Abstract
Objective:
To examine the association between the American Heart Association (AHA) Life's Simple 7 (LS7) metric and brain structure.
Methods:
We determined cardiovascular health (CVH) according to the AHA LS7, assigning 0, 1, or 2 points for meeting poor, intermediate, or ideal criteria for the 7 components (range 0–14) at baseline (aged 18–30 years in 1985–1986) and year 25 follow-up examination for 518 participants of the Coronary Artery Risk Development in Young Adults (CARDIA) brain MRI substudy. Visit-based CVH score and average score was assessed in relation to percent of intracranial volume of normal tissue of the whole brain, gray matter, and white matter, and abnormal tissue volume of white matter at year 25 using multivariable linear, logistic, and quantile regression, after adjustment for age, sex, race, field center, educational attainment, and alcohol consumption.
Results:
Mean percentage of whole brain volume, normal gray matter, and normal white matter was 81.3% (±2.5), 42.9% (±2.0), and 38.4% (±2.0). Greater CVH score at baseline (per each additional point at year 0: 0.1%, 95% confidence limits 0.01–0.3; p < 0.05) and average CVH score were associated with greater percentage of whole brain volume (per each additional point in average score: 0.2%, 95% confidence limits 0.04–0.3; p < 0.05). Visit-based or average CVH score was not significantly associated with normal gray or white matter volume or abnormal white matter volume.
Conclusions:
Maintaining ideal levels of cardiovascular health, determined by the LS7, in young adulthood is associated with greater whole brain volume in middle age but not regional differences in structure.
In 2010, the American Heart Association (AHA) introduced the Life's Simple 7 (LS7), defining cardiovascular health (CVH) according to meeting poor, intermediate, or ideal criteria for 7 modifiable behaviors and factors.1 Epidemiologic research has shown that meeting ideal status for a greater number of LS7 components is associated with better scores on neurocognitive tests and lower incidence of cognitive impairment.2–4 Adverse levels for individual components of the LS7 score are associated with worse concurrent brain structure.5 However, the CVH profile integral to maintaining healthy brain structure is not well-understood. Our objective was to assess the association between CVH during young adulthood and brain structure in middle adulthood. Our hypothesis was that meeting more favorable levels of CVH at distinct junctures and on average during young adulthood would be associated with greater percentage of intracranial volume of normal brain tissue and lower percentage of intracranial volume of abnormal brain tissue.
METHODS
Coronary Artery Risk Development in Young Adults study design.
Coronary Artery Risk Development in Young Adults (CARDIA) is a longitudinal cohort study of 5,115 black and white men and women from 4 US metropolitan populations. Participants were 18–30 years of age at enrollment in 1985–1986 (year [Y] 0).6 Since enrollment, participants have been invited to participate in 8 follow-up examinations roughly 2, 5, 7, 10, 15, 20, 25, and 30 years (Y2–Y30) after baseine.6
Standard protocol approvals, registrations, and patient consents.
Participants have provided written informed consent and institutional review boards have granted approval for all examinations.
Assessment of demographics, health behaviors, and clinical examination.
At each clinical examination, questionnaires were used to collect information on sociodemographic factors, medical and family history, use of medications, and behavioral/lifestyle characteristics. Venous blood was drawn in the morning after an overnight fast and plasma and serum separation was performed before aliquots were stored at −70°C and shipped on dry ice to central laboratories. Total cholesterol was measured enzymatically within 6 weeks of collection.7,8 At Y0, serum glucose was measured using the hexokinase ultraviolet method by American BioScience Laboratories (Van Nuys, CA) and at subsequent examinations using hexokinase coupled to glucose-6-phosphate dehydrogenase by Linco Research (St. Louis, MO).9
At examination Y0, seated blood pressure (BP) was measured in triplicate after 5 minutes of rest using a random-zero sphygmomanometer. At Y25, BP was measured with an automated oscillometric BP monitor (Omron HEM-907XL; Online Fitness, Santa Monica, CA) and Y25 BP values were standardized to the sphygmomanometric measures.10 The average of the last 2 BP measurements was used for this analysis. Body weight was measured using a calibrated balance beam scale and height was measured using a vertical ruler.
