Significance Statement
Sickle cell disease (SCD) is accompanied by chronic pain that is difficult to treat. There is evidence that increasing levels of endocannabinoids in the periphery can modulate pain by decreasing excitability of nociceptors. Using a humanized mouse model of SCD, we show that blocking hydrolysis of the endocannabinoid anandamide peripherally with URB597 decreased mechanical hyperalgesia in HbSS-BERK mice. In parallel studies conducted in vivo, it was found that cutaneous nociceptors in sickle mice were sensitized as evidenced by increased spontaneous activity and increased responses to mechanical, heat and cold stimuli. Injection of URB597 decreased sensitization of nociceptors. Thus, decreasing the hydrolysis of endocannabinoids in the periphery may be a novel approach to decrease pain in SCD.
1. Introduction
Sickle cell disease (SCD) describes a group of disorders resulting from a point mutation in the beta chain of hemoglobin. This mutation leads to the creation of sickle hemoglobin (HbS) and causes distortion of erythrocytes through polymerization under low oxygen, resulting in characteristic sickle red blood cells (sRBC). Vaso-occlusion (VOC) caused by accumulation of sRBCs results in ischemia-reperfusion injury, reduced oxygen supply to organs, oxidative stress, organ damage and severe pain [7,9,25,31] and often requires long hospital stays [39,50]. Patients also suffer from chronic pain that includes increased sensitivity to touch, heat, and cold stimuli as well as spontaneous pain [10,16]. Pain may result from inflammation as well as neuropathy, which creates a complex pain syndrome that may not respond well to currently available therapies [5]. Opioids are the mainstay of treatment for severe pain associated with SCD [5], but high doses are needed due to altered pharmacokinetics of morphine [15,17,49] which increases undesirable side effects such as sedation, nausea, constipation, and pruritis as well as the development of tolerance and dependence [5,16].
Progress towards the development of new and effective treatments for pain in SCD has been impeded by a lack of insight into the mechanisms underlying the pain in SCD [9]. Mouse models of SCD have been developed which express human hemoglobin (normal HbA or sickle HbS) [53]. Homozygous HbSS-BERK sickle mice express exclusively human beta-sickle hemoglobin whereas HbAA-BERK control mice express normal human hemoglobin A. We [12,38,61] and others [22,24,65] have shown that HbSS-BERK mice exhibit robust hyperalgesia as compared to age-matched HbAA-BERK control mice. Electrophysiological studies revealed that sensitization of nociceptors [27,65] and spinal dorsal horn neurons [14] contribute to hyperalgesia in HbSS sickle mice.
Transgenic sickle mice are excellent models for studying mechanisms underlying chronic pain in SCD and for assessing the potential efficacy of novel analgesic treatment strategies, including those which target the endocannabinoid system. Systemic administration of a relatively low-dose of the non-selective cannabinoid receptor agonist CP 55940 decreased hyperalgesia in HbSS-BERK sickle mice with an effect comparable to that of high dose of morphine [38]. The use of receptor-selective compounds showed that activation of cannabinoid receptors, both type 1 (CB1) and type 2 (CB2), decreased hyperalgesia. Interestingly, the CB2 receptor agonist JWH-133 reduced deep tissue hyperalgesia and attenuated mast cell degranulation [60]. Degranulation of mast cells releases inflammatory mediators that sensitize nociceptors (see reviews [2,3,26,40]).
There is growing evidence that increasing local levels of endocannabinoids can decrease hyperalgesia in models of chronic pain (for reviews, see [23,28,47,63,64]) including bone cancer pain [32,33,54,57] and chemotherapy-induced neuropathic pain [35,59]. Importantly, targeting cannabinoid receptors in the periphery can limit the incidence of unwanted side effects associated with the activation of CB1 receptors in the CNS. We therefore examined whether URB597, a fatty acid amine hydrolase (FAAH) inhibitor that blocks the breakdown of the endogenous cannabinoid anandamide (AEA), reduced mechanical hyperalgesia and nociceptor sensitization in HbSS-BERK mice and whether this occurred through CB1 or CB2 receptors. A preliminary report has appeared [58].
2. Materials and Methods
2.1. Animals
The current study used adult (5–8 mo) male HbSS-BERK sickle mice, HbSS/CB2R−/− sickle mice, and HbAA-BERK control mice bred in-house and maintained in groups of 1–4 mice per cage maintained on a 12 h light/dark cycle with ad libitum access to food and water. BERK transgenic mice are murine alpha and beta globin knockouts that express human sickle hemoglobin (S) or normal human hemoglobin (A) [53]. These mice show characteristic features of pain observed clinically in patients [10,12,16,38,46]. CB2 receptor knockout mice (Jackson Laboratory, Bar Harbor, ME, USA) were backcrossed with BERK mice to create sickle mice that do not express CB2 receptors (HbSS/CB2R−/−)[60]. Sickle mice with CB2R−/− were identified by PCR with primers specific for CB2R (Cnr2) gene (Jackson Laboratory). HbSS sickle and HbAA control mice were bred and phenotyped for sickle and normal human hemoglobin by iso-electric focusing and genotyped for the knockout of mouse alpha and beta globins and the presence of human alpha and beta-S and hemoglobin transgenes (Transnetyx, Cordova, TN, USA)[38]. All behavioral experiments occurred between 8:00 am and 5:00 pm. A total of 121 mice were used over the course of experiments in this study. For behavioral studies, HbSS-BERK (n = 40), and HbSS-BERK/CBR2−/− (n = 6) were used, while HbSS-BERK (n = 77) and HbAA-BERK (n = 32) mice were used for electrophysiological studies. All protocols and procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and were conducted according to the guidelines established by the International Association for the Study of Pain [66].
2.2. Behavioral measurement of mechanical hyperalgesia
Mice were placed beneath individual glass containers (11 cm L x 6.5 cm W x 5.5 cm H) on a raised wire mesh surface and allowed to habituate for 30–40 min. Mechanical hyperalgesia was evaluated using a calibrated von Frey monofilament (0.4 g, 3.9 mN) applied to the plantar surface of the hind paw through the mesh screen. Pressure was applied until the filament bent slightly, and it was held against the skin for 1–2 s. A withdrawal response was indicated by rapid removal of the hind paw from the monofilament, which was occasionally followed by rapid flinching and/or licking of the paw. The monofilament was applied to each hind paw ten times at intervals of at least 5 s, and the total number of withdrawal responses was recorded for each hind paw.
Baseline measurements were taken over a three day period prior to experiments in order to establish a consistent pattern of hyperalgesia (≥60% withdrawal responses on each hind paw) for HbSS-BERK sickle mice and in order to show that HbAA-BERK control mice did not exhibit high levels of withdrawal responses. Sickle mice that did not exhibit baseline withdrawal response frequencies of at least 60% were not used (4.2% of mice).
2.3. Surgical procedures and electrophysiological recording
Mice were anesthetized using inhaled isoflurane (2–3% induction, 1–2% maintenance). The level of anesthesia was evaluated by applying pressure to the right hind paw or tail and/or testing for corneal reflexes. Once anesthetized, the hair around the left hind leg was removed and an incision was made in the skin over the gastrocnemius muscle, which was dissected and removed to access the tibial nerve. The skin was then sutured to a stainless steel ring (1.3 cm inner diameter) to form a pool that was filled with warm mineral oil. Dental impression material (COE-FLEX, GC America) was applied to the skin and around the ring to prevent oil from leaking out of the pool and to stabilize the hind paw. After the impression material had cured (~10 min), the nerve was gently dissected from surrounding tissue and placed on a small mirror platform in order to perform fine dissection of nerve fibers. The epineurium was cut and removed allowing small bundles of fibers to be cut proximally, teased into fine filaments using fine forceps, and placed on a silver wire recording electrode. Action potentials from individual fibers were amplified, audiomonitored, visualized on an oscilloscope, and stored on a PC using Spike 2.0 software (CED, Cambridge, UK) for off-line analyses.
Nociceptors were initially identified by mild pinching and/or applying pressure to the glabrous skin of the hind paw. Von Frey monofilaments were used to identify the precise location of the receptive field (RF), which was marked on the skin with a felt-tip pen. Conduction velocity (CV) was determined for each fiber. The fiber was stimulated electrically by inserting two fine pin electrodes under the skin outside the RF. Beginning with a voltage below threshold, electrical pulses (200 μs) were delivered every 2 s until the response threshold was reached, and the conduction velocity was calculated using a stimulus of 1.5 times the threshold value. The CV for each fiber was determined by dividing the conduction distance (distance from RF to recording electrode in mm) by the latency to the action potential. Fibers with CV of 1.3 m/s or less were classified as C-fibers, while those with CV between 1.3 m/s and 13.6 were classified as Aδ-fibers and those with CV > 13.6 were classified as Aβ-fibers. For this study, C-fiber and Aδ-fiber nociceptors were studied.
