Abstract
Eukaryotic parasites possess complex life cycles and utilize an assortment of molecular mechanisms to overcome physical barriers, suppress and/or bypass the host immune response, including invading host cells where they can replicate in a protected intracellular niche. Protein S-palmitoylation is a dynamic post-translational modification in which the fatty acid palmitate is covalently linked to cysteine residues on proteins by the enzyme palmitoyl acyltransferase (PAT), and can be removed by lysosomal palmitoyl-protein thioesterase (PPT) or cytosolic acyl-protein thioesterase (APT). In addition to anchoring proteins to intracellular membranes, functions of dynamic palmitoylation include – targeting proteins to specific intracellular compartments via trafficking pathways, regulating the cycling of proteins between membranes, modulating protein function, and regulating protein stability. Recent studies in the eukaryotic parasites—Plasmodium falciparum, Toxoplasma gondii, Trypanosoma brucei, Cryptococcus neoformans, and Giardia lamblia—have identified large families of PATs and palmitoylated proteins. Many palmitoylated proteins are important for diverse aspects of pathogenesis, including differentiation into infective life cycle stages, biogenesis and tethering of secretory organelles, assembling the machinery powering motility, and targeting virulence factors to the plasma membrane. This review aims to summarize our current knowledge of palmitoylation in eukaryotic parasites, highlighting five exemplary mechanisms of parasite virulence dependent on palmitoylation.
Keywords: palmitoylation, palmitoyl acyltransferase, Trypanosoma, Giardia, Plasmodium, Toxoplasma, pathogenesis
Graphical abstract
Introduction
Lipid modifications of proteins are an important class of post-translational modifications, typically facilitating the anchoring of proteins to hydrophobic membranes. Covalent lipid modifications include N-myristoylation, N-palmitoylation, S-palmitoylation, and prenylation (Nadolski and Linder, 2007, Aicart-Ramos et al., 2011). N-myristoylation is the co-translational attachment of a 14-carbon saturated fatty acid chain to an N-terminal glycine residue in the MGXXXS/T motif catalyzed by N-myristoyltransferase (NMT) (Figure 1A). Prenylation is the addition either a farnesyl or geranylgeranyl moiety to the cysteine residue of the CAAX box motif (cysteine-aliphatic-aliphatic-any) at the C-terminus of a protein (Figure 1B) (Wang and Casey, 2016). These isoprenoid modifications are catalyzed by farnesyltransferases and geranylgeranyltransferases. Both N-myristoylation and prenylation are irreversible. S-palmitoylation refers to the covalent addition of a 16-carbon fatty acid chain to a cysteine residue in a protein via a thioester linkage (Figure 1C) (Linder and Jennings, 2013). Palmitate (16:0) is the predominant and archetypal lipid species, although several other heterogeneous saturated or unsaturated fatty acids can also be incorporated, including oleic acid (18:1) and arachidonic acid (20:4) (Liang et al., 2001). A recognition sequence for palmitoylation has not been precisely defined, although palmitoylation sites are categorized based on simple patterns including Type I (-CC-), Type II (CXXC) and Type III (other sites), which an online algorithm called CSS-Palm 2.0 uses to predict sites in silico (Ren et al., 2008). N-palmitoylation is a rare irreversible modification of cysteine with palmitate utilizing a stable amide-linkage, and will not be discussed again (Buglino and Resh, 2008). Notably, individual proteins often possess combinations of these lipid moieties, attached concurrently, which confer increased hydrophobicity, and can strengthen their interaction with intracellular membranes, thus altering their targeting and/or function in the cell (Aicart-Ramos et al., 2011).
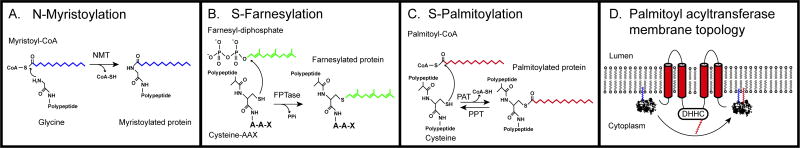
Figure 1.
Three classes of lipid modifications of proteins occur in eukaryotes: N-myristoylation, S-prenylation, and S-palmitoylation. A. N-myristoylation is catalyzed by N-myristoyltransferase (NMT) which transfers the 14-carbon saturated fatty acid myristate from myristoyl-CoA to the amino terminal glycine residue of the MGXXXS/T motif, releasing coenzyme A (CoA-SH). This reaction forms an amide bond, and is irreversible. B. S-farnesylation is catalyzed by a farnesyl protein transferase (FPTase) which transfers the 15-carbon farnesyl isoprenoid from farnesyl diphosphate to the cysteine residue of the carboxyl terminal CAAX motif (A is any aliphatic amino acid; X is one of several amino acids), releasing pyrophosphate (PPi). S-farnesylation is one of two kinds of irreversible protein S-prenylation, the other is S-geranylgeranylation which is catalyzed by geranylgeranyl protein transferase (GGTase) (not shown). C. S-palmitoylation is a reversible modification catalyzed in the forward reaction by palmitoyl acyltransferase (PAT) which transfers 16-carbon palmitate from palmitoyl-CoA to a cysteine residue in one of three types of simple motif (CXXC, XCCX, or other), releasing CoA-SH. Palmitoyl-protein thioesterase (PPT) or acyl- protein thioesterase (APT) catalyze the reverse reaction. D. Palmitoyl acyltransferase proteins typically possess 4–6 transmembrane domains (red cylinders). The canonical membrane topology is represented here with the loop containing the DHHC motif exposed in the cytoplasm, along with the amino and carboxyl termini. Two loop regions between TM1/TM2 and TM3/4 are exposed (i.e. to the outside of the cell or to the lumen of an organelle). In this panel, a myristoylated protein (left) associates with the phospholipid bilayer and is palmitoylated by a PAT, thus anchoring it firmly in the membrane (right).
Studies of S-palmitoylation in parasites are not as advanced as they are in humans or yeast, although several palmitoyl proteomes (palmitoylomes) have been characterized in recent years, and dozens of palmitoylated proteins with parasite-specific functions have been identified (Emmer et al., 2011, Jones et al., 2012a, Frenal et al., 2014, Foe et al., 2015). This review aims to summarize our basic knowledge of palmitoylation, and describe five unique examples of palmitoylation contributing to parasite virulence.
The discovery of dynamic S-palmitoylation in eukaryotes
Palmitoylation performs many functions in eukaryotic cells, not only anchoring protein in lipid membranes, but also trafficking of proteins to subcellular compartments, stabilizing proteins by preventing their ubiquitination and degradation, and regulating protein activity (Linder and Deschenes, 2007, Shipston, 2011). Palmitoylation is known to preferentially anchor proteins in detergent-resistant membranes called lipid rafts - membrane microdomains containing high concentrations of sterols and sphingolipids and possessing high liquid order, enriched in signaling proteins (Simons and Ikonen, 1997). This is a crucial phenomenon for the specific trafficking of proteins to ciliary membranes (Emmer et al., 2010b).
Palmitoyl acyltransferases (PATs) were first characterized in S. cerevisiae and were found to contain a novel cysteine-rich domain (CRD), similar to a zinc-finger metal-binding site, and a short consensus motif, aspartate-histidine-histidine-cysteine, hence referred to as the DHHC-CRD (Lobo et al., 2002, Roth et al., 2002). This motif was previously identified in eukaryotic genomes (Putilina et al., 1999, Mitchell et al., 2006). The completion of many genome sequences has allowed the facile identification of PAT families in many other eukaryotes, including seven PATs in S. cerevisiae and twenty-four PATs in humans (Greaves and Chamberlain, 2011). Notably, PAT proteins possess a distinct topology with four to six transmembrane domains, and the DHHC motif facing the cytoplasm on an inter-transmembrane-domain loop (Figure 1D) (Linder and Jennings, 2013). Many PATs are located to the endoplasmic reticulum (E.R.) or Golgi apparatus in yeast and humans, although a few are found in the plasma membrane or specialized vacuoles (Ohno et al., 2006).
Two classes of palmitoyl thioesterases catalyze the reverse reaction - acyl-protein thioesterase (APT) and palmitoyl-protein thioesterase (PPT) (Das et al., 2000, Duncan and Gilman, 2002). As palmitoylation is a reversible lipid modification, it often functions in dynamic cellular processes, similar to protein phosphorylation (Salaun et al., 2010). The classic example is the Ras family of small GTPases, which are palmitoylated in the Golgi, targeting them to the plasma membrane, where they are depalmitoylated, thus creating a continuous cycle which spatially organizes these peripheral membrane proteins without the need for targeting receptors (Rocks et al., 2005, Rocks et al., 2010). Crosstalk between palmitoylation of proteins and other modification like phosphorylation also occurs via two possible mechanisms. Either the palmitate restricts access of a protein kinase to its substrate, or the phosphate prevents a PAT from palmitoylating its substrate (Salaun et al., 2010).
The development of protocols for palmitoyl proteomics has enabled the rapid characterization of whole cell palmitoylomes, typically composed of hundreds of proteins for any cell type. Acyl-biotinyl exchange was the original method, which in simple terms involves replacement of palmitate with a biotin moiety that permits protein purification on streptavidin and identification by mass spectrometry. Palmitoyl proteomics employing ABE measures the steady-state palmitoylome at the time ABE is performed and revealed the functional redundancy of the PAT family in yeast (Roth et al., 2006, Wan et al., 2007). Subsequently, a protocol to measure dynamic palmitoylation was invented, involving metabolic labeling of palmitoylated proteins in vivo with the palmitate analog 17-octadecanoic acid (17-ODYA), followed by addition of a biotin moiety via click chemistry, streptavidin purification and identification by mass spectrometry (Martin and Cravatt, 2009, Martin et al., 2012). For more details on these methods, we recommend a recent review summarizing these technologies and other improvements, that now allow specific palmitoylation sites to be mapped (Zhou et al., 2014).
In humans, aberrant palmitoylation plays a role in many diseases, including the adult-onset neurodegenerative disease Huntington disease, and also many cancers expressing N- and H-Ras oncogenes (Ducker et al., 2004, Sanders and Hayden, 2015). Palmitoylation can be inhibited by 2-bromopalmitate (2BP), although proteomics analysis showed that there may be some off-target effects of this drug. New inhibitors have been developed with better selectivity (Jennings et al., 2009, Davda et al., 2013).
The innovations described above have prompted parasitologists to explore palmitoylation using proteomics and characterize its role in a variety of processes from protein targeting to virulence factor biogenesis. In this context, the role of palmitoylation in parasite biology is unsurprising, as it is fundamental to the functioning of so many membrane proteins in other eukaryotes.
Apicomplexan parasites regulate pathogenic processes by palmitoylation
Three epidemiologically significant diseases in humans are caused by unicellular parasites from the Apicomplexan phylum. Malaria in humans is caused by five Plasmodium species transmitted by Anopheles mosquitoes in tropical latitudes, but P. falciparum causes the majority of the estimated 700,000–1,000,000 global annual fatalities, making P. falciparum by far the most devastating parasitic infection of humans in terms of mortality (Winzeler, 2008, Murray et al., 2014). Death is caused by severe anemia from erythrocyte lysis or CNS inflammation. Toxoplasma gondii is a widespread zoonotic coccidian responsible for a largely asymptomatic latent infection called toxoplasmosis. Toxoplasma is acquired by consumption of meat containing toxoplasma cysts and leads to a mild illness in healthy people, or a potentially fatal neurologic disease in immunocompromised individuals (Black and Boothroyd, 2000, Dubey, 2009). Congenital transmission of T. gondii can cause spontaneous abortions and devastating neurologic disease in newborns (Black and Boothroyd, 2000). Cryptosporidium species cause an acute enteric disease in humans called intestinal cryptosporidiosis, the primary symptom being watery diarrhea, as well as causing respiratory cryptosporidiosis, with C. hominis and C. parvum species responsible for the majority of cases (Tzipori and Widmer, 2008). Cryptosporidiosis is the second most common cause of diarrheal disease and death in infants, and is also common in immunocompromised patients (Striepen, 2013). Apicomplexan parasites possess complex life cycles, and pathogenesis in human host tissues is primarily caused by their obligate intracellular stages. Post-translation modifications are critical to all aspects of the life cycles of apicomplexans, as highlighted in an excellent recent review of post-translational modifications in malaria parasites (Doerig et al., 2015).
