Abstract
During the development of the peripheral nervous system, Schwann cells, the myelin-forming glia, migrate along axons before initiating myelination. We previously demonstrated that endogenous neurotrophin-3 (NT3) acting through the TrkC tyrosine kinase receptor enhances migration of premyelinating Schwann cells. This signaling pathway is mediated by the c-Jun N-terminal kinase (JNK) cascade regulated by the Rho GTPases Rac1 and Cdc42. However, missing is the link between TrkC and the GTPases. Here, we show that a guanine-nucleotide exchange factor (GEF), Dbl's big sister (Dbs), couples with TrkC to activate Cdc42 in Schwann cells. Furthermore, TrkC directly phosphorylates Dbs, thereby inducing the Cdc42-GEF activity. Taken together, activation of TrkC triggers Schwann cell migration by regulating Dbs upon direct tyrosine phosphorylation, providing a mechanism whereby a membrane receptor tyrosine kinase can induce the activation of Rho GTPase-GEFs.
Keywords: tyrosine phosphorylation, Rho GTPase, cell motility
Cell migration is crucial for early development and is initiated in response to extracellular guidance cues such as diffusible factors or signals present on neighboring cells (1). During the development of the peripheral nervous system, Schwann cells migrate along the axonal surface to their final destination, where they differentiate to form the myelin sheath (2). There is growing evidence that neurotrophins have multiple functions other than just supporting the survival and differentiation of neurons (3, 4). Recently, we showed that endogenous neurotrophin-3 (NT3) enhances migration of premyelinating Schwann cells (5, 6) and inhibits myelination (7, 8) by interacting with the receptor-type tyrosine kinase, full-length TrkC, on Schwann cells. Furthermore, NT3 binding to TrkC enhances Schwann cell migration by activating the Rho GTPases Rac1 and Cdc42 and the downstream c-Jun N-terminal kinase (JNK) cascade (5). However, it remains to be clarified how TrkC activates these GTPases and which intermediate signaling molecule or molecules exist in the signaling pathway between TrkC and the GTPases.
Like other small GTPases, the Rho GTPases cycle between active (GTP-bound) and inactive (GDP-bound) conformations. Guanine-nucleotide exchange factors (GEFs) stimulate the exchange reaction of GDP for free cytoplasmic GTP to generate the active form of the GTPases (9, 10). Despite identification of >60 GEFs for the Rho GTPases by several human genome-sequencing projects, there have been relatively few studies on the activating mechanism(s), with the exception of the non-receptor-type tyrosine kinases such as the Src family (11–16). This observation prompted us to examine the possibility that the TrkC receptor phosphorylates and stimulates the catalytic activity of one particular GEF, Dbl's big sister (Dbs)/Ost/KIAA0362 (17, 18), the major Cdc42-GEF expressed in primary Schwann cells (Fig. 1C) and Schwannoma cell lines (data not shown), leading to JNK activation and induction of cell migration. In this study, we provide an example of a Rho GTPase-GEF as the functional substrate of a receptor-type tyrosine kinase, as well as a role for the Dbs/Cdc42/JNK cascade in the nervous system.
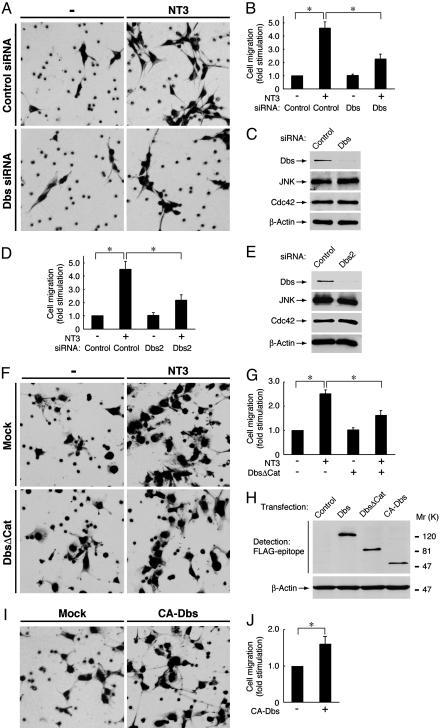
Fig. 1.