At the completion of the experiment, mice were euthanized via an intraperitoneal injection of Euthasol® (sodium pentobarbital, 390 mg/ml and phenytoin sodium, 90 mg/ml).
2.4. Electrophysiological responses of nociceptors
Once a nociceptor was identified, the rate of spontaneous activity was determined for a period of two minutes before any testing. Mechanical response thresholds were obtained using a set of calibrated von Frey monofilaments. The RF was stimulated multiple times with a single filament, and if no response was elicited, the next higher force (or lower force if there was a response) was applied. Response threshold was defined as the lowest force eliciting a response on 50% or more of the trials. Responses evoked by suprathreshold mechanical stimuli were determined using a single von Frey monofilament that delivered a force of 147 mN. This monofilament was applied three times for 5 s with an inter-stimulus interval of 60 sec. The response to the von Frey stimulus was defined as the mean number of evoked action potentials from the three trials.
A Peltier device (contact area 5 mm2) was used to deliver heat and cold stimuli to the skin. For heat testing, from a base temperature of 32°C, stimuli of 34°C to 50°C were delivered in ascending increments of 2°C. Each stimulus was applied for 5 s every 60 s. The temperature at which the fiber first responded was considered the heat threshold. For cold testing, from a base temperature of 32°C, stimuli of 28°C to 0°C were delivered in descending increments of 4°C. Each stimulus was applied for 10 s every 120 s. Nociceptors that did not respond to any heat or cold stimuli were classified as either C- or Aδ-mechanonociceptors (CM or AδM). Fibers that responded to mechanical, heat, and cold stimuli were classified as C- or Aδ-mechanoheatcold nociceptors (CMHC or AδMHC). Fibers that responded to mechanical and heat, but not cold were classified as either C-mechanoheat nociceptors (CMH) or Aδ-mechanoheat nociceptors (AδMH). Finally, fibers that responded to mechanical and cold, but not heat, were classified as either C-mechanocold nociceptors (CMC) or Aδ-mechanocold nociceptors (AδMC). We were unable to apply heat or cold to some fibers due to the location of the RF. For a certain proportion of fibers, the response to stimuli of 50°C and 0°C was used to classify the fiber type, but additional testing was not performed in order to prevent further sensitization of the fiber prior to drug administration.
2.5. Drug preparation and administration
The FAAH inhibitor (3′-(aminocarbonyl)[1,1′-biphenyl]-3-yl)-cyclohexylcarbamate (URB597, Cayman Chemical) was prepared in a 10 μg/μl stock solution of 100% ethanol and diluted to 1 μg/μl with Tween-80 and saline for vehicle consisting of 89.4% saline, 10% ethanol, and 0.6% Tween-80. The CB1 receptor antagonist 1-(2,4-Dichlorophenyl)-5-(4- iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide (AM 281, Tocris Bioscience) and the CB2 receptor antagonist 6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4- methoxyphenyl)methanone (AM 630, Tocris Bioscience) were prepared in stock solutions of 10 μg/μl in DMSO and diluted to 1 μg/μl in saline. URB597 was administered at a dose of 10 μg in 10 μl and the antagonists were administered at a dose of 10 μg in 10 μl. Vehicle injections were also given in a volume of 10 μl. All injections were administered into the plantar surface of the hind paw using a 0.3 ml syringe with a 30 g needle. For the electrophysiology studies, care was taken to insert the needle just outside the RF.
2.6. Experimental design
The frequency of paw withdrawal responses was determined for three days prior to the start of experiments. HbSS-BERK sickle mice and HbAA-BERK control mice were assigned to behavioral and/or electrophysiological experiments. HbSS-BERK/CB2R−/− sickle mice were used for behavioral experiments only.
In order to assess the anti-hyperalgesic effect of URB597, HbSS-BERK sickle mice with consistent mechanical hyperalgesia were assigned to one of four treatment groups: URB597 (1μg/μl), AM281 + URB597 (both 1 μg/μl), AM630 + URB597 (both 1 μg/μl) or the vehicle for URB597 (89.4:10:0.6 of saline:ethanol:Tween-80). The CB1 receptor antagonist AM281 and the CB2 receptor antagonist AM630 were injected 5 min prior to URB597. Each injection was given in a 10 μl volume into the plantar region of the right hind paw using a 30 g needle and insulin syringe. Following injection, paw withdrawal responses were assessed for both the ipsilateral and contralateral hind paws every 30 min for 2 h. For experiments using the antagonists, post-injection time is relative to the final injection (URB597). The experimenter was blinded to the treatment condition.
HbSS-BERK/CBR2−/− sickle mice with consistent hyperalgesia received a 10 μl i.pl. injection of either URB597 (1 μg/μl) or its vehicle into the right hind paw and both hind paws were assessed for paw withdrawal responses every 30 min for 2 h. After a 24 h washout period, paw withdrawal responses were assessed again to confirm the presence of hyperalgesia, followed by a 10 μl i.pl. injection into the left hind paw and paw withdrawal assessments every 30 min for 2 h. Mice that had been injected with URB597 in the right paw on the previous day received vehicle in the left paw, and vice versa, so that each mouse was exposed to both treatment conditions.
Electrophysiological studies were performed in order to compare the characteristics of peripheral nociceptors in HbSS-BERK sickle mice with HbAA-BERK control mice. Mice were assessed for mechanical hyperalgesia prior to electrophysiology experiments, and any subjects used for behavioral experiments were allowed at least 72 h for a washout period prior to this assessment. Further, electrophysiological experiments were conducted on the non-injected left hind paw.
For the first part of the study, all well-isolated C- and Aδ-fibers encountered during electrophysiological experiments were studied. Once a fiber was isolated, the presence or absence of spontaneous activity was determined first by recording 2 min of activity in the absence of stimulation. This was followed by determining the mechanical response threshold, responses evoked by a suprathreshold mechanical stimulus (147 mN), heat threshold (if applicable), cold threshold (if applicable), and conduction velocity (CV).
The second part of the study focused on the effects of URB597 on responses of sensitized C-fiber nociceptors in HbSS-BERK sickle mice only. For these experiments, only C-fibers with spontaneous discharge during the initial 2 min of recording were studied. Once a fiber had been isolated, spontaneous activity and evoked responses were determined. Then drug or vehicle was injected into the RF and responses were determined every 30 min for 2 h. Injections consisted of the FAAH inhibitor URB597 alone or with CB1 (AM281) or CB2 (AM630) receptor antagonists and the vehicle for URB597. Antagonists were administered 5 min prior to injection of URB597. For experiments using the antagonists, post-injection time is relative to the final injection (URB597).
2.7. Data analysis
The frequency of withdrawal responses evoked by the 3.9 mN von Frey monofilament was expressed as the number of positive responses out of 10 trials. A two-way analysis of variance (ANOVA) with repeated measures was used to compare the difference between withdrawal responses before and after i.pl. administration of URB597, AM281 + URB597, AM630 + URB597 or vehicle (for HbSS-BERK sickle mice) and URB597 or vehicle (for HbSS-BERK/CBR2−/− sickle mice). Fisher’s LSD post-hoc tests were used to determine differences between the groups at specific time points.
Non-parametric analyses (Chi-Square tests) were used to compare the distribution of spontaneously active C-fiber and Aδ-fiber nociceptors in nociceptors isolated from HbSS-BERK sickle in comparison to those isolated in HbAA-BERK control mice. Median mechanical response thresholds (mN) of C-fiber and Aδ-fiber nociceptors from HbSS-BERK sickle and HbAA-BERK control mice were also compared using non-parametric analyses (Mann-Whitney U test).
Evoked responses of each fiber to the suprathreshold von Frey monofilament were determined by subtracting the number of spontaneous impulses during the 5 sec immediately preceding each stimulus from the number of impulses evoked during the stimulus. The average number of impulses over three trials was determined. The mean numbers of evoked impulses were compared between HbSS-BERK sickle mice and HbAA control mice for both C-fiber and Aδ-fiber nociceptors using an independent t-test.
