Abstract
Germline heterozygous gain-of-function (GOF) mutations of NFKBIA, encoding IκBα, cause an autosomal dominant (AD) form of anhidrotic ectodermal dysplasia with immunodeficiency (EDA-ID). Fourteen unrelated patients have been reported since the identification of the first case in 2003. All mutations enhance the inhibitory activity of IκBα, by preventing its phosphorylation on serine 32 or 36 and its subsequent degradation. The mutations certainly or probably occurred de novo in 13 patients, whereas it was inherited from a parent with somatic mosaicism in one patient. Eleven mutations, belonging to two groups, have been identified: (i) missense mutations affecting S32, S36, or neighboring residues (8 mutations, 11 patients) and (ii) nonsense mutations upstream from S32 associated with the reinitiation of translation downstream from S36 (3 mutations, 3 patients). Thirteen patients had developmental features of EDA, the severity and nature of which differed between cases. All patient cells tested displayed impaired NF-κB-mediated responses to the stimulation of various surface receptors involved in cell-intrinsic (fibroblasts), innate (monocytes), and adaptive (B and T cells) immunity, including TLRs, IL-1Rs, TNFRs, TCR, and BCR. All patients have profound B-cell deficiency. Specific immunological features, found in some, but not all patients, include a lack of peripheral lymph nodes, lymphocytosis, dysfunctional α/β T cells, and a lack of circulating γ/δ T cells. The patients had various pyogenic, mycobacterial, fungal, and viral severe infections. Patients with a missense mutation tend to display more severe phenotypes, probably due to higher levels of GOF proteins. In the absence of hematopoietic stem cell transplantation (HSCT), this condition can cause death before the age of 1 year (one child). Two survivors are on prophylaxis (at 9 and 22 years). Six children died after HSCT. Five survived, four of whom are on prophylaxis (3 to 21 years post HSCT), whereas one is well with no prophylaxis. Heterozygous GOF mutations in IκBα underlie a severe and syndromic immunodeficiency, the inter-individual variability of which might partly be ascribed to the dichotomy of missense and nonsense mutations, and the hematopoietic component of which can be rescued by HSCT.
Keywords: NFKBIA, gain-of-function, combined immunodeficiency, pediatrics, Hematopoietic stem cell transplantation
Introduction
Anhidrotic ectodermal dysplasia with immunodeficiency (EDA-ID, OMIM#300291) was first described by Mario Abinun [1, 2]. Its pathogenesis was clarified in 2001, with the discovery of hypomorphic mutations of IKBKG (NEMO) impairing NF-κB activation and accounting for its X-linked recessive (XR) form [3–5]. Complete loss-of-function mutations of IKBKG (encoding NEMO) underlie X-linked dominant (XD) incontinentia pigmenti (IP) in females and are lethal in male fetuses [6, 7]. Hypomorphic IKBKG mutations in male children underlie typical features of EDA (e.g. conical teeth, sparse hair, hypohidrosis) and various immunological and infectious phenotypes. The most common immunological abnormality is a poor antibody (Ab) response to glycans, including pneumococcal capsular glycans [3]. Consistently, although not necessarily due to this mechanism, invasive pneumococcal disease is the most common infectious disease in these patients. Inter-individual variability is observed for immunological and infectious phenotypes in patients with XR-EDA-ID, but also for developmental features, as some patients display mild or even no signs of EDA, even well into their twenties [8–15] (unpublished data). By contrast, others display not only full-blown EDA, but also additional features of osteopetrosis and lymphedema [3]. Over the last 14 years, at least a hundred patients with IKBKG mutations have been reported [14, 15]. The identification of hypomorphic IKBKG mutations in 2001 led to the discovery, in 2003, of a hypermorphic mutation in NFKBIA, encoding IκBα, in a child with an AD form of EDA-ID [16]. This de novo mutation defined the first AD PID caused by a GOF allele, following on from the discovery of neutropenia-causing GOF mutations of the X-linked WASP gene in 2001 [17]. Hypomorphic IKBKG mutations and hypermorphic NFKBIA mutations share a common pathogenic mechanism, involving inhibition of the canonical NF-κB pathway [18–21]. We review here the genetic, biochemical, immunological, and clinical features of the 14 patients with germline GOF NFKBIA mutations described since 2003.
1. Molecular genetic basis: mutant NFKBIA alleles
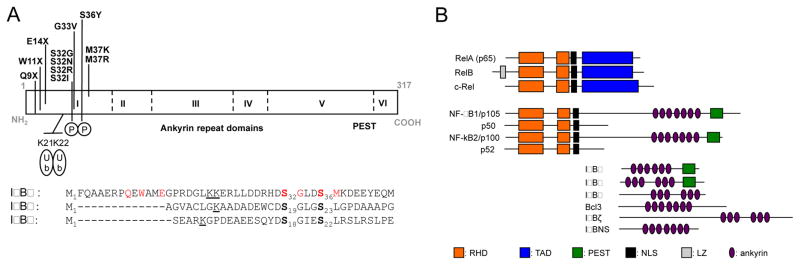
The molecular basis of AD EDA-ID was first elucidated in 2003, with the discovery of a de novo heterozygous GOF germline mutation of NFKBIA in a male infant [16]. Thirteen other patients from thirteen other kindreds have since been identified [22–32]. The patients originated from six countries on three continents: Asia (Japan, 4; Singapore, 1), Europe (England, 1; Germany, 1; Italy, 1; the Netherlands, 2,) and America (USA; 2) [16, 22–32] (Table 1). Seven of the 14 patients carried a de novo mutation (P1, P4, P6, P9, P10, P11, P12), whereas the father of P2 had related clinical manifestations and harbored himself a de novo mosaic mutation. The remaining seven patients probably also carried de novo mutations, but genotyping data were not obtained for one or both parents (Table 1). Eleven different mutations of NFKBIA (three of which are recurrent due to a mutation hotspot) have been identified. All the mutations identified affect the codons of exon 1 encoding the first 76 N-terminal amino acids. The mutations are missense (S32I: P1, P2, P13; S32G: P10; S32R: P11; S32N: P12; G33V: P14; S36Y: P6, P9; M37K: P7; M37R: P8) or nonsense (Q9X: P5; W11X: P3; E14X: P4) (Figure 1A). The missense mutations affect S32, S36, or neighboring residues (8 mutations, 11 patient), whereas the nonsense mutations are upstream of S32 (3 mutations, 3 patients). As explained below, these mutations are GOF. There are no such variations in databases of healthy individuals, such as gnomAD. In contrast, there are both missense and nonsense rare variations predicted to be loss-of-function elsewhere in the gene, strongly suggesting that there is no haplo-insufficiency at the NFKBIA locus. The N-terminal sequence of IκBα is highly conserved across species, especially the six-amino acid degron (DSGLDS) and the two serine residues located at positions 32 and 36 in humans (Figure 1A). Once these serine residues have been phosphorylated, the degron becomes a substrate for β-TrCP, a component of the Skp1-Cullin-1-F-box proteasomal degradation complex that adds poly-ubiquitin chains (K48) to lysines K21 and K22, targeting IκBα for degradation [18, 33–35]. This results in the release of NF-κB dimers from their IκBα inhibitor, and the translocation of these dimers into the nucleus. The first mutant allele to be described, S32I, was recently introduced into the nfkbia locus of mice, by knock-in techniques. Most of the phenotypes of these mice reproduce those reported for patients, but the mutant mice also display several new features not described in humans, including lack of Peyer’s patches, spleen follicles, marginal zone and germinal centers [36].
Table 1.
Genetic and clinical features of patients with heterozygous NFKBIA mutations
| Kindred | Patient | Sex | Origin | Heterozygous NFKBIA mutation | Inheritance | HSCT | Outcome | EDA | ID | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| I | P1 | M | Italian | S32I | de novo | Yes | Alive | Yes | Yes | [16, 49] |
| II | P2 | M | Dutch | S32I | Inherited from his father | Yes | Dead*,** | Yes | Yes | [22] |
| III | P3 | F | American | W11X | Mother: WT Father: nr |
Not scheduled | Alive | Yes | Yes | [23] |
| IV | P4 | M | American | E14X | de novo | Yes | Dead* | Yes | Yes | [24] |
| V | P5 | M | Japanese | Q9X | nr | Yes | Alive | Yes | Yes | [25] |
| VI | P6 | M | Japanese | S36Y | de novo | Yes | Dead* | Yes | Yes | [28] |
| VII | P7 | M | German | M37K | Mother: WT Father: nr |
Yes | Dead* | Yes | Yes | [27] |
| VIII | P8 | F | Italian | M37R | nr | No | Dead | Yes | Yes | [26] |
| IX | P9 | F | Chinese | S36Y | de novo | Not scheduled | Alive | No | Yes | [29] |
| X | P10 | F | Caucasian/Thai | S32G | de novo | Yes | Alive | Yes | Yes | [31] |
| XI | P11 | M | Japanese | S32R | de novo | Yes | Dead* | Yes | Yes | [30] |
| XII | P12 | M | Japanese | S32N | de novo | Yes | Dead* | Yes | Yes | [30] |
| XIII | P13 | nr | nr | S32I | ? | Yes | Alive | Yes | Yes | [32] |
| XIV | P14 | nr | nr | G33V | ? | Yes | Alive | Yes | Yes | [32] |
Note: P2′ father with S32Im mutation is alive, do not have EDA but had ID during his childhood nr: not reported
during or after HSCT
personal communication
Figure 1. Human homologs of NFKBIA and NFKBIA mutations.
