Abstract
The stability of ecological communities largely depends on the strength of interactions between predators and their prey. Here we show that these interaction strengths are structured nonrandomly in a large Caribbean marine food web. Specifically, the cooccurrence of strong interactions on two consecutive levels of food chains occurs less frequently than expected by chance. Even when they occur, these strongly interacting chains are accompanied by strong omnivory more often than expected by chance. By using a food web model, we show that these interaction strength combinations reduce the likelihood of trophic cascades after the overfishing of top predators. However, fishing selectively removes predators that are overrepresented in strongly interacting chains. Hence, the potential for strong community-wide effects remains a threat.
Keywords: community stability, omnivory, trophic chain, trophic cascade, quantitative networks
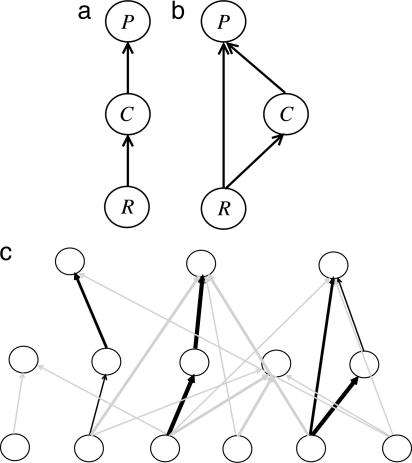
Quantification of the strength of interactions between species is essential for understanding how ecological communities are organized and how they respond to human exploitation. Food webs are characterized by many weak interactions and a few strong interactions (1–6), which appears to promote community persistence and stability (7–9). However, little is known about how interaction strengths are combined to form the simplest components of complex food webs (10, 11). An example of such a component is a tritrophic food chain (TFC) in which a top predator P eats a consumer C, which in turn eats a resource R (Fig. 1).
Fig. 1.
The building blocks of complex food webs. (a) TFC. (b) TFC with omnivory. Nodes from top to bottom represent the top predator (P), the consumer (C), and the resource (R). Arrows represent trophic links. (c) Schematic representation of a food web highlighting three TFCs (one of them with omnivory). The central food chain shows cooccurrence of two strong interaction strengths, the combination explored in this paper.
The cooccurrence of strong interactions on two consecutive levels of a trophic chain has the potential to modify the structure and dynamics of entire food webs through trophic cascades (12–16). Trophic cascades are predator–prey effects that alter biomass or abundance of a species across more than one trophic link (12, 16). Reductions in the abundance of a predator through fishing would propagate through the food chain resulting in increased consumer abundance and fewer resources (13).
The role of omnivory (the top predator also feeds on the resource) (Fig. 1b) in food web stability has been debated for decades. Although previous results concluded that omnivory destabilizes food webs (17), recent studies have shown the opposite trend (18, 19). It is unclear whether omnivory can mitigate the effect of trophic cascades when top predators and consumers are strong interactors (20). Thus, although the overfishing of top predators has the potential to cause trophic cascades, we still do not know how general the community-wide effects of overfishing are.
Here we analyze a real, large food web to describe the patterns of interaction strength combinations and explore their implications for food web dynamics. Specifically, we first quantified per-capita interaction strengths (interaction strengths hereafter) between all predator–prey links. Then, we determined how strong interactions are combined within TFCs with and without omnivory. Finally, we linked structure and dynamics by using a biologically parameterized food web model. This model allows us to assess the implications of the observed interaction strength combinations on trophic cascades.
Materials and Methods
The Food Web. We compiled from published studies (21, 22) a large quantitative food web: 249 species/trophic groups and 3,313 interactions. The food web depicts the trophic interactions of a Caribbean marine ecosystem covering ≈1,000 km2 and comprises all benthic and pelagic communities from the surface to a 100-m depth, including detritus, 4 primary producer groups, 35 invertebrate taxa, and 208 fish species, as well as sea turtles and sea birds (see Data Set 1, which is published as supporting information on the PNAS web site, for the food web and a list of species and Supporting Materials and Methods, which is published as supporting information on the PNAS web site, for the strengths and limitations of data) (21, 22).