Response thresholds and evoked responses to heat and/or cold stimuli were assessed in C-fiber nociceptors only due to the small number of Aδ-fibers that were found to be responsive to heat and/or cold. Heat thresholds (in °C) were compared between HbSS-BERK sickle mice and HbAA-BERK control mice for C-fiber nociceptors using non-parametric analysis (Mann-Whitney U test). Evoked responses were calculated for heat stimuli by subtracting the number of spontaneous impulses during the 5 s immediately preceding the stimulus from the number of impulses evoked during the stimulus. Differences in heat responses between HbSS-BERK sickle mice and HbAA-BERK control mice for C-fiber nociceptors were determined using repeated measures ANOVA with temperature (36, 38, 40, 42, 44, 46, and 50°C) as the within-subjects factor. Cumulative evoked response values were calculated using the sum of all evoked responses from 36°C to 50°C for each individual fiber. These values were compared between HbSS-BERK sickle mice and HbAA control mice for C-fiber nociceptors using independent t-tests.
Cold response thresholds (°C) were compared between HbSS-BERK sickle mice and HbAA-BERK control mice for C-fiber nociceptors using non-parametric analysis (Mann-Whitney U test). Evoked responses were calculated for cold stimuli by subtracting the number of spontaneous impulses during the 10 s immediately preceding the stimulus from the number of impulses evoked during the stimulus. Differences in cold responses between HbSS-BERK sickle mice and HbAA control mice for C-fiber nociceptors were determined using repeated measures ANOVA with temperature (28, 24, 20, 16, 12, 8, 4, and 0°C) as the within-subjects factor. Cumulative evoked responses were calculated by determining the sum of all evoked responses from 28°C to 0°C for each individual fiber. These values were compared between HbSS-BERK sickle mice and HbAA-BERK control mice for C-fiber nociceptors using an independent t-test.
To determine changes in response characteristics produced by URB597 (alone or in combination with AM281 or AM630) in comparison to vehicle control, we compared spontaneous activity, mechanical response thresholds, and responses evoked by a 147 mN von Frey monofilament before and after injection. A log transformation was performed on mechanical response thresholds (mN) to correct for positively skewed data among post-injection time points in order for parametric analyses to be performed. Data for the evoked response to a suprathreshold mechanical stimulus (147 mN) were expressed as percent change from pre-injection values. Log-transformed mechanical response thresholds, the percent change in response to suprathreshold mechanical stimulus, and the rate of spontaneous discharge were evaluated using two-way ANOVA with repeated measures. Post-hoc evaluations (Fisher’s LSD) were performed to determine differences between the groups at specific time points and changes within each group over time. All data are expressed as mean ± SEM or median [25th percentile, 75th percentile].
3. Results
3.1. Sensitization of nociceptors in HbSS-BERK sickle mice
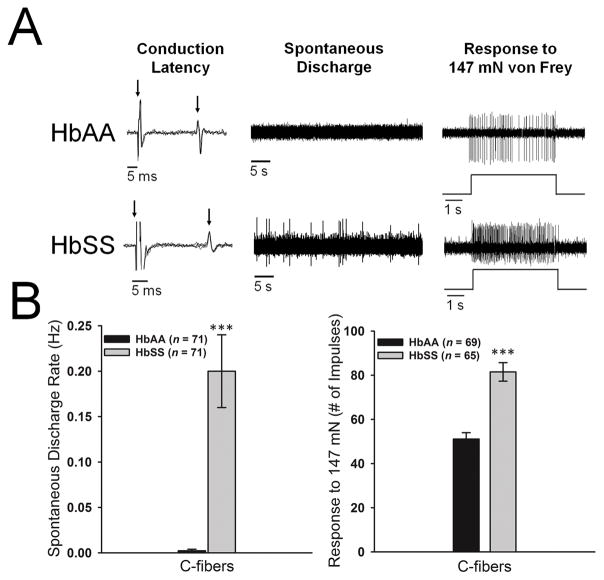
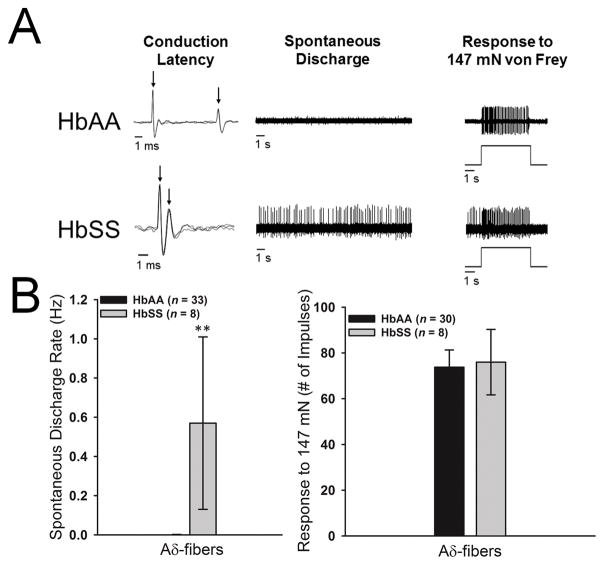
A total of 153 C-fiber and 41 Aδ-fiber nociceptors were studied for comparison between HbSS-BERK sickle mice (n = 77) and HbAA-BERK control mice (n = 32). Examples of conduction latency, spontaneous activity, and evoked responses of individual C- and Aδ-fibers from HbAA-BERK and HbSS-BERK mice are shown in Figures 1A and 2A. There were no differences between the groups in nerve fiber conduction velocities for C-fibers (0.57±0.03 m/s vs 0.60±0.02 m/s) or Aδ-fibers (6.2±1.0 m/s vs 6.0±0.7 m/s). A large proportion of C-fibers (70 of 81, 86.4%) and Aδ-fibers (4 of 9, 44.4%) in HbSS-BERK sickle mice exhibited spontaneous discharge, while only 2.8% (2 of 72) of C-fibers and 3.0% (1 of 33) of Aδ-fibers in HbAA-BERK control mice had spontaneous discharge. The proportion of fibers with spontaneous activity was higher in HbSS-BERK sickle mice than HbAA-BERK control mice for both C-fibers (χ2[1] = 96.36, p<.001) and Aδ-fibers (χ2 [1] = 13.99, p<.001). The mean rate of spontaneous discharge was also higher in HbSS-BERK sickle mice than HbAA-BERK control mice for both C-fibers (t137= 5.0, p<.001) and Aδ-fibers (t39= 2.9, p<.01; see Figures 1B and 2B).
Figure 1.
A: Representative examples of C-fiber nociceptors isolated from HbAA-BERK control (top row) and HbSS-BERK (bottom row) mice. Left column: overlapping traces of conduction latency. Left arrow indicates onset of the stimulus and the right arrow indicates the evoked action potential. Middle column: examples of spontaneous activity for a period of 45 s. Right column: example of a response evoked by 147 mN applied for 5 s. Time of stimulation is illustrated below the evoked response. B: The mean spontaneous discharge rate and responses to 147 mN were higher in C-fibers isolated from HbSS-BERK sickle mice in comparison to HbAA-BERK control mice. ***p<.001 vs HbAA-BERK mice.
Figure 2.
A: Representative examples of Aδ-fiber nociceptors isolated from HbAA-BERK control (top row) and HbSS-BERK (bottom row) mice. Left column: overlapping traces of conduction latency. Left arrow indicates onset of the stimulus and the right arrow indicates the evoked action potential. Middle column: examples of spontaneous activity for a period of 45 s. Right column: example of a response evoked by 147 mN force for 5 s. Time of stimulation is illustrated below the evoked response. B: The mean spontaneous discharge rate was significantly higher in Aδ-fibers isolated from HbSS-BERK sickle mice in comparison to HbAA-BERK control mice. ***p<.01 vs HbAA-BERK mice.
Among C-fibers, the median mechanical response threshold was lower in HbSS-BERK sickle mice (9.8[7.9,13.7] mN vs 16.7[9.8,39.2] mN; Mann-Whitney U = 1321.0, n1 = 70, n2 = 61, p<.001), and responses evoked by 147 mN stimulation were higher in HbSS-BERK mice compared to HbAA-BERK control mice (81.5±4.2 impulses vs. 51.1±2.9 impulses; t128 = 6.0, p<.001, see Figure 1B). For Aδ-fibers, the median mechanical response threshold was lower in HbSS-BERK sickle mice compared to HbAA-BERK control mice (13.7[3.9,13.7] mN vs 39.2[19.6,58.8] mN; Mann-Whitney U = 25.00, n1 = 31, n2 = 7, p=.001, see Figure 2B); however, responses evoked by 147 mN stimulation did not differ between the groups (76.0±14.3 impulses vs 73.8±7.5 impulses; t36 = 0.1, n.s., see Figure 2B). Spontaneous activity, lowered response thresholds, and elevated responses to noxious stimuli are all hallmarks of nociceptor sensitization and occur in models of inflammation, bone cancer pain and chemotherapy-induced peripheral neuropathy [4,13,59].
