Abstract
Objective:
To identify gadolinium-enhancing lesions affecting the cortex of patients with early multiple sclerosis (MS) and to describe the frequency and evolution of these lesions.
Methods:
We performed a retrospective, observational, longitudinal analysis of MRI scans collected as part of the Betaseron vs Copaxone in Multiple Sclerosis with Triple-Dose Gadolinium and 3T MRI Endpoints (BECOME) study. Seventy-five patients with early-stage MS were scanned monthly, over a period of 12–24 months, using 3T MRI after administration of triple-dose gadolinium. A total of 1,188 scans were included in the analysis. A total of 139 were selected using an image pipeline algorithm that integrated the image information from cortical gray matter masks and gadolinium-enhancing lesion masks. These scans were evaluated to identify gadolinium-enhancing lesions affecting the cortex.
Results:
The total number of gadolinium-enhancing lesions was 2,044. The number of gadolinium-enhancing lesions affecting the cortex was 120 (6%), 95% of which were leukocortical. The number of patients who showed gadolinium-enhancing lesions affecting the cortex was 27 (36%). The number of gadolinium-enhancing lesions affecting the cortex at baseline was 25 (21%) and the number of new lesions that developed in follow-up scans was 49 (41%). The number of persistent lesions was 46 (38%).
Conclusions:
The presence of enhancing lesions affecting the cortex and adjacent white matter, although transient and not frequent, suggests that at least some cortical lesions are related to blood–brain barrier disruption. Our data support the concept that there may be an acute inflammatory phase in the development of leukocortical MS lesions.
Clinicaltrials.gov identifier:
Multiple sclerosis (MS) is an inflammatory and neurodegenerative disease of the CNS that affects both white matter (WM) and gray matter (GM).1 The presence of MS pathology in the cortical GM in vivo is generally underappreciated due to its relative invisibility on MRI.2–4
MS cortical lesions (CLs) have received increased attention over the last 2 decades due to several ex vivo histologic studies showing a substantial CL load.5–7 Most studies have used autopsy material from patients with end-stage, progressive MS, where CLs are generally believed to be noninflammatory.6,8 In contrast, there have been conflicting reports of some CLs also showing rims of activated microglia that could be interpreted as a sign of active inflammation.9 However, the presence of activated microglia alone does not necessarily implicate acute, peripherally driven inflammation accompanied by blood–brain barrier (BBB) leakage and MRI gadolinium enhancement.10
Finally, studies of MS biopsy tissue from early-stage MS have shown that early cortical pathology may occur on a classical inflammatory background.11,12 These varied observations leave open the question of whether cortical pathology is related to a primarily neurodegenerative process, an innate more active microglial phenomenon, or a peripherally driven mechanism similar to that of inflammatory demyelination in WM plaques.9,13
Our study specifically aimed to determine whether cortical gadolinium enhancement, which provides MRI evidence of BBB impairment,14 a marker of peripherally driven acute inflammatory disease activity, occurs in early MS.
METHODS
Study design.
Our study is a retrospective, longitudinal, observational analysis that aims to assess the prevalence of gadolinium-enhancing lesions affecting the cortex in patients with early relapsing-remitting MS (RRMS) and clinically isolated syndromes (CIS) suggestive of MS. We used an existing MRI dataset15 acquired in the context of a previous randomized clinical trial that assessed treatment response to interferon and glatiramer acetate (the Betaseron vs Copaxone in Multiple Sclerosis with Triple-Dose Gadolinium and 3T MRI Endpoints [BECOME] study) in early-stage, treatment-naive patients with MS. This study uniquely employed a triple dose of gadolinium and a longer delay postcontrast administration (20–40 minutes) using frequent monthly 3T MRI scanning for up to 2 years. Combining triple-dose contrast administration, delayed scanning, and 3T MRI has been reported to more than double the enhancing lesion detection rate in WM.16–18
Participants.
Sixty-one patients with RRMS and 14 patients with CIS participated in the BECOME study15 (protocol M167-2002). All patients (52 women and 23 men, mean age 36 years, range 18–55, median EDSS score 2 at baseline, range 0–5.5, and median time since onset of 1.1 years) were randomized to receive approved doses of immunomodulating treatment (36 interferon-β-1b; 39 glatiramer acetate). Patients were scanned monthly during the first year of the study and given the possibility of continuing on a monthly schedule for an additional second year. Sixty-four patients (85%) remained in the study for the full 2 years. Patients showed a high compliance rate for the monthly scanning employed in the study, with 88% of the patients completing at least 9 monthly scans during the first year. When all time points for all patients were taken together, a total of 1,188 scans were included in the analysis.