The study of S-palmitoylation in apicomplexan parasites is more advanced than for other infectious microorganisms, as numerous palmitoylated proteins have been characterized that are important for life cycle progression, cell viability, gametocytogenesis, trafficking, gliding motility, host-cell invasion, and egress from host cells (Beck et al., 2013, Frenal et al., 2013, Santos et al., 2015, Hopp et al., 2016, Santos et al., 2016, Tay et al., 2016). Importantly, palmitoyl proteomes have been generated using the complementary ABE and metabolic labelling strategies, in combination with stable isotope labelling with amino acids in cell culture (SILAC) for highly accurate quantification by mass spectrometry. More than 280 palmitoylated proteins in T. gondii and more than 400 palmitoylated proteins in P. falciparum asexual schizonts have been identified (Jones et al., 2012a, Foe et al., 2015). Informed speculation about the significance of these palmitoylated proteins in erythrocyte invasion and drug resistance is detailed in a fascinating review (Jones et al., 2012b). However, only a handful of these candidates have been experimentally validated as palmitoylated in P. falciparum: 45 kDa gliding-associated protein (PfGAP45), calcium-dependent protein kinase 1 (PfCDPK1), calpain, chloroquine resistance transporter (PfCRT), PfRab5b, adenylate kinase 2 (PfAK2), inner membrane complex (IMC) sub-compartment proteins (PfISP1 and 3), and myosin tail interacting protein (PfMTIP) (Moskes et al., 2004, Rees-Channer et al., 2006, Russo et al., 2009, Jones et al., 2012a, Ezougou et al., 2014, Thavayogarajah et al., 2015, Wetzel et al., 2015).
Families of twelve PATs have been identified in P. falciparum (PfDHHC1–12), eleven PATs in P. berghei (PbDHHC1–11), and eighteen PATs in T. gondii (TgDHHC1–18), five of which are orthologous among the species (Frenal et al., 2013, Hodson et al., 2015). Also, Cryptosporidium parvum possesses ten PATs (CpDHHC1–10), although none has been studied biochemically to date (Frenal et al., 2013). Concomitantly, a bona fide orthologue of human acyl protein thioesterase (APT1) was identified in T. gondii by two independent groups (named TgPPT or TgASH1), and three other related putative active serine hydrolases (TgASH2, 3, 4) were also identified as binding APT1 inhibitors (Child et al., 2013, Kemp et al., 2013). All twelve PfDHHC-PATs and eighteen TgDHHC-PATs are expressed near ubiquitously throughout the life cycle, although some are upregulated in specific stages (Jones et al., 2012b, Frenal et al., 2013). Apicomplexan PATs are primarily found in the Golgi apparatus and ER, as is typical in other eukaryotes, although these studies often only image T. gondii tachyzoites and Plasmodium schizonts (Seydel et al., 2005, Frenal et al., 2013, Tay et al., 2016). A few others are found in the plasma membrane (TgDHHC4 and TgDHHC13), or are dually located in the E.R. and vesicles or the nuclear membrane (Frenal et al., 2013). However, many PATs in P. falciparum, P. berghei, and T. gondii are localized to unique apicomplexan organelles, including the inner membrane complex (IMC) of vesicles underlies the plasma membrane (PfDHHC1, PfDHHC9, PfDHHC3, TgDHHC2, TgDHHC14), which is important for gliding motility, the rhoptries, which are involved in secretion of factors necessary for invasion (PfDHHC7, PbDHHC7, TgDHHC7), and the crystalloid bodies in Plasmodium ookinete an oocyst stages (PbDHHC10) (Beck et al., 2013, Frenal et al., 2013, Wetzel et al., 2015, Hopp et al., 2016, Mueller et al., 2016, Santos et al., 2016, Tay et al., 2016).
Functional studies by gene disruption have been used to interrogate apicomplexan PATs with great success, with several studies in the last year highlighting the role of these proteins in pathogenesis or life cycle progression (Beck et al., 2013, Frenal et al., 2013, Santos et al., 2015, Wetzel et al., 2015, Hopp et al., 2016, Santos et al., 2016, Tay et al., 2016). In T. gondii, eleven PATs are dispensable in tachyzoites with no effect on the lytic cycle or intracellular growth, whereas five others could not be disrupted (TgDHHC2, 5, 7, 9 and 4) (Frenal et al., 2013). Similarly, seven P. berghei PATs are non-essential in blood stage parasites, and two resistant are to knockout attempts (PbDHHC4 and 8). In P. falciparum, PfDHHC5 and PfDHHC9 genes are dispensable by knockout in schizonts with no effect on cell growth, whereas PfDHHC3, 7 and 8 genes could not be replaced by double cross-over recombination (Tay et al., 2016). These results suggest functional redundancy for the majority of individual apicomplexan PATs in the life cycle stages tested, or alternatively life cycle stage-specific roles, with a small number of PATs essential for palmitoylation of a subset of substrates. Nevertheless, individual PATs and their palmitoylated substrates have been characterized with irreplaceable roles in apicomplexan life cycles and the biological processes involved in establishing and maintaining an intracellular infection, as described below.
Example 1: Dual acylation is required for the tethering of rhoptries to the apical complex and secretion of virulence factors
The T. gondii armadillo repeats-only protein (ARO) is the most thoroughly studied example of a palmitoylated protein connecting parasite cell architecture with host-cell invasion through an acylation-dependent mechanism. Apicomplexan parasites possess an apical complex consisting of the motile conoid and a suite of specialized secretory organelles required for host-cell invasion and establishment of a parasitophorous vacuole (PV) (Baum et al., 2008). These organelles include micronemes, exonemes, rhoptries, and dense granules, which are derived from the trans-Golgi network, and sequentially release their cargo that are required for host cell invasion (Figure 2A). In P. falciparum, merozoites, ookinetes, and sporozoites, are the three life cycle stages capable of host cell invasion, while in T. gondii only tachyzoites are invasive. Upon contact with the host cell, the zoites reorient themselves with their apices contacting the host cell, and cell invasion is initiated with the secretion of proteins including proteases and adhesins surface of the parasite plasma membrane (Figure 2B). Rhoptries are bulbous shaped organelles with an elongated neck orientated towards the conoid. There are 6–14 lipid- and protein-containing rhoptries in T. gondii tachyzoites, which are released by exocytosis. The moving junction is formed by proteins secreted by the micronemes (AMA1) and rhoptries, and is functionally equivalent to a tight junction between the plasma membranes of the motile zoite and host cell, facilitating parasite entry, and initially establishing the PV (Tyler et al., 2011). Through this mechanism, Plasmodium parasites secrete hundreds of proteins into the red blood cell cytoplasm. The dense granules are last to secrete their contents, and these proteins reinforce the PV and remodel the infected host cell.
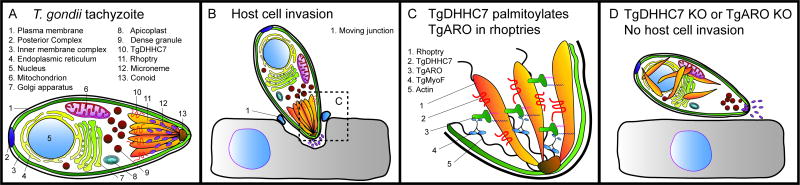
Figure 2.
The rhoptries are tethered to the actin cytoskeleton in T. gondii tachyzoites by palmitoylation of TgARO by TgDHHC7, and host cell invasion is impaired in parasites lacking either gene. A. Schematic diagram of a T. gondii tachyzoite showing its primary organelles, including three classes of secretory organelles at the apical end (micronemes, rhoptries, dense granules). TgDHHC7 with its four transmembrane domains is localized to the rhoptry membrane. B. Host cell invasion occurs when a tachyzoite attaches to the surface of the target cell, and the micronemes secrete their contents. Invasion is powered by gliding motility, and a moving junction is formed around the invading cell. The rhoptries secrete their proteins to form the parasitophorous vacuole, and the dense granules are secreted last. A tachyzoite is represented secreting its micronemes. C. The apical end of a tachyzoite contains six to fourteen rhoptries (only three are shown). TgDHHC7 is localized to the rhoptry membrane where it palmitoylates previously myristoylated TgARO, and the dual acylation firmly anchors TgARO to the rhoptry membrane. TgARO binds myosin F (TgMyoF) which is a motor protein that binds actin, thus tethering the rhoptries to the apical end of the cell. The C-terminal domain of TgARO also binds other rhoptries, clustering them together. D. Host cell invasion is impaired in TgDHHC7 or TgARO knockout cells. Palmitoylation of TgARO is necessary for its localization to the rhoptry membrane. In cells lacking either TgARO or TgDHHC7, rhoptry tethering is compromised, and the rhoptries are dispersed in the cytoplasm. Secretion of proteins from the micronemes is not affected by rhoptry dispersal in these knockouts.
ARO is dual acylated at its N-terminus and is exclusively targeted to the cytoplasmic face of the rhoptry membrane in both P. falciparum and T. gondii (Cabrera et al., 2012). Expression of PfARO is restricted to the invasive late blood stages (schizonts and merozoites) in P. falciparum, and tachyzoites in T. gondii. Two groups published studies demonstrating that TgARO tethers rhoptries to the apical complex (Figure 2C) (Beck et al., 2013, Mueller et al., 2013). Depletion of TgARO caused rhoptries to disperse in the cytoplasm, although the protein cargo was processed and loaded correctly in the sub-compartments of the rhoptries. The micronemes, dense granules, and apicoplast were correctly positioned in the TgAROcKO knockdown parasites, and microneme secretion was normal. TgARO contains six armadillo repeats, and all of them are required for tethering the rhoptries, but only ARM6 at the C-terminus is required for clustering the rhoptries (Mueller et al., 2016).
Tethering of the rhoptries at the apical end of the tachyzoites by TgARO is necessary for virulence during the lytic cycle of T. gondii (Figure 2B) (Beck et al., 2013, Mueller et al., 2013). Without TgARO, invasion of fibroblast cells is impaired due to defective attachment to host cells. The few parasites that are able to enter replicate, exit the PV and exhibit gliding motility normally. TgARO-deficient parasites also do not kill mice like normal parasites. After rhoptry secretion, wild type cells have empty vacuoles called evacuoles. In contrast, TgAROcKO parasites retain rhoptry cargo and do not produce evacuoles. Acylation by myristate and/or palmitate is critical for TgARO function; complementation with wild type TgARO restored apical rhoptry tethering and evacuole formation, while complementation with a G2A mutant defective in both myristoylation and palmitoylation did not.
TgARO covers the surface of the rhoptries, and is concentrated at their apices. Three proteins were identified and confirmed as TgARO binding partners: myosin F, adenylate cyclase β, and a conserved hypothetical protein, armadillo interacting protein (Figure 2C) (Mueller et al., 2013, Mueller et al., 2016). Tethering of the rhoptries via TgARO is an actomyosin-dependent process since treatment of cells with either an actin polymerization inhibitor or a myosin ATPase inhibitor resulted in dispersion of rhoptries, with no effect on the micronemes, mimicking the TgAROcKO phenotype (Mueller et al., 2013).
