ABSTRACT
Acetylene (C2H2) is a trace constituent of the present Earth's oxidizing atmosphere, reflecting a mixture of terrestrial and marine emissions from anthropogenic, biomass-burning, and unidentified biogenic sources. Fermentation of acetylene was serendipitously discovered during C2H2 block assays of N2O reductase, and Pelobacter acetylenicus was shown to grow on C2H2 via acetylene hydratase (AH). AH is a W-containing, catabolic, low-redox-potential enzyme that, unlike nitrogenase (N2ase), is specific for acetylene. Acetylene fermentation is a rare metabolic process that is well characterized only in P. acetylenicus DSM3246 and DSM3247 and Pelobacter sp. strain SFB93. To better understand the genetic controls for AH activity, we sequenced the genomes of the three acetylene-fermenting Pelobacter strains. Genome assembly and annotation produced three novel genomes containing gene sequences for AH, with two copies being present in SFB93. In addition, gene sequences for all five compulsory genes for iron-molybdenum N2ase were also present in the three genomes, indicating the cooccurrence of two acetylene transformation pathways. Nitrogen fixation growth assays showed that DSM3426 could ferment acetylene in the absence of ammonium, but no ethylene was produced. However, SFB93 degraded acetylene and, in the absence of ammonium, produced ethylene, indicating an active N2ase. Diazotrophic growth was observed under N2 but not in experimental controls incubated under argon. SFB93 exhibits acetylene fermentation and nitrogen fixation, the only known biochemical mechanisms for acetylene transformation. Our results indicate complex interactions between N2ase and AH and suggest novel evolutionary pathways for these relic enzymes from early Earth to modern days.
IMPORTANCE Here we show that a single Pelobacter strain can grow via acetylene fermentation and carry out nitrogen fixation, using the only two enzymes known to transform acetylene. These findings provide new insights into acetylene transformations and adaptations for nutrient (C and N) and energy acquisition by microorganisms. Enhanced understanding of acetylene transformations (i.e., extent, occurrence, and rates) in modern environments is important for the use of acetylene as a potential biomarker for extraterrestrial life and for degradation of anthropogenic contaminants.
KEYWORDS: acetylene hydratase, nitrogenase, acetylene fermentation, nitrogen fixation, Pelobacter, genomics, diazotrophy
INTRODUCTION
Acetylene (C2H2) is a trace constituent (∼20 to 40 parts per trillion [ppt]) of the present Earth's oxidizing atmosphere (1–3), reflecting a mixture of terrestrial and marine emissions from anthropogenic, biomass-burning, and unidentified biogenic sources (4–7). Acetylene in the putative anaerobic atmosphere of early Earth (8, 9) might have reached an abundance of 5 ppm (10, 11) due to its formation from bombardment of simple molecules (e.g., methane, ammonia, hydrogen sulfide, and phosphine) with high-energy photons. Such reactive atmospheric chemistry derived from simple constituents formed the basis of the complex prebiotic chemistry thought to have led to the origin of life (12–14). In the outer solar system, acetylene has been detected in the atmosphere of Titan (15, 16), where it participates in the formation of complex organics (17, 18). It has been proposed that a form of extraterrestrial life on Titan could be sustained energetically by hydrogen's reductive splitting of acetylene to form methane at ∼90 K (19). The presence of acetylene catabolism on Earth and the potential for it to support life in our solar system raise the need to understand the mechanisms by which acetylene is biologically processed.
On Earth there are two known biochemical mechanisms whereby acetylene is transformed, namely, the low-electrochemical-potential and oxygen-sensitive enzymes nitrogenase (N2ase) and acetylene hydratase (AH); these enzymes are not structurally related. The well-studied N2ase, which is possessed by an array of diazotrophic (nitrogen-fixing) prokaryotes, is promiscuous in the sense that it not only reduces dinitrogen to ammonia but also reduces other triple-bonded molecules, such as cyanide and acetylene. The latter forms the basis of the well-known bioassay in which acetylene serves as a proxy for dinitrogen and the resultant ethylene (C2H4) (Fig. 1A) represents the amount of ammonia produced (20). In contrast, the much less studied AH initiates exergonic hydration of acetylene to acetaldehyde in a reaction catalyzed by its tungsten pyranopterin cofactor (21). Acetaldehyde undergoes further dismutation to ethanol and acetate (22) (Fig. 1A). Unlike N2ase, the AH enzyme, encoded by the ahy gene, is specific for acetylene. Characterization of AH has focused on the acetylene-fermenting enzymes from the anaerobic bacterium Pelobacter acetylenicus (23, 24). However, aerobic acetylene-degrading bacteria have been reported to use acetylene as their sole carbon and energy source (25–29). The aerobic acetylene-metabolizing enzymes have not been fully characterized, but experiments by Rosner et al. showed that aerobic AHs are structurally distinct, because of their lack of cross-reactivity with antibodies raised to the AH of P. acetylenicus (25), indicating that this is a heterogeneous group of enzymes.
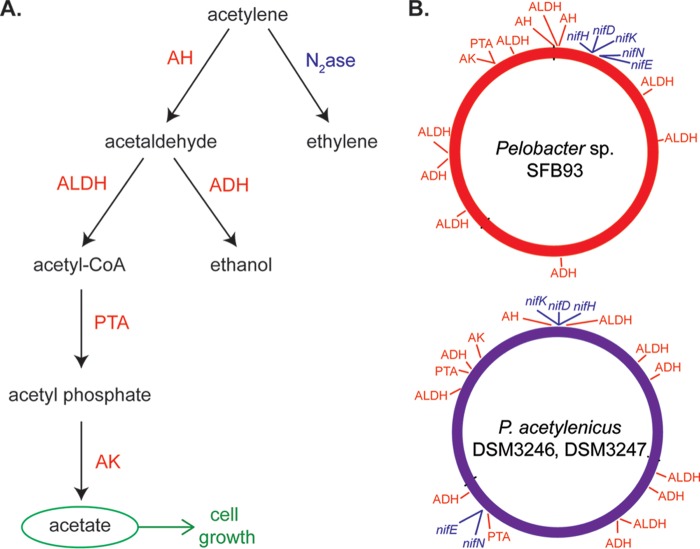
FIG 1.