Standard protocol approvals, registrations, and participant consents.
This work was approved by the Research Ethics Board of the Montreal Neurologic Institute. The BECOME study was approved by the University of Medicine and Dentistry of New Jersey (now Rutgers–New Jersey Medical School) (institutional review board no. M167-2002), and written informed consent was obtained from all patients.
MRI acquisition.
All patients were imaged using a 3T Allegra MRI scanner15 (Siemens Medical Systems, Malvern, PA). The MRI protocol consisted of the following: (1) a 3-plane localizer, (2) a sagittal T2-weighted turbo spin echo scan specifically designed to produce an internal frame of reference for reproducible axial images, (3) an axial T1-weighted spin echo scan without and with fat suppression (echo time [TE] 8 ms, repetition time [TR] 700 ms, voxel size 0.7 × 0.7 × 3 mm), (4) an injection of 0.1 mmol/kg (standard dose) gadopentetate dimeglumine (Magnevist, Bayer Healthcare, Wayne, NJ), (5) a postcontrast, axial turbo dual spin echo scan (used to obtain proton density and T2-weighted images: TE 10/100 ms, TR 4,800 ms, turbo factor 5, voxel size 0.7 × 0.7 × 3 mm), (6) an axial fluid-attenuated inversion recovery (FLAIR) scan (TE 80 ms, TR 8,530 ms, inversion time [TI] 2,500 ms, voxel size 0.7 × 0.7 × 3mm), (7) an injection of 0.2 mmol/kg (double dose) gadopentetate dimeglumine (delay between the first and second injections: 15–20 minutes), (8) an axial diffusion-weighted imaging scan, (9) a cumulatively triple-dose, delayed T1 coronal conventional spin echo, and (10) a cumulatively triple-dose, delayed T1 axial conventional spin echo with and without fat suppression (TE 8 ms, TR 700 ms, voxel size 0.7 × 0.7 × 3 mm). These T1-weighted images were obtained with delays of 40 minutes from the first injection and 20 minutes from the second injection. The fat-suppressed images precontrast and postcontrast were used to identify gadolinium-enhancing lesions.
MRI analysis.
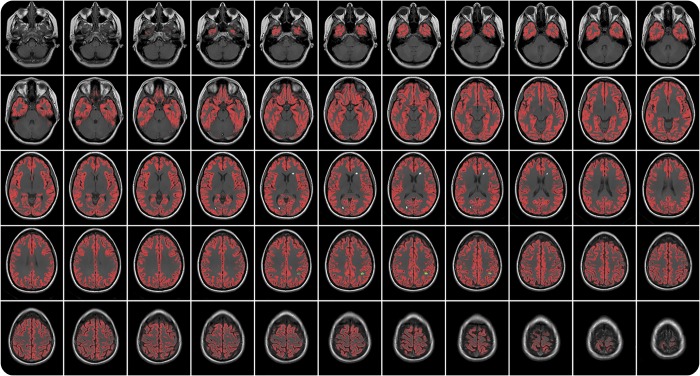
A single rater with >10 years of research experience with MRI of patients with MS (L.W.) identified all gadolinium-enhancing lesions.15 Next, scans with potential cortical enhancing lesions were selected based on the following pipeline, applied at each time point for each patient: (1) intensity uniformity correction of the T1-weighted images using the N3 method,19 (2) linear registration of the precontrast T1-weighted image to the International Consortium for Brain Mapping (ICBM) template space using full affine registration,20 (3) image denoising using a patch-based method,21 (4) segmentation of the precontrast T1-weighted image using statistical parametric mapping,22 producing a probability map of GM, (5) nonlinear registration of the preprocessed, precontrast T1-weighted image to the ICBM template using Advanced Normalization Tools,23 (6) inverse transformation of the ICBM cortical GM mask into native precontrast T1-weighted image space using trilinear interpolation and treating it as a probability map, (7) coregistration of the precontrast T1-weighted image and the postcontrast T1-weighted image using rigid-body registration, (8) transformation of the gadolinium-enhancing lesion mask from the postcontrast T1-weighted image space to the precontrast T1-weighted image space using nearest neighbor interpolation, (9) multiplication of the statistical parametric mapping–derived GM probability map, nonlinearly transformed cortical GM probability map, and transformed gadolinium-enhancing lesion mask (10), thresholding the result at 0.5 to identify the cortical component of enhancing lesions, and (11) connected component analysis (seed-fill) to detect the gadolinium-enhancing lesions that affected the cortex. Figure 1 displays an example of the cortical masks employed in our study.