Quantification of Predator–Prey Interaction Strengths. To investigate the structure of the food web we calculated a per-capita, standardized measure of the strength of the interaction of predators on their prey (2, 5). This measure is estimated as the proportion of prey biomass consumed per capita (per unit of biomass of the predator), per day, i.e.,
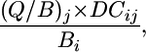 |
[1] |
where (Q/B)j is the number of times an age-structured population of predator j consumes its own weight per day (23), DCij is the proportion of prey i in the diet of predator j, and Bi is biomass of prey i (see Supporting Materials and Methods for the detailed derivation of the above expression). Parameter values were obtained from many individual studies compiled by Opitz (22). From the above parameters, (Q/B)j is probably the most reliable, and it is estimated as the metabolic efficiency of an average individual during its growth (23). Information on this metabolic efficiency comes from experimental studies for almost all species considered in this food web (see Supporting Materials and Methods for details) (22).
DCij values were obtained primarily from fish stomach contents in the U.S. Virgin Islands (21). Specifically, stomach contents of a total of 5,526 specimens of 212 fish species were analyzed (21, 22). The average number of individuals per species is 27.1 (SD = 29.03).
The biomass (measured in grams per square meter) of the species used here are average estimates for the U.S. Virgin Islands–Puerto Rico region (22). Body mass is estimated as the average body mass of adults multiplied by a correction factor describing the age structure of the population (see ref. 22 for details). Density estimates were made by Opitz (22) synthesizing several sources. She quantified previously qualitative measures of density. The only exception are fire corals, for which no biomass data were available. We estimated the average biomass of fire corals on Caribbean coral reefs from ref. 24. This approach takes advantage of the logic of the mass balance model ECOPATH (25), which assumes that, at equilibrium, consumption equals production plus respiration plus unassimilated food and provides “instantaneous” snapshot estimates of biomasses and biomass flow between species or trophic groups. Our goal, however, was not to produce a mass balance model but to use estimates of consumption/biomass ratios at equilibrium to obtain measures of energy transfer between taxa that we then standardized to average per-capita effects of predators on prey populations.
With the above measure, we first described the frequency distribution of interaction strengths. Second, we moved beyond this statistical distribution by studying how interaction strengths are combined in TFCs (Fig. 1a) and TFCs with omnivory (Fig. 1b). These basic chains can be viewed as the building blocks of complex food webs (Fig. 1c) (10) or the simplest representation of multitrophic relationships frequently used in theoretical studies (8, 26, 27). All TFCs were considered, from those with primary producers at the base to those with fishes at the base.
Interaction Strength Combinations in Food Chains. Per-capita interaction strengths (IS) were classified in four quartile classes: class 1, IS < 10–7; class 2, 10–7 ≤ IS < 10–5; class 3, 10–5 ≤ IS < 10–3; and class 4, IS ≥ 10–3. We defined class 4 as strong interactions. We looked at combinations of interaction strength values within class 4 in TFCs and food chains with omnivory. Other classifications did not qualitatively change the results presented here.
To determine whether two strong interactions cooccur more often than expected by chance within TFCs and chains with omnivory, we randomized the original food web by randomly exchanging predator–prey pairs of interaction strengths. These pairs were kept intact to preserve the topological structure of the matrix (10). That is, if two species interact in the real food web, they also interact in each replicate, but the algorithm assigns to this link a weight randomly chosen from the pool of interaction strength values. Similarly, if two species do not interact in the real food web, they will not interact in any replicate. We generated a total of 50,000 replicate food webs. For each replicate food web, we classified interaction strengths in the previous four classes and measured the number of food chains with two strong interactions. We then used the distribution of the number of food chains with two strong interactions to determine the probability that a random food web has a smaller or larger number of such food chains than that in the real food web. Because not all interaction strength values necessarily form a TFC (e.g., a basal species A may be eaten by species B, which is not eaten by any other species), we used a second null model in which only the interaction strengths that belong to at least a TFC are randomized. Results were qualitatively similar.
Linking Structural and Dynamical Measures of Interaction Strength. There are two main approaches to calculate interaction strength. First, Paine's seminal paper (2) was based on a dynamical assessment of the “absolute prey response standardized by some measure of prey abundance.” This measure has been empirically calculated for only a few species. In contrast, observational, indirect (static) information has been used to estimate interaction strength for larger communities (5). Our measure of interaction strength builds from the last approach. This calculation, unfortunately, precludes any inference about dynamical implications. The reason is that it is a static measure, i.e., it cannot describe changes in biomass. There is a lack of direct estimates of other parameters that affect such changes. For this reason, we bridge between static and dynamic measures when relating the results on structure to the dynamical model, a model built in a way that maximizes the use of observational information. This synthesis facilitates comparisons. As a first step, we have parameterized a bioenergetic model with biologically realistic values. Combinations of the structural interaction strengths are incorporated in the dynamical model by combining preference, nonlinear functional response, metabolic parameters, and body mass ratios. The above static and dynamic measures of interaction strength have the following similarities.