Diet history was assessed at Y0, Y7, and Y20 examinations wherein interviewers asked open-ended questions about food consumption in the previous month including 100 food categories, referencing 1,609 unique food items.11 This instrument shows better validity and stronger reliability for habitual food intake in white compared to black participants.12 Physical activity was assessed as the frequency of participation over the previous 12 months for 8 vigorous intensity and 5 moderate intensity sports-related activities, demonstrating high 2-week test-retest reliability (0.77–0.84).13 A physical activity exercise units (EU) score was derived, with a score of 300 EU corresponding to 30 minutes of moderate physical activity 5 times per week.14
Determination of ideal, intermediate, and poor CVH status and overall CVH status score.
We determined each participant's CVH status at CARDIA examinations Y0 and Y25 according to AHA criteria.1 We calculated CVH scores at each examination, assigning points (0, 1, or 2) for meeting poor, intermediate, or ideal criteria for each of the 7 components. Modifications were made for physical activity and diet based on the data available in CARDIA. We used a physical activity equivalent to the AHA criteria14 and diet data from Y20 to determine diet status for Y25. CVH scores could range from 0 to 14, with a CVH score of 0 interpreted as meeting poor criteria for all 7 components and a CVH score of 14 interpreted as meeting ideal criteria for all 7 components. We also modeled CVH score categorically according to cutpoints of 0–7, 8–11, and 12–14, chosen for consistency of score definition across time. We also calculated an individual average CVH score, the average of Y0 and Y25.
Brain MRI substudy methods.
The brain MRI substudy was limited to a defined subset of CARDIA participants (n = 719) from the Birmingham, Minneapolis, and Oakland field centers at examination Y25 (2010–2011). Exclusion criteria for sample selection included contraindication to MRI, possible pregnancy, or a body size that was too large for the MRI tube bore. MRI acquisition and processing have been described previously.5 Briefly, 3T magnetic resonance scanners were used to acquire structural imaging, standardized across machines using a common machine head phantom (Oakland: Siemens [Munich, Germany] 3T Tim Trio/VB 15 platform; Minneapolis: Siemens 3T Tim Trio/VB 15 platform; and Birmingham: Philips [Best, the Netherlands] 3T Achieva/2.6.3.6 platform).5 Using sagittal 3D T1 sequence, total intracranial volume (TICV) was estimated as the sum of gray matter (GM), white matter (WM), and CSF volumes, and whole brain volume (WBV) as the sum of GM and WM volumes. Structural image processing was based on an automated multispectral computer algorithm that classified all supratentorial brain tissue into GM, WM, and CSF. WM was further characterized as normal (NWM) and abnormal (AWM) and into specific regions of interest.5 AWM tissue was estimated from the sagittal 3D fluid-attenuated inversion recovery, T1, and T2 sequences. Volume of abnormal GM was small and therefore not assessed. All image processing was done at the Section of Biomedical Image Analysis, Department of Radiology, University of Pennsylvania. Quality assurance protocols, developed for the Functional Bioinformatics Research Network, and the Alzheimer's Disease Neuroimaging Initiative, included the evaluation of scanner stability and image distortion prior to each site's acceptance and subsequent quarterly quality control evaluations including additional checks for motion artifacts or other quality issues before image processing.
Statistical analysis.
Our analytic sample for this analysis draws from the 719 individuals who underwent brain MRI at Y25. Participant exclusions are noted in figure e-1 at Neurology.org. We excluded individuals from analysis due to missing data for imaging (n = 9), LS7 component data (n = 185 total, 140 of which were missing Y20 diet data), and covariate data (n = 7), leaving 518 for analysis. Participant characteristics for those with and without brain MRI are presented in table e-1. Our outcomes were WBV, NGM, NWM, and AWM. We divided each specific brain tissue volume by TICV, converting absolute volumes to % relative to TICV (% TICV). In separate models, we used multivariable linear regression to assess the association between CVH at Y0, Y25, and the average of the 2 examinations and each normal tissue. For reasons of small and skewed AWM volumes, we assessed the odds of being in the top quartile of AWM vs being in the lower 3 quartiles of AWM according to CVH using multivariable logistic regression and used quantile regression to estimate median differences in absolute AWM volume according to CVH. For each outcome, we modeled CVH as an ordinal CVH score and as a categorical predictor. We included adjustment for age, sex, race, MRI field center, educational attainment (any up to high school graduate, any college, or more than 4 years of college), and alcohol consumption (no regular, moderate consumption: up to 1 drink daily for women/2 drinks daily for men, or heavy consumption: greater than 1 drink daily for women/2 drinks daily for men), measured concurrently with CVH. We assessed for evidence effect modification of the association between CVH and each brain tissue by sex, race, and education. Finally, we examined the strength of association between each of the 7 components and each of the brain structures. SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC) was used for statistical analysis.