(A) Pathways of acetylene transformation via acetylene fermentation (left process) and N2ase (right process). (B) BRIG plots showing the locations of genes in the acetylene fermentation pathway (red) and those encoding nitrogenase (blue) in the genomes of Pelobacter sp. SFB93 and P. acetylenicus DSM3246 and DSM3247, generated in this study. Genes in the acetylene fermentation pathway (red) include acetylene hydratase (AH), aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), phosphate acetyltransferase (PTA), and acetate kinase (AK). AH catalyzes the reaction of C2H2 + H2O → CH3CHO, providing free energy of ΔG = −111.9 kJ per mol (22). Acetaldehyde is disproportionated to acetate and ethanol by aldehyde dehydrogenase and alcohol dehydrogenase, respectively. Together, these enzymes catalyze the reaction of 2CH3CHO + H2O → CH3CH2OH + CH3COO− + H+, yielding free energy of ΔG = −17.3 kJ per mol acetaldehyde (22). The nitrogenase genes nifHDKEN are highlighted (blue), and additional genes (nifBX and fdxN) are also found in all genomes (Fig. 3B; also see Table S3 in the supplemental material).
The specificity of AH for acetylene as a growth substrate has prompted speculation with respect to its early origin, when Earth's atmosphere was comparatively rich in this gas (30, 31). It was speculated by Postgate (32) that N2ase may have coappeared to detoxify noxious substances such as cyanide, reducing N2 only when cyanide and ammonia became limiting as life emerged. We now report the unusual circumstance of gene sequences for both of these enzymes, N2ase and AH, being present in the annotated genomes of two deposited type reference strains of P. acetylenicus (DSM3246 and DSM3247), as well as in Pelobacter strain SFB93, isolated from San Francisco Bay (30, 33) (Fig. 1B). We further present experimental evidence that Pelobacter strain SFB93 is capable of diazotrophic growth, during which both enzymes are active.
RESULTS AND DISCUSSION
Comparison of Pelobacter genomes.
Genome sequencing of the three Pelobacter strains produced complete genomes ranging in size from 3,175,390 bp for DSM3247 to 3,191,564 bp for DSM3246 (34) and 3,218,469 bp for strain SFB93 (35). Interestingly, DSM3246 also contained a 13,658-bp plasmid. Strain DSM3246 has four small-subunit (SSU) rRNA genes, whereas DSM3247 and SFB93 have three copies, but the two DSM strains share 100% sequence identity at the SSU rRNA gene sequence level. The SSU rRNA genes of SFB93 share 95.8 to 97.4% sequence identity with the two type strains, indicating that SFB93 is likely a new species within the Pelobacter genus (see Fig. S1 in the supplemental material). The divergence in SSU rRNA genes is also seen across the whole genome, with the levels of nucleotide identity between the three species being quite low except for the region containing the gene for AH (ahy) (Fig. S2). Average nucleotide identity (ANI) values for SFB93 versus DSM3246 and DSM3247 were 62.27% and 60.32%, respectively, suggesting that DNA-DNA hybridization would be below the 70% standard threshold for designating a new species.
Acetylene fermentation and diazotrophy genes present in Pelobacter genomes.
The genomes of all three Pelobacter strains contain the genes for complete conversion of acetylene to ethanol and acetate, including genes for AH, aldehyde dehydrogenase, phosphate acetyltransferase, acetate kinase, and alcohol dehydrogenase (Fig. 1B; also see Table S1) (22). However, the genes are not located within a single locus but are scattered throughout the genomes. Most interestingly, SFB93 contains two copies of the ahy gene, located ∼18,000 bp apart. The AH amino acid sequences from the three genomes are closely related to previously published AH sequences (Fig. 2; also see Table S1) (33). The two ahy copies in the Pelobacter SFB93 genome are most closely related to each other (96.9% amino acid sequence similarity) but demonstrate only 88.4 and 90.4% amino acid sequence similarity to the AHs of strains DSM3246 and DSM3247, respectively (Table S2). No genes related to ahy are found in the existing genomes of other Pelobacter strains.
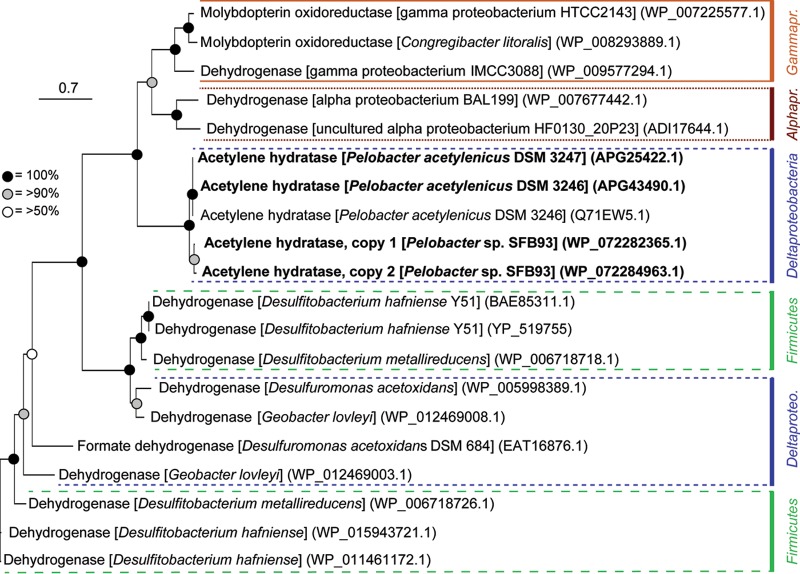
FIG 2.
Phylogenetic tree of acetylene hydratase and related dehydrogenase and oxidoreductase amino acid sequences. All AH sequences are from organisms known to couple anaerobic growth to acetylene fermentation. Sequences from this study are indicated in bold. Bootstrap values (100 replicates) are indicated by node markers. The scale bar indicates 0.7 changes per amino acid.
Phylogenetic reconstruction of AH and closely related molybdopterin oxidoreductase and dehydrogenase sequences suggests that AH evolved from an ancestral dehydrogenase (Fig. 2). The phylogenies of these amino acid sequences do not reflect phylogeny at the SSU rRNA gene level. In addition, the ahy genes are flanked by group II intron reverse transcriptases/maturases and integrases (Fig. 3). These observations suggest the potential for ahy genes to undergo horizontal gene transfer (HGT) into or out of the Pelobacter genomes or movement within the genomes. Currently, the National Center for Biotechnology Information (NCBI) has over 1,600 keyword hits for AH, but it is not clear whether those annotations indicate functional genes or represent accurately annotated proteins. The only demonstrated anaerobic AH activity occurs in P. acetylenicus DSM3246 and DSM3247 and in Pelobacter strain SFB93. These observations imply either that there is an abundance of genes poorly annotated as ahy or that the genes code for the structurally distinct AHs from aerobic acetylene-metabolizing bacteria observed by Rosner et al. (25).