A total of 59 nociceptors exhibited sensitivity to heat and/or cold, with 12 classed as CMH, 30 as CMC, 9 as CMHC, 7 as AMC and 1 as AMHC. A proportion of heat and/or cold responsive fibers were only tested for classification purposes and thresholds were not assessed in order to prevent further sensitization prior to drug testing. In addition, since the number of Aδ–fibers that responded to heat and/or cold was very low (data not shown), we did not conduct analyses on thresholds or evoked responses.
Median heat response thresholds did not differ between C-fibers from HbSS-BERK sickle mice (40[38,43]°C) and those from HbAA-BERK control mice (42[40,42]°C; Mann-Whitney U = 37.0, n1 = 9, n2 = 11, n.s.). In C-fibers exposed to the entire range of heat stimuli, those from HbSS-BERK mice exhibited higher cumulative responses (118.9±16.2 impulses vs. 65.7±18.2 impulses; t18 = 2.1, p<.05), but responses to individual temperatures did not differ between the groups (F1,18 = 0.5, n.s., Figure 3A). Among cold-sensitive C-fibers, those isolated from HbSS-BERK sickle mice had higher median response thresholds compared to fibers from HbAA-BERK control mice (24.0[20.0,28.0]°C vs. 14.0[8.0,20.0]°C; Mann-Whitney U = 41.00, n1 = 22, n2 = 15, p<.001). The number of impulses was greater for all stimuli from 28°C to 0°C in C-fibers isolated from HbSS-BERK sickle mice (group: F1,32 = 12.1, p<.001; temperature: F7,224 = 15.6, p<.001, Figure 3B). The cumulative number of impulses evoked by stimuli of 28°C to 0°C were higher among C-fibers from HbSS-BERK sickle mice (166.0±27.8 impulses) as compared to fibers from HbAA-BERK control mice (63.1±15.4 impulses; t32 = 3.5, p<.005). Sensitization of nociceptors to heat and cold stimuli parallels hyperalgesia to heat and cold described in sickle mice and in patients [10,38].
Figure 3.
A: The mean (±SEM) discharge rate evoked by heat stimuli of 36°C to 50°C in C-fibers isolated from HbAA-BERK control mice and HbSS-BERK sickle mice. B: The mean (±SEM) discharge rate evoked by cold stimuli of 28°C to 0°C for C-fibers isolated from HbAA-BERK control mice and HbSS-BERK sickle mice. *p<.05, **p<.01, ***p≤.005 indicates differences from HbAA-BERK mice.
3.2. URB597 decreased spontaneous activity and responses to mechanical stimuli in sensitized C-fiber nociceptors
Based on the proportion of spontaneous active fibers, lower mechanical response thresholds and elevated responses to mechanical stimuli, sensitization of nociceptors in HbSS-BERK mice appeared to be more robust in C-fibers than in Aδ-fibers. We therefore focused on C-fibers in order to determine whether URB597 would attenuate sensitization in HbSS-BERK sickle mice. Fibers were divided into separate treatment groups that received an intraplantar injection (10 μl) of vehicle, URB597 (1 μg/μl), AM281 + URB597 (both 1 μg/μl), or AM630 + URB597 (both 1 μg/μl) into their RF. A total of 37 C-fiber nociceptors were studied before and after drug treatment. Prior to any injection, the mean rate of spontaneous discharge was 0.34±0.11 Hz, the median mechanical response threshold was 11.8[9.8,13.73] mN, and the mean number of impulses evoked by the suprathreshold von Frey stimulus was 83.3±5.1 impulses for all fibers. These values did not differ among the treatment groups. Once a C-fiber was characterized, vehicle or drug was injected into the RF. Injections of the antagonists preceded the injection of URB597 by 5 min.
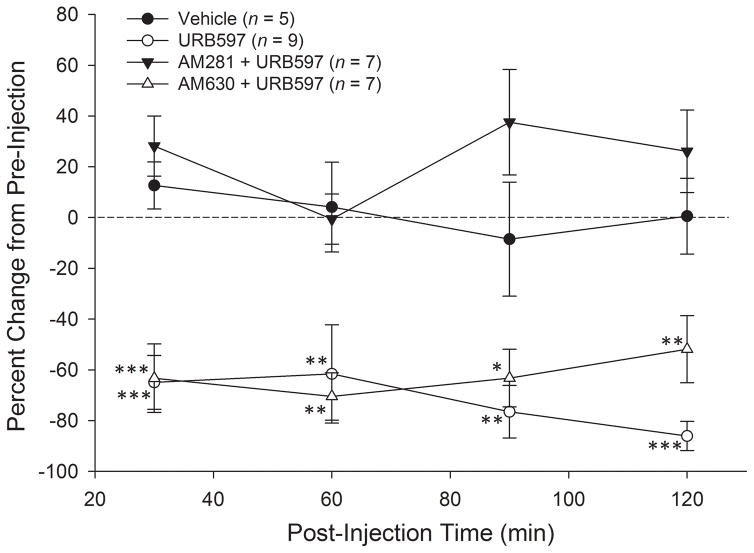
Spontaneous activity was decreased following injection of URB597 (Figure 4). Fibers with a baseline rate of spontaneous discharge less than 0.05 Hz were not included (n = 8). Spontaneous discharge rates for individual fibers at 30, 60, 90 and 120 min after injection were converted to percent change scores relative to pre-injection values. Two-way ANOVA revealed a significant difference among the groups in percent change of spontaneous activity following injection (F3,24 = 25.3, p<.001). Prior to injection there were no differences in spontaneous discharge rate among the four treatment groups. Administration of URB597 decreased spontaneous activity relative to the vehicle-treated group at 30, 60, 90, and 120 min after injection. Injection of vehicle did not cause any change in spontaneous discharge at any time after injection. Pre-treatment with CB1 receptor antagonist, AM281, blocked the effect of URB597, and it did not differ from vehicle at any time point. Pre-treatment with AM630 (CB2 receptor antagonist) did not alter the effect of URB597 at any time point, and treatment with AM630 + URB597 resulted in a decrease in spontaneous discharge relative to vehicle at all time points post-injection.
Figure 4.
URB597 attenuated spontaneous activity in C-fiber nociceptors in HbSS-BERK sickle mice. Data illustrate the mean (±SEM) percent change in spontaneous activity at 30, 60, 90 and 120 min after various treatments. Intraplantar administration of URB597, which inhibits the breakdown of anandaminde (AEA) by fatty acid amide hydrolase (FAAH), decreased spontaneous activity relative to vehicle treatment at 30, 60, 90, and 120 min post-injection, and this was blocked by the CB1 receptor antagonist AM281. Pre-treatment with the CB2 receptor antagonist, AM630, failed to block the effect of URB597 and these fibers showed significantly lower rates of spontaneous activity relative to vehicle at 30, 60, 90, and 120 min post-injection. *p<.05, **p≤.01, ***p<.001 indicates significant differences from the vehicle-treated group.
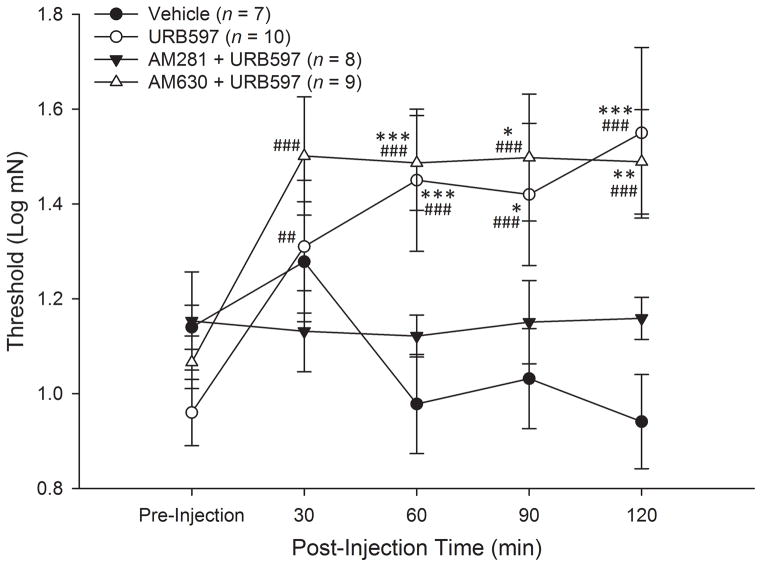
Log-transformations of mechanical response thresholds were determined before and at 30, 60, 90 and 120 min after injection. Two-way ANOVA with repeated measures indicated a significant group x time interaction (F12,120 = 5.3, p<.001) and a main effect for time (F4,120 = 7.2, p<.001), but not for treatment group (F3,30 = 2.5, p=.08). Post-hoc comparisons showed that while there were no differences in response thresholds among the four treatment groups before injection, there were differences between the groups at 60, 90 and 120 min after injection. Vehicle treatment did not alter mechanical response thresholds at any time whereas injection of URB597 increased thresholds at 60, 90 and 120 min after injection (Figure 5). This effect was completely blocked by pre-treatment with the CB1 receptor antagonist AM281. In contrast, pre-treatment with the CB2 receptor antagonist AM630 did not alter the effect of URB597 and mechanical response thresholds for these fibers and were increased relative to pre-injection values at 60, 90 and 120 min after injection.
