Abstract
Chlorophyll biosynthesis is a process involving ≈20 different enzymatic steps. Half of these steps are common to the biosynthesis of other tetrapyrroles, such as heme. One of the least understood enzymatic steps is formation of the isocyclic ring, which is a characteristic feature of all (bacterio)chlorophyll molecules. In chloroplasts, formation of the isocyclic ring is an aerobic reaction catalyzed by Mg-protoporphyrin IX monomethyl ester cyclase. An in vitro assay for the aerobic cyclase reaction required membrane-bound and soluble components from the chloroplasts. Extracts from barley (Hordeum vulgare L.) mutants at the Xantha-l and Viridis-k loci showed no cyclase activity. Fractionation of isolated plastids by Percoll gradient centrifugation showed that xantha-l and viridis-k mutants are defective in components associated with chloroplast membranes. The Xantha-l gene, corresponding to Arabidopsis thaliana CHL27, Rubrivivax gelatinosus acsF, Chlamydomonas reinhardtii CRD1, and CTH1 and situated at the short arm of barley chromosome 3 (3H), was cloned, and the mutations in xantha-l35, xantha-l81, and xantha-l82 were characterized. This finding connected biochemical and genetic data because it demonstrated that Xantha-l encodes a membrane-bound cyclase subunit. The evidence suggests that the aerobic cyclase requires at least one soluble and two membrane-bound components.
Keywords: barley, acsF, CHL27, CRD1, mutant
The chlorophyll biosynthetic pathway, like many other metabolic routes, has been explored by mutants. Because mutants often accumulate the substrate of the enzyme affected by the mutation, it is possible to sort out the consecutive order of intermediates in a pathway. A systematic search of barley (Hordeum vulgare L.) mutants defective in chlorophyll biosynthesis was initiated in the 1920s (1). Today, the collection, one of the oldest, contains 357 mutants representing 105 loci. Feeding experiments with 5-aminolevulinate, the chlorophyll biosynthetic precursor, showed that barley mutants at the Xantha-l and Viridis-k loci accumulated Mg-protoporphyrin IX monomethyl ester (MPE) (2), which suggests that they are involved in formation of the isocyclic ring that is found in all chlorophylls and bacteriochlorophylls. The enzyme responsible for this reaction is MPE cyclase, and it catalyses the synthesis of protochlorophyllide from MPE (Fig. 1). The cyclase reaction remains one of the least understood in the pathway, and there have been considerable problems with attempts to connect genetic and biochemical data. The sequence of the Rhodobacter capsulatus and Rhodobacter sphaeroides photosynthetic gene clusters revealed many genes involved in chlorophyll biosynthesis (GenBank accession no. Z11165 and AJ010302). Systematic transposon mutagenesis demonstrated that MPE accumulated in a bchE mutant (3), which suggested that the bchE gene product is a MPE cyclase. Bioinformatic analyses indicated that this anaerobic-induced enzyme requires a cobalamin cofactor. Experiments with two vitamin B12 requiring R. capsulatus mutants showed that they were indeed blocked at the cyclase stage. Provision of external adenosylcobalamin or methylcobalamin restored cyclase activity in permeabilized cells (4). It was shown in Rhodovulum sulfidophilum and Rubrivivax gelatinosus that two distinct cyclases, an anaerobic enzyme system and an aerobic enzyme system, exist in the same organism (5–7). The anaerobic cyclase depends on a functional bchE gene. In contrast, the aerobic cyclase depends on acsF, which has homologs only in photosynthetic species. In Chlamydomonas reinhardtii, two homologs of acsF, CRD1 and CTH1, had previously been found. CRD1 was required for accumulation of photosystem I during hypoxia or copper deficiency. CTH1 was expressed primarily during copper sufficiency and, hence, could not cover the loss of CRD1 function in crd1 mutants (8, 9). An acsF homolog, CHL27, was also found in the genome of Arabidopsis thaliana. Antisense A. thaliana plants with reduced amounts of CHL27 accumulate MPE, and the plants have chlorotic leaves with reduced levels of all chlorophyll-binding proteins (10). Enzymatic work on the aerobic cyclase has been performed with cell extract of cucumber (11, 12). Biochemical characterization has shown that the oxidative cyclase needs NADPH, Fe2+, and O2 (13), and it has been suggested that the cyclization reaction goes through β-oxidation of the 6-methylpropionate side chain to a methyl-β-ketopropionate group and cyclization to protochlorophyllide (12, 14, 15).
Fig. 1.
The cyclase catalyses the conversion of MPE to protochlorophyllide. In nature, an aerobic and an anaerobic pathway exist. In the aerobic reaction, NADPH is the cofactor used as an electron donor in the reaction and molecular oxygen is incorporated into protochlorophyllide.