TgDHHC7 was found to be the sole PAT that palmitoylates TgARO (Beck et al., 2013, Frenal et al., 2013). TgDHHC7 is found along the length of the rhoptries. The TgDHHC7 knockdown emulated the TgAROcKO phenotype, causing dispersal of the untethered rhoptries in the cytoplasm resulting in similar impaired invasion and propagation. Rhoptry subdomain organization, positioning of other organelles, secretion from the micronemes, host cell attachment, gliding motility, host cell egress, were all normal. Complementation of the TgDHHC7 conditional knockdown with an ectopic wild type copy rescued the induced cells, whereas an inactive copy with a mutagenized DHHC motif (DHHA) localized to the rhoptries, but did not rescue rhoptry tethering. These results explain an earlier observation in which the rhoptries of P. falciparum were mislocalized after the treatment of late trophozoite/early schizont parasites with 2BP, and reduced invasion efficiency of erythrocytes in a dose-dependent manner (Jones et al., 2012a).
This remarkable example of secretory organelle tethering in a parasite by palmitoylation of a protein connected to the cytoskeleton could still yield more secrets into rhoptry biogenesis, exocytosis, and recycling. Positioning of other organelles may also rely on this mechanism in apicomplexans or other parasites, and the identification and localization of the PAT responsible for the palmitoylation also can provide clues about substrate localization and trafficking of palmitoyl proteins generally.
Example 2: Palmitoylation at the inner membrane complex is essential for gliding motility and host cell invasion
Apicomplexan parasites present another splendid example of palmitoylation in virulence, specifically relating to their unusual mode of motility called gliding motility, which requires specialized adaptations to cell anatomy and is essential for host cell invasion. The IMC is a peripheral network of flattened membrane vesicles called alveoli underlying the plasma membrane, assembled from clathrin-coated vesicles from the ER-Golgi secretory pathway (Harding and Meissner, 2014). The IMC is supported on its cytoplasmic side by the subpellicular network (SPN) of intermediate filaments and the microtubule cytoskeleton, which functions as a scaffolding platform, thus giving the cell its shape as well as being essential to invasion and gliding motility (Baum et al., 2008, Harding and Meissner, 2014). The IMC is found in all apicomplexan parasites, as well as in other organisms from related phyla within the superphylum Alveolata. There are more than 40 IMC resident proteins in apicomplexans (Chen et al., 2015). Many IMC proteins possess myristoylation and/or palmitoylation sites, and fourteen were identified in the P. falciparum blood stage palmitoylome (Jones et al., 2012a). IMC sub-compartment proteins ISP1–4 constitute a novel family of proteins specifically targeted to sub-domains in this organelle by multiple acylations in several apicomplexans (Beck et al., 2010, Fung et al., 2012, Poulin et al., 2013, Wetzel et al., 2015). In contrast, HSP20 only requires palmitoylation to localize to the IMC membrane (De Napoli et al., 2013). The IMC is remodeled throughout the Plasmodium life cycle (Figure 3A) and is required for the invasion of hepatocytes by sporozoites, erythrocytes by merozoites, and traversal of the peritrophic matrix and epithelial cells of the mosquito midgut by the ookinete (Harding and Meissner, 2014).
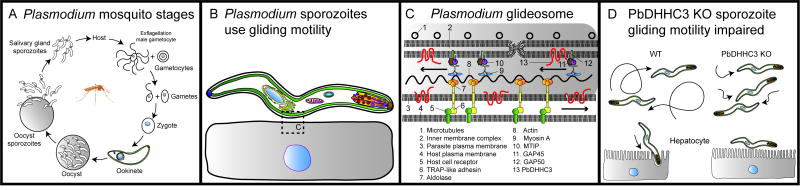
Figure 3.
Gliding motility is dependent on palmitoylation in the Plasmodium (P. berghei) insect stages. A. Life cycle of Plasmodium mosquito stages and initial invasion of host hepatocytes. The mosquito consumes a blood meal containing male and female gametocytes (not shown), which differentiate into gametes. Fertilization of the female gamete by a male gamete yields a diploid zygote, which develops into a motile ookinete that then develops into an oocyst, in which sporozoites are generated by sporogony. The sporozoites burst out of the oocyst and migrate to the salivary glands, which are injected into the human host during a blood meal. Sporozoites invade host hepatocytes to initiate infection. The liver and blood stages are not shown. B. Plasmodium zoites are the motile stages; a sporozoite is shown here adhering to the surface of a host cell and gliding. Gliding motility is powered by the glideosome (box), which is expanded in Panel C. C. The Plasmodium glideosome is shown here (top) interacting with the host cell membrane (bottom) and represents a generalized model for other apicomplexans. Microtubules stabilize the inner membrane complex (top), and contain the glideosome components GAP45 and MTIP which are palmitoylated by a PbDHHC, anchoring them to the membrane. Another major membrane protein GAP50 is not palmitoylated. GAP45 and MTIP interact with the myosin A motor protein that traverses actin filaments, generating the force which is transduced via the TRAP-like adhesin complex in the plasma membrane. The TRAP-like adhesins interact with host cell receptors in the plasma membrane during attachment. PbDHHC3 is located in the IMC. Apicomplexans possess plasma membrane DHHCs, which are probably responsible for palmitoylating these proteins. D. Gliding motility is impaired in ookinetes and sporozoites lacking PbDHHC3 or PbDHHC3/9 (double knockout). Wild type cells glide on surfaces in vitro and invade hepatocytes, whereas the gliding speed and distance is attenuated in the mutants, and they cannot invade host cells.
The glideosome powers motility, migration, host cell invasion, and egress in zoite stages, and is located between the plasma membrane and the IMC (Figure 3B) (Frenal et al., 2010). Palmitoylation is necessary for normal gliding motility and effective host cell invasion in T. gondii tachyzoites (Alonso et al., 2012). Conversely, inhibition of depalmitoylation also affects host cell invasion in vitro, although conflicting enhanced or reduced phenotypic effects were reported in two independent studies, possibly due to differences in the inhibitor classes tested or treatment duration prior to the assay (Child et al., 2013, Kemp et al., 2013). The gliding motor complex powers the glideosome, and consists of the myosin motor firmly anchored by its tail in the cholesterol-rich IMC and bound by its head to actin filaments that underlie the plasma membrane, which in turn are attached to extracellular adhesins that specifically bind host cells (Figure 3C) (Baum et al., 2008). The cyclical activity of the myosin motors elicits propulsion by transporting these adhesins to the posterior end of the cell generating torque from its contact with the host-cell surface at the moving junction. Disengagement of the adhesins is required for directional movement, and this is performed by surface proteases which digest specific substrate adhesins.
The glideosome is a motor complex composed of several proteins – the atypical myosin A (myoA), its light chains MLC1 (named PfMTIP) and ELC1, and the glideosome-associated proteins GAP40, GAP45, and GAP50 (Figure 3C) (Harding and Meissner, 2014). The glideosome-associated protein 45 (PfGAP45) in P. falciparum merozoites is dual acylated and localized to the IMC (Rees-Channer et al., 2006). Surprisingly, the lipid moieties anchor the T. gondii homologue of PfGAP45 (TgGAP45) to the plasma membrane, not the IMC membrane (Frenal et al., 2010). Palmitoylation is required in P. falciparum schizonts for the stability of PfGAP45 and PfMTIP, but not GAP50 and myoA, to prevent their degradation in the proteasome (Jones et al., 2012a). The PATs responsible for palmitoylating these IMC proteins have not been determined, but as already noted, there are three PATs in the IMC membrane in malaria parasites (PfDHHC1, 3, 9), so these are logical candidates (Wetzel et al., 2015, Hopp et al., 2016). However, localization of PbDHHC3 is life cycle stage dependent – in ookinetes it is in the IMC, whereas in oocyst sporozoites it exhibits punctate and near-nuclear staining, and is not detected in salivary gland sporozoites.
Gliding motility does indeed depend on IMC-resident PATs in P. berghei parasites, particularly the infective asexual stages in the mosquito which invade liver cells after transmission (Hopp et al., 2016). Gliding motility was impaired in ookinetes depleted of PbDHHC3, but the combined effect of knocking out both PbDHHC3/9 was greater than the sum of the effects in the individual mutants, decreasing speed and distance travelled (Figure 3D). Diminished gliding motility probably affected the ability of the parasites to traverse the peritrophic matrix of the midgut, thus explaining the precipitous reduction in oocysts and salivary gland sporozoites in PbDHHC3 knockout parasites. Notably, there was a disproportionate decrease in oocysts in the PbDHHC3/9 double knockout, and ookinetes were arrested with aberrant morphology. These results strongly suggest the functional redundancy of these two PATs in P. berghei, likely due to the fact that one or more substrates of PbDHHC3 with critical function in morphogenesis are able to be palmitoylated by PbDHHC9. The PbDHHC3 knockout salivary gland sporozoites were severely impaired in trail assays, and were unable to glide as far as wild type parasites continuously before detaching. The PbDHHC3/9 double knockout cells behaved similarly, whereas the PbDHHC9 knockout cells displayed healthy gliding behavior. Crucially, the infectivity of PbDHHC3 knockout hemolymph or salivary gland sporozoites in mice was compromised, causing a significant reduction in infected hepatocytes and blood parasitemia. PbDHHC9 knockout sporozoites were as effective as wild type cells in these mice infection experiments.
In summary, PbDHHC3 and PbDHHC9 are essential to gliding motility in P. berghei ookinetes and salivary gland sporozoites, as well as invasion of hepatocytes. It is almost certain that this is primarily due to the palmitoylation activity of PbDHHC3, even though the precise mechanism is still enigmatic. The IMC and glideosome possesses several proteins which require palmitoylation to function, so these proteins are likely PbDHHC substrates.
Example 3: Palmitoylation is necessary for crystalloid biogenesis in ookinetes and oocysts and differentiation into infectious sporozoites
The role of several Plasmodium PATs (PbDHHC2, PbDHHC3, PfDHHC9, and PbDHHC10) in life cycle progression and morphogenesis in the mosquito has been described during the past year, with implications for transmission and infectivity (Santos et al., 2015, Hopp et al., 2016, Santos et al., 2016, Tay et al., 2016). However, only one of these represents an exceptional model for the role of palmitoylation in organelle biogenesis, namely the formation of crystalloid bodies in the asexual stages in the mosquito.
The crystalloid bodies are transient structures assembled in ookinetes and consisting of an aggregation of irregular particles or vesicles in symmetrical crystalline patterns, surrounded by hemozoin clusters (Figure 4A) (Dessens et al., 2011). They are retained in early oocysts, but are absent from mature oocysts. Crystalloids have an elusive function, but are required for the development, transmission, and virulence of sporozoites. It is thought that they provide a reservoir of proteins and lipids for oocyst biogenesis or alternatively act as temporary store of molecules from the female gametocyte to be trafficked to their final destination in mature oocysts (Dessens et al., 2011). Crystalloid biogenesis is dependent on crystalloid-resident LCCL-lectin adhesive-like proteins (LAPs), which are localized to these structures in the ookinete stages (Carter et al., 2008, Saeed et al., 2013, Saeed et al., 2015). However, the temporal expression of LAP4, 5, and 6 is regulated by translational repression in the gametocytes (Saeed et al., 2013). Translational repression of mRNAs in female gametocytes is a well characterized mechanism controlling the sexual development of malaria parasites, and a subset of maternal mRNAs in ribonucleoprotein complexes are quiescent until after fertilization (Mair et al., 2006). Nevertheless, the molecular events involve in crystalloid biogenesis are poorly understood.
