Abstract
Background
The hippocampus and amygdala have been repeatedly implicated in the psychopathology of posttraumatic stress disorder (PTSD). While numerous structural neuroimaging studies examined these two structures in PTSD, these analyses have largely been limited to volumetric measures. Recent advances in vertex-based neuroimaging methods have made it possible to identify specific locations of subtle morphometric changes within a structure of interest.
Methods
In this cross-sectional study, we used high-resolution magnetic resonance imaging to examine the relationship between PTSD symptomatology, as measured using the Clinician Administered PTSD Scale for the DSM-IV, and structural shape of the hippocampus and amygdala using vertex-wise shape analyses in a group of combat-exposed U.S. Veterans (N = 69).
Results
Following correction for multiple comparisons and controlling for age and cranial volume, we found that participants with more severe PTSD symptoms showed an indentation in the anterior half of the right hippocampus and an indentation in the dorsal region of the right amygdala (corresponding to the centromedial amygdala). Post hoc analysis using stepwise regression suggest that among PTSD symptom clusters, arousal symptoms explain most of the variance in the hippocampal abnormality, whereas reexperiencing symptoms explain most of the variance in the amygdala abnormality.
Conclusion
The results provide evidence of localized abnormalities in the anterior hippocampus and centromedial amygdala in combat-exposed U.S. Veterans suffering from PTSD symptoms. This novel finding provides a more fine-grained analysis of structural abnormalities in PTSD and may be informative for understanding the neurobiology of the disorder.
Keywords: posttraumatic stress disorder, structural magnetic resonance imaging, hippocampus, amygdala, anterior hippocampus, hippocampal shape, vertex-wise analysis, shape analysis, veterans, morphometry
Introduction
Posttraumatic stress disorder (PTSD) is a common mental illness, with an estimated lifetime prevalence rate of 6.8% in the general population in the United States,1 and an estimated 23% among post-9/11 U.S. Veterans.2 Yet, the neurobiological mechanisms underlying the disorder are not fully understood, and the availability of effective pharmacotherapies is scarce.3–5 Better understanding of the underlying pathophysiology may contribute to the development of novel rational therapeutics. In this article, we investigated the relationship between PTSD symptoms and the shape (vertex-based) of two subcortical structures critical to stress response and emotion regulation that have been repeatedly implicated in the pathophysiology of PTSD:6–9 the hippocampus and amygdala. In contrast to the presence of numerous vertex-based cortical PTSD studies,10–25 structural analyses of the hippocampus and amygdala have largely been limited to volumetric measures (i.e., total or voxel-based volumes). This vertex-based approach complements previous volumetric findings and might provide enhanced localization of the structural abnormalities within the hippocampus and amygdala.
Multiple neuroimaging studies have examined brain regions involved in PTSD symptomatology in an effort to characterize the mechanisms of the disorder and ultimately inform treatment. In animal models, chronic stress has been shown to have opposing effects on synaptic plasticity in the hippocampus and the amygdala. Trauma and stress induce synaptic degeneration and neuronal atrophy in the hippocampus but result in trophic changes and synaptogenesis in the amygdala.26 These preclinical findings of atrophy have been paralleled by clinical evidence of reduced hippocampal volumes in PTSD patients. A large number of studies, including several meta-analyses, have reported total volume reduction in the hippocampi of PTSD patients16,27–39 (note that citations do not represent an exhaustive list; for further details refer to recent meta-analyses by Kühn et al.36 and Li et al.37). Nevertheless, it should be noted that a number of studies and at least one meta-analysis have failed to replicate these volumetric changes.40–43 In the case of the amygdala, the increased synaptogenesis in preclinical models has so far mostly been captured from the purview of functional imaging, where findings of a hyperactive amygdala are prevalent.44 The evidence from structural imaging is inconclusive, as both reduced6,39,45,46 and increased7 amygdala volumes have been reported. Other studies40–42 including one meta-analysis47 failed to identify amygdala volumetric abnormalities associated with PTSD.
The vast majority of structural neuroimaging studies investigating these two structures have so far made use of volume measurements, i.e., region of interest (ROI) or voxel-based morphometry (VBM). Despite their value, volumetric measurements are unable to capture abnormalities related to the shape of a structure and possess limited ability to localize abnormalities within regions of interest. Furthermore, several questions have been raised regarding the sensitivity of VBM towards subtle morphometric changes, particularly with regard to subcortical nuclei.48–50 The hippocampus and amygdala are heterogeneous structures with different subregions having unique cellular architectures and developmental and functional properties.51,52 For example, the anterior hippocampus is thought to perform stress- and emotion-related functions while the posterior hippocampus is associated with various cognitive functions.53 In addition, differing functional PTSD-related abnormalities have been found in the anterior versus posterior hippocampus.9,54 Similarly, the basolateral amygdala (BLA) is involved in sensory integration with afferents from the various sensory and association cortices; in contrast, the centromedial amygdala (CMA) mediates efferent fear response.52,55 In animal models, trauma and stress-induced amygdala hypertrophy has been most evident in the BLA.56,57 It is therefore conceivable that the inconsistent findings in the literature could be partially due to an inherent limitation in gross volumetric measurement, namely the inability to localize abnormalities within the structure in question or to detect structural changes other than total volumes. Additionally, the multifaceted phenotype observed in PTSD, which consists of dimensions of depressive as well as hyperarousal and reexperiencing symptoms,58 could be each linked to a particular abnormality within the hippocampus or amygdala.