Barley xantha-l35, viridis-k23, and viridis-k170 are recessive leaky mutants that synthesize some chlorophyll when grown in the light. In the dark, these mutants produce reduced levels of protochlorophyllide. MPE accumulation was observed when the mutants were fed 5-aminolevulinate. MPE accumulates when the xantha-l35 mutation was combined with the mutation tigrina-d12, which, in homozygous form, causes the overproduction of protochlorophyllide (16). Accordingly, the tigrina-d12 mutant was mutagenized, and more double mutants accumulating MPE were selected. From these mutants, the xantha-l81, xantha-l82, xantha-l83, and xantha-l84 mutants were isolated. All these mutants are completely blocked at the Xantha-l locus and show a yellow phenotype when grown in the light (Fig. 2).
Fig. 2.
Phenotypes of 7-day-old wild-type, viridis-k23, viridis-k170, xantha-l35, xantha-l81, and xantha-l82 seedling leaves. The two viridis mutants and the xantha-l35 mutant are leaky and are able to synthesize a limited amount of chlorophyll, which gives them a pale green color. The xantha-l81 and xantha-l82 mutants are nonleaky and unable to make chlorophyll; therefore, they are only colored yellow from carotenoids.
In this work, we measured aerobic cyclase activity in barley chloroplast extracts. We showed that activity required soluble and membrane-bound fractions and that membrane components are defective in the xantha-l and the viridis-k mutants. We further cloned and sequenced the acsF homolog from barley. Mutations were only found in the cloned DNA from the barley mutants xantha-l35, xantha-l81, and xantha-l82 but not in that of viridis-k23 and viridis-k170. This finding demonstrated that the barley Xantha-l gene is a homolog of acsF. We connected the genetic and biochemical data, and we conclude that Xantha-l, acsF, CRD1, CTH1, and CHL27 encode the same membrane-bound component of the aerobic cyclase. Most likely the aerobic cyclase is composed of three gene products, a soluble protein, a membrane-bound component encoded by Xantha-l, and another membrane-bound component encoded by Viridis-k.
Methods
Chloroplast Isolation and Fractionation. Barley (Hordeum vulgare L. cv. Bonus) wild type and mutants xantha-l35, xantha-l81, xantha-l82, viridis-k23, and viridis-k170 were grown on moist vermiculite in darkness at 22°C for 7 days. Seedlings were illuminated for 4 h (23 μE·m–2·s–1) before chloroplast isolation. Barley seedlings were cut above the coleoptile and chopped into l-cm lengths in ice-cold buffer A {0.5 M sorbitol/1 mM MgCl2/1 mM EDTA/20 mM N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid/10 mM Hepes/1 mM DTT/0.5% (wt/vol) fatty acid-poor BSA, pH 7.8}. Chopped seedlings were homogenized in buffer A in a blender fitted with fresh razor blades. The homogenate was forced through one layer of nylon cloth (31-μm mesh), filtered through another layer, and immediately centrifuged at 3,000 × g for 3 min in a swinging bucket rotor to sediment the chloroplasts. The crude chloroplast pellet was resuspended in a small volume of buffer A and layered on top of a 45% Percoll pad also in buffer A but lacking BSA. Percoll gradients were spun at 13,000 × g for 9 min to sediment intact chloroplasts, which were resuspended in buffer A lacking BSA and centrifuged again at 3,000 × g for 4 min to remove remaining Percoll. Intact chloroplasts were resuspended in sufficient volume of lysis buffer B {11 mM MgCl2/1 mM EDTA/20 mM N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid/10 mM Hepes/1 mM DTT/1 mM 4-(2-aminoethyl)-benzenesulfonylfluoride, pH 7.8} to give a uniform suspension corresponding to 10 μl per g of leaves (fresh weight). The chloroplasts were allowed to lyse for 10 min on ice, resuspended with a l-ml automatic pipette tip, and then spun at 16,000 × g for 20 min. The supernatant (S) was reserved, and the pellet (P) was resuspended again in half the original volume of buffer B and spun at 16,000 × g for 20 min. The supernatants were combined, and the pellet fraction was again resuspended in half the original volume of buffer B. At all stages, samples were kept on ice or at 4°C. If not used immediately, fractions were frozen in liquid nitrogen and stored at –80°C.
