Abstract
Although it is known that calcium is a very important messenger involved in many eukaryotic cellular processes, much less is known about calcium's role in bacteria. CcbP, a Ca2+-binding protein, was isolated from the heterocystous cyanobacterium Anabaena sp. PCC 7120, and the ccbP gene was cloned and inactivated. In the absence of combined nitrogen, inactivation of ccbP resulted in multiple contiguous heterocysts, whereas overexpression of ccbP suppressed heterocyst formation. Calmodulin, which is not present in Anabaena species, could also suppress heterocyst formation in both Anabaena sp. PCC 7120 and Anabaena variabilis. HetR induction upon nitrogen step-down was slow in the strain overexpressing ccbP. The Ca2+ reporter protein obelin was used to show that mature heterocysts had a high intracellular free Ca2+concentration {[Ca2+]i}, and immunoblotting showed that CcbP was absent from heterocysts. A regular pattern of cells with higher [Ca2+]i was established during heterocyst differentiation before the appearance of proheterocysts. A rapid increase of [Ca2+]i could be detected 4 h after the removal of combined nitrogen, and this increase was suppressed by excessive CcbP. These results suggest that Ca2+ ions play very important roles in hetR induction and heterocyst differentiation.
Keywords: cyanobacteria, hetR, pattern formation
In eukaryotes, Ca2+ ions play very important roles in cellular processes such as cell differentiation (1, 2). The intracellular free calcium ion concentration {[Ca2+]i} is tightly regulated through Ca2+ channels, Ca2+ pumps, and Ca2+-binding proteins, and [Ca2+]i is generally maintained in the nanomolar range. In bacteria, the role of Ca2+ in cellular activities is less clear (3–5). It has been demonstrated that [Ca2+]i is tightly regulated in some bacteria (6), and there is evidence indicating that calcium is critical to some bacterial cellular processes such as sporulation of Bacillus (7), chemotaxis of Escherichia coli (8), and heterocyst differentiation of cyanobacteria (9). Heterocyst development may represent one of the earliest examples of pattern formation in evolution (10). Heterocysts are specialized cells for nitrogen fixation of some filamentous cyanobacteria (11–14). When heterocystous cyanobacteria are subjected to nitrogen step-down, some vegetative cells differentiate and become heterocysts. Many genes are specifically involved in heterocyst differentiation. The genes that are required for initiation of cell differentiation include ntcA (15, 16) and hetR (17). HetR is a serine-type protease that plays an important role in heterocyst formation (18).
Evidence obtained by manipulating extracellular calcium concentration and by using inhibitors of Ca2+-binding proteins suggested that Ca2+ could be involved in heterocyst differentiation (9). Here we describe a Ca2+-binding protein, CcbP, from Anabaena sp. PCC 7120. Our study shows that free calcium accumulates in differentiating cells and mature heterocysts, correlated with a drop in the level of the Ca2+-binding protein. The free Ca2+concentration appears to be critical for the differentiation process.
Materials and Methods
The following protocols can be found in Supporting Text, which is published as supporting information on the PNAS web site: growth of the strains of Anabaena; isolation of the Ca2+-binding protein CcbP; isolation of the ccbP gene from total genomic DNA; expression of recombinant ccbP in Escherichia coli; construction of plasmids for inactivation of the ccbP gene, for copper-regulated expression of ccbP, for copper-regulated expression of rat calmodulin (CaM) gene, for ccbP promoter regulation of GFP expression, and for expression of obelin, a reporter for the level of free Ca2+.
Assays for Ca2+-Binding Proteins. 45Ca2+ overlay assay was performed as follows: Proteins were first separated by SDS/PAGE and then transferred to a poly(vinylidene difluoride) membrane. It was then washed three times with buffer C (20 mM Tris·HCl, pH 7.2/5 mM MgCl2/60 mM KCl/10 mM imidazole). The membrane was then soaked in 25 ml of buffer C containing 50 μCi 45CaCl2 for 10 min before washing briefly with buffer C containing 5% ethanol and blotted to dry. The radioactive bands were detected with Kodak x-ray film. For the Ca2+-dependent electrophoretic mobility-shift assay, Ca2+-binding proteins were separated by SDS/PAGE (12%) in the presence of 2 mM CaCl2 or 2 mM EGTA (19).
