Abstract
Neuropathic pain remains a prevalent and persistent clinical problem because of our incomplete understanding of its pathogenesis. This study demonstrates for the first time, to our knowledge, a critical role for CNS innate immunity by means of microglial Toll-like receptor 4 (TLR4) in the induction phase of behavioral hypersensitivity in a mouse and rat model of neuropathy. We hypothesized that after L5 nerve transection, CNS neuroimmune activation and subsequent cytokine expression are triggered by the stimulation of microglial membrane-bound TLR4. To test this hypothesis, experiments were undertaken to assess tactile and thermal hypersensitivity in genetically altered (i.e., TLR4 knockout and point-mutant) mice after L5 nerve transection. In a complementary study, TLR4 antisense oligodeoxynucleotide (ODN) was administered intrathecally to L5 spinal nerve injured rats to reduce the expression of spinal TLR4. Both the genetically altered mice and the rats treated with TLR4 antisense ODN displayed significantly attenuated behavioral hypersensitivity and decreased expression of spinal microglial markers and proinflammatory cytokines as compared with their respective control groups. This finding shows that TLR4 contributes to the initiation of CNS neuroimmune activation after L5 nerve transection. Further understanding of this early, specific, innate CNS/microglial response and how it leads to sustained glial/neuronal hypersensitivity may point to new therapies for the prevention and treatment of neuropathic pain syndromes.
Keywords: astrocytes, behavioral hypersensitivity, microglial activation, nerve injury, neuropathic pain
Neuropathic pain remains a prevalent, persistent, and debilitating problem. Attempts to elucidate its mechanisms have focused principally on peripheral nerves, dorsal root ganglion, and CNS neurons. Recently, however, research has expanded into the burgeoning field of glial/neuronal transmission and CNS immunologic responses to nerve injury. CNS glia display immune cell functions in both normal and pathologic conditions, and there is increasing evidence that neuropathic pain arising from nerve injury has a CNS neuroimmune component (1, 2). Spinal glial activation triggers rapid, graded CNS expression of proinflammatory cytokines (including TNF-α, IL-1β, and IL-6) that contributes to the initiation and maintenance of behavioral hypersensitivity after L5 nerve transection (1, 3, 4). The onset of proinflammatory cytokine expression correlates with microglial activation and the initiation of behavioral hypersensitivity (5–8), and neuroimmune activation in painful neuropathy has been established (3, 9, 10). However, the mechanistic links between L5 nerve transection, microglial activation, and the genesis of behavioral hypersensitivity have remained unknown.
Cells of the innate immune system, including monocytes/macrophages, natural killer cells, neutrophils, and microglia recognize invariant molecular structures of pathogens (termed pathogen-associated molecular patterns, PAMP) by means of stable, genetically conserved, pattern-recognition receptors on the cell surface. The genes producing these receptors are homologous to the Toll gene in Drosophila and are therefore termed Toll-like receptors (11). Toll-like receptor 4 (TLR4), the focus of the present study, is a transmembrane receptor protein with extracellular leucine-rich repeat domains and a cytoplasmic signaling domain. TLR4 expression has been demonstrated in the rodent CNS (12, 13), where in vivo and in vitro studies show that TLR4 is exclusively expressed by microglia (14, 15). LPS (a well known exogenous ligand for TLR4) and potential endogenous ligands for TLR4 (e.g., members of the heat shock protein family and proteoglycans) lead to NF-κB activation and subsequent induction of proinflammatory cytokines (16, 17). Thus, there is a plausible linkage of TLR4 to the production of proinflammatory cytokines, which, in turn, contribute to behavioral hypersensitivity. However, prior research has not conclusively demonstrated a role for TLR4 in the onset of behavioral hypersensitivity. Our previous results show that increased spinal microglial TLR4 activation correlates with the onset of behavioral hypersensitivity in rats after injury to the L5 spinal nerve, even in the absence of exogenous TLR4 ligands such as LPS (18).
The current study was undertaken to determine the mechanistic contribution of TLR4 and CNS innate immunity to behavioral hypersensitivity. We postulated that TLR4 responds immediately to stressors induced by nerve or cell-body damage, including ATP, heat shock protein, fragments of proteoglycans, and cations.
Our approach to investigating the functional links between TLR4, microglial activation, and the initiation of behavioral hypersensitivity was twofold. First, we assessed glial activation and behavioral hypersensitivity after spinal L5 nerve transection in wild-type mice and in genetically manipulated mice lacking normal TLR4 expression. Two mouse strains with distinct genetic defects at the TLR4 loci were used: the TLR4 knockout (KO) mouse (C57BL/10ScNJ), which has a complete deletion of the TLR4 gene and thus cannot synthesize TLR4 mRNA or protein, and the TLR4 point-mutant mouse (C3H/HeJ), which expresses only a mutated (nonfunctional) TLR4 protein because of an amino acid substitution at position 712 in the third exon of the TLR4 gene (19, 20). Second, we assessed glial activation and behavioral hypersensitivity after L5 nerve transection in normal rats and in rats injected intrathecally with antisense oligodeoxynucleotide (ODN) to decrease CNS expression of TLR4. Both series of experiments established a role for TLR4 and CNS innate neuroimmune activation in the onset of behavioral hypersensitivity.
