Abstract
The gliotransmitter d-serine is released upon (S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate and metabotropic glutamate receptor stimulation, but the mechanisms involved are unknown. Here, by using a highly sensitive bioassay to continuously monitor extracellular d-serine levels, we have investigated the pathways used in its release. We reveal that d-serine release is inhibited by removal of extracellular calcium and augmented by increasing extracellular calcium or after treatment with the Ca2+ ionophore A23187. Furthermore, release of the amino acid is considerably reduced after depletion of thapsigargin-sensitive intracellular Ca2+ stores or chelation of intracellular Ca2+ with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate–acetoxymethyl ester. Interestingly, d-serine release also was markedly reduced by concanamycin A, a vacuolar-type H+-ATPase inhibitor, indicating a role for the vesicular proton gradient in the transmitter storage/release. In addition, agonist-evoked d-serine release was sensitive to tetanus neurotoxin. Finally, immunocytochemical and sucrose density gradient analysis revealed that a large fraction of d-serine colocalized with synaptobrevin/VAMP2, suggesting that it is stored in VAMP2-bearing vesicles. In summary, our study reveals the cellular mechanisms subserving d-serine release and highlights the importance of the glial cell exocytotic pathway in influencing CNS levels of extracellular d-serine.
Keywords: glia, synaptobrevin, d-amino acid, vesicles, tetanus neurotoxin
Astrocytes play pivotal roles in synaptic transmission by controlling transmitter diffusion and concentration in the extracellular space (1) and also by back-signaling to neurons directly through the release of neuroactive substances (2). Although glutamate and ATP are the most recognized chemical transmitters that mediate astrocyte–neuron signaling (2), other cell–cell mediators also are involved in this pathway. d-Serine has recently been identified as a major gliotransmitter in the central nervous system that serves as an endogenous ligand for the glycine site of NMDA receptors (3–6).
However, many aspects regarding d-serine release still need to be addressed. Although it has been suggested that astrocytes may release d-serine, through the reverse operation of a sodium-dependent transporter (7), the precise molecular mechanisms underlying d-serine release are currently unknown. To unravel the functional consequences of d-serine-mediated astrocyte-to-neuron signaling, it is essential to shed light on the mechanisms controlling the gliotransmitter storage and release pathways.
For this purpose, we have devised a previously undescribed bioassay to continuously monitor the release of d-serine from cultured glial cells. In this work, we show that astrocytes and C6 glioma cells synthesize and contain a large amount of d-serine that can be released upon glutamate receptor (GluR) stimulation. Moreover, our observations demonstrate that d-serine release, evoked by glutamatergic agonists, is linked to a [Ca2+]i increase, because efflux of the transmitter is altered by manipulations of intracellular and extracellular calcium ion concentrations. Furthermore, treatment of primary astrocytes or glioma cells with tetanus neurotoxin (TeNT), which selectively cleaves the vesicle-associated SNARE proteins VAMP2 and VAMP3 (8, 9), or with concanamycin A, a compound that inhibits the vacuolar-type H+-ATPase (10), strongly reduced the release of d-serine. Finally, we demonstrate that a large part of d-serine is stored in VAMP2-bearing vesicles, as revealed by sucrose-gradient analysis and confocal microscopy. We thus provide, to our knowledge, the first evidence for vesicular storage and Ca2+-dependent exocytotic release of d-serine from glial cells.
Materials and Methods
Cell Culture. Cultured astrocytes were prepared from cerebral cortex of 1- to 3-day-old postnatal rats, as described in ref. 11, and used after 1–3 weeks in culture. Rat glioma C6 cells were purchased from American Type Culture Collection and cultured in DMEM/Nut mix F-12 (Invitrogen) supplemented with 2 mM glutamine and 10% FCS. Cells were grown at 37°C in a 5% CO2 incubator.
Bioassay for d-Serine Release. d-Serine levels were detected by using an enzymatic assay (12) (Fig. 1A). After the addition of d-amino acid oxidase (dAAO) and horseradish peroxidase (HRP)/luminol to astrocytes bathed in saline solution, any detectable levels of d-serine released in the medium were visualized by an emission of light. For this bioassay, dAAO purified from the yeast Rhodotorula gracilis (RgdAAO) was used. RgdAAO displays peculiar features in comparison with mammalian enzyme, such as the tight binding of the coenzyme FAD (Kd = 2.0 × 10–8 M), a strict stereospecificity for d-amino acids, and a significant higher catalytic efficiency for its substrates (13). Recombinant wild-type and mutant Arg-285 → Ala RgdAAOs were expressed and purified from Escherichia coli cells by using the pT7-dA AO expression system in BL21(DE3)pLysS cells (14). The mutant enzyme shows a residual activity of <0.1% of that determined for the wild-type dAAO (general properties of Arg-285 → Ala RgdAAO are described in ref. 15). The presence of the HRP and luminol does not affect the activity of the RgdAAO (see Fig. 7, which is published as supporting information on the PNAS web site).
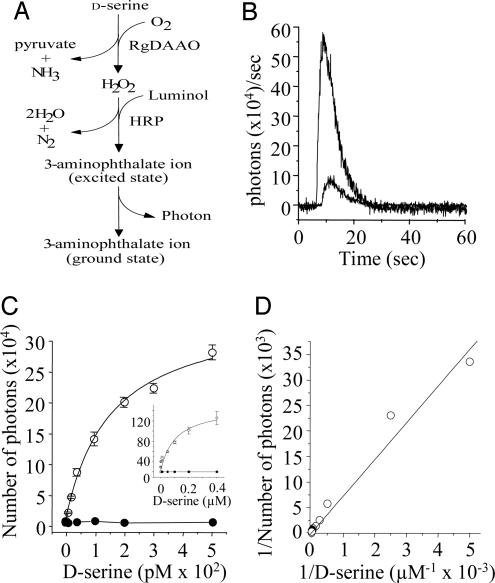
Fig. 1.
