastric volume reduction as intervention for weight management: 12-month follow-up of TRIM trial. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2012;8(3):296–303. doi: 10.1016/j.soard.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Koehestanie P, de Jonge C, Berends FJ, Janssen IM, Bouvy ND, Greve JW. The effect of the endoscopic duodenal-jejunal bypass liner on obesity and type 2 diabetes mellitus, a multicenter randomized controlled trial. Ann Surg. 2014;260(6):984–92. doi: 10.1097/SLA.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 40.Stimac D, Klobucar Majanovic S, Licina M. Recent Trends in Endoscopic Management of Obesity. Surgical Innovation. 2016;23(5):525–37. doi: 10.1177/1553350616643615. [DOI] [PubMed] [Google Scholar]
- 41.Forssell H, Noren E. A novel endoscopic weight loss therapy using gastric aspiration: results after 6 months. Endoscopy. 2015;47(1):68–71. doi: 10.1055/s-0034-1378097. [DOI] [PubMed] [Google Scholar]
- 42.Behary J, Kumbhari V. Advances in the Endoscopic Management of Obesity. Gastroenterology Research and Practice. 2015;(2015):757821. doi: 10.1155/2015/757821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Apovian CM, Shah SN, Wolfe BM, Ikramuddin S, Miller CJ, Tweden KS, Billington CJ, Shikora SA. Two-Year Outcomes of Vagal Nerve Blocking (vBloc) for the Treatment of Obesity in the ReCharge Trial. Obes Surg. 2017;27(1):169–176. doi: 10.1007/s11695-016-2325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 45.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132(6):2116–30. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 46.Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8(7):643–4. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 47.Arepally A, Barnett BP, Montgomery E, Patel TH. Catheter-directed gastric artery chemical embolization for modulation of systemic ghrelin levels in a porcine model: initial experience. Radiology. 2007;244(1):138–43. doi: 10.1148/radiol.2441060790. [DOI] [PubMed] [Google Scholar]
- 48.Arepally A, Barnett BP, Patel TH, Howland V, Boston RC, Kraitchman DL, Malayeri AA. Catheter-directed gastric artery chemical embolization suppresses systemic ghrelin levels in porcine model. Radiology. 2008;249(1):127–33. doi: 10.1148/radiol.2491071232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paxton BE, Alley CL, Crow JH, Burchette J, Weiss CR, Kraitchman DL, Arepally A, Kim CY. Histopathologic and immunohistochemical sequelae of bariatric embolization in a porcine model. Journal of Vascular and Interventional Radiology: JVIR. 2014;25(3):455–61. doi: 10.1016/j.jvir.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paxton BE, Kim CY, Alley CL, Crow JH, Balmadrid B, Keith CG, Kankotia RJ, Stinnett S, Arepally A. Bariatric embolization for suppression of the hunger hormone ghrelin in a porcine model. Radiology. 2013;266(2):471–9. doi: 10.1148/radiol.12120242. [DOI] [PubMed] [Google Scholar]
- 51.Bawudun D, Xing Y, Liu WY, Huang YJ, Ren WX, Ma M, Xu XD, Teng GJ. Ghrelin suppression and fat loss after left gastric artery embolization in canine model. Cardiovasc Intervent Radiol. 2012;35(6):1460–6. doi: 10.1007/s00270-012-0362-8. [DOI] [PubMed] [Google Scholar]
- 52.Diana M, Pop R, Beaujeux R, Dallemagne B, Halvax P, Schlagowski I, Liu YY, Diemunsch P, Geny B, Lindner V, Marescaux J. Embolization of arterial gastric supply in obesity (EMBARGO): an endovascular approach in the management of morbid obesity. proof of the concept in the porcine model. Obes Surg. 2015;25(3):550–8. doi: 10.1007/s11695-014-1535-0. [DOI] [PubMed] [Google Scholar]
- 53.Gunn AJ, Oklu R. A preliminary observation of weight loss following left gastric artery embolization in humans. Journal of Obesity. 2014;(2014):185349. doi: 10.1155/2014/185349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kipshidze N, Archvadze A, Bertog S, Leon MB, Sievert H. Endovascular Bariatrics: First in Humans Study of Gastric Artery Embolization for Weight Loss. JACC Cardiovascular Interventions. 2015;8(12):1641–4. doi: 10.1016/j.jcin.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 55.Syed MI, Morar K, Shaikh A, Craig P, Khan O, Patel S, Khabiri H. Gastric Artery Embolization Trial for the Lessening of Appetite Nonsurgically (GET LEAN): Six-Month Preliminary Data. Journal of Vascular and Interventional Radiology: JVIR. 2016;27(10):1502–8. doi: 10.1016/j.jvir.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Weiss CR, Akinwande O, Paudel K, Cheskin LJ, Holly B, Hong K, Fischman AM, Patel RS, Shin EJ, Steele KE, Moran TH, Kaiser K, Park A, Shade DM, Kraitchman DL, Arepally A. Clinical Safety of Bariatric Arterial Embolization: Preliminary Results of the BEAT Obesity Trial. Radiology. 2017:160914. doi: 10.1148/radiol.2016160914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