Materials and Methods
Animals. Mice. C57BL/10ScNJ mice (TLR4-deleted; n = 21) have a homozygous deletion of 74 kb at the tlr4 locus that removes all three TLR4 exons (19). C3H/HeJ mice (TLR4-deficient; n = 8) possess a dominant-negative point mutation Pro → His at position 712 in the third exon of the TLR4 gene (19). C57BL/10ScSnJ (n = 15) and C3H/HeN (n = 7) mice are the respective wild-type controls. The C57BL/10ScNJ, C57BL/10ScSnJ, and C3H/HeJ strains were purchased from The Jackson Laboratory, and the C3H/HeN strain was purchased from Charles River Laboratories. All mice weighed 25–30 g at the time of surgery. Rats. Male Sprague–Dawley rats (n = 48) purchased from Harlan (Indianapolis) were used. All weighed 200–250 g at the time of surgery.
The Institutional Animal Care and Use Committee at Dartmouth College approved all experimental protocols. In accordance with the guidelines set forth by the International Association for the Study of Pain, efforts were made throughout to minimize animal discomfort and to use the fewest animals needed for statistical significance. Animals were housed on a 12-h light, 12-h dark cycle with food and water available ad libitum.
Surgery. Animals were anesthetized with halothane in an O2 carrier (induction, 4% and maintenance, 2%). A small incision to the skin overlaying L5-S1 was made, followed by retraction of the paravertebral musculature from the vertebral transverse processes. The L6 transverse process was partially removed, exposing the L4 and L5 spinal nerves. The L5 spinal nerve was identified, lifted slightly, and transected. The wound was irrigated with saline and closed in two layers with 3-0 polyester suture (fascial plane) and surgical skin staples.
Behavioral Testing. For mice, mechanical sensitivity was assessed by applying 0.008- and 0.015-g von Frey filaments (Stoelting, Wood Dale, IL) on the plantar surface of the ipsilateral hind paw. For rats, 2- and 12-g von Frey filaments were used. These stimuli are normally nonnoxious. Allodynia was characterized as the number of paw withdrawals in three sets of 10 stimulations each, and was tested on days 1, 3, 5, 7, 10, and 14 after surgery for mice and on days 1, 3, 5, and 7 for rats. Thermal sensitivity was determined by using paw-withdrawal latencies to radiant heat and tail-flick latencies to tail immersion in hot water (49°C). Three tail-flick and paw-withdrawal latencies were obtained per animal for each testing session. Animals were tested for baseline responses three times before undergoing the L5 spinal nerve transection surgery.
Intrathecal Oligodeoxynucleotide Administration. We conducted a set of experiments by using the L5 spinal nerve transection model in rats. TLR4 antisense ODN, mismatch ODN, and a random control oligonucleotide labeled with FITC were custom-made by Biognostik (Göttingen, Germany). The oligonucleotide was resuspended in sterile PBS at a final concentration of 1 μg/μl for mismatch ODN and 2 μg/μl for antisense ODN. The rats were divided into three groups based on the final amount of ODN intrathecally administered. In the first group, rats were administered antisense ODN (10 μg/day; n = 8), mismatch ODN (10 μg/day; n = 8), or saline (n = 8), and tested separately. In the second group, rats were administered antisense ODN (20 μg/day; n = 8), mismatch ODN (20 μg/day; n = 8), or saline (n = 8). All rats in both groups underwent L5 nerve transection surgery, and, after daily behavioral testing that took place between 8:00 and 10:00 a.m., were administered ODN intrathecally by means of lumbar puncture under brief inhalational anesthesia for 7 days starting 1 day before surgery. In the third group, 20 μg of FITC-labeled random oligonucleotide was injected intrathecally by means of lumbar puncture in three rats. These rats were killed at 4, 8, or 12 h after injection to determine the optimal cellular uptake time of FITC-labeled oligonucleotide. The injection volume was 10 μl followed by a 15-μl sterile PBS wash.
The ODN solutions were aliquoted in sterile 1.5-ml Eppendorf tubes. The aliquots were stored at –20°C, and three aliquots were taken out daily during the 7 days of intrathecal administration. The experimenter was blind to their content throughout the study. Behavioral testing was carried out 1 h before the administration of TLR4 antisense ODN, mismatch ODN, or saline. All rats in groups 1 and 2 were killed on day 7 after surgery, 1 day after the final intrathecal administration of TLR4 antisense ODN, mismatch ODN, or saline.
Real-Time RT-PCR. To test for a direct link between TLR4 expression, microglial activation, and proinflammatory cytokine expression, we used quantitative RT-PCR to measure levels of mRNA for TLR4, CD11b, CD14, IL-1β, IL-6, and TNF-α in homogenates from lumbar spinal cord tissue. The method has been described (18). Briefly, the animals were killed by carbon dioxide asphyxiation followed by decapitation on days 3, 7, and 14 after surgery for mice and on day 7 for rats. The spinal cord tissue was flushed out with PBS by using a 15-ml syringe and 18-gauge needle. The harvested cords were immediately snap-frozen to –51°C and stored at –80°C before RNA isolation. Total RNA was isolated from the L5 lumbar spinal cord tissue and processed for RT-PCR. The primers and probes selected for this experiment met the G+C content requirement and had melting temperatures of 60°C and 70°C, respectively (see Tables 1 and 2, which are published as supporting information on the PNAS web site).