Schematic illustration and standard curves of the d-serine-induced reaction pathway leading to luminol-derived chemiluminescence (LDCL) with the RgdAAO/HRP enzymatic system. (A) Recombinant RgdAAO catalyzes the oxidative deamination of d-serine, producing ammonia, hydroxypyruvate, and hydrogen peroxide (H2O2). The latter product is used by HRP to oxidize luminol, thus yielding a photon. The emitted photon then is captured by the photomultiplier tube. (B) Examples of LDCL spectra induced by d-serine at 40 pM (smaller trace) and 50 nM (larger trace). (C) Dose-response curve for integrated photons for low and high (Inset) d-serine concentrations obtained with the active RgdAAO (open circles) or with the inactive mutant (filled circles). (D) Michaelis–Menten plot of the bioassay. Each point is the mean (n ≥ 8 independent experiments) of light emission above background light production.
Experiments were performed at room temperature (22–26°C) by using a laboratory-made photon counter. The photons emitted during the bioassay were detected by an ultra-low-noise photomultiplier tube (R464SS, Hamamatsu Photonics, Hamamatsu City, Japan; dark counts, 0.5 cps) running at 800 V. The luminol-derived signal was analyzed offline by using origin 7.0 software (Microcal Software, Northampton, MA). Emitted photons were counted on a 32-ms-interval time period. Fig. 1B represents two spectra obtained with 40 pM and 50 nM d-serine. Each drug used in the present study was checked for potential interference with the d-serine assay.
Levels of d-serine released by cells were calibrated with fixed amounts of d-serine that were added at the end of each test. The area of the spectra represents the quantity of d-serine release; scales on ordinate were defined by the number of indicated photons per 5-s integration period. Total content of d-serine in cells was determined by using the chemiluminescence assay in offline mode after free amino acids were extracted with ice-cold 5% trichloroacetic acid, as described in refs. 4 and 12.
Subcellular Fractionation. Glial fractions were isolated by ultracentrifugation of postnuclear supernatant on continuous sucrose gradient (0.4–1.2 M). Each fraction was resuspended in denaturating sample buffer (pH 6.8) and separated by SDS/PAGE before gel electrophoresis and immunoblotting. Glutamate levels were measured by using the glutamate dehydrogenase/ NAD+ assay (16), and d-serine content was measured by using the chemiluminescent assay in offline mode.
Protein Electrophoresis and Immunoblotting. Protein extracts resuspended in denaturating sample buffer were subjected to SDS/PAGE (12%) analysis, followed by blotting onto poly(vinylidene difluoride) membrane. Immunodetection was performed by using the ECL amplification system (Amersham Pharmacia Biotech) following the manufacturer's protocol. Protein content was determined by the Lowry method using the DC protein Bio-Rad assay.
Immunostaining. Subconfluent cell cultures prepared as described above were fixed in 4% paraformaldehyde/0.1% glutaraldehyde for 60 min before being treated with blocking solution containing 4% horse serum and 0.2% Triton X-100 for 1 h at room temperature. Cultures then were probed with anti-glial fibrillary acidic protein (1:2,000, DAKO), conjugated anti-d-serine (1:2,000, GEMAC, Cenon, France), anti-VAMP2 (1:1,000, Synaptic System, Gottingen, Germany), anti-VAMP3 antibody (1:100, Santa Cruz Biotechnology), or anti-chromogranin B (CgB) (1:100, courtesy of J. Meldolesi, San Raffaele Scientific Institute, Milan) overnight at 4°C. After washing to remove excess primary antibodies, the cultures were incubated for 1 h at room temperature with Alexa secondary antibodies (Molecular Probes). Cells were imaged by using an upright laser-scanning confocal microscope (TCS SP2, Leica, Mannheim, Germany). Controls were performed by antisera preadsorption with 0.5 mM liquid d-serine-glutaraldehyde conjugate or by emitting the primary antibody. Colocalization of d-serine with different cellular markers was quantified with imagej software (http://rsb.info.nih.gov/ij) by using the colocalization option. Colocalization analysis was performed on each z-optical section for a cell, and values from 10–15 different cells from three independent experiments were used to calculate the colocalization frequencies.
Statistics. All data were expressed as mean ± SEM, and n refers to the number of independent experiments. Statistical differences were calculated by one-way ANOVA followed by post hoc Scheffé test using origin 7.0.
Results
Enzyme-Linked Assay for Monitoring d-Serine Release from Cultured Cells. Fig. 1C shows the dose-response curves obtained with standard amounts of d-serine. The assay shows a limit of sensitivity of 2 pM and a dynamic response over several orders of magnitude (Fig. 1C). The [d-serine] dependence of the observed signal follows a classic Michaelis–Menten behavior (Fig. 1D). Control experiments showed that no light emission was detected in the absence of any components of the physiological reaction mix or when the wild-type RgdAAO was replaced by the catalytic inactive Arg-285 → Ala mutant (Fig. 1C).