Schematic representation of NFKBIA. A) All the reported mutations are in the upper part of the protein. The different domains are indicated in the lower part of the protein. Alignment of the N-terminal amino-acid sequence of IκBα with IκBβ and IκBε. The mutations are indicated in red, the two phosphorylated serine residues are shown in bold, and the two lysine residues polyubiquitinated with K48 chains are underlined. B) Human IκB and NF-κB family member (Adapted from Ghosh, G. et al., 2012 in Immunological Review). RHD: relative homology domain, TAD: transactivation domain, PEST: rich in proline, glutamic acid, serine, and threonine, NLS: nuclear localization site, LZ: leucine zipper.
2. Biochemical phenotypes: IκBα expression, phosphorylation, and degradation
A hallmark of the NF-κB pathway is its regulation by IκB proteins. Typical (IκBα, IκBβ, IκBε) and atypical (IκBζ, BCL-3 (B-cell lymphoma 3), IκBNS, and the precursor proteins p100 (NF-κB2) and p105 (NF-κB1)) IκB proteins are characterized by the presence of multiple ankyrin repeat domains (Figure 1B) [37, 38]. In mice and humans, the three typical IκBs are expressed constitutively and control NF-κB retention in the cytoplasm (Figure 2) [38]. Knock-out approaches in mice have demonstrated that IκBα is essential, as its absence leads to death in the perinatal period [39], whereas knockout mice for the genes encoding IκBβ and IκBε are viable, suggesting they are dispensable for development [40, 41]. These observations are consistent with the higher level of expression (10 times as much mRNA as for IκBβ and IκBε, although all three genes are ubiquitously expressed) and the strong and rapid activation of IκBα (phosphorylation and degradation) [42, 43] (http://www.gtexportal.org). For a gain of function, the mutant NFKBIA allele must encode a mutant IκBα protein satisfying three conditions: the protein must be synthesized (although possibly at lower than normal levels), it must bind the Rel homology domain (RHD) of NF-κB heterodimers (p50, p65, c-REL) and it must not be degraded following the activation of cellular pathways that normally signal via NF-κB (Figure 2). The amounts of mutant IκBα within the cell, its binding affinity to NF-κB, and its resistance to degradation therefore determine the degree of GOF and, consequently, the levels of NF-κB inhibition in heterozygous cells.
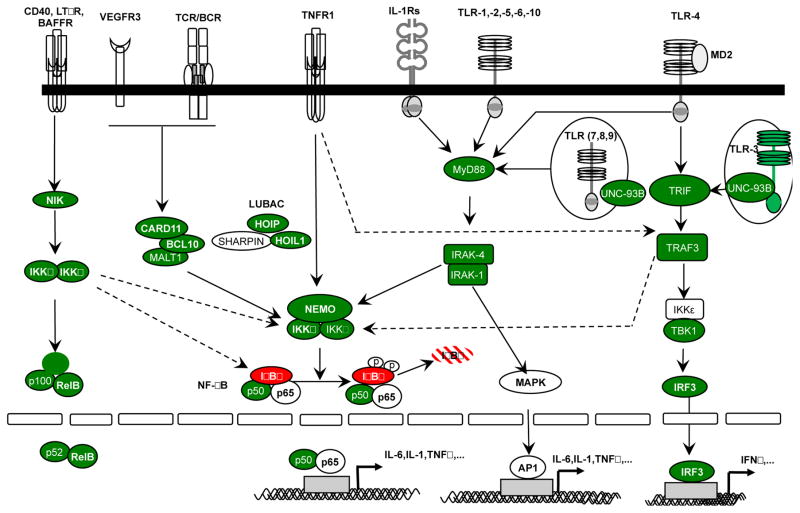
Figure 2. The NF-κB signaling pathways.
Immune receptor signaling pathways leading to NF-κB activation can be grouped into four categories on the basis of the surface receptors involved: members of the TIR superfamily (IL-1Rs/TLRs), antigen receptors (TCR and BCR), members of the TNF receptor superfamily (TNF-Rs), RANK, VEGFR3 and EDAR receptors. The proteins for which mutations have been reported in patients with primary immunodeficiency are shown in green.
The total amounts of IκBα protein expression in the patients’ cells have been quantified by Western blotting [32]. Since the total amount of IκBα in the fibroblastic cell lines carrying heterozygous missense alleles (S32I: P1, P2, G33V: P14, M37K: P7) is similar to that in control WT/WT cell lines, and the abundance of missense proteins was similar with that of WT proteins when over-expressed, these missense alleles are probably expressed in normal amounts in the patients’ cells. Indeed, the S32I, G33V, S36Y and M37K missense alleles have all been stably overexpressed in vitro. In contrast, the truncated alleles (Q9X: P5, W11X: P3) are clearly less expressed than the WT in patients’ fibroblasts [32]. The in vitro overexpression of individual Q9X, W11X, or E14X nonsense alleles resulted also in the production of a mutant protein, despite the N-terminal stop codon, due to the re-initiation of translation. There are three downstream methionine (M) residues, at positions 13, 37, and 45. For the Q9X and W11X mRNAs, two proteins of lower molecular weight (MW) than the wild-type (WT) protein were produced, whereas only the smaller protein was generated from the E14X mRNA. The M13 residue may therefore be responsible for at least a partial re-initiation of translation for both the Q9X and W11X alleles. The second M residue used for translation of the lowest MW protein for the three alleles has not been identified definitively, but M37 and M45 are both plausible candidates. A re-initiation of translation has also been observed in leukocytes (Q9X), fibroblasts (W11X), and EBV-B cells (E14X) from the patients [23–25].
The capacity of mutant IκBα to bind p65 or c-REL has been assessed only for the mutant protein encoded by the W11X allele. This protein has been shown to retain its capacity to bind p65 in fibroblasts after the immunoprecipitation of IκBα [23]. Phosphorylation of the S32 and S36 residues is a prerequisite for the degradation of IκBα. In fibroblasts from patients heterozygous for the S32I mutation (P1), and in those from KI nfkbiaWT/S32I mice, phosphorylation of the S32 residue is delayed and occurs at lower levels than in WT cells; the phosphorylation of S36 has not yet been analyzed [16, 36]. By contrast, IκBα has been shown to be phosphorylated, at least on S32, in fibroblasts heterozygous for the S36Y mutation (P9). The phosphorylation of S32 of the W11X IκBα protein is undetectable. The phosphorylation of S32/S36 in cells from patients with S32R, S32N, G33V, M37K or M37R mutations has not been studied. Finally, the specific degradation of missense mutant proteins (S32I, S32G, S32R, S32N, G33V, S36Y, M37R, M37K) cannot be investigated in patients’ cells, due to the impossibility of distinguishing between the WT and mutant alleles. Incomplete IκBα degradation is nevertheless observed in all heterozygous cell lines tested (see below). Moreover, experiments with tagged-IκBα proteins have shown that the S32I- and M37K-IκBα mutant proteins are not degraded [16, 27]. Experiments where the same approach was used directly in patients’ cells, in which the mutant proteins encoded by nonsense alleles have a lower MW, showed that the W11X and E14X proteins were stable, whereas the WT protein was degraded normally [23, 24].
Finally, cells from nine patients carrying heterozygous missense mutations tested (S32I: P1, P2; S32G: P10; S32N: P12; G33V: P14; S36Y: P9, M37K: P7 in fibroblasts, S32R: P11 in EBV-B cells or S36Y: P6 in T cell blasts) displayed incomplete IκBα degradation at early time points after stimulation (TNF-α, CD40L, IL-1β, LPS or PAM3CSK4). The nfkbiaWT/S32I mice also displayed incomplete IκBα degradation [36]. The GOF observed for the mutant alleles was due to their expression, leading to the formation of stable complexes with NF-κBs dimers, and escape from proteasomal degradation. However, none of the mutant proteins has been studied in depth in terms of the recognition of its degron by β-TrCP or its capacity to be polyubiquitinated with K48 chains.