NT3 activation of TrkC enhances migration of Schwann cells and Cos-7 cells through Dbs. (A, B, and D) Schwann cells were transfected with control or Dbs siRNA. After incubation with NT3, migration was assayed by using Boyden chambers. (C and E) To confirm the effects of the siRNAs, the lysates from transfected cells were immunoblotted with anti-Dbs, JNK, Cdc42, or β-actin antibody. (F and G) Cos-7 cells were transfected with TrkC ± FLAG-DbsΔCat. After incubation with NT3, migration was assayed. (H) To confirm the expression of FLAG-tagged wild-type Dbs or the mutants, the lysates from transfected cells were immunoblotted with anti-FLAG or β-actin antibody. (I and J) Cos-7 cells were transfected with or without FLAG-CA-Dbs, and migration was assayed. Data were evaluated by using Student's t test. *, P < 0.01.
Methods
For details, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Short Interfering RNA (siRNA) Transfection. The siRNAs were transfected into primary Schwann cells by using Oligofectamine reagent (Invitrogen) according to the manufacturer's protocol. The medium was replaced 24 h after transfection, and cells were cultured in Sato medium containing 1 mg/ml BSA for 24 h. The efficiencies of Dbs depletion were 91 ± 3.3% for the first Dbs siRNA and 89 ± 5.7% for the second Dbs siRNA.
Plasmid Transfection. pCMV-FLAG-CA-Dbs (in which “CA” indicates constitutively active) was transfected into Schwann cells by using Lipofectamine Plus reagent (Invitrogen) according to the manufacturer's protocol. Cos-7 cells were transfected by the method of calcium phosphate precipitation.
Guanine Nucleotide-Releasing Assay for the Rho GTPases. The [3H]GDP-bound GST-Rho GTPases were obtained by incubation with reaction buffer (20 mM Hepes-NaOH, pH 7.5/150 mM NaCl/5 mM MgCl2/1 mM DTT/1 mM phenylmethane sulfonylfluoride/1 μg/ml leupeptin/1 mM EDTA) containing 125 ng/μl each Rho GTPase, 250 ng/μl BSA, 5 mM EDTA, and 0.3 μM [3H]GDP [0.3 μCi/μl (1 Ci = 37 GBq)] at 37°C for 90 min, as described in refs. 14 and 18. The reaction was stopped by adding 5 mM MgCl2, and mixtures were immediately cooled on ice. In the case of measuring the GEF activity of Dbs, transfected Cos-7 cells were pretreated with or without 100 nM K252a for 45 min and were stimulated with 10 ng/ml NT3 for 30 min. The immunoprecipitated FLAG-Dbs, FLAG-Vav2 (168–379), and HA-Stef (464–1,715) were incubated in 30 μl of reaction buffer containing 16 ng/μl GST-Rho GTPase·[3H]GDP, 33 ng/μl BSA, and 3 μM cold GDP at 30°C for 0, 10, 20, and 30 min. The reactions were stopped by adding 1 ml of ice-cold wash buffer (20 mM Hepes-NaOH, pH 7.5/10 mM MgCl2) and were filtered through nitrocellulose membranes. The membranes were immediately washed with ice-cold wash buffer and air-dried. The radioactivity remaining on each membrane was measured by a liquid scintillation counter. Three to 10 separate experiments were performed, and activities were normalized to the amount of Dbs in the immunoprecipitates.
In Vitro Tyrosine Phosphorylation and Guanine Nucleotide-Releasing Assay for Cdc42. The immobilized, purified FLAG-Dbs (250 ng) was incubated with 3 ng/μl GST-TrkC kinase domain in 30 μlof reaction buffer containing 20 μM ATP with or without 100 nM K252a at 30°C for 15 min and was then chilled on ice. The immobilized tyrosine-phosphorylated FLAG-Dbs was washed with reaction buffer and was used for a guanine nucleotide-releasing assay for Cdc42 (30°C for 15 min).