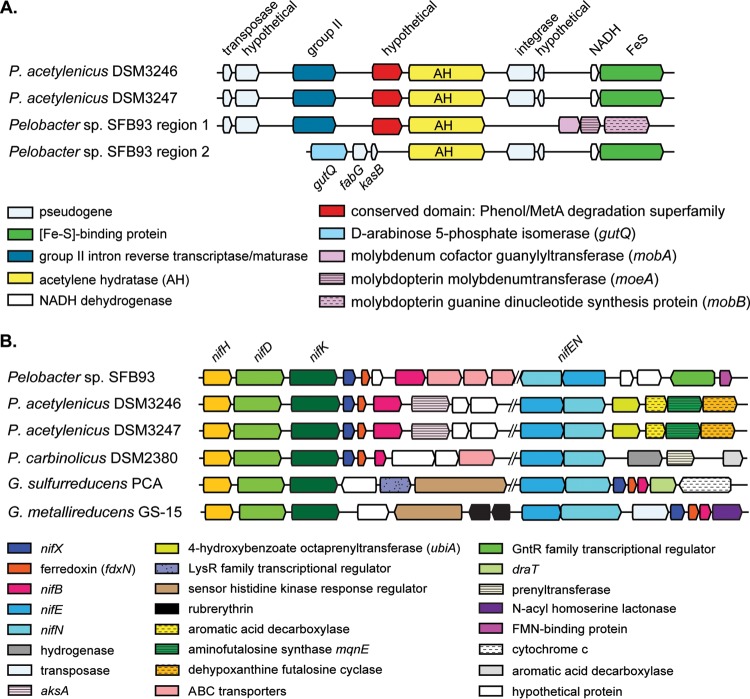
FIG 3.
Syntenic map of acetylene hydratase (ahy) and flanking genes (A) and nitrogenase genes (B) in Pelobacter sp. SFB93 and P. acetylenicus DSM3246 and DSM3247. Pelobacter sp. SFB93 contains two ahy genes in two regions, separated by ∼18,000 bp. Geobacter sulfurreducens PCA and G. metallireducens GS-15, known nitrogen-fixing organisms, are included for reference in panel B. Nitrogenase genes are separated by gaps (indicated by double slashes) of different lengths, ranging from ∼36,000 bp to 1.2 Mbp, between nifHDKXB and nifEN in SFB93 and DSM3247, respectively (see Table S4 in the supplemental material).
The genes essential for encoding an iron-molybdenum N2ase (nifHDKEN) were found in the genomes of SFB93, DSM3246, and DSM3247 (Fig. 1B). Interestingly, nifHDK genes are located adjacent to ahy in all three of our sequenced Pelobacter genomes (∼166 kb for SFB93 and ∼40 kb for both DSM3246 and DSM3247). Nitrogenase genes in SFB93 are closely grouped (nifHDKXB genes are ∼36,000 bp from nifEN genes), compared to DSM3246 and DSM3247 (nifHDKXB genes are ∼1 to 2 Mbp from nifEN genes) (Fig. 3B; also see Table S4). The nifHDKEN genes from SFB93 are similar to those from Pelobacter carbinolicus, although the nifEN genes in SFB93 have undergone an inversion. In contrast, the nifXBRS genes in SFB93 are most similar to the nif genes in the genomes of other members of the Desulfuromonadales (Table S3 and Fig. S3). Although nitrogen fixation genes are known to occur on single or dispersed operons (36), the relatively small gap in SFB93 is more similar to the reference genomes of Geobacter sulfurreducens PCA (GenBank accession no. NC_002939) and Geobacter metallireducens GS-15 (GenBank accession no. NC_007517), in which all nif genes are closely grouped (nifHDK genes are ∼8,000 bp and ∼5,000 bp, respectively, from nifEN genes) (Fig. 3B; also see Table S4). The Pelobacter genus is within the order Desulfuromonadales (Fig. S2), and the nifH genes from our genomes are most closely related to nif genes from members of that order (Fig. S3). To date, G. sulfurreducens and G. metallireducens are the only organisms in that order that have been definitively shown to fix nitrogen (37, 38) (Fig. S3). Although the reference genomes of P. carbinolicus DSM2380 (GenBank accession no. NC_007498) (Fig. 3B) and Pelobacter propionicus DSM2379 (GenBank accession no. CP000482) contain annotated nif genes, to date there are no reports of those organisms being tested for nitrogen fixation.
Nitrogen fixation and acetylene fermentation in Pelobacter sp. SFB93.
To ascertain whether the annotated nif genes for nitrogen fixation were expressed in vivo and were functional, we carried out acetylene reduction assays with strains DSM3246 and SFB93. Washed cells of acetoin-grown P. acetylenicus DSM3246 and pyruvate-grown SFB93 were tested for nitrogen fixation. Based on their growth preferences, different substrates were used to grow the organisms; strain DSM3246 grows best using acetoin but cannot utilize pyruvate (22), and strain SFB93 grows best on pyruvate but does not grow on acetoin (data not shown). P. acetylenicus DSM3246 cells consumed acetylene when incubated with or without ammonium present, but in neither case was any ethylene detected (Fig. S4). These results show that, under these conditions, strain DSM3246 lacks the capacity for nitrogen fixation, despite having a full complement of nif genes; based on these results, it is not clear whether the nif genes are functional. DSM3247 was not tested for diazotrophic growth, due to its high level of genetic similarity to DSM3246.
SFB93 cells incubated with or without ammonium ions demonstrated steady consumption of acetylene, which necessitated headspace replenishment after 4 days of incubation (Fig. 4). A buildup of ethylene (33 ± 3 μmol) occurred only in the absence of ammonium, however, and there was no significant accumulation (1.0 ± 0.5 μmol) in the presence of ammonium. Because ammonium represses the expression of N2ase (39), these results clearly demonstrate that SFB93 possesses an active N2ase. The cumulative amounts of acetylene consumed (∼1,386 ± 34 μmol without ammonium and 1,425 ± 52 μmol with ammonium) exceeded by ∼43-fold the amount of ethylene recovered under ammonium-depleted conditions. This further illustrates that the observed acetylene consumption was primarily a function of AH rather than N2ase.