Detection of Intracellular Free Ca2+. Ca2+-dependent fluorescence emission by obelin was detected as follows. One milliliter of Anabaena sp. PCC 7120 (Anabaena 7120 from hereon) culture at an optical density (750 nm) of 0.3 was washed with BG11 or BG110 media before coelenterazine was added to a final concentration of 2 μM from a stock solution of 1 mM in methanol. The culture was incubated in darkness for 30 min at 28°C. The fluorescence images with UV light for excitation were recorded as described by Huang et al. (20). For measurement of the fluorescence spectra of Anabaena 7120 cells containing obelin, the cell cultures were prepared as above, and the spectra were recorded by a PTI (South Brunswick, NJ) fluorescence spectrophotometer. The excitation wavelength was 340 nm with a slit width of 1 nm. Changes of fluorescence emission at 460 nm were used to determine changes of [Ca2+]i in Anabaena 7120.
Other Methods. SDS/PAGE was performed according to Laemmli (21). Heterocyst isolation and immunoblotting for detection of HetR and CcbP were performed according to Zhou et al. (22). Protein concentration was determined as described (23). Localization of GFP on Anabaena 7120 filaments was carried out according to Yoon and Golden (24).
Results
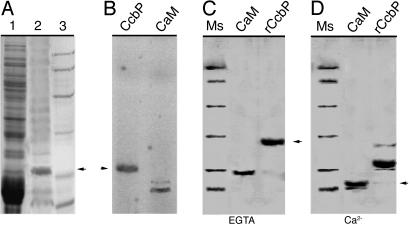
Characterization of CcbP from Anabaena 7120. We tried several methods to isolate Ca2+-binding proteins from Anabaena 7120. A method used for calsequestrin isolation (25) was found suitable, and we were able to partially purify a Ca2+-binding protein from Anabaena 7120 (Fig. 1A). A protein band was labeled with Ca2+ in 45Ca2+ overlay (Fig. 1B). The N-terminal sequence of the protein was determined (ASVERDETREHRIETEIIV). It matched a hypothetical protein encoded by an ORF (alr1010) in the genome sequence without the initial Met residue. The gene was named ccbP (cyanobacterial calcium-binding protein). The ccbP gene was overexpressed in E. coli, and the gene product was purified (Fig. 1C). A Ca2+-dependent gel-shifting assay showed that CcbP had an apparent molecular mass of 31 kDa in the presence of EGTA, whereas it had an apparent molecular mass of 21 kDa in the presence of Ca2+. This mobility shift was larger than that of rat CaM, which showed a shift of ≈4 kDa (Fig. 1 C and D).
Fig. 1.
Partial purification and characterization of CcbP from Anabaena 7120. (A) SDS/PAGE analysis of isolated CcbP. Lane 1, total cellular extract from Anabaena 7120; lane 2, partially purified CcbP after (NH4)2SO4 precipitation, a DEAE column, and a gel filtration column; lane 3, protein molecular mass standards (in kDa, from top): 97, 64, 45, 31, 21.5, and 14. (B) 45Ca2+ overlay. Partially purified CcbP (lane 1) and rat CaM (lane 2) were transferred to a poly(vinylidene difluoride) membrane after SDS/PAGE and labeled with 45Ca2+ before exposure to x-ray film. (C and D) Characterization of the recombinant CcbP (rCcbP) by electrophoretic mobility in SDS/PAGE in the presence of 2 mM EGTA (C) or 2 mM CaCl2 (D). The proteins were treated with 1 mM DTT before electrophoresis. The rat CaM was used as control. Molecular mass standards (Ms) are as described in A.
CcbP from Anabaena 7120 has 126-aa residues (14.75 kDa) with a pI of 4.1. The CcbP contains no EF hand, and it does not have motifs or domains known to bind Ca2+. There are two hydrophobic stretches flanking an Asp-rich area located in the middle of the protein.
CcbP Regulates Calcium Availability for Heterocyst Formation. To study the functions of CcbP, an insertion mutant of ccbP was constructed in Anabaena 7120 (CCBP-M). The mutant was confirmed by Southern hybridization and immunoblotting as shown in Fig. 8, which is published as supporting information on the PNAS web site. We also constructed a plasmid (pPpetE-ccbP) that had the ccbP gene under control of the petE promoter (26). This plasmid was used to transform Anabaena 7120 (CCBP-IE) so that the expression of ccbP was inducible with copper.