Results
The GEF Dbs Is Essential for Schwann Cell Migration Regulated by NT3 Activation of TrkC. To examine whether Dbs is involved in Schwann cell migration after stimulation with NT3, we used siRNAs to knock down endogenous Dbs. Expression of Dbs in primary Schwann cells was markedly down-regulated by transfection with two nonoverlapping Dbs siRNA, whereas expression of JNK, Cdc42, and β-actin was unaffected, as revealed by immunoblotting (Fig. 1 C and E). Migration assays were carried out in the Boyden chambers, where Schwann cells were plated on the filters in the upper well and were allowed to migrate out onto the lower compartment through 8-μm pores (5, 6). NT3 was placed in the lower wells of Boyden chambers to form a concentration gradient that extended into the upper wells. The knockdown of Dbs abolished the NT3-induced migration of Schwann cells by ≈50% (Fig. 1 A, B, and D). To address the issue of cell motility versus a directed migration, we performed migration assays using reaggregated Schwann cells on fasciculated dorsal root ganglion axons to mimic more physiological conditions, as described in ref. 6. Consistent with the Boyden chamber assay, Dbs depletion significantly inhibited Schwann cell migration from the reaggregates after stimulation with NT3 (Fig. 6, which is published as supporting information on the PNAS web site). To further confirm that NT3 activation of TrkC enhances migration through Dbs, a transient transfection system using Cos-7 cells was used. When transfected into Cos-7 cells, FLAG-tagged constructs of Dbs were detectable with an anti-FLAG antibody (Fig. 1H). Cotransfection of the plasmid encoding the catalytic Dbl-homology (DH) domain-deficient Dbs (DbsΔCat), which displays a dominant-inhibitory effect (6, 14, 18), inhibited the NT3-induced migration of Cos-7 cells expressing TrkC by ≈50% (Fig. 1 F and G). Conversely, transfection of a CA-Dbs, containing the isolated DH and phosphoinositide-bound pleckstrin-homology domains (14, 17, 18), enhanced migration of Cos-7 cells by ≈1.5-fold (Fig. 1 I and J). These results indicate that Dbs mediates the signaling pathway linking NT3 activation of TrkC to migration.
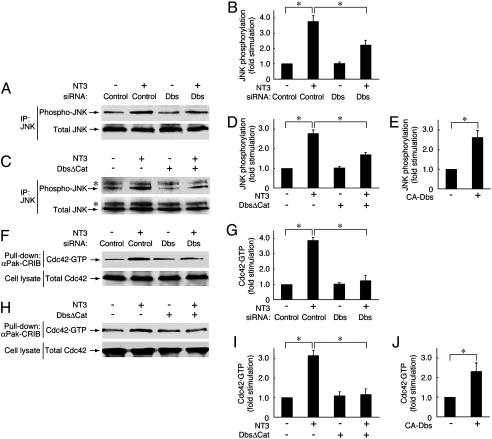
Dbs Is Involved in the Activation of Cdc42 and the JNK Cascade. Because activation of TrkC induces migration through the JNK signaling pathway (5), we investigated whether Dbs participates in JNK activation. Immunoprecipitated JNK was immunoblotted with an antiphosphorylated JNK antibody, which recognizes the active state. Transfection of Dbs siRNA into Schwann cells inhibited the NT3-induced JNK activation by ≈50% (Fig. 2 A and B). Similarly, DbsΔCat also inhibited the NT3-induced JNK activation in Cos-7 cells transfected with JNK and TrkC by ≈50% (Fig. 2 C and D). Additionally, CA-Dbs had the ability to activate JNK by ≈2.5-fold (Fig. 2E). We further examined the involvement of Dbs in Cdc42 activation, because Dbs is the preferred GEF for Cdc42 (17, 18), and Cdc42 acts upstream of JNK in the TrkC-JNK signaling pathway (5). The activity of Cdc42 was measured by the pull-down assay with αPak-CRIB, which specifically associates with GTP-bound Cdc42 or Rac1. The knockdown of Dbs blocked the NT3-induced Cdc42 activation in Schwann cells (Fig. 2 F and G), as did DbsΔCat in Cos-7 cells transfected with Cdc42 and TrkC (Fig. 2 H and I). In contrast, inhibition of Dbs had no effect on Rac1 activation (Fig. 7, which is published as supporting information on the PNAS web site). In addition, CA-Dbs promoted the formation of Cdc42·GTP by >2-fold (Fig. 2 J). These results demonstrate that Dbs plays a key role in activating Cdc42 and the downstream JNK cascade in the TrkC signaling pathway.