They represent a property of each individual link (28).
They provide a top-down measure of consumption intensity (28).
They do not measure prey response (28).
Prey preference is used in both measures: Ωij in the model and DCij in the static measure of interaction strength.
(Q/B)j is essentially identical to the maximum ingestion rate Yj, although the first variable is per unit biomass and the second is per unit metabolic rate.
The same static and dynamic measures of interaction strength also differ. Our static measure ignores functional responses (it is based on fixed biomass of prey). In contrast, our model considers functional responses, which captures the fact that interaction strength varies with prey and predator density. The first measure can be calculated for many species, whereas the latter can only be calculated for a small subset of species (the real form of the functional response is unknown for the bulk of species).
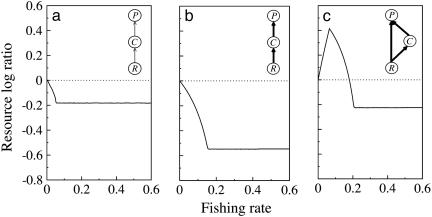
The Food Web Model. To determine the consequences of the interaction strength combinations on trophic cascades, we used a bioenergetic model of a simple TFC and a food chain with omnivory (8, 19, 29). Because overfishing tends to eliminate the species in the higher levels of food chains (30, 31), we simulated the fishing of top predators and explored the subsequent change in resource biomass. As in related studies, the magnitude of the trophic cascade was measured as the log ratio of resource biomass without fishing of the top predator to resource biomass with fishing of the top predator (15).
Although the model describes independent trophic modules, the modules studied in this paper are embedded within the entire food web. A first step in addressing this difference is by adding allochthonous inputs A to the model, which captures the fact that resources and consumers feed on other species (see Supporting Materials and Methods for more details). The model can be written as
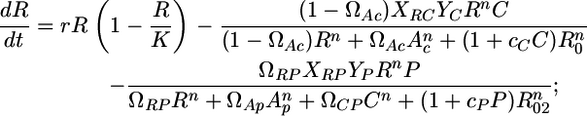 |
[2] |
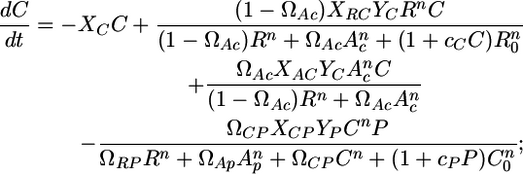 |
[3] |
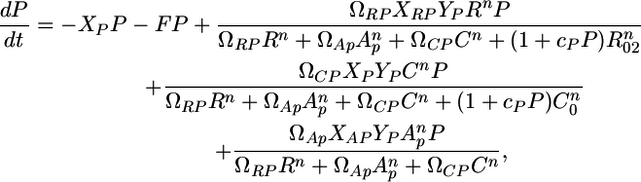 |
[4] |
where R is the resource (basal species) biomass, C is the consumer (intermediate species) biomass, and P is the top predator biomass. r is the resource intrinsic growth rate (its production-to-biomass ratio, see ref. 29). K is the resource-carrying capacity, and R0, R02, and C0 are the half saturation densities of the resource when consumed by C, by P, and of the consumer itself when consumed by P, respectively. Xij is a relative specific rate of respiration, normalized to the production-to-biomass ratio of the consumer population (see ref. 29 and Supporting Materials and Methods for a detailed derivation). Yj is the ingestion rate per unit metabolic rate of species j (29). The two previous metabolic parameters are estimated by using information for vertebrate ectotherms (29) and biomass estimated from this study (see Supporting Materials and Methods). F is the fishing rate of the top predator.
Ωij represents the species j preference for species i. Thus, Eqs. 2–4 represent a simple TFC when ΩRP = 0 and an omnivory food web when ΩRP > 0.
c is a positive constant describing the magnitude of interference among predators (see ref. 32 for details), and n is the number of encounters a predator must have with its prey before the predator is maximally efficient at feeding on that prey item (33). The role of n is to shift the functional response from type II to type III. The type II response is a special case of the type III response (for n = 1, where a predator is always maximally efficient on the prey item) (33).