Cyclase Assay. MPE and protochlorophyllide were isolated as described in refs. 4 and 17. All procedures described below were performed under dim light with special precautions to avoid light when quenching the reaction to prevent photoconversion of the product of the reaction, protochlorophyllide, to chlorophyllide. In a typical assay, P (80 μg of protein) and S (200 μg of protein) were incubated in a total volume of 100 μl in buffer B with 4 μM MPE and 5 mM NADPH. MgCl2 was added to a 25 mM final concentration. Samples were shaken at 1.1 × g for 20 min at 30°C in darkness. Then 80 μl of sample was transferred to a 1.5-ml tube on ice containing 750 μl of acetone, 170 μl of 0.12 M NH4OH, and 300 μl of hexane. Samples were shaken and spun at 12,000 × g for 2 min to sediment precipitated protein. The lower phase was collected, and the amount of protochlorophyllide was quantitated fluorometrically by comparison with authentic standards. To minimize interference from fluorescence of the substrate, an excitation of 440 nm was used for protochlorophyllide (11). The emission at 635 nm was monitored in a 1-ml quartz cuvette by using a FluoroMax-2 fluorometer (Spex spectrofluorometer system, Jobin Yvon, Longjumeau, France). Protochlorophyllide is used as a generic term for both the monovinyl and divinyl species.
Cloning and DNA Sequencing. Chromosomal DNA was isolated from barely leaves with cetyltrimethylammonium bromide (18) or according to the GenElute Plant Genomic DNA Kit (Sigma) and used as template in PCR. Three sets of oligonucleotides were used as primers to obtain DNA fragments of the Xantha-l gene. Oligonucleotides KRCycF27 (5′-CCTCGGAGGATGGCGTCCGCCATGGAGCTCTCC-3′) and KRCycR18 (5′-CTGCGACCGAGCTCCTTGTAGAGG-3′) generated a fragment corresponding to base pairs 1–500 of Xantha-l. Oligonucleotides KRCycF7 (5′-CAGCTCAACCAGGACGAGTTCGAC-3′) and KRCycR10 (5′-CATTCAGGTACATGGTTATATACACC-3′) generated a fragment corresponding to base pairs 298–1389. Oligonucleotides KRCycF14 (5′-CAGCCGCAGTTCCTCAATGACTGGAAG-3′) and KRCycR15 (5′-CAGGTGAGCACAGGAAGATGTAGATC-3′) generated a fragment corresponding to base pairs 1194–2097. After an initial denaturation step at 96°C for 2 min, 35 cycles consisted of 30 s at 96°C, 30 s at 58°C, and 1 min at 68°C. Obtained DNA fragments were purified from agarose gels according to the JetSorb Extraction kit (Genomed, Bad Oeynhausen, Germany) and cloned into the pGEM-T easy vector (Promega). DNA sequencing was performed directly on PCR products or on plasmid DNA with a Prism 3100 Genetic Analyzer and the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems).
Western Blot and Southern Blot Analyses. Extraction of total proteins from barley leaves and Western blot analysis were performed as described in ref. 19. Polyclonal antibodies against A. thaliana CHL27 were used as primary antibodies (20). Chromosomal DNA used in Southern blot analysis was isolated from 13 wheat-barley disomic addition lines as well as from the wheat and barley parent strains (21, 22). The DNA was digested overnight with PstI and HindIII in combination, separated by 0.8% agarose gel electrophoresis, and transferred by capillary blotting to a Biodyne B Transfer membrane (Pall). A proximal 1,092-bp EcoRI fragment of the barley Xantha-l gene was labeled with [α-32P]CTP and used as probe in the hybridization. Labeling of the DNA and the hybridization was performed as described in ref. 19.