FIG 4.
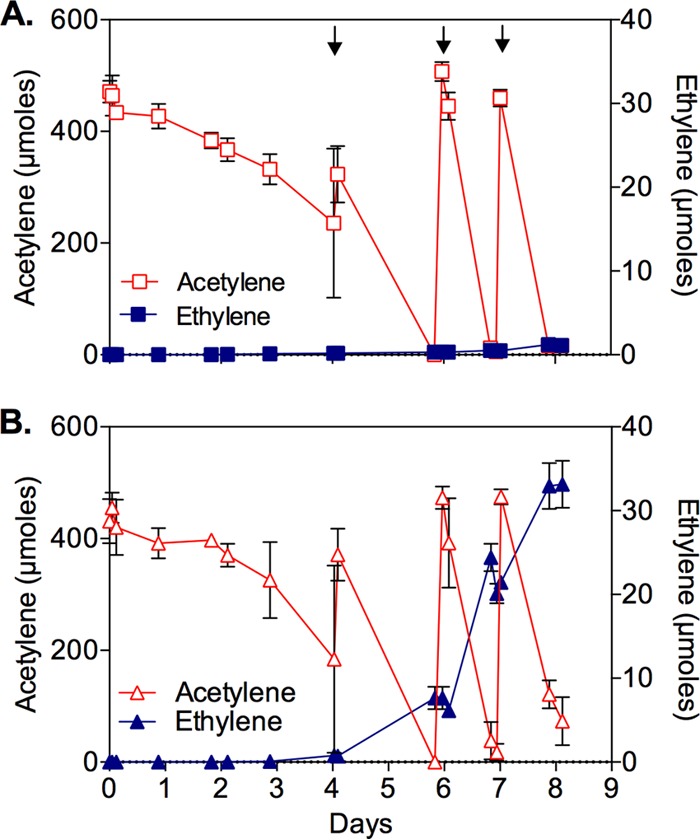
Consumption of acetylene (red) and production of ethylene (blue) by washed cell suspensions of pyruvate-grown Pelobacter strain SFB93 incubated under N2. The starting cell density was ∼4.5 × 108 cells/ml. (A) Cells incubated with 2 mM NH4Cl. (B) Cells incubated without NH4Cl. Symbols represent the means of three different cell suspensions, and bars indicate ±1 standard deviation. Arrows indicate the times at which acetylene was added again to the gas phases of all samples.
A diazotrophic growth experiment was conducted with strain SFB93, in which acetylene was employed as the sole carbon and energy source in lieu of pyruvate (Fig. 5). Growth, measured as either optical density at 680 nm (OD680) or cell counts, was far more extensive in an atmosphere of N2, compared to one of argon (Fig. 5A). Acetylene uptake (Fig. 5B) and ethylene formation (Fig. 5C) were noted under both conditions but were always more extensive in the N2 atmosphere, clearly indicating that N2 was being fixed and used to meet cellular growth requirements. The small amount of growth observed in the Ar-incubated samples was attributable to NH4+ carryover from the inoculum, as well as to cell scavenging of nitrogen contained in the amino acid-reducing agent employed (cysteine) in the medium. It is relevant to note that, because acetylene is a competitive substrate that inhibits nitrogen fixation, diazotrophic growth under N2 required the removal of acetylene through AH activity; this allowed electrons to be shifted from reduction of C2H2 to C2H4 to reduction of N2 to NH3.
FIG 5.
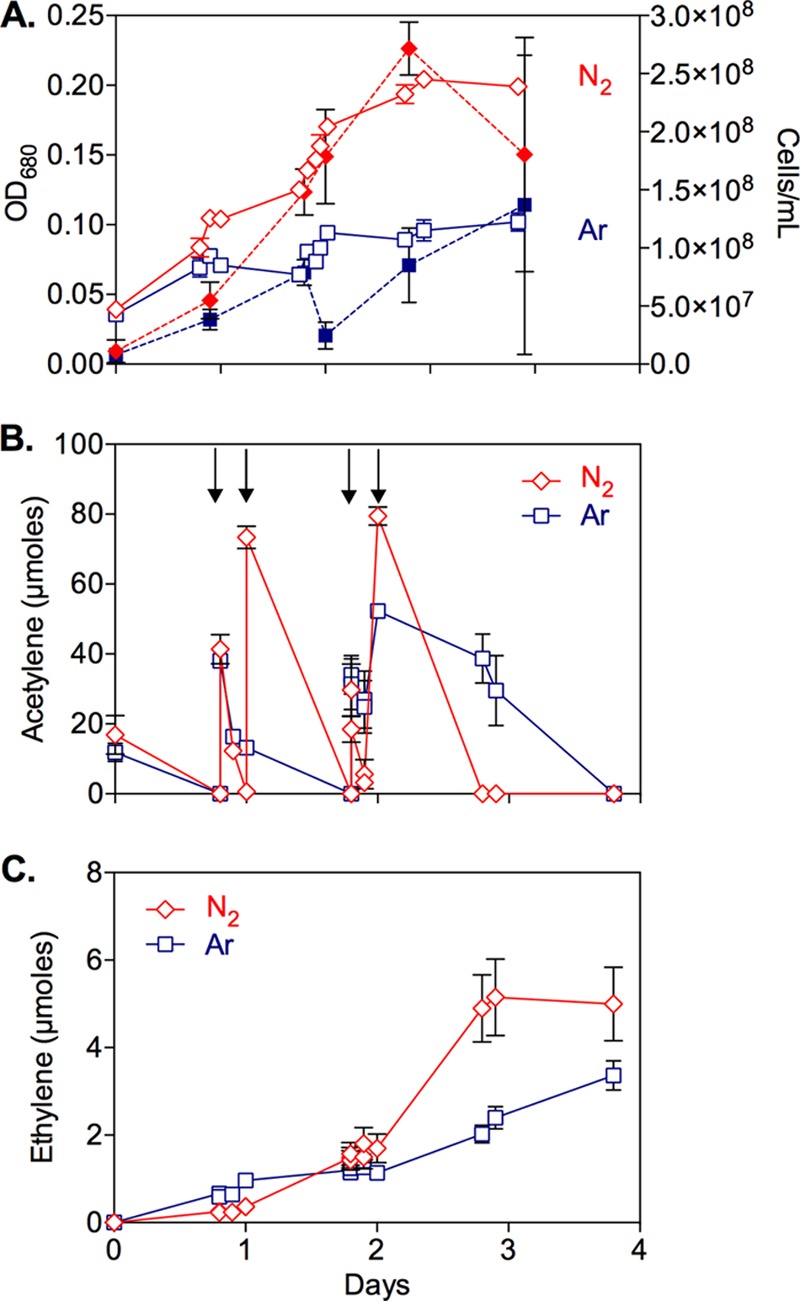
Diazotrophic growth of Pelobacter strain SFB93 under N2 (red) versus Ar (blue), with acetylene as the carbon and energy source. (A) Growth measured by optical density (solid lines) and cell counts (dashed lines). (B) Consumption of acetylene, which, after depletion, was added again to the gas phase at four times, as indicated by the arrows. (C) Ethylene accumulation. Symbols represent the means of three separate cultures, and bars indicate ±1 standard deviation.
