Abstract
Background
Hypophosphatemia is one of the common disorders that develop in critically ill patients. It has potential complications and is often unrecognized in those patients.
Objective
Determining the incidence of hypophosphatemia in critically ill children, its association with clinical outcomes and the possible risk factors.
Methods
50 patients were enrolled in the study. Levels of serum phosphate were measured on day 1 and day 7 of PICU (Pediatric intensive care unit) stay. The following variables were analyzed: age, gender, diagnosis on admission, malnutrition, phosphorus intake, clinical severity score on admission OFI (Organ Failure Index) and daily scores PELOD (Pediatric Logistic Organ Dysfunction), sepsis, use of dopamine, furosemide and steroids and assessment of nutrition by z scores.
Results
The incidence of hypophosphatemia on admission was 42%. On seventh day of admission incidence of hypophosphatemia was 62%. Malnutrition was present in 24% of patients, serum phosphorus level was significantly lower in malnourished than in well-nourished children (p value = 0.018). Hypophosphatemia was associated with prolonged PICU length of stay (p < 0.001) but was not associated with increased mortality (p = 0.13). Cases on parenteral nutrition and insufficient oral intake while on mechanical ventilator significantly showed hypophosphatemia (p = 0.017). Hypophosphatemia was associated with the use of furosemide, dopamine, steroids and β2 agonist.
Conclusion
Hypophosphatemia was common in the first 7 days of PICU hospitalization and was associated with prolonged PICU stay, Significant association between hypophosphatemia and duration of use of mechanical ventilation, use of furosemide, dopamine, steroids and β2 agonist.
Keywords: Hypophosphatemia, Critical illness, Malnutrition, Phosphorus
Highlights
-
•
Hypophosphatemia was common in the first 7 days of pediatric intensive care unit hospitalization.
-
•
Significant association between hypophosphatemia and duration of use of mechanical ventilation.
-
•
Hypophosphatemia was associated with prolonged pediatric intensive care unit length of stay.
-
•
Hypophosphatemia was associated with use of furosemide, dopamine, steroid and β2 agonist.
-
•
Good nutrition of critically ill children has an important role in improving their clinical condition.
1. Introduction
Phosphate is a constituent of various intermediate compounds involved in key physiological processes such as adenosine triphosphate, 2,3-diphosphoglycerate and intracellular chemical messengers (e.g., cyclical adenosine monophosphate, cyclical guanosine monophosphate) [1], [2]. Electrolyte disorders frequently develop in critically ill patients during the course of stay in PICU [3]. Phosphate disturbance is one of those frequently encountered electrolyte disorders These are at increased risk of morbidity [4] In general hospital populations, the prevalence of moderate hypophosphatemia ranges between 0.43% and 3.1% [5], [6], and 45% of all hospital hypophosphatemia cases occur in the ICU population in this prospective study, hypophosphatemia was common in critically ill children that incidence of hypophosphatemia was 42% at admission. At seventh day incidence of hypophosphatemia was 62% and Potential risk factors in most patients with phosphate disturbance include malnutrition, which was present in 24% cases, sepsis was present in 34% of cases, drugs like catecholamine's 24% of cases, antacids 10% of cases, diuretics 36% of cases, steroid therapy 76% of cases [7].
Symptoms of hypophosphatemia tend to be nonspecific in the majority of cases and include fatigue and irritability. Lower level (less than 1.0 mg/dl) may lead to more hypophosphatemia serious problems such as reduced diaphragmatic contractility, cardiac arrhythmias, myocardial reduction and severe congestive cardiac insufficiency [8].
Phosphate disturbance has been reported to be associated with increased morbidity and mortality in critically ill patients such as respiratory failure, increase the duration of stay on mechanical ventilation, and increased PICU length of stay [9].
To date, the majority of studies have involved adults, but hypophosphatemia is generally underdiagnosed in children, where frequency and predisposing factors are not yet fully understood. Based on the premise that hypophosphatemia is a frequent, often undiagnosed disturbance in children, the aim of this study was to identify the incidence and risk factors for hypophosphatemia in critically ill children. .
1.1. Aim of work
The aim of the study was to estimate the incidence of phosphate disturbance in critically ill children, study its clinical effects and risk factors in patients during their stay in PICU.