Results
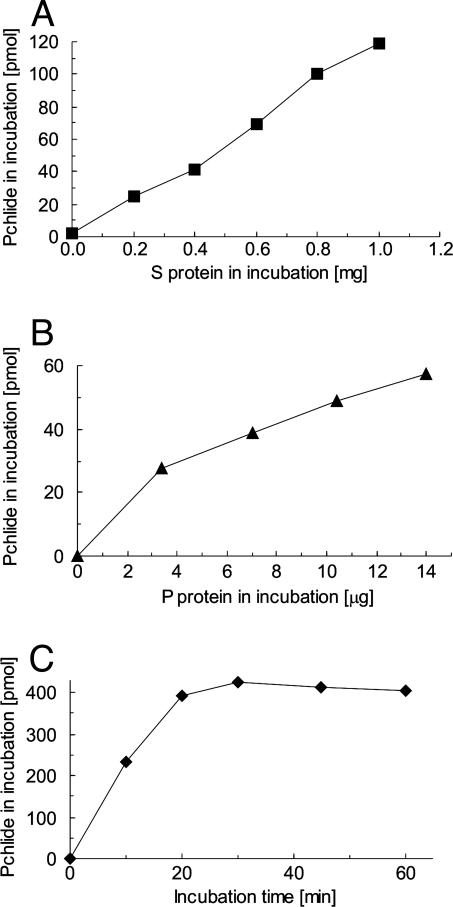
In Vitro Assay for Barley Cyclase. Barley mutants at the Xantha-l and Viridis-k loci accumulate MPE upon feeding with 5-aminolevulinate and are, therefore, potential cyclase mutants (16). To measure activity directly, a biochemical assay was developed for the cyclase in this species. The cucumber in vitro assay (11) was used as a starting point. A Percoll gradient was used to isolate intact barley plastids, which were subsequently lysed by osmolysis in a small volume of hypotonic buffer. The Percoll step was important to the removal of material from the crude plastid fraction, which was inhibitory for the cyclase. Furthermore, addition of a protease inhibitor, 4-(2-aminoethyl)-benzenesulfonylfluoride, and MgCl2 in the lysis buffer doubled the recovery of cyclase activity. Still, the specific activity between different preparations varied. Centrifugation of lysed chloroplasts yielded a P and a S. No cyclase activity was observed with the S or P fraction alone. However, activity increased in a linear fashion when the P was incubated with increasing amounts of S. Similarly, activity increased when the S was incubated with increasing amounts of P (Fig. 3). This demonstrated that the barley cyclase, like that of cucumber, required at least two components.
Fig. 3.
Cyclase activity measurements. (A) A fixed amount of P fraction (60 μg of protein) was incubated with various amounts of S fraction for 20 min. The maximal specific activity in this preparation was 112 pmol of protochlorophyllide per mg of protein for every 20-min period. (B) A fixed amount of S (230 μg of protein) was incubated with various amounts of P for 20 min. The maximal specific activity was 237 pmol of protochlorophyllide per mg of protein for every 20-min period. (C) The protochlorophyllide formation was followed by time. P (50 μg of protein) and S (240 μg of protein) were incubated under standard assay conditions except that samples were quenched at the various times indicated. The specific cyclase activity at 20 min was 393 pmol of protochlorophyllide per mg of protein for every 20-min period.
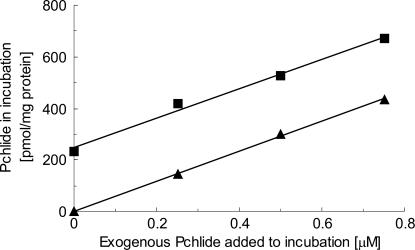
Under optimal conditions, cyclase activity was linear for ≈30 min, after which the activity slowed and stopped (Fig. 3). An incubation time of 20 min was therefore selected as optimal for estimating the specific activity of preparations. After 30 min, there was no further drop in MPE, indicating that the enzyme had become inactive, rather than the protochlorophyllide being further processed. Although it was possible that the enzyme became unstable after this short incubation, it was also possible that the cyclase was subject to product inhibition by protochlorophyllide. To test for product inhibition, known amounts of protochlorophyllide were added to incubations. The amount of additional protochlorophyllide synthesized during the incubation was unaffected by protochlorophyllide concentrations up to 0.75 μM, which was the typical concentration of protochlorophyllide present at the end of an incubation (Fig. 4). Thus, no evidence was found for product inhibition under these conditions.
Fig. 4.
Effect of the presence of exogenous protochlorophyllide on cyclase activity. P (28 μg of protein) and S (140 μg of protein) were set up under standard assay conditions, with various concentrations of protochlorophyllide. One set of samples was quenched at time zero, and the protochlorophyllide extracted was quantitated (▴). The second set of samples was incubated for 20 min, and the protochlorophyllide present in these samples is indicated (▪). The cyclase activity at each concentration of added protochlorophyllide is the difference between the zero time and incubated samples. At all protochlorophyllide concentrations, this difference was ≈230 pmol of protochlorophyllide per mg of protein for every 20-min period.
The cofactor requirements for the barley cyclase were similar to those described for other photosynthetic organisms (23–25). Cyclase activity depended on high concentrations of NADPH. NADPH (5 mM) saturated the activity and gave a specific activity of 320 ± 16 pmol of protochlorophyllide per milligram of protein for every 20-min period with 30 μg of P protein and 200 μg of S protein. No activity was observed when 5 mM NADP+ was used instead of NADPH. The substrate, MPE, is methylated on the 6-propionate side chain and is the physiological substrate for the cyclase. In cucumber, this methyl group can be removed to form Mg-protoporphyrin during the incubation. The addition of S-adenosyl-l-methionine (AdoMet) allowed the physiological methyl transferase to regenerate MPE and, thus, stimulated activity by 50% (25). In barley, the same methyl transferase is active because Mg-protoporphyrin and AdoMet can be used as alternative substrates for the cyclase (C. J. Walker, unpublished observation). However, no significant stimulation was observed on the addition of 2 mM AdoMet with 5 mM NADPH in the assay (331 ± 15 pmol protochlorophyllide per milligram of protein for every 20-min period). The same observation was made with wheat etioplasts (26). AdoMet was not routinely included in our incubations.