Recent advances in neuroimaging methods have made it possible to identify specific locations of subtle morphometric changes within a structure of interest. This morphometric approach, known as shape analysis or vertex-wise analysis, aims to measure shape differences by analyzing surface representation rather than individual voxels.59 Here, we report on vertex-wise shape analyses of the hippocampus and amygdala and the relationship with PTSD symptomatology, as measured with the Clinician Administered PTSD Scale for the DSM-IV (CAPS).60 Rather than using a dichotomy consisting of PTSD patients and controls, we employ a single-group dimensional approach in a sample of combat-exposed U.S. Veterans to capture a continuous spectrum of PTSD symptoms for the primary analysis. We attempt to answer additional questions relating to confounds, symptom clusters, and sex differences in post hoc analyses.
Methods
Participants and Clinical Assessments
A total of 69 combat-exposed U.S. Veterans (aged 21–60) participated in this study. Details of the study sample and procedures were previously reported.61 Briefly, inclusion criteria required at least one combat tour deployment, and exclusion criteria included psychotic disorder or bipolar disorder, attention-deficit/hyperactivity disorder, learning disorder, moderate or severe traumatic brain injury (TBI), brain tumor, epilepsy or other neurological disorders, current benzodiazepine use, and magnetic resonance imaging contraindication. To ensure external validity and generalizability of the findings to the target population, the following were not considered exclusionary due to their high co-occurrence in Veterans with PTSD: depression, anxiety, substance/alcohol use disorder, and stable antidepressant regimens.
PTSD diagnosis, overall symptom severity, and symptom cluster severity (i.e., numbing-avoidance, hyperarousal, re-experiencing) were assessed using CAPS.62 Combat exposure severity was assessed using the Combat Exposure Scale.63 Depressive and anxiety symptoms were assessed using the Beck Depression Inventory64 and Beck Anxiety Inventory,65 respectively. A measure of estimated pre-exposure intellectual functioning was determined using the Wechsler Test of Adult Reading.66 Psychiatric comorbidities were assessed using the Structured Clinical Interview for the DSM-IV.62
The study was approved by Institutional Review Boards at the VA Connecticut Healthcare System and Yale University. Written informed consent was obtained from all participants.
Neuroimaging Methods
A Siemens TIM Trio 3.0 Tesla magnet with a 32-channel head coil was used. magnetic resonance imaging acquisition included a T1-weighted MPRAGE (voxel size = 1 × 1 × 1 mm; TR = 2530 ms; TE = 2.71 ms; Flip = 7°). Shape processing for the hippocampus and amygdala was conducted using the FSL FIRST toolbox.59 Briefly, the processing included image reorientation, cropping, bias-field correction, nonlinear registration to standard space, FNIRT-based brain extraction, and structural segmentation. FIRST automated segmentation uses shape models constructed from manually segmented images; for technical details, refer to a detailed description of the method by Patenaude et al.59 In the standard space, the mesh representation of each structure and their mode parameters were then used to generate a study specific surface standard for the hippocampus and the amygdala (i.e., the average of all subjects). Per subject, each vertex anatomical location was projected onto the standard surface. The signed perpendicular distance between each projected vertex and the standard surface represent the projection values (negative = depression/indentation & positive = inflation), which were used in the study statistical analysis.
Statistical Analyses
Vertex-wise shape for the hippocampus and amygdala was correlated with CAPS score using FSL Randomise with nonparametric permutations (number of permutations = 5000) and cluster-based thresholding (z > 2.3, corrected α = 0.05),67 controlling for age and cranial volume.
For post hoc analyses, we extracted the average of the vertices in the segment showing significant abnormality in the vertex-wise analysis as a measure of average abnormality. It is important here to note that the post hoc analyses should not be considered as independent evidence, but rather an exploratory assessment to inform future studies and meta-analyses, and to better characterize the variables associated with the abnormalities identified by the vertex-wise results. We first conducted partial correlation analyses between CAPS severity and average abnormality in the hippocampus or amygdala, covarying for each of the following putative confounds separately: sex, Major Depressive Disorder diagnosis, substance/alcohol abuse, other psychiatric diagnosis, combat exposure severity, pre-exposure intelligence, education, medication status, and TBI status. Before proceeding to test the symptom cluster scores of numbing-avoidance, hyperarousal, and re-experiencing, the variance inflation factor (VIF) was used to assess for problematic multicollinearity, by entering the three subtype scores simultaneously in a multiple regression with either the hippocampus or amygdala abnormality as dependent variables. Next, we conducted a stepwise multiple regression to assess which of the PTSD symptom clusters contribute most to the abnormalities. For each structure separately, the average abnormality was entered as a dependent variable, and the three symptom cluster scores were entered into the model (p-value thresholds: entry = 0.05, removal = 0.1). Considering the known sex differences in PTSD,68,69 we assessed whether one group was disproportionally influencing the results. Correlation analyses were used to examine the relationship between the shape abnormalities and CAPS scores for females and males separately. Finally, to facilitate the interpretation and integration of our results by other groups, we included a group comparison between Veterans with PTSD and without PTSD (non-PTSD), controlling for age and intracranial volume.