Figure 1. Candidate enhancing cortical lesion selection: example output.
Example of the application of our automated algorithm for selecting candidate patient images with potential enhancing lesions affecting the cortex. Red: cortical gray matter ribbon. White lesion mask: enhancing lesion not touching the cortical gray matter. Green lesion mask: enhancing lesion touching the cortical gray matter.
From the 1,188 scans processed based on the steps outlined above, 139 were selected as candidate scans that could contain gadolinium-enhancing lesions affecting the brain cortex. Specifically, scans were selected if there was an intersection of the gadolinium-enhancing lesion mask and the cortical GM mask.
All 139 selected scans were then evaluated by a single rater (J.M.) with >10 years of experience in quantification procedures on MRI research scans of patients with MS, to identify gadolinium-enhancing lesions affecting the cortex and generate binary voxel masks for these lesions.
The segmentation of lesions was performed using the interactive software package Display,24 part of the minc toolkit (github.com/BIC-MNI) developed at the McConnell Brain Imaging Center of the Montreal Neurologic Institute. Display allows simultaneous visualization and segmentation of lesions in the axial, coronal, and sagittal planes as well as easy cycling between each image modality. The images in our study were coregistered so that, when assessing a given voxel or region and switching from one contrast (e.g., T1-weighted image, precontrast injection) to another (e.g., T1-weighted image, postcontrast injection), the rater assessed the image intensity of the same region of the brain on each contrast. All lesions identified in one plane were simultaneously shown in the other 2 orientations. Marked voxels were then saved in a separate label file that could be loaded on its own or superimposed on the brain images.
Gadolinium-enhancing lesions with a clear cortical portion to the enhancement were segmented if they met the following criteria: (1) minimum size of 3 contiguous voxels, (2) hyperintense in relation to the surrounding GM and WM on the postcontrast T1-weighted image, (3) isointense to hypointense in relation to the surrounding GM on the precontrast T1-weighted image, (4) hyperintense on proton density or T2-weighted or FLAIR images. These criteria were adopted in an attempt to minimize false-positive identifications, particularly the T2-weighted/FLAIR characteristics that are typical of MS lesions, but not, for example, of blood vessels, which are characteristically dark on T2-weighted/FLAIR images.
RESULTS
A total of 1,188 scans from 75 different patients showing 2,044 gadolinium-enhancing lesions were included in the analysis. A total of 139 scans were selected as potentially containing gadolinium-enhancing lesions affecting the cortex. Two scans were excluded from the analysis due to motion artifact affecting the postcontrast T1-weighted image. Seven scans, not selected initially by the automated pipeline, were added when assessing lesion enhancement duration in follow-up scans.
In the 144 cases selected for detailed visual inspection, the total number of gadolinium-enhancing lesions was 1,134 (55% of the total number of gadolinium-enhancing lesions in all participants). The number of gadolinium-enhancing lesions affecting the cortex in these 144 cases was 120: 6% of the total number of enhancing lesions in all subject time points. Cortical enhancement was present in 27 of the 75 patients, i.e., 36% of the patient population showed gadolinium enhancement affecting the cortex at some point during the study.
The majority (95%) of the gadolinium-enhancing lesions affecting the cortex presented as type I leukocortical lesions25 (figure 2) with clear involvement of the subcortical WM. Only 5% presented as type II intracortical lesions, restricted to the cortex. However, we acknowledge that involvement of the subcortical WM cannot be ruled out completely at the acquired image resolution (figures 3 and 4).
Figure 2. Gadolinium-enhancing leukocortical lesion with persistent T2-weighted hyperintensity.