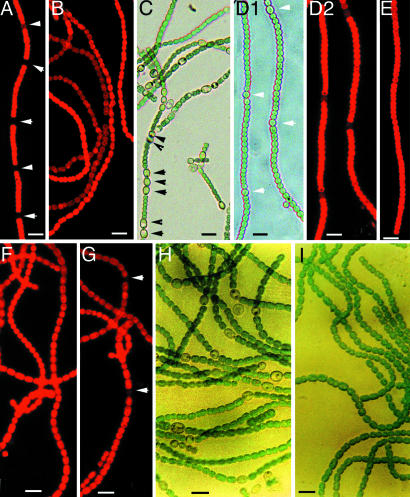
When combined nitrogen was removed, wild-type filaments formed heterocysts within 24 h (Fig. 2A). CCBP-M was able to grow at a reduced rate (70%) and did not form heterocysts in BG11 (Fig. 2B). When CCBP-M was subjected to nitrogen step-down, its heterocyst frequency was higher than that of the wild type, and multiple contiguous heterocysts (MCH) were formed (Fig. 2C). The strain CCBP-IE was also able to grow in BG11. In the absence of added copper, CCBP-IE could form heterocysts with a slightly reduced heterocyst frequency under nitrogen-limiting condition (Fig. 2D). When copper was added to the growth medium, heterocyst formation was completely suppressed in CCBP-IE (Fig. 2E), suggesting that CcbP negatively regulates heterocyst differentiation.
Fig. 2.
The effects of inactivation of ccbP and overexpression of ccbP and cam on heterocyst differentiation in Anabaena 7120. (A) Fluorescence image of a wild-type filament. (B) Fluorescence image of CCBP-M grown in the presence of nitrate. (C) Bright-field image of CCBP-M in the absence of combined nitrogen. (D) Bright-field (D1) and fluorescence (D2) images of CCBP-IE grown in the absence of combined nitrogen without copper induction. (E) Fluorescence image of CCBP-IE grown in the absence of combined nitrogen with copper induction. (F and G) Fluorescence images of the wild type expressing a rat CaM gene (cam) and CCBP-M expressing cam in the absence of combined nitrogen with copper induction, respectively. (H and I) Bright-field images of A. variabilis carrying pPpetE-ccbP grown in the absence of combined nitrogen without and with copper induction, respectively. Arrows indicate the positions of heterocysts. (Bar, 10 μm.)
Ca2+-binding proteins might regulate gene expression either through Ca2+-dependent protein–protein interactions or through regulation of [Ca2+]i (calcium homeostasis). To understand the mechanism by which CcbP regulates heterocyst differentiation, we studied the effect of expressing a rat CaM gene (cam) in Anabaena. When the wild type carrying pPpetE-cam was induced to express cam with copper, it failed to form heterocysts 48 h after nitrogen step-down (Fig. 2F). Expression of cam in CCBP-M by copper induction suppressed MCH in the absence of combined nitrogen (Fig. 2G). A few heterocysts could be observed, but the frequency was <4%. Transformation of CCBP-M with the plasmid pPpetE-ccbP(eryR) could also suppress the MCH phenotype (data not shown). Anabaena variabilis is another species that also forms heterocysts under nitrogen-limiting conditions. When pPpetE-cam was transformed into A. variabilis, it suppressed heterocyst formation completely in the absence of combined nitrogen with copper induction (Fig. 2I). However, the strain could grow under this condition, probably because of the presence of the alternative nitrogenase present in vegetative cells (27). Thus, the inhibition of heterocyst formation in Anabaena by CcbP and CaM was likely to result from Ca2+ sequestration. These results strongly indicate that Ca2+ is required for heterocyst differentiation and predict that [Ca2+]i would increase in differentiating cells and mature heterocysts of Anabaena.
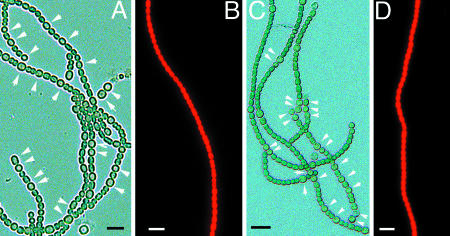
patS and hetR are critical to heterocyst formation in Anabaena (17, 18, 26, 28). The strain patS– formed MCH in the absence of combined nitrogen (Fig. 3A). However, when patS– was transformed with pPpetE-ccbP(eryR), it did not form heterocysts when induced with copper (Fig. 3B). When the hetR gene was present on a multicopy plasmid (pRL25C-hetR), Anabaena 7120 formed MCH in BG110 (Fig. 3C). When the strain with pRL25C-hetR also contained the pPpetE-ccbP(eryR) and was induced with copper, it did not form heterocysts in the absence of combined nitrogen (Fig. 3D). This result suggests that CcbP could act at an early stage of heterocyst differentiation, possibly by preventing the HetR activation of transcription.