Results
Demographic variables and clinical characteristics are presented in Table 1. On average, participants had a moderate level of PTSD symptoms, and 51% met DSM-IV criteria for PTSD.
Table 1.
Demographic and clinical characteristics.
| Mean ± SEM or % | |
|---|---|
| Age (years) | 34.4 ± 1.1 |
| Sex (% female) | 11% |
| WTAR standard score | 103 ± 1.0 |
| Education (years) | 14.0 ± 0.2 |
| CAPS | 44.7 ± 3.6 |
| CES | 18.0 ± 1.2 |
| BDI | 18.9 ± 1.5 |
| BAI | 13.4 ± 1.3 |
| DSM-IV axis I | 65% |
| PTSD | 51% |
| MDD | 18% |
| SUD | 23% |
| Anxiety disorder | 7% |
| Psychotropic medication | 33% |
| Mild TBI | 59% |
Note: SEM: Standard Error of Means; WTAR: Wechsler Test of Adult Reading; CAPS: Clinician Administered PTSD Scale for the DSM-IV; CES: Combat Exposure Scale; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory; PTSD: Posttraumatic Stress Disorder; MDD: Major Depressive Disorder; SUD: Substance/Alcohol Use Disorder; Anxiety: Panic Disorder, Generalized Anxiety Disorder, Obsessive Compulsive Disorder; TBI: Traumatic Brain Injury.
Vertex-Wise Analysis
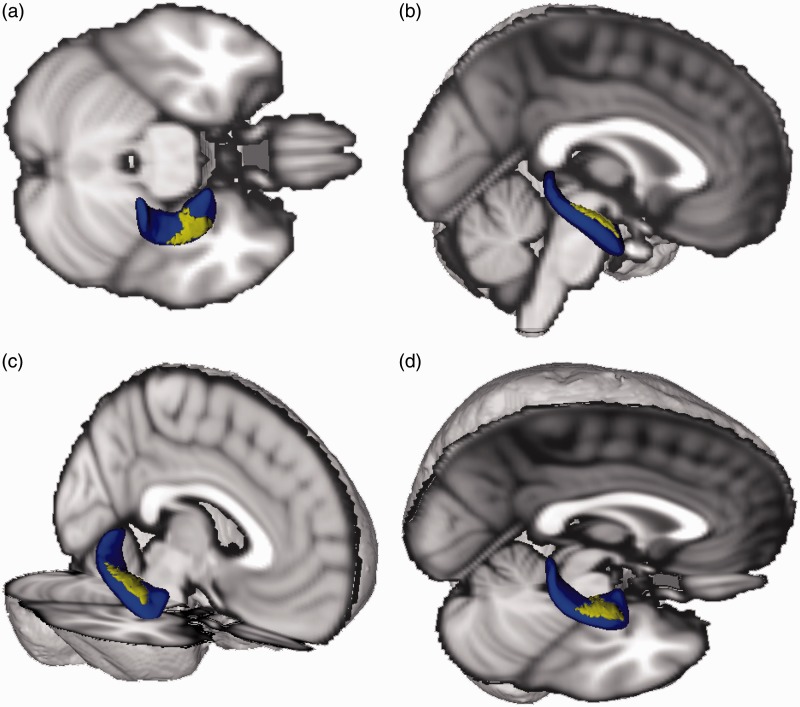
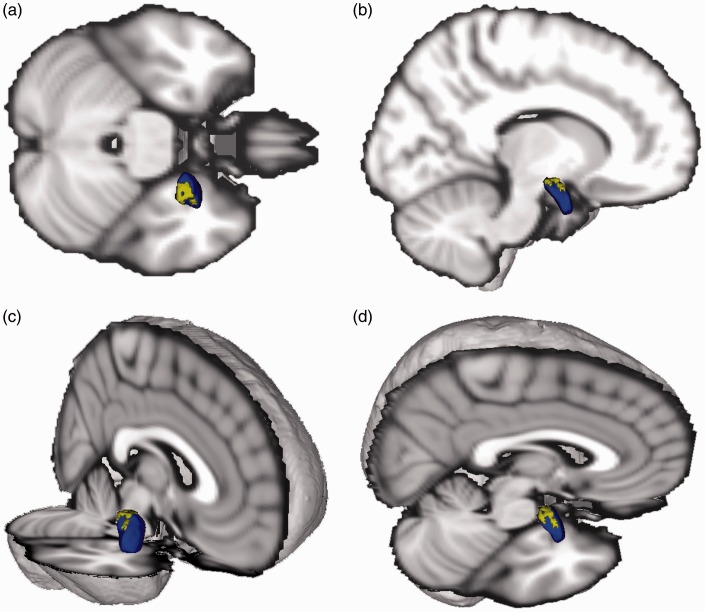
Following correction for multiple comparisons using Randomise and controlling for age and cranial volume, the vertex-wise analysis revealed a negative correlation between CAPS severity and hippocampal vertices, such that participants with more severe PTSD symptoms showed a depression/indentation in the anterior half of the right hippocampus (Figure 1), but no significant alterations in the posterior right hippocampus or all of the left hippocampus. Similarly, we found a negative correlation between CAPS severity and amygdala vertices, such that participants with more severe PTSD symptoms showed a depression/indentation in the dorsal region (corresponding to the CMA) of the right amygdala, but no significant alterations elsewhere in the amygdala (Figure 2).