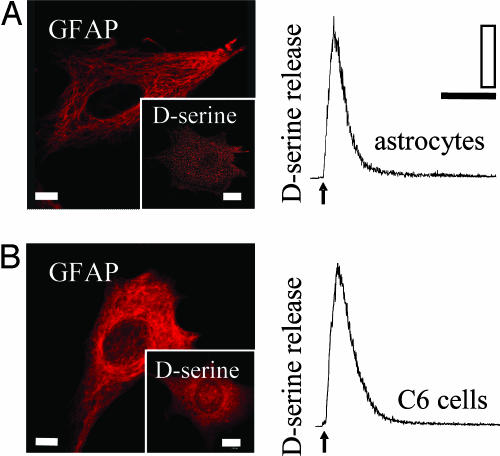
Glial Cells Synthesize and Release d-Serine upon GluRs Stimulation. Indirect immunofluorescence confocal microscopy analysis of primary cultured astrocytes indicated that these cells contain a significant amount of d-serine (see Fig. 2A Inset). By using our bioassay in offline mode, we estimated the astrocyte content of d-serine to be 142 ± 23.0 amol per cell (n = 3). To analyze the molecular mechanisms of astrocytic d-serine release, we also pursued our studies on the rat glioma-derived cell line C6, which is a glial cell strain that expresses most ion channels and receptors found in primary rat astrocytes, notably the GluRs (17, 18). Indirect immunofluorescence analysis of these cells clearly shows the presence of the specific astrocyte marker, glial fibrillary acidic protein (Fig. 2B Inset). In addition, the C6 cells contain large amount of d-serine as revealed by indirect immunofluorescence microscopy (Fig. 2B Inset). The C6 cells content of d-serine was measured at 196 ± 17.5 amol per cell (n = 8), a value in agreement with the levels found in primary astrocytes in the present study and with previously published values (7, 11). A 24-h time course analysis with subconfluent astrocytes and C6 glioma cultures showed large accumulation of d-serine in the extracellular medium from undetectable levels to 2.78 ± 0.92 nmol/mg for astrocytes and 2.01 ± 0.63 nmol/mg for C6 cells, which presupposes the spontaneous release of d-serine from glial cells.
Fig. 2.
Online detection of d-serine release from glial cells. Indirect immunofluorescence of glial fibrillary acidic protein and d-serine (Left Insets) for astrocytes (A) and C6 glioma cells (B). (Scale bars: 20 μm.) LDCL spectra of glutamate (1 mM) evoked d-serine release detected from astrocytes (A Right) and C6 glioma cells (B Right). The release of d-serine was assayed from 10,000 cells for each condition. Traces represent mean average from four independent experiments. (Scales, 4 fmol per 5 and 20 s). Arrows indicate the onset of glutamate application.
We next examined the effect of glutamate (1 mM) application on d-serine. In astrocytes, glutamate consistently evoked a rapid release of the gliotransmitter (Fig. 2A), a response also seen in C6 cells (Fig. 2B). Because of possible endogenous interfering substances released by the responsive cells upon stimulation of GluRs, two sets of control experiments were performed. First, no photon emission was observed in experiments performed on astrocytes and C6 cells in which RgdAAO or HRP was omitted from the reaction buffer (data not shown). Second, no light was detected when 1 mM glutamate or 60–90 mM KCl were applied to primary neurons or the neuroblastoma N18 cell line. Importantly, only primary astrocytes and C6 glioma cells expressed the serine racemase enzyme for d-serine biosynthesis (see Fig. 8, which is published as supporting information on the PNAS web site); therefore, only these cells contain significant amounts of the amino acid, further confirming the specificity of our assay for d-serine.
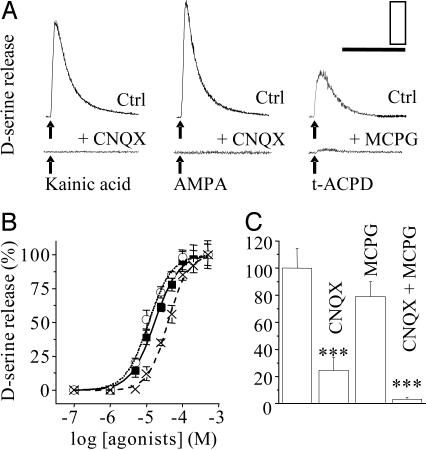
d-Serine Is Released from Glial Cells in Response to Stimulation of (S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA)/Kainate Receptors (KARs) and Metabotropic GluRs (mGluRs). Given that C6 cells conserve the properties of cultured astrocytes, we next verified whether these cells can release d-serine when challenged with different GluRs agonists. Application of AMPA, kainic acid, and (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD), agonists of AMPA/kainate and mGluRs, respectively, induced a potent and rapid release of d-serine from C6 cells (Fig. 3A) in a dose-dependent manner (Fig. 3B); t-ACPD was the least effective. d-Serine release was evoked by AMPA/KARs and mGluRs activation because it was occluded by 6-cyano-7-nitroquinoxaline-2,3-dione and (S)-α-methyl-4-carboxyphenylglycine, two selective blockers for AMPA/KARs and mGluRs, respectively (Fig. 3 A and C). Calculated from the standard dose-response curve for d-serine and the estimated content of d-serine in glioma cells, the actual fractional release of d-serine by AMPA and kainic acid at 100 μM was ≈5% of the total cell content, which corresponds to ≈6 × 106 molecules of d-serine released per cell. These results confirmed precedent works (4, 7, 11) showing effectiveness of GluR agonists on d-serine release from cultured astrocytes.
Fig. 3.
Non-NMDA glutamatergic agonists induce d-serine release from glial cells. (A) Traces representing d-serine release elicited by AMPA, kainic acid, or t-ACPD (upper traces; each at 100 μM) or in the presence of antagonist (lower traces; scales, 9 fmol per 5 and 20 s). (B) Dose-response curves for kainic acid-evoked (▪), AMPA-evoked (○), and t-ACPD-evoked (x) d-serine release. Values represent mean ± SEM (n ≥ 5). (C) Quantitative analysis of the different pharmacological manipulations on d-serine release evoked by glutamate (1 mM). Values represent percent changes (mean ± SEM, n ≥ 4) from controls. The mean GluRs-evoked LDCL is 128,023 photons for kainic acid, 156,270 for AMPA, and 84,783 for t-ACPD. ***, P < 0.001, one-way ANOVA with post hoc Scheffé test.
