Summary
This short review focuses on the importance of nonhuman primate nutrition and aging studies and makes the case that a targeted expansion of the use of this highly translatable model would be advantageous to the biology of aging field. First, we describe the high degree of similarity of the model in terms of aging phenotypes including incidence and prevalence of common human age-related diseases. Second, we discuss the importance of the nonhuman primate nutrition and aging studies and the extent to which the outcomes of two ongoing long-term studies of caloric restriction are congruent with short-term equivalent studies in humans. Third, we showcase a number of pharmacological agents previously employed in nonhuman primate studies that display some potential as caloric restriction mimetics. Finally, we present nonhuman primates as an important model for translation of mechanisms of delayed aging identified in studies of shorter-lived animals. Proof of efficacy and safety of candidate longevity agents in nonhuman primates would be a cost-effective means to bring these exciting new avenues a step closer to clinical application.
Keywords: Aging, nutrition, nonhuman primates, caloric restriction, mimetics, translational research
Rhesus monkeys as a model for human health and disease
Nonhuman primate species are an excellent model for human biology due to their genetic and physiological similarity to humans. Nonhuman primate studies bring the promise that the insights into aging biology gleaned will be highly translatable to human aging biology. The rhesus monkey (Macaca mulatta) genome shares ~93% sequence identity with the human genome1. Similarity between monkeys and humans at the genomic level extends to numerous aspects of anatomy, physiology, neurology, endocrinology, immunology, and behavior2. Rhesus monkeys develop and age in similar ways to humans but on a compressed time-scale3,4. In captivity, the median lifespan for rhesus monkeys is ~26 years of age and the maximum lifespan of a captive rhesus monkey is ~40 years. A reasonable rule of thumb considers macaques aging at a rate of two and a half to three times that of humans5,6, with the caveat that not all aging and developmental milestones are paralleled. For example, females are reproductively fit relatively early and maintain menarche relatively longer than humans. Nonhuman primate studies have considerable advantages over human studies in terms of experimental design; the environment, dietary intake, and medical oversight can be fully defined, thus limiting confounding issues arising due to lack of uniformity in these parameters. Unlike rodents, rhesus monkeys display patterns of eating and sleeping behavior that mirror those of humans. Yet unlike human subject studies, rhesus monkey studies can be designed to facilitate comprehensive monitoring of subjects and strict adherence to the study protocol. Given the high degree of translatability and the tractability in study design, nonhuman primates are a vital link between basic research and clinical application. The links between aging and adiposity in nonhuman primates has been reviewed recently7, so here we will focus on caloric restriction (CR) and putative CR mimetics. We present that increased understanding of the biology of aging in rhesus monkeys will be extremely illuminating for human aging, and efforts to understand causative elements in rhesus monkey aging and age-related disease vulnerability are highly likely to reveal novel approaches for application in preventative human health care.
Delayed aging by caloric restriction in rhesus monkeys
Caloric restriction is the only environmental intervention that repeatedly and strongly increases maximum lifespan and delays biological aging in laboratory rodents8. Over the last 20 years, astonishing progress has been made in defining longevity pathways and identifying factors that contribute to age-related changes in short lived species9. In many of these studies CR is viewed as the gold-standard model of delayed aging and the reference to which other models of delayed aging are compared. The translatability of mechanistic insights from the study of CR in shorter-lived species hinges on the effects of CR being conserved in primates including humans and nonhuman primates. To address this, three independent rhesus monkey studies were initiated in the late 1980’s. Two of these studies are ongoing: one at the National Institute on Aging (NIA)10 and the other at the Wisconsin National Primate Research Center based at the University of Wisconsin (UW)-Madison11. The third study, performed at the University of Maryland reported favorable effects of CR, although the study was focused on obesity and glucoregulation with only a small cohort designated to CR12. At the UW, the CR intervention in a cohort of 76 adult monkeys was associated with significant improvements in morbidity and mortality13. These findings contrasted with the report from the parallel NIA study, where a difference in survival was not observed between groups within the cohort of 121 monkeys, although a trend towards lower morbidity was reported for CR monkeys compared to controls14. Two major differences in study design included the timing of onset of CR where CR was implemented in adults at UW and in juveniles and advanced-age animals at NIA, and in the implementation of the diet including feeding protocols and diet composition. Subsequent analysis indicated that a direct comparison of longitudinal data from both studies is warranted15. A joint initiative from both UW and NIA research teams has been developed to directly compare the two studies with a view to uncovering the basis for differences in outcome and the publication of this work is highly anticipated.