Fig. 2.
Dbs participates in the TrkC-mediated JNK and Cdc42 activation in Schwann cells and Cos-7 cells. (A and B) Schwann cells were transfected with control or Dbs siRNA. (C and D) Cos-7 cells were transfected with HA-JNK and TrkC ± FLAG-DbsΔCat. After stimulation with NT3, JNK was immunoprecipitated (IP) with anti-JNK antibody and immunoblotted with antiphosphorylated JNK or JNK antibody. (E) Cos-7 cells were transfected with HA-JNK ± FLAG-CA-Dbs. The JNK phosphorylation was measured. (F and G) Schwann cells were transfected with control or Dbs siRNA. (H and I) Cos-7 cells were transfected with Cdc42 and TrkC ± FLAG-DbsΔCat. After stimulation with NT3, the Cdc42 activity was measured with the pull-down assay by using GST-αPak-CRIB in the lysates. The total Cdc42 in the cell lysates is also shown. (J) Cos-7 cells were transfected with Cdc42 ± FLAG-CA-Dbs. The Cdc42 activity was measured. Data were evaluated by using Student's t test. *, P < 0.01.
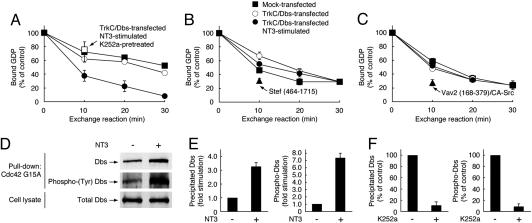
TrkC Activation Stimulates Dbs Activity in a Tyrosine Phosphorylation-Dependent Manner. To clarify whether Dbs is activated after stimulation with NT3, we followed the intrinsic GEF activity in Cos-7 cells transfected with Dbs and TrkC. The GEF activities of immunoprecipitated Dbs were measured by dissociation of [3H]GDP from recombinant RhoA, Rac1, and Cdc42 proteins. Upon stimulation with NT3, the Cdc42-GEF activity of Dbs is specifically activated (Fig. 3A), whereas no change was observed in Rac1 and RhoA (Fig. 3 B and C). As positive controls, N-terminal-deleted Stef (Fig. 3B) and Src kinase-phosphorylated Vav2 (Fig. 3C) were able to activate Rac1 and RhoA, respectively (5, 6, 9, 10). Additionally, the TrkC-induced activation of Cdc42-GEF activity of Dbs was blocked by pretreatment with K252a, an inhibitor of Trk tyrosine kinases (Fig. 3A). To further confirm the activation of Dbs upon stimulation of TrkC, we used a pull-down assay to detect active Dbs by using Cdc42G15A, a guanine nucleotide-free form of Cdc42 (19). A glycine-to-alanine point mutation of residue 15 in Cdc42 decreases its nucleotide binding, and active GEFs preferentially interact with guanine nucleotide-free forms of small GTPases (9, 10, 19). After stimulation with NT3 in Cos-7 cells transfected with Dbs and TrkC, Dbs precipitated with Cdc42G15A was increased and was also strikingly tyrosine-phosphorylated (Fig. 3 D and E). Additionally, pretreatment with K252a decreased Dbs precipitated with Cdc42G15A and the tyrosine phosphorylation (Fig. 3F). Together, these results indicate that activation of TrkC stimulates the Cdc42-GEF activity of Dbs in a tyrosine phosphorylation-dependent manner.
Fig. 3.