Implications.
Our study showed not only that three Pelobacter strains contain multiple genes for acetylene transformation but also that a single microbe, SFB93, has the ability to transform acetylene via two separate reactions, catalyzed by the enzymes AH and N2ase. The presence of both enzymatic pathways in a single anaerobic organism likely represents a competitive advantage, with AH providing a mechanism for the organism to obtain carbon and energy and N2ase providing a mechanism to obtain nitrogen. In addition, SFB93 may benefit by having N2ase available for detoxification of other triple-bonded carbon compounds (30). Understanding acetylene transformations in modern environments (i.e., extent, occurrence, and rates) may allow better interpretation of acetylene as a potential biomarker for extraterrestrial life.
Given the very low concentrations of acetylene in the natural environment (e.g., parts per billion in seawater [5]) and the presence of flanking HGT elements, it is surprising that ahy has persisted in these organisms. Acetylene fermentation is thought to be a rare route of metabolism but is not constrained to a single habitat type, with activity being found under fresh water and seawater conditions (33). Indeed, it was during the routine application of acetylene as a bioassay tool to quantify nitrogen transformations by diverse soils and sediments that the unexpected consumption of acetylene was often noted (40–44). The variety of habitats reported in those early serendipitous observations suggests a broad occurrence of ahy-like genes in nature. HGT may also be functioning to maintain metabolic versatility for the rare encounters of these organisms with acetylene. It is possible that anthropogenic inputs of acetylene, e.g., from pollutant degradation (45–48) or motor vehicles (49), are continuing to select for this metabolic pathway. Abiotic dehalogenation of trichloroethylene (TCE), a ubiquitous groundwater pollutant, with reduced iron minerals produces acetylene (45–48), thereby yielding an anthropogenic source for acetylene fermenters. In fact, Miller et al. (33) demonstrated acetylene fermentation in a TCE-contaminated subsurface aquifer in New Jersey. Further, TCE bioremediation efforts are aimed at stimulating complete dechlorination, resulting in the accumulation of ethylene (C2H4) as the desired innocuous end product (50, 51). Recent laboratory studies have shown that acetylene-fermenting organisms can be used to overcome acetylene-linked inhibition of TCE bioremediation (52). Considering that Pelobacter N2ase reduction of in situ acetylene may be a source of ethylene, production of any ethylene arising from N2ase activity versus dehalogenation could complicate interpretation of the use of ethylene buildup to assess the efficacy of TCE dehalogenation. This work highlights the need to examine acetylene transformations in more depth and to understand the interactions between acetylene and nitrogen cycling through coexpression of AH and N2ase.
MATERIALS AND METHODS
Growth of Pelobacter strains.
Pelobacter strain SFB93 was grown in the bicarbonate-buffered anaerobic bay water (ABW) medium described by Miller et al. (33). Pelobacter acetylenicus strains DSM3246 and DSM3247 were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) (Braunschweig, Germany). Freshwater strain DSM3246 was grown in DSMZ medium 298 using acetoin (CH3COCHOHCH3) (0.01 mM). Estuarine strain DSM3247 was grown in DSMZ medium 293 using either acetoin or C2H2 (1.5%).
Genome sequencing and annotation.
Cultures of DSM3246, DSM3247, and SFB93 were grown to high density and then pelleted by centrifugation. DNA was extracted from multiple pellets using the DNeasy blood and tissue kit (Qiagen, Valencia, CA, USA), according to the manufacturer's protocol, with DNA shearing being limited by mixing through inversion instead of vortex-mixing. The DNA concentrations and purity of the extracts were then measured with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and the extracts were visualized on a 1% agarose gel. DNA was concentrated from multiple extractions using either the PowerClean Pro kit (Mo Bio Laboratories, Carlsbad, CA, USA) or a Zymo genomic DNA spin concentrator (Zymo Research Corp., Irvine, CA, USA), according to the manufacturer's instructions. The pooled and concentrated DNA was quantified with a Qubit 3.0 fluorometer (Life Technologies) and visualized on a 1% agarose gel, to verify the recovery of high-molecular-weight DNA.
DNA extracts from DSM3246, DSM3247, and SFB93 were sent to the University of California, Davis, Genome Center (http://genomecenter.ucdavis.edu) for PacBio long-read sequencing (Pacific Biosciences of California, Inc., Menlo Park, CA, USA); sequencing on a PacBio RS instrument yielded 71,385, 62,650, and 63,968 sequence reads, respectively, for the strains. The sequence reads were subsequently assembled using the hierarchical genome assembly process (HGAP) developed by PacBio (53). The HGAP was performed through the PacBio SMRT portal using version RS_HGAP_Assembly.3. Annotation was performed with the prokaryotic genome annotation pipeline (PGAP) from the NCBI (54).
SSU rRNA, nifH, and ahy genes were extracted from genomes using Geneious R9.1.8 (Biomatters, Ltd.) (55). The SSU rRNA genes of DSM3246 and DSM3247 were identical, and one representative copy was imported in ARB (56) for phylogenetic analysis. The SSU rRNA genes of SFB93 were more variable; therefore, all 3 copies were included in the phylogenetic analysis. All sequences were aligned to the SILVA release 123 database (57) in ARB. To determine the relationship of SFB93 to published Pelobacter strains and its placement within the Deltaproteobacteria, a maximum likelihood phylogenetic tree of those strains and a selection of related Deltaproteobacteria was computed using RAxML (58) within ARB, with the best tree being selected based on 100 iterations. The nucleic acid sequences of nifH and ahy were translated to amino acid sequences from genomic nucleotide sequences with Geneious R9.1.8, using the genetic code for bacteria and archaea (11). Amino acid sequences were aligned using MUSCLE, and phylogenetic trees were constructed using PhyML with 100 bootstrap replicates within Geneious.