Caloric restriction impacts health indices in rhesus monkeys
Similar to humans, rhesus monkeys undergo changes in body composition with age including increased adiposity and a redistribution of body fat16. Not surprisingly, animals on caloric restriction tend to be smaller than their control fed counterparts and this is disproportionately evident in the reduction in adiposity17–19. Along with these favorable changes in body composition, improved glucoregulatory function was one of the first identified benefits of CR in rhesus monkeys, including lower circulating glucose levels and improved insulin sensitivity12,20,21. Incidence of insulin resistance and diabetes are significantly lower in CR animals14,22. Similar to humans, obesity in rhesus monkeys is associated with a number of risk factors for disease including insulin resistance and elevated serum triglycerides and cholesterol23–25. CR lowers circulating levels of triglycerides and improves lipoprotein profiles where levels of HDLs are higher with CR and levels of VLDLs are lower26–28. These outcomes are consistent with improved metabolic homeostasis and reduced risk for diabetes and cardiovascular disease.
Further evidence for delayed aging in rhesus monkeys on CR comes from studies focused on specific tissues including skeletal muscle, brain, bone, and the immune system. Sarcopenia is the age-related loss in skeletal muscle mass and function and begins in middle age in rhesus monkeys29,30. The onset and progression of this age-related condition is delayed in CR monkeys31, where cellular atrophy and muscle fibrosis are both attenuated32,33. Aging of skeletal muscle in rhesus monkeys is gradual, similar to humans, and the onset set of aging phenotypes is linked to changes in mitochondrial activity and redox metabolism34. The age-related decline in physical activity is also attenuated in rhesus monkeys on CR and the intervention is associated with improved metabolic cost of movement35. Measures of resting metabolic rate suggest that it is lower with CR35,36, however, analysis of the data is complicated by overt difference in body composition, and it is unclear how meaningful the small differences reported might be. Brain aging is also delayed by CR. MRI based studies of brain volume reveal preservation of white matter and neuronal volume, and markers of inflammation are significantly lower13,37,38. The caudate nucleus and putamen regions are vulnerable to age-associated atrophy and are protected by CR13,39. The age related increase in iron deposition in the globus pallidus and the substantia nigra is also attenuated by CR40. Correlation analysis of brain volume against peripheral insulin sensitivity suggests a role for systemic homeostasis in protection against age-related atrophy41. CR has long been associated with lower bone mass and lower bone mineral density that, until quite recently, was viewed as a potentially negative outcome42. It has become clear that bone density is markedly influenced by body weight and in this light the lower bone density measured in CR animals could be viewed as an adaptive rather than a pathological outcome of the diet43,44. Although the starting point is different for control and CR monkeys, the rate of decline in bone mass and bone density is greater in the controls suggesting a protective effect of CR. The ability of CR to delay aging of the immune system has also been investigated and reports suggest that CR delays senescence of T cells and preserves the naïve population and repertoire diversity45,46. Other reports indicate that certain types of age-related skin damage are lowered and wound healing capacity is somewhat augmented in monkeys on CR47. Finally, there is some indication that age-related changes in circadian rhythm are prevented by CR where a youthful 24 hour cortisol periodicity is preserved in old monkeys on CR although this outcome was observed in males only48. These disparate studies together indicate that the impact of CR on aging is pervasive, with evidence of multiple indicators of disease vulnerability being either attenuated or abrogated in monkeys fed a CR diet.