RESULTS
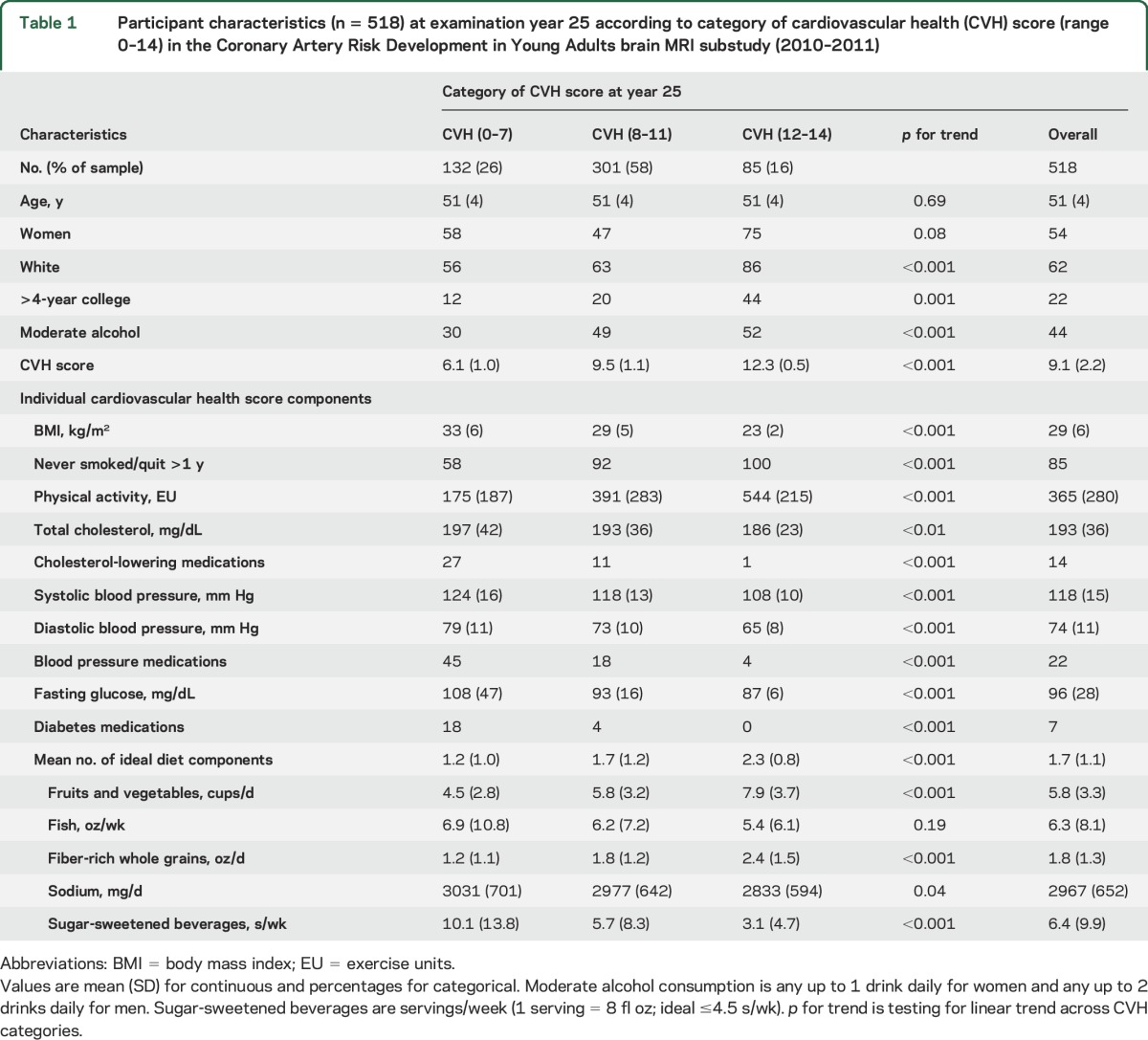
The final study population consisted of 518 participants (mean age 51 ± 4 years, 54% female, 62% white). At examination Y0, 5% of the sample had a CVH score 0–7, 62% had a CVH score 8–11, and 33% had a CVH score 12–14. At Y25, 26% of the sample had a CVH score of 0–7, 58% had a score of 8–11, and 16% had a score of 12–14. Table 1 presents the participant characteristics at Y25 according to category of cardiovascular score. Individuals who had a higher CVH score at Y25 were more likely to be female, white, highly educated, and moderate consumers of alcohol. Fasting glucose was the individual metric with the greatest proportion of participants obtaining ideal status, while diet was the metric with the lowest percentage of individuals attaining ideal status. The distribution of ordinal CVH score for each examination is presented in table e-2.