Because considerable evidence supports that astrocytes release gliotransmitters in response to elevated internal Ca2+ ([Ca2+]i) (19–23), we therefore next analyzed the effect of agonists on intracellular calcium concentration. Addition of AMPA and t-ACPD (100 μM each) to cells caused a large increase in [Ca2+]i (see Fig. 9, which is published as supporting information on the PNAS web site).
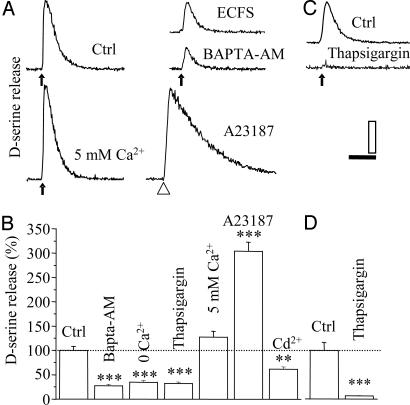
Evoked Release of d-Serine Is Calcium-Dependent. When primary astrocytes were pharmacologically stimulated with glutamatergic agonists in extracellular calcium-free solution or after treatment with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-acetoxymethyl ester, d-serine release was markedly reduced (Fig. 4 A and B), indicating that the release of d-serine was Ca2+-dependent. By contrast, an extracellular Ca2+ concentration ([Ca2+]o) increase from 2 to 5 mM augmented d-serine release (Fig. 4 A and B). Consistent with a [Ca2+]o-dependent release of d-serine, the Ca2+ ionophore A23187 by itself triggered the release of the amino acid (Fig. 4 A and B). Because activation of AMPA/KARs can lead to cell depolarization (24), and because of the fact that astrocytes display Ca2+ currents typical of low-voltage- and high-voltage-dependent channels (24–26), we thus asked whether activation of these channels could contribute to the calcium influx pathway after AMPA/KARs activation and hence triggers d-serine release. When astrocytes were treated with Cd2+, a broad-spectrum antagonist of Ca2+ channels, the AMPA-evoked d-serine release was reduced (Fig. 4B). The existence of both ryanodine-sensitive and inositol 1,4,5-trisphosphate-sensitive intracellular calcium stores in glial cells (24, 27) led us to examine their physiological relevance in triggering d-serine release upon GluRs activation. Thus, we preincubated astrocytes with the sarco-endoplasmic reticulum Ca2+-ATPase inhibitor, thapsigargin (10 μM; 20 min), to deplete the intracellular stores, before a challenge with either AMPA or t-ACPD. As predicted, thapsigargin greatly reduced the GluR agonist-evoked d-serine release (Fig. 4 B–D).
Fig. 4.
Ca2+-dependent release of d-serine from astrocytes. (A) LDCL trace shows d-serine release in response to AMPA (black arrow) in control conditions (normal calcium), in extracellular calcium-free solution (ECFS) (0 Ca2+/2 mM EGTA), in high Ca2+-containing medium (5 mM), or after intracellular Ca2+chelation with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-acetoxymethyl ester (5 μM; 1 h). Application of the ionophore A23187 (open arrowhead; 10 μM) induced a slow and long increase of chemiluminescence. (Scales: 9 fmol per 5 and 20 s.) (B) Statistical histogram of the different pharmacological manipulations during AMPA stimulation. (C and D) Effect of thapsigargin (10 μM; 20 min) on the response induced by t-ACPD (100 μM). Data are mean ± SEM of n ≥ 3 independent experiments. **, P < 0.01; ***, P < 0.001, one-way ANOVA plus Scheffé test.
All together, these results not only strongly support the hypothesis that the evoked d-serine release is triggered by calcium but also show that both extracellular and intracellular Ca2+ sources are necessary for GluRs-evoked d-serine release.
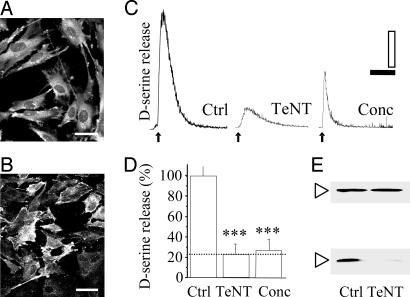
SNARE Proteins Are Required for Ca2+-Dependent d-Serine Release. Our data reveal the existence of a Ca2+-dependent release of d-serine from glial cells, suggesting the involvement of a vesicular mechanism. Several proteins of the synaptic vesicle exocytotic machinery, including the v-SNAREs VAMP2 and VAMP3, are expressed in astrocytes (28–30) or in astrocytoma-derived cell lines (31). Accordingly, immunostaining revealed high levels of VAMP2 and VAMP3 in primary astrocytes (Fig. 5 A and B), as well as in C6 cells (data not shown).
Fig. 5.
Effect of TeNT and vacuolar H+-ATPase inhibition on agonist-evokedd-serine release. (A and B) Immunofluorescence confocal microscopy analysis for VAMP2 and cellubrevin. (Scales: 40 μm.) (C) Effect of TeNT (50 nM; 24 h) and concanamycin A (1 μM; 1 h) on AMPA/t-ACPD-evoked d-serine release. (Scales: 10 fmol per 5 and 20 s). (D) Histogram summarizing the effect of TeNT and concanamycin A on the cells. Values are mean percent changes (±SEM) from four or more independent experiments. ***, P < 0.001, one-way ANOVA plus Scheffé test. (E) Immunoblot analysis of TeNT-treated and control cell extracts showing that VAMP2 (lower bands) is cleaved by TeNT, whereas α-tubulin (upper bands) immunoreactivity is not altered by toxin application.