Figure 5.
URB597 increased mechanical response thresholds in C-fibers from HbSS-BERK sickle mice. Data show the mean (±SEM) log-transformed mechanical thresholds before and at 60, 90, and 120 min post-injection. Mechanical thresholds were elevated following URB597 and this was blocked by the CB1 receptor antagonist AM281. In contrast, the CB2 receptor antagonist AM630 failed to block the effect of URB597, and thresholds following AM630 + URB597 were decreased at 60, 90, and 120 min after injection. *p<.05, **p<.01, ***p≤.005 indicates a significant difference from the vehicle-treated group. ##p<.005, ###p≤.001 indicates significant differences from pre-injection.
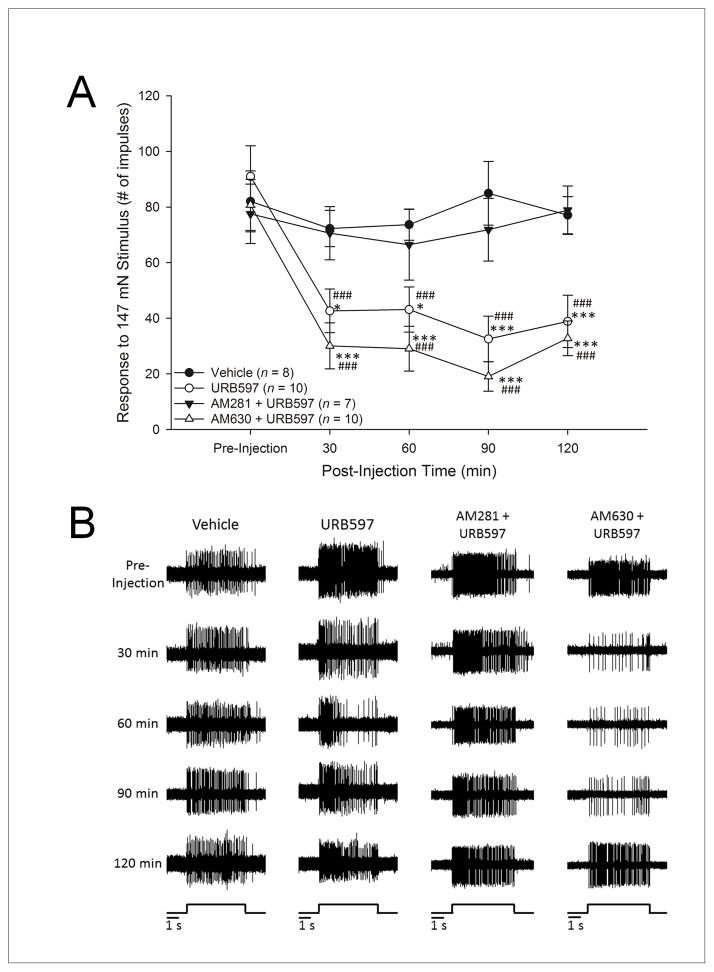
Responses to the suprathreshold mechanical stimulus (147 mN for 5 s) were assessed by counting the number of evoked action potentials. Prior to any injection, the number of evoked impulses did not differ among the four treatment groups. Two-way ANOVA revealed a significant difference among the groups in their evoked number of impulses (F3,31 = 6.5, p<.005) and over time (F4,124 = 22.4, p<.001). Post-hoc comparisons (Fisher’s LSD) revealed that responses were decreased following the injection of URB597 at 30, 60, 90, and 120 min post-injection relative to responses obtained prior to injection and to responses following vehicle treatment (Figure 6A). Pre-treatment with AM281 blocked the effect of URB597, and responses did not differ at any time from those following vehicle. The decrease in evoked responses produced by URB597 was not altered by AM630 and the responses following treatment with AM630 + URB597 were lower at 30, 60, 90 and 120 min after injection relative to responses prior to injection and to responses following vehicle treatment (Figure 6A). Representative examples of individual C-fibers showing the effects of vehicle, URB597, AM281 + URB597 and AM630 + URB597 on the number of evoked impulses over time are provided in Figure 6B.
Figure 6.
A: Intraplantar administration of URB597 decreased evoked responses in C-fiber nociceptors isolated from HbSS-BERK sickle mice. Data show the mean (±SEM) number of impulses evoked by 147 mN before and at 30, 60, 90 and 120 min after various drug treatments. The number of evoked impulses was reduced at 30, 60, 90, and 120 min following intraplantar administration of URB597, and this effect was blocked by the CB1 receptor antagonist AM281. Pre-treatment with the CB2 receptor antagonist AM630 did not alter the effect of URB597, and these fibers exhibited lower responses to 147 mN at 30, 60, 90, and 120 min after injection. *p<.05, **p<.005, ***p≤.001 vs. the vehicle-treated group. ###p<.001 indicates significant differences from pre-injection value.
B: Representative examples of responses of individual C-fibers evoked by 147 mN for 5 s before (pre-injection) and at 30, 60, 90 and 120 min after intraplantar injection of either vehicle, URB597, URB597 + AM281, or URB597 + AM630. The time of mechanical stimulation is illustrated at the bottom of each column. Evoked activity was reduced by URB597 and this was blocked by the CB1 receptor antagonist AM281.
3.3. Intraplantar administration of URB597 reduced mechanical hyperalgesia in HbSS-BERK sickle mice
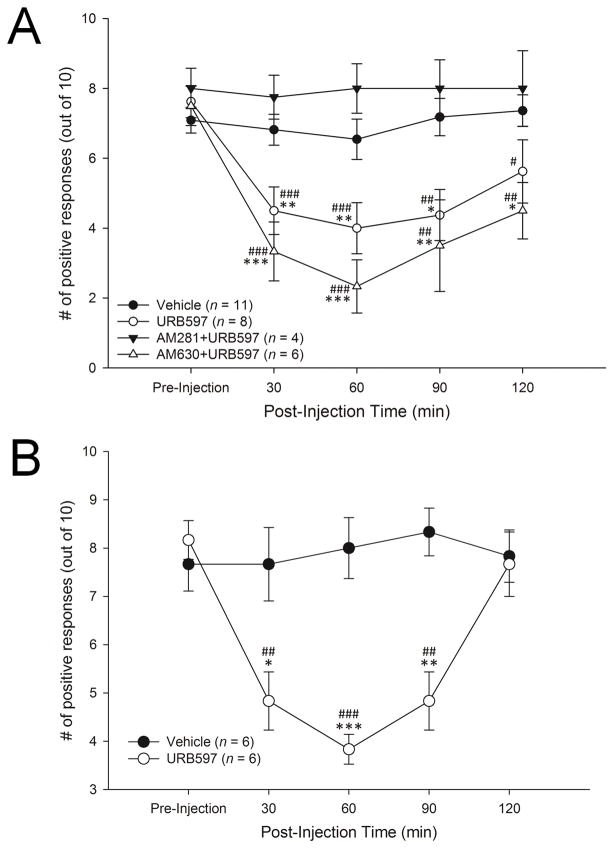
Twenty-nine HbSS-BERK sickle mice were used to assess the anti-hyperalgesic effect of URB597. The mean (±SEM) frequency of paw withdrawal responses was reduced by URB597 (group: F3,25 = 11.33, p<.001; time: F4,100 = 8.6, p<.001). Post-hoc comparisons (Fisher’s LSD) indicated that while withdrawal responses of URB597- and vehicle-treated mice did not differ before injection, URB597 decreased paw withdrawal frequency at 30, 60 and 90 min after injection (Figure 7A). Pre-treatment with the CB1 receptor antagonist AM281 completely blocked this effect and responses in these mice did not differ from pre-injection or vehicle at any time point. In contrast, the effect of URB597 was not altered by pre-treatment with CB2 receptor antagonist AM630, and these mice exhibited decreased response frequencies from 30 to 120 min post-injection. Response frequencies among vehicle-treated mice remained constant throughout the treatment period (Figure 7A).