Figure 1.
Localized right hippocampus abnormality. Three-dimensional (3D) depiction (blue) of the right hippocampus showing the location of the indentation (yellow) in participants with severe PTSD symptoms. Standard brain added to aid visualization. Superior (a), lateral (b), anterolateral (c), superolateral (d) views.
Figure 2.
Localized right amygdala abnormality. Three-dimensional (3D) depiction (blue) of the right amygdala showing the location of the indentation (yellow) in participants with severe PTSD symptoms. Standard brain added to aid visualization. Superior (a), lateral (b), anterolateral (c), superolateral (d) views.
Post Hoc Analyses
The correlation between hippocampus/amygdala shape and CAPS remained significant (p < 0.05) after controlling for each of the following variables separately: age, gender, Major Depressive Disorder diagnosis, other psychiatric diagnosis, combat exposure severity, estimated pre-exposure intelligence, education, substance/alcohol abuse, medication status, and TBI.
All symptom cluster scores were significantly associated with the identified indentations in both structures (bivariate models are presented in Tables 2 and 3, respectively). The multiple regression model revealed a moderate level of multicollinearity between the symptom clusters (VIF < 4 for all cases). In the stepwise model relating to the hippocampus, initially all three terms that were entered were significantly correlated with the indentation (p < 0.01). Subsequently, only the hyperarousal score survived and demonstrated a significant effect, with the model explaining 12% of the variance of the abnormality (R2 = 0.12; p = 0.02). Neither the numbing-avoidance nor re-experiencing scores were found to have significant effect in the model as a whole and were excluded (p > 0.4). Similarly, in the stepwise model relating to the amygdala, all three terms were significantly correlated with the indentation (p < 0.01), but only the re-experiencing score subsequently survived and demonstrated a significant effect, with the model explaining 15% of the amygdala abnormality variance (R2 = 0.15; p = 0.01). Neither the numbing-avoidance nor re-experiencing scores were found to have significant effect in the model as a whole and were excluded (p > 0.7). A summary of the stepwise models can be found in Table 4.
Table 2.
Bivariate correlation matrix for PTSD symptom clusters and the hippocampal abnormality.
| Right hippocampus | Re-experiencing | Hyperarousal | Numbing-avoidance | ||
|---|---|---|---|---|---|
| Right hippocampus | r | 1 | |||
| p | * | ||||
| Re-experiencing | r | −0.339 | 1 | ||
| p | 0.002 | * | |||
| Hyperarousal | r | −0.359 | 0.785 | 1 | |
| p | 0.001 | * | * | ||
| Numbing-avoidance | r | −0.245 | 0.791 | 0.825 | 1 |
| p | 0.021 | * | * | * | |
p < 0.001.
Table 3.
Bivariate correlation matrix for PTSD symptom clusters and the amygdala abnormality.
| Right amygdala | Re-experiencing | Hyperarousal | Numbing-avoidance | ||
|---|---|---|---|---|---|
| Right amygdala | r | 1 | |||
| p | * | ||||
| Re-experiencing | r | −0.382 | 1 | ||
| p | 0.001 | * | |||
| Hyperarousal | r | −0.319 | 0.785 | 1 | |
| p | 0.004 | * | * | ||
| Numbing-avoidance | r | −0.289 | 0.791 | 0.825 | 1 |
| p | 0.008 | * | * | * | |
p < 0.001.
Table 4.
Stepwise regression models with PTSD symptom clusters and the localized abnormalities.
| Structure | Predictor | β | SE | Standardized β | t | p | R2 |
|---|---|---|---|---|---|---|---|
| Right hippocampus | Arousal | −.041 | 0.013 | −0.359 | −3.153 | 0.002 | 0.129 |
| Right amygdala | Re-experiencing | −.032 | 0.009 | −0.382 | −3.381 | 0.001 | 0.146 |
Note: The original model in both cases included CAPS numbing-avoidance, arousal, reexperiencing.