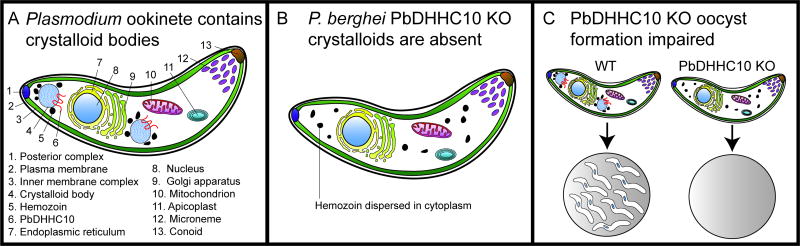
Figure 4.
The crystalloid bodies form in ookinetes and are present in oocysts. A. P. berghei ookinetes are motile stages that traverse the peritrophic matrix in the mosquito midgut and epithelial cells before forming oocysts. This stage does not form a parasitophorous vacuole in the host cells so only contains micronemes, but not rhoptries or dense granules. There are 1–3 crystalloid bodies per cell, which have an unknown function, but contain proteins and lipids and possess the PAT PbDHHC10 in their membranes. The crystalloids are also surrounded by hemozoin granules. B. In the PbDHHC10 knockout parasites the crystalloids do not form, and hemozoin is dispersed in the cytoplasm. C. The wild type ookinetes normally form oocysts containing many sporozoites, but the PbDHHC10 knockout parasites form empty oocysts.
An important role for palmitoylation in crystalloid biogenesis became apparent from investigations of PbDHHC10 function (Santos et al., 2016). Similar to transcription of the three LAPs, transcription of PbDHHC10 is upregulated in gametocytes, and the mRNA is stored in the cytoplasm as a translationally repressed messenger ribonucleoprotein complex. Furthermore, PbDHHC10 protein expression only occurs in ookinetes, where it is exclusively localized to crystalloids. A PbDHHC10 knockout line was generated in asexual blood stages, which was able to proceed through the life cycle in mosquitoes (Santos et al., 2016). There was no striking phenotype during the asexual erythrocytic or sexual stages (Santos et al., 2016). Unexpectedly, crystalloids are absent from PbDHHC10 knockout cells, even though the number of oocysts in the mosquito midgut was comparable to those in wild type cells (Figure 4B) (Santos et al., 2016). Moreover, the PbDHHC10 knockout line was found to be defective in sporulation (Figure 4c), colonization of the salivary glands, and transmission to naïve mice (Figure 4d) (Santos et al., 2016). This failure of crystalloid biogenesis mimicked a phenotype previously described for crystalloid-resident LAP family mutants, which are also impaired in oocyst sporulation (Carter et al., 2008, Saeed et al., 2015). Examination of ookinetes by electron microscopy revealed that the hemozoin clusters are dispersed in PbDHHC10 knockouts. Also, a crystalloid-resident protein (LAP2) was mislocalized in the cytoplasm of the mutant ookinetes, although it was not ascertained whether LAP2 is a substrate of PbDHHC10, or if this was an indirect effect of crystalloid biogenesis failure.
In summary, the role of PbDHHC10 in crystalloid formation is an exciting new paradigm of palmitoylation mediating the biogenesis of a subcellular compartment important for virulence in parasites. Although the mechanism is still a mystery, it is likely that PbDHHC10 palmitoylates one or more substrates involved in crystalloid formation, perhaps sequestering them in the nascent membrane by kinetic trapping, thus facilitating its assembly.
Palmitoylation and virulence in trypanosomatid parasites
The trypanosomatids are another large family of unicellular parasites which cause a great toll on human health, and are considered neglected tropical diseases (Hotez et al., 2016). Three human diseases are caused by these parasites – African sleeping sickness (African trypanosomiasis), Chagas disease (American trypanosomiasis), and leishmaniasis (Stuart et al., 2008). African sleeping sickness is a hemolymphatic fever that kills thousands of people every year in sub-Saharan Africa, and is caused by two sub-species of Trypanosoma brucei (T. brucei gambiense and T. brucei rhodesiense), transmitted in the saliva of the tsetse fly during a blood meal (Welburn et al., 2016). Chagas disease is a chronic inflammatory heart disease that affects millions throughout the Americas, and is caused by Trypanosoma cruzi parasites, primarily transmitted by blood-sucking triatomine bugs, although congenital and foodborne transmission is frequent (Robertson et al., 2016). Three pathologically distinct diseases are caused by several Leishmania species transmitted by sandflies in tropical and sub-tropical latitudes. Visceral leishmaniasis is a fatal lymphatic fever that kills hundreds of thousands of people every year, cutaneous leishmaniasis is a self-healing skin infection, and mucocutaneous leishmaniasis is a disfiguring, but not fatal infection (McGwire and Satoskar, 2014). Treatments for all of these diseases are available but suboptimal, due to inherent toxicity, poor oral bioavailability, poor dosing regimen, and emergence of drug resistant parasites (Baker et al., 2013). Myristoylation is already a validated drug target in trypanosomatids, and pyrazole sulfonamide inhibitors of N-myristoyltransferase are efficacious in animal models of infection (Frearson et al., 2010, Herrera et al., 2016). Palmitoyl acyltransferases (PATs) offer alternative targets for inhibition, and results from our lab show 2-BP kills T. brucei bloodstream forms in vitro (Emmer et al., 2011).
The history of palmitoylation in trypanosomatids precedes our understanding of the mechanisms involved, with several palmitoylated proteins of biological significance under investigation before the discovery of any eukaryotic PATs (Godsel and Engman, 1999, Denny et al., 2000, Hertz-Fowler et al., 2001). We determined that palmitoylation is essential in T. brucei and identified a family of 12 PATs in the T. brucei genome, and performed a phenotypic RNAi screen to analyze the function of each individual PAT in vitro (Emmer et al., 2011). However, no striking phenotype was found in any knockdown line, suggesting functional redundancy. We also characterized the steady state palmitoylome of procyclic forms using the ABE method, identifying 124 proteins by mass spectrometry, including all known palmitoylated proteins in T. brucei (Emmer et al., 2011). The palmitoylome was enriched in membrane transporters, proteases, surface proteins, lipid metabolism enzymes, trafficking proteins, and signaling proteins. Recently, the myristoylome and palmitoylome were both characterized and compared in bloodstream forms by metabolic labeling and click chemistry (Wright et al., 2016). The palmitoylomes of T. cruzi and Leishmania parasites have not been reported, but are actively being pursued in our laboratory.
Currently, about a dozen palmitoylated proteins have been studied in detail in trypanosomatids, whereas the majority of the palmitoylome is still uncharacterized. Our recent review summarized acylation in trypanosomatids these in detail (Goldston et al., 2014). Palmitoylation is necessary for the targeting of these proteins to various organelles including: the flagellar membrane (LmjSMP-1, Ld/TbFlabarin, FCaBP/Calflagins, TbMCA4, TbGPI-PLC, LmjHASPB), the flagellar tip (TbCALP1.3), the flagellar pocket and endosomes (LmjPPEF), the plasma membrane (TcPI-PLC, TbGPI-PLC, LmjHASPB), the sub-pellicular microtubule cytoskeleton (TbCAP5.5), and the mitochondrion (TbPOMP39) (Godsel and Engman, 1999, Hertz-Fowler et al., 2001, Tull et al., 2004, Mills et al., 2007, Emmer et al., 2009, de Paulo Martins et al., 2010, Liu et al., 2010, Proto et al., 2011, Maclean et al., 2012, Lefebvre et al., 2013, Sunter et al., 2013, Albisetti et al., 2015, Tetaud et al., 2016). Several palmitoylated proteins are essential for virulence in trypanosomatids, but the specific PATs have not yet been attributed that palmitoylate these proteins. Also, their mechanisms of virulence are still obscure, thus leaving only one outstanding model to discuss from trypanosomatids.
Example 4: Palmitoylation is essential for targeting a virulence factor to the flagellum in trypanosomes
Our laboratory has studied the flagellar calcium-binding protein (FCaBP) in T. cruzi for almost three decades, which has proven to be a fascinating endeavor. Antibodies to this immunogenic protein are produced by essentially all humans and animals infected with T. cruzi, and FCaBP was named for its calcium-binding properties and flagellar membrane enrichment (Engman et al., 1989). FCaBP is a dual acylated calcium-acyl switch protein that possesses four EF-hand domains, two of which are functional in binding calcium, and associates with the flagellar membrane in a calcium-dependent manner (Godsel and Engman, 1999, Maldonado et al., 1999). We proposed a model in which FCaBP associates with the flagellar membrane and undergoes a massive calcium-dependent conformational change which alters its binding properties to a partner protein (Wingard et al., 2008).
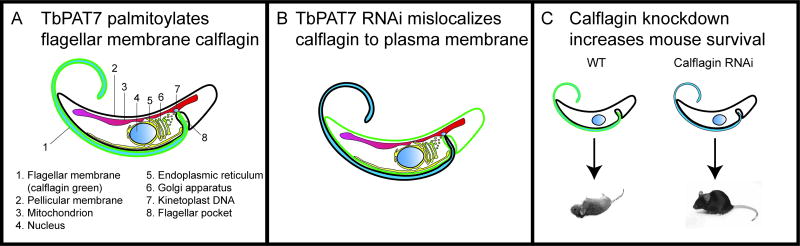
In recent years, we have explored the function of the orthologous calflagin family in T. brucei, and the role of dual acylation for lipid raft association in the flagellum (Emmer et al., 2009, Tyler et al., 2009). This led to the discovery of the first example in trypanosomatids of a palmitoylation enzyme-substrate pair, namely TbPAT7 and calflagin (Figure 5A) (Emmer et al., 2009). Depletion of TbPAT7 inhibits calflagin palmitoylation, resulting in calflagin mislocalization to the plasma membrane (Figure 5B). This localization is identical to that of a C3A calflagin mutant; thus prevention of calflagin palmitoylation either by depleting its PAT or by mutating its palmitoylation site causes the same protein mislocalization. Calflagins bind calcium with a high affinity and are upregulated 10-fold in bloodstream forms (Emmer et al., 2010a). Furthermore, calflagin is required for virulence in a mouse model of infection, since depletion of calflagin leads to the clearance of parasites after remitting and relapsing waves of parasitemia, and prolonged survival of infected animals (Figure 5C). This mutant phenotype depends on host-parasite interactions, as there is no change in parasite growth or flagellar motility in vitro. The relapsing parasitemia in mutants is associated with VSG-mediated antigenic variation, but surface antibody clearance in the mutant parasites is normal. The nature of this virulence function of calflagin is still unknown. We are currently investigating the role of other TbPATs and their individual palmitoylomes, as well as depalmitoylation enzymes, with the intention of defining the rules for the targeting of proteins to different membranes by palmitoylation. We also intend to determine in the near future whether FCaBP is essential for virulence in mice, and whether TcPAT7 is responsible for FCaBP palmitoylation, using CRISPR-Cas9 genome editing tools (Peng et al., 2014, Lander et al., 2015). The evolutionary conservation of this virulence factor across trypanosome species with very different modes of pathogenesis in the mammalian host (intracellular vs. extracellular) presents the question of whether FCaBP and calflagin utilize the same mechanism in both parasites. This example highlighting the role of palmitoylation in mediating the flagellar targeting of a virulence factor will continue to evolve.
Figure 5.
T. brucei calflagin is a flagellar membrane calcium binding protein that requires myristoylation and palmitoylation for membrane targeting and is required for virulence. A. Calflagin (green) is located in the flagellum of T. brucei parasites. The PAT that palmitoylates calflagin (TbPAT7) has been placed between Golgi and flagellar pocket; its location is currently being determined. B. Calflagin (green) is mislocalized to the pellicular (cell body) membrane upon TbPAT7 depletion by RNAi. C. Mice infected with wild type T. brucei suffer unremitting parasitemia and die within 10 days, whereas in vivo depletion of calflagin by RNAi permits the mice to survive.