Table 1.
Participant characteristics (n = 518) at examination year 25 according to category of cardiovascular health (CVH) score (range 0–14) in the Coronary Artery Risk Development in Young Adults brain MRI substudy (2010–2011)
Brain imaging outcomes.
Table 2 presents separate results for multivariable adjusted mean percentage WBV, NGM, and NWM and odds ratios for being in the top quartile of percentage AWM compared to being in the lower 3 quartiles of percentage AWM per unit increase in CVH score. None of the associations varied significantly by race, sex, or education (p for interaction >0.1, for all).
Table 2.
Multivariable adjusted mean percentage of intracranial volume (% ICV) of normal tissue volume and odds ratio (OR) for abnormal tissue at year 25 (95% confidence limits) for 518 individuals according to cardiovascular health (CVH) score in the Coronary Artery Risk Development in Young Adults brain MRI substudy (1985–1986 to 2010–2011)
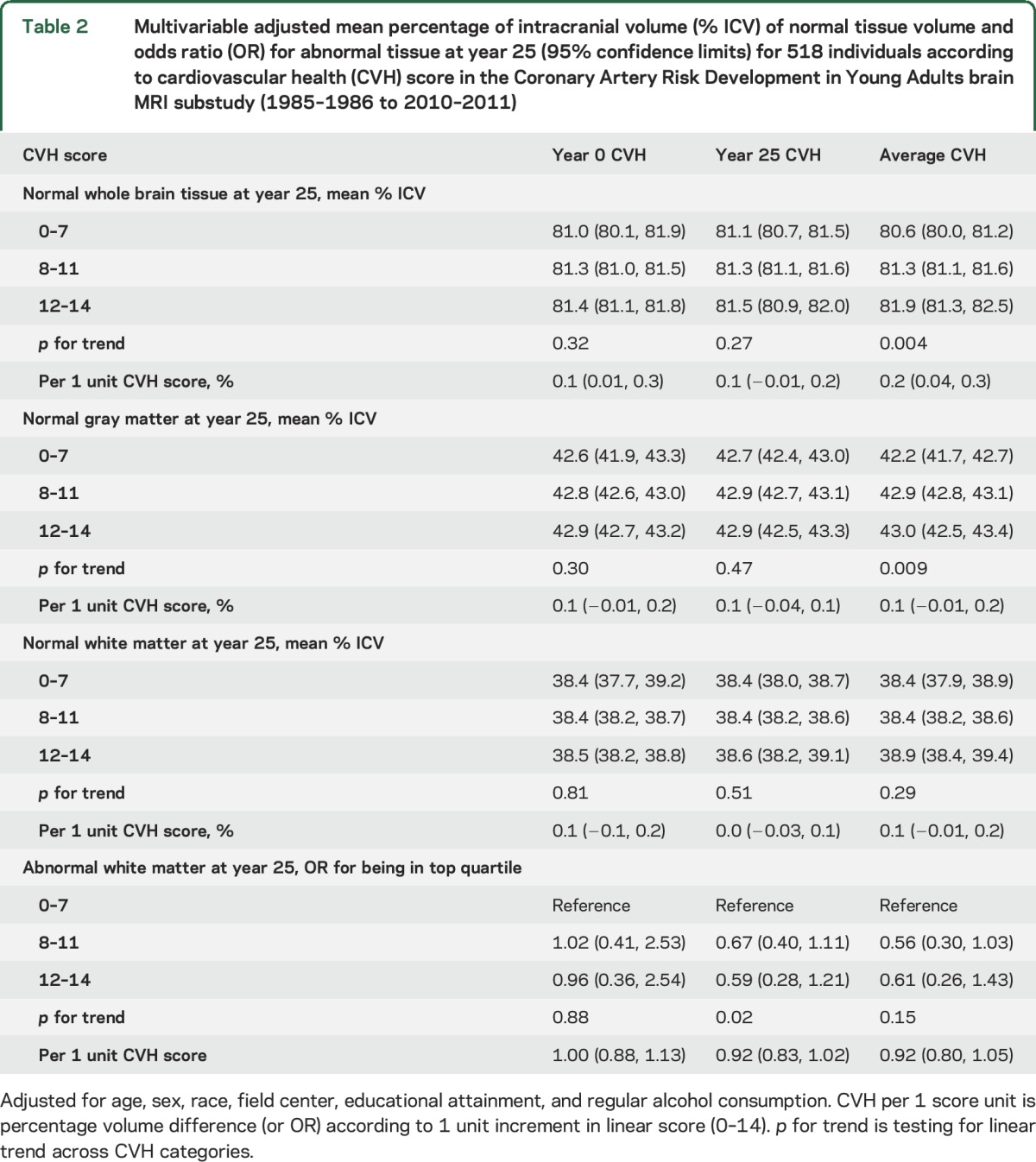
Whole brain volume, % of TICV.