In the male group, abnormality in both structures correlated with the total CAPS score (hippocampus: r = −0.4, p = 0.02; amygdala: r = −0.4, p = 0.02), whereas in the female group, none of the two structures were found to be significantly correlated with the total CAPS score (hippocampus: r = 0.1, p = 0.9; amygdala: r = −0.23, p = 0.7). It is important to note that women only constituted 11% (8 participants) of the total sample.
Compared to Veterans with no PTSD, the abnormality in the PTSD group was as follows: hippocampus (PTSD: mean ± SEM = −0.417 ± 0.175; non-PTSD: mean ±SEM = 0.454 ± 0.183; p = 0.001) and amygdala (PTSD: mean ± SEM = −0.258 ± 0.121; CC: mean ± SEM =0.282 ± 0.127; p = 0.003).
Discussion
Using a vertex-wise approach, we demonstrated shape abnormalities in the right anterior hippocampus and dorsal amygdala associated with increased PTSD symptom severity. In the hippocampus, the direction of the change—namely, indentation/depression—is consistent with previous volumetric literature showing shrinkage of this structure in PTSD.16,27–35 In the amygdala, where prior evidence in PTSD has been inconclusive, we found an indentation grossly overlapping with the CMA area, but no changes in the BLA, an area that is particularly sensitive to stress-related hypertrophy in animal models.56,57
The hippocampus has long been identified as an important structure in PTSD; its failure to recognize contextual cues in the absence of threat, and to relay that information to the amygdala and the vmPFC, is believed to contribute to the observed exaggerated states of fear and hyperarousal.3 A smaller hippocampal volume in PTSD has been widely reported in the literature16,27–35; however, the majority of these studies considered the hippocampus as a single entity. Evidence from animal studies demonstrating functional segmentation within the hippocampus is accumulating; according to such models, the anterior portion of the hippocampus plays a central role in stress, emotion, and affect, while the posterior portion is primarily involved in spatial memory and other cognitive functions.53 Converging evidence suggests a gradient along longitudinal axis of the hippocampus where coarse field representations take place anteriorly while more fine-grained representations are encoded posteriorly.70 It has been suggested that this “sparse” representation in the anterior hippocampus is optimized for cross-environment generalization (i.e., pattern completion); in contrast, denser and more fine-grained segments present posteriorly enable filtering out the interference, particularly useful in more similar environments (i.e., pattern separation).70 An altered balance between pattern completion and pattern separation is hypothesized to underline overgeneralization of fear and context-inappropriate fear response in PTSD.71,72 Our analyses identified an indentation in the hippocampus associated with increasing PTSD severity that is localized to the right anterior half of the hippocampus, which could be reflecting a dysfunctional dynamic between these two hippocampal functions.9 Reduction in the anterior but not posterior hippocampal volume has also been previously described in Veterans with PTSD.73 Future studies can further assess the anterior hippocampal abnormalities by examining the role of hippocampal subfields (e.g., CA3) in the pathophysiology of PTSD. Finally, it is noticeable that in the current study, the shape abnormalities were detected in the right hippocampus, whereas in several previous PTSD studies, volumetric differences were reported either bilaterally or on the left.36 It remains to be seen whether this observation is limited to our sample or whether our results represent a lateralization of hippocampal shape abnormalities to the right hemisphere.
The amygdala has a crucial role in fear learning and expression, as well as the detection of threat.55 While numerous functional neuroimaging studies have shown heightened amygdala reactivity in PTSD patients in various experimental paradigms, the evidence for amygdala structural abnormalities has been contradictory.44 This may have been due to the suboptimal sensitivity of the employed volumetric and VBM approaches. Using vertex-wise analyses, we identified a region of indentation in the dorsal area of the right amygdala associated with increased PTSD severity—which anatomically corresponds to the central nucleus of the amygdala (CEA). The amygdala consists of multiple nuclei with distinct roles. The CMA, which comprises the CEA and the medial nucleus, plays a major role in affect expression and anxiety-related behavior, while the BLA is involved in perception and emotional modulation.55 In animal models, the BLA, but not the CMA, is a site of synaptogenesis under conditions of chronic stress.26,56,57 It is therefore conceivable that inconsistent results in previous volumetric studies of the amygdala reflect mixed changes that could include concomitant CMA atrophy and BLA hypertrophy. While we were not able to identify regions of hypertrophy in the BLA, this approach highlights the importance of pursuing similar analyses in larger samples of PTSD patients.