Immunohistochemistry. To assess the time course of CD11b/CR3 immunoreactivity, a separate group of mice consisting of C57BL/10ScNJ (KO) mice and C57BL/10ScSnJ (wild-type) mice, four injured and two uninjured per strain, was perfused transcardially with 0.1 M PBS followed by 4% paraformaldehyde in PBS on days 3, 7, and 14 after surgery. The L5 segment of the spinal cord was harvested by means of a laminectomy. Immunohistochemistry was performed on transverse 20-μm L5 spinal cord sections by using an avidin-biotin complex technique previously described (21). A monoclonal antibody for CD11b was used to label the time-course expression of CD11b/CR3 on activated microglia (1:500 working dilution, Serotec).
Statistical Analysis. The statistical significance of differences between TLR4 KO, point-mutant, TLR4 antisense ODN-treated, and control animals was determined by using multivariate two-way ANOVA followed by post hoc Bonferroni analysis. All statistical analyses were performed by using prism 4.01 (GraphPad, San Diego). A P value of <0.05 was considered significant.
Results
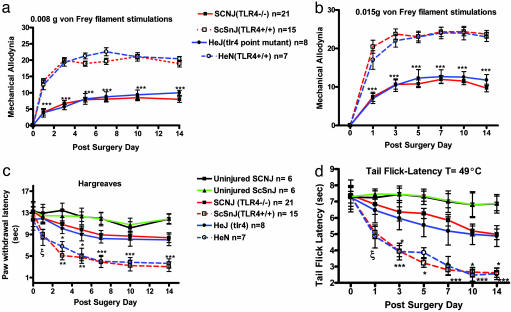
TLR4 Is Critical for Pain Induction After Nerve Injury. The TLR4 KO mice (C57BL/10SCNJ; n = 21) and point-mutant mice (C3H/HeJ; n = 8) displayed significantly attenuated mechanical allodynia compared with their respective wild-type controls (C57BL10/ScSnJ; n = 15 and C3H/HeN; n = 7) for 0.008-g (P < 0.001) and 0.015-g (P < 0.001) von Frey filament stimulations beginning at day 1 after surgery (Fig. 1 a and b). Fig. 1c shows that TLR4 KO and point-mutant mice also displayed a significantly attenuated response to heat for days 1–7 (P < 0.05) and 10–14 after surgery (P < 0.001). Fig. 1d shows that the tail-flick response to immersion in 49°C water was also significantly attenuated for these mice for days 1–7 (P < 0.05) and 10–14 after surgery (P < 0.001).
Fig. 1.
Mechanical allodynia and thermal hyperalgesia in the L5 spinal nerve-transected mice. Shown are responses measured by foot-lift response frequency to stimulation with 0.008-g (a) and 0.015-g (b) von Frey filament. Paw-withdrawal latency (c) and tail-flick latency (d) are shown postoperatively. Mechanical allodynia and thermal hyperalgesia were significantly attenuated in KO mice (C57BL/10SCNJ; n = 21) and TLR4 mutant mice (C3H/HeJ; n = 8) relative to their respective controls, i.e., TLR4 wild-type mice (C57BL10/ScSnJ; n = 15) and C3H/HeN mice (n = 7). In a and b, asterisks indicate significant attenuation in tactile allodynia compared with the wild-type controls (***, P < 0.001; two-way ANOVA followed by Bonferroni post hoc test). In c and d, asterisks and ξ indicate significant decreases in paw-withdrawal and tail-flick latencies compared with wild type (***, P < 0.001; **, P < 0.01; *, P < 0.05; ξ, P < 0.05 vs. ScSnJ, two-way ANOVA followed by Bonferroni post hoc test). For a and b, results are reported as the mean response frequency from three trials of 10 stimulations each ± SEM. For c and d, the results are reported as the mean latency response time of three stimulations ± SEM.
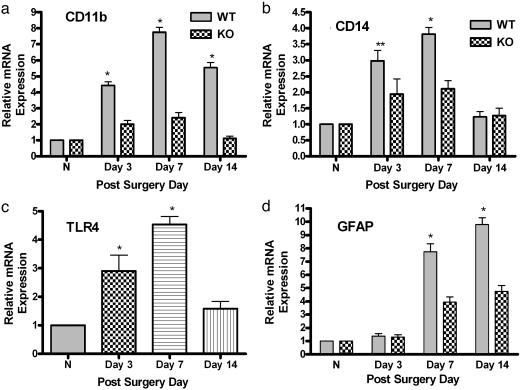
Knockout of TLR4 Leads to Attenuation in Spinal Glial Activation. A3- to 5-fold decrease in expression of mRNA for CD11b and CD14 was observed in TLR4 KO mice compared with the wild-type control group beginning at day 3 after surgery (Fig. 2 a and b; P < 0.001). Fig. 2c shows graded, significant up-regulation of TLR4 mRNA in the wild-type control group (P < 0.001). No TLR4 mRNA was detected in the TLR4 KO mice. Messenger RNA for the astrocytic activation marker glial fibrillary acid protein (GFAP) also decreased in TLR4 KO mice relative to the wild-type control group, starting at day 7 after surgery (Fig. 2d; P < 0.001).
Fig. 2.