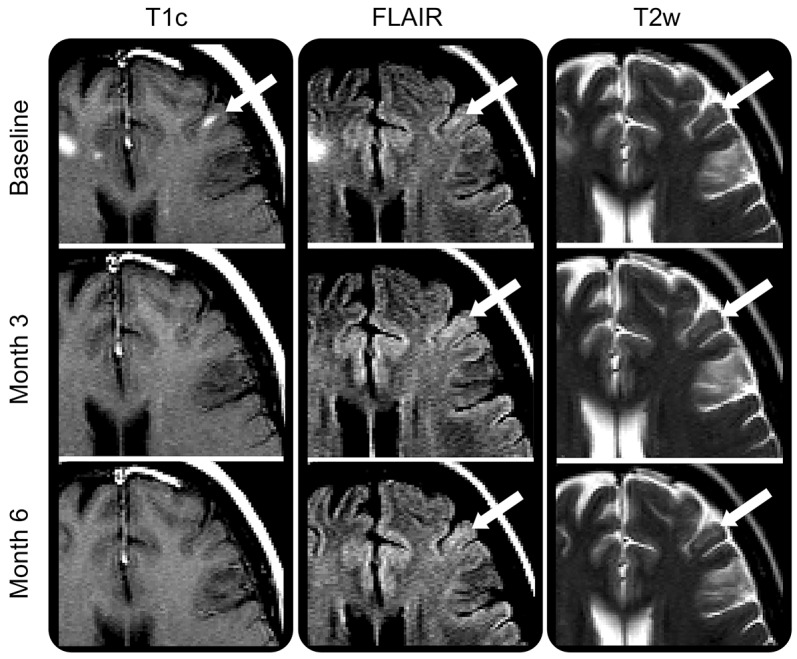
Leukocortical lesion affecting the cortex (white arrow) shown on contrast-enhanced T1-weighted imaging, fluid-attenuated inversion recovery (FLAIR), and T2-weighted imaging. For this leukocortical lesion, enhancement appeared at baseline and resolved leaving a subtle but persistent hyperintense signal on T2-weighted and FLAIR images. T1c = T1-weighted image postgadolinium injection; T2w = T2-weighted imaging.
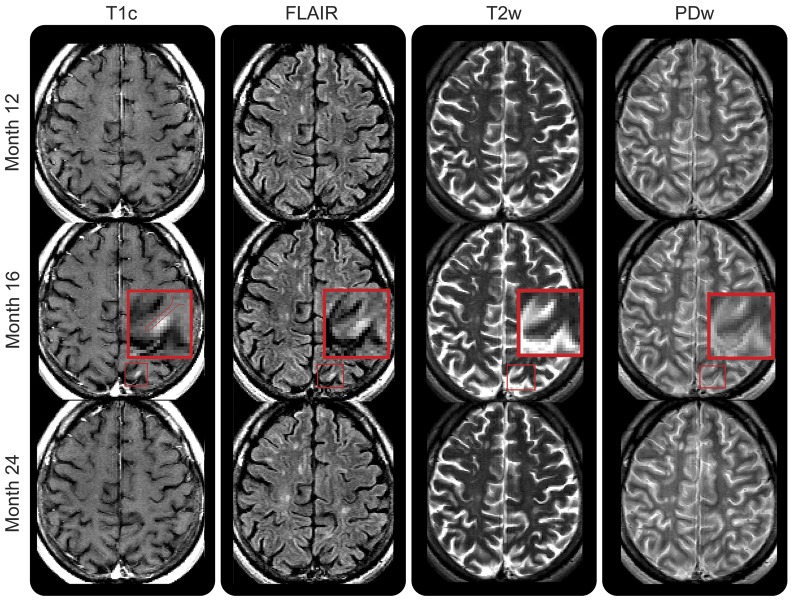
Figure 3. Gadolinium-enhancing intracortical lesion with resolving T2-weighted hyperintensity.
Small enhancing lesion affecting the cortex (red box indicates the magnified area) that appears at month 16 on contrast-enhanced T1-weighted imaging, fluid-attenuated inversion recovery (FLAIR), and T2/proton density (PD)–weighted imaging, and resolves by month 24. Enhancement appears limited to the cortex, but due to the resolution of the postgadolinium T1-weighted image, the possibility that the enhancement involves the white matter cannot be excluded. PDw = proton density-weighted imaging; T1c = T1-weighted image postgadolinium injection; T2w = T2-weighted imaging.
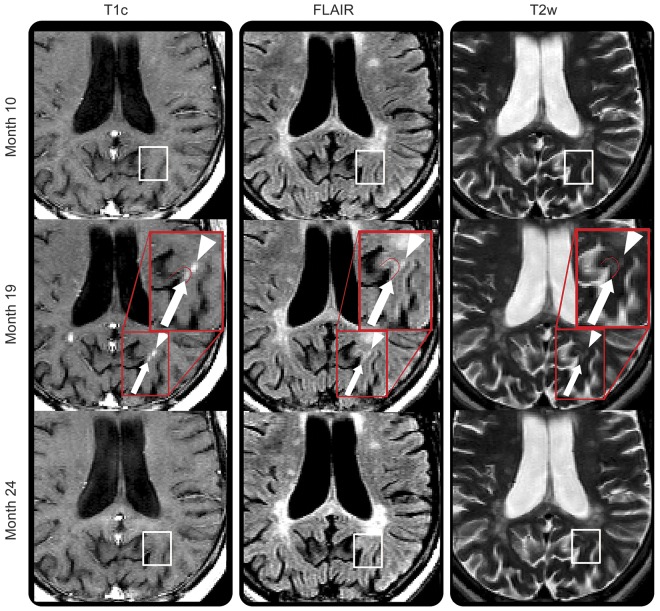
Figure 4. Cortical and subcortical gadolinium-enhancing lesions.