Using CAPS symptom cluster scores for numbing-avoidance, hyperarousal, and re-experiencing, we probed the association between the identified abnormalities in the hippocampus and amygdala and these symptoms. The results of the stepwise analysis support a model in which PTSD arousal and re-experiencing symptoms contribute most to the variance of the localized abnormalities in the hippocampus and amygdala, respectively. Although the amygdala plays a central role in arousal,55 one possibility that could explain this apparent discrepancy could be related to the location of the abnormality in the CMA area. Recent evidence selectively implicates the BLA but not the CMA in the afferent aspect of arousal in functional neuroimaging studies.74
Women have a higher life-time risk of developing PTSD, and the manifestation of the disorder is often more pronounced.68,69,75 Although female participants only constituted 11% (8 participants) of our total sample, we assessed how the results of this subgroup relate to the predominantly male sample; particularly, whether in this subgroup the relationship between PTSD severity and structural abnormality would be stronger. In the female group, we failed to show a statistically significant relationship between PTSD severity and the shape abnormalities that we detected at the level of the whole sample. It is likely that this is due to small number of observations, and should not be interpreted as a confirmatory negative finding.
There are several limitations in our approach. Our study was cross-sectional, which precludes conclusions regarding causality between brain abnormalities and symptomatology. It remains to be determined whether these localized structural abnormalities represent premorbid vulnerability or trauma-related changes. Another limitation is related to our sample, which consisted of predominantly male participants, with women only comprising 11% of the total sample. This could limit the generalizability of our findings to women. Medication status as well as co-morbid psychiatric and substance abuse disorders were not excluded so that our sample was representative of the PTSD population, although this limits internal validity. Additionally, the sample size did not permit the inclusion of all 11 potential confounds in the primary analysis. Thus, we only included the two main putative confounds (age and intracranial volume), while the remaining variables were assessed in a post hoc fashion which should be interpreted with caution. All post hoc analyses were conducted on the average volume of indentation detected in the primary vertex analysis. Attempting to control for multiple potential confounds at this level would have reduced the statistical power. It is therefore important to reiterate that the post hoc analyses are not independent76 and were included solely for the purpose of providing preliminary data to better understand the different factors that might have contributed to these shape changes. The study strengths include the use of a validated neuroimaging approach that allows identification of morphological abnormalities beyond total volume59 and a dimensional approach to examine the effects of PTSD symptoms across the target population of combat-exposed U.S. Veterans.
In sum, this study makes a unique contribution to the literature by demonstrating shape abnormalities in the anterior part of the hippocampus and the dorsal amygdala, which future studies might use to further explore the neurobiology of PTSD and ultimately guide treatment development. By identifying these abnormalities, our study extends prior research on structural brain abnormalities in PTSD that can be detected using neuroimaging methods.
Acknowledgments
The authors would like to thank the Veterans who participated in this study for their invaluable contribution.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Abdallah has served as a consultant or on advisory boards for Genentech and Janssen. He also serves as editor for the journal Chronic Stress published by SAGE Publications, Inc. Dr. Krystal is a consultant for AbbVie, Inc., Amgen, Astellas Pharma Global Development, Inc., AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol-Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Inc., Neurovance, Inc., FORUM Pharmaceuticals, Janssen Research & Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Inc., Sage Therapeutics, Inc., Sunovion Pharmaceuticals, Inc., and Takeda Industries; is on the Scientific Advisory Board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., and Pfizer; serves as the Associate Editor for the journal Chronic Stress; is a stockholder in Biohaven Medical Sciences; holds stock options in Mnemosyne Pharmaceuticals, Inc.; holds patents for Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia, U.S. Patent No. 5,447,948 (issued Sep 5, 1995), and Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued Jul 15, 2014); and filed a patent for Intranasal Administration of Ketamine to Treat Depression. U.S. Application No. 14/197,767 (filed on Mar 5, 2014); U.S. application or Patent Cooperation Treaty international application No. 14/306,382 (filed on Jun 17, 2014). Dr. L. A. Averill serves as the Managing Editor for Chronic Stress. Rest of the authors report no competing interests.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding support was provided by the U.S. Department of Veterans Affairs National Center for PTSD, NIH (MH-101498), and the Brain and Behavior Foundation (NARSAD). Dr. Scott’s participation was supported by a Department of Veterans Affairs Career Development Award (IK2CX000772). Support for Dr. L. A. Averill was provided by a NARSAD/Brain & Behavior Research Foundation Young Investigator Award and the NY Women’s Committee who named Dr. L. A. Averill as the Woman of the Year Breaking the Silence Against Mental Illness. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005; 62: 593–602. [DOI] [PubMed] [Google Scholar]