3. Cellular phenotypes: NF-κB activation in heterozygous fibroblasts
The human NF-κB protein family has five members: p65 (RelA), RelB, c-Rel, p105/p50 (NFKB1), and p100/p52 (NFKB2). These transcription factors operate as homodimers or heterodimers [19, 44] and regulate multiple physiological facets of cells, tissues, organs, and individuals [6, 37] (Figure 1B). These five proteins are involved in the canonical (p65, p50, c-Rel, p52) or non-canonical pathway (p100/p52, RelB) [19, 44, 45]. The canonical pathway is dependent on the kinase activity of the IKK complex, whereas the non-canonical pathway depends on that of NIK [45]. In the canonical pathway, p65/p50, p50/p50, and c-Rel/p50 dimers are sequestered in the cytoplasm through association with inhibitors of NF-κB (IκBs) proteins that mask the p50 nuclear localization sequence (NLS) [33, 34]. Upon stimulation, IκBs are degraded, and the p50 NLS is exposed, driving the translocation of homo- or heterodimers containing this sequence to the nucleus, where they bind DNA at cognate binding sites, thereby driving gene transcription (Figure 2). Most of the receptors signaling through the canonical pathway play key roles in immunity. They include Toll Like Receptors (TLR), IL-1Rs, and TNF-Rs [33–35] (Figure 2). In patients with missense mutations (S32I: P1, P2; S36Y: P6), the translocation of p50/p65 and p50/p50 NF-κB dimers is impaired, but not abolished in fibroblasts (Table 2). A similar effect has been observed in fibroblasts from patients carrying a nonsense allele (W11X). Activation of the TLR/IL-1Rs (TIR) signaling pathway leads to the synthesis of various cytokines, including IL-1β, IL-6, IL-8 and TNFα. Activation of the TIR signaling pathway in non-hematopoietic cells (fibroblasts) from patients with missense mutations, in response to treatment with IL-1β (via IL-1R) (S32I: P1; S36Y: P9) or LPS (TLR4) (S32I: P1, P2; G33V: P14; M37K: P7) and PAM3CSK4 (TLR1/2) (M37K: P7), have been investigated. The TIR pathway is strongly impaired or abolished as shown by evaluations of the levels of proteins encoded by genes known to be targeted by NF-κB, such as IL-6 and TNFα (Table 2). Impaired responses were also observed upon treatment with IL-1β or LPS, in cells from patients with a nonsense mutation (Q9X, W11X). A comparison of LPS-driven IL-6 production by patients’ fibroblasts showed a stronger phenotype for missense (S32I: P1, P2; G33V: 15; M37K: P7) than nonsense mutations (Q9X: P5, W11X: P3) [32]. Moreover, the LPS-driven induction of p100 (NFKB2) and RelB is more impaired in fibroblasts with missense (S32I: P1, P2; G33V; M37K) than nonsense mutations (Q9X: P5, W11X: P3) [32]. TNFα production in response to similar LPS and PAM3CSK4 treatments is impaired in bone marrow-derived dendritic cells from nfkbiaWT/S32I mice [36]. TNFR1 and LTBR are also expressed in non-hematopoietic cells and signal through the canonical pathways [45]. The fibroblasts of patients with missense mutations (S32I: P1; S36Y: P6, P9; M37K: P7) have impaired responses to TNFα (TNFR1) and LTα1β2 (LTBR). None of the patients with nonsense mutations has undergone assessments of TNFR activation (Table 2). Fibroblasts of patients with missense (S32I: P1, P2; G33V: P14 M37K: P7) and nonsense mutations (Q9X: P5, W11X: P3) have also been co-stimulated with LPS and α-LTBR. RNA-Seq and qPCR data showed a broader and more profound phenotype in fibroblasts with missense mutations, when compared with nonsense mutations. Overall, missense mutations seem to underlie more severe cellular phenotypes than nonsense mutations, probably because of the more abundant levels of missense proteins in heterozygous cells, when compared with proteins resulting from re-initiation of translation. None of the patients with NFKBIA mutations has been tested for cellular responses to the stimulation of other TNFRs, including EDA-R and RANK (active in the ectoderm and bone, respectively) or VEGFR3 (lymphatic development). These cellular phenotypes are fully reproduced in nfkbiaWT/S32I mice [36] and mimic the phenotype of non-hematopoietic cells carrying hypomorphic alleles of the X-linked NEMO/IKBKG gene.
Table 2.
Molecular and cellular phenotypes of patients with heterozygous NFKBIA mutation
| Patient | IκBα degradation (agonist-cell type) | NF-κBs translocation (dimer-agonists-cells) | IL-1R/TLRs pathways activation (agonist-cell type) | TNFR pathway activation (agonist-cell type) | T-cell response in PBMCs (stimulus) | B cell prolif. (stimulus) |
|---|---|---|---|---|---|---|
| P1-S32I | Impaired (TNFα, LPS -fibroblast) | Impaired (p50/p65; p50/p50–TNFα-fibroblast) | Impaired (LPS-PBMC) Impaired (LPS, IL-1β-fibroblast) |
Impaired (TNFα; LTα1β2 fibroblast) | Absent prolif. (low αCD3, recall Ags) Normal prolif.(αCD3/αCD28, PMA, allogeneic cells) Normal IFN-γ prod. (αCD3, αCD3/αCD28) |
nr |
| P2-S32I | Impaired (LPS – fibroblasts | Impaired (p65 -LPS - MdM) | Impaired (LPS, PAM3, zymosan-WB) Impaired (LPS-MdM) Impaired (LPS-Fibroblast) |
nr | Low prolif! (low αCD3, PHA) Absence prolif. (recall Ags) |
Normal (CD40L+IL4) |
| P3-W11X | Impaired (IL1β-fibroblast) | Impaired (p50/p65 -IL1β - fibroblast) | Impaired (IL-1β, LPS-fibroblast) Impaired (poly( I:C), LPS, flagellin, CpG-PBMC) Normal (3M-13-PBMC) |
nr | Normal prolif. (low αCD3, αCD3/αCD28, PMA/iono, PHA, recall antigens) | nr |
| P4-E14X | Impaired (CD40L – EBV-B) | Impaired (p50; p65; cREL - CD40L - EBV-B) | Impaired (LPS, SAC OspA – PBMC) | Impaired (CD40L-EBV B cells) | Normal prolif. (PHA, ConA and recall Ags) Impaired IFN-γ and TNFα prod. (αCD3) |
nr |
| P5-Q9X | nr | nr | Impaired (LPS-monocyte) Impaired (LPS-Fibroblast) |
nr | Low prolif. (PHA; ConA) | nr |
| P6-S36Y | Impaired (TNFα – T blast cells) | Impaired (p50/p65 – TNFα - fibroblast) | Impaired (LPS, IL-18-PBMC) | Impaired (TNFα, LTα1β2 - fibroblast) Impaired (CD40L - PBMC) |
Low prolif. (low dose of αCD3) Normal prolif. (high dose of αCD3) Normal prolif. (PHA; PMA, recall Ags)! |
nr |
| P7-M37K | Impaired (TNFα, LPS, PAM3 - fibroblast) | Impaired (p50/p65 – TNFα-HeLa cells) | Impaired (LPS, PAM3-fibroblasts) Normal (SAC-WB); impaired (IL-1β, SAC, LPS, PAM2, PMA/Iono-WB) |
Impaired (TNFα - fibroblast) Impaired (TNFα - WB) |
Low prolif. (OKT3, SAC) Normal prolif. (PHA, PWM, ConA, recall Ags, diphtheria, tetanus/streptolysin O/mumps) |
nr |
| P8-M37R | nr | nr | nr | nr | Normal prolif. (PHA, PMA, αCD3/αCD28) | Decreased (CpG) |
| P9-S36Y | Impaired (TNFα, IL1β-fibroblast) | nr | Impaired (IL-1β-fibroblast) | Impaired (TNFα - fibroblast) | Impaired prolif. (high αCD3) Normal prolif. αCD3/αCD28, PHA, ConA, PMA/iono) Impaired (IFN-γ and IL-12 production; BCG, BCG/IL-12;BCG/IFN-γ)* |
nr |
| P10-S32G | Impaired (TNFα-fibroblasts) | nr | Impaired (LPS-WB) | nr | Normal prolif. (PHA) | nr |
| P11-S32R | Impaired (TNFα-fibroblast) | nr | nr | nr | nr | nr |
| P12-S32N | Impaired (CD40L – EBV-B) | nr | nr | nr | nr | nr |
| P13-S32I | nr | nr | nr | nr | nr | nr |
| P14-G33V | Impaired (LPS – fibroblasts) | nr | Impaired (LPS-fibroblast) | nr | nr | nr |
Note: P2′ father with S32I mosaic mutation had normal or mildly impaired molecular or cellular response.
nr: not reported; Whole blood: WB; Mdm: macrophage-derived monocytes, Ags: antigen, prolif: proliferation; !: thymidine incorporation;
on whole blood; low or high α-CD3: < or > 1 μg/mL α-CD3
4. Cellular phenotypes: NF-κB activation in heterozygous leukocytes
The TIRs (e.g. TLR4, IL-1R), TNFRs (e.g. TNFR1, CD40), BCR, and TCR in hematopoietic cells operate via the canonical NF-κB pathway, in an IκBα-dependent manner (Figure 2). In patients heterozygous for NFKBIA GOF, investigations of TIR leukocyte responses to stimuli have been carried out for IL-1β (IL-1Rs), IL-18 (IL-18R), PAM3CSK4 (TLR1/2), Zymozan A (TLR2), poly(I:C) (TLR3), LPS (TLR4), flagellin (TLR5), and ODN2216 (TLR9). In patients with missense mutations (S32I: P2), the translocation of p50/p65 is impaired, but not abolished, in macrophage-derived monocytes stimulated with LPS. Similarly, leukocytes (from PBMC or whole blood) from patients with missense (S32I: P1, P2; S32G; S36Y: P6; M37K) or nonsense (W11X: P3; E14X: P4; Q9X: P5) mutations have impaired cytokine production in response to IL-1β, IL-18, LPS, PAM3CSK4, Zymozan, and poly(I:C). Even P2′ father with heterozygous S32I mosaicism had an impaired response to LPS, PAM3CSK4 and Zymosan. However, the TLR7 pathway seems to be intact, based on the normal activation observed after stimulation with the synthetic and TLR7-specific agonist 3M-13, but this pathway has been tested only in the cells of P3 (W11X). Investigations of leukocyte TNFR responses have been carried out with TNFα (TNFR1) and CD40L (CD40). Cytokine production in response to TNFα was impaired in leukocytes from P7 (M37K; whole blood). PBMCs from the individual with S32I mosaicism also had impaired responses to TNFα. The cooperation between B and T cells is dependent on the interaction between CD40 and CD40L. Following CD40L stimulation, EBV-B cells from the patient (P4) with the E14X mutation displayed impaired translocation of p50/p65 to the nucleus. Consistent with this finding, the EBV-B cell response to CD40L was also impaired in a patient with a heterozygous S36Y mutation (P6), as demonstrated by the lower surface induction of NF-κB-dependent co-stimulatory molecules (such as CD23, CD54) on B cells [28]. A similar activation defect has also been detected in nfkbiaWT/S32I mice [36]. The induction of NF-κB target genes has not been investigated in any of the patients with heterozygous nonsense mutations. TCR and BCR also signal through IκBα via an indirect pathway controlled by a signalosome consisting of a CARD11, BCL-10, and MALT-1 (CBM) complex [46] (Figure 2). Only two patients with missense mutations (S32I: P1; S36Y: P9) have been tested for T-cell activation in response to TCR stimulation (anti-CD3 (α-CD3), α-CD3/α-CD28, Bacille Calmette Guérin (BCG)). In cells from the patient with the S32I mutation, stimulation with α-CD3 or α-CD3/α-CD28 led to normal IFN-γ production. However, in the presence of BCG, cells from the patient with the S36Y mutation (P9) displayed low levels of IL-12 or IFN-γ production (Table 2). In purified splenic T cells from nfkbiaWT/S32I mice produced normal amounts of IL-2 upon α-CD3 stimulation, but half the normal amount upon stimulation with α-CD3/α-CD28. Finally, B-cell activation in response to BCR engagement was not investigated in any of the patients. Overall, despite methodological differences between studies (stimulation or read-out), all the patients display impaired induction of NF-κB-dependent target genes, with the exception of the response to TCR engagement for the patient with the S32I mutation. However, as this assay was not used in any of the other reports, and only few patients and experimental approaches tested, it is too early to conclude that TCR pathways are potentially intact in these patients.