Real-time quantitative RT-PCR analyses of mRNA temporal expression of microglial and astrocytic activation markers in mouse lumbar spinal cord. TaqMan real-time RT-PCR was performed. Total mRNA was isolated from L5 lumbar spinal cord tissue of KO mice (C57BL/10SCNJ) and TLR4 wild-type control mice (C57BL10/ScSnJ). Data are shown for normal, uninjured mice (N; n = 3 per strain) or KO mice (n = 4) and wild-type mice (n = 4) subjected to L5 nerve transection surgery at days 3, 7, and 14 for CD11b (a), CD14 (b), TLR4 (c) and GFAP (d) in the presence of their respective primers and probes. No PCR amplification was observed in the TLR4-deficient mice with TLR4 primers and probe (data not shown). (c) The time-course expression of TLR4 in wild-type mice after injury. The real-time PCR was performed in duplicate for both target gene and GAPDH. The level of gene expression was calculated after normalizing against GAPDH in each sample and is presented as relative mRNA expression units. Values are mean ± SEM. (**, P < 0.01 vs. KO; *, P < 0.05 vs. KO; two-way ANOVA followed by Bonferroni post hoc test.)
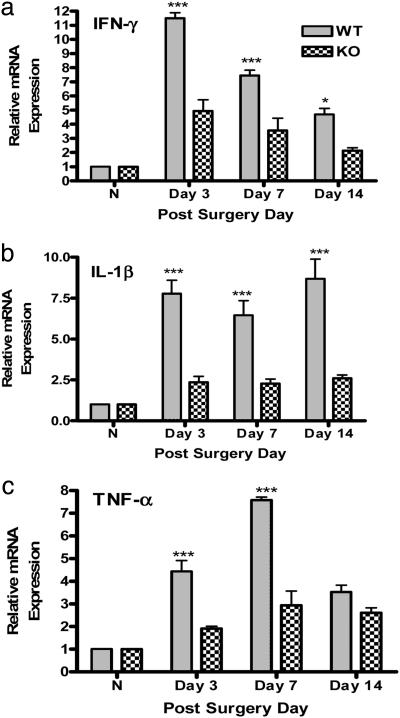
TLR4: Link Between Innate Immunity and Spinal Proinflammatory Cytokine Expression. Significantly (3- to 5-fold) lower spinal expression of mRNA for IFN-γ, IL-1β, and TNF-α was observed after injury in the TLR4 KO mice as compared with the wild-type control group (Fig. 3; P < 0.001).
Fig. 3.
Real-time RT-PCR analyses of the temporal expression of proinflammatory cytokine mRNA in mouse lumbar spinal cord. Total mRNA was isolated from L5 lumbar spinal cord tissue of normal, uninjured mice (N) (n = 3 per strain), TLR4 KO mice (n = 4), and wild-type mice (n = 4) subjected to L5 nerve transection surgery at days 3, 7, and 14 for INF-γ (a), IL-1β (b) and TNF-α (c). The level of gene expression was calculated after normalizing against GAPDH in each sample and is presented as relative mRNA expression units. Values are mean ± SEM. (***, P < 0.001 vs. KO; *, P < 0.05 vs. KO; two-way ANOVA followed by Bonferroni post hoc test.)
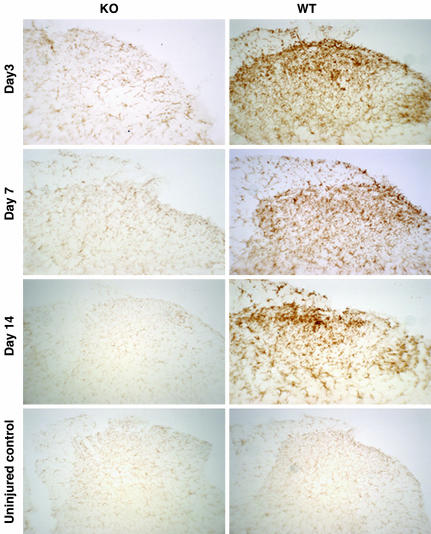
Relationship Between Microglial Activation and TLR4. In injured wild-type mice, we observed robust immunoreactive staining of CD11b/CR3 throughout the dorsal horn ipsilateral to the L5 nerve transection (laminae I-IV) on days 3, 7, and 14 after surgery. By contrast, CD11b/CR3 immunoreactivity was reduced almost to baseline levels in the ipsilateral dorsal horn of injured TLR4 KO mice (Fig. 4). These immunohistochemical protein data support the mRNA findings for CD11b (see Fig. 2a).
Fig. 4.
Immunohistochemical staining for CD11b/CR3 of microglia in the ipsilateral L5 lumbar spinal cord dorsal horns 3, 7, and 14 days after surgery. Shown are representative photomicrographs depict microglial activation by using CD11b/CR3 immunoreactivity in uninjured TLR4 KO and its wild-type control mice and in L5 nerve transected KO and WT mice at 3, 7, and 14 days after surgery. Microglial activation in the dorsal horn of the spinal cord demonstrated a baseline staining in uninjured mice, a mild response at 3, 7, and 14 days after surgery for KO mice, and a moderate to intense response at day 3 and an intense response at 7 and 14 days after surgery in WT mice.