Giardia lamblia possesses a sizable PAT family despite genomic minimalism
Giardiasis is a zoonotic waterborne and foodborne intestinal infection caused by the unicellular eukaryote Giardia lamblia (Adam, 2001). Diarrhea is the primary symptom of giardiasis and chronic infections are especially debilitating in developing countries. G. lamblia has two major life cycle stages, a highly motile flagellated trophozoite and relatively inert cyst. Encystation occurs in response to bile fluid in the jejunum, and the cysts are passed in the host feces into the environment (Adam, 2001). Cysts can survive for months in cold water, and transmission occurs when they are ingested by a new host. The morphology of the trophozoite is extraordinary compared to other protists. Giardia possesses eight flagella organized into four bilaterally symmetrical pairs, and a ventral adhesive disk to attach to host intestinal villi, as well as other unique cytoskeletal structures (Figure 6A) (Dawson and House, 2010). Despite this morphological complexity, the endomembrane system of Giardia parasites is simpler than other eukaryotes; notably, the Golgi apparatus appears to be absent (Faso and Hehl, 2011). Genomic minimalism is a characteristic of this parasite, and remnant mitochondria called mitosomes are also an example of secondary reduction (Tovar et al., 2003, Morrison et al., 2007).
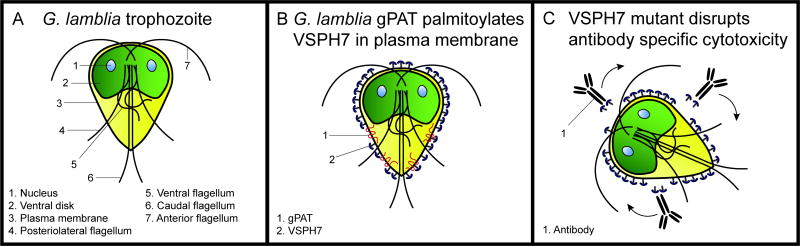
Figure 6.
G. lamblia trophozoites express variant surface proteins (VSPs) which localize to the plasma membrane, and undergo antigenic variation to mediate immune evasion. A. Schematic diagram of G. lamblia trophozoite showing its eight flagella, two nuclei, and ventral disk. B. VSPH7 is exclusively expressed on the plasma membrane surface in this trophozoite clone. VSPH7 is palmitoylated by a Giardia palmitoyl acyltransferase (gPAT) in the plasma membrane. C. A C-terminal cysteine to alanine mutant of VSPH7 did not disrupt protein localization but does lead to resistance to complement-independent antibody-mediated cytotoxicity through an unknown mechanism.
There are nine PATs in G. lamblia that are all expressed in trophozoites and cysts, but each life cycle stage contains a different repertoire of palmitoylated proteins (Merino et al., 2014). Palmitoylation is required for cyst formation, as 2-BP inhibits encystation, whereas over-expression of four DHHC proteins is associated with greater production of cyst wall proteins and cysts. The DHHCs are localized in different membranes of trophozoites—three are in the ER and nuclear envelope, and one is in the plasma membrane. However, only two palmitoylated proteins are known in G. lamblia. One is α19-giardin, a cytoskeletal protein that exclusively localizes to the ventral pair of flagella in trophozoites (Saric et al., 2009). The other constitutes our final and arguably most direct example for the palmitoylation-mediated virulence of parasites, and represents the first PAT-substrate pair identified in parasites.
Example 5: Palmitoylation anchors variant surface antigens to the plasma membrane in Giardia parasites
G. lamblia has a large repertoire of more than 200 variant-specific surface proteins (VSP) which mediate antigenic variation, and expression of surface coat composed of a single VSP is controlled by the RNA interference machinery, which silences all other VSP transcripts until spontaneous antigen switching occurs (Prucca et al., 2008). Antibodies bind to surface-exposed regions of specific VSPs, causing trophozoite aggregation, detachment from host cells, and complement-independent cytotoxicity (Touz et al., 2005). Palmitoylation and glycosylation are frequent modifications of VSPs which have an unusually high threonine and cysteine content (Hiltpold et al., 2000). VSPH7 is a clone-specific VSP localized to lipid rafts in the plasma membrane, and palmitoylation of VSPH7 occurs at the C-terminus on a cysteine-containing motif conserved in all VSPs (Figure 6B) (Touz et al., 2005). The cysteine to alanine mutants in the C-terminal motif of VSPH7 are resistant to complement-independent antibody specific cytotoxicity, but plasma membrane localization did not require these features (Figure 6C). Fortuitously, a Giardia PAT (gPAT) was identified and localized to the plasma membrane. Depletion of gPAT expression led to a reduction in palmitoylation of VSPH7 and two other VSPs, demonstrating an enzyme-substrate relationship. This concise yet persuasive model of the palmitoylation of G. lamblia variant surface antigens represents a novel association between lipid modifications and antigenic variation, which could have greater significance in vivo. The mechanism for the antibody-dependent cytotoxicity of palmitoylated VSPH7 is cryptic, but perhaps conformational changes expose surface epitopes altering their recognition. Determining the effect of a VSPH7 mutant in the host intestines is paramount.
Concluding remarks
The discovery of protein palmitoylation and the determination of the palmitoylomes of cells from a variety of organisms have extended our understanding of the roles of lipids in protein function and regulation (Lobo et al., 2002, Roth et al., 2002, Rocks et al., 2005, Roth et al., 2006, Martin and Cravatt, 2009). The application of these methods to parasites has illuminated the important roles of palmitoyl proteins in parasite biology and disease pathogenesis (Emmer et al., 2011, Jones et al., 2012a, Foe et al., 2015, Santiago-Tirado et al., 2015). Palmitoylation has a general function of anchoring proteins to membranes, targeting them to the correct subcellular compartments, regulating enzyme activity, and mediating protein stability (Rocks et al., 2010, Salaun et al., 2010, Shipston, 2011). However, in parasites this modification has added significance, since the endomembrane networks are dynamically remodeled in response to changing environmental conditions and life cycle transitions; some changes are mediated by palmitoyl proteins (Field and Carrington, 2009, Faso and Hehl, 2011, Harding and Meissner, 2014). Reversible, or dynamic, palmitoylation provides an additional mechanism for the post-translational regulation of membrane proteins in parasites (Doerig et al., 2015).
Acylation is a promising drug target in apicomplexans and trypanosomatids and there has been significant success employing inhibitors of protein myristoylation (Price et al., 2003, Frearson et al., 2010, Emmer et al., 2011, Wright et al., 2014, Wright et al., 2015). Opportunities exist for the specific targeting of individual parasite PATs for chemotherapy and libraries of small molecular inhibitors of palmitoylation have been generated (Ducker et al., 2006, Jennings et al., 2009). Given that PAT families are large in parasites, their sequences are highly divergent, and palmitoylation is known to be essential, palmitoylation is an attractive target for the development of novel trypanocidal agents with high parasite selectivity and low human toxicity (Emmer et al., 2011, Alonso et al., 2012, Jones et al., 2012a).
In this review, we highlighted five examples by which S-palmitoylation modulates virulence, including: (i) correct tethering of secretory organelles required for host cell invasion by palmitoylation in T. gondii, (ii) dependency of cell motility on palmitoylation for host cell invasion in malaria parasites, (iii) the biogenesis of a putative storage structure/organelle required for differentiation into infective life cycle stages in malaria parasites, (iv) targeting of a virulence factor to a motility organelle in trypanosomes and (v) the anchoring of a variant surface protein for antigenic variation in G. lamblia. The identification of the PAT responsible for modifying each of these virulence factors has helped to illuminate its function, and it will be important to identify the PAT involved in the biogenesis of additional palmitoylated virulence factors mentioned above, such as TbGPI-PLC, TbMCA4, TcPI-PLC, LmjHASPB, and TgCDPK3 (Webb et al., 1997, McKean et al., 2001, Okura et al., 2005, Proto et al., 2011, McCoy et al., 2012). Importantly, there are also numerous palmitoylated proteins essential for viability but not virulence per se, such as TbCAP5.5 which is required for cytoskeleton assembly and cell morphogenesis in procyclic form trypanosomes (Olego-Fernandez et al., 2009). Similarly, in the fungal pathogen Cryptococcus neoformans, pathogenesis is also dependent on a palmitoyl acyltransferase (PFA4) activity, required for normal cell wall morphology and virulence (Santiago-Tirado et al., 2015). Clearly, palmitoylation serves many important targeting and regulatory roles in parasites.
With this insight, we have completed a comprehensive phylogenetic analysis of the PAT family in kinetoplastids, and found T. cruzi and Leishmania species have several novel family members (manuscript in preparation). In contrast, the study of depalmitoylation in parasites by two different classes of enzymes the acyl-protein thioesterases (APTs) and palmitoyl-protein thioesterases (PPTs) still lags the PATs (Duncan and Gilman, 1998, Das et al., 2000). Interestingly, increasing palmitoylation in T. gondii by the inhibition of a PPT protein enhances parasite invasion in vitro, an unexpected finding if one assumes that the equilibrium between palmitoylation and depalmitoylation states is optimal (Child et al., 2013). Our preliminary results on a T. brucei APT are similar (manuscript in preparation).
Several unresolved questions need to be explored experimentally to integrate these advances into a broadly applicable model to understand palmitoylation in parasite biology and pathogenesis. What are the features of the PAT active sites or their domain architecture which mediate substrate specificity? What are the recognition sequences on substrates which determine PAT specificity? Are interactions with other proteins needed for PAT recognition of substrates? There is precedent for this from yeast, in which Erf4 stabilizes the PAT Erf2 to palmitoylate Ras (Mitchell et al., 2012). What features other than acylation serve to specifically traffic palmitoylated substrates to their target membranes? For instance, dual acylation of T. cruzi PI-PLC targets the protein to the extracellular face of the plasma membrane, whereas dual acylation of T. cruzi FCaBP or T. brucei calflagin targets these proteins to the inner aspect of the flagellar membrane (Emmer et al., 2009, de Paulo Martins et al., 2010, Maric et al., 2011). Are all of the answers to these questions applicable across different parasites or eukaryotes in general, or are there different rules for different organisms?
Finally, there are examples in bacteria of secreted virulence factors that are palmitoylated by the host cell, such as effector proteins in Salmonella enterica and Legionella pneumophila, which are targeted to the plasma membrane and Golgi where they mediate their effects (Hicks et al., 2011, Lin et al., 2015, Schroeder et al., 2015). Palmitoylation is required for the cell surface expression and sensitivity of the receptor which binds Bacillus anthracis anthrax toxin (Abrami et al., 2006). Palmitoylation is also necessary for the viral lytic cycle, particularly during the entry of and budding from host cells (Blanc et al., 2013). It will be important to test whether secreted proteins from other intracellular pathogens, including protozoan parasites, might be palmitoylated by the host and constitute yet another type of virulence factor generated by the palmitoylation process.
Acknowledgments
We wish to thank Ben Falk for suggestions on the manuscript and Cheryl Olson and Conrad Epting for helpful discussions.