5. Immunological phenotypes: innate and intrinsic immunity in heterozygous patients
The impaired activation of NF-κB in these patients leads to a broad immunological phenotype. In the myeloid lineage, normal monocyte counts have been reported for patients with missense (S32I: P1, P2; S32G: P10; S36Y: P6) or nonsense (Q9X: P5) mutations (Table 3). Neutrophil levels were high, whereas eosinophil and basophil levels were not, in P9 (S36Y) but in the normal ranges for P10 (S32G); no data have been reported for the other patients. Granulocytes were normal in nfkbiaWT/S32I mice. Dendritic cell counts were not obtained for any of the patients. In nfkbiaWT/S32I mice, myeloid and plasmacytoid dendritic cell counts were similar to those in their WT littermates, but lymphoid DC counts were lower. NK cell counts were low in two (S36Y: P6; E14X: P4) and high in one (W11X: P3) of the nine patients tested (S32I: P2; S32G: P10; S32R: P11, S32N: P12, S36Y: P9, M37R: P8). Only one patient (S32I: P1) has been assessed for NK function and which was normal (Table 3). No proportions or counts have been reported for innate lymphoid cells in these patients. Given the ubiquitous expression of IκBα and NF-κB, and the strong phenotype of dermal fibroblasts, it is reasonable to assume that most, if not all, cells and tissues in these patients display some form of cell-intrinsic disorder that may facilitate the development of certain infections. However, the ability of fibroblasts to control invading microorganisms, such as viruses, was not tested. Moreover, as discussed below, the apparently favorable outcome of HSCT suggests that immunodeficiency may result mostly from innate or adaptive leukocyte disorders.
Table 3.
Immunological features of patients with heterozygous NFKBIA mutation
| Patient | Total monocytes /granulocytes (counts) |
Total lymphocytes (counts) |
T-cell count (count; %) naïve–memory (subset) ϒ/δ T cells |
NK (count; %) |
Total B-cell (count;%) Memory B cells |
Igs | Production of specific Ab |
Other |
|---|---|---|---|---|---|---|---|---|
| P1-S32I | nr | High (18,000–22,900) | High High–Low (both) Low |
In range (nr; 10%) | High (6,412, 28%) nr |
High IgM Low IgG |
Anti-diphtheria (−) Anti-tetanus (−) |
Anemia Chronic diarrhea |
| P2-S32I | In range (1,400) nr |
High (14,300) | High (13,600; 95%) High – in range (both) Low |
nd | Low (400;2.7%) nr |
High IgM Low IgG |
Anti-diphtheria (−) Anti-tetanus (−) Anti-Hib (−), Anti-poliovirus (−) |
Chronic diarrhea Neuromotor development |
| P3-W11X | nr | High (12,000) | High (6360; 58%) nr nr |
High (424; 4%) | High (4,240;38%) nr |
Low IgM High IgA |
Anti-glycans (−) Anti-tetanus (+) |
nr |
| P4-E14X | nr | In range (nr) | In range In range - in range Low |
Low (nr; 0.06%) | In range (nr) nr |
In range | Negative | Chronic diarrhea |
| P5-Q9X | In range (1.270) nr |
In range (11,230) | In range (nr; 59%) nr nr |
nd | High (nr; 34%) nr |
High IgA | nr | Inflammatory bowel disease |
| P6-S36Y | In range (987) High neutrophils (15,848) |
In range (2,457) | In range (2246; 66%) In range-Low (CD8) Low |
Low (63; 1.8%) | High (1,075; 31%) Low |
High IgG High IgA High IgD |
nr | Systemic inflammation, |
| P7-M37K | nr | High (nr) | High (4,973;94%) High (CD4)-in range (both) Low (30; 0.6%) |
nd | In range (331; nr) No switched memory |
High IgM Low IgG Low IgA |
Anti-diphtheria (−) Anti-tetanus (−), Anti-Hib (−) Anti-pneumococcus (−) |
Auto-immune thyroidis Hypopituitarism Chronic diarrhea |
| P8-M37R | nr | Not interpretable | nr (nr; 67%) nr nr |
In range | High (nr, 32%) Low |
High IgM High IgA |
Anti-tetanus (−) Anti-Hib (−) |
Chronic diarrhea |
| P9-S36Y | nr | In range (7,496) | In range (4556,62%) In range - in range In range |
In range | In range nr |
High IgG High IgA |
nr | Lupus |
| P10-S32G | In range (1,410) In range (neutrophils; 4,680) |
High (9,860) | In range (3,884) In range – Low (CD8) nr |
In range | High (2,529) Low No switched |
In range IgG No IgA In range IgM |
Anti-tetanus (+) Anti-Hib (−) Anti-pneumococcus (+) |
Chronic diarrhea Erythematous and purple skin rash |
| P11-S32R | nr | High (26,300) | (nd; 78%) High– (CD4) Low (CD4) Low |
In range (nr; 1.1%) | nd (nr;19.8%) Low (1.4%) |
Low IgM Low IgG Low IgA |
nr | Fever Erythema Auto-inflammation |
| P12-S32N | nr | High (20,700) | (nd; 91%) High– (CD4) Low (CD4) Low |
In range (nr; 1.1%) | nd (nr;2.4%) Low (1.1 %) |
Low IgM Low IgA |
nr | Fever Diarrhea Auto-inflammation Inflammatory bowel disease |
| P13-S32I | nr | nr | nr | nr | nr | nr | nr | nr |
| P14-G33V | nr | nr | nr | nr | nr | nr | nr | nr |
Note: P2′ father with S32I mosaic mutation has normal cell counts. He developed systemic juvenile idiopathic arthritis at 6 yo. All the values are expressed per mm3. nr: not reported ; Hib: Haemophilus influenzae b
6. Immunological phenotypes: adaptive immunity in heterozygous patients
Whole-blood formula determinations and immunophenotyping showed that six patients had T lymphocytosis (S32I: P1, P2; S32G: P10; S32R: P11; S32N: P12; M37K: P7; Table 3). Not coincidentally, these same patients excepted P10 (S32G) also had an excess of naïve CD4 and CD8 T cells, with low proportions of memory T cells in three patients (S32I: P1; S32R: P11; S32N: P12) and a normal memory T-cell count in the remaining patient (P2: S32I). A similar phenotype was observed in nfkbiaWT/S32I mice. One of the three patients with a nonsense mutation (W11X: P3) also presented T lymphocytosis with a high count of CD4 T cells. Six of the eight patients with missense mutations tested also had low proportions or a complete absence of γ/δ T cells (S32I: P1, P2; S32R: P11; S32N: P12; S36Y: P6; M37K: P7; Table 3), as did the only patient with a nonsense mutation (E14X: P4) tested. One patient (M37K: P7) had a low proportion of Th17 cells in an ex vivo experiment, but this aspect was not investigated for any of the other patients. Interestingly, naïve CD4 T-cell polarization to Th1 and Th17 in vitro was impaired for nfkbiaWT/S32I mice [36].
The in vitro proliferation of T cells following TCR engagement has been studied in detail. The stimulation with α-CD3 of PBMCs from patients showed proliferation to be impaired in all studied patients with missense mutations (S32I: P1, P2; S36Y: P6, P9; M37K: P7), even at low doses of α-CD3 in four cases (<1 μg/mL α-CD3; S32I: P1, P2; S36Y: P6; M37K: P7; Table 2). The patient with the S36Y mutation (P6) had normal T-cell proliferation rates at high doses (>1 μg/mL α-CD3). The addition of a co-stimulatory signal (α-CD28) to α-CD3 stimulation abolished the T cell proliferation defect for all patients with missense mutations (S32I: P1; M37R: P8; S36Y: P9). A similar pattern has been observed in nfkbiaWT/S32I mice, with a slight decrease in T-cell proliferation following stimulation with α-CD3, but normal T cell proliferation in the presence of α-CD3 and α-CD28. Only one patient with a nonsense mutation (W11X: P3) was tested, and found to have a normal T-cell proliferation upon stimulation with α-CD3, with or without α-CD28. Following incubation with lectin phytohemagglutinin A (PHA) or concanavalin A (ConA), the PBMC from one of the eight patients with missense mutations (S32I: P2) tested displayed a proliferation defect. T cells from three patients with nonsense mutations were studied. Only those from the patient with the Q9X mutation had a lower rate of proliferation than control cells. T-cell proliferation in response to recall antigen stimulation was abolished in two patients (S32I: P1, P2) and occurred at an intermediate rate in P2′ father (S32I mosaic). T-cell proliferation in response to allogeneic cell stimulation was normal in the only patient tested (S32I: P1). Patients with other missense (S36Y: P6; M37K: P7) or nonsense (W11X: P3: E14X: P4) mutations display normal T-cell proliferation under these stimulation conditions. Overall, these data strongly suggest that the T-cell function mediated by TCR engagement is impaired, but not abolished in these patients. However, comparisons of individuals with the same mutation have revealed no obvious genotype/phenotype correlations.