1.2. Patients and methods
The current cross sectional study was conducted at the Departments of Pediatrics and Clinical Pathology, Faculty of Medicine, Benha University Hospital, during the period from January 2016 to September 2016. After approval of the study protocol by the local research and ethical Committee of Faculty of Medicine, Benha University and after obtaining parents' written fully informed consent; 50 critically ill children were enrolled in the study. The study included all critically ill children from both sexes from 1 month and up to 18 years admitted to PICU in Benha university hospital. The cases included patients attending with pneumonia, central nervous system infection, acute heart failure, status asthmatics, and sepsis. Exclusion criteria: In our study, we excluded patients outside age group, PICU stay less than 7 days, patients refused to be involved in the study.
Blood specimens of serum phosphorus levels on admission and the second sample on seventh after admission were collected [ 5 ml whole blood collected in Red top tube (clot activator) from subjects], the specimens were centrifuged and the serum or plasma removed from the cells within 2 h of collection. Samples were frozen at −15 °C to −20 °C. Frozen samples were thawed only once. Analyze deterioration may occur in samples that are repeatedly frozen and thawed. DIALAB and EASYLYTE was used for samples analysis.
1.3. Clinical data collection
The study group was subjected to: Complete history taking including history of chronic illness, primary admission diagnosis and history of medications used especially that having significant effect on phosphate level. General examination and systemic review was done. Presence of infection or sepsis according to center for disease control and prevention [10]. Detecting severity of illness using (OFI) score and (PELOD) score [11].
Nutritional state classification, the weight-for-age (W/A) percentile is used as the anthropometric indicator for children younger than 2 years, whereas body mass index is used for children older than 2 years. By using percentiles developed for Egyptian children.
The target caloric requirements are calculated according to the recommended daily allowance and the route of administration based on age, sex and weight.
1.4. Laboratory analysis
Laboratory investigations including: CBC, CRP, ABG, random blood sugar, serum electrolytes, renal and liver function tests were done. Blood specimens of serum phosphorus levels on admission and the second sample on seventh day after admission.
Statistical analysis: Data were analyzed by (SPSS)
-
•
Description of quantitative variables as mean, SD for normally distributed data and median value for abnormally distributed data.
-
•
Description of qualitative variables as number & percentage.
-
•
Student t-test was used to assess the statistical significance of the difference between two population means in study involving independent samples.
-
•
Chi-square test was used to compare qualitative variable between independent groups samples.
-
•
Mann-Whitney U test (Z value) for two independent samples of abnormal data distribution.
-
•Level of significance
-
-Probability level (P-value) ≥ 0.05 = Non significant (NS).
-
-P-value < 0.05 = Significant.
-
-P-value < 0.001 = highly significant
-
-
2. Results
In our study 50 critically ill children admitted to the PICU were included in the study. The baseline characteristics of the patients are shown in Table 1.
Table 1.
Demographic and clinical characteristics of study population.
| Variable | No. (N = 50) | % (100.0) | |
|---|---|---|---|
| Age (m) | Mean ± SD (Median) | 14.3 ± 13.9 (9) | |
| Range | 3–84 | ||
| Sex | Male | 26 | 52.0 |
| Female | 24 | 48.0 | |
| Weight (kg) | Mean ± SD | 7.5 ± 3.2 | |
| Range | 3–20 | ||
| Length (cm) | Mean ± SD | 72.3 ± 12.4 | |
| Range | 55–120 | ||
| BMI (kg/m2) | Mean ± SD | 13.8 ± 2.4 | |
| Range | 7.1–19.6 | ||
| Admission diagnosis | |||
| Respiratory disorder | 30 | 60% | |
| Heart failure | 7 | 14% | |
| Sepsis | 15 | 30% | |
| Malnutrition | 9 | 18% | |
| Mechanical ventilated | 18 | 36% | |
| Acute encephalopathy | 8 | 16% | |
| Septic shock | 3 | 6% | |
| Trauma | 1 | 2% | |
SD Standard deviation.
During the study period 42 patients of the studied group had been discharged and 8 patients died. On admission, incidence of hypophosphatemia was 42% (n 21) and incidence of normophosphatemia was 58% (n 19). While on seventh day of admission incidence of hypophosphatemia was 62% (n 31) and incidence of normophosphatemia was 38% (n 19) Table 2 Fig. 1.
Table 2.
Matching serum phosphorus levels on admission and on the 7th day among the studied group.
| Hypophosphatemia 7TH Day |
Normophosphatemia 7th Day |
Total Count % within Ps | |||
|---|---|---|---|---|---|
| Count | % | count | % | ||
| Normophosphatemia on admission | 18 | 62.1% | 11 | 37.9% | 29 |
| 100% | |||||
| Hypophosphatemia on admission | 1 | 4.8% | 20 | 95.2% | 21 |
| 100% | |||||
| Total | 19 | 38.0% | 31 | 62.0% | 50 |
Mc Nemar's test was used P = 0.006 (S).