Figure 7.
A: URB597 decreased withdrawal response frequency in hyperalgesic HbSS-BERK sickle mice. Intraplantar administration of 10 μg URB597 decreased mean (±SEM) paw withdrawal frequency at 30, 60, 90, and 120 min after injection. This effect was blocked by pretreatment with the CB1 receptor antagonist AM281 but not the CB2 receptor antagonist AM630, and response frequencies decreased at 30, 60, 90, and 120 min post-injection. *p<.05, **p<.01, ***p<.001 vs. vehicle-treated group. #p<.05, ##p<.01, ###p<.001 indicates significant differences from pre-injection.
B: Intraplantar administration of URB597 decreased paw withdrawal frequency in hyperalgesic HbSS-BERK/CBR2−/− mice. URB597 decreased paw withdrawal frequency at 30, 60, and 90 min after administration in sickle mice lacking CB2 receptors. *p<.05, **p<.01, ***p<.001 vs. vehicle-treated group. ##p<.01, ###p<.001 indicates significant differences from pre-injection.
We also conducted a set of control experiments with the antagonists and their vehicle. We injected the vehicle for AM281 and AM630 (90:10, saline:DMSO) 5 min prior to the vehicle for URB597 in order to assess the effect of multiple injections and to determine whether the vehicle for the antagonists had an impact on mechanical hyperalgesia. These treatments did not alter paw withdrawal frequency at 30, 60, 90 or 120 min after injection (n = 6, pre-injection withdrawal frequency 7.2±0.5; F4,20 =0.2, n.s.; data not shown). Further, we determined whether the CB1 receptor antagonist AM281 or the CB2 receptor antagonist AM630, given in the absence of URB597, altered mechanical hyperalgesia. Neither AM281 (n = 10) nor AM630 (n = 6) had any impact on paw withdrawal frequency at 30, 60, 90 or 120 min post-injection (AM281: pre-injection withdrawal frequency 7.5±0.3; F4,36 = 0.5, n.s., AM630: pre-injection withdrawal frequency 7.0±0.3; F4,16 = 1.3, n.s., data not shown). Since neither the antagonists nor their vehicle altered withdrawal responses, these were not tested in the electrophysiology experiments and we used the vehicle for URB597 as a control for these studies.
3.4. Intraplantar administration of URB597 reduced mechanical hyperalgesia in HbSS-BERK/CB2R−/− sickle mice
Twelve HbSS-BERK/CB2R−/− sickle mice were used to further determine the role of CB1 and CB2 receptors in the anti-hyperalgesic effect of URB597. The frequency of paw withdrawal responses differed between treatments (Group: F1,10 = 23.00, p=.001; Time: F4,40 = 5.52, p=.001). Post-hoc comparisons (Fisher’s LSD) showed that injection of URB597, but not vehicle, decreased mean (±SEM) paw withdrawal frequency 30, 60, and 90 min after injection (Figure 7B).
4. Discussion
This study shows for the first time in vivo that nociceptors recorded from sickle mice exhibit sensitization characterized as an increase in the proportion of nociceptors with spontaneous activity and enhanced responses evoked by mechanical, heat and cold stimuli. These results are consistent with earlier studies showing nociceptor sensitization in sickle mice using a skin-nerve preparation [27,65]. It is likely that spontaneous activity of nociceptors contributes to ongoing pain and the increase in responses to mechanical, heat and cold stimuli contributes to mechanical and cold hyperalgesia seen in patients with SCD [10,16]. We also showed that blocking the enzyme that breaks down the endocannabinoid anandamide decreased sensitization of nociceptors and hyperalgesia in HbSS-BERK sickle mice. Thus, increasing AEA in the periphery by blocking its hydrolysis may have beneficial effects in managing pain in SCD.
Sickle cell disease involves a complex pain syndrome that is often resistant to treatment [5]. Growing concern over the chronic use of opioids as primary therapy for pain has highlighted the importance of alternative therapies and complementary treatment strategies. The use of cannabinoids for the treatment of pain in SCD and other chronic pain disorders has been recognized [30,38,60,61], and elucidating the mechanisms underlying their anti-nociceptive properties is vital for the development of cannabinoid-based treatment strategies. While more than one-third of SCD patients have self-reported the use of cannabis for relief of pain and other symptoms, the majority also reported experiencing sedation and effects on mood [30]. Precisely targeted cannabinoid therapy could minimize or eliminate unwanted side effects while still achieving desired pain relief [21].
Transgenic mouse models of SCD provide an opportunity for developing new mechanism-based pain therapies in a model that replicates the genotype and phenotype of this disease. As previously reported, HbSS-BERK mice exhibit robust mechanical hyperalgesia relative to HbAA-BERK control mice [12,38]. In the present study, mechanical hyperalgesia was attenuated by intraplantar administration of the FAAH inhibitor URB597 starting at 30 min after injection and lasting for about 2 hours. This effect was blocked by the CB1 receptor antagonist AM281, but not the CB2 receptor antagonist AM630. Further, the anti-hyperalgesic effect of URB597 was not altered in CB2 receptor knockout sickle mice. Thus, the primary mechanism for the acute anti-hyperalgesic effect of URB597 appears to be the activation of CB1 receptors by increased levels of AEA. This could occur via AEA binding to CB1 receptors located either on peripheral nociceptor terminals or on cells in the vicinity of nociceptors (i.e., keratinocytes). Previous studies showed that activation of CB1 receptors using cannabinoid receptor agonists reduced hyperalgesia in HbSS-BERK mice [38,60]. Moreover, chronic administration of the non-selective cannabinoid receptor agonist CP 55,940 attenuated hyperalgesia in sickle mice over a 3-week period with no decrease in efficacy [60]. Also, cannabinoid receptor-selective agonists decreased cutaneous or deep tissue hyperalgesia over a 7-day treatment period. It appears that these antihyperalgesic effects were due, at least in part, to decreased activity of nociceptors. The efficacy of drugs that target endocannabinoid activity in the periphery of sickle mice could be related to altered endocannabinoid tone which could alter both the level of endocannabinoids (including AEA) and the expression and/or function of cannabinoid receptors, which may explain why CB2 does not appear to contribute to the anti-hyperalgesic effect of URB597.
Characterization of response properties of primary afferent nociceptors in HbSS-BERK mice showed a clear pattern of sensitization among both C- and Aδ-fiber nociceptors. HbSS-BERK mice had a higher proportion of nociceptors with spontaneous discharge, lower response thresholds to mechanical and cold stimuli, and enhanced responses to suprathreshold mechanical and cold stimuli as compared to HbAA-BERK control mice. Abnormal spontaneous discharge along with lower response thresholds and/or increased evoked responses are consistent with nociceptor sensitization observed in other chronic pain conditions, including inflammation, diabetic neuropathy, post-herpetic neuralgia, bone cancer pain and chemotherapy-induced peripheral neuropathy [4,6,13,52,59]. Increased responses to mechanical stimuli have been observed in C-fiber and high-threshold Aδ-fiber nociceptors, as well as in non-nociceptive Aβ and rapidly adapting Aδ D-hair afferent fibers in sickle mice [22,27]. Increased activity in nociceptors and mechanoreceptors likely contributes to enhanced responses of nociceptive dorsal horn neurons in HbSS-BERK mice [14]. Collectively, these studies indicate that ongoing pain and hyperalgesia in SCD involves the sensitization of multiple fiber types.
Hyperalgesia to thermal stimuli, particularly cold, is also characteristic of SCD. Patients with SCD had decreased pain thresholds for heat and cold stimuli [10]. Consistent with patient reports, hyperalgesia to cold and heat occurs in HbSS-BERK sickle mice [12,38,27,48,65]. In our study, C-fiber nociceptors sensitive to heat stimuli did not show lower thresholds but cumulative responses were higher than those in HbAA mice. We also showed that C-fiber nociceptors in HbSS-BERK mice had increased responses to cold stimuli and elevated response thresholds as compared to control mice, which is consistent with earlier reports [65]. Changes in response characteristics in thermal-sensitive nociceptors could be mediated by altered expression and function of ion channels involved in the detection of thermal stimuli, including TRPV1. Inhibition of TRPV1 in sickle mice attenuated mechanical hyperalgesia and decreased mechanical responses in DRG neurons [27].