We have tested Holling type II (29, 33) (n = 1, ci = 0), Holling type III (29, 33) (n = 2, ci = 0), and predation interference (34) (n = 1, ci > 0) functional responses. All three functional responses and a range of realistic parameter combinations showing stable dynamics have given similar qualitative results (except for predator interference for certain parameter combinations, see Fig. 4, which is published as supporting information on the PNAS web site). The specific parameter combination used in Fig. 3 is the following. Functional response, Holling type II; ΩAC = 0.6, ΩCP = 0.4, and ΩAP = 0.6 for Fig. 3 a and b; ΩAP = 0.2 and ΩRP = 0.4 for Fig. 3c. Xij = 0.1 and Yi = 3, corresponding to weak interactions as depicted in Fig. 3a Inset. Xij = 0.2 and Yi = 4, corresponding to strong interactions in Fig. 3 b and c. Other parameter values are r = 1, K = 1, R0 = R02 = C0 = 0.75, n = 1, cC = 0.005, cP = 0.35, and AC = AP = 0.01.
Fig. 3.
Response of the resource as a function of the fraction of predators fished in TFCs with two weak interactions (a), two strong interactions (b), and food chains with omnivory and three strong interactions (c), based on a bioenergetic model (see Materials and Methods). The magnitude of the trophic cascade (measured as the resource log ratio) is greater for food chains with two strong interactions (compare a with b), and it is reduced when there is a similarly strong omnivory link (compare b with c). The dotted line is used as a reference. Parameter combinations are specified in Materials and Methods.
Results and Discussion
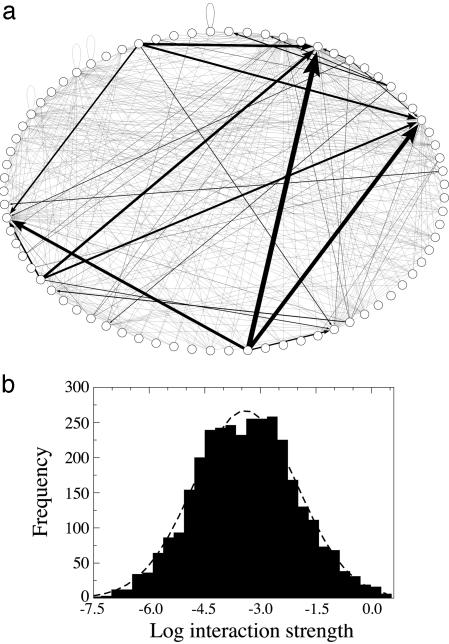
Fig. 2a shows a random sample of the whole food web for representation purposes. Link width represents interaction strength. There are a few strong interaction strengths distributed within a matrix of weak interactions, confirming previous results (1–6). The frequency distribution of per-capita interaction strengths fits a lognormal distribution with marginal significance (P = 0.06, Lilliefors' test) (Fig. 2b) and spans seven orders of magnitude, highlighting the extreme variability of predator–prey interaction strengths.
Fig. 2.
Interaction strength variability. (a) Random sample of the Caribbean food web containing 30% of the species and 11% of the interactions. Each node represents a species or taxon. Arrows represent trophic interactions between predators and their prey. Arrow thickness is proportional to the interaction strength. Loops represent cannibalism. (b) Frequency distribution of interaction strengths (n = 3,313) spanning seven orders of magnitude. The line represents the best fit to a lognormal distribution.
The fish species involved in most strongly interacting TFCs were sharks as top predators (see Data Set 1), groupers (family Serranidae) as consumers, and fishes of the Blenniidae, Clupeidae, Engraulidae, Pomacentridae, and Scaridae families as the base of the TFC.
Our analysis showed that the cooccurrence of two strong interactions in TFCs is less frequent than expected by chance (n = 3,086, P = 0.0018). When two strong interactions cooccur, strong TFCs have a strong omnivory link more often that expected by chance (n = 585, P = 0.0001).
The food web model indicated that the cooccurrence of two strong interactions in the basic TFC increases the magnitude of the trophic cascade (Fig. 3, compare a with b). However, the magnitude of the trophic cascade is reduced in the presence of strong omnivory (Fig. 3, compare b with c). In addition, omnivory qualitatively changes the response of the resource, which may first increase with moderate fishing of the top predator (Fig. 3, compare b with c). These results indicate that the interaction strength combinations here reported reduce the likelihood of trophic cascades, with important implications for food web dynamics. Future work is needed to explore how these results are affected by the use of trophic modules more explicitly embedded within the whole food web. This challenge remains because models describing an increasing number of species become increasingly unstable for biologically realistic parameter combinations (35).