Fig. 1.
Incidence of Hypophosphatemia among the studied sample.
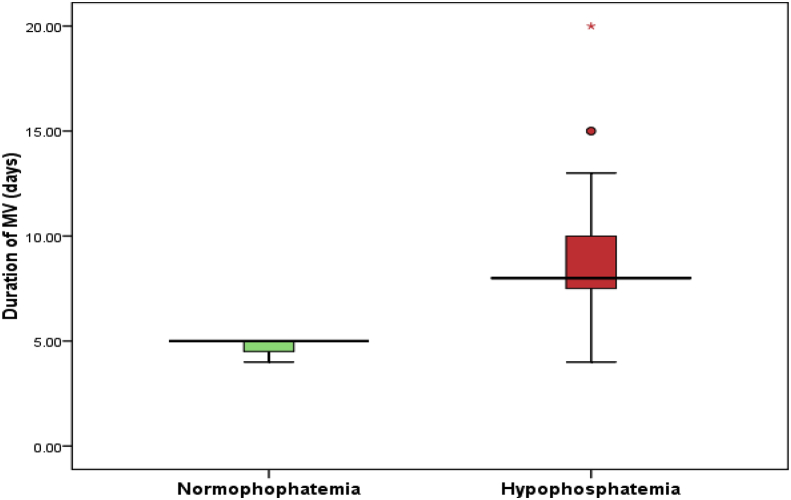
On admission the majority of cases were suffering of respiratory problems as pneumonias, bronchiolitis, bronchial asthma, pneumothorax, pleural effusion and stridor. Thirty six percent (n = 18) of all cases were mechanically ventilated (p = .02). Fifteen patients of them were hypophosphatemic (n = 15 vs. n = 3) and spent more days being ventilated (8days vs. 5 days) (P = 0.017) Table 3, Table 4.
Table 3.
Comparing serum phosphorus level according to use of mechanical ventilation.
| P |
Total | ||||
|---|---|---|---|---|---|
| Normophophatemia | Hypophosphatemia | ||||
| MV: | No | Count | 16 | 16 | 32 |
| % within P7s | 84.2% | 51.6% | 64.0% | ||
| Yes | Count | 3 | 15 | 18 | |
| % within P7s | 15.8% | 48.4% | 36.0% | ||
| Total | Count | 19 | 31 | 50 | |
| % within P7s | 100.0% | 100.0% | 100.0% | ||
X2 = 5.43 P = 0.02 (S).
MV, mechanical ventilator. S, significant.
Table 4.
Comparing duration of MV and PICU stay according to serum phosphorus level.
|
Variable |
Normophosphatemia (N = 3) |
Hypophosphatemia (N = 15) |
MWU test |
P value |
||
|
Median |
Range |
Median |
Range |
|||
| Duration of MV (days) |
5.0 |
4–5 |
8.0 |
4–19 |
2.39 |
0.017 (S) |
|
Normophosphatemia (N = 19) |
Hypophosphatemia (N = 31) |
MWU test |
P |
|||
| Duration of PICU stay (days) | 8.0 | 7–13 | 10.0 | 7–20 | 3.82 | <0.001 (HS) |
MV, mechanical ventilator. PICU, pediatric intensive care unit. HS, highly significant.
MWU test, Mann Whitney U test.
Box plot 1: for the median and range of duration of MV according to phosphorus level.
MV, mechanical ventilator.
Regarding PICU stay there was highly significant difference between normophosphatemic group and hypophosphatemic group (P < 0.001) Table 4.
Malnutrition was present in 24% (n = 12) of cases. There was significant association between malnutrition and hypophosphatemia (p = 0.018) Table 6.
Table 6.
Risk factor for hypophosphatemia.
| Variable | N | Percentage of patients | Hypo-phosphatemia | Normo-phosphatemia | P value |
|---|---|---|---|---|---|
| Malnutrition | 12 | 24 | 11 | 1 | 0.018 |
| Drugs | |||||
| Furosemide | 18 | 36 | 15 | 3 | 0.002 |
| Dopamine | 12 | 24 | 11 | 1 | 0.018 |
| Steroid | 38 | 76 | 28 | 10 | 0.005 |
| β2 agonist | 29 | 58 | 22 | 7 | 0.018 |
| Ranitidine/omeprazole | 5 | 10 | 4 | 1 | 0.31 |
| Sepsis | 17 | 34 | 10 | 7 | 0.74 |
Also there was a significant association between hypophosphatemia and the time to attain target caloric requirements by enteral route (P = 0,005). The median times to attain target caloric requirements by enteral route were 3/5 days in normo/hypo phospatemic groups respectively (P = 0.005) Table 5.