- 2.Fulton JJ, Calhoun PS, Wagner HR, et al. The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans: a meta-analysis. J Anxiety Disord. 2015; 31: 98–107. [DOI] [PubMed] [Google Scholar]
- 3.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012; 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, Abdallah CG. Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neurosci Lett. 2016; 649: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelmendi B, Adams TG, Yarnell S, Southwick S, Abdallah CG, Krystal JH. PTSD: from neurobiology to pharmacological treatments. Eur J Psychotraumatol. 2016; 7: 31858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morey RA, Gold AL, LaBar KS, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012; 69: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo JR, Kaloupek DG, Woodward SH. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Arch Gen Psychiatry. 2012; 69: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 8.Sripada RK, King AP, Garfinkel SN, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012; 37: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdallah CG, Wrocklage KM, Averill CL, et al. Anterior hippocampal dysconnectivity in posttraumatic stress disorder: a dimensional and multimodal approach. Transl Psychiatry. 2017; 7: e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodward SH, Schaer M, Kaloupek DG, Cediel L, Eliez S. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 2009; 66: 1373–1382. [DOI] [PubMed] [Google Scholar]
- 11.Bing X, Ming-Guo Q, Ye Z, et al. Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res. 2013; 1490: 225–232. [DOI] [PubMed] [Google Scholar]
- 12.Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008; 41: 675–681. [DOI] [PubMed] [Google Scholar]
- 13.Hunter M, Villarreal G, McHaffie GR, et al. Lateralized abnormalities in auditory M50 sensory gating and cortical thickness of the superior temporal gyrus in post-traumatic stress disorder: preliminary results. Psychiatry Res. 2011; 191: 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Li YJ, Luo EP, Lu HB, Yin H. Cortical thinning in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. PLoS One. 2012; 7: e39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller SG, Ng P, Neylan T, et al. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Res. 2015; 234: 194–201. [DOI] [PubMed] [Google Scholar]
- 16.Qi S, Mu Y, Liu K, et al. Cortical inhibition deficits in recent onset PTSD after a single prolonged trauma exposure. NeuroImage Clin. 2013; 3: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landre L, Destrieux C, Baudry M, et al. Preserved subcortical volumes and cortical thickness in women with sexual abuse-related PTSD. Psychiatry Res. 2010; 183: 181–186. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Huang X, Li L, et al. Posttraumatic stress disorder: structural characterization with 3-T MR imaging. Radiology. 2016; 280: 537–544. [DOI] [PubMed] [Google Scholar]
- 19.Sadeh N, Spielberg JM, Logue MW, et al. SKA2 methylation is associated with decreased prefrontal cortical thickness and greater PTSD severity among trauma-exposed veterans. Mol Psychiatry. 2016; 21: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. Neuroimage Clin. 2013; 2: 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corbo V, Salat DH, Amick MM, Leritz EC, Milberg WP, McGlinchey RE. Reduced cortical thickness in veterans exposed to early life trauma. Psychiatry Res. 2014; 223: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyoo IK, Kim JE, Yoon SJ, Hwang J, Bae S, Kim DJ. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Arch Gen Psychiatry. 2011; 68: 701–713. [DOI] [PubMed] [Google Scholar]
- 23.Dickie EW, Brunet A, Akerib V, Armony JL. Anterior cingulate cortical thickness is a stable predictor of recovery from post-traumatic stress disorder. Psychol Med. 2013; 43: 645–653. [DOI] [PubMed] [Google Scholar]
- 24.Nilsen AS, Hilland E, Kogstad N, et al. Right temporal cortical hypertrophy in resilience to trauma: an MRI study. Eur J Psychotraumatol. 2016; 7: 31314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helpman L, Papini S, Chhetry BT, et al. PTSD remission after prolonged exposure treatment is associated with anterior cingulate cortex thinning and volume reduction. Depress Anxiety. 2016; 33: 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011; 13: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossini L, Tavanti M, Calossi S, et al. Magnetic resonance imaging volumes of the hippocampus in drug-naïve patients with post-traumatic stress disorder without comorbidity conditions. J Psychiatr Res. 2008; 42: 752–762. [DOI] [PubMed] [Google Scholar]
- 28.Felmingham K, Williams LM, Whitford TJ, et al. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. NeuroReport. 2009; 20: 1402. [DOI] [PubMed] [Google Scholar]
- 29.Papagni S, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A. Effects of stressful life events on human brain structure: A longitudinal voxel-based morphometry study. Stress. 2010; 14: 227–232. [DOI] [PubMed] [Google Scholar]
- 30.Sussman D, Pang EW, Jetly R, Dunkley BT, Taylor MJ. Neuroanatomical features in soldiers with post-traumatic stress disorder. BMC Neurosci. 2016; 17: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villarreal G, Hamilton DA, Petropoulos H, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002; 52: 119–125. [DOI] [PubMed] [Google Scholar]
- 32.Wignall EL, Dickson JM, Vaughan P, et al. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol Psychiatry. 2004; 56: 832–836. [DOI] [PubMed] [Google Scholar]