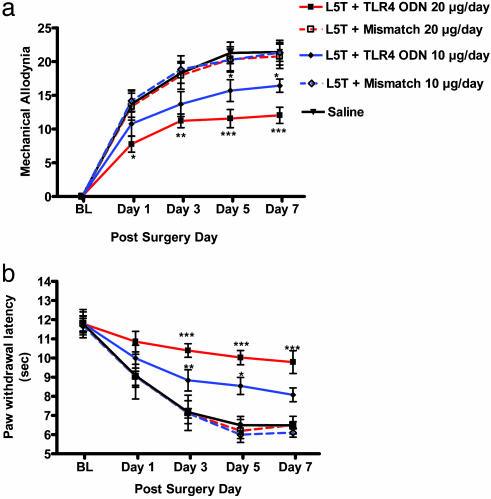
Spinal TLR4 Antisense ODN-Attenuated Behavioral Hypersensitivity in Rats. We confirmed that FITC-labeled random control ODN was incorporated into the spinal cord. Colabeling with DAPI demonstrated the proximity of the FITC-labeled ODN to the nucleus, showing that the modified ODN was incorporated into the CNS parenchyma (see Fig. 7, which is published as supporting information on the PNAS web site). Daily intrathecal injection of 10 μg of TLR4 antisense ODN resulted in moderate but significant attenuation of both mechanical allodynia and thermal hyperalgesia; more significant attenuation was observed with 20-μg daily injections (Fig. 5; P < 0.001). Daily injections of saline or of 10 or 20 μg of mismatch ODN did not alter mechanical or thermal hypersensitivity.
Fig. 5.
Dose–response relationship after daily intrathecal administration of TLR4 antisense ODN in L5 spinal nerve-transected rats. The effect of a daily intrathecal injection of two doses of TLR4 antisense ODN, mismatch ODN, or saline solution (10 or 20 μg per day) on mechanical allodynia (a) and paw-withdrawal latencies (b) is shown. Thermal and tactile hypersensitivity were significantly attenuated in a dose-dependent manner compared with saline and mismatch ODN-treated rats with marked attenuation observed with 10-μg daily injections of TLR4 antisense ODN starting at days 3–7 (P < 0.05 and P < 0.01) and day 1 (*, P < 0.05), day 3 (**, P < 0.01), and through day 7 (***, P < 0.001) for 20-μg daily injection of TLR4 antisense ODN (two-way ANOVA followed by Bonferroni post hoc test).
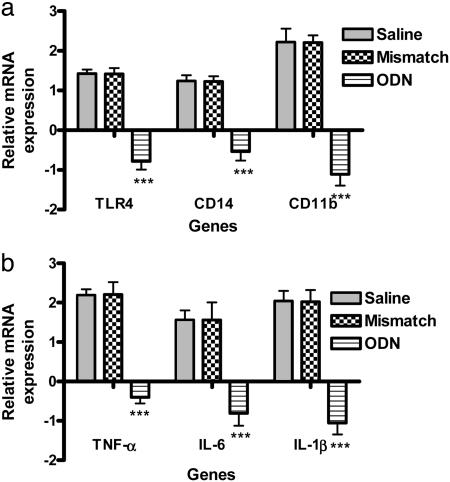
TLR4 Antisense ODN Leads to Decreased Microglial Activation and Proinflammatory Cytokine Expression. Because of the potential for TLR4 antisense ODN backbone toxicity, the maximum daily intrathecal administration of TLR4 antisense ODN was 20 μg. This dosage provided a 56% decrease in spinal TLR4 mRNA expression, as shown in Fig. 6a (P < 0.001). Decreased expression of TLR4 paralleled the decreased expression of the microglial activation markers CD11b (51%) and CD14 (44%) (P < 0.001). The decrease in TLR4 also led to a significant decrease in proinflammatory cytokines, i.e., TNF-α (17%), IL-6 (52%), and IL-1β (53%); P < 0.001 in all cases in TLR4 antisense ODN-treated rats as compared with rats treated with saline or mismatch ODN (Fig. 6b).
Fig. 6.
Effect of TLR4 antisense ODN on microglial activation and proinflammatory cytokine mRNA expression. A daily dose of 20 μg of TLR4 antisense ODN, mismatch ODN, or saline was injected intrathecally for 8 days, starting 1 day before L5 spinal nerve transection. The rats were killed on day 7 after surgery, and the lumbar spinal cord was processed for RT-PCR. RT-PCR revealed a significant down-regulation of TLR4 (P < 0.001). The down-regulation of TLR4 paralleled a significant decrease in the expression of two microglial activation markers, CD14 (***, P < 0.001) and CD11b (*** P < 0.001) (a), and in that of proinflammatory cytokines TNF-α, IL-6, and IL-1β (***, P < 0.001) (b). The level of gene expression was presented as a fold decrease relative to saline treated rats. Values were mean ± SEM. (n = 8 per treatment group; ***, P < 0.001; two-way ANOVA followed by Bonferroni post hoc test.)
Discussion
The data presented herein demonstrate for the first time, to our knowledge, a key role for microglial TLR4 in the induction of behavioral hypersensitivity in rodent models of neuropathy. Our data also show that the mRNA expression of the microglial activation markers, TLR4, CD11b/CR3, and CD14, were only elevated at the initiation phase of behavioral hypersensitivity (days 3 and 7). Conversely, the astrocytic activation marker GFAP showed an opposite pattern with a graded increase in the mRNA expression level at later time points (days 7 and 14). These results are consistent with our previous finding that suggests that microglia are involved in the initiating phase of behavioral hypersensitivity, whereas astrocytes are involved in the maintenance phase (18, 22). Many factors have been postulated to activate microglia after injury, but our finding that microglial TLR4 plays a crucial part as a receptor in the induction phase of behavioral hypersensitivity in rodent models of neuropathy may be novel.