Table 5.
Time to attain target caloric requirement.
| Variable | Normophosphatemia (N = 19) |
Hypophosphatemia (N = 31) |
MWU test | P value | ||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | |||
| Time to attain target caloric requirement (days) | 3.0 | 0.25–9 | 5.0 | 0.5–12 | 2.98 | 0.005 (S) |
MWU, Mann Whitney u test.
Seventeen cases (34%) were septic 0.10 of them had hypophosphatemia with no significant association (P = 0.74) Table 6.
Hypophosphatemia was associated with the use of furosemide (P = .002), steroids (P = 0.005), β2 agonist (P = . 018) and dopamine (P = , 018) Table 6.
3. Discussion
In this prospective study, hypophosphatemia was common in critically ill children (prevalence 62%) during the first seventh days of PICU admission. It was independently associated with prolonged PICU length of stay. It was also associated with increased duration of stay on mechanical ventilator, but was not associated with increased mortality.
The prevalence of hypophosphatemia in this study was slightly higher than previous reports in critically ill children which showed the prevalence of 60% [12], [13]. This observation may be due to increased frequency of serum phosphate measurement in our study. However, from all the reports, it is clear that there is higher prevalence of hypophosphatemia in PICU patients.
This study showed an independent association between hypophosphatemia and prolonged PICU length of stay (P < 0.001) the median time was 10 days in hypophosphatemic compared to 8 days in normophosphatemic group. Similar observation has been reported by Kilic et al. [13] (P = 0.001), Shah et al. [14] (P = 0.005) who reported that hypophosphatemia was associated with prolonged PICU length of stay > 6 d). Many children were admitted in PICU for respiratory support. It was shown that hypophosphatemia may impair diaphragmatic contractility during acute respiratory failure and may be one of the most important causes for difficult weaning from mechanical ventilator [15]. Hypophosphatemic patients spent more time on mechanical ventilator. In our study we found independent association between hypophosphatemia and prolonged mechanical ventilation as most patients was hypophosphatemic before the need for mechanical ventilation so hypophosphatemia lead to prolonged ventilator support.
Malnutrition is one of the risk factors for hypophosphatemia [16], [17]; In our study there was a significant association between hypophosphatemia and malnutrition (P = 0.018). This agree with Souza de Meneses et al. [18] (P = 0.04) and in severe malnutrition (P = 0.0182). Santana e Meneses et al. [12] also reported significant association between malnutrition and hypophosphatemia (p = 0.01). In contrast Kilic et al. [13], (p = 0.2) and Shah et al. [14] (p = 0.5) reported that there was no significant association between malnutrition and hypophosphatemia.
In our study the time to attain target caloric requirements by enteral route demonstrated correlation with hypophosphatemia (P = 0.005) this agree with Kilic et al. [13], (P = 0.006).
Sepsis is considered as an important risk factor for hypophosphatemia [19], [20], [21]. Although, greater proportion of septic patient were hypophosphatemic but this observation did not reach statistical significance.
In our study there was a significant association between hypophosphatemia and furosemide use (P = 0.002). Santana e Meneses et al. [12] (P = 0.09) and Kilic et al. [13] (P = 0.04). Agreed with us this may result from the fact that Loop diuretics have effects on phosphate excretion, probably due to the fact that these drugs act on the loop of Henle where phosphate reabsorption is minimal. However, these drugs also exhibit a weak carbonic anhydrase activity, which can explain their weak phosphaturic effect [22]. In contrast Souza de Meneses et al. [18] reported that no significant association was found (P = 0.7).
In our study there was a significant association between hypophosphatemia and dopamine use (P = 0.018) This agree with Santana e Meneses et al. [12] (P = 0.01). This occur due to the movement of Pi from the extracellular to intracellular compartment is common. It is related to the formation of Pi-containing intermediates of glycolytic metabolism [23]. In contrast Shah et al. [14] reported no significant association between dopamine use and hypophosphatemia (P = 0.37).