The reduced tendency for trophic cascades resulting from the reported interaction strength combinations, however, does not imply that this community is buffered from the effects of exploitation. Fishing selectively targets a biased sample of species belonging to upper trophic levels (30, 31). These species, which include top predators, are overrepresented in the relatively rare strongly interacting TFCs. For example, 10 heavily fished top predators (sharks from seven families; see Data Set 1) account for 48% of the strongly interacting TFCs in the Caribbean food web. The likelihood of trophic cascades after the depletion of these strong interactors will thus depend on the relative fraction of strong omnivory. Of these strongly interacting TFCs, 31% have the buffering effect of strong omnivory, still leaving roughly two of three strongly interacting TFCs susceptible to trophic cascades.
The dynamic consequences of the structural patterns reported here provide a framework to assess the community-level impacts of overfishing. Although the web appears to be well protected against the overfishing of randomly picked species, it is susceptible to the effects of selective fishing. Strongly interacting TFCs include species at the base, such as parrotfishes (Scaridae) and other herbivores (see Data Set 1), which are important grazers of macroalgae (21). The removal of herbivores by fishing has been partly responsible for the shift of Caribbean reefs from coral- to algae-dominated (36). Our results suggest that overfishing of sharks may have also contributed to the depletion of herbivorous fishes through trophic cascades, thus enhancing the degradation of Caribbean reefs. The community-wide impacts of fishing are stronger than expected because fishing preferentially targets species whose removal can destabilize the food web.
Supplementary Material
Acknowledgments
This paper is dedicated to the memory of Ramon Margalef (1919–2004). We thank J. Alroy, F. Ballantyne, L.-F. Bersier, P. Buston, J. Jackson, P. Jordano, N. Knowlton, N. Martinez, R. M. May, R. Paine, A. G. Sáez, S. Sandin, E. Seabloom, G. Sugihara, D. Vázquez, and several anonymous referees for comments on a previous draft and interesting discussions. J.B. and C.J.M. thank the faculty at the Scripps Institution of Oceanography for hospitality during a 3-month visit in 2002. Funding was provided by Spanish Ministry of Science and Technology Grant REN2003-04774 (to J.B.), Ph.D. Fellowship FP2000-6137 (to C.J.M.), and a grant from the History of Marine Animal Populations Program of the Census of Marine Life, which was sponsored by the Alfred P. Sloan Foundation (to E.S. and J. Jackson). Part of this work was conducted at the National Center for Ecological Analysis and Synthesis while J.B. was a visiting scientist (summer 2003).
Author contributions: J.B. and E.S. designed research; J.B. and C.J.M. performed research; C.J.M. contributed new reagents/analytic tools; J.B. and C.J.M. analyzed data; and J.B. and E.S. wrote the paper.
Abbreviation: TFC, tritrophic food chain.
References
- 1.Ulanowicz, R. E. & Wolff, W. F. (1991) Math. Biosci. 103, 45–68. [DOI] [PubMed] [Google Scholar]
- 2.Paine, R. T. (1992) Nature 355, 73–75. [Google Scholar]
- 3.Fagan, W. F. & Hurd, L. E. (1994) Ecology 75, 2022–2032. [Google Scholar]
- 4.Raffaelli, D. & Hall, S. (1995) in Food Webs: Integration of Patterns and Dynamics, eds. Polis, G. & Winemiller, K. (Chapman & Hall, New York), pp. 185–191.
- 5.Wootton, J. T. (1997) Ecol. Monogr. 67, 45–64. [Google Scholar]
- 6.Goldwasser, L. & Roughgarden, J. (1997) Ecology 78, 41–54. [Google Scholar]
- 7.May, R. M. (1973) Stability and Complexity in Model Ecosystems (Princeton Univ. Press, Princeton). [PubMed]
- 8.McCann, K., Hastings, A. & Huxel, G. R. (1998) Nature 395, 794–798. [Google Scholar]
- 9.Kokkoris, G. D., Troumbis, A. Y. & Lawton, J. H. (1999) Ecol. Lett. 2, 70–74. [Google Scholar]
- 10.Neutel, A., Heesterbeek, J. A. P. & Ruiter, P. C. (2002) Science 296, 1120–1123. [DOI] [PubMed] [Google Scholar]
- 11.Milo, R., Shen-Orr, S., Itzkovitz, S., Kashtan, N., Chklovskii, D. & Alton, U. (2002) Science 298, 824–827. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter, S. R. & Kitchell, J. F. (1993) The Trophic Cascade in Lake Ecosystems (Cambridge Univ. Press, New York).