Several pathophysiological mechanisms in SCD have been shown to promote hyperalgesia and nociceptor sensitization. Immunoreactivity for SP and CGRP, two neuropeptides known to play a role in the transmission of pain and neurogenic inflammation, were increased in the skin of HbSS mice [38]. Sickling also releases pro-inflammatory cell components which activate immune and endothelial cells, triggering the release of additional pro-inflammatory proteins and cytokines [25]. For example, degranulation of mast cells contributes to neurogenic inflammation and pain in sickle mice, and cannabinoid agonists inhibited mast cell degranulation and hyperalgesia in HbSS-BERK mice [60,61].
Consistent with our behavioral studies, administration of URB597 into the RF of C-fiber nociceptors decreased spontaneous discharge, increased mechanical response thresholds, and attenuated mechanically-evoked responses; effects that were blocked by pre-treatment with the CB1 antagonist AM281 but not the CB2 antagonist AM630. These results demonstrate that the anti-hyperalgesia produced by the inhibition of FAAH occurred through activation of CB1 receptors. In earlier studies we showed that URB597 elevated levels of AEA in the skin without altering levels of other endocannabinoids [36,59], suggesting that AEA binding to CB1 receptors in the periphery is the primary mechanism by which URB597 produced anti-hyperalgesia. There are several possible sources of AEA in the periphery, including keratinocytes, sensory neurons and macrophages. Human keratinocytes have been shown to possess all of the necessary components for binding and metabolizing AEA: the CB1 receptor, AEA-specific membrane transporter (AMT), and enzymes for the synthesis (phospholipase D) and breakdown (FAAH) of AEA [41]. Neurons and macrophages have been shown to produce AEA in response to membrane depolarization [8,19,20,62]. These studies suggest that URB597 increases tissue levels of AEA from a variety of sources.
There are multiple mechanisms by which AEA could reduce nociceptor sensitization and pain in sickle mice. Endocannabinoids including AEA have been shown to inhibit TRPV1 via CB1 receptors in primary sensory neurons [45]. CB1 receptor mRNA is expressed predominately in intermediate to large neurons [11,29], whereas CB1 receptor-immunoreactivity has been localized to small [1] and large neurons [37]. AEA binding to CB1 receptors would modulate the release of peptides and other neuromodulators that sensitize nociceptors, possibly through G-proteins which inhibit N- and P/Q-type calcium channels [42–44,56] and increase K+ conductance [18,44]. There is also evidence that cannabinoid receptors modulate the activity of voltage-gated potassium channels and have an inhibitory effect on calcium channels in sensory neurons [34,37,51].
One concern that has been raised with FAAH inhibitors such as URB597 is that while AEA has a CB1 receptor-mediated inhibitory effect on cultured primary sensory neurons at concentrations below 500 nM, it has also been shown to sensitize cultured primary sensory neurons via TRPV1 at concentrations higher than 1 uM, and this effect may actually be enhanced under inflammatory conditions [1,55]. Based on our previous experience with URB597, we do not believe that the increase in the concentration of AEA produced is sufficient to reach the threshold for TRPV1-mediated sensitization. While we did not explore the effects of higher doses of URB597 on AEA levels in the current study, the impact of AEA binding TRPV1 should be further examined in future studies with this drug. Another potential concern is that inhibition of FAAH not only increases the level of AEA, but also impacts the level of other fatty acid amides. These compounds may not be endogenous ligands for cannabinoid receptors, but alterations in their tone could cause non-specific effects that are not captured by studies focusing on the endocannabinoid system.
In conclusion, this study demonstrates that blocking the hydrolysis of the endocannabinoid AEA reduces hyperalgesia and nociceptor sensitization in mice with SCD. Since opioids are commonly used to treat pain in SCD, it will be important to determine whether elevating levels of endocannabinoids will augment opioid analgesia in SCD. This approach may reduce the doses of opioids needed and thereby reduce their associated side effects while maintaining effective pain control.
Acknowledgments
This work was supported by NIH grants HL103773, HL117664 and HL135895. The authors would like to thank Barbara Benson, Catherine Harding-Rose, Abraham Freybler, Ritu Jha, Katherine NH Johnson, Susan Thompson and Huy Tran for breeding, genotyping, phenotyping mice and/or technical assistance.
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100(4):685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- 2.Aich A, Afrin LB, Gupta K. Mast cell-mediated mechanisms of nociception. Int J Mol Sci. 2015;16(12):29069–29092. doi: 10.3390/ijms161226151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand P, Singh B, Jaggi AS, Singh N. Mast cells: an expanding pathophysiological role from allergy to other disorders. Naunyn-Schmiedeberg’s Arch Pharmacol. 2012;385(7):657–670. doi: 10.1007/s00210-012-0757-8. [DOI] [PubMed] [Google Scholar]
- 4.Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82(5):2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- 5.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120(18):3647–3656. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- 6.Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000;16(2):S12–S20. doi: 10.1097/00002508-200006001-00004. [DOI] [PubMed] [Google Scholar]
- 7.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, Vercellotti GM. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisogno T, Maurelli S, Melck D, De Petrocellis L, Di Marzo V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem. 1997;272(6):3315–3323. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- 9.Brandow AM, Farley RA, Panepinto JA. Early insights into the neurobiology of pain in sickle cell disease: A systematic review of the literature. Pediatr Blood Cancer. 2015;62(9):1501–1511. doi: 10.1002/pbc.25574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol. 2013;88(1):37–43. doi: 10.1002/ajh.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridges D, Rice ASC, Egertova M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 2003;119(3):803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 12.Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness. Brit J Haematol. 2012;156(4):535–544. doi: 10.1111/j.1365-2141.2011.08977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cain DM, Wacnik PW, Eikmeier L, Beitz A, Wilcox GL, Simone DA. Functional interactions between tumor and peripheral nerve in a model of cancer pain in the mouse. Pain Med. 2001;2(1):15–23. doi: 10.1046/j.1526-4637.2001.002001015.x. [DOI] [PubMed] [Google Scholar]
- 14.Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. PAIN®. 2015;156(4):722–730. doi: 10.1097/j.pain.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dampier CD, Setty BNY, Logan J, Ioli JG, Dean R. Intravenous morphine pharmacokinetics in pediatric patients with sickle cell disease. J Pediatr. 1995;126(3):461–467. doi: 10.1016/s0022-3476(95)70472-8. [DOI] [PubMed] [Google Scholar]
- 16.Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. Eur J Haematol. 2014;93(2):89–95. doi: 10.1111/ejh.12340. [DOI] [PubMed] [Google Scholar]
- 17.Darbari DS, Minniti CP, Rana S, van den Anker J. Pharmacogenetics of morphine: Potential implications in sickle cell disease. Am J Hematol. 2008;83(3):233–236. doi: 10.1002/ajh.21027. [DOI] [PubMed] [Google Scholar]
- 18.Deadwyler SA, Hampson RE, Mu J, Whyte A, Childers S. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J Pharmacol Exp Ther. 1995;273(2):734–743. [PubMed] [Google Scholar]
- 19.Di Marzo V, De Petrocellis L, Nunzio S, Buono A. Biosynthesis of anandamide and related acylethanolamides in mouse J774 macrophages and N18 neuroblastoma cells. Biochem J. 1996;316(3):977–984. doi: 10.1042/bj3160977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372(6507):686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 21.Elikottil J, Gupta P, Gupta K. The analgesic potential of cannabinoids. J Opioid Manag. 2009;5(6):341. [PMC free article] [PubMed] [Google Scholar]
- 22.Garrison SR, Kramer AA, Gerge NZ, Hillery CA, Stucky CL. Sickle cell mice exhibit mechanical allodynia and enhanced responsiveness in light touch cutaneous mechanoreceptors. Mol Pain. 2012;8(1):1. doi: 10.1186/1744-8069-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guindon J, Beaulieu P. The role of the endogenous cannabinoid system in peripheral analgesia. Curr Mol Pharmacol. 2009;2(1):134–139. doi: 10.2174/1874467210902010134. [DOI] [PubMed] [Google Scholar]