Enhancing lesion affecting the cortex (white arrow) next to an area of enhancement in the white matter (WM) (white arrowhead). The cortical enhancement appears distinct from the WM enhancement. Both areas of enhancement are observed first at month 19 in an area of normal-appearing tissue (white box on month 10 scan: first row), and regress, leaving a subtle hyperintense signal on T2-weighted imaging (white box on month 24 scan: last row). FLAIR = fluid-attenuated inversion recovery; T1c = T1-weighted image postgadolinium injection; T2w = T2-weighted imaging.
The number of gadolinium-enhancing lesions affecting the cortex at baseline was 25 (21%) and the number of new lesions that developed in follow-up scans was 49 (41%). The number of persistent enhancing lesions visible on one or more subsequent scans was 46 (38%).
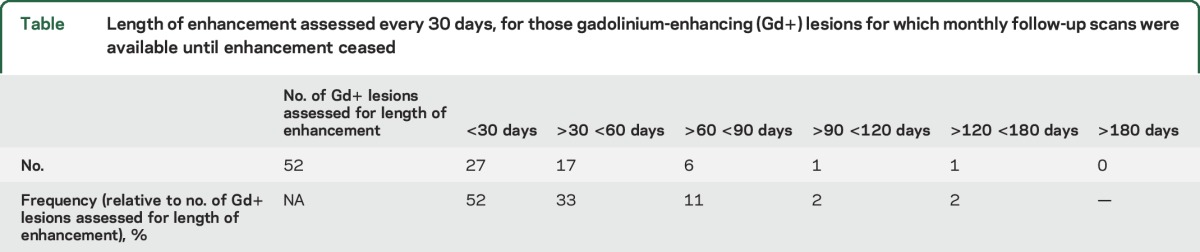
We assessed the enhancement duration for the lesions where subsequent monthly scans were available to follow the evolution of the enhancement every 30 days. The cases that lacked monthly subsequent scans were excluded to avoid inaccurate estimation of the enhancement duration. A total of 52 lesions were evaluated: 27 (52%) enhanced for less than 30 days, 17 (33%) between 30 and 60 days, 6 (11%) between 60 and 90 days, 1 (2%) between 90 and 120 days, and 1 (2%) between 120 and 180 days (table).
Table.
Length of enhancement assessed every 30 days, for those gadolinium-enhancing (Gd+) lesions for which monthly follow-up scans were available until enhancement ceased
Finally, we analyzed the evolution of enhancing lesions with images other than T1-weighted spin-echo (e.g., T2-weighted/proton density–weighted/FLAIR) on serial scans. We assessed the evolution of the 25 lesions at baseline and the 49 new enhancing lesions that developed during the trial. Five of the new enhancing lesions could not be assessed on follow-up because they appeared at the last visit (month 24). Twenty (29%) of the enhancing lesions resolved completely, leaving no signal abnormality (figure 3) when visually assessing all the different MRI modalities of follow-up scans. Such resolving lesions were characterized by a disappearance of gadolinium-enhancing voxels on postgadolinium T1-weighted imaging and the associated hyperintensity on FLAIR, T2-weighted, and proton density–weighted sequences. Twelve lesions (17%) resolved almost completely, leaving a subtle and small hyperintense signal that could only be scored as an MS lesion in a retrospective analysis, when comparing with the previous scan where the larger and gadolinium-enhancing lesion was visible. The remainder of the lesions (37 lesions: 54%) were still visible as a hyperintensity on T2-weighted/proton density–weighted/FLAIR once the gadolinium enhancement had resolved.