In our study there was a significant association between hypophosphatemia and steroids use (P = 0.005) this agree with Loudenot et al. [24] (P = 0.001) and Kilic et al. [13] (p = 0.04). In contrast with our study Souza de Meneses et al. [18] reported no association between steroid and hypophosphatemia (p = 0.5).
In our study there was a significant association between hypophosphatemia and β2 agonist use (P = 0.018). This agrees with Kilic et al. [13] (p = 0.026).
The strength of the study is that it was a prospective study. Serum phosphate measurements were done at two time points to get an exact idea of serum phosphate level. In addition, the concept of the study might give some research ideas to the intensivists practicing in the resource limited settings, where total parenteral nutrition with phosphate is not available.
3.1. Conclusion
Hypophosphatemia is frequent in children admitted to PICU if they cannot be fed early by enteral route. More length of stay and ventilator support was associated with hypophosphatemia. Drugs like furosemide, dopamine, β2 agonist and steroids lead to hypophosphatemia.
3.2. Recommendation
Our recommendations are the following:
1-Early detection of hypophosphatemia in critically ill children is important to reduce duration of use mechanical ventilation and decrease time of pediatric intensive care unit stay.
2-Good nutrition of critically ill children has an important role in improving their clinical condition.
3-Further studies may be performed on a large sample size of children with critical illness to evaluate the magnitude of the problem.
Ethical approval
The study was approved by the research and ethical committees of the contributing hospital.
Funding
There was no funding.
Author contribution
Ahmed Nabih El Shazly: Substantial contributions to the conception and design of the work and acquisition of data.
Doaa Refaey Soliman: Drafting the work.
Conflicts of interest
There was no conflict of interest.
Guarantor
Ibrahim Abd El Naby Gad.
Consent
Written consent was taken from all patient.
Registration of research studies
Researchregistry 2501.
References
- 1.Bugg N.C., Jones J.A. Hypophosphataemia. Pathophysiology, effects and management on the intensive care unit. Anaesthesia. 1998;53:895–902. doi: 10.1046/j.1365-2044.1998.00463.x. [DOI] [PubMed] [Google Scholar]
- 2.Lichtman M.A., Miller D.R., Cohen J., Waterhouse C. Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann. Intern Med. 1971;74:562–568. doi: 10.7326/0003-4819-74-4-562. [DOI] [PubMed] [Google Scholar]
- 3.Barak V., Schwartz A., Kalickman I. Prevalence of hypophosphatemia in sepsis and infection: the role of cytokines. Am. J. Med. 1998;104:40–47. doi: 10.1016/s0002-9343(97)00275-1. [DOI] [PubMed] [Google Scholar]
- 4.Berndt T.J., Schiavi S., Kumar R. “Phosphatonins” and the regulation of phosphorus homeostasis. Am. J. Physiol.-Renal Physiol. 2005 Dec 1;289(6):F1170–F1182. doi: 10.1152/ajprenal.00072.2005. [DOI] [PubMed] [Google Scholar]
- 5.Larsson L., Rebel K., Sorbo B. Severe hypophosphatemia: a hospital survey. Acta Med. Scand. 1993;214:221–223. doi: 10.1111/j.0954-6820.1983.tb08598.x. 6- Larsson L, Rebel K, Sorbo B. Severe hypophosphatemia: a hospital survey. Acta Med Scand 1993;214:221-3. [DOI] [PubMed] [Google Scholar]
- 6.Aubier M., Murciano D., Lecocguic Y., Viires N., Jacquens Y., Sqaura P., Pariente R. Effect of hypophosphatemia on diaphragmatic contractility in patients with acute respiratory failure. N. Engl. J. Med. 1985;313:420–424. doi: 10.1056/NEJM198508153130705. [DOI] [PubMed] [Google Scholar]
- 7.Afzal N.A., Addai S., Fagbemi A., Murch S., Thomson M., Heuschkel R. Refeeding syndrome with enteral nutrition in children: a case report, literature review and clinical guidelines. Clin. Nutr. 2002 Dec 1;21(6):515–520. doi: 10.1054/clnu.2002.0586. [DOI] [PubMed] [Google Scholar]
- 8.Amanzadeh J., Reilly R.F. Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat. Clin. Pract. Nephrol. 2006 Mar 1;2(3):136–148. doi: 10.1038/ncpneph0124. [DOI] [PubMed] [Google Scholar]