Fig. 3.
The effect of expression of ccbP in patS– and the wild-type strain carrying pRL25C-hetR. (A) patS– formed MCH in the absence of combined nitrogen. (B) patS– with pPpetE-ccbP did not form heterocysts in the absence of combined nitrogen when induced with copper. (C) The wild type with pRL25C-hetR has MCH in the absence of combined nitrogen. (D) The MCH phenotype shown in C was suppressed when the strain also carried pPpetE-ccbP and induced with copper. Arrows indicate the positions of heterocysts. (Bar, 10 μm.)
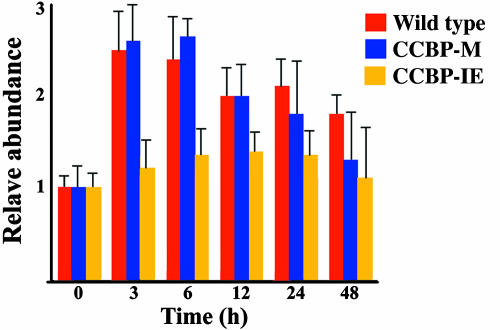
The expression of hetR was investigated by immunoblotting with the results shown in Fig. 4. The amount of HetR reached its maximum level within 3 h after nitrogen step-down in the wild type. The expression of ccbP induced by copper prevented the fast induction of hetR in CCBP-IE. The HetR level in this strain increased gradually and reached a low plateau 12 h after the removal of combined nitrogen (Fig. 4). The induction of hetR in CCBP-M was similar to that of the wild type within 24 h after nitrogen step-down. The HetR level in CCBP-M declined faster after 24 h of nitrogen step-down than that of the wild type, probably because the cells were under a more stressed condition.
Fig. 4.
Determination of HetR level by immunoblotting during the process of heterocyst differentiation. Cells of the wild type, CCBP-M, and CCBP-IE were collected at the times indicated. Total proteins were separated by SDS/PAGE and transferred to a poly(vinylidene difluoride) membrane. The amount of HetR was determined with antibodies against HetR. The values are the average of triplicate measurements.
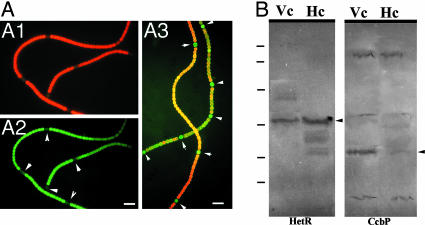
Localization of CcbP. The spatial pattern of ccbP expression in Anabaena 7120 was studied by using gfp as a reporter gene under control of the ccbP promoter (800-bp fragment upstream of ccbP). Fig. 5 A1 and A2 show that, in the strain carrying pPccbP-gfp, the GFP was mostly located in vegetative cells, whereas the green fluorescence was much weaker in heterocysts, suggesting that transcription of ccbP was down-regulated in heterocysts. As a control, the green fluorescence in the strain expressing gfp under control of the patS promoter (24) was almost exclusively located in heterocysts (Fig. 5A3). Immunoblotting the proteins from isolated heterocysts (Fig. 5B) showed that, whereas HetR was more abundant in heterocysts, CcbP could not be detected in heterocysts, suggesting that the weak green fluorescence observed in heterocysts in Fig. 5 A1 and A2 could be due to incomplete degradation of GFP during differentiation. We also studied the level of CcbP from entire filaments during the process of heterocyst differentiation and found that it was largely unchanged (data not shown).
Fig. 5.
Analysis of the ccbP promoter in Anabaena 7120 with gfp as reporter gene (A) and localization of CcbP by immunoblotting (B). (A1 and A2) Anabaena 7120 carrying pPccbP-gfp was subjected to nitrogen step-down for 24 h. The fluorescence image was obtained with blue light excitation without (A1) and with (A2) the red fluorescence blocked. (A3) GFP fluorescence from Anabaena 7120 carrying plasmid pAM1951 that contained a PpatS-gfp fusion (24) after nitrogen step-down for 24 h. Arrows indicate the positions of heterocysts. (B) Immunoblotting analysis of CcbP in vegetative cells (Vc) and isolated heterocysts (Hc). Antibodies against HetR and CcbP were used as primary antibodies in these reactions. Arrows indicate the positions of HetR or CcbP. The molecular mass standards shown on the left side of the blots are the same as in Fig. 1. (Bar, 10 μm.)