Stimulation of the Cdc42-GEF activity of Dbs by NT3 interaction with TrkC. Cos-7 cells transfected with FLAG-Dbs plus TrkC (A–C), HA-Stef (464–1,715) (B), or FLAG-Vav2 (168–379) plus CA-Src (C) were pretreated with or without K252a. After stimulation with NT3, release of [3H]GDP from [3H]GDP-occupied GST-Cdc42, Rac1, and RhoA by the GEF immunoprecipitates was measured. (D and E) After stimulation with NT3, active, tyrosine-phosphorylated FLAG-Dbs was detected by the pull-down assay by using GST-Cdc42G15A from the lysates of Cos-7 cells transfected with FLAG-Dbs and TrkC. The total FLAG-Dbs in the cell lysates is also shown. (F) Effect of K252a on Dbs precipitated from the lysates of Cos-7 cells stimulated with NT3. Active, tyrosine-phosphorylated FLAG-Dbs was detected by the pull-down assay by using GST-Cdc42G15A.
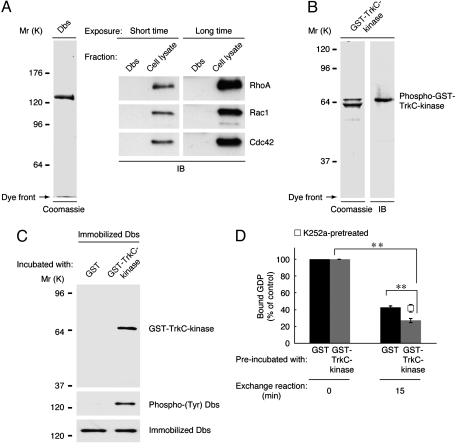
Dbs Associates Directly with TrkC. To test the possibility that TrkC directly associates with Dbs, we used recombinant proteins of Dbs and the TrkC kinase domain. FLAG-tagged full-length Dbs was purified from the lysates of 293T cells transiently transfected with FLAG-Dbs by using resin preabsorbed with an anti-FLAG antibody (Fig. 4A). The purified Dbs preparation contained none of the three Rho GTPases (Fig. 4A) nor the active, phosphorylated form of Dbs (Fig. 4C, GST lane) as detected by immunoblotting. Although degraded forms of GST-tagged TrkC kinase domain were observed, only the intact kinase domain possessed activity, as recognized by an antiphosphorylated Trk antibody (Fig. 4B). Because GST forms a dimer (20), GST-tagged TrkC kinase domain is thought to be autophosphorylated and activated. When immobilized Dbs was incubated with GST or GST-TrkC kinase domain, GST-TrkC kinase domain was coprecipitated with Dbs (Fig. 4C). Additionally, GST-TrkC kinase domain had the ability to phosphorylate Dbs (Fig. 4C). Therefore, we next investigated whether TrkC directly stimulates the Cdc42-GEF activity of Dbs. Dbs preincubated with GST alone or with GST-TrkC kinase domain was mixed with Cdc42·[3H]GDP. The phosphorylated Dbs increased the dissociation of [3H]GDP from Cdc42 (Fig. 4D). In contrast, pretreatment with K252a blocked this effect (Fig. 4D), indicating that TrkC tyrosine phosphorylation of Dbs stimulates the Cdc42-GEF activity. The basal activity of purified Dbs (Fig. 4D) is higher than that of immunoprecipitated Dbs alone (Fig. 3A). This result might be due to dissociation of the possible inhibitory phospholipids (21) and/or the associating proteins that could inhibit the basal activity of Dbs. So far, several Dbs-binding phospholipids and proteins have been identified in a lipid array (21) or our analysis (data not shown) and other yeast two-hybrid analyses (22).
Fig. 4.
TrkC binds, phosphorylates, and activates Dbs in vitro. (A) Purified FLAG-Dbs (2 μg) was applied to SDS/PAGE, stained with Coomassie brilliant blue R-250, and immunoblotted (IB) with antibodies against the Rho GTPases. (B) Purified GST-TrkC kinase domain (2 μg) was resolved by SDS/PAGE, stained, and immunoblotted with antiphosphorylated Trk antibody. (C) Immobilized FLAG-Dbs (250 ng) was incubated with 20 μM cold ATP and 3 ng/μl GST or GST-TrkC kinase domain in 30 μl of reaction buffer, washed, and immunoblotted with anti-GST, pTyr, or FLAG antibody. (D) Release of [3H]GDP from 16 ng/μl GST-Cdc42·[3H]GDP by Dbs preincubated with ATP and GST or GST-TrkC kinase domain (with or without K252a) was measured. Data were evaluated by using Student's t test. *, P < 0.01; **, P < 0.005.