DISCUSSION
Various studies have described CLs histopathologically as primarily noninflammatory.6,7,26 Furthermore, CLs are most commonly observed in the progressive stages of MS. However, the majority of studies conducted to date are based on autopsy material of patients with end-stage MS, leaving the early stages of cortical lesion pathophysiology largely unexplored. Conversely, there has recently been MRI evidence that CLs occur early in the course of the disease,11,12 with studies reporting CLs in pediatric MS,27–29 CIS, and radiologically isolated syndrome.30 An associated histology study, based on biopsy material of patients with early-stage MS, showed CLs can occur on a background of acute inflammation.11 However, since the cortical samples were obtained in passing while performing biopsies of intensely inflammatory WM lesions with needles that passed through cortex, WM inflammation could possibly have biased the findings. In addition, there has been one report of an MS case presenting with a single cortical MRI gadolinium-enhancing lesion,12 where the biopsy material was obtained surgically, showing an acute demyelinating lesion typical of inflammation-induced BBB damage. This evidence suggests that CLs may be present from the early stages of MS, but that their transient periods of enhancement and the largely reversible nature of the associated T2- or T1-weighted signal abnormality makes MRI detection difficult.14 Longitudinal studies scoring CLs have reported variable gadolinium enhancement behavior, likely due, in part, to differences in MRI protocol and patient characteristics. An early study by Kidd et al.5 reported 41 leukocortical gadolinium-enhancing lesions out of a total of 258 (16%), using monthly scanning and postcontrast images with 5-mm-thick slices and 1.25-mm gap. Another study reported lack of enhancement in CLs followed over 3 years,31 while in a later study featuring postcontrast images with 1-mm-thick slices the same group described 42 gadolinium-enhancing lesions out of 1,103 affecting the cortex (3.8%).32
These 42 lesions were identified at the first scan of the 212 participants, but the number of patients presenting with gadolinium-enhancing lesions was not assessed. Interestingly, the 42 enhancing lesions appeared in pediatric MS and RRMS cases. Such enhancement was not observed in primary or secondary progressive MS. A more recent study, performed using 7T MRI and focused on the BBB behavior in MS, found 1 gadolinium-enhancing leukocortical lesion.14 Our study provides additional MRI evidence that an acute phase in MS leukocortical pathology may be present, and quantifies the prevalence of acute leukocortical pathology in a cohort of patients with early RRMS. Because the detection of CLs in vivo using noncontrast MRI has proven difficult, in this study we employed a dataset with scans acquired at 3T following a cumulative triple dose of gadolinium contrast agent with a prolonged delay (combined delay of 20 and 40 minutes).15 This method is known to increase detection efficiency for enhancing lesions in WM.16 We also used coregistered images to allow the assessment of the suspected enhancing voxels in all available MRI contrasts.24 We were not able to assess the prevalence of nonenhancing CLs. This was due to the MRI modalities acquired in the BECOME study, which was not designed to measure cortical pathology. Specifically, a double inversion recovery sequence33 or high-resolution FLAIR paired with a high-resolution T1-weighted image24,34,35 were not acquired. Given the lack of appropriate images, we chose not to include nonenhancing CLs in our analysis.
We did not observe enhancement in the leptomeningeal compartment using the T1-weighted postcontrast scans. We consider this finding consistent with the fact that high-resolution, delayed postcontrast, 3D FLAIR is currently the sequence of choice for observing the leptomeningeal enhancement phenomenon.36 The original BECOME MRI protocol did not include such a sequence.36–38
Other groups have recently reported a high incidence of leptomeningeal enhancing foci without associated leukocortical enhancing lesions.38 The patient population of this study had a considerably longer disease duration (mean of 16.5 years of duration at baseline), were 50% secondary progressive patients, and the MRI protocol had a single standard dose of gadolinium administered to the patients. In addition, leukocortical lesion enhancement was only assessed at 2 time points, while we performed monthly scanning over 12–24 months. These important differences in MS phenotype and MRI protocol, particularly the scanning frequency and contrast dose, could account for the discrepancies with our findings.
We found that 6% of all gadolinium-enhancing lesions affected the cortex. Gadolinium-enhancing lesions with cortical involvement were almost exclusively leukocortical lesions. Since our postcontrast T1-weighted images had a voxel size of 0.7 × 0.7 × 3 mm, it is possible that some of the observed cortical involvement was due to partial volume from juxtacortical lesions abutting the cortex rather than true leukocortical lesions. However, all cortical lesion candidates were carefully reviewed and only included if there was clear (i.e., sufficiently large) involvement of the cortex, in order to limit the number of false-positives. Among the enhancing cortical lesions, 29% fully disappear in follow-up scans, leaving no visible residual signal abnormalities on any of the standard MRI contrasts. In addition, 17% of the lesions experience partial resolution, leaving a subtle hyperintense signal that could only be scored as a lesion retrospectively, via comparison with the previous scan that showed the larger gadolinium-enhancing lesion. This behavior reflects an early but transient acute leukocortical inflammation, which makes the MRI identification of acute, early CLs extremely difficult. We speculate that the resolution of the T1/T2 abnormalities that accompanies resolution of gadolinium enhancement is largely related to resolution of the acute inflammation, although future studies should include quantitative MRI modalities, such as magnetization transfer imaging, to assess to what extent remyelination may contribute to the CL resolution. In addition, it is not clear whether the observed gadolinium enhancement in leukocortical lesions originates in the WM and spreads to the cortex, or originates in the cortex and spreads to the WM, or arises simultaneously in both. Further work is necessary to elaborate the dynamic aspects of this phenomenon.