In cultured astrocytes, ATP and glutamate release are completely dependent on the functional integrity of the proteins of this core complex (16, 19, 21, 23, 32). To provide further insight into the mechanism of d-serine release, glial cells were incubated with TeNT, a specific clostridial toxin that blocks the exocytotic release of neurotransmitters by proteolysis of VAMP2 (8) and VAMP3 (9). When cells were treated with TeNT (50 nM; 24 h), the AMPA/t-ACPD-evoked d-serine release was significantly impaired by 77.1 ± 10% (Fig. 5 C and D), when compared with untreated cells. As expected, Western blotting analysis revealed that this effect of the clostridial neurotoxin is due to cleavage of VAMP2 (Fig. 5E) and VAMP3 (data not shown).
Importantly, the effect of TeNT on the release of d-serine was highly specific, because neither the amplitude and pattern of t-ACPD/AMPA-induced [Ca2+]i elevations, nor the physiological integrity of the glial cells was affected by the neurotoxin (see Fig. 10, which is published as supporting information on the PNAS web site).
These observations further favor the hypothesis of a d-serine release process mediated primarily through vesicle exocytosis.
Concanamycin A Reduces the Release of d-Serine. Vesicular uptake of neurotransmitters requires a transmembrane electrochemical proton gradient maintained by a H+-ATPase (32) that is potently and selectively inhibited by concanamycin A or bafilomycin A1 (10). It has been shown that these compounds are effective in dissipating the electrochemical proton gradient of acidic organelles in astrocytes and thus in reducing glutamate (22, 26, 32) or ATP (21) release.
Concanamycin A (1 μM; 1 h) significantly inhibited the AMPA/t-ACPD-evoked d-serine release (Fig. 5 C and D) without affecting the agonist-induced Ca2+ signal (control mean dF/F0 = 48.0 ± 5.3%; concanamycin A mean dF/F0 = 42.7 ± 2.4%; P > 0.05; n = 6), consistent with impaired storage of d-serine in secretory organelles caused by concanamycin A.
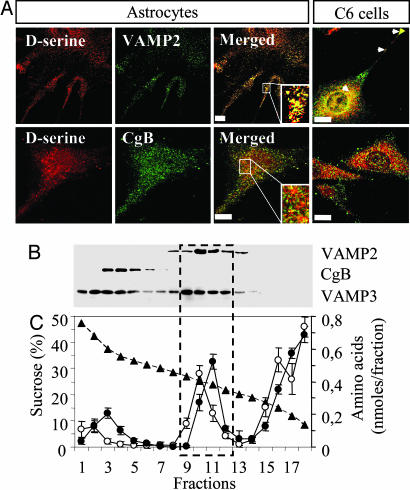
Subcellular Distribution of d-Serine. Indirect immunofluorescence confocal microscopy using specific antibodies against d-serine revealed punctate immunoreactivity (Fig. 6A), prominently localized in the perinuclear region of the cell. To define the nature of the d-serine-containing compartment, colocalization with different known markers of vesicular structures were analyzed. Small synaptic-like vesicles were detected with antibodies against VAMP2, whereas secretory granules were identified by the presence of CgB, a granin present in cultured astrocytes (21, 33). Immunocytochemical analysis revealed punctuate VAMP2 immunoreactivity in both astrocytes and C6 cells, with a high degree of colocalization with d-serine, particularly in the perinuclear regions and in the processes (Fig. 6A). Colocalization was quantified on single z-section images for all astrocytes and revealed that 42.5 ± 15.1% (59.4 ± 15.6% for C6 cells) of the SNARE protein systematically overlapped with d-serine immunostaining. Accordingly, 33.8 ± 14.6% of total cell d-serine in astrocytes (38.6 ± 22.4% for C6 cells) overlapped with VAMP2 immunostaining. Immunolabeling performed with antibodies against CgB revealed a punctate immunoreactivity throughout the cell cytoplasm (Fig. 6A). Double immunolabeling performed by using anti-d-serine and anti-CgB (Fig. 6A) or anti-SgII (data not shown) revealed a distinct distribution for each protein (d-serine/CgB overlap: 7.0 ± 4.5% in astrocytes and 5.4 ± 3.0% in C6 cells), suggesting that d-serine is not stored in secretory granules. Taken together, these results strongly support the idea that a part of the endogenous d-serine is stored in small synaptic-like vesicles.
Fig. 6.
Subcellular distribution of d-serine in glial cells. (A) Astrocytes and C6 cells were double-immunolabeled with pairs of antibodies against d-serine and VAMP2 or d-serine and CgB. A stack of z-section images that cover the entire depth of the cell is represented. Colocalization analysis for paired antibodies was performed on one single z-section image as described in Materials and Methods. The stack image is representative of the staining obtained for each single z-section. Arrows indicate regions of high colocalization. (Scale bars: 10 μm.) (B) Equilibrium sucrose density gradient analysis of d-serine storage. The membrane was probed with VAMP2 (top line), CgB (middle line), and VAMP3 (bottom line) antibodies. Blots are representative of three independent experiments. (C) Plot of d-serine (○) and glutamate (•) content in the sucrose gradient fractions. Values are the means of three independent experiments with duplicate measurements. Sucrose concentration (▴) was determined by refractometry. Note the presence of a d-serine peak overlapping the VAMP2 positive fractions and the glutamate peak.