When CVH score was modeled as an ordinal variable, a higher CVH score at Y0 was associated with significantly greater percentage WBV at Y25 after adjustment (table 2: per 1 unit increment in score: 0.1%; 95% confidence limits [CL] 0.01–0.3; p < 0.05). Alternatively, ordinal CVH at Y25 was not associated with percentage WBV at Y25. Categorical CVH at Y0 and Y25 were not associated with percentage WBV at Y25. However, higher average CVH at Y0 and Y25 combined was associated with significantly greater percentage WBV at Y25 (per each additional point in average CVH: 0.2%; 95% CL 0.04–0.3; p < 0.05). For reference, in each model a 1-year higher increment in age was associated with 0.1% less percentage WBV (95% CL −0.2 to −0.01; p < 0.05 all models).
Normal GM, % of TICV.
After adjustment for age, sex, race, field center, education, and alcohol consumption, CVH score at Y0 or Y25 was not associated with percentage NGM (table 2). Average CVH was not associated with % NGM at Y25. We did observe a positive linear trend across categories of average CVH for increasing mean percentage of NGM at Y25 (p for trend = 0.009). However, pairwise comparisons with the lowest average CVH group were not significant (p > 0.05). For reference, a 1-year higher increment in age was associated with 0.1% lower percentage NGM (95% CL −0.1 to −0.01; p < 0.05 all models).
Normal WM, % of TICV.
CVH score, modeled ordinal or categorical, at Y0, Y25, or the average of both examinations was not associated with percentage NWM at Y25, before or after adjustment.
Abnormal WM.
The top quartile of AWM was ≥0.5 cm3. When CVH was modeled in ordinal fashion, visit-based or average CVH score was not associated with being in the top quartile of AWM at Y25. We did observe category of higher CVH score at Y25 and the average of both examinations was associated with lower odds of being in the top quartile of AWM at Y25 compared to the lowest CVH category at Y25; however, these pairwise associations did not attain statistical significance (α 0.05, shown in figure e-2). When AWM was modeled as a continuous variable, individuals with greater average CVH at both examinations had lower AWM volume compared to individuals with the lowest CVH, but these differences were not statistically significant (median difference in AWM for CVH 8–11 −0.11, 95% confidence interval [CI] −0.23 to 0.02; median difference in AWM for CVH 12–14 −0.09, 95% CI −0.24 to 0.06).
Individual CVH metrics.
The multivariable-adjusted estimates for each continuous CVH component with each brain tissue outcome are presented in table e-3. Compared to never smokers at Y0, current smokers at Y0 had significantly less percentage WBV at Y25. In this analysis, no other individual metrics assessed at Y0 were independently associated with brain structure at Y25. Greater level of fasting glucose at Y25 was associated with significantly lower percentage WBV and NGM at Y25 (p < 0.05 each). Similarly, greater total cholesterol measured at Y25 was associated with significantly lower percentage WBV and NWM at Y25 (p < 0.05 each).