We observed acute gadolinium-enhancing lesions affecting the cortex and subcortical WM in patients with early MS undergoing treatment with first-line disease-modifying treatments. Although the prevalence of leukocortical gadolinium enhancement is low over relatively short intervals, our data suggest that peripherally driven inflammation associated with transient BBB leakage may be one mechanism underlying cortical lesion formation over the long term. It remains to be determined whether acute, inflammatory leukocortical lesions that appear to resolve on conventional MRI eventually evolve into other types of cortical lesions defined by histopathology, and what the link is between acute inflammation and cortical neurodegeneration.
GLOSSARY
- BBB
blood–brain barrier
- BECOME
Betaseron vs Copaxone in Multiple Sclerosis with Triple-Dose Gadolinium and 3T MRI Endpoints
- CIS
clinically isolated syndrome
- CL
cortical lesion
- FLAIR
fluid-attenuated inversion recovery
- GM
gray matter
- ICBM
International Consortium for Brain Mapping
- MS
multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
- TE
echo time
- TI
inversion time
- TR
repetition time
- WM
white matter
AUTHOR CONTRIBUTIONS
Josefina Maranzano: study concept and design, analysis and interpretation of the data, drafting and revising the manuscript. David A. Rudko: study concept and design, revising the manuscript. Kunio Nakamura: analysis of the data, revising the manuscript. Stuart Cook: revising the manuscript, obtaining the MRI, designing the original BECOME study. Diego Cadavid: revising the manuscript, obtaining the MRI, designing the original BECOME study. Leo Wolansky: revising the manuscript, designing the original BECOME study. Douglas L. Arnold: study concept and design, revising the manuscript. Sridar Narayanan: study concept and design, interpretation of the data, drafting and revising the manuscript.
STUDY FUNDING
The BECOME study was supported by Bayer Schering Pharma, distributors of IFN, but was investigator-initiated and remains the intellectual property of Rutgers–New Jersey Medical School, Newark, New Jersey. J.M. acknowledges personal support from a David G. Guthrie Fellowship from the Faculty of Medicine of McGill University. D.A.R. was supported by a postdoctoral fellowship from the MS Society of Canada.
DISCLOSURE
J. Maranzano, D. Rudko, and K. Nakamura received personal compensation from NeuroRx Research for consulting services. S. Cook participated in the original BECOME study. D. Cadavid is a full-time employee of Fulcrum Therapeutics, to which the work on the BECOME study is not related, and participated in the original BECOME study. L. Wolansky participated in the original BECOME study and received salary support from Bayer. D. Arnold is president and CEO of NeuroRx Research. S. Narayanan received personal compensation from NeuroRx Research for consulting services unrelated to the current work. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–1517. [DOI] [PubMed] [Google Scholar]
- 2.Geurts JJ, Bo L, Pouwels PJ, Castelijns JA, Polman CH, Barkhof F. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol 2005;26:572–577. [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen AS, Kinkel RP, Tinelli E, Benner T, Cohen-Adad J, Mainero C. Focal cortical lesion detection in multiple sclerosis: 3 Tesla DIR versus 7 Tesla FLASH-T2. J Magn Reson Imaging 2012;35:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daams M, Geurts JJ, Barkhof F. Cortical imaging in multiple sclerosis: recent findings and 'grand challenges'. Curr Opin Neurol 2013;26:345–352. [DOI] [PubMed] [Google Scholar]
- 5.Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain 1999;122:17–26. [DOI] [PubMed] [Google Scholar]
- 6.Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler 2003;9:323–331. [DOI] [PubMed] [Google Scholar]
- 7.Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 2006;67:960–967. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese M, Filippi M, Gallo P. Cortical lesions in multiple sclerosis. Nat Rev Neurol 2010;6:438–444. [DOI] [PubMed] [Google Scholar]
- 9.Kooi EJ, Strijbis EM, van der Valk P, Geurts JJ. Heterogeneity of cortical lesions in multiple sclerosis: clinical and pathologic implications. Neurology 2012;79:1369–1376. [DOI] [PubMed] [Google Scholar]