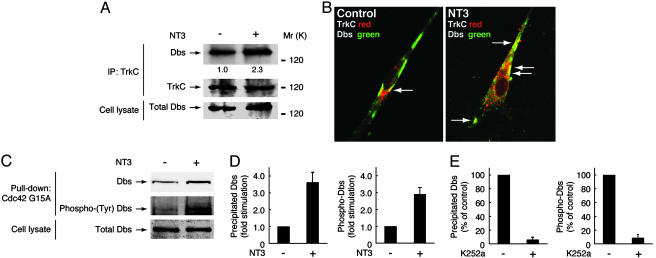
We examined whether endogenous Dbs associates with TrkC in Schwann cells. Because Schwann cells express the full-length form of TrkC but not of TrkA or TrkB (7, 8), anti-pan-Trk antibody was used to immunoprecipitate TrkC. Dbs was immunoprecipitated with TrkC, and their interaction was induced by stimulation with NT3 (Fig. 5A). Additionally, NT3 stimulation of Schwann cells transfected with FLAG-Dbs enhanced colocalization of Dbs with TrkC in punctate structure (Fig. 5B). We further tried to detect endogenous, active Dbs in Schwann cells. After stimulation with NT3, Dbs precipitated with Cdc42G15A was increased and was tyrosine-phosphorylated (Fig. 5 C and D), consistent with the result from Cos-7 cells (Fig. 3). Pretreatment with K252a decreased Dbs precipitated with Cdc42G15A and the tyrosine phosphorylation (Fig. 5E), indicating that Dbs is an endogenous linker between TrkC and Cdc42 in a tyrosine phosphorylation-dependent manner.
Fig. 5.
NT3 interaction with TrkC mediates phosphorylation and activation of endogenous Dbs in Schwann cells. (A) After stimulation with NT3, the Trk immunoprecipitates were immunoblotted with antibodies against Dbs and pan-Trk. Immunoprecipitated Dbs was quantified and normalized to the total amount of immunoprecipitated Trk, indicated by “1.0” and “2.3” below the Dbs bands. The total Dbs in the cell lysates is also shown. (B) Schwann cells were transfected with FLAG-Dbs and stained with antibodies against FLAG (green) and pan-Trk (red). Structures where Dbs and TrkC colocalize are indicated by arrows. (C and D) Active, tyrosine-phosphorylated Dbs was detected by the pull-down assay by using GST-Cdc42G15A from the cell lysates after incubation with NT3. The total Dbs in the cell lysates is also shown. (E) Effect of K252a on Dbs precipitated from the lysates of Schwann cells stimulated with NT3. Active, tyrosine-phosphorylated Dbs was detected by the pull-down assay by using GST-Cdc42G15A.
Discussion
Although the mechanism(s) by which GEFs for the Rho GT-Pases are activated is still not fully understood, it is becoming apparent that the non-receptor-type tyrosine kinases directly activate the GEFs (11–16). These GEFs include the Vav subfamily GEFs (Vav, Vav2, and Vav3), Ras-GRF1/Cdc25Mm as a Rac1-GEF, Frg/Fir/KIAA0793 (activated by the Src family), leukemia-associated RhoGEF (activated by Tec), and Sos-1 as a Rac1-GEF (activated by Abl). Our present study provides a mechanism whereby a membrane receptor-type tyrosine kinase can directly activate the GEF Dbs and subsequently Cdc42 and shows that this activation is ligand-dependent. These findings remind us that tyrosine phosphorylation is a crucial event for activation of GEFs for the Rho GTPases. Support for this principle comes from the structural analysis of the DH domain and the N-terminal extension of Vav (11), where a tyrosine residue positioned in the N-terminal helical region preceding the DH domain is essential for the activation mechanism. In this scenario, tyrosine phosphorylation of the residue relieves the autoinhibitory helical region from the GTPase-association site of the DH domain. Because Dbs also has tyrosine residues in the helical region [predicted computationally by a papia program (http://www.cbrc.jp/papia)] preceding the DH domain, it is possible that tyrosine phosphorylation of this site by TrkC helps to enhance the interaction of Dbs with Cdc42, increasing the Cdc42-GEF activity. Further experiments concerning the identification of the tyrosine phosphorylation site(s) on Dbs should allow us to provide a more detailed mechanism for activating Dbs in Schwann cells.