Nitrogen fixation assays.
P. acetylenicus strain DSM3246 was grown in 100 ml of anaerobic DSMZ m298 medium that was modified by using a HEPES buffer (5.2 g/liter) instead of sodium bicarbonate and 5 mM acetoin instead of butanediol as the carbon source. Late-log-phase cells were harvested by centrifugation and brought back to 30-ml volumes with ammonium-free medium. Cell suspensions were dispensed (5 ml) into 13-ml serum bottles and sealed under a N2 headspace. Cultures were amended with 5 mM acetoin and injected with 0.1 ml C2H2. One-half of the prepared bottles were also supplemented with NH4Cl to a final concentration of 2 mM. The starting cell density was 5.2 × 107 cells/ml.
Strain SFB93 was grown in anaerobic bicarbonate-buffered CM medium, as described by Visscher and Taylor (59), which was modified by including 5.2 g/liter HEPES for additional buffering capacity. For washed cell experiments, cells were grown on 10 mM pyruvate, harvested at late log phase (4 days), concentrated by centrifugation under a N2 atmosphere, resuspended to an OD680 of ∼0.2 in 200 ml of ammonium-free CM medium, and subsequently divided into two portions (100 ml each), with one portion supplemented with 0.2 ml of 2 M NH4Cl. Thirty milliliters of this cell suspension was then dispensed into 70-ml serum bottles, after which all bottles were injected with 10 ml of C2H2. All manipulations were performed in anaerobic chambers, as outlined previously (33). Headspace analyses of C2H2 and C2H4 were carried out by gas chromatography with flame ionization detection, as described previously (33). The Henry's law KH values employed were 9.78 × 10−1 for C2H2 and 1.17 × 10−1 for C2H4, as calculated previously by Miller et al. (60). Growth experiments were conducted in Balch tubes sealed under N2 or Ar (10), with ammonium-free CM medium and 1.0 ml of added C2H2. Growth was determined by measuring OD680 values spectrophotometrically, as well as by assessing cell densities. Cell densities were determined by direct cell counting using acridine orange epifluorescence microscopy, as described by Miller et al. (60).
Accession number(s).
The genomes reported in this paper are available from the NCBI GenBank database under BioProject number PRJNA319824 and accession numbers CP015455 (P. acetylenicus DSM3246 genome), CP015456 (P. acetylenicus DSM3246 plasmid), CP015518 (P. acetylenicus DSM3247 genome), and CP015519 (Pelobacter sp. strain SFB93 genome). Full descriptions of the genomes are also available as genome announcements (34, 35). Data from nitrogen fixation assays are available (61).
Supplementary Material
ACKNOWLEDGMENTS
We thank Robert Andrews, Charlie Quinn, Shelly Hoeft-McCann, and Laurence Miller for laboratory assistance and helpful discussions. We also thank the anonymous reviewers for their valuable comments and suggestions to improve the manuscript.
This work was supported by a NASA Research Opportunities in Space and Earth Science (ROSES-2013), Astrobiology: Exobiology and Evolutionary Biology Program Element grant (grant 13-EXO13-0001) to D.M.A. and R.S.O. Funding was also provided by the U.S. Geological Survey Toxic Substances Hydrology Program and the Water Mission Area. J.M.S. and J.L.F. were supported by University of Alabama startup funds, a University of Alabama RGC level 2 grant, and NIH grant R01 GM102511 to J.L.F.
Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. government.
We declare no competing financial interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01198-17.
REFERENCES
- 1.Cronn D, Robinson E. 1979. Tropospheric and lower stratospheric vertical profiles of ethane and acetylene. Geophys Res Lett 6:1–10. doi: 10.1029/GL006i008p00641. [DOI] [Google Scholar]
- 2.Goldman A, Murcray FJ, Blatherwick RD, Gillis JR, Bonomo FS, Murcray FH, Murcray DG, Cicerone RJ. 1981. Identification of acetylene (C2H2) in infrared atmospheric absorbtion spectra. J Geophys Res 86:12143–12146. doi: 10.1029/JC086iC12p12143. [DOI] [Google Scholar]
- 3.Rudolph J, Ehhalt DH, Khedim A. 1984. Vertical profiles of acetylene in the troposphere and stratosphere. J Atmos Chem 2:117–124. doi: 10.1007/BF00114125. [DOI] [Google Scholar]
- 4.Arnts RR, Meeks SA. 1981. Biogenic hydrocarbon contribution to the ambient air of selected areas. Atmos Environ 15:1643–1651. doi: 10.1016/0004-6981(81)90149-9. [DOI] [Google Scholar]
- 5.Bonsang B, Martin D, Lambert G, Kanakidou M, Le Roulley JC, Sennequier G. 1991. Vertical distribution of non methane hydrocarbons in the remote marine boundary layer. J Geophys Res 96:7313–7324. doi: 10.1029/90JD02539. [DOI] [Google Scholar]
- 6.Cofer WR, Levine JS, Winstead EL, LeBel PJ, Koller AM, Hinkle CR. 1990. Trace gas emissions from burning Florida wetlands. J Geophys Res 95:1865–1870. doi: 10.1029/JD095iD02p01865. [DOI] [Google Scholar]
- 7.Kanakidou M, Bonsang B, Roulley JCL, Lambert G, Martin D, Sennequier G. 1988. Marine source of atmospheric acetylene. Nature 333:51–52. doi: 10.1038/333051a0. [DOI] [Google Scholar]
- 8.Kasting J. 1993. Earth's early atmosphere. Science 259:920–926. doi: 10.1126/science.11536547. [DOI] [PubMed] [Google Scholar]
- 9.Kasting JF. 2004. When methane made climate. Sci Am 291:78–85. doi: 10.1038/scientificamerican0704-78. [DOI] [PubMed] [Google Scholar]
- 10.Zahnle KJ. 1986. Photochemistry of methane and the formation of hydrocyanic acid (HCN) in the Earth's early atmosphere. J Geophys Res 91:2819–2834. doi: 10.1029/JD091iD02p02819. [DOI] [Google Scholar]