GOF NFKBIA mutations also have a major impact on the B-cell compartment (Table 2). Six of the patients with these mutations had an abnormally large total number of B cells (S32I: P1; S32G: P10; S36Y: P6; M37R: P8; W11X: P3; Q9X: P5) and those tested for memory B cells were found to have low counts, unswitched or a total absence of these cells (S32G: P10; S32R: P11; S32N: P12; S36Y: P6; M37K: P7; M37R: P8) (Table 3). Dysgammaglobulinemia was detected in eight of 10 patients (S32I: P1, P2; S32G: P10; S36Y: P6, P9; M37K: P7; M37R: P8; W11X: P3; Q9X: P5), and, when reported, these patients displayed only low levels or no Ab against vaccine antigens (Table 3). Diagnostically, GOF NFKBIA mutations thus underlie a combined immunodeficiency [47, 48] with an additional layer of impaired innate immunity, affecting myeloid cells in particular, but also lymphoid cells. It is a combined immunodeficiency in another sense, in that it affects both innate and adaptive immunity.
7. Developmental manifestations in heterozygous patients
The disruption of NF-κB activity downstream from TNFR family members such as EDAR and RANK leads to developmental phenotypes. All but one of the fourteen patients with a germline NFKBIA mutation (the exception being P9, with the S36Y mutation) have some features of EDA (Table 4). The S32I mosaic individual (P2′ father) displayed no signs of EDA. Hypohidrosis or anhidrosis, resulting from the absence of a functional sweat gland, has been observed in nine of the eleven documented patients (S32I: P1; S32R: P11; S32N: P12; S36Y: P6, M37K: P7, M37R: P8, Q9X: P5, W11X: P3, E14X: P4). An absence of teeth, their late eruption and a peg-shaped or pointed appearance of the teeth were also documented in 8 (S32I: P1, P2, S32G: P10, S32R: P11; S32N: P12; M37K: P7, Q9X: P5, W11X: P3) of 10 documented patients (Table 4). Finally, seven of nine documented patients had sparse hair (S32I: P1; S32R: P11; S32N: P12; S36Y: P6, M37K: P7, M37R: P8, W11X: P3). The absence of EDA in father’ P2 (S32Im), who presented a complex mosaicism of the GOF mutation, resulted from the higher proportion of cells with the WT/WT genotype than of those with the WT/S32I genotype (4:1). However, it remains unclear why P9, who has the same mutation as P6 (S36Y), has not developed signs of EDA. Finally, four patients with missense mutations (S32I: P1, P2; S36Y: P6; M37K: P7) and one with a nonsense mutation (E14X: P4) also had growth retardation (SD-1 to SD-2.5); growth data were not reported for the other patients. The nfkbiaWT/S32I mice have most of the developmental features of EDA, although they do not have osteopetrosis or lymphedema [36]. Mice and humans heterozygous for NFKBIA GOF mutations do not seem to display other developmental features (e.g. neurological features), at least early in life.
Table 4.
Developmental and infection features of patients with heterozygous GOF NFKBIA mutations
| Patient | Growth dev. | EDA | Sparse hair | Hypo hidrosis | Conical teeth | Peripheral nodes | First infection (age in months) | Infection | Chronic diarrhea | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| P1-S32I | Retardation (SD-2.5) | Y | +/− | Y | Y | N | 2 | Recurrent LRTI: P. aeruginosa, Klebsiella, Serratia, S. aureus CMC |
Y | Alive |
| P2-S32I | Retardation (SD-1) | Y | nr | nr | Y | N | 2 | Meningitis: β-hemolytic group A Streptococcus: sepsis respiratory infection pneumonia Pneumocystis jirovecii, mild CMC | Y | Dead*** |
| P3-W11X | nr | Y | Y | Y | Y | Y | 2 | Recurrent pneumonia | N | Alive |
| P4-E14X | Retardation | Y | nr* | Y | nr* | Y | 2 | Pyogenic bacteria sepsis, CMC Parainfluenza virus, Pneumocystis jirovecii : Pneumonia, respiratory distress |
Y | Dead** |
| P5-Q9X | nr | Y | nr | Y | Y | Y | 1 | Bacterial : pneumonia, respiratory syncytial virus Bronchiolitis, acute otitis media, urinary tract infection Cytomegalovirus : hepatitis, Rotavirus: enteritis Bronchiolitis with respiratory syncytial virus |
Y | Alive*** |
| P6-S36Y | Retardation | Y | Y | Y but presence of sweat gland | N | small | 4 | Unknown: perianal abscess BCG: skin abscess |
Y | Dead** |
| P7-M37K | Retardation | Y | Y | Y | Y | N | 6 | Haemophilus influenza: pneumonia, CMC | Y | Dead** |
| P8-M37R | nr | Y | Y | Y | nr* | nr | 5 | Recurrent LRTI sepsis: K. Klebsiella pneumonia, Candida parapsilosis: Stenotrophomonas maltophilia, Osteomyelitis of skull and limb, CMC |
Y | Dead |
| P9-S36Y | nr | N | N | N | N | nr | 1 | LRTI : P. aeruginosa: bronchiectasis and sinusitis Mycobacterium tuberculosis: abdominal lymphadenopathy M. abscessus : septic arthritis of the knee, osteomyelitis Urinary tract infection: K. pneumoniae |
N | Alive |
| P10-S32G | N | Y | N | N | Y | nr | 20 | Salmonella enteritidis: osteomyelitis, hematochezia Candida : Oesophagitis Mycobacterium malmoense : blood and skin Sapovirus and norovirus : stool |
Y | Alive |
| P11-S32R | nr | Y | Y | Y | Y | N | 2 | S. aureus sepsis, CMC, Recurrent pneumonia | nr | Dead** |
| P12-S32N | nr | Y | Y | Y | Y | N | 1 | S. aureus and P. aeruginosa sepsis | Y | Dead** |
| P13-S32I | nr | Y | nr | nr | nr | N | nr | Recurrent infections | nr | Alive |
| P14-G33V | nr | Y | nr | nr | nr | N | nr | Recurrent infections | nr | Alive |
Note: P2′ father with S32I mosaic mutation developed at 1.5 yo Salmonella typhimurium enteritis.
Too young for an evaluation of the teeth.
after HSCT; nr : not reported, LRTI: lower respiratory tract infection (LRTI),
personal communication, CMC: chronic mucocutaneous candidiasis
8. Infectious manifestations in heterozygous patients
These patients presented a broad spectrum of severe infectious diseases, consistent with the breadth and depth of the immunological and cellular phenotypes. Most patients presented multiple, severe infections caused by bacteria, fungi and viruses, beginning at an early age, typically before the age of three months (Table 4). Invasive and non-invasive bacterial infections have been reported in 11 of the 12 documented patients. Recurrent upper respiratory tract infections or pneumonia due to Klebsiella, Pseudomonas aeruginosa or Haemophilus influenzae have been reported in nine of the 12 patients (S32: P1, P2; S32R: P11; S32N: P12; S36Y: P9; M37K: P7; M37R: P8; Q9X: P5; W11X: P3), sepsis or meningitis due to Streptococcus A, K. pneumoniae or Staphylococcus aureus has been reported in five cases (S32I: P2; S32R: P11; S32N: P12; M37R: P8; E14X: P4), (Table 4) [22–24, 49]. This susceptibility may be due to impairment of the ability of naïve B cells to switch and differentiate into memory B cells, leading to low levels of Ab production [50]. These patients display impairments of both T-independent and T-dependent B-cell immunity; they failed to mount strong antibody responses to vaccine antigens. This high susceptibility may also be associated with the impaired response to TIR pathways, as observed in patients with MyD88 or IRAK-4 deficiency [51–54]. The P2′ father with mosaicism for the heterozygous S32I mutation had enteritis caused by Salmonella typhimurium at the age of 20 years. S32G (P10) also had recurrent Salmonella enteritidis extra-intestinal infection. Three patients had mycobacterial infection caused by the BCG vaccine (skin abscess in P6: S36Y), Mycobacterium abscessus, and M. tuberculosis (septic arthritis of the knee, osteomyelitis and abdominal lymphadenopathy, P9: S36Y) or M. malmoense (in the blood and in the skin culture, P10: S32G) [28, 29]. Interestingly, two of the patients carried the same mutation (S36Y). Susceptibility to mycobacteria may be related to a defect of T-dependent IL-12-producing monocytes, leading to impaired IFN-γ secretion by T cells, as shown in NEMO-deficient patients [55–58]. Impaired Th1 cell differentiation as observed in nfkbiaWT/S32I mice may also be relevant [58]. The patients are also prone to fungal infections; seven patients have chronic mucocutaneous candidiasis (CMC) (S32I: P1, P2; S32G: P10; S32R: P11; M37K: P7; M37R: P8; E14X: P4) and two of these patients have also had pulmonary pneumocystosis (S32I: P2; E14X: P4) (Table 4). Two non-exclusive factors may underlie CMC in these patients. The response of endothelial cells to IL-17A/F may be defective, although this has not been explored [59]. Alternatively, a T-cell activation defect detected in some patients and low Th17 cell counts in one patient (M37K: P7) may be involved [36]. Pneumocystosis is probably more likely to occur in patients with impaired CD40 responses, as this infection is commonly seen in patients with CD40 or CD40L deficiency [60, 61]. Three patients had severe viral infections caused by rotavirus, norovirus, sapovirus, parainfluenza virus, RSV, and CMV (S32G: P10; E14X: P4; Q9X: P5). The production of type I and type II IFN has not been investigated, but the key role of NF-κB in inducing IFNs [62], and the results of studies conducted in patients with hypormorphic NEMO deficiency [63] suggest that NFKBIA GOF may decrease the production of anti-viral IFNs [64, 65].