It is clear that TrkC phosphorylates and activates Dbs in vitro, but is it enough to evoke the full activation of Dbs in primary cells? The pleckstrin-homology (PH) domain of Dbs interacts with various phosphoinositides (23), which include phosphatidylinositol-3, 4, 5-tri-phosphate (PIP3), the product of phosphatidylinositol 3-kinases. Like other Trk receptors, TrkC has the ability to activate phosphatidylinositol 3-kinases (3). It is unlikely that PIP3 modulates the catalytic activity of the isolated DH and PH domains of Dbs; however, it remains unclear whether PIP3 has an effect on full-length Dbs, as seen in the other GEFs for Rho GTPases (10). Second, the PH domain of Dbs is also associated with Rac1·GTP, leading to activation of Dbs in cells (24). Because activation of TrkC increases the formation of Rac1·GTP (5), activation of Dbs may be amplified by the accumulation of Rac1·GTP as an active feedback. Third, as mentioned above, several genes have been isolated as the binding partners of Dbs by using a yeast two-hybrid system (22). Among them, overexpression of the gene Ostip2 shows modest proliferation-stimulating activity similar to the oncogenic form of the Dbs gene (22). Although Ostip2 displays limited expression in skeletal muscle, this possible activator of Dbs may exist in Schwann cells.
Recently, the Ras guanine-releasing factor 1 (RasGrf1), a GEF for the members of the Ras and Rho family of GTPases, was shown to be tyrosine-phosphorylated by Trk receptors in a neurotrophin-dependent manner. This activation was associated with neurite outgrowth and identifies the RasGrf1 as a previously unrecognized target of neurotrophin activation (25). Similarly, in our study, we demonstrate that NT3 activation of TrkC enhances the Cdc42-GEF activity of Dbs, which stimulates the JNK cascade and migration of premyelinating Schwann cells. On the basis of these findings, we summarize the proposed signaling pathway for Schwann cell migration in Fig. 8, which is published as supporting information on the PNAS web site. It should be noted that inhibition of Dbs only partially impairs the Schwann cell migration, as well as the JNK activation (Figs. 1 and 2). Importantly, concomitant with the Cdc42 activation, Rac1 activation is required for Schwann cell migration and JNK activation (5). Elucidation of the responsible Rac1-GEF(s) and its activation mechanism should provide further valuable information. Furthermore, NT3 interaction with TrkC leads to potent inhibition of myelination by Schwann cells in vivo as well as in Schwann cell/neuronal cocultures (7, 8). It will be of interest to examine whether TrkC inhibits myelination through the Dbs/Cdc42/JNK signaling pathway. Further studies are needed to promote our understanding not only of how Schwann cells migrate before initiation of myelination, but also of how the remyelination program proceeds after injury. Such studies could allow for development of potential therapeutic strategies after injury.
Supplementary Material
Acknowledgments
We thank Drs. M. Hoshino, S. Takashima, and H. Abe for helpful discussions. This work was supported by National Institute of Neurological Disorders and Stroke Grant NS 04270, grants from the Muscular Dystrophy Association and the McGowan Charitable Trust (to E.M.S.), and the National Multiple Sclerosis Society Career Transition Fellowship (to J.R.C).
Author contributions: J.Y. designed research; J.Y., J.R.C., and Y.M. performed research; J.Y., J.R.C., Y.M., G.T., and E.M.S. analyzed data; G.T. contributed new reagents/analytic tools; and J.Y., J.R.C., and E.M.S. wrote the paper.
Abbreviations: NT3, neurotrophin-3; GEF, guanine-nucleotide exchange factor; JNK, c-Jun N-terminal kinase; Dbs, Dbl's big sister; siRNA, short interfering RNA; DH, Dbl homology; CA, constitutively active.