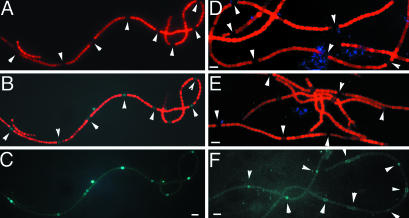
Measurement of Intracellular Free Calcium with Obelin. It was reported that a transient increase of [Ca2+]i occurred after nitrogen step-down in Anabaena 7120 based on whole-filament measurements (29). The level of [Ca2+]i in individual cells in Anabaena has not been reported. To monitor [Ca2+]i in individual cells, we used recombinant obelin as an indicator of [Ca2+]i in Anabaena 7120. In the presence of coelenterazine, the intensities of luminescence and fluorescence generated by obelin are linearly related to Ca2+ concentration (30). The blue fluorescence of obelin-generated coelenteramide is excellent for studying cyanobacterial [Ca2+]i, because the fluorescence from phycobiliproteins (PBP) and chlorophyll in this wavelength range is much lower. The obe gene on pAM505-ob was controlled by an E. coli promoter, Ptac, and the blue fluorescence generated by obelin was observed in Anabaena 7120 (Fig. 6). When the filaments were excited by PBP-absorbing light, red fluorescence was observed only from vegetative cells (Fig. 6A). When the filaments were excited with near-UV light, vegetative cells showed red fluorescence, whereas heterocysts were blue (Fig. 6B). Fig. 6C shows the same filament with the red fluorescence blocked. The level of blue fluorescence in vegetative cells was comparable in BG11 and BG110 (not shown). The blue fluorescence depended upon coelenterazine, because none was observed in its absence (Fig. 6D). The heterocysts from the wild type without obelin showed no blue fluorescence even when coelenterazine was added (Fig. 6E). Fig. 6F shows the pattern of blue-fluorescent cells along the filaments 12 h after the removal of combined nitrogen.
Fig. 6.
Increase of intracellular free Ca2+ concentration as revealed by recombinant obelin. Anabaena 7120 carrying pAM505-ob (A–D, F) or without pAM505-ob (E) was subjected to nitrogen step-down for 24 h (A–E) or for 12 h (F). Except for D, coelenterazine was added to 1 ml of cultures of Anabaena 7120 at an optical density (750 nm) of 0.3 to a final concentration of 2 μM and incubated for 30 min before they were observed with the fluorescence microscope. (A) Fluorescence image with green-light excitation that excited phycobiliproteins (PBP). (B and C) Fluorescence images with near-UV light excitation with the red fluorescence from chlorophyll and PBP unblocked (B) and blocked (C). (D) Fluorescence image in the absence of coelenterazine when excited with near-UV light. (E) Fluorescence image of the wild-type Anabaena 7120 without obelin in the presence of coelenterazine with near-UV light excitation. (F) Fluorescence image of Anabaena 7120 carrying pAM505-ob 12 h after the removal of combined nitrogen. The filament was excited with near-UV light, and the image was obtained as in C. (Bar, 10 μm.)
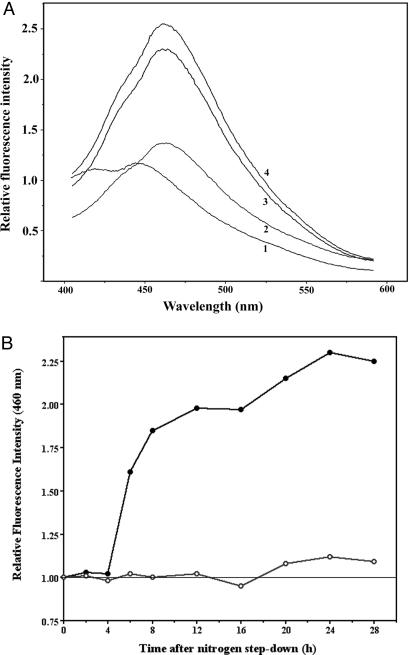
The emission spectrum generated by Ca2+–obelin during heterocyst differentiation was measured (Fig. 7). The spectrum showed an emission peak at 460 nm that depended upon the presence of coelenterazine (Fig. 7A). The change of fluorescence intensity at 460 nm (Fig. 7B) showed a fast increase of [Ca2+]i 4h after nitrogen step-down, and a higher [Ca2+]i was maintained in the following period of heterocyst differentiation. Expression of ccbP with copper induction in CCBP-IE completely suppressed the increase of [Ca2+]i after nitrogen step-down. Because the fluorescence levels in vegetative cells were similar, a doubling of fluorescence intensity in whole filaments (Fig. 7B) suggests that [Ca2+]i in heterocysts or differentiating cells would be ≈10 times higher than in vegetative cells, assuming that their frequency is ≈10%.