The translatability of the benefits of CR to humans is suggested in outcomes of a multicenter clinical study designed to evaluate CR in humans in the CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) study. The study outcomes are reviewed elsewhere in this issue; however, in terms of the findings reported above a short-term 6 month trial indicated that CR induced favorable changes in body weight, body composition, glucoregulatory function and serum risk factors for cardiovascular disease49–54, consistent with the findings in nonhuman primates, and the beneficial metabolic effects were sustained out to 2 years of CR55–58. These clinical studies demonstrate the general translatability of CR’s effects with parallel outcomes in human and nonhuman primate studies, but issues related to compliance in humans studies suggest that in depth mechanistic studies might best be performed in the nonhuman primate model, where tightly controlled environmental conditions and adherence to a uniform study protocol are ensured. Studies using tissues and data from the longitudinal rhesus monkey studies will provide crucial understanding of the mechanisms behind the beneficial effects of CR and these insights gleaned will almost certainly prove to be highly translatable to human health and human aging. The ideal design would identify mechanisms of CR using data and specimens from nonhuman primate studies and then seek confirmation of their conserved impact in the outcomes of CR in humans.
Translational intervention studies in rhesus monkeys
In addition to investigation of the mechanisms of CR, a major line of inquiry in aging research is to identify CR mimetics59. These are pharmacological agents that are intended to mimic the delayed aging effects of CR without requiring a reduction in caloric intake. The Interventions Testing Program (ITP) is an NIH/NIA initiative launched in 2000 with the initial goal of uncovering pharmacological agents that extend lifespan in laboratory mice60. The intent was to engage the aging-focused research community to propose novel or repurposed existing agents for trials in mice to determine their ability to promote longevity. This program has been a resounding success and has identified several novel longevity agents, including rapamycin, an immunosuppressant; acarbose, an inhibitor of complex carbohydrate absorption in the gut; 17-alpha estradiol, a non-feminizing estrogen ortholog; and nordihydroguaiaretic acid, an antioxidant61–63. Another compound of considerable interest to the aging community is resveratrol, a polyphenol that has been shown to positively regulate longevity in non-mammalian species and positively influence health in mammalian species64.
There have been several difficulties in translating rodent treatments directly to humans, including issues related to differences in genetics and physiology, dosing, and species specificity in the pharmacokinetics. This translational gap might be bridged by rhesus monkey studies that could confirm the ability of agents identified in shorter-lived species to regulate longevity in long-lived nonhuman primates. The absence of beneficial effects might not necessarily indicate that the intervention is not of value in a primate species, rather it may point to the need for primate specific adaptation or optimization in the approach. Here we select a few agents with proven efficacy in nonhuman primates that are strong candidates as CR mimetics due to their target regulatory pathways (Figure 1).
Figure 1.
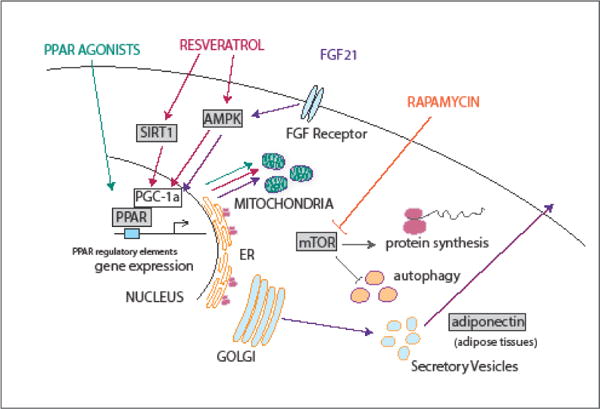
Target pathways and processes for putative CR mimetic agents resveratrol, rapamycin, PPAR agonists, and FGF21. Apart from rapamycin, nuclear receptor activation and mitochondrial activation are shared outcomes of treatments. Key signaling molecules include AMPK, SIRT1, PGC-1a, and mTOR, all of which have been associated with regulation of longevity in the context of CR.