After L5 nerve transection injury, mediators are released or activated, causing secondary swelling and damage to neurons. Such mediators include: ATP, glutamate, acidosis, free saturated or unsaturated fatty acids, high-extracellular potassium, serotonin, bradykinin, substance P, histamine, and products from the cyclooygenase and lipoxygenase pathway of the arachidonic acid metabolism (23). At the spinal cord level, these alterations lead to a hyperexcitable state with intense nociceptive input to the dorsal horn and the chemical sensitization of high-threshold nociceptors to transmit low-intensity nonnoxious stimuli.
One specific example of a TLR4 ligand released after nerve injury are saturated fatty acids, which have been shown to induce the activation of NF-κb and the up-regulation of cyclooxygenase 2 (COX-2) and other inflammatory markers upon interacting with membrane bound TLR4 of monocyte/macrophage such as microglia (24, 25). The up-regulation of COX-2 and its contribution to central sensitization after various types of peripheral nerve injury has been well established (26–28). It has also been shown that the induction of NF-κb and the up-regulation of COX-2 was inhibited by dominant-negative TLR4 (25, 29, 30). These previous findings suggest that the modulation of COX-2 expression by fatty acids via the TLR4-derived signaling pathways is one potentially important pathway that links peripheral nerve injury to behavioral hypersensitivity after microglial TLR4 activation.
We used two strategies to investigate the role of TLR4 in the induction of behavioral hypersensitivity after L5 nerve transection. First, two strains of genetically altered mice were used to examine the effects of complete TLR4 gene deletion and of functionally mutated TLR4 protein, respectively. Second, intrathecal injection of antisense ODN in rats was used to examine the effects of reduced CNS/spinal TLR4 expression. We demonstrated that after L5 nerve transection, functional knockout, or point mutation of the TLR4 gene in mice (i) significantly attenuates L5 nerve transection-induced tactile and thermal hypersensitivity, (ii) reduces spinal microglial activation as assayed by using CD11b/CR3 immunoreactivity and expression of mRNA for microglial activation markers, (iii) reduces expression of mRNA for spinal proinflammatory cytokine, and (iv) significantly decreases expression of mRNA for GFAP. These results agree with the previously observed correlation between microglial and astrocytic activation and the initiation and maintenance of behavioral hypersensitivity in rats (18, 22). Furthermore, we have previously shown that expression of mRNA for CD11b precedes sustained up-regulation of GFAP in the lumbar spinal cord in a similar neuropathic pain model in rat (18).
We have also shown that TLR4 antisense ODN intrathecally administered in rats (i) produces a significant and dose-related attenuation of L5 nerve transection-induced tactile and thermal hypersensitivity, (ii) reduces expression of mRNA for microglial markers, and (iii) reduces expression of mRNA for spinal proinflammatory cytokine.
It is important to note, however, that neither set of experiments in this study completely reversed L5 nerve transection-induced behavioral hypersensitivity. This finding suggests a potential role for other glial receptors or glial mediators such as CD14/MD-2, CD11b/CR3, or unique glial/neuronal signals like fractalkine in the initiation of behavioral hypersensitivity (31, 32). Thus, our results underscore the complexity of CNS cascades and mediators that may underlie neuronal sensitization, the pathological correlate to chronic pain.
The involvement of TLR4 in the CNS innate immune response is now well established (12, 14). Previous studies by our laboratory and others have shown that TLR4 is mainly expressed by microglia (14, 15) and that TLR4 participates in the innate immune response after CNS exposure to LPS (2, 14, 15, 33). Importantly, we have previously shown that spinal mRNA for TLR4 is increased after L5 nerve transection in the absence of LPS (18, 22). These data, together with the results presented herein, provide information related to a component of a mechanistic link between microglial activation and behavioral hypersensitivity.
The CNS innate immune response includes rapid activation of immune effectors cells and the release of proinflammatory cytokines such as TNF-α, IL-1β, and IFN-γ through the activation of TLR4 MyD88-dependent or -independent pathways (34). Furthermore, the expression of proinflammatory cytokines by activated glia after L5 nerve transection has also been shown to be a major factor contributing to the establishment of behavioral hypersensitivity (4). Given that the knockout or knockdown of TLR4 expression leads (as shown in the present study) to attenuation of behavioral hypersensitivity, decreased glial activation, and decreased expression of proinflammatory cytokines, our picture of this aspect of the etiology of behavioral hypersensitivity is significantly clarified. Microglial activation occurs through TLR4 and leads to the enhanced expression of spinal proinflammatory cytokines. Cytokines, in turn, whether alone or by inducing the expression of known algesic mediators such as substance P, nitric oxide, nerve growth factor, glutamate, and prostaglandins, produce central sensitization (i.e., enhanced excitation and responsiveness of spinal neurons due to activity-dependent changes in those neurons), a prerequisite for behavioral hypersensitivity.