Fig. 7.
Fluorescence emission spectra in vivo and time course of the increase of emission at 460 nm during heterocyst differentiation. (A) Selected fluorescence spectra from coelenteramide-obelin in Anabaena 7120 carrying pAM505-ob during heterocyst differentiation. Cells were collected, and coelenterazine was added as described in Fig. 6. The cultures were then excited with UV light at 340 nm, and four emission spectra were obtained and averaged. Spectrum 1, emission spectrum without coelenterazine; spectra 2–4, emission spectra obtained at 0, 12, and 24 h after the removal of combined nitrogen, with coelenterazine. (B) Increase of fluorescence emission at 460 nm of Anabaena 7120 carrying pAM505-ob during heterocyst differentiation. Cells were collected at the times indicated and incubated with coelenterazine before emission spectra were obtained as in A. Each point represents an average of four independent measurements. Solid circles: Anabaena 7120 carrying pAM505-ob. Open circles: CCBP-IE(eryR) carrying pAM505-ob in the presence of 2.5 μM CuCl2.
Discussion
A low [Ca2+]i is normally maintained in Anabaena 7120 (31). The detection of a transient increase of [Ca2+]i during heterocyst differentiation (29) and the results obtained with manipulation of extracellular Ca2+ concentrations (9) suggested that Ca2+ might be a signal for the differentiation process. In this report, we describe a Ca2+-binding protein, CcbP, from Anabaena 7120 and suggest a role of CcbP in regulation of heterocyst differentiation. CcbP was isolated by using a procedure similar to that for calsequestrin (25). CcbP is rich in acidic amino acids and lacks an EF hand. So it is possible that CcbP binds Ca2+ similarly to calsequestrin, which also lacks an EF hand and binds Ca2+ by protein surface charge (32). The binding of Ca2+ to calsequestrin generates a more compact structure of the protein (33). The mobility shift of CcbP in SDS/PAGE induced by Ca2+ (Fig. 1 C and D) suggests that CcbP could at least partially maintain its structure when treated with SDS, as does calsequestrin (33). It also suggests that CcbP has a more compact structure with bound Ca2+ as calsequestrin and CaM, although the possibility that the band of CcbP in SDS/PAGE in the presence of EGTA is a stable noncovalent dimer could not be completely ruled out.
Our study shows that lack of ccbP results in a MCH phenotype, and overexpression of ccbP suppresses heterocyst formation (Fig. 2). Overexpression of cam in both Anabaena 7120 and A. variabilis also suppressed heterocyst formation (Fig. 2). Because CaM is absent from both Anabaena strains, the effect we observed in the strains overexpressing cam is most likely a result of Ca2+ sequestration that reduces [Ca2+]i rather than a result of protein–protein interactions. That excessive CcbP suppresses the increase of [Ca2+]i during heterocyst differentiation (Fig. 7B) supports this suggestion. This suggestion is also supported by the results of CcbP localization, which showed that it was present in vegetative cells but absent from heterocysts (Fig. 5). These results indicate that an increase of [Ca2+]i is required for cells to differentiate.
To measure [Ca2+]i, we expressed the obelin gene from Obelia geniculata (30) in Anabaena 7120. Obelin is an excellent indicator for Ca2+ and is less sensitive to Mg2+ (30). Although the maximum fluorescence emission of recombinant obelin–Ca2+ in vitro is at 517 nm (30), the peak of obelin-Ca2+ fluorescence emission is at 460 nm in Anabaena cells (Fig. 7). This result suggests that the emission is contributed mostly by an ion pair state and the amide anion of coelenteramide (30). The total cellular-free Ca2+ concentration increases rapidly 4 h after nitrogen step-down (Fig. 7B), and this increase is due to the increase of [Ca2+]i in mature heterocysts and differentiating cells (Fig. 6). The results in Fig. 6 suggest that Ca2+ plays an important role early in heterocyst differentiation, because a pattern of cells with high blue fluorescence was established earlier than the appearance of proheterocysts. That overexpression of ccbP prevents heterocyst formation in the strain patS– or the strain with pRL25C-hetR (Fig. 3) also suggests that Ca2+ functions early in the process of differentiation. HetR is an important protein in regulation of heterocyst differentiation and pattern formation (17, 18, 20, 26, 34, 35), and hetR is up-regulated early in the process of heterocyst differentiation (17, 22). Sequestration of Ca2+ with CcbP prevented the initial hetR induction (Fig. 4), indicating that hetR up-regulation could not be accomplished when [Ca2+]i is too low. Because HetR is possibly a Ca2+-dependent protease (18), the increased [Ca2+]i is likely to be important to the functions of HetR in the differentiating cells.