- 33.Winter H, Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. Am J Psychiatry. 2004; 161: 2194–2200. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Tan Q, Yin H, et al. Decreased gray matter volume in the left hippocampus and bilateral calcarine cortex in coal mine flood disaster survivors with recent onset PTSD. Psychiatry Res Neuroimaging. 2011; 192: 84–90. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Zhuo C, Lang X, Li H, Qin W, Yu C. Structural impairments of hippocampus in coal mine gas explosion-related posttraumatic stress disorder. PLoS One. 2014; 9: e102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kühn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol. Psychiatry. 2013; 73: 70–74. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Wu M, Liao Y, et al. Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neurosci Biobehav Rev. 2014; 43: 163–172. [DOI] [PubMed] [Google Scholar]
- 38.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005; 88: 79–86. [DOI] [PubMed] [Google Scholar]
- 39.Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006; 30: 1004–1031. [DOI] [PubMed] [Google Scholar]
- 40.Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol. Psychiatry. 2002; 52: 1089–1101. [DOI] [PubMed] [Google Scholar]
- 41.Herringa R, Phillips M, Almeida J, Insana S, Germain A. Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res Neuroimaging. 2012; 203: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sui S, Wu M, King ME, et al. Abnormal grey matter in victims of rape with PTSD in Mainland China: a voxel-based morphometry study. Acta Neuropsychiatrica. 2010; 22: 118–126. [DOI] [PubMed] [Google Scholar]
- 43.Meng Y, Qiu C, Zhu H, et al. Anatomical deficits in adult posttraumatic stress disorder: a meta-analysis of voxel-based morphometry studies. Behav Brain Res. 2014; 270: 307–315. [DOI] [PubMed] [Google Scholar]
- 44.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010; 35: 169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Depue BE, Olson-Madden JH, Smolker HR, Rajamani M, Brenner LA, Banich MT. Reduced amygdala volume is associated with deficits in inhibitory control: a voxel- and surface-based morphometric analysis of comorbid PTSD/Mild TBI. BioMed Res Int. 2014; 2014: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veer IM, Oei N, van Buchem MA, Spinhoven P, Elzinga BM, Rombouts S. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res Neuroimaging. 2015; 233: 436–442. [DOI] [PubMed] [Google Scholar]
- 47.Woon FL, Hedges DW. Amygdala volume in adults with posttraumatic stress disorder: a meta-analysis. J Neuropsychiatry Clin Neurosci. 2009; 21: 5–12. [DOI] [PubMed] [Google Scholar]
- 48.Menke RAL, Krolikowski K, Jbabdi S, et al. Comprehensive morphometry of subcortical grey matter structures in early stage Parkinson’s disease. Hum. Brain Mapp. 2014; 35: 1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bookstein FL. “Voxel-Based Morphometry” should not be used with imperfectly registered images. NeuroImage. 2001; 14: 1454–1462. [DOI] [PubMed] [Google Scholar]
- 50.Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. NeuroImage. 2004; 23: 17–20. [DOI] [PubMed] [Google Scholar]
- 51.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014; 15: 655–669. [DOI] [PubMed] [Google Scholar]
- 52.Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010; 11: 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010; 65: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013; 38: 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000; 23: 155–184. [DOI] [PubMed] [Google Scholar]
- 56.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002; 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995; 69: 89–98. [DOI] [PubMed] [Google Scholar]
- 58.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.). Washington, DC: Author, 2000.
- 59.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011; 56: 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blake D, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD Scale. J Trauma Stress. 1995; 8: 75–90. [DOI] [PubMed] [Google Scholar]
- 61.Wrocklage KAL, Scott C, Averill C, et al. Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur Neuropsychopharmacol. 2017; 27: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.First M SR, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). http://www.scid4.org/info/refscid.html (2002).
- 63.Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA. Clinical evaluation of a measure to assess combat exposure. Psychol Assess J Consult Clin Psychol. 1989; 1: 53–55. [Google Scholar]
- 64.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II 1996; Vol. 1, San Antonio, TX: Psychological Corporation, pp. 82. [Google Scholar]
- 65.Beck AT, Steer RA. Manual for the Beck Anxiety Inventory, San Antonio, TX: Psychological Corporation, 1990. [Google Scholar]
- 66.Holdnack HA. Wechsler Test of Adult Reading: WTAR, San Antonio: The Psychological Corporation, 2001. [Google Scholar]
- 67.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009; 44: 83–98. [DOI] [PubMed] [Google Scholar]
- 68.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006; 132: 959–992. [DOI] [PubMed] [Google Scholar]
- 69.Street AE, Gradus JL, Giasson HL, Vogt D, Resick PA. Gender differences among veterans deployed in support of the wars in Afghanistan and Iraq. J Gen Intern Med. 2013; 28(Suppl 2): S556–S562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016; 17: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016; 92: 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012; 15: 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vythilingam M, Luckenbaugh DA, Lam T, et al. Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psychiatry Res. 2005; 139: 89–99. [DOI] [PubMed] [Google Scholar]
- 74.Patel R, Girard TA, Pukay-Martin N, Monson C. Preferential recruitment of the basolateral amygdala during memory encoding of negative scenes in posttraumatic stress disorder. Neurobiol Learn Mem. 2016; 130: 170–176. [DOI] [PubMed] [Google Scholar]
- 75.Vogt D, Smith BN, Fox AB, Amoroso T, Taverna E, Schnurr PP. Consequences of PTSD for the work and family quality of life of female and male U.S. Afghanistan and Iraq War veterans. Soc Psychiatry Psychiatr Epidemiol. 2017; 52: 341–352. [DOI] [PubMed] [Google Scholar]
- 76.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009; 12: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]