Resveratrol
Resveratrol is a polyphenolic compound known for its anti-aging and anti-tumorigenic properties64. The biological effects of resveratrol are attributed to activation of a variety of mediators including, but not limited to, SIRT1 (nicotinamide adenine dinucleotide (NAD)-dependent histone deacetylase) and AMPK (AMP-activated protein kinase), which in turn leads to activation of PGC1a and inhibition of NF-kB and mTOR signaling65. Resveratrol is thought to ameliorate dietary and age-related metabolic complications through modulation of these diverse signaling factors and transcriptional regulators that are nutritionally regulated and nutrient responsive. Studies in rhesus monkeys have demonstrated pleiotropic effects of resveratrol. Resveratrol supplementation improved insulin sensitivity, decreased adipocyte size and attenuated inflammation in the visceral adipose tissue of monkeys fed a high-fat, high-sugar diet fed monkeys66. In these monkeys, resveratrol treatment also prevented dedifferentiation of β-cells in islets67. In the blood vessels, resveratrol supplementation prevented high fat diet-induced macrophage and lipid accumulation, preserved endothelial cell integrity and decreased inflammation-driven stiffening of arterial wall68. Resveratrol treatment also counteracted high fat diet-induced fiber type switching from type I to II fibers in the soleus muscle69. In terms of the central nervous system, resveratrol conferred neuroprotection against diet-induced neuroinflammation and cerebral vascular dysfunction70. Together these studies are strongly supportive of a beneficial impact of resveratrol on health in over-nourished primates, boding well for its application in humans as preliminary studies suggest71.
Rapamycin
Rapamycin, an inhibitor of the nutrient sensitive kinase mTOR, has been demonstrated to extend lifespan and delay the onset of age associated diseases in rodents72 Rapamycin is FDA-approved as an immunosuppressant in organ transplant patients and some forms of cancer. Even so, extending the translational potential of rapamycin as an anti-aging intervention in humans is met with resistance due to adverse metabolic changes such as glucose intolerance, insulin resistance and hyperlipidemia that occurred with chronic treatment in mice73–75. Furthermore, recent studies suggest that the metabolic effects of rapamycin are quite variable depending on the duration of treatment (transient vs long term), dosing regimen (intermittent vs continuous), age and sex of the animals being treated76–78. Clarification of the long-term effects of rapamycin in nonhuman primates could be a valuable next step before attempting to broaden the clinical applications of rapamycin in humans. A recent study in common marmosets, Callithrix jacchus, showed that long term (14 months) oral treatment of rapamycin did not affect body weight, blood lipids or glucose tolerance79. The authors did report a transient decrease in fat mass during the initial months of rapamycin treatment, but there was no difference in fat mass at the conclusion of the intervention period. The authors also reported evidence of induced autophagy in the muscle and adipose tissue, but not in the liver of rapamycin treated marmosets, suggesting rapamycin modulates proteostasis80 It will be extremely informative to extend these studies that we might know whether prolonged rapamycin treatment can impact healthspan or other indices of longevity in nonhuman primates.
PPAR agonists
Peroxisome proliferator-activated receptors (PPARs) are known to regulate lipid and glucose homeostasis in a variety of metabolically active tissues like adipose tissue, liver, skeletal muscle, and heart81. Synthetic ligands for PPARs such as thiazolidinediones (PPARγ agonists, also known as insulin sensitizers), fibrates (PPARα agonists, lipid lowering drugs) and dual agonists (PPARα/γ) are currently used in type 2 diabetic patients to manage hyperglycemia and hyperlipidemia to reduce cardiovascular risk factors associated with diabetes. Nonhuman primates have served as an excellent preclinical model for testing and validating the safety and efficacy of many of these existing PPAR agonists. Rosiglitazone or pioglitazone (both PPARγ agonists) treatment in obese diabetic rhesus monkeys improved peripheral insulin sensitivity, increased insulin clearance, decreased circulating triglyceride and cholesterol levels, and increased HDL and adiponectin levels82–86. The insulin sensitizing action of thiazolidinediones in monkeys is attributed to increase in muscle AMPK activity and atypical protein kinase C (aPKC). PPARα agonists such as fenofibrate or K-111 also improve circulating lipid profiles and insulin sensitivity25,87,88. Dual peroxisome proliferator-activated receptor α/γ agonists may be even more efficient in reducing cardiovascular events in diabetic patients as they confer both the insulin sensitizing effects of PPARγ agonism and lipid lowering effects associated with PPARα agonism. In diabetic rhesus monkeys, dual PPAR α/γ agonists like Aleglitazar and TAK559 have been shown to correct hyperinsulinemia and result in a less atherogenic lipoprotein profile without adversely affecting liver function, body weight or fluid retention as observed with other selective PPAR agonists89,90. The beneficial effects of these classes of drugs on lipid profiles and glucoregulatory function in the context of metabolic dysfunction are reminiscent of the outcomes of CR91,92. Unfortunately, long-term treatments with these types of compounds have fallen into disfavor clinically due to the occurrence of unappealing side effects, including increased risk for bone fractures, congestive heart failure, edema, and inflammation of the liver81. It may be possible to design PPAR agonists that impinge on only a subset of downstream functions avoiding these confounding side effects. With a better understanding of the underlying biology, a PPAR-based strategy might prove to be the ultimate CR mimetic.