- 11.Trainer MG, Pavlov AA, Curtis DB, McKay CP, Worsnop DR, Delia AE, Toohey DW, Toon OB, Tolbert MA. 2004. Haze aerosols in the atmosphere of early Earth: manna from heaven. Astrobiology 4:409–419. doi: 10.1089/ast.2004.4.409. [DOI] [PubMed] [Google Scholar]
- 12.Miller SL. 1953. A production of amino acids under possible primitive Earth conditions. Science 117:528–529. doi: 10.1126/science.117.3046.528. [DOI] [PubMed] [Google Scholar]
- 13.Miller SL, Urey HC. 1959. Organic compound synthesis on the primitive earth. Science 130:245–251. doi: 10.1126/science.130.3370.245. [DOI] [PubMed] [Google Scholar]
- 14.Scheidler C, Sobotta J, Eisenreich W, Wächtershäuser G, Huber C. 2016. Unsaturated C3,5,7,9-monocarboxylic acids by aqueous, one-pot carbon fixation: possible relevance for the origin of life. Sci Rep 6:27595. doi: 10.1038/srep27595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shemansky DE, Stewart AIF, West RA, Esposito LW, Hallett JT, Liu X. 2005. The Cassini UVIS stellar probe of the Titan atmosphere. Science 308:978–982. doi: 10.1126/science.1111790. [DOI] [PubMed] [Google Scholar]
- 16.Clark RN, Curchin JM, Barnes JW, Jaumann R, Soderblom L, Cruikshank DP, Brown RH, Rodriguez S, Lunine J, Stephan K, Hoefen TM, Le Mouélic S, Sotin C, Baines KH, Buratti BJ, Nicholson PD. 2010. Detection and mapping of hydrocarbon deposits on Titan. J Geophys Res 115:1–28. doi: 10.1029/2009JE003369. [DOI] [Google Scholar]
- 17.Sagan C, Thompson WR. 1984. Production and condensation of organic gases in the atmosphere of Titan. Icarus 59:133–161. doi: 10.1016/0019-1035(84)90018-6. [DOI] [Google Scholar]
- 18.Derenne S, Coelho C, Anquetil C, Szopa C, Rahman AS, McMillan PF, Corà F, Pickard CJ, Quirico E, Bonhomme C. 2012. New insights into the structure and chemistry of Titan's tholins via 13C and 15N solid state nuclear magnetic resonance spectroscopy. Icarus 221:844–853. doi: 10.1016/j.icarus.2012.03.003. [DOI] [Google Scholar]
- 19.McKay CP, Smith HD. 2005. Possibilities for methanogenic life in liquid methane on the surface of Titan. Icarus 178:274–276. doi: 10.1016/j.icarus.2005.05.018. [DOI] [Google Scholar]
- 20.Stewart WD, Fitzgerald GP, Burris RH. 1967. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A 58:2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seiffert GB, Ullmann GM, Messerschmidt A, Schink B, Kroneck PM, Einsle O. 2007. Structure of the non-redox-active tungsten/[4Fe:4S] enzyme acetylene hydratase. Proc Natl Acad Sci U S A 104:3073–3077. doi: 10.1073/pnas.0610407104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schink B. 1985. Fermentation of acetylene by an obligate anaerobe, Pelobacter acetylenicus sp. nov. Arch Microbiol 142:295–301. doi: 10.1007/BF00693407. [DOI] [Google Scholar]
- 23.Boll M, Einsle O, Ermler U, Kroneck PM, Ullmann GM. 2016. Structure and function of the unusual tungsten enzymes acetylene hydratase and class II benzoyl-coenzyme A reductase. J Mol Microbiol Biotechnol 26:119–137. doi: 10.1159/000440805. [DOI] [PubMed] [Google Scholar]
- 24.Kroneck PM. 2016. Acetylene hydratase: a non-redox enzyme with tungsten and iron-sulfur centers at the active site. J Biol Inorg Chem 21:29–38. doi: 10.1007/s00775-015-1330-y. [DOI] [PubMed] [Google Scholar]
- 25.Rosner BM, Rainey FA, Kroppenstedt RM, Schink B. 1997. Acetylene degradation by new isolates of aerobic bacteria and comparison of acetylene hydratase enzymes. FEMS Microbiol Lett 148:175–180. doi: 10.1111/j.1574-6968.1997.tb10285.x. [DOI] [PubMed] [Google Scholar]
- 26.Kanner D, Bartha R. 1982. Metabolism of acetylene by Nocardia rhodochrous. J Bacteriol 150:989–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birch-Hirschfeld L. 1932. Die Umsetzung von Acetylen durch Mycobacterium lacticola. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 2 86:113–129. [Google Scholar]
- 28.Germon JC, Knowles R. 1988. Metabolism of acetylene and acetaldehyde by Rhodococcus rhodochrous. Can J Microbiol 34:242–248. doi: 10.1139/m88-045. [DOI] [PubMed] [Google Scholar]
- 29.de Bont JAM, Peck MW. 1980. Metabolism of acetylene by Rhodococcus A1. Arch Microbiol 127:99–104. doi: 10.1007/BF00428012. [DOI] [Google Scholar]
- 30.Culbertson CW, Strohmaier FE, Oremland RS. 1988. Acetylene as a substrate in the development of primordial bacterial communities. Orig Life Evol Biosphere 18:397–407. doi: 10.1007/BF01808218. [DOI] [PubMed] [Google Scholar]
- 31.Oremland RS, Voytek MA. 2008. Acetylene as fast food: implications for development of life on anoxic primordial Earth and in the outer solar system. Astrobiology 8:45–58. doi: 10.1089/ast.2007.0183. [DOI] [PubMed] [Google Scholar]
- 32.Postgate JR. 1974. Evolution within nitrogen-fixing systems. In Carlile MJ, Skehel JJ (ed), Evolution in the microbial world: proceedings of the 24th symposium of the Society for General Microbiology, p 263–292 Cambridge University Press, New York, NY. [Google Scholar]
- 33.Miller LG, Baesman SM, Kirshtein J, Voytek MA, Oremland RS. 2013. A biogeochemical and genetic survey of acetylene fermentation by environmental samples and bacterial isolates. Geomicrobiol J 30:501–516. doi: 10.1080/01490451.2012.732662. [DOI] [Google Scholar]
- 34.Sutton JM, Baesman SM, Fierst JL, Poret-Peterson AT, Oremland RS, Dunlap DS, Akob DM. 2017. Complete genome sequences of two acetylene fermenting Pelobacter acetylenicus strains. Genome Announc 5:e01572-16. doi: 10.1128/genomeA.01572-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton JM, Baesman SM, Fierst JL, Poret-Peterson AT, Oremland RS, Dunlap DS, Akob DM. 2017. Complete genome sequence of the acetylene fermenting Pelobacter strain SFB93. Genome Announc 5:e01573-16. doi: 10.1128/genomeA.01573-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raymond J, Siefert JL, Staples CR, Blankenship RE. 2004. The natural history of nitrogen fixation. Mol Biol Evol 21:541–554. doi: 10.1093/molbev/msh047. [DOI] [PubMed] [Google Scholar]