To further characterize the intracellular organelles that accumulate d-serine in glial cells, we performed subcellular fractionation on sucrose gradients. Following procedures described in refs. 21 and 32, the postnuclear supernatant prepared from C6 cells was subjected to a sucrose equilibrium gradient. Aliquots of each collected fraction were analyzed by immunoblotting using various protein markers (Fig. 6B) and by quantification of d-serine content (Fig. 6C). VAMP2 immunoreactivity was mainly present in intermediate fractions (fractions 8–13; see Fig. 6B, first line), where the synaptic vesicles are known to migrate, whereas dense-core secretory granules (immunoreactive for CgB) were mainly present in the bottom fractions (3 to 6; Fig. 6B, middle line). Under these experimental conditions, VAMP3 was found in fractions 1–14 with an overall distribution overlapping VAMP2 and CgB (Fig. 6B, third line). Analysis of the d-serine content for each fraction revealed a peak of the amino acid in intermediate fractions 9–12 (Fig. 6C), where immunoreactivity for VAMP2 is higher. These data also indicate that at least a portion of d-serine may be packaged in VAMP2-bearing vesicles. A significant amount of d-serine also is observed in lighter fractions (Fig. 6C), which may represent the cytosolic fraction of the amino acid. Interestingly, when the fractions were probed for the presence of glutamate (Fig. 6C), another astrocyte-derived messenger, the analysis revealed the presence of a glutamate peak overlapping that of d-serine, thus suggesting that d-serine and glutamate may be stored partly in the same compartments. To provide further evidence to the latter hypothesis, we immunostained astrocytes for the vesicular glutamate transporter subtype 3, which is present in astrocytes (22), and compared its distribution with that of d-serine. We found that 45.3 ± 16.1% of vesicular glutamate transporter subtype 3 immunoreactivity overlapped with d-serine staining (see Fig. 11, which is published as supporting information on the PNAS web site).
Discussion
We show that astrocytes and C6 glioma cells contain high micromolar concentrations of d-serine, and part of this pool (≈40%) is vesicular and thus available for receptor-mediated secretion. The levels of [d-serine]i found here are in accordance with concentrations found in previous studies for this amino acid (11) and also for other amino acids in astrocytes (34, 35). By using a previously undescribed highly sensitive bioassay, we demonstrate that GluRs-induced d-serine release operates by means of a Ca2+-dependent and SNARE protein-dependent process. Our conclusion is based on several compelling arguments as follows: (i) disrupting [Ca2+]i homeostasis affects d-serine release; (ii) inhibiting the vacuolar H+-ATPase considerably reduces d-serine release, most probably by affecting its storage in acidic compartments; (iii) treatment with TeNT markedly reduces d-serine release by cleavage of synaptobrevins; and (iv) confocal imaging and sucrose gradient analysis reveal a significant colocalization of d-serine positive compartments with the vesicle marker VAMP2, suggesting that d-serine may be stored in VAMP2-bearing vesicles.
One of the most striking observations of this study is that receptor-mediated d-serine release is calcium-dependent (Fig. 4), a feature also shared by the gliotransmitters glutamate and ATP (19, 21, 32). Our data also indicate that intracellular calcium stores play an important role in the regulation of d-serine release, because depletion of these stores greatly affects the AMPA-evoked d-serine release (Fig. 4) and prevents the effect of t-ACPD on changes in [Ca2+]i and d-serine release. It will be important to determine the respective contribution of the inositol 1,4,5-trisphosphate-sensitive and caffeine/ryanodine-sensitive stores in controlling the Ca2+-dependent d-serine release. Interestingly, a recent work has shown that these Ca2+ stores are involved in the signaling pathway leading to glutamate release from glial cells (36). Our data also show that [Ca2+]o is important in controlling the release of d-serine. Activation of GluRs may induce a Ca2+ influx either sufficient to directly trigger d-serine release or to depolarize the astrocytes with the subsequent activation of voltage-gated Ca2+ channels expressed in glial cells (24, 27). Classically, neurotransmitter release from secretory cells is mediated by the opening of voltage-gated Ca2+ channels. Indeed, neuroligand-induced d-serine release was affected in the presence of Cd2+, a Ca2+-channel blocker (Fig. 4). In addition, we observed that the AMPA/kainate-induced [Ca2+]i elevation was greatly reduced in extracellular calcium-free solution, suggesting that part of the Ca2+ signal originates from an extracellular source. Calcium ions may permeate through voltage-gated Ca2+ channels, which have been shown to be present in cultured astrocytes (24, 27). However, the nature of the subtypes of voltage-gated Ca2+ channels activated after stimulation of astrocytes with GluRs agonists remains elusive. Another possibility is that a capacitative Ca2+ entry (27) is activated, induced by the depletion of internal Ca2+ stores. It is unlikely that the effects observed with Cd2+ are due to inhibition of the AMPA receptors because they are not readily permeable to calcium in primary cortical or hippocampal astrocytes (27).