This work was supported in part by NIH grant GM102689 (DME) and AHA predoctoral fellowship 16PRE26400009 (AIS). The paper was written by us.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- Abrami L, Leppla SH, Van Der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–20. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–75. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicart-Ramos C, Valero RA, Rodriguez-Crespo I. Protein palmitoylation and subcellular trafficking. Biochim Biophys Acta. 2011;1808:2981–94. doi: 10.1016/j.bbamem.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Albisetti A, Wiese S, Schneider A, Niemann M. A component of the mitochondrial outer membrane proteome of T. brucei probably contains covalent bound fatty acids. Exp Parasitol. 2015;155:49–57. doi: 10.1016/j.exppara.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Alonso AM, Coceres VM, De Napoli MG, Nieto Guil AF, Angel SO, Corvi MM. Protein palmitoylation inhibition by 2-bromopalmitate alters gliding, host cell invasion and parasite morphology in Toxoplasma gondii. Mol Biochem Parasitol. 2012;184:39–43. doi: 10.1016/j.molbiopara.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N, De Koning HP, Maser P, Horn D. Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol. 2013;29:110–8. doi: 10.1016/j.pt.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J, Gilberger TW, Frischknecht F, Meissner M. Host-cell invasion by malaria parasites: insights from Plasmodium and Toxoplasma. Trends Parasitol. 2008;24:557–63. doi: 10.1016/j.pt.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Beck JR, Fung C, Straub KW, Coppens I, Vashisht AA, Wohlschlegel JA, Bradley PJ. A Toxoplasma palmitoyl acyl transferase and the palmitoylated armadillo repeat protein TgARO govern apical rhoptry tethering and reveal a critical role for the rhoptries in host cell invasion but not egress. PLoS Pathog. 2013;9:e1003162. doi: 10.1371/journal.ppat.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JR, Rodriguez-Fernandez IA, De Leon JC, Huynh MH, Carruthers VB, Morrissette NS, Bradley PJ. A novel family of Toxoplasma IMC proteins displays a hierarchical organization and functions in coordinating parasite division. PLoS Pathog. 2010;6:e1001094. doi: 10.1371/journal.ppat.1001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–23. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc M, Blaskovic S, Van Der Goot FG. Palmitoylation, pathogens and their host. Biochem Soc Trans. 2013;41:84–8. doi: 10.1042/BST20120337. [DOI] [PubMed] [Google Scholar]
- Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J Biol Chem. 2008;283:22076–88. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera A, Herrmann S, Warszta D, Santos JM, John Peter AT, Kono M, Debrouver S, Jacobs T, Spielmann T, Ungermann C, Soldati-Favre D, Gilberger TW. Dissection of minimal sequence requirements for rhoptry membrane targeting in the malaria parasite. Traffic. 2012;13:1335–50. doi: 10.1111/j.1600-0854.2012.01394.x. [DOI] [PubMed] [Google Scholar]
- Carter V, Shimizu S, Arai M, Dessens JT. PbSR is synthesized in macrogametocytes and involved in formation of the malaria crystalloids. Molecular Microbiology. 2008;68:1560–1569. doi: 10.1111/j.1365-2958.2008.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AL, Kim EW, Toh JY, Vashisht AA, Rashoff AQ, Van C, Huang AS, Moon AS, Bell HN, Bentolila LA, Wohlschlegel JA, Bradley PJ. Novel components of the Toxoplasma inner membrane complex revealed by BioID. MBio. 2015;6:e02357–14. doi: 10.1128/mBio.02357-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child MA, Hall CI, Beck JR, Ofori LO, Albrow VE, Garland M, Bowyer PW, Bradley PJ, Powers JC, Boothroyd JC, Weerapana E, Bogyo M. Small-molecule inhibition of a depalmitoylase enhances Toxoplasma host-cell invasion. Nat Chem Biol. 2013;9:651–6. doi: 10.1038/nchembio.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AK, Bellizzi JJ, 3rd, Tandel S, Biehl E, Clardy J, Hofmann SL. Structural basis for the insensitivity of a serine enzyme (palmitoyl-protein thioesterase) to phenylmethylsulfonyl fluoride. J Biol Chem. 2000;275:23847–51. doi: 10.1074/jbc.M002758200. [DOI] [PubMed] [Google Scholar]
- Davda D, El Azzouny MA, Tom CT, Hernandez JL, Majmudar JD, Kennedy RT, Martin BR. Profiling targets of the irreversible palmitoylation inhibitor 2-bromopalmitate. ACS Chem Biol. 2013;8:1912–7. doi: 10.1021/cb400380s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SC, House SA. Life with eight flagella: flagellar assembly and division in Giardia. Curr Opin Microbiol. 2010;13:480–90. doi: 10.1016/j.mib.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Napoli MG, De Miguel N, Lebrun M, Moreno SN, Angel SO, Corvi MM. N-terminal palmitoylation is required for Toxoplasma gondii HSP20 inner membrane complex localization. Biochim Biophys Acta. 2013;1833:1329–37. doi: 10.1016/j.bbamcr.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paulo Martins V, Okura M, Maric D, Engman DM, Vieira M, Docampo R, Moreno SN. Acylation-dependent export of Trypanosoma cruzi phosphoinositide-specific phospholipase C to the outer surface of amastigotes. J Biol Chem. 2010;285:30906–30917. doi: 10.1074/jbc.M110.142190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny PW, Gokool S, Russell DG, Field MC, Smith DF. Acylation-dependent protein export in Leishmania. J. Biol. Chem. 2000;275:11017–11025. doi: 10.1074/jbc.275.15.11017. [DOI] [PubMed] [Google Scholar]
- Dessens JT, Saeed S, Tremp AZ, Carter V. Malaria crystalloids: specialized structures for parasite transmission? Trends Parasitol. 2011;27:106–10. doi: 10.1016/j.pt.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerig C, Rayner JC, Scherf A, Tobin AB. Post-translational protein modifications in malaria parasites. Nat Rev Microbiol. 2015;13:160–72. doi: 10.1038/nrmicro3402. [DOI] [PubMed] [Google Scholar]
- Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol. 2009;39:877–82. doi: 10.1016/j.ijpara.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Ducker CE, Griffel LK, Smith RA, Keller SN, Zhuang Y, Xia Z, Diller JD, Smith CD. Discovery and characterization of inhibitors of human palmitoyl acyltransferases. Mol Cancer Ther. 2006;5:1647–59. [Google Scholar]
- Ducker CE, Stettler EM, French KJ, Upson JJ, Smith CD. Huntingtin interacting protein 14 is an oncogenic human protein: palmitoyl acyltransferase. Oncogene. 2004;23:9230–7. doi: 10.1038/sj.onc.1208171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JA, Gilman AG. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS) J Biol Chem. 1998;273:15830–7. doi: 10.1074/jbc.273.25.15830. [DOI] [PubMed] [Google Scholar]
- Duncan JA, Gilman AG. Characterization of Saccharomyces cerevisiae acyl-protein thioesterase 1, the enzyme responsible for G protein alpha subunit deacylation in vivo. J Biol Chem. 2002;277:31740–52. doi: 10.1074/jbc.M202505200. [DOI] [PubMed] [Google Scholar]
- Emmer BT, Daniels MD, Taylor JM, Epting CL, Engman DM. Calflagin inhibition prolongs host survival and suppresses parasitemia in Trypanosoma brucei infection. Eukaryot Cell. 2010a;9:934–42. doi: 10.1128/EC.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J. Cell Sci. 2010b;123:529–36. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Nakayasu ES, Souther C, Choi H, Sobreira TJ, Epting CL, Nesvizhskii AI, Almeida IC, Engman DM. Global analysis of protein palmitoylation in African trypanosomes. Eukaryot Cell. 2011;10:455–63. doi: 10.1128/EC.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Souther C, Toriello KM, Olson CL, Epting CL, Engman DM. Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J. Cell Sci. 2009;122:867–74. doi: 10.1242/jcs.041764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engman DM, Krause K-H, Blumin JH, Kim KS, Kirchhoff LV, Donelson JE. A novel flagellar Ca2+-binding protein in trypanosomes. J Biol Chem. 1989;264:18627–18631. [PubMed] [Google Scholar]
- Ezougou CN, Ben-Rached F, Moss DK, Lin JW, Black S, Knuepfer E, Green JL, Khan SM, Mukhopadhyay A, Janse CJ, Coppens I, Yera H, Holder AA, Langsley G. Plasmodium falciparum Rab5B is an N-terminally myristoylated Rab GTPase that is targeted to the parasite’s plasma and food vacuole membranes. PLoS One. 2014;9:e87695. doi: 10.1371/journal.pone.0087695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faso C, Hehl AB. Membrane trafficking and organelle biogenesis in Giardia lamblia: use it or lose it. Int J Parasitol. 2011;41:471–80. doi: 10.1016/j.ijpara.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Field MC, Carrington M. The trypanosome flagellar pocket. Nat Rev Microbiol. 2009;7:775–86. doi: 10.1038/nrmicro2221. [DOI] [PubMed] [Google Scholar]
- Foe IT, Child MA, Majmudar JD, Krishnamurthy S, Van Der Linden WA, Ward GE, Martin BR, Bogyo M. Global Analysis of Palmitoylated Proteins in Toxoplasma gondii. Cell Host Microbe. 2015;18:501–11. doi: 10.1016/j.chom.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson JA, Brand S, Mcelroy SP, Cleghorn LA, Smid O, Stojanovski L, Price HP, Guther ML, Torrie LS, Robinson DA, Hallyburton I, Mpamhanga CP, Brannigan JA, Wilkinson AJ, Hodgkinson M, Hui R, Qiu W, Raimi OG, Van Aalten DM, Brenk R, Gilbert IH, Read KD, Fairlamb AH, Ferguson MA, Smith DF, Wyatt PG. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature. 2010;464:728–32. doi: 10.1038/nature08893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenal K, Kemp LE, Soldati-Favre D. Emerging roles for protein S-palmitoylation in Toxoplasma biology. Int J Parasitol. 2014;44:121–31. doi: 10.1016/j.ijpara.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Frenal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D. Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe. 2010;8:343–57. doi: 10.1016/j.chom.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Frenal K, Tay CL, Mueller C, Bushell ES, Jia Y, Graindorge A, Billker O, Rayner JC, Soldati-Favre D. Global analysis of apicomplexan protein S-acyl transferases reveals an enzyme essential for invasion. Traffic. 2013;14:895–911. doi: 10.1111/tra.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung C, Beck JR, Robertson SD, Gubbels MJ, Bradley PJ. Toxoplasma ISP4 is a central IMC sub-compartment protein whose localization depends on palmitoylation but not myristoylation. Mol Biochem Parasitol. 2012;184:99–108. doi: 10.1016/j.molbiopara.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsel LM, Engman DM. Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J. 1999;18:2057–65. doi: 10.1093/emboj/18.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldston AM, Sharma AI, Paul KS, Engman DM. Acylation in trypanosomatids: an essential process and potential drug target. Trends Parasitol. 2014;30:350–360. doi: 10.1016/j.pt.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves J, Chamberlain LH. DHHC palmitoyl transferases: substrate interactions and (patho)physiology. Trends Biochem Sci. 2011;36:245–53. doi: 10.1016/j.tibs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Harding CR, Meissner M. The inner membrane complex through development of Toxoplasma gondii and Plasmodium. Cell Microbiol. 2014;16:632–41. doi: 10.1111/cmi.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera LJ, Brand S, Santos A, Nohara LL, Harrison J, Norcross NR, Thompson S, Smith V, Lema C, Varela-Ramirez A, Gilbert IH, Almeida IC, Maldonado RA. Validation of N-myristoyltransferase as Potential Chemotherapeutic Target in Mammal-Dwelling Stages of Trypanosoma cruzi. PLoS Negl Trop Dis. 2016;10:e0004540. doi: 10.1371/journal.pntd.0004540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Fowler C, Ersfeld K, Gull K. CAP5.5, a life-cycle-regulated, cytoskeleton-associated protein is a member of a novel family of calpain-related proteins in Trypanosoma brucei. Mol. Biochem. Parasit. 2001;116:25–34. doi: 10.1016/s0166-6851(01)00296-1. [DOI] [PubMed] [Google Scholar]