9. Other immunological manifestations
Seven patients with missense mutations (S32I: P1, P2, S32R: P11; S32N: P12; P13; S32G: P10; M37K: P7) apparently lacked peripheral nodes (LNs), even during regional infection, whereas another had very small LNs (S36Y: P6). In contrast, all patients with nonsense mutations (Q9X, W11X, E14X) apparently had LNs. Similarly, in nfkbiaWT/S32I mice, a complete absence of LNs was associated with an inability to respond to T-independent and T-dependent antigens and to form germinal centers [36]. Eight patients presented recurrent diarrhea and/or colitis, the inflammatory or infectious nature of which was unclear (Table 3) [16, 22–29]. It may be no coincidence that nfkbiaWT/S32I mice have no Peyer’s patches in small intestine, but these mice have not been reported to have diarrhea or colitis. This lymphoid structure has not been investigated in patients with NFKBIA GOF. Three patients have been found to display auto-inflammation (S36Y: P6; S32R: P11; S32N: P12), and the relative with complex mosaicism (S32Im) had juvenile idiopathic arthritis. One of the patients has been reported to have auto-immunity (M37K: P7) but none to have cancer. The broad clinical and infectious phenotype of these patients reflects the large impact of IκBα on innate, adaptive and non-hematopoietic cells.
10. Treatment and outcome of heterozygous patients
Seven of the fourteen patients had already died (6 after HSCT) and seven were still alive (5 after HSCT) at the time at which they were reported (Table 5). Eleven of the fourteen patients received HSCT (S32I: P1; P2, P13; S32G: P10; S32R: P11; S32N: P12; G33V: P14; S36Y: P6; M37K: P7; Q9X: P5; E14X: P4). Six died during or after the transplantation period, from bacterial sepsis (S32R: P11; M37K: P7; E14X: P4), progressive neurodegenerative disease (Dr Lankester, personal communication; S32I: P2), acute respiratory distress (S32N: P12), or cerebellar hemorrhage (S36Y: P6). Five cases of successful HSCT have been reported: the first of these patients (S32I: P1) is now 21 years old and underwent haplo-identical HSCT at the age of one year ([49] and Dr Cancrini, personal communication); the second patient (Q9X: P5) is 7 years old and received HSCT from an unrelated donor at the age of two years [66]; the third one (S32G: P10) is 6 years old and received HSCT from an unrelated donor at the age of four years [31]. The two last patients (S32I: P13; G33V: P14) are both 10 years old and received the HSCT 3 and 8 years earlier, respectively [32]. All still have the EDA phenotype. P1, P13 (S32I), P10 (S32G) and P14 (G33V) still have persistent partial immunodeficiency (recurrent infections, diffuse cutaneous warts and chronic diarrhea) on intravenous Immunoglobulins (IVIG), whereas P5 (Q9X) is free of treatment. The remaining two patients, now aged 9 and 21 years (W11X: P3, S36Y: P9), are not scheduled to undergo HSCT, in one case because the clinical features were not severe enough (P3), and in another because of the family’s reservations (P9). However, these two patients are on IVIG, and P9 (S36Y) has been receiving anti-tuberculosis treatment, IFN-γ injections and anti-bacterial treatment since a recurrence of infection when she was off all treatment. The patient chimeric for the S32I allele is not on treatment.
Table 5.
Outcome of patients with heterozygous NFKBIA mutations
| Patient | Status (year of the report) | HSCT (age) | HSCT | Outcome and treatment |
|---|---|---|---|---|
| P1-S32I | Alive at 21 y (2017*) | Yes (1 y) | Haplo-identical (mother) HSCT | IVIG With diarrhea, warts, recurrent respiratory infection |
| P2-S32I | Dead (2005*) | Yes (2.5 y) | Matched unrelated donor. Died 4 months post-transplant, from progressive tetraplegia and axial hypertonia, and progressive developmental regression; generalized atrophy without focal or structural abnormalities was observed pre-transplant | |
| P3-W11X | Alive at 22 y (2016*) | No | IVIG | |
| P4-E14X | Dead (2008) | Yes (7 mo) | Allogeneic cord blood Died during the peri-transplant period from Gram-negative sepsis |
|
| P5-Q9X | Alive at 7 y (2016*) | Yes (1.4 y) | Unrelated donor: mixed chimera (80 :20) | Off treatment, no medication |
| P6-S36Y | Dead at 6 y (2013) | Yes (6 y) | Allogeneic BMT from an HLA-DRB1 mismatched unrelated donor Full donor chimerism at day 25 (graft versus host disease and ITP), Died on day 211, from cerebellar hemorrhage |
|
| P7-M37K | Dead (2013) | Yes (4.9 y) | Full donor chimerism Died 18 months post HSCT, from bacterial sepsis and pericarditis due to E. faecium and A. lwofii |
|
| P8-M37R | Dead at 1 y (2013) | No | Died just before the scheduled HSCT | |
| P9-S36Y | Alive at 9 y (2015) | No | Subcutaneous rIFN-γ (50 μg/m2), cotrimoxazole, IVIG, anti-tuberculosis treatment | |
| P10-S32G | Alive at 6 y (2017) | Yes (4 y) | Unrelated donor (9/10) ; Chimerism dropped from 100% to 1% (CD15) and 40% (CD3) | Severe auto-immunity IVIG |
| P11-S32R | Dead* | Yes (8 mo) | Cord blood - Died 1y 6mo later from sepsis | |
| P12-S32N | Dead* | Yes (1 y) | Cord blood – Died on day 11 from acute respiratory distress and sepsis | |
| P13-S32I | Alive at 10 y (2017) | Yes (7 y) | nr | IVIG |
| P14-G33V | Alive at 10y (2017) | Yes (2 y) | nr | IVIG |
personal communication Dr Cancrini (P1), Dr Lankester (P2) Drs McDouglas and Geha (P3), Drs Onhishi and Okada (P5), Drs Moriya and Morio (P11, P12), nr: not reported
Antibiotic prophylaxis with cotrimoxazole and/or penicillin and anti-fungal treatment with fluconazole should be proposed (in the absence of allergy), to prevent bacterial and fungal infections. Moreover, despite the impairment of B-cell immunity in these patients, they should be immunized with S. pneumoniae conjugated and non-conjugated vaccines, H. influenzae conjugated vaccine and N. meningitidis conjugated and non-conjugated vaccines. If no specific Abs are produced, intravenous or subcutaneous IgG substitution should be carried out. These patients should not receive live vaccines (BCG, attenuated Polio, Measles, Mumps, Rotavirus, Rubella and Varicella viruses); one patient developed BCG-osis [28]. Finally, patients with heterozygous NFKBIA mutations should be started on empiric parenteral antibiotic treatment against K. pneumoniae, S. aureus, P. aeruginosa and β–hemolytic Streptococcus as soon as an infection is suspected. A secondary adaptation of antibiotic treatment should be carried out once the causal bacterium has been documented. In cases of mycobacterial infection, long-term anti-mycobacterial treatment associated with rIFN-γ should be initiated. The success of HSCT in five patients (P1, P5, P10, P13 and P14) suggests that this treatment can be proposed [32, 49, 66]. However, HSCT should correct the hematopoietic immunity, both innate and adaptive, but cannot correct defects of non-hematopoietic compartments, including those involved in cell-autonomous, intrinsic immunity and the development of certain lymphoid organs, not to mention the signs of EDA. This point has been elegantly demonstrated by the engraftment of WT cells into nfkbiaWT/S32I mice. After transplantation, the mice had normal hematopoietic cell counts and function, but no LNs (and germinal centers). The premature death of two of the patients from bacterial infection (including by commensals) after HSCT and the lifelong need for IV-IgG treatment in three of the patients in whom transplantation was successful support this view. It is therefore important to keep these patients on long-term prophylaxis after HSCT. In EDA-ID patients mutated in NEMO, whose clinical features are extremely variable, the indication of HSCT should be discussed on a case-by-case basis, depending on the patient’s genotype and phenotype. Patients with GOF mutations in IκBα are clinically heterogenous too, although to a much lesser degree. They indication of HSCT should be discussed on a case-by-case basis, depending on the patient’s genotype (missense vs. nonsense mutation) and phenotype (immunological and clinical).
Concluding remarks
NFKBIA germline mutations have been reported in 14 patients. The 14 patients displayed a broad spectrum of severe infections. With hindsight, the initial description of the first patient proved to be both accurate, in terms of immunological and clinical phenotypes, and thus helpful, for the diagnosis and management of subsequent cases [67, 68]. This description proved to be accurate, because the principal findings of the first paper were confirmed to be robust in all the other patients identified later on, highlighting the utility and importance of single-patient genetic studies [67]. However, as expected, the description of other patients revealed some degree of inter-individual variability (Table 6). The clinical and immunological features of these patients seem to depend in part on the type of mutation (missense versus nonsense). The nonsense mutations, especially those encoding by re-initiation of translation proteins that are GOF but poorly expressed, seem to underlie less severe phenotypes at the cellular and clinical levels, when compared with missense mutations (Table 6). The broad and profound immunological phenotypes of patients with heterozygous NFKBIA mutations are responsible for the broad susceptibility to infection with invasive pyogenic bacteria (meningitis, sepsis, arthritis, osteomyelitis and abscesses), environmental mycobacteria, fungi, and, to a lesser extent, parasites and viruses. The clinical consequences of their immunological phenotypes also appear to be largely restricted to infectious diseases. The patients display no major signs of allergy, autoinflammation, virus-induced cancer or autoimmunity, although this may be due to premature death or treatment by HSCT. Other extra-hematopoietic manifestations, reflecting the breadth of NF-κB physiology are also present. With the recognition of infectious diseases as monogenic inborn errors of immunity, the description of mutations in other genes, upstream or downstream from IκBα, should gradually elucidate the pathogenesis of other infectious and immunological phenotypes affecting EDA-ID patients [69–71], as illustrated by the discovery of mutations in the BCL10-CARD core complex [72] or in the TIR pathway [51–53, 73–75]. These mutations provide explanations for some of the immunological and clinical phenotypes common to patients with hypomorphic IKBKG/NEMO mutations or hypermorphic NFKBIA/IκBα mutations, such as excessively high numbers of naïve B and T cells. Future discoveries of RelA, c-REL, or RelB deficiency, or deficiencies of other IκBs, IL-1Rs, TLRs or TNFRs should clarify the contribution of each of these molecules to human immunity and physiology.