That TLR4 provides a mechanistic link between microglial activation, innate immunity, and the initiation of behavioral hypersensitivity is further supported by previous data from our laboratory. In this previous study, when given before (preemptively) to L5 nerve transection, the specific microglial inhibitor minocycline, resulted in decreased expression of mRNA for microglial activation markers, including ITGAM (CD11b/CR3) and TLR4 mRNA for proinflammatory cytokines and in the attenuation of behavioral hypersensitivity (22). Recent work has shown direct involvement of TLR4 in the expression of costimulatory molecules, MHC class II, chemokines, integrins, and adhesion molecules, all of which contribute to central sensitization, and thus, to behavioral hypersensitivity (31, 32, 35–41). The present study has also demonstrated that a decrease of TLR4 leads to a decrease in CD11b/CR3 and in proinflammatory cytokine expression. That is, TLR4 is a microglial sensor that triggers glial activation and the dynamic CNS immune response that ensues in response to L5 nerve transection. This finding agrees with previous studies showing that a deficiency in CD11b/CR3 seriously compromises innate immunity through a syndrome clinically known as leukocyte adhesion deficiency. This syndrome provides compelling evidence for the importance of β2-integrins, including CD11b/CR3, in mediating leukocyte adhesive, migratory, and phagocytic activities in response to inflammatory stimuli, and underlines their role in facilitating first-line host defense (42–44).
All these findings support a CNS role for TLR4 and innate immunity in the etiology of neuropathic pain. The ability of TLR4 to activate a pathway leading to central sensitization and nerve injury-induced behavioral hypersensitivity may provide an opportunity for regulating glial activation, and thus, alleviating chronic pain due to nerve damage.
Supplementary Material
Acknowledgments
We thank Tammy Kielian, Ph.D., for scientific consultations and Larry Gilman, Tracy Wynkoop, and Vivianne Tawfik for editorial assistance. This work was supported by National Institute of Drug Abuse Grant DA11276 (to J.A.D.).
Author contributions: F.Y.T. and J.A.D. designed research; F.Y.T. and N.N.-M. performed research; F.Y.T. analyzed data; and F.Y.T., N.N.-M., and J.A.D. wrote the paper.
Abbreviations: TLR4, Toll-like receptor 4; ODN, oligodeoxynucleotide; KO, knockout; GFAP, glial fibrillary acid protein; COX-2, cyclooxygenase 2.
References
- 1.DeLeo, J. A. & Yezierski, R. P. (2001) Pain 90, 1–6. [DOI] [PubMed] [Google Scholar]
- 2.DeLeo, J. A., Tanga, F. Y. & Tawfik, V. L. (2004) Neuroscientist 10, 40–52. [DOI] [PubMed] [Google Scholar]
- 3.Vuong, C., Voyich, J. M., Fischer, E. R., Braughton, K. R., Whitney, A. R., DeLeo, F. R. & Otto, M. (2004) Cell. Microbiol. 6, 269–275. [DOI] [PubMed] [Google Scholar]
- 4.Raghavendra, V., Tanga, F. Y. & DeLeo, J. A. (2004) Neuropsychopharmacology 29, 327–334. [DOI] [PubMed] [Google Scholar]
- 5.Sommer, C., Galbraith, J. A., Heckman, H. M. & Myers, R. R. (1993) J. Neuropathol. Exp. Neurol. 52, 223–233. [DOI] [PubMed] [Google Scholar]
- 6.Sommer, C. & Myers, R. R. (1995) Acta Neuropathol. 90, 478–485. [DOI] [PubMed] [Google Scholar]
- 7.Popovich, P. G., Wei, P. & Stokes, B. T. (1997) J. Comp. Neurol. 377, 443–464. [DOI] [PubMed] [Google Scholar]
- 8.Popovich, P. G., Yu, J. Y. & Whitacre, C. C. (1997) J. Neuropathol. Exp. Neurol. 56, 1323–1338. [DOI] [PubMed] [Google Scholar]
- 9.Bennett, G. J. (1999) Proc. Natl. Acad. Sci. USA 96, 7737–7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett, G. J. (2000) Clin. J. Pain 16, S139–S143. [DOI] [PubMed] [Google Scholar]
- 11.Akira, S. & Sato, S. (2003) Scand J. Infect. Dis. 35, 555–562. [DOI] [PubMed] [Google Scholar]
- 12.Laflamme, N. & Rivest, S. (2001) FASEB J. 15, 155–163. [DOI] [PubMed] [Google Scholar]
- 13.Eklind, S., Mallard, C., Leverin, A. L., Gilland, E., Blomgren, K., Mattsby-Baltzer, I. & Hagberg, H. (2001) Eur. J. Neurosci. 13, 1101–1106. [DOI] [PubMed] [Google Scholar]