Cyclase Activity in Barley Mutants. Lethal chlorophyll biosynthetic mutants are not viable in the homozygous state beyond the seedling stage after depletion of the storage material in the grain endosperm. Mutant lines are therefore maintained as heterozygotes, and the homozygous mutant seedling progenies are selected from green segregating heterozygous individuals. In a tray of green seedlings the yellow/pale green mutants are selected by eye, and mutant plastids are prepared from them. P and S fractions were prepared in parallel with wild-type barley fractions. As expected, reconstitution of the wild-type P and S was active (Table 1). Combinations of wild-type P and mutant S were also active, indicating that the cyclase component in the S fractions was not affected by the xantha-l35, xantha-l81, and viridis-k23 mutations. However, combinations involving the P fraction from the three mutants were all inactive, which suggested that a cyclase component in the P fraction was inactive in xantha-l and viridis-k mutants. The absence of activity in the extracts of the leaky mutants xantha-l35 and viridis-k23 is noteworthy but not exceptional. We have observed this phenomenon before with leaky mutants of the enzyme magnesium chelatase, which is also a multisubunit enzyme (27). The cyclase reaction is complex and proceeds by means of two stable intermediates before protochlorophyllide is formed (12, 15). It was therefore possible that fractions from mutants at the Xantha-l and Viridis-k loci may complement each other. However, when the mutant P fractions were combined and assayed in the presence of wild-type S, there was no detectable activity. No inhibition or stimulation of cyclase activity was observed when mutant P fractions were added to an incubation of wild-type P and S.
Table 1. Cyclase activity upon reconstitution of plastid P and S fractions from xantha-l35, xantha-l81, and viridis-k23 mutants with wild-type fractions.
| Fractions in incubation
|
||||
|---|---|---|---|---|
| Exp. no. | P fraction | S fraction | Cyclase activity | |
| 1 | xan-l35 | + | xan-l35 | 0 |
| WT | + | xan-l35 | 99 ± 0 | |
| xan-l35 | + | WT | 0 | |
| WT | + | WT | 76 ± 3 | |
| 2 | xan-l81 | + | xan-l81 | 0 |
| WT | + | xan-l81 | 284 ± 8 | |
| xan-l81 | + | WT | 0 | |
| WT | + | WT | 309 ± 3 | |
| 3 | vir-k23 | + | vir-k23 | 0 |
| WT | + | vir-k23 | 113 ± 12 | |
| vir-k23 | + | WT | 0 | |
| WT | + | WT | 134 ± 2 | |
Primary leaves from wild-type and homozygous mutant seedlings were harvested simultaneously, and chloroplast fractions were prepared. Cyclase assays were under standard incubation conditions, with the combinations of fractions shown. Protein amounts in the assay for each experiment were as follows. Experiment 1: wild-type P, 70 μg; xantha-l35 P, 90 μg; wild-type S, 300 μg; xantha-l35 S, 400 μg. Experiment 2: wild-type P, 110 μg; xantha-l81 P, 80 μg; wild-type S, 230 μg; xantha-l81 S, 240 μg. Experiment 3: wild-type P, 80 μg; viridis-k23 P, 70 μg; wild-type S, 300 μg; viridis-k23 S, 400 μg. Cyclase activity is given in pmol of protochlorophyllide formed per mg of protein for every 20-min period.