Table 6.
Summary of the clinical and immunological features with heterozygous NFKBIA mutations
| Patients with features/Total studied patients | Percentage | |||
|---|---|---|---|---|
| Missense mutation | Non-sense mutation | Total patients | ||
| Clinical features | ||||
| Ectodermal dysplasia | 10/11 | 3/3 | 13/14 | 93 % |
| Anhidrosis or hypohidrosis | 6/8 | 3/3 | 9/11 | 81 % |
| Conical teeth | 6/8 | 2/2 | 8/10 | 80 % |
| Sparse hair | 6/8 | 1/1 | 7/9 | 78 % |
| Growth retardation | 4/5 | 1/1 | 5/6 | 83 % |
| Absence of peripheral nodes | 7/7 | 0/3 | 7/10 | 70 % |
| Auto-inflammation | 3/9 | 0/2 | 3/11 | 27 % |
| Chronic diarrhea | 6/9 | 1/2 | 7/11 | 64 % |
| Pyogenic bacteria | 8/9 | 3/3 | 11/12 | 91 % |
| Mycobacteria | 3/9 | 0/3 | 3/12 | 27 % |
| Fungi | 6/9 | 1/3 | 7/12 | 58 % |
| Viruses | 1/9 | 2/3 | 3/12 | 27 % |
| Other clinical manifestations | ||||
| Auto-immunity | 1/9 | 0/2 | 1/11 | 9 % |
| Inflammatory bowel disease | 1/9 | 1/2 | 2/11 | 18 % |
| Immunological features | ||||
| Lymphocytosis | 6/8 | 1/3 | 7/11 | 64 % |
| T-cell lymphocytosis | 6/8 | 1/3 | 6/11 | 54 % |
| B-cell lymphocytosis | 4/9 | 2/3 | 6/12 | 50 % |
| Abnormal Ig level | 9/9 | 2/3 | 11/12 | 92 % |
| High IgM (and/or) low IgG/A | 5/9 | 0/3 | 5/12 | 42 % |
| Low IgM (and/or) high IgG/A | 4/9 | 2/3 | 6/12 | 50 % |
| Low or absence of antibody production after immunization | 5/5 | 2/2 | 7/7 | 100 % |
Acknowledgments
We would first like to thank Dr. Cancrini (P1), Dr. Lankester (P2), Drs. Geha and McDouglas (P3), Drs. Onhishi and Okada (P5), and Drs. Moriya and Morio (P11, P12) for taking the time to answer our questions and sharing information about the reported patients with us. We also thank Dr. Alain Israel for careful review of the manuscript. We thank Stéphanie Boisson-Dupuis, Jacinta Bustamante, Michael Ciancanelli, Emmanuelle Jouanguy, and Shen-Ying Zhang of the Human Genetics of Infectious Diseases Laboratory for helpful discussions. We also thank Maya Chrabieh, Yelena Nemiroskaya, Lahouari Amar, Dominick Papandrea, Mark Woollett, Cécile Patissier and Céline Desvallees for their assistance. This work was supported by the St. Giles Foundation, the Rockefeller University, INSERM, Paris Descartes University, Howard Hughes Medical Institute, National Institutes of Health (NIH 5P01AI061093), and the French National Research Agency (ANR 14-CE15-0009-01).
Footnotes
Conflict of interest statement
The authors declared that they have no conflict of interest.
References
- 1.Abinun M. Ectodermal dysplasia and immunodeficiency. Arch Dis Child. 1995;73(2):185. doi: 10.1136/adc.73.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abinun M, Spickett G, Appleton AL, Flood T, Cant AJ. Anhidrotic ectodermal dysplasia associated with specific antibody deficiency. European journal of pediatrics. 1996;155(2):146–7. doi: 10.1007/BF02075774. [DOI] [PubMed] [Google Scholar]
- 3.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27(3):277–85. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 4.Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67(6):1555–62. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain A, Ma CA, Liu S, Brown M, Cohen J, Strober W. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2(3):223–8. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 6.Smahi A, Courtois G, Vabres P, Yamaoka S, Heuertz S, Munnich A, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature. 2000;405(6785):466–72. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- 7.Israel A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harbor perspectives in biology. 2010;2(3):a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niehues T, Reichenbach J, Neubert J, Gudowius S, Puel A, Horneff G, et al. A NEMO-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:1456–62. doi: 10.1016/j.jaci.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Orange JS, Jain A, Ballas ZK, Schneider LC, Geha RS, Bonilla FA. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. J Allergy Clin Immunol. 2004;113(4):725–33. doi: 10.1016/j.jaci.2004.01.762. [DOI] [PubMed] [Google Scholar]
- 10.Ku CL, Dupuis-Girod S, Dittrich AM, Bustamante J, Santos OF, Schulze I, et al. NEMO mutations in 2 unrelated boys with severe infections and conical teeth. Pediatrics. 2005;115(5):e615–9. doi: 10.1542/peds.2004-1754. [DOI] [PubMed] [Google Scholar]
- 11.Puel A, Reichenbach J, Bustamante J, Ku CL, Feinberg J, Doffinger R, et al. The NEMO mutation creating the most-upstream premature stop codon is hypomorphic because of a reinitiation of translation. Am J Hum Genet. 2006;78(4):691–701. doi: 10.1086/501532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ku CL, Picard C, Erdos M, Jeurissen A, Bustamante J, Puel A, et al. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J Med Genet. 2007;44(1):16–23. doi: 10.1136/jmg.2006.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson EP, Monaco-Shawver L, Solt LA, Madge LA, Banerjee PP, May MJ, et al. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122(6):1169–77. e16. doi: 10.1016/j.jaci.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clinical microbiology reviews. 2011;24(3):490–7. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fusco F, Pescatore A, Conte MI, Mirabelli P, Paciolla M, Esposito E, et al. EDA-ID and IP, two faces of the same coin: how the same IKBKG/NEMO mutation affecting the NF-kappaB pathway can cause immunodeficiency and/or inflammation. International reviews of immunology. 2015;34(6):445–59. doi: 10.3109/08830185.2015.1055331. [DOI] [PubMed] [Google Scholar]
- 16.Courtois G, Smahi A, Reichenbach J, Doffinger R, Cancrini C, Bonnet M, et al. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. The Journal of clinical investigation. 2003;112(7):1108–15. doi: 10.1172/JCI18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devriendt K, Kim AS, Mathijs G, Frints SG, Schwartz M, Van Den Oord JJ, et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Genet. 2001;27(3):313–7. doi: 10.1038/85886. [DOI] [PubMed] [Google Scholar]
- 18.Puel A, Picard C, Ku CL, Smahi A, Casanova JL. Inherited disorders of NF-kappaB-mediated immunity in man. Current opinion in immunology. 2004;16(1):34–41. doi: 10.1016/j.coi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor perspectives in biology. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courtois G, Israel A. IKK regulation and human genetics. Curr Top Microbiol Immunol. 2011;349:73–95. doi: 10.1007/82_2010_98. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-kB: a blossoming of relevance to human pathobiology. Cell. 2017 doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen R, van Wengen A, Hoeve MA, ten Dam M, van der Burg M, van Dongen J, et al. The same IkappaBalpha mutation in two related individuals leads to completely different clinical syndromes. The Journal of experimental medicine. 2004;200(5):559–68. doi: 10.1084/jem.20040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald DR, Mooster JL, Reddy M, Bawle E, Secord E, Geha RS. Heterozygous N-terminal deletion of IkappaBalpha results in functional nuclear factor kappaB haploinsufficiency, ectodermal dysplasia, and immune deficiency. J Allergy Clin Immunol. 2007;120(4):900–7. doi: 10.1016/j.jaci.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Granados E, Keenan JE, Kinney MC, Leo H, Jain N, Ma CA, et al. A novel mutation in NFKBIA/IKBA results in a degradation-resistant N-truncated protein and is associated with ectodermal dysplasia with immunodeficiency. Human mutation. 2008;29(6):861–8. doi: 10.1002/humu.20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi H, Miyata R, Suzuki T, Nose T, Kubota K, Kato Z, et al. A rapid screening method to detect autosomal-dominant ectodermal dysplasia with immune deficiency syndrome. J Allergy Clin Immunol. 2012;129(2):578–80. doi: 10.1016/j.jaci.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 26.Giancane G, Ferrari S, Carsetti R, Papoff P, Iacobini M, Duse M. Anhidrotic ectodermal dysplasia: a new mutation. J Allergy Clin Immunol. 2013;132(6):1451–3. doi: 10.1016/j.jaci.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 27.Schimke LF, Rieber N, Rylaarsdam S, Cabral-Marques O, Hubbard N, Puel A, et al. A novel gain-of-function IKBA mutation underlies ectodermal dysplasia with immunodeficiency and polyendocrinopathy. Journal of clinical immunology. 2013;33(6):1088–99. doi: 10.1007/s10875-013-9906-1. [DOI] [PubMed] [Google Scholar]
- 28.Yoshioka T, Nishikomori R, Hara J, Okada K, Hashii Y, Okafuji I, et al. Autosomal dominant anhidrotic ectodermal dysplasia with immunodeficiency caused by a novel NFKBIA mutation, p.Ser36Tyr, presents with mild ectodermal dysplasia and non-infectious systemic inflammation. Journal of clinical immunology. 2013;33(7):1165–74. doi: 10.1007/s10875-013-9924-z. [DOI] [PubMed] [Google Scholar]
- 29.Lee AJ, Moncada-Velez M, Picard C, Llanora G, Huang CH, Abel L, et al. Severe Mycobacterial Diseases in a Patient with GOF IkappaBalpha Mutation Without EDA. Journal of clinical immunology. 2016;36(1):12–5. doi: 10.1007/s10875-015-0223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriya K, Tanita K, Ohnishi H, Ono S, Niizuma H, Rikiishi T, et al. IκBα S32 mutation underlies Ectodermal Dysplasia with Immunodeficiency and severe Non-Infectious Systemic Inflammation. European Society for Immunodeficiencies. 2016 [Google Scholar]