DISCUSSION
In this subsample of CARDIA participants who underwent brain MRI, individuals with better CVH scores at baseline and an average of the baseline and Y25 examinations had greater mean percentage of WBV as percent of TICV in midlife. The observed differences in percentage WBV for a 1-unit increment in CVH score was roughly equivalent to an increment in age of 1 year older age. A greater number of LS7 in young adulthood was not significantly associated with normal tissue volumes of GM and WM or AWM in midlife. These findings suggest overall CVH in young adulthood is associated with early differences in whole brain structure, but no differences by tissue class.
Our findings complement previous work from CARDIA on this topic investigating individual CVH metrics.5 We observed that current smoking was most strongly associated with brain MRI outcomes and we did not observe an association between systolic BP and WBV, findings in agreement with previous reports from CARDIA.5 However, prior investigation in CARDIA observed an association between higher systolic BP and greater volume of AWM and that individuals with hypertension had significantly lower WBV and greater AWM relative to those with normal BP. While in the expected direction in our sample, the association between systolic BP and being in the top quartile of AWM did not attain statistical significance in the present study, possibly the result of our smaller sample size and different adjustment schemes.
Attaining a greater number of ideal metrics for the LS7 in middle adulthood is strongly and consistently associated with a variety of favorable health outcomes in older age.15–19 Our current findings add to the literature by highlighting the potential structural underpinnings of the previously observed positive association between ideal CVH and better cognitive profile.2–4,20 Data from the Framingham Heart Study Offspring demonstrated adherence to a greater number of LS7 components was associated with lower risk for incident stroke and dementia and less frontal brain atrophy, but not with whole brain or lateral ventricular atrophy or WM hyperintensity volume.21 Our current work is unique from that previously assessing brain structure, namely with the determination of ideal CVH during young adulthood and brain structure in middle adulthood. This is noteworthy for multiple reasons. First, estimates for the attributable risk of cardiovascular risk factors to the development of Alzheimer disease and other dementias generally focus on midlife levels of these factors.22,23 Second, our work suggests differences in CVH at a young age are related to early structural differences in brain tissue. These structural differences are concomitant with cognitive differences.2 This may support a mechanism linking CVH to cognitive function that originates early in life. This speculation awaits confirmation.
From a methodologic standpoint, we focused on whole brain, normal GM and WM, and AWM volume because these would provide insights on the picture of overall brain structure in middle adulthood. Epidemiologic investigation of structural changes of the brain is traditionally noted in cohorts older than CARDIA, where differences in brain structure appear to be more prominent. A curvilinear association for age-related differences in WBV was observed in Framingham, demonstrating little difference in brain volumes before the age of 55 years and pronounced steepening after the age of 55.24 Individuals in our analysis were all under the age of 55 yet we were able to observe differences in percentage WBV related to our CVH score independent of age.
Our results may be generalizable to other communities of similar racial, age, and CVH composition. Study strengths include the standardized measurement of cardiovascular risk factors antecedent to and concurrent with brain imaging in a large biracial community-based cohort. Study limitations should also be considered. First, brain imaging was assessed at a single time in midlife and we observed a low prevalence of AWM. We are unable to determine if ideal CVH through young adulthood is associated with change in brain volume or pathology or if early-life development of brain pathology led to changes in health behaviors (reverse causation). Second, in determining CVH status, we ascribe equal weight to each LS7 metric. In our data, current smoking status and higher continuous fasting glucose was associated with lower cross-sectional whole brain and GM volumes and higher total cholesterol was associated with lower WBV and WMV. Our objective was to determine the application and association of the global CVH status as determined by comprehensive LS7 score rather than focus on individual metrics. Third, the analytic sample accounted for only 10% of the original CARDIA population and was more likely than the overall cohort to be white, more educated, and showed trends for being healthier with regard to LS7 scores, smoking status, and BMI at Y0 as well as BP, glucose, and physical activity at Y25, potentially limiting generalizability. Persons who did not return at 25 years had less favorable levels for each of the LS7 CVH factors, particularly greater smoking and more cardiovascular disease events, and understandably experienced more mortality. As a result, the observed associations may underestimate associations of CVH with brain structure. Finally, this is an observational epidemiologic study; residual confounding and false-positive associations cannot be excluded, and our findings should be validated in other cohorts. The current study suggests individuals with better CVH in young adulthood have greater WBV in middle adulthood. This underscores the importance of achieving and maintaining ideal CVH at a young age and favorable cerebral structure in later adulthood. Our findings support strategies in achieving AHA 2020 Goals and Beyond for improving and maintaining global health including CVH and promoting healthy brain aging for individuals in young adulthood.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the other investigators, the staff, and the participants of the CARDIA study for their contributions.