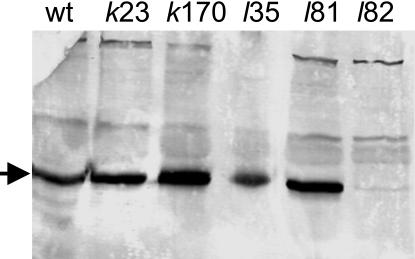
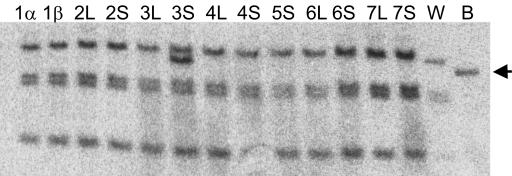
Characterization of xantha-l Mutations. Accumulation of MPE in acsF/chl27 suggested that these mutants are deficient in the cyclase (6, 10). Analysis of the CHL27 gene products showed that it was located in the chloroplast thylakoid and inner-envelope membranes (10). Furthermore, a diiron center predicted from conserved sequence residues (9) suggested that they were involved in an oxidation, which is required in the cyclase reaction and fits with the iron requirement of the enzyme (13). Because the barley xantha-l and viridis-k mutants also accumulate MPE and have no cyclase activity because of deficient membrane-bound components of the cyclase, it was likely that the Xantha-l or Viridis-k gene is homologous to acsF/CRD1/CTH1/CHL27. A blast search in the Entrez EST database using the rice full-length mRNA homolog (accession no. AK069333) of acsF as a query revealed that the barley Unigene-collected ESTs Hv.584 contained 227 homologous and overlapping cDNA fragments. From these fragments, a full-length cDNA sequence could be assembled. This sequence was used to design complementary oligonucleotides that allowed the amplification of the barley gene by PCR. Chromosomal DNA of mutants xantha-l35, xantha-l81, xantha-l82, viridis-k23, and viridis-k170 were used as a template. The DNA fragments obtained were sequenced from both strands, generating a consensus sequence of 2,097 bp (Fig. 5). The barley gene consisted of five exons. Pre-mRNA splicing sites were identified according to ref. 28 and by comparisons to the polypeptides deduced from R. gelatinosus acsF, C. reinhardtii CRD1, and A. thaliana CHL27 DNA sequences. The polypeptide of 417 aa residues deduced from the barley gene corresponded theoretically to a molecular mass of 48,161 Da and to an isoelectric point of 8.9. Mutations were found in the amplified DNA of the three xantha-l mutants but not in those of viridis-k mutants. These mutations clearly identified the cloned barley acsF/CRD1/CTH1/CHL27 homolog as Xantha-l. In the leaky mutant xantha-l35, a C-to-T point mutation resulted in an exchange of amino acid residue Ser-181 to Phe (Fig. 5). In the nonleaky mutant xantha-l81, a G-to-A point mutation resulted in the exchange of Gly-155 to Glu. A multiple sequence alignment of the polypeptides deduced from Xantha-l/acsF/CRD1/CTH1/CHL27 sequences showed that Gly-155 is a strictly conserved residue, whereas Ser-181 corresponds to an Ala residue in acsF of R. gelatinosus and R. sphaeroides. A point mutation was also found in the nonleaky mutant xantha-l82, but this change resulted in truncation as a TGG codon corresponding to Trp-291 turned into a TGA stop codon. The truncated protein in the non-sense mutant xantha-l82 was not stable because it could not be detected in a Western blot analysis of crude cell extract. In contrast, the wild-type level of the protein was found in the xantha-l35 and xantha-l81 mutants, as well as in viridis-k23 and viridis-k170 (Fig. 6). The Xantha-l gene was further characterized by determination of its chromosomal location. This characterization was achieved by the Southern blot analysis of wheat strains, which contained the short or long arm from one of the seven barley chromosomes (22). A proximal fragment of the barley Xantha-l gene was used as a probe in the Southern blot. The Xantha-l gene was shown to be located on the short arm of barley chromosome 3 [Burnham and Hagberg (29) nomenclature] or 3H [nomenclature according to Linde-Laursen et al. (30)] (Fig. 7).
Fig. 5.
The barley Xantha-l gene encoding a membrane-bound component of the aerobic cyclase (GenBank accession no. AY887063). Below the exon DNA (underlined) is the derived amino acid sequence of the polypeptide. The nucleotides affected by the xantha-l35, xantha-l81, and xantha-l82 mutations and the corresponding amino acid residue are shown in bold type. In mutant xantha-l35, a TCC codon has been changed to TTC (Ser-181 to Phe). In mutant xantha-l81, a GGG codon has been changed to GAG (Gly-155 to Glu). In mutant xantha-l82, a TGG codon encoding Trp-291 has been changed to a TGA stop codon.
Fig. 6.
Western blot analysis of the Xantha-l encoded cyclase component in barley wild type (wt) and mutants viridis-k23, viridis-k170, xantha-l35, xantha-l81, and xantha-l82. The arrow indicates the cyclase protein antigen. Five micrograms of total cell protein was loaded in each lane. Antibodies raised against the A. thaliana CHL27 protein were used.
Fig. 7.
Southern blot analysis of genomic DNA extracted from barley (B), wheat (W), and wheat–barley disomic additional lines containing arms of the barley chromosomes 1–7 (nomenclature according to ref. 29). The DNA was digested with PstI and HindIII. A proximal part of the barley Xantha-l gene was used as probe. The arrow indicates the barley DNA fragment. The barley Xantha-l gene is located on the short arm of chromosome 3 (3H).