References
- 1.Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., Parsons, J. T. & Horwitz, A. R. (2003) Science 302, 1704–1709. [DOI] [PubMed] [Google Scholar]
- 2.Bunge, R. P., Bunge, M. B. & Eldridge, C. F. (1986) Annu. Rev. Neurosci. 9, 305–328. [DOI] [PubMed] [Google Scholar]
- 3.Huang, E. J. & Reichardt, L. F. (2003) Annu. Rev. Biochem. 72, 609–642. [DOI] [PubMed] [Google Scholar]
- 4.Barker, P. A. (2004) Neuron 42, 529–533. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi, J., Chan, J. R. & Shooter, E. M. (2003) Proc. Natl. Acad. Sci. USA 100, 14421–14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamauchi, J., Chan, J. R. & Shooter, E. M. (2004) Proc. Natl. Acad. Sci. USA 101, 8774–8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, J. R., Cosgaya, J. M., Wu, Y. J. & Shooter, E. M. (2001) Proc. Natl. Acad. Sci. USA 95, 10459–10464. [Google Scholar]
- 8.Cosgaya, J. M., Chan, J. R. & Shooter, E. M. (2002) Science 298, 1245–1248. [DOI] [PubMed] [Google Scholar]
- 9.Kaibuchi, K., Kuroda, S. & Amano, M. (1999) Annu. Rev. Biochem. 68, 459–486. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt, A. & Hall, A. (2002) Genes Dev. 16, 1587–1609. [DOI] [PubMed] [Google Scholar]
- 11.Aghazadeh, B., Lowry, W. E., Huang, X. Y. & Rosen, M. K. (2000) Cell 102, 625–633. [DOI] [PubMed] [Google Scholar]
- 12.Bustelo, X. R. (2000) Mol. Cell. Biol. 20, 1461–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiyono, M., Kaziro, Y. & Satoh, T. (2000) J. Biol. Chem. 275, 5441–5446. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto, Y., Yamauchi, J. & Itoh, H. (2003) J. Biol. Chem. 278, 29890–29900. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki, N., Nakamura, S., Mano, H. & Kozasa, T. (2003) Proc. Natl. Acad. Sci. USA 100, 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sini, P., Cannas, A., Koleske, A. J., Di Fiore, P. P. & Scita G. (2004) Nat. Cell Biol. 6, 268–274. [DOI] [PubMed] [Google Scholar]
- 17.Rossman, K. L., Worthylake, D. K., Snyder, J. T., Siderovski, D. P., Campbell, S. L. & Sondek, J. (2002) EMBO J. 21, 1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamauchi, J., Hirasawa, A., Miyamoto, Y., Kokubu, H., Nishii, H., Okamoto, M., Sugawara, Y., Tsujimoto, G. & Itoh, H. (2002) Biochem. Biophys. Res. Commun. 296, 85–92. [DOI] [PubMed] [Google Scholar]
- 19.Arthur, W. T., Ellerbroek, S. M., Der, C. J., Burridge, K. & Wennerberg, K. (2002) J. Biol. Chem. 277, 42964–42972. [DOI] [PubMed] [Google Scholar]
- 20.McTigue, M. A., Williams, D. R. & Tainer, J. A. (1995) J. Mol. Biol. 246, 21–27. [DOI] [PubMed] [Google Scholar]
- 21.Ueda, S., Kataoka, T. & Satoh, T. (2004) Cell. Signalling 16, 899–906. [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka, R., Blumenthal, R., Lorenzi, M. V., Tatsumoto, T. & Miki, T. (2001) DNA Cell Biol. 20, 383–390. [DOI] [PubMed] [Google Scholar]
- 23.Snyder, J. T., Rossman, K. L., Baumeister, M. A., Pruitt, W. M., Siderovski, D. P., Der, C. J., Lemmon, M. A. & Sondek, J. (2001) J. Biol. Chem. 276, 45868–45875. [DOI] [PubMed] [Google Scholar]
- 24.Cheng, L., Mahon, G. M., Kostenko, E. V. & Whitehead, I. P. (2004) J. Biol. Chem. 279, 12786–12793. [DOI] [PubMed] [Google Scholar]
- 25.Robinson, K. N., Manto, K., Buchsbaum, R. J., MacDonald, J. I. S. & Meakin, S. O. (2005) J. Biol. Chem. 280, 225–235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.