- 9.Schiffl H., Lang S.M. Severe acute hypophosphatemia during renal replacement therapy adversely affects outcome of critically ill patients with acute kidney injury. Int. Urol. Nephrol. 2013 Feb 1;45(1):191–197. doi: 10.1007/s11255-011-0112-x. [DOI] [PubMed] [Google Scholar]
- 10.Garner J.S., Jarvis W.R., Emori T.G., Horan T.C., Hughes J.M. CDC definitions for nosocomial infections. In: Olmsted R.N., editor. APIC Infection Control and Applied Epidemiology: Principles and Practice. 1996. A-1–A-20. [Google Scholar]
- 11.Leteurtre S., Martinot A., Duhamel A., Proulx F., Grandbastien B., Cotting J., Gottesman R., Joffe A., Pfenninger J., Hubert P., Lacroix J. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003 Jul 19;362(9379):192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 12.Santana e Meneses J.F., Leite H.P., de Carvalho W.B., Lopes E., Jr. Hypophosphatemia in critically ill children: prevalence and associated risk factors. Pediatr. Crit. Care Med. 2009;10:234–238. doi: 10.1097/PCC.0b013e3181937042. [DOI] [PubMed] [Google Scholar]
- 13.Kilic O., Demirkol D., Ucsel R., Citiak A., Karabocuoglu M. Hypophosphatemia and its clinical implications in critically ill children: a retrospective study. J. Crit. Care. 2012;27:474–479. doi: 10.1016/j.jcrc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Shah S.K., Irshad M., Gupta N., Kabra S.K., Lodha R. Hypophosphatemia in critically ill children: risk factors, outcome and mechanism. Indian J. Pediatr. 2016 Nov 1;83(12–13):1379–1385. doi: 10.1007/s12098-016-2188-x. [DOI] [PubMed] [Google Scholar]
- 15.Gravelyn T.R., Brophy N., Siegert C., Peters-Golden M. Hypophosphatemia and phosphorous depletion in respiratory muscle weakness in general inpatient population. Am. J. Med. 1988;84:870–876. doi: 10.1016/0002-9343(88)90065-4. [DOI] [PubMed] [Google Scholar]
- 16.Manary M.J., Hart C.A., Whyte M.P. Severe hypophosphatemia in children with kwashiorkor is associated with increased mortality. J. Pediatr. 1998;133:789–791. doi: 10.1016/s0022-3476(98)70153-2. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimatsu S., Hossain M.I., Islam M.M. Hypophosphatemia among severely malnourished children with sepsis in Bangladesh. Pediatr. Int. 2013;55:79–84. doi: 10.1111/j.1442-200X.2012.03724.x. [DOI] [PubMed] [Google Scholar]
- 18.de Menezes F.S., Leite H.P., Fernandez J., Benzecry S.G., de Carvalho W.B. Hypophosphatemia in children hospitalized within an intensive care unit. J. intensive care Med. 2006 Jul;21(4):235–239. doi: 10.1177/0885066606287081. [DOI] [PubMed] [Google Scholar]
- 19.Shor R., Halabe A., Rishver S. Severe hypophosphatemia in sepsis as a mortality predictor. Ann. Clin. Lab. Sci. 2006;36:67–72. [PubMed] [Google Scholar]
- 20.Riedler G.F., Scheitlin W.A. Hypophosphataemia in septicaemia:higher incidence in gram-negative than in gram-positive infections. Br. Med. J. 1969;1:753–756. doi: 10.1136/bmj.1.5646.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antachopoulos C., Margeli A., Giannaki M., Bakoula C., Liakopoulou T., Papassotiriou I. Transient hypophosphataemia associated withacute infectious disease in paediatric patients. Scand. J. Infect. Dis. 2002;34:836–839. doi: 10.1080/0036554021000026960. [DOI] [PubMed] [Google Scholar]
- 22.DiMeglio L.A., White K.E., Econs M.J. Disorders of phosphate metabolism. Endocrinol. Metab. Clin. N. Am. 2000 Sep 1;29(3):591–609. doi: 10.1016/s0889-8529(05)70152-3. [DOI] [PubMed] [Google Scholar]
- 23.Brautbar N., Leibovici H., Massry S.G. On the mechanism of hypophosphatemia during acute hyperventilation: evidence for increased muscle glycolysis. Min. Electrolyte Metab. 1983;9(1):45. [PubMed] [Google Scholar]
- 24.Loudenot A., Michot C., Alberti C., Armoogum P., Tsapis M., Dauger S. High prevalence of hypophosphataemia at PICU admission in non-malnourished children. Intensive care Med. 2010 Aug 1;36(8):1443–1444. doi: 10.1007/s00134-010-1898-1. [DOI] [PubMed] [Google Scholar]