- 14.Lehnardt, S., Lachance, C., Patrizi, S., Lefebvre, S., Follett, P. L., Jensen, F. E., Rosenberg, P. A., Volpe, J. J. & Vartanian, T. (2002) J. Neurosci. 22, 2478–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehnardt, S., Massillon, L., Follett, P., Jensen, F. E., Ratan, R., Rosenberg, P. A., Volpe, J. J. & Vartanian, T. (2003) Proc. Natl. Acad. Sci. USA 100, 8514–8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vabulas, R. M., Ahmad-Nejad, P., Ghose, S., Kirschning, C. J., Issels, R. D. & Wagner, H. (2002) J. Biol. Chem. 277, 15107–15112. [DOI] [PubMed] [Google Scholar]
- 17.Tsan, M. F. & Gao, B. (2004) Am. J. Physiol. 286, C739–C744. [DOI] [PubMed] [Google Scholar]
- 18.Tanga, F. Y., Raghavendra, V. & DeLeo, J. A. (2004) Neurochem. Int. 45, 397–407. [DOI] [PubMed] [Google Scholar]
- 19.Poltorak, A., He, X., Smirnova, I., Liu, M. Y., Van Huffel, C., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., et al. (1998) Science 282, 2085–2088. [DOI] [PubMed] [Google Scholar]
- 20.Poltorak, A., Ricciardi-Castagnoli, P., Citterio, S. & Beutler, B. (2000) Proc. Natl. Acad. Sci. USA 97, 2163–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colburn, R. W., Rickman, A. J. & DeLeo, J. A. (1999) Exp. Neurol. 157, 289–304. [DOI] [PubMed] [Google Scholar]
- 22.Raghavendra, V., Tanga, F. & DeLeo, J. A. (2003) J. Pharmacol. Exp. Ther. 306, 624–630. [DOI] [PubMed] [Google Scholar]
- 23.Kempski, O. S. & Volk, C. (1994) Acta Neurochir., Suppl. 60, 7–11. [DOI] [PubMed] [Google Scholar]
- 24.Hwang, D. (2001) FASEB J. 15, 2556–2564. [DOI] [PubMed] [Google Scholar]
- 25.Lee, J. Y., Sohn, K. H., Rhee, S. H. & Hwang, D. (2001) J. Biol. Chem. 276, 16683–16689. [DOI] [PubMed] [Google Scholar]
- 26.Ma, W., Du, W. & Eisenach, J. C. (2002) Brain Res. 937, 94–99. [DOI] [PubMed] [Google Scholar]
- 27.Ma, W. & Eisenach, J. C. (2002) Eur. J. Neurosci. 15, 1037–1047. [DOI] [PubMed] [Google Scholar]
- 28.Broom, D. C., Samad, T. A., Kohno, T., Tegeder, I., Geisslinger, G. & Woolf, C. J. (2004) Neuroscience 124, 891–900. [DOI] [PubMed] [Google Scholar]
- 29.Lee, J. Y., Zhao, L., Youn, H. S., Weatherill, A. R., Tapping, R., Feng, L., Lee, W. H., Fitzgerald, K. A. & Hwang, D. H. (2004) J. Biol. Chem. 279, 16971–16979. [DOI] [PubMed] [Google Scholar]
- 30.Lee, J. Y., Ye, J., Gao, Z., Youn, H. S., Lee, W. H., Zhao, L., Sizemore, N. & Hwang, D. H. (2003) J. Biol. Chem. 278, 37041–37051. [DOI] [PubMed] [Google Scholar]
- 31.Fan, J. & Malik, A. B. (2003) Nat. Med. 9, 315–321. [DOI] [PubMed] [Google Scholar]
- 32.Proost, P., Verpoest, S., De Borne, K. V., Schutyser, E., Struyf, S., Put, W., Ronsse, I., Grillet, B., Opdenakker, G. & Damme, J. V. (2004) J. Leukocyte Biol. 75, 777–784. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen, M. D., D'Aigle, T., Gowing, G., Julien, J. P. & Rivest, S. (2004) J. Neurosci. 24, 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janeway, C. A., Jr., & Medzhitov, R. (2002) Annu. Rev. Immunol. 20, 197–216. [DOI] [PubMed] [Google Scholar]
- 35.Sweitzer, S. M., White, K. A., Dutta, C. & DeLeo, J. A. (2002) J. Neuroimmunol. 125, 82–93. [DOI] [PubMed] [Google Scholar]
- 36.Khatri, S., Lass, J. H., Heinzel, F. P., Petroll, W. M., Gomez, J., Diaconu, E., Kalsow, C. M. & Pearlman, E. (2002) Invest. Ophthalmol. Visual Sci. 43, 2278–2284. [PubMed] [Google Scholar]
- 37.Wang, M. J., Jeng, K. C. & Shih, P. C. (2000) Cell. Immunol. 204, 88–95. [DOI] [PubMed] [Google Scholar]
- 38.Michelsen, K. S., Aicher, A., Mohaupt, M., Hartung, T., Dimmeler, S., Kirschning, C. J. & Schumann, R. R. (2001) J. Biol. Chem. 276, 25680–25686. [DOI] [PubMed] [Google Scholar]
- 39.Cecil, A. A. & Klemsz, M. J. (2004) J. Leukocyte Biol. 75, 560–568. [DOI] [PubMed] [Google Scholar]
- 40.Hoebe, K. & Beutler, B. (2004) J. Endotoxin. Res. 10, 130–136. [DOI] [PubMed] [Google Scholar]
- 41.Kaisho, T., Hoshino, K., Iwabe, T., Takeuchi, O., Yasui, T. & Akira, S. (2002) Int. Immunol. 14, 695–700. [DOI] [PubMed] [Google Scholar]
- 42.Yefenof, E. (2000) Adv. Exp. Med. Biol. 479, 15–25. [DOI] [PubMed] [Google Scholar]
- 43.Rosenkranz, A. R., Coxon, A., Maurer, M., Gurish, M. F., Austen, K. F., Friend, D. S., Galli, S. J. & Mayadas, T. N. (1998) J. Immunol. 161, 6463–6467. [PubMed] [Google Scholar]
- 44.Ehlers, M. R. (2000) Microbes Infect. 2, 289–294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.