- 24.He Y, Wilkie DJ, Nazari J, Wang R, Messing RO, DeSimone J, Molokie RE, Wang ZJ. PKCδ-targeted intervention relieves chronic pain in a murine sickle cell disease model. J Clin Invest. 2016;126(8) doi: 10.1172/JCI86165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11(2):129–151. [PubMed] [Google Scholar]
- 26.Héron A, Dubayle D. A focus on mast cells and pain. J Neuroimmunol. 2013;264(1):1–7. doi: 10.1016/j.jneuroim.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118(12):3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem Phys Lipids. 2002;121(1):173–190. doi: 10.1016/s0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 29.Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 30.Howard J, Anie KA, Holdcroft A, Korn S, Davies SC. Cannabis use in sickle cell disease: a questionnaire study. Brit J Haematol. 2005;131(1):123–128. doi: 10.1111/j.1365-2141.2005.05723.x. [DOI] [PubMed] [Google Scholar]
- 31.Kassim AA, DeBaun MR. Sickle cell disease, vasculopathy, and therapeutics. Annu Rev Med. 2013;64:451–466. doi: 10.1146/annurev-med-120611-143127. [DOI] [PubMed] [Google Scholar]
- 32.Khasabova IA, Chandiramani A, Harding-Rose C, Simone DA, Seybold VS. Increasing 2-arachidonoyl glycerol signaling in the periphery attenuates mechanical hyperalgesia in a model of bone cancer pain. Pharmacol Res. 2011a;64:60–67. doi: 10.1016/j.phrs.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khasabova IA, Gielissen J, Chandiramani A, Harding-Rose C, Odeh DA, Simone DA, Seybold VS. CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain. Behav Pharmacol. 2011b;22(5–6):607. doi: 10.1097/FBP.0b013e3283474a6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khasabova IA, Harding-Rose C, Simone DA, Seybold VS. Differential effects of CB1 and opioid agonists on two populations of adult rat dorsal root ganglion neurons. J Neurosci. 2004;24(7):1744–1753. doi: 10.1523/JNEUROSCI.4298-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khasabova IA, Khasabov S, Paz J, Harding-Rose C, Simone DA, Seybold VS. Cannabinoid type-1 receptor reduces pain and neurotoxicity produced by chemotherapy. J Neurosci. 2012;32(20):7091–7101. doi: 10.1523/JNEUROSCI.0403-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khasabova IA, Khasabov SG, Harding-Rose C, Coicou LG, Seybold BA, Lindberg AE, Steevens CD, Simone DA, Seybold VS. A decrease in anandamide signaling contributes to the maintenance of cutaneous mechanical hyperalgesia in a model of bone cancer pain. J Neurosci. 2008;28(44):11141–11152. doi: 10.1523/JNEUROSCI.2847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khasabova IA, Simone DA, Seybold VS. Cannabinoids attenuate depolarization-dependent Ca 2+ influx in intermediate-size primary afferent neurons of adult rats. Neuroscience. 2002;115(2):613–625. doi: 10.1016/s0306-4522(02)00449-9. [DOI] [PubMed] [Google Scholar]
- 38.Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, Nguyen J, Gupta V, Hebbel RP, Simone DA, Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116(3):456–465. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin RJ, Evans AT, Wakeman K, Unterbrink M. A Mixed-Methods Study of Pain-related Quality of Life in Sickle Cell Vaso-Occlusive Crises. Hemoglobin. 2015;39(5):305–309. doi: 10.3109/03630269.2015.1055576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo J, Feng J, Liu S, Walters ET, Hu H. Molecular and cellular mechanisms that initiate pain and itch. Cell Mol Life Sci. 2015;72(17):3201–3223. doi: 10.1007/s00018-015-1904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maccarrone M, Di Rienzo M, Battista N, Gasperi V, Guerrieri P, Rossi A, Finazzi-Agrò A. The Endocannabinoid System in Human Keratinocytes: Evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activating protein-1, and transglutaminase. J Biol Chem. 2003;278(36):33896–33903. doi: 10.1074/jbc.M303994200. [DOI] [PubMed] [Google Scholar]
- 42.Mackie K, Devane WA, Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. 1993;44(3):498–503. [PubMed] [Google Scholar]
- 43.Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Nat Acad Sci USA. 1992;89(9):3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15(10):6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahmud A, Santha P, Paule CC, Nagy I. Cannabinoid 1 receptor activation inhibits transient receptor potential vanilloid type 1 receptor-mediated cationic influx into rat cultured primary sensory neurons. Neuroscience. 2009;162(4):1202–1211. doi: 10.1016/j.neuroscience.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 46.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood. 2006;107(4):1651–1658. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mbvundula EC, Rainsford KD, Bunning RA. Cannabinoids in pain and inflammation. Inflammopharmacology. 2004;12(2):99–114. doi: 10.1163/1568560041352275. [DOI] [PubMed] [Google Scholar]
- 48.Mittal A, Gupta M, Lamarre Y, Jahagirdar B, Gupta K. Quantification of pain in sickle mice using facial expressions and body measurements. Blood Cell Mol Dis. 2016;57:58–66. doi: 10.1016/j.bcmd.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagar S, Remmel RP, Hebbel RP, Zimmerman CL. Metabolism of opioids is altered in liver microsomes of sickle cell transgenic mice. Drug Metab Dispos. 2004;32(1):98–104. doi: 10.1124/dmd.32.1.98. [DOI] [PubMed] [Google Scholar]
- 50.Nouraie M, Gordeuk VR. Blood transfusion and 30-day readmission rate in adult patients hospitalized with sickle cell disease crisis. Transfusion. 2015;55(10):2331–8. doi: 10.1111/trf.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ocaña M, Cendán CM, Cobos EJ, Entrena JM, Baeyens JM. Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol. 2004;500(1):203–219. doi: 10.1016/j.ejphar.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Ørstavik K, Weidner C, Schmidt R, Schmelz M, Hilliges M, Jørum E, Handwerker H, Torebjörk E. Pathological C-fibres in patients with a chronic painful condition. Brain. 2003;126(3):567–578. doi: 10.1093/brain/awg060. [DOI] [PubMed] [Google Scholar]
- 53.Pászty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 54.Potenzieri C, Harding-Rose C, Simone DA. The cannabinoid receptor agonist, WIN 55,212-2, attenuates tumor-evoked hyperalgesia through peripheral mechanisms. Brain Res. 2008;1215:69–75. doi: 10.1016/j.brainres.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tahim AS, Santha P, Nagy I. Inflammatory mediators convert anandamide into a potent activator of the vanilloid type 1 transient receptor potential receptor in nociceptive primary sensory neurons. Neuroscience. 2005;136(2):539–548. doi: 10.1016/j.neuroscience.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N-and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78(1):43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- 57.Uhelski ML, Cain DM, Harding-Rose C, Simone DA. The non-selective cannabinoid receptor agonist WIN 55,212-2 attenuates responses of C-fiber nociceptors in a murine model of cancer pain. Neuroscience. 2013;247:84–94. doi: 10.1016/j.neuroscience.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uhelski ML, Gupta K, Simone DA. Sensitization of C-fiber nociceptors in a murine model of sickle cell disease (SCD) is decreased by the inhibition of anandamide hydrolysis through local administration of URB597 [abstract]. Journal of Sickle Cell Disease and Hemoglobinopathies; 10th Annual Sickle Cell Disease Research & Educational Symposium and National Sickle Cell Disease Scientific Meeting; April 15–18, 2016; Ft. Lauderdale, FL. Pembroke Pines, FL. Apr 15, 2016. JSCDH-D-16-00050. [Google Scholar]
- 59.Uhelski ML, Khasabova IA, Simone DA. Inhibition of anandamide hydrolysis attenuates nociceptor sensitization in a murine model of chemotherapy-induced peripheral neuropathy. J Neurophysiol. 2015;113(5):1501–1510. doi: 10.1152/jn.00692.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vincent L, Vang D, Nguyen J, Benson B, Lei J, Gupta K. Cannabinoid receptor-specific mechanisms to ameliorate pain in sickle cell anemia via inhibition of mast cell activation and neurogenic inflammation. Haematologica. 2016;101(5):566–77. doi: 10.3324/haematol.2015.136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122(11):1853–1862. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390(6659):518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- 63.Walker JM, Huang SM. Cannabinoid analgesia. Pharmacol & Ther. 2002;95:127–135. doi: 10.1016/s0163-7258(02)00252-8. [DOI] [PubMed] [Google Scholar]
- 64.Walker JM, Huang SM, Strangman NM, Tsou K, Sañudo-Peña MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Nat Acad Sci USA. 1999;96(21):12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zappia KJ, Garrison SR, Hillery CA, Stucky CL. Cold hypersensitivity increases with age in mice with sickle cell disease. PAIN®. 2014;155(12):2476–2485. doi: 10.1016/j.pain.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zimmerman M. Ethical guidelines for investigation of experimental pain in conscious animals. PAIN®. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]