The results shown in Figs. 6 and 7 demonstrate that [Ca2+]i in mature heterocysts is 10 times higher than in the vegetative cells. The increase of [Ca2+]i in mature heterocysts and differentiating cells could result from specific degradation of CcbP and the release of the bound Ca2+, which could occur 4 h after nitrogen step-down (Fig. 7B). The phase of fast [Ca2+]i increase (Fig. 7B) was ≈1–2 h behind the phase of HetR increase (Fig. 4). The timing and kinetics of HetR induction (Fig. 4) and [Ca2+]i increase (Fig. 7) after nitrogen step-down are suggestive that HetR can be a candidate responsible for CcbP degradation, although experimental data are lacking at the moment. Anabaena 7120 has at least one potential P-type calcium-pump (all3245) based on surveys of its genome. It is possible that the activity of the pump is down-regulated in differentiating cells and mature heterocysts.
The increase of [Ca2+]i in heterocysts strongly suggests that Ca2+ is important for heterocyst functions. It has been shown that GlnB modification is inhibited by Ca2+ (K. Forchhammer, personal communication). Laurent et al. (36) have shown that GlnB in heterocysts is present in an unmodified form, which is required for the activation of N-acetyl glutamate kinase (37, 38), a key enzyme involved in arginine synthesis. Thus, a higher [Ca2+]i might be critical for nitrogen assimilation in heterocysts.
Supplementary Material
Acknowledgments
We thank Dr. J. Golden (Texas A&M University, College Station, TX) for providing useful plasmids, Dr. X. Xu (Institute of Hydrobiology, Wuhan, China) for providing the patS– strain, and Dr. J. Brosius (Mount Sinai School of Medicine, New York) for providing the rat CaM gene. We also thank Dr. K. Forchhammer (University of Giessen, Giessen, Germany) for communicating his experimental results before publication. This research is supported by the National Natural Science Federation of China (30230040) and by grants from the Ministry of Science of Technology of China (01CB108903 and 2004AA626021).
Author contributions: J.B. and J.Z. designed research; Y.Z., Y.S., W.Z., X.H., D.W., and N.B. performed research; J.Z. analyzed data; and J.B. and J.Z. wrote the paper.
Abbreviations: CaM, calmodulin; MCH, multiple contiguous heterocysts.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY919604).
References
- 1.Campbell, A. K. (1983) Intracellular Calcium: Its Universal Role as Regulator (Wiley, New York).
- 2.Clapham, D. E. (1995) Cell 80, 259–268. [DOI] [PubMed] [Google Scholar]
- 3.Youatt, J. (1993) Crit. Rev. Microbiol. 19, 87–93. [DOI] [PubMed] [Google Scholar]
- 4.Michiels, J., Xi, C., Verhaert, J. & Vanderleyden, J. (2002) Trends Microbiol. 10, 83–97. [DOI] [PubMed] [Google Scholar]
- 5.Dominguez, D. C. (2004) Mol. Microbiol. 54, 291–297. [DOI] [PubMed] [Google Scholar]
- 6.Gangola, P. & Rosen, B. P. (1987) J. Biol. Chem. 262, 12570–12574. [PubMed] [Google Scholar]
- 7.Herbaud, M. L., Guiseppi, A., Denizot, F., Haiech, J. & Kihoffer, M. C. (1998) Biochim. Biophys. Acta 1448, 212–226. [DOI] [PubMed] [Google Scholar]
- 8.Tisa, L. S., Olivera, B. M. & Adier, J. (1993) J. Bacteriol. 175, 1235–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith, R. L., Hobson, S. & Ellis, I. R. (1987) New Phytol. 105, 531–541. [Google Scholar]
- 10.Cloud, P., Moorman, M. & Pierce, D. (1975) Q. Rev. Biol. 50, 131–150. [Google Scholar]
- 11.Buikema W. J. & Haselkorn, R. (1993) Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 33–52. [Google Scholar]
- 12.Wolk, C. P. (2000) in Prokaryotic Development, eds. Brun, Y. V. & Shimkets, L. J. (Academic, Washington, DC), pp. 83–104.