- 37.Bazylinski DA, Dean AJ, Schuler D, Phillips EJ, Lovley DR. 2000. N2-dependent growth and nitrogenase activity in the metal-metabolizing bacteria, Geobacter and Magnetospirillum species. Environ Microbiol 2:266–273. doi: 10.1046/j.1462-2920.2000.00096.x. [DOI] [PubMed] [Google Scholar]
- 38.Coppi MV, Leang C, Sandler SJ, Lovley DR. 2001. Development of a genetic system for Geobacter sulfurreducens. Appl Environ Microbiol 67:3180–3187. doi: 10.1128/AEM.67.7.3180-3187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helber JT, Johnson TR, Yarbrough LR, Hirschberg R. 1988. Effect of nitrogenous compounds on nitrogenase gene expression in anaerobic cultures of Anabaena variabilis. J Bacteriol 170:558–563. doi: 10.1128/jb.170.2.558-563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe I, De Guzman MR. 1980. Effect of nitrate on acetylene disappearance from anaerobic soil. Soil Biol Biochem 12:193–194. doi: 10.1016/0038-0717(80)90058-9. [DOI] [Google Scholar]
- 41.Culbertson CW, Zehnder AJB, Oremland RS. 1981. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol 41:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeomans JC, Beauchamp EG. 1982. Acetylene as a possible substrate in the denitrification process. Can J Soil Sci 62:139–144. doi: 10.4141/cjss82-015. [DOI] [Google Scholar]
- 43.Tam TY, Mayfield CI, Inniss WE. 1983. Aerobic acetylene utilization by stream sediment and isolated bacteria. Curr Microbiol 8:165–168. doi: 10.1007/BF01568851. [DOI] [Google Scholar]
- 44.Payne WJ. 1984. Influence of acetylene on microbial and enzymatic assays. J Microbiol Methods 2:117–133. doi: 10.1016/0167-7012(84)90001-0. [DOI] [Google Scholar]
- 45.Roberts AL, Totten LA, Arnold WA, Burris DR, Campbell TJ. 1996. Reductive elimination of chlorinated ethylenes by zero-valent metals. Environ Sci Technol 30:2654–2659. doi: 10.1021/es9509644. [DOI] [Google Scholar]
- 46.Arnold WA, Roberts AL. 2000. Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles. Environ Sci Technol 34:1794–1805. doi: 10.1021/es990884q. [DOI] [Google Scholar]
- 47.Han YS, Hyun SP, Jeong HY, Hayes KF. 2012. Kinetic study of cis-dichloroethylene (cis-DCE) and vinyl chloride (VC) dechlorination using green rusts formed under varying conditions. Water Res 46:6339–6350. doi: 10.1016/j.watres.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 48.Schaefer CE, Towne RM, Lippincott DR, Lacombe PJ, Bishop ME, Dong H. 2015. Abiotic dechlorination in rock matrices impacted by long-term exposure to TCE. Chemosphere 119:744–749. doi: 10.1016/j.chemosphere.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Seinfeld JH, Pandis SN. 1998. Atmospheric chemistry and physics, 1st ed, p 49–124 John Wiley and Sons, Inc., New York, NY. [Google Scholar]
- 50.Revesz KM, Lollar BS, Kirshtein JD, Tiedeman CR, Imbrigiotta TE, Goode DJ, Shapiro AM, Voytek MA, Lacombe PJ, Busenberg E. 2014. Integration of stable carbon isotope, microbial community, dissolved hydrogen gas, and 2HH2O tracer data to assess bioaugmentation for chlorinated ethene degradation in fractured rocks. J Contam Hydrol 156:62–77. doi: 10.1016/j.jconhyd.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Mao X, Stenuit B, Polasko A, Alvarez-Cohen L. 2015. Efficient metabolic exchange and electron transfer within a syntrophic trichloroethene-degrading coculture of Dehalococcoides mccartyi 195 and Syntrophomonas wolfei. Appl Environ Microbiol 81:2015–2024. doi: 10.1128/AEM.03464-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao X, Oremland RS, Liu T, Gushgari S, Landers AA, Baesman SM, Alvarez-Cohen L. 2017. Acetylene fuels TCE reductive dechlorination by defined Dehalococcoides/Pelobacter consortia. Environ Sci Technol 51:2366–2372. doi: 10.1021/acs.est.6b05770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 54.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Ciufo S, Li W. 2013. Prokaryotic genome annotation pipeline. In The NCBI handbook [internet], ed 2 National Center for Biotechnology Information, Bethesda, MD: https://www.ncbi.nlm.nih.gov/books/NBK174280. [Google Scholar]
- 55.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westram R, Bader K, Pruesse E, Kumar Y, Meier H, Glockner FO, Ludwig W. 2011. ARB: a software environment for sequence data, p 399–406. In de Bruijn F-J. (ed), Handbook of molecular microbial ecology I: metagenomics and complementary approaches. John B. Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- 57.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Visscher PT, Taylor BF. 1994. Demethylation of dimethylsulfoniopropionate to 3-mercaptopropionate by an aerobic marine bacterium. Appl Environ Microbiol 60:4617–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller LG, Baesman SM, Oremland RS. 2015. Stable carbon isotope fractionation during bacterial acetylene fermentation: potential for life detection in hydrocarbon-rich volatiles of icy planet(oid)s. Astrobiology 15:977–986. doi: 10.1089/ast.2015.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akob DM, Baesman SM, Bennett SC, Oremland RS. 2017. Discovery of two biological mechanisms for acetylene metabolism in a single organism. US Geological Survey data release. USGS, Reston, VA. doi: 10.5066/F70Z71JH. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.