- 13.Pace, M. L., Cole, J. J., Carpenter, S. R. & Kitchell, J. F. (1999) Trends Ecol. Evol. 14, 483–488. [DOI] [PubMed] [Google Scholar]
- 14.Paine, R. T. (1980) J. Anim. Ecol. 49, 667–685. [Google Scholar]
- 15.Shurin, J. B., Borer, E. T., Seabloom, E. W., Anderson, K., Blanchette, C. A., Broitman, B., Cooper, S. D. & Halpern, B. S. (2002) Ecol. Lett. 5, 785–791. [Google Scholar]
- 16.Pinnegar, J. K., Polunin, N. V. C., Francour, P., Badalamenti, F., Chemello, R., Harmelin-Vivien, M.-L., Hereu, B., Milazzo, M., Zabala, M., D'Anna, G. & Pipitone, C. (2000) Environ. Conserv. 27, 179–200. [Google Scholar]
- 17.Pimm, S. L. & Lawton, J. H. (1978) Nature 275, 542–544. [Google Scholar]
- 18.Fagan, W. F. (1997) Am. Nat. 150, 554–567. [DOI] [PubMed] [Google Scholar]
- 19.McCann, K. & Hastings, A. (1997) Proc. R. Soc. London Ser. B 264, 1249–1254. [Google Scholar]
- 20.Polis, G. A. & Holt, R. D. (1992) Trends Ecol. Evol. 7, 151–154. [DOI] [PubMed] [Google Scholar]
- 21.Randall, J. E. (1967) Stud. Trop. Oceanogr. 5, 665–847. [Google Scholar]
- 22.Opitz, S. (1996) Trophic Interactions in Caribbean Coral Reefs, International Center for Living Aquatic Resources Management Technical Reports (Int. Cent. Living Aquat. Resour. Manage., Makati City, Philippines), Vol. 43.
- 23.Pauly, D.A. (1986) Fish. Bull. 84, 827–840. [Google Scholar]
- 24.McField, M. (1998) The Influence of Disturbance and Management on Coral Reef Communities in Belize. Ph.D. Thesis. (Univ. of Newcastle, Newcastle, U.K.).
- 25.Christensen, V, Walters, C. J. & Pauly, D. (2004). Ecopath with Ecosim: A User's Guide, Fisheries Centre Research Reports (Univ. of British Columbia, Vancouver), Vol. 12.
- 26.Pimm, S. L. & Lawton, J. H. (1997) Nature 268, 329–331. [Google Scholar]
- 27.Holt, R. D. (1996) in Multitrophic Interactions, eds. Begon, M., Gange, A. & Brown, V. (Chapman & Hall, London), pp. 333–350.
- 28.Berlow, E. L., Neutel, A-M., Cohen, J. E., De Ruiter, P. C., Ebenman, B., Emmerson, M., Fox, J. W., Jansen, V. A. A., Jones, J. I., Kokkoris, G. D., et al. (2004) J. Anim. Ecol., 73, 585–598. [Google Scholar]
- 29.Yodzis, P. & Innes, S. (1992) Am. Nat. 139, 1151–1175. [Google Scholar]
- 30.Myers, R. A. & Worm, B. (2003) Nature 423, 280–283. [DOI] [PubMed] [Google Scholar]
- 31.Pauly, D., Christensen, V., Dalsgaard, J., Froese, R. & Torres, F., Jr. (1998) Science 279, 860–863. [DOI] [PubMed] [Google Scholar]
- 32.Skalski, G. T. & Gilliam, J. F. (2001) Ecology 82, 3083–3092. [Google Scholar]
- 33.Real, L. A. (1997) Am. Nat. 111, 289–300. [Google Scholar]
- 34.Beddington, J. R. (1975) J. Anim. Ecol. 51, 331–340. [Google Scholar]
- 35.Chen, X. & Cohen, J. E. (2001) J. Theor. Biol. 212, 223–235. [DOI] [PubMed] [Google Scholar]
- 36.Hughes, T. P. (1994) Science 265, 1547–1551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.