Discussion
Genetic studies of the photosynthetic gene cluster of R. capsulatus and R. sphaeroides revealed many of the genes involved in bacteriochlorophyll biosynthesis. Given that the reactions involved in bacteriochlorophyll synthesis and chlorophyll biosynthesis are often similar, the studies have also been extremely important for the understanding of chlorophyll synthesis in vascular plants. However, isotopic labeling studies involving the enzymatic cyclase step indicated that vascular plants, algae, and an aerobic chemotrophic bacterium use molecular oxygen as a substrate in the reaction (13, 31–33). Certain photosynthetic bacteria do not form bacteriochlorophyll under aerobic conditions. R. sphaeroides uses water as an oxygen source for the anaerobic cyclase reaction (33, 34). Additionally, the anaerobic cyclase coded by the bchE gene requires a cobalamin cofactor, which cannot be formed by plants (4). Therefore, evolutionary walk techniques based on the Rhodobacter bchE gene have not been successful for identification of the cyclase in vascular plants. In this work, we connect genetic and biochemical data; three mutations in the barley Xantha-l gene were identified at the DNA level, and xantha-l mutants are devoid of cyclase activity. Our results show that Xantha-l/acsF/CRD1/CTH1/CHL27 encode a component of the aerobic cyclase. Previously, accumulation of MPE in mutants of these genes was the primary evidence that the Xantha-l, acsF, and CHL27 loci encode a subunit of the aerobic cyclase. A multiple alignment of the five gene products reveals 35% identical amino acid residues among bacteria, algae, and dicot and monocot plants. Thus, the genes encode the same cyclase subunit. Fractionation of chloroplast extracts demonstrated that the aerobic cyclase should consist of at least two components, as S and P fractions were required to restore cyclase activity (11). Therefore, nonallelic cyclase mutants could be expected in vascular plants. To our knowledge, the barley xantha-l and viridis-k mutants are the only nonallelic cyclase mutants that have been reported thus far. xantha-l and viridis-k mutants produced a functional soluble component of the cyclase. In the viridis-k mutants, the Xantha-l gene does not contain any mutation, and the Western blot gave the expected amount of protein. However, attempts to reconstitute cyclase activity by using the soluble component from wild-type and membrane components from xantha-l and viridis-k mutants were unsuccessful. Because genetic complementation functions in vivo, we suggest that these two groups of mutant loci encode two different membrane components. Our evidence indicates that both components are required in addition to the soluble component to convert MPE into protochlorophyllide. The cyclase reaction is known to involve the consecutive reactions of an O2-dependent oxidation, a dehydrogenation, and a ring-closing oxidative dehydrogenation to give protochlorophyllide. In light of this complex biochemistry, it is not surprising that the number of components now increases from at least two to at least three. Further work aims to characterize the second membrane-bound protein encoded by Viridis-k and to identify the unknown soluble component.
Acknowledgments
K.R. and C.J.W. are grateful for fellowships from the Wenner–Grenska Samfundet and the European Molecular Biology Organisation, respectively. This work was supported by the Magnus Bergvall Foundation, the O. E. and Edla Johansson Foundation, and the Swedish Research Council (M.H.) and National Research Initiative Competitive Grants Program 2002-35318-12673 from the U.S. Department of Agriculture (to S.M.). This is scientific paper 0201-05 from the College of Agricultural, Human, and Natural Resource Sciences Research Center, Washington State University.
Author contributions: K.R., C.J.W., and M.H. designed research; K.R., C.J.W., T.W., C.G.K., S.P.G., and M.H. performed research; K.R., C.J.W., S.P.G., and M.H. analyzed data; and D.v.W., S.M., S.P.G., and M.H. wrote the paper.
Abbreviations: MPE, Mg-protoporphyrin IX monomethyl ester; P, membrane fraction from broken chloroplasts; S, soluble fraction from broken chloroplasts; AdoMet, S-adenosyl-l-methionine.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession no. AY887063).