- 13.Meeks, J. C. & Elhai, J. (2002) Microbiol. Mol. Biol. Rev. 66, 94–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden, J. W. & Yoon, H. S. (2003) Curr. Opin. Microbiol. 6, 557–563. [DOI] [PubMed] [Google Scholar]
- 15.Frias, J. E., Flores, E. & Herrero, A. (1994) Mol. Microbiol. 14, 823–832. [DOI] [PubMed] [Google Scholar]
- 16.Wei, T.-F., Ramasubramanian, T. S. & Golden, J. W. (1994) J. Bacteriol. 176, 4473–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buikema, W. J. & Haselkorn, R. (1991) Genes Dev. 5, 321–330. [DOI] [PubMed] [Google Scholar]
- 18.Zhou, R., Wei, X., Jiang, N., Li, H., Dong, Y., Hsi, K. L. & Zhao, J. (1998) Proc. Natl. Acad. Sci. USA 95, 4959–4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess, W. H., Jemiolo, D. K. & Kretsinger, R. H. (1980) Biochim. Biophys. Acta 623, 257–270. [DOI] [PubMed] [Google Scholar]
- 20.Huang, X., Dong, X. & Zhao, J. (2004) Proc. Natl. Acad. Sci. USA 101, 4848–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. (1970) Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- 22.Zhou, R., Cao, Z. & Zhao, J. (1998) Arch. Microbiol. 169, 417–423. [DOI] [PubMed] [Google Scholar]
- 23.Peterson, G. L. (1977) Anal. Biochem. 83, 346–356. [DOI] [PubMed] [Google Scholar]
- 24.Yoon, H. S & Golden, J. W. (1998) Science 282, 935–938. [DOI] [PubMed] [Google Scholar]
- 25.Campbell, K. P., McLennan, D. H., Jorgensen, A. O. J. & Mintzer, M. C. (1983) J. Biol. Chem. 258, 1197–1204. [PubMed] [Google Scholar]
- 26.Buikema, W. J. & Haselkorn, R. (2001) Proc. Natl. Acad. Sci. USA. 98, 2729–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiel, T., Lyons, E. M. & Erker, J. C. (1997) J. Bacteriol. 179, 5222–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon, H. S. & Golden, J. W. (2001) J. Bacteriol. 183, 2605–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torrecilla, I., Leganes, F., Bonilla, I. & Femandez-Pinas, F. (2004) Microbiology 150, 3731–3739. [DOI] [PubMed] [Google Scholar]
- 30.Markova, S. V., Vysotski, E. S., Blinks, J. R., Burakova, L. P., Wang, B. C. & Lee, J. (2002) Biochemistry 41, 2227–2236. [DOI] [PubMed] [Google Scholar]
- 31.Torrecilla, I., Leganes, F., Bonilla, I. & Femandez-Pinas, F. (2000) Plant Physiol. 123, 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maclennan, D. H., Abu-Abed, M. & Kang, C. (2002) J. Mol. Cell Cardiol. 34, 897–918. [DOI] [PubMed] [Google Scholar]
- 33.Cozens, B. & Reithmeier, R. A. F. (1984) J. Biol. Chem. 259, 6248–6252. [PubMed] [Google Scholar]
- 34.Black, T. A. & Wolk, C. P. (1994) J. Bacteriol. 176, 2282–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khudyakov, I. & Golden, J. W. (2004) Proc. Natl. Acad. Sci. USA 101, 16040–16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurent, S., Forchhammer, K., Gonzalez, L., Heulin, T., Zhang, C. C. & Bedu, S. (2004) FEBS Lett. 576, 261–265. [DOI] [PubMed] [Google Scholar]
- 37.Heinrich, A., Maheswaran, M., Ruppert, U. & Forchhammer, K. (2004) Mol. Microbiol. 52, 1225–1228. [DOI] [PubMed] [Google Scholar]
- 38.Burillo, S., Luque, I., Fuentes, I. & Contreras, A. (2004) J. Bacteriol. 186, 3346–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.