- 10.Popescu BF, Pirko I, Lucchinetti CF. Pathology of multiple sclerosis: where do we stand? Continuum 2013;19:901–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. New Engl J Med 2011;365:2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popescu BF, Bunyan RF, Parisi JE, Ransohoff RM, Lucchinetti CF. A case of multiple sclerosis presenting with inflammatory cortical demyelination. Neurology 2011;76:1705–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herranz E, Gianni C, Louapre C, et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol 2016;80:776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaitan MI, Sati P, Inati SJ, Reich DS. Initial investigation of the blood-brain barrier in MS lesions at 7 tesla. Mult Scler 2013;19:1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 2009;72:1976–1983. [DOI] [PubMed] [Google Scholar]
- 16.Wolansky LJ, Finden SG, Chang R, et al. Gadoteridol in multiple sclerosis patients: a comparison of single and triple dose with immediate vs. delayed imaging. Clin Imaging 1998;22:385–392. [DOI] [PubMed] [Google Scholar]
- 17.Leppert IR, Narayanan S, Araujo D, et al. Interpreting therapeutic effect in multiple sclerosis via MRI contrast enhancing lesions: now you see them, now you don't. J Neurol 2014;261:809–816. [DOI] [PubMed] [Google Scholar]
- 18.Wolansky LJ, Bardini JA, Cook SD, Zimmer AE, Sheffet A, Lee HJ. Triple-dose versus single-dose gadoteridol in multiple sclerosis patients. J Neuroimaging 1994;4:141–145. [DOI] [PubMed] [Google Scholar]
- 19.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998;17:87–97. [DOI] [PubMed] [Google Scholar]
- 20.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 1994;18:192–205. [PubMed] [Google Scholar]
- 21.Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging 2008;27:425–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 23.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maranzano J, Rudko DA, Arnold DL, Narayanan S. Manual segmentation of ms cortical lesions using MRI: a comparison of 3 MRI reading protocols. AJNR Am J Neuroradiol 2016;37:1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol 2003;62:723–732. [DOI] [PubMed] [Google Scholar]
- 26.Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 2001;50:389–400. [DOI] [PubMed] [Google Scholar]
- 27.Absinta M, Rocca MA, Moiola L, et al. Cortical lesions in children with multiple sclerosis. Neurology 2011;76:910–913. [DOI] [PubMed] [Google Scholar]
- 28.Calabrese M, Seppi D, Romualdi C, et al. Gray matter pathology in MS: a 3-year longitudinal study in a pediatric population. AJNR Am J Neuroradiol 2012;33:1507–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocca MA, De Meo E, Amato MP, et al. Cognitive impairment in paediatric multiple sclerosis patients is not related to cortical lesions. Mult Scler 2015;21:956–959. [DOI] [PubMed] [Google Scholar]
- 30.Giorgio A, Stromillo ML, Rossi F, et al. Cortical lesions in radiologically isolated syndrome. Neurology 2011;77:1896–1899. [DOI] [PubMed] [Google Scholar]
- 31.Calabrese M, Filippi M, Rovaris M, et al. Morphology and evolution of cortical lesions in multiple sclerosis: a longitudinal MRI study. Neuroimage 2008;42:1324–1328. [DOI] [PubMed] [Google Scholar]
- 32.Calabrese M, Poretto V, Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 2012;135:2952–2961. [DOI] [PubMed] [Google Scholar]
- 33.Geurts JJ, Roosendaal SD, Calabrese M, et al. Consensus recommendations for MS cortical lesion scoring using double inversion recovery MRI. Neurology 2011;76:418–424. [DOI] [PubMed] [Google Scholar]
- 34.Nelson F, Poonawalla A, Hou P, Wolinsky JS, Narayana PA. 3D MPRAGE improves classification of cortical lesions in multiple sclerosis. Mult Scler 2008;14:1214–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sethi V, Yousry TA, Muhlert N, et al. Improved detection of cortical MS lesions with phase-sensitive inversion recovery MRI. J Neurol Neurosurg Psychiatry 2012;83:877–882. [DOI] [PubMed] [Google Scholar]
- 36.Absinta M, Vuolo L, Rao A, et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology 2015;85:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011;134:2755–2771. [DOI] [PubMed] [Google Scholar]
- 38.Zivadinov R, Ramasamy DP, Vaneckova M, et al. Leptomeningeal contrast enhancement is associated with progression of cortical atrophy in MS: a retrospective, pilot, observational longitudinal study. Mult Scler Epub 2016 Nov 1. [DOI] [PubMed] [Google Scholar]