- 31.Staples E, Morillo-Gutierrez B, Davies J, Slatter M, Doffinger R, Hackett S, et al. Disseminated Mycobacterium malmoense and Salmonella infections associated with a novel variant in NFKBIA. Journal of clinical immunology. 2017 doi: 10.1007/s10875-017-0390-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersheim D, Massad MJ, Lee L, Cancrini C, Morio T, Sasahara Y, et al. Genotype-phenotype correlation in autosomal dominant ectodermal dysplasia with immune deficiency: More severe disease and greater impairment of NF-κB activation in IκBα point mutants versus truncation mutants. J Allergy Clin Immunol. 2017 Submitted. [Google Scholar]
- 33.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh S, Hayden MS. New regulators of NF-kappa B in inflammation. Nat Rev Immunol. 2008;8(11):837–48. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 35.Liang Y, Zhou Y, Shen P. NF-kappaB and its regulation on the immune system. Cell Mol Immunol. 2004;1(5):343–50. [PubMed] [Google Scholar]
- 36.Mooster JL, Le Bras S, Massaad MJ, Jabara H, Yoon J, Galand C, et al. Defective lymphoid organogenesis underlies the immune deficiency caused by a heterozygous S32I mutation in IkappaBalpha. The Journal of experimental medicine. 2015;212(2):185–202. doi: 10.1084/jem.20140979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annual review of immunology. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 38.Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes & development. 2012;26(3):203–34. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes & development. 1995;9(22):2736–46. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298(5596):1241–5. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 41.Memet S, Laouini D, Epinat JC, Whiteside ST, Goudeau B, Philpott D, et al. IkappaBepsilon-deficient mice: reduction of one T cell precursor subspecies and enhanced Ig isotype switching and cytokine synthesis. Journal of immunology. 1999;163(11):5994–6005. [PubMed] [Google Scholar]
- 42.Kanarek N, London N, Schueler-Furman O, Ben-Neriah Y. Ubiquitination and degradation of the inhibitors of NF-kappaB. Cold Spring Harbor perspectives in biology. 2010;2(2):a000166. doi: 10.1101/cshperspect.a000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanarek N, Ben-Neriah Y. Regulation of NF-kappaB by ubiquitination and degradation of the IkappaBs. Immunological reviews. 2012;246(1):77–94. doi: 10.1111/j.1600-065X.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 44.Smale ST. Dimer-specific regulatory mechanisms within the NF-kappaB family of transcription factors. Immunological reviews. 2012;246(1):193–204. doi: 10.1111/j.1600-065X.2011.01091.x. [DOI] [PubMed] [Google Scholar]
- 45.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21(1):71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wegener E, Krappmann D. CARD-Bcl10-Malt1 signalosomes: missing link to NF-kappaB. Sci STKE. 2007;2007(384):pe21. doi: 10.1126/stke.3842007pe21. [DOI] [PubMed] [Google Scholar]
- 47.Notarangelo LD, Kim MS, Walter JE, Lee YN. Human RAG mutations: biochemistry and clinical implications. Nat Rev Immunol. 2016;16(4):234–46. doi: 10.1038/nri.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bousfiha A, Jeddane L, Al-Herz W, Ailal F, Casanova JL, Chatila T, et al. The 2015 IUIS Phenotypic Classification for Primary Immunodeficiencies. Journal of clinical immunology. 2015;35(8):727–38. doi: 10.1007/s10875-015-0198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupuis-Girod S, Cancrini C, Le Deist F, Palma P, Bodemer C, Puel A, et al. Successful allogeneic hemopoietic stem cell transplantation in a child who had anhidrotic ectodermal dysplasia with immunodeficiency. Pediatrics. 2006;118(1):e205–11. doi: 10.1542/peds.2005-2661. [DOI] [PubMed] [Google Scholar]
- 50.Fried AJ, Bonilla FA. Pathogenesis, diagnosis, and management of primary antibody deficiencies and infections. Clinical microbiology reviews. 2009;22(3):396–414. doi: 10.1128/CMR.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321(5889):691–6. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299(5615):2076–9. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 53.Picard C, von Bernuth H, Ghandil P, Chrabieh M, Levy O, Arkwright PD, et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 2010;89(6):403–25. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Bernuth H, Picard C, Puel A, Casanova JL. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. Eur J Immunol. 2012;42(12):3126–35. doi: 10.1002/eji.201242683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Filipe-Santos O, Bustamante J, Haverkamp MH, Vinolo E, Ku CL, Puel A, et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. The Journal of experimental medicine. 2006;203(7):1745–59. doi: 10.1084/jem.20060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bustamante J, Picard C, Boisson-Dupuis S, Abel L, Casanova JL. Genetic lessons learned from X-linked Mendelian susceptibility to mycobacterial diseases. Annals of the New York Academy of Sciences. 2011;1246:92–101. doi: 10.1111/j.1749-6632.2011.06273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Seminars in immunology. 2014;26(6):454–70. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boisson-Dupuis S, Bustamante J, El-Baghdadi J, Camcioglu Y, Parvaneh N, El Azbaoui S, et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunological reviews. 2015;264(1):103–20. doi: 10.1111/imr.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12(6):616–22. doi: 10.1097/ACI.0b013e328358cc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen RC, Armitage RJ, Conley ME, Rosenblatt H, Jenkins NA, Copeland NG, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259(5097):990–3. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 61.Ferrari S, Giliani S, Insalaco A, Al-Ghonaium A, Soresina AR, Loubser M, et al. Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12614–9. doi: 10.1073/pnas.221456898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conzelmann KK. Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. J Virol. 2005;79(9):5241–8. doi: 10.1128/JVI.79.9.5241-5248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Audry M, Ciancanelli M, Yang K, Cobat A, Chang HH, Sancho-Shimizu V, et al. NEMO is a key component of NF-kappaB- and IRF-3-dependent TLR3-mediated immunity to herpes simplex virus. J Allergy Clin Immunol. 2011;128(3):610–7. e1–4. doi: 10.1016/j.jaci.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ciancanelli MJ, Huang SX, Luthra P, Garner H, Itan Y, Volpi S, et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348(6233):448–53. doi: 10.1126/science.aaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciancanelli MJ, Itan Y, Herman M, Audry M, Byun M, Sancho-Shimizu V, et al. Human Tlr3 Controls Constitutive Interferon-B Immunity. Journal of clinical immunology. 2012;32:22. [Google Scholar]
- 66.Nagasawa M, Ohkawa T, Takagi M, Imai K, Morio T. A stable mixed chimera after SCT with RIC in an infant with IkappaBa hypermorphic mutation. Journal of clinical immunology. 2017 doi: 10.1007/s10875-017-0375-9. in press. [DOI] [PubMed] [Google Scholar]
- 67.Casanova JL, Conley ME, Seligman SJ, Abel L, Notarangelo LD. Guidelines for genetic studies in single patients: lessons from primary immunodeficiencies. The Journal of experimental medicine. 2014;211(11):2137–49. doi: 10.1084/jem.20140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boisson B, Quartier P, Casanova JL. Immunological loss-of-function due to genetic gain-of-function in humans: autosomal dominance of the third kind. Current opinion in immunology. 2015;32:90–105. doi: 10.1016/j.coi.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Casanova JL. Severe infectious diseases of childhood as monogenic inborn errors of immunity. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(51):E7128–37. doi: 10.1073/pnas.1521651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casanova JL. Human genetic basis of interindividual variability in the course of infection. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(51):E7118–27. doi: 10.1073/pnas.1521644112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Su HC, Lenardo MJ. Genomics is rapidly advancing precision medicine for immunological disorders. Nat Immunol. 2015;16(10):1001–4. doi: 10.1038/ni.3275. [DOI] [PubMed] [Google Scholar]
- 72.Perez de Diego R, Sanchez-Ramon S, Lopez-Collazo E, Martinez-Barricarte R, Cubillos-Zapata C, Ferreira Cerdan A, et al. Genetic errors of the human caspase recruitment domain-B-cell lymphoma 10-mucosa-associated lymphoid tissue lymphoma-translocation gene 1 (CBM) complex: Molecular, immunologic, and clinical heterogeneity. J Allergy Clin Immunol. 2015;136(5):1139–49. doi: 10.1016/j.jaci.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. The Journal of experimental medicine. 2007;204(10):2407–22. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Della Mina E, Borghesi A, Zhou H, Bougarn S, Boughorbel S, Israel L, et al. Inherited human IRAK-1 deficiency selectively impairs TLR signaling in fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(4):E514–E23. doi: 10.1073/pnas.1620139114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Israel L, Wang Y, Bulek K, Della Mina E, Zhang Z, Pedergnana V, et al. Human Adaptive Immunity Rescues an Inborn Error of Innate Immunity. Cell. 2017;168(5):789–800. e10. doi: 10.1016/j.cell.2017.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]