References
- 1.Gustafsson, Å. (1938) Hereditas 24, 33–93. [Google Scholar]
- 2.Gough, S. (1972) Biochim. Biophys. Acta 286, 36–54. [DOI] [PubMed] [Google Scholar]
- 3.Bollivar, D. W., Suzuki, J. Y., Beatty, J. T., Dobrowolski, J. M. & Bauer, C. E. (1994) J. Mol. Biol. 237, 622–640. [DOI] [PubMed] [Google Scholar]
- 4.Gough, S. P., Petersen, B. O. & Duus, J. O. (2000) Proc. Natl. Acad. Sci. USA 97, 6908–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porra, R. J., Urzinger, M., Winkler, J., Bubenzer, C. & Scheer, H. (1998) Eur. J. Biochem. 257, 185–191. [DOI] [PubMed] [Google Scholar]
- 6.Pinta, V., Picaud, M., Reiss-Husson, F. & Astier, C. (2002) J. Bacteriol. 184, 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouchane, S., Steunou, A. S., Picaud, M. & Astier, C. (2004) J. Biol. Chem. 279, 6385–6394. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson, M., Moseley, J. L., Tottey, S., Del Campo, J. A., Quinn, J., Kim, Y. & Merchant, S. (2004) Genetics 168, 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moseley, J., Quinn, J., Eriksson, M. & Merchant, S. (2000) EMBO J. 19, 2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tottey, S., Block, M. A., Allen, M., Westergren, T., Albrieux, C., Scheller, H. V., Merchant, S. & Jensen, P. E. (2003) Proc. Natl. Acad. Sci. USA 100, 16119–16124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong, Y.-S. & Castelfranco, P. A. (1984) Plant Physiol. 75, 658–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker, C. J., Mansfield, K. E., Rezzano, I. N., Hanamoto, C. M., Smith, K. M. & Castelfranco, P. A. (1988) Biochem. J. 255, 685–692. [PMC free article] [PubMed] [Google Scholar]
- 13.Walker, C. J., Mansfield, K. E., Smith, K. M. & Castelfranco, P. A. (1989) Biochem. J. 257, 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granick, S. (1950) Harvey Lect. 44, 220–245. [PubMed] [Google Scholar]
- 15.Wong, Y.-S., Castelfranco, P. A., Goff, D. A. & Smith, K. M. (1985) Plant Physiol. 79, 725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Wettstein, D., Kahn, A., Nielsen, O. F. & Gough, S. (1974) Science 184, 800–802. [DOI] [PubMed] [Google Scholar]
- 17.Walker, C. J., Castelfranco, P. A. & Whyte, B. J. (1991) Biochem. J. 276, 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray, H. G. & Thompson, W. F. (1980) Nucleic Acids Res. 9, 4321–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson, M., Gough, S. P., Kannangara, C. G. & von Wettstein, D. (1997) Plant Physiol. Biochem. 35, 827–836. [Google Scholar]
- 20.Moseley, J. L., Page, M. D., Alder, N. P., Eriksson, M., Quinn, J., Soto, F., Theg, S. M., Hippler, M. & Merchant, S. (2002) Plant Cell 14, 673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson, M., Gough, S. P., Kannangara, C. G. & von Wettstein, D. (1998) Plant Physiol. Biochem. 36, 545–554. [Google Scholar]
- 22.Islam, A. K. M. R., Shepherd, K. W. & Sparrow, D. H. B. (1981) Heredity 46, 161–174. [Google Scholar]
- 23.Bollivar, D. W. & Beale, S. I. (1995) Photosynth. Res. 43, 113–124. [DOI] [PubMed] [Google Scholar]
- 24.Bollivar, D. W. & Beale, S. I. (1996) Plant Physiol. 112, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chereskin, B. M., Wong, Y.-S. & Castelfranco, P. A. (1982) Plant Physiol. 70, 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasrulhaq-Boyce, A., Griffiths, W. T. & Jones, O. T. (1987) Biochem. J. 243, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson, U., Sirijovski, N. & Hansson, M. (2004) Plant Physiol. Biochem. 42, 557–564. [DOI] [PubMed] [Google Scholar]
- 28.Goodall, G. J. & Filipowicz, W. (1991) EMBO J. 10, 2635–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnham, C. R. & Hagberg, A. (1956) Hereditas 42, 467–482. [Google Scholar]
- 30.Linde-Laursen, I., Heslop-Harrison, J. S., Shepherd, K. W. & Taketa, S. (1997) Hereditas 126, 1–16. [Google Scholar]
- 31.Schneegurt, M. A. & Beale, S. I. (1992) Biochemistry 31, 11677–11683. [DOI] [PubMed] [Google Scholar]
- 32.Porra, R. J., Schafer, W., Cmiel, E., Katheder, I. & Scheer, H. (1993) FEBS Lett. 323, 31–34. [DOI] [PubMed] [Google Scholar]
- 33.Porra, R. J., Schafer, W., Gad'on, N., Katheder, I., Drews, G. & Scheer, H. (1996) Eur. J. Biochem. 239, 85–92. [DOI] [PubMed] [Google Scholar]
- 34.Porra, R. J., Schafer, W., Katheder, I. & Scheer, H. (1995) FEBS Lett. 371, 21–24. [DOI] [PubMed] [Google Scholar]