- Hicks SW, Charron G, Hang HC, Galan JE. Subcellular targeting of Salmonella virulence proteins by host-mediated S-palmitoylation. Cell Host Microbe. 2011;10:9–20. doi: 10.1016/j.chom.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltpold A, Frey M, Hulsmeier A, Kohler P. Glycosylation and palmitoylation are common modifications of Giardia variant surface proteins. Mol Biochem Parasitol. 2000;109:61–5. doi: 10.1016/s0166-6851(00)00229-2. [DOI] [PubMed] [Google Scholar]
- Hodson N, Invergo B, Rayner JC, Choudhary JS. Palmitoylation and palmitoyl-transferases in Plasmodium parasites. Biochem Soc Trans. 2015;43:240–5. doi: 10.1042/BST20140289. [DOI] [PubMed] [Google Scholar]
- Hopp CS, Balaban AE, Bushell E, Billker O, Rayner JC, Sinnis P. Palmitoyl Transferases have Critical Roles in the Development of Mosquito and Liver Stages of Plasmodium. Cell Microbiol. 2016 doi: 10.1111/cmi.12601. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Pecoul B, Rijal S, Boehme C, Aksoy S, Malecela M, Tapia-Conyer R, Reeder JC. Eliminating the Neglected Tropical Diseases: Translational Science and New Technologies. PLoS Negl Trop Dis. 2016;10:e0003895. doi: 10.1371/journal.pntd.0003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings BC, Nadolski MJ, Ling Y, Baker MB, Harrison ML, Deschenes RJ, Linder ME. 2-Bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo[b]thiophen-3-one inhibit DHHC-mediated palmitoylation in vitro. J Lipid Res. 2009;50:233–42. doi: 10.1194/jlr.M800270-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML, Collins MO, Goulding D, Choudhary JS, Rayner JC. Analysis of protein palmitoylation reveals a pervasive role in Plasmodium development and pathogenesis. Cell Host Microbe. 2012a;12:246–58. doi: 10.1016/j.chom.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML, Tay CL, Rayner JC. Getting stuck in: protein palmitoylation in Plasmodium. Trends Parasitol. 2012b;28:496–503. doi: 10.1016/j.pt.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Kemp LE, Rusch M, Adibekian A, Bullen HE, Graindorge A, Freymond C, Rottmann M, Braun-Breton C, Baumeister S, Porfetye AT, Vetter IR, Hedberg C, Soldati-Favre D. Characterization of a serine hydrolase targeted by acyl-protein thioesterase inhibitors in Toxoplasma gondii. J Biol Chem. 2013;288:27002–18. doi: 10.1074/jbc.M113.460709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander N, Li Z-H, Niyogi S, Docampo R. CRISPR/Cas9-induced disruption of paraflagellar rod proteins 1 and 2 genes in Trypanosoma cruzi reveals their role in flagellar attachment. mBio. 2015;6:e01012–01015. doi: 10.1128/mBio.01012-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre M, Tetaud E, Thonnus M, Salin B, Boissier F, Blancard C, Sauvanet C, Metzler C, Espiau B, Sahin A, Merlin G. LdFlabarin, a new BAR domain membrane protein of Leishmania flagellum. PLoS One. 2013;8:e76380. doi: 10.1371/journal.pone.0076380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Nazarian A, Erdjument-Bromage H, Bornmann W, Tempst P, Resh MD. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J Biol Chem. 2001;276:30987–94. doi: 10.1074/jbc.M104018200. [DOI] [PubMed] [Google Scholar]
- Lin YH, Doms AG, Cheng E, Kim B, Evans TR, Machner MP. Host Cell-catalyzed S-Palmitoylation Mediates Golgi Targeting of the Legionella Ubiquitin Ligase GobX. J Biol Chem. 2015;290:25766–81. doi: 10.1074/jbc.M115.637397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- Linder ME, Jennings BC. Mechanism and function of DHHC S-acyltransferases. Biochem Soc Trans. 2013;41:29–34. doi: 10.1042/BST20120328. [DOI] [PubMed] [Google Scholar]
- Liu W, Apagyi K, Mcleavy L, Ersfeld K. Expression and cellular localisation of calpain-like proteins in Trypanosoma brucei. Mol Biochem Parasitol. 2010;169:20–6. doi: 10.1016/j.molbiopara.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Lobo S, Greentree WK, Linder ME, Deschenes RJ. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2002;277:41268–73. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- Maclean LM, O’toole PJ, Stark M, Marrison J, Seelenmeyer C, Nickel W, Smith DF. Trafficking and release of Leishmania metacyclic HASPB on macrophage invasion. Cell Microbiol. 2012;14:740–61. doi: 10.1111/j.1462-5822.2012.01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, Khan SM, Dimopoulos G, Janse CJ, Waters AP. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667–9. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RA, Mirzoeva S, Godsel LM, Lukas TJ, Goldenberg S, Watterson DM, Engman DM. Identification of calcium binding sites in the trypanosome flagellar calcium acyl switch protein. Mol Biochem Parasitol. 1999;101:61–70. doi: 10.1016/s0166-6851(99)00055-9. [DOI] [PubMed] [Google Scholar]
- Maric D, Mcgwire BS, Buchanan KT, Olson CL, Emmer BT, Epting CL, Engman DM. Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein. J. Biol. Chem. 2011;286:33109–17. doi: 10.1074/jbc.M111.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Cravatt BF. Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods. 2009;6:135–8. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Wang C, Adibekian A, Tully SE, Cravatt BF. Global profiling of dynamic protein palmitoylation. Nat Methods. 2012;9:84–9. doi: 10.1038/nmeth.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccoy JM, Whitehead L, Van Dooren GG, Tonkin CJ. TgCDPK3 regulates calcium-dependent egress of Toxoplasma gondii from host cells. PLoS Pathog. 2012;8:e1003066. doi: 10.1371/journal.ppat.1003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgwire BS, Satoskar AR. Leishmaniasis: clinical syndromes and treatment. QJM. 2014;107:7–14. doi: 10.1093/qjmed/hct116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckean PG, Denny PW, Knuepfer E, Keen JK, Smith DF. Phenotypic changes associated with deletion and overexpression of a stage-regulated gene family in Leishmania. Cell Microbiol. 2001;3:511–23. doi: 10.1046/j.1462-5822.2001.00135.x. [DOI] [PubMed] [Google Scholar]
- Merino MC, Zamponi N, Vranych CV, Touz MC, Ropolo AS. Identification of Giardia lamblia DHHC proteins and the role of protein S-palmitoylation in the encystation process. PLoS Negl Trop Dis. 2014;8:e2997. doi: 10.1371/journal.pntd.0002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E, Price HP, Johner A, Emerson JE, Smith DF. Kinetoplastid PPEF phosphatases: dual acylated proteins expressed in the endomembrane system of Leishmania. Mol Biochem Parasitol. 2007;152:22–34. doi: 10.1016/j.molbiopara.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Hamel LD, Ishizuka K, Mitchell G, Schaefer LM, Deschenes RJ. The Erf4 subunit of the yeast Ras palmitoyl acyltransferase is required for stability of the Acyl-Erf2 intermediate and palmitoyl transfer to a Ras2 substrate. J Biol Chem. 2012;287:34337–48. doi: 10.1074/jbc.M112.379297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res. 2006;47:1118–27. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- Morrison HG, Mcarthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, Best AA, Cande WZ, Chen F, Cipriano MJ, Davids BJ, Dawson SC, Elmendorf HG, Hehl AB, Holder ME, Huse SM, Kim UU, Lasek-Nesselquist E, Manning G, Nigam A, Nixon JE, Palm D, Passamaneck NE, Prabhu A, Reich CI, Reiner DS, Samuelson J, Svard SG, Sogin ML. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–6. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- Moskes C, Burghaus PA, Wernli B, Sauder U, Durrenberger M, Kappes B. Export of Plasmodium falciparum calcium-dependent protein kinase 1 to the parasitophorous vacuole is dependent on three N-terminal membrane anchor motifs. Molecular Microbiology. 2004;54:676–91. doi: 10.1111/j.1365-2958.2004.04313.x. [DOI] [PubMed] [Google Scholar]
- Mueller C, Klages N, Jacot D, Santos JM, Cabrera A, Gilberger TW, Dubremetz JF, Soldati-Favre D. The Toxoplasma protein ARO mediates the apical positioning of rhoptry organelles, a prerequisite for host cell invasion. Cell Host Microbe. 2013;13:289–301. doi: 10.1016/j.chom.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Mueller C, Samoo A, Hammoudi PM, Klages N, Kallio JP, Kursula I, Soldati-Favre D. Structural and functional dissection of Toxoplasma gondii armadillo repeats only protein. J Cell Sci. 2016;129:1031–45. doi: 10.1242/jcs.177386. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, Dansereau EA, Graetz N, Barber RM, Brown JC, Wang H, Duber HC, Naghavi M, Dicker D, Dandona L, Salomon JA, Heuton KR, Foreman K, Phillips DE, Fleming TD, Flaxman AD, Phillips BK, Johnson EK, Coggeshall MS, Abd-Allah F, Abera SF, Abraham JP, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Achoki T, Adeyemo AO, Adou AK, Adsuar JC, Agardh EE, Akena D, Al Kahbouri MJ, Alasfoor D, Albittar MI, Alcala-Cerra G, Alegretti MA, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allen PJ, Alsharif U, Alvarez E, Alvis-Guzman N, Amankwaa AA, Amare AT, Amini H, Ammar W, Anderson BO, Antonio CA, Anwari P, Arnlov J, Arsenijevic VS, Artaman A, Asghar RJ, Assadi R, Atkins LS, Badawi A, Balakrishnan K, Banerjee A, Basu S, Beardsley J, Bekele T, Bell ML, Bernabe E, Beyene TJ, Bhala N, Bhalla A, Bhutta ZA, Abdulhak AB, Binagwaho A, Blore JD, Basara BB, Bose D, Brainin M, Breitborde N, Castaneda-Orjuela CA, Catala-Lopez F, Chadha VK, Chang JC, Chiang PP, Chuang TW, Colomar M, Cooper LT, Cooper C, Courville KJ, Cowie BC, Criqui MH, Dandona R, Dayama A, De Leo D, Degenhardt L, Del Pozo-Cruz B, Deribe K, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–70. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadolski MJ, Linder ME. Protein lipidation. FEBS J. 2007;274:5202–10. doi: 10.1111/j.1742-4658.2007.06056.x. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 2006;1761:474–83. doi: 10.1016/j.bbalip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Okura M, Fang J, Salto ML, Singer RS, Docampo R, Moreno SN. A lipid-modified phosphoinositide-specific phospholipase C (TcPI-PLC) is involved in differentiation of trypomastigotes to amastigotes of Trypanosoma cruzi. J. Biol. Chem. 2005;280:16235–43. doi: 10.1074/jbc.M414535200. [DOI] [PubMed] [Google Scholar]
- Olego-Fernandez S, Vaughan S, Shaw MK, Gull K, Ginger ML. Cell morphogenesis of Trypanosoma brucei requires the paralogous, differentially expressed calpain-related proteins CAP5.5 and CAP5.5V. Protist. 2009;160:576–90. doi: 10.1016/j.protis.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Peng D, Kurup SP, Yao PY, Minning TA, Tarleton RL. CRISPR-Cas9-mediated single-gene and gene family disruption in Trypanosoma cruzi. mBio. 2014;6:e02097–14. doi: 10.1128/mBio.02097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin B, Patzewitz EM, Brady D, Silvie O, Wright MH, Ferguson DJ, Wall RJ, Whipple S, Guttery DS, Tate EW, Wickstead B, Holder AA, Tewari R. Unique apicomplexan IMC sub-compartment proteins are early markers for apical polarity in the malaria parasite. Biol Open. 2013;2:1160–70. doi: 10.1242/bio.20136163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price HP, Menon MR, Panethymitaki C, Goulding D, Mckean PG, Smith DF. Myristoyl-CoA:protein N-myristoyltransferase, an essential enzyme and potential drug target in Kinetoplastid parasites. J. Biol. Chem. 2003;278:7206–7214. doi: 10.1074/jbc.M211391200. [DOI] [PubMed] [Google Scholar]