GLOSSARY
- AHA
American Heart Association
- AWM
abnormal white matter
- BP
blood pressure
- CARDIA
Coronary Artery Risk Development in Young Adults
- CI
confidence interval
- CL
confidence limits
- CVH
cardiovascular health
- EU
exercise units
- GM
gray matter
- LS7
Life's Simple 7
- NWM
normal white matter
- TICV
total intracranial volume
- WBV
whole brain volume
- WM
white matter
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
M. Bancks is the guarantor of this work, had full access to the data, and takes responsibility for the integrity of the data and the accuracy of the data analysis. M. Bancks and P. Schreiner conceived and designed the project. M. Bancks performed statistical analyses and drafted the manuscript. N. Allen, P. Dubey, J. Reis, S. Sidney, and Y. Yano contributed to the interpretation of the data and manuscript review. L. Launer designed and obtained funding for the brain MRI substudy and contributed to the interpretation of the data and manuscript review. D. Lloyd-Jones and P. Schreiner contributed to data acquisition, interpretation of the data, and manuscript review. All authors supplied significant intellectual contributions and read and approved the final version of this manuscript.
STUDY FUNDING
M.P.B. was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the NIH under award numbers T32HL007779 and T32HL069771 to conduct the current work. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the NHLBI in collaboration with the University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 2.Reis JP, Loria CM, Launer LJ, et al. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol 2013;73:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thacker EL, Gillett SR, Wadley VG, et al. The American Heart Association Life's Simple 7 and incident cognitive impairment: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc 2014;3:e000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crichton GE, Elias MF, Davey A, Alkerwi A. Cardiovascular health and cognitive function: the Maine-Syracuse Longitudinal Study. PLoS One 2014;9:e89317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Launer LJ, Lewis CE, Schreiner PJ, et al. Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS One 2015;10:e0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 7.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol 1986;129:101–123. [DOI] [PubMed] [Google Scholar]
- 8.Bild DE, Jacobs DR, Liu K, et al. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: the CARDIA Study. Ann Epidemiol 1996;6:235–245. [DOI] [PubMed] [Google Scholar]
- 9.Odegaard AO, Jacobs DR Jr, Steffen LM, Van Horn L, Ludwig DS, Pereira MA. Breakfast frequency and development of metabolic risk. Diabetes Care 2013;36:3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hozawa A, Jacobs DR Jr, Steffes MW, Gross MD, Steffen LM, Lee DH. Circulating carotenoid concentrations and incident hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Hypertens 2009;27:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald A, Van Horn L, Slattery M, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc 1991;91:1104–1112. [PubMed] [Google Scholar]
- 12.Liu K, Slattery M, Jacobs D Jr, et al. A study of the reliability and comparative validity of the CARDIA dietary history. Ethn Dis 1994;4:15–27. [PubMed] [Google Scholar]
- 13.Jacobs DJ, Hahn L, Haskell W, Pirie P, Sidney S. Validity and reliability of a short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil 1989;9:448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Sports Medicine. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness in healthy adults: position stand of the American College of Sports Medicine. Schweiz Z Sportmed 1993;41:127–137. [PubMed] [Google Scholar]
- 15.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2012;125:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson NC, Cushman M, Judd SE, et al. American Heart Association's Life's Simple 7 and risk of venous thromboembolism: the reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc 2015;4:e001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulshreshtha A, Vaccarino V, Judd SE, et al. Life's Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke 2013;44:1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folsom AR, Shah AM, Lutsey PL, et al. American Heart Association's Life's Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med 2015;128:970–976 e972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen-Torvik LJ, Shay CM, Abramson JG, et al. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk in Communities Study. Circulation 2013;127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardener H, Wright CB, Dong C, et al. Ideal cardiovascular health and cognitive aging in the Northern Manhattan Study. J Am Heart Assoc 2016;5:e002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pase MP, Beiser A, Enserro D, et al. Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke 2016;47:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol 2014;13:788–794. [DOI] [PubMed] [Google Scholar]
- 23.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