We demonstrate that proteolysis of SNAREs with TeNT significantly affects the agonist-evoked release of d-serine. After a 24-h treatment with 50 nM TeNT, we observed an almost complete cleavage of VAMP2, whereas VAMP3 was less affected. In this context, it is possible that some vSNAREs are still functional, permitting exocytosis of vesicular d-serine, which may explain the residual release of the amino acid we observed after TeNT treatment (Fig. 5). In neurons, the effects of TeNT depend on receptor-mediated endocytosis of the toxin and the subsequent release of the active subunit into the cytoplasm (37). Prolonged incubations and high toxin concentration are required to inhibit glutamate or ATP release from cultured astrocytes (19, 21, 38). The lack of specific membrane receptors for TeNT in astrocytes (39) may explain the long incubations (24 h) that are necessary to accumulate an efficient intracellular concentration of TeNT to significantly reduce d-serine release. In this context, the combined conclusions based on our studies with concanamycin A and TeNT strongly implicate a vesicular compartment in mediating GluRs-dependent release of d-serine from astrocytes. Whether a novel vesicular d-serine transporter exists or whether the amino acid is cotranslocated by an already identified carrier is not yet known. To date, four different activities have been distinguished for acetylcholine, monoamines, GABA/glycine, and glutamate. All transporters depend on a proton electrochemical gradient for active transport, generated by H+-ATPases that electrogenically pump protons into the vesicle. The effectiveness of concanamycin A in disrupting the release of d-serine suggests that the mechanism of vesicular transport for d-serine shares some bioenergetic features with the other vesicular transporters. It seems unlikely that d-serine is stored in dense-core secretory granules as the distribution of the amino acid does not parallel that of CgB (Fig. 6). By contrast, d-serine colocalized with the small vesicular marker, VAMP2 and, to a lesser degree, VAMP3, supporting the idea that the amino acid is stored in synaptic-like vesicles. However, the two synaptobrevins are also present in endosomal sorting and recycling systems, which may represent an alternative vesicular source for d-serine, because these structures are known to be involved in Ca2+-dependent exocytosis in both secretory and nonsecretory cells (40).
Although our data support Ca2+-dependent vesicular release of d-serine from glial cells, it is highly probable that the TeNT-insensitive releasable pools of d-serine may be under the control of an alternative storage/release pathway. Indeed, the large fraction of d-serine present in the cytosol may represent a source for nonvesicular release through connexin hemichannels or volume anion channels, as reported for glutamate or ATP (21, 34, 41). A more widespread analysis of factors controlling d-serine compartmentalization in astrocytes and of the physiological stimuli controlling its release are necessary to define the conditions leading to its synaptic availability in the active brain.
Supplementary Material
Acknowledgments
We thank Dr. J. Meldolesi for the generous supply of the monoclonal CgB antibody; Dr. P. Rozza (Institute of Neuroscience, Milan) for the monoclonal SgII antibody; Dr. J. Molgo (Centre National de la Recherche Scientifique) for the kind gift of purified TeNT; and Drs. J. Barbier and N. Morel for experimental help. We are grateful to Dr. J. M. Billard for critical evaluation and to Dr. K. J. Mitchell for assistance in correcting the manuscript. This work was supported by Centre National de la Recherche Scientifique Grant “Jeune équipe” (to J.-P.M.) and a grant from Fondo d'Ateneo per la Ricerca 2003 (to L.P.). G.O. is supported by an Association Française Contre les Myopathies Ph.D. fellowship, and M.M. is supported by a Ministère de l'Education Nationale, de la Recherche, et de la Technologie studentship.
Author contributions: J.-P.M., P.F., and G.B. designed research; J.-P.M., L.P., G.O., and M.M. performed research; J.-P.M. and L.P. contributed new reagents/analytic tools; J.-P.M., G.O., and M.M. analyzed data; and J.-P.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMPA, (S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; CgB, chromogranin B; dAAO, d-amino acid oxidase; HRP, horseradish peroxidase; KAR, kainate receptor; LDCL, luminol-derived chemiluminescence; GluR, glutamate receptor; mGluR, metabotropic GluR; RgdAAO, dAAO purified from Rhodotorula gracilis; t-ACPD, (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid.
References
- 1.Oliet, S. H., Piet, R. & Poulain, D. A. (2001) Science 292, 923–926. [DOI] [PubMed] [Google Scholar]
- 2.Bezzi, P. & Volterra, A. (2001) Curr. Opin. Neurobiol. 11, 387–394. [DOI] [PubMed] [Google Scholar]
- 3.Schell, M. J., Brady, R. O., Jr., Molliver, M. E. & Snyder, S. H. (1997) J. Neurosci. 17, 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mothet, J. P., Parent, A. T., Wolosker, H., Brady, R. O., Jr., Linden, D. J., Ferris, C. D., Rogawski, M. A. & Snyder, S. H. (2000) Proc. Natl. Acad. Sci. USA 97, 4926–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens, E. R., Esguerra, M., Kim, P. M., Newman, E. A., Snyder, S. H., Zahs, K. R. & Miller, R. F. (2003) Proc. Natl. Acad. Sci. USA 100, 6789–6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang, Y., Ge, W., Chen, Y., Zhang, Z., Shen, W., Wu, C., Poo, M. & Duan, S. (2003) Proc. Natl. Acad. Sci. USA 100, 15194–15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro, C. S., Reis, M., Panizzutti, R., de Miranda, J. & Wolosker, H. (2002) Brain Res. 929, 202–209. [DOI] [PubMed] [Google Scholar]