- Proto WR, Castanys-Munoz E, Black A, Tetley L, Moss CX, Juliano L, Coombs GH, Mottram JC. Trypanosoma brucei metacaspase 4 is a pseudopeptidase and a virulence factor. J Biol Chem. 2011;286:39914–25. doi: 10.1074/jbc.M111.292334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prucca CG, Slavin I, Quiroga R, Elias EV, Rivero FD, Saura A, Carranza PG, Lujan HD. Antigenic variation in Giardia lamblia is regulated by RNA interference. Nature. 2008;456:750–4. doi: 10.1038/nature07585. [DOI] [PubMed] [Google Scholar]
- Putilina T, Wong P, Gentleman S. The DHHC domain: a new highly conserved cysteine-rich motif. Mol Cell Biochem. 1999;195:219–26. doi: 10.1023/a:1006932522197. [DOI] [PubMed] [Google Scholar]
- Rees-Channer RR, Martin SR, Green JL, Bowyer PW, Grainger M, Molloy JE, Holder AA. Dual acylation of the 45 kDa gliding-associated protein (GAP45) in Plasmodium falciparum merozoites. Mol Biochem Parasitol. 2006;149:113–6. doi: 10.1016/j.molbiopara.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein Eng Des Sel. 2008;21:639–44. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LJ, Devleesschauwer B, De Noya BA, Gonzalez ON, Torgerson PR. Trypanosoma cruzi: Time for International Recognition as a Foodborne Parasite. Plos Neglected Tropical Diseases. 2016;10:e0004656. doi: 10.1371/journal.pntd.0004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocks O, Gerauer M, Vartak N, Koch S, Huang ZP, Pechlivanis M, Kuhlmann J, Brunsveld L, Chandra A, Ellinger B, Waldmann H, Bastiaens PI. The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell. 2010;141:458–71. doi: 10.1016/j.cell.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PI. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–52. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- Roth AF, Feng Y, Chen L, Davis NG. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol. 2002;159:23–8. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR, 3rd, Davis NG. Global analysis of protein palmitoylation in yeast. Cell. 2006;125:1003–13. doi: 10.1016/j.cell.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I, Oksman A, Goldberg DE. Fatty acid acylation regulates trafficking of the unusual Plasmodium falciparum calpain to the nucleolus. Mol Microbiol. 2009;72:229–45. doi: 10.1111/j.1365-2958.2009.06639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed S, Carter V, Tremp AZ, Dessens JT. Translational repression controls temporal expression of the Plasmodium berghei LCCL protein complex. Mol Biochem Parasitol. 2013;189:38–42. doi: 10.1016/j.molbiopara.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed S, Tremp AZ, Dessens JT. Biogenesis of the crystalloid organelle in Plasmodium involves microtubule-dependent vesicle transport and assembly. Int J Parasitol. 2015;45:537–47. doi: 10.1016/j.ijpara.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun C, Greaves J, Chamberlain LH. The intracellular dynamic of protein palmitoylation. J Cell Biol. 2010;191:1229–38. doi: 10.1083/jcb.201008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SS, Hayden MR. Aberrant palmitoylation in Huntington disease. Biochem Soc Trans. 2015;43:205–10. doi: 10.1042/BST20140242. [DOI] [PubMed] [Google Scholar]
- Santiago-Tirado FH, Peng T, Yang M, Hang HC, Doering TL. A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome. PLoS Pathog. 2015;11:e1004908. doi: 10.1371/journal.ppat.1004908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JM, Duarte N, Kehrer J, Ramesar J, Avramut MC, Koster AJ, Dessens JT, Frischknecht F, Chevalley-Maurel S, Janse CJ, Franke-Fayard B, Mair GR. Maternally supplied S-acyl-transferase is required for crystalloid organelle formation and transmission of the malaria parasite. Proc Natl Acad Sci U S A. 2016;113:7183–8. doi: 10.1073/pnas.1522381113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JM, Kehrer J, Franke-Fayard B, Frischknecht F, Janse CJ, Mair GR. The Plasmodium palmitoyl-S-acyl-transferase DHHC2 is essential for ookinete morphogenesis and malaria transmission. Sci Rep. 2015;5:16034. doi: 10.1038/srep16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saric M, Vahrmann A, Niebur D, Kluempers V, Hehl AB, Scholze H. Dual acylation accounts for the localization of {alpha}19-giardin in the ventral flagellum pair of Giardia lamblia. Eukaryot Cell. 2009;8:1567–74. doi: 10.1128/EC.00136-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder GN, Aurass P, Oates CV, Tate EW, Hartland EL, Flieger A, Frankel G. Legionella pneumophila Effector LpdA Is a Palmitoylated Phospholipase D Virulence Factor. Infect Immun. 2015;83:3989–4002. doi: 10.1128/IAI.00785-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydel KB, Gaur D, Aravind L, Subramanian G, Miller LH. Plasmodium falciparum: characterization of a late asexual stage golgi protein containing both ankyrin and DHHC domains. Exp Parasitol. 2005;110:389–93. doi: 10.1016/j.exppara.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Shipston MJ. Ion channel regulation by protein palmitoylation. J Biol Chem. 2011;286:8709–16. doi: 10.1074/jbc.R110.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Striepen B. Parasitic infections: Time to tackle cryptosporidiosis. Nature. 2013;503:189–91. doi: 10.1038/503189a. [DOI] [PubMed] [Google Scholar]
- Stuart K, Brun R, Croft S, Fairlamb A, Gurtler RE, Mckerrow J, Reed S, Tarleton R. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 2008;118:1301–10. doi: 10.1172/JCI33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunter J, Webb H, Carrington M. Determinants of GPI-PLC localisation to the flagellum and access to GPI-anchored substrates in trypanosomes. PLoS Pathog. 2013;9:e1003566. doi: 10.1371/journal.ppat.1003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay CL, Jones ML, Hodson N, Theron M, Choudhary JS, Rayner JC. Study of Plasmodium falciparum DHHC palmitoyl-transferases identifies a role for PfDHHC9 in gametocytogenesis. Cell Microbiol. 2016 doi: 10.1111/cmi.12599. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetaud E, Lefebvre M, M’bang-Benet DE, Crobu L, Blancard C, Sterkers Y, Pages M, Bastien P, Merlin G. TbFlabarin, a flagellar protein of Trypanosoma brucei, highlights differences between Leishmania and Trypanosoma flagellar-targeting signals. Exp Parasitol. 2016;166:97–107. doi: 10.1016/j.exppara.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Thavayogarajah T, Gangopadhyay P, Rahlfs S, Becker K, Lingelbach K, Przyborski JM, Holder AA. Alternative Protein Secretion in the Malaria Parasite Plasmodium falciparum. PLoS One. 2015;10:e0125191. doi: 10.1371/journal.pone.0125191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touz MC, Conrad JT, Nash TE. A novel palmitoyl acyl transferase controls surface protein palmitoylation and cytotoxicity in Giardia lamblia. Mol Microbiol. 2005;58:999–1011. doi: 10.1111/j.1365-2958.2005.04891.x. [DOI] [PubMed] [Google Scholar]
- Tovar J, Leon-Avila G, Sanchez LB, Sutak R, Tachezy J, Van Der Giezen M, Hernandez M, Muller M, Lucocq JM. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature. 2003;426:172–6. doi: 10.1038/nature01945. [DOI] [PubMed] [Google Scholar]
- Tull D, Vince JE, Callaghan JM, Naderer T, Spurck T, Mcfadden GI, Currie G, Ferguson K, Bacic A, Mcconville MJ. SMP-1, a member of a new family of small myristoylated proteins in kinetoplastid parasites, is targeted to the flagellum membrane in Leishmania. Mol. Biol. Cell. 2004;15:4775–4786. doi: 10.1091/mbc.E04-06-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler JS, Treeck M, Boothroyd JC. Focus on the ringleader: the role of AMA1 in apicomplexan invasion and replication. Trends Parasitol. 2011;27:410–20. doi: 10.1016/j.pt.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, Hazlett TL, Engman DM. Flagellar membrane localization via association with lipid rafts. J. Cell Sci. 2009;122:859–66. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S, Widmer G. A hundred-year retrospective on cryptosporidiosis. Trends Parasitol. 2008;24:184–9. doi: 10.1016/j.pt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: purification and identification. Nat. Protocols. 2007;2:1573–84. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- Wang M, Casey PJ. Protein prenylation: unique fats make their mark on biology. Nat Rev Mol Cell Biol. 2016;17:110–22. doi: 10.1038/nrm.2015.11. [DOI] [PubMed] [Google Scholar]
- Webb H, Carnall N, Vanhamme L, Rolin S, Van Den Abbeele J, Welburn S, Pays E, Carrington M. The GPI-phospholipase C of Trypanosoma brucei is nonessential but influences parasitemia in mice. J Cell Biol. 1997;139:103–14. doi: 10.1083/jcb.139.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn SC, Molyneux DH, Maudlin I. Beyond Tsetse--Implications for Research and Control of Human African Trypanosomiasis Epidemics. Trends Parasitol. 2016;32:230–41. doi: 10.1016/j.pt.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Wetzel J, Herrmann S, Swapna LS, Prusty D, John Peter AT, Kono M, Saini S, Nellimarla S, Wong TW, Wilcke L, Ramsay O, Cabrera A, Biller L, Heincke D, Mossman K, Spielmann T, Ungermann C, Parkinson J, Gilberger TW. The role of palmitoylation for protein recruitment to the inner membrane complex of the malaria parasite. J Biol Chem. 2015;290:1712–28. doi: 10.1074/jbc.M114.598094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard JN, Ladner J, Vanarotti M, Fisher AJ, Robinson H, Buchanan KT, Engman DM, Ames JB. Structural insights into membrane targeting by the flagellar calcium-binding protein (FCaBP), a myristoylated and palmitoylated calcium sensor in Trypanosoma cruzi. J. Biol. Chem. 2008;283:23388–96. doi: 10.1074/jbc.M803178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler EA. Malaria research in the post-genomic era. Nature. 2008;455:751–6. doi: 10.1038/nature07361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MH, Clough B, Rackham MD, Rangachari K, Brannigan JA, Grainger M, Moss DK, Bottrill AR, Heal WP, Broncel M, Serwa RA, Brady D, Mann DJ, Leatherbarrow RJ, Tewari R, Wilkinson AJ, Holder AA, Tate EW. Validation of N-myristoyltransferase as an antimalarial drug target using an integrated chemical biology approach. Nat Chem. 2014;6:112–21. doi: 10.1038/nchem.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MH, Paape D, Price HP, Smith DF, Tate EW. Global Profiling and Inhibition of Protein Lipidation in Vector and Host Stages of the Sleeping Sickness Parasite Trypanosoma brucei. ACS Infect Dis. 2016;2:427–441. doi: 10.1021/acsinfecdis.6b00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MH, Paape D, Storck EM, Serwa RA, Smith DF, Tate EW. Global analysis of protein N-myristoylation and exploration of N-myristoyltransferase as a drug target in the neglected human pathogen Leishmania donovani. Chem Biol. 2015;22:342–54. doi: 10.1016/j.chembiol.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, An M, Freeman MR, Yang W. Technologies and Challenges in Proteomic Analysis of Protein S-acylation. J Proteomics Bioinform. 2014;7:256–263. doi: 10.4172/jpb.1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]