- 8.Schiavo, G., Benfenati, F., Poulain, B., Rossetto, O., Polverino de Laureto, P., DasGupta, B. R. & Montecucco, C. (1992) Nature 359, 832–835. [DOI] [PubMed] [Google Scholar]
- 9.McMahon, H. T., Ushkaryov, Y. A., Edelmann, L., Link, E., Binz, T., Niemann, H., Jahn, R. & Sudhof, T. C. (1993) Nature 364, 346–349. [DOI] [PubMed] [Google Scholar]
- 10.Huss, M., Ingenhorst, G., Konig, S., Gassel, M., Drose, S., Zeeck, A., Altendorf, K. & Wieczorek, H. (2002) J. Biol. Chem. 277, 40544–40548. [DOI] [PubMed] [Google Scholar]
- 11.Schell, M. J., Molliver, M. E. & Snyder, S. H. (1995) Proc. Natl. Acad. Sci. USA 92, 3948–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolosker, H., Sheth, K. N., Takahashi, M., Mothet, J. P., Brady, R. O., Jr., Ferris, C. D. & Snyder, S. H. (1999) Proc. Natl. Acad. Sci. USA 96, 721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollegioni, L., Falbo, A. & Pilone, M. S. (1992) Biochim. Biophys. Acta 1120, 11–16. [DOI] [PubMed] [Google Scholar]
- 14.Molla, G., Vegezzi, C., Pilone, M. S. & Pollegioni, L. (1998) Protein Expression Purif. 14, 289–294. [DOI] [PubMed] [Google Scholar]
- 15.Molla, G., Porrini, D., Job, V., Motteran, L., Vegezzi, C., Campaner, S., Pilone, M. S. & Pollegioni, L. (2000) J. Biol. Chem. 275, 24715–24721. [DOI] [PubMed] [Google Scholar]
- 16.Innocenti, B., Parpura, V. & Haydon, P. G. (2000) J. Neurosci. 20, 1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brismar, T. (1995) Glia 15, 231–243. [DOI] [PubMed] [Google Scholar]
- 18.Cotrina, M. L., Lin, J. H., Alves-Rodrigues, A., Liu, S., Li, J., Azmi-Ghadimi, H., Kang, J., Naus, C. C. & Nedergaard, M. (1998) Proc. Natl. Acad. Sci. USA 95, 15735–15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezzi, P., Carmignoto, G., Pasti, L., Vesce, S., Rossi, D., Rizzini, B. L., Pozzan, T. & Volterra, A. (1998) Nature 391, 281–285. [DOI] [PubMed] [Google Scholar]
- 20.Bezzi, P., Gundersen, V., Galbete, J. L., Seifert, G., Steinhauser, C., Pilati, E. & Volterra, A. (2004) Nat. Neurosci. 7, 613–620. [DOI] [PubMed] [Google Scholar]
- 21.Coco, S., Calegari, F., Pravettoni, E., Pozzi, D., Taverna, E., Rosa, P., Matteoli, M. & Verderio, C. (2003) J. Biol. Chem. 278, 1354–1362. [DOI] [PubMed] [Google Scholar]
- 22.Montana, V., Ni, Y., Sunjara, V., Hua, X. & Purpura, V. (2004) J. Neurosci. 24, 2633–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, Q., Fukuda, M., Van Bockstaele, E., Pascual, O. & Haydon, P. G. (2004) Proc. Natl. Acad. Sci. USA 101, 9441–9446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmignoto, G. (2000) Prog. Neurobiol. 62, 561–581. [DOI] [PubMed] [Google Scholar]
- 25.Newman, E. A. & Zahs, K. R. (1998) J. Neurosci. 18, 4022–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Ascenzo, M., Vairano, M., Andreassi, C., Navarra, P., Azzena, G. B. & Grassi, C. (2004) Glia 45, 354–363. [DOI] [PubMed] [Google Scholar]
- 27.Verkhratsky, A., Orkand, R. K. & Kettenmann, H. (1998) Physiol. Rev. 78, 99–141. [DOI] [PubMed] [Google Scholar]
- 28.Jeftinija, S. D., Jeftinija, K. V. & Stefanovic, G. (1997) Brain Res. 750, 41–47. [DOI] [PubMed] [Google Scholar]
- 29.Hepp, R., Perraut, M., Chasserot-Golaz, S., Galli, T., Aunis, D., Langley, K. & Grant, N. J. (1999) Glia 27, 181–187. [DOI] [PubMed] [Google Scholar]
- 30.Maienschein, V., Marxen, M., Volknandt, W. & Zimmermann, H. (1999) Glia 26, 233–244. [DOI] [PubMed] [Google Scholar]
- 31.Volknandt, W., Kuster, F., Wilhelm, A., Obermuller, E., Steinmann, A., Zhang, L. & Zimmermann, H. (2002) Cell. Mol. Neurobiol. 22, 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araque, A., Li, N., Doyle, R. T. & Haydon, P. G. (2000) J. Neurosci. 20, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calegari, F., Coco, S., Taverna, E., Bassetti, M., Verderio, C., Corradi, N., Matteoli, M. & Rosa, P. (1999) J. Biol. Chem. 274, 22539–22547. [DOI] [PubMed] [Google Scholar]
- 34.Levi, G. & Patrizio, M. (1992) J. Neurochem. 58, 1943–1952. [DOI] [PubMed] [Google Scholar]
- 35.Nedergaard, M., Takano, T. & Hansen, A. J. (2002) Nat. Rev. Neurosci. 3, 748–755. [DOI] [PubMed] [Google Scholar]
- 36.Hua, X., Malarkey, E. B., Sunjara, V., Rosenwald, S. E., Li, W. H. & Parpura, V. (2004) J. Neurosci. Res. 76, 86–97. [DOI] [PubMed] [Google Scholar]
- 37.Matteoli, M., Verderio, C., Rossetto, O., Iezzi, N., Coco, S., Schiavo, G. & Montecucco, C. (1996) Proc. Natl. Acad. Sci. USA 93, 13310–13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasti, L., Zonta, M., Pozzan, T., Vicini, S. & Carmignoto, G. (2001) J. Neurosci. 21, 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verderio, C., Coco, S., Rossetto, O., Montecucco, C. & Matteoli, M. (1999) J. Neurochem. 73, 372–379. [DOI] [PubMed] [Google Scholar]
- 40.Jaiswal, J. K., Andrews, N. W. & Simon, S. M. (2002) J. Cell Biol. 159, 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stout, C. E., Costantin, J. L., Naus, C. C. & Charles, A. C. (2002) J. Biol. Chem. 277, 10482–10488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.