Fibroblast growth factor-21 analogs
Fibroblast growth factor-21 (FGF21) is a peptide hormone secreted by metabolic tissues like liver, adipose tissue, pancreas and skeletal muscle with both paracrine and endocrine actions. Rodent studies have shown that FGF21 administration decreases body weight, corrects hyperglycemia, improves insulin sensitivity, and reduces circulating triglyceride and LDL-cholesterol levels93. FGF21 signaling occurs through AMPK and its mechanism of action involves the adipokines, adiponectin94–96 Interestingly, overexpression of the FGF21 transgene confers longevity in mice97. Complementary to rodent data, studies in NHPs have also documented the beneficial metabolic effects of FGF21. In diabetic rhesus monkeys, administration of human recombinant FGF21 improved fasting glucose, insulin, glucagon and triglyceride levels98. LY2405319 (an engineered FGF21 variant) also showed a similar metabolic effect, in addition to decreasing leptin and increasing adiponectin levels, this compound had a lipid-lowering effects in late stage diabetic monkeys99. Elevated circulating levels of adiponectin are consistently observed in animals on CR100–103 and may be mechanistically important due to its established ability to stimulate AMPK and PPARs104–106 To circumvent the dosing difficulties associated with a shorter half-life of FGF21, several long-acting analogs have been developed and tested in nonhuman primates. Fc-FGF21 (RG), the first-long acting analog of FGF21 generated by fusion of Fc to human recombinant FGF21, showed greater efficacy than human recombinant FGF21 in improving the metabolic status of obese rhesus monkeys107. A monoclonal antibody mimAb1 that mimics FGF21 and activates βKlotho/FGFR1c (FGF receptor 1c) receptor signaling mimicked FGF21’s metabolic actions and resulted in an improved glycemic and lipid profile in obese cynomolgus monkeys108. Obese cynomolgus monkeys treated with the bispecific Avimer polypeptides anti-FGFR1c/β-Klotho protein decreased body weight, lowered triglyceride levels and lowered fasting insulin levels109. A complicating issue with the use of FGF21 to treat metabolic disease is that circulating FGF21 levels are higher in the obese suggesting the possibility of FGF21 resistance110. This is unlikely to be a problem in healthy individuals and places renewed emphasis on the impact of FGF21 on aging in otherwise healthy humans and nonhuman primates.
The concept that aging itself might be a suitable drug target in a clinical context is very new but there is considerable interest in bringing this idea to fruition111,112. Until now, the emphasis for each of the interventions described above, apart from rapamycin, has been on correcting metabolic dysfunction. Mechanistically, each one is predicted to show at least some similarity to CR, making a strong case for their use as agents for anti-aging. With a clearer set of biomarkers derived from studies of aging and delayed aging by CR, it should be possible to test these promising agents in nonhuman primate studies to determine their ability to impinge on the aging process.
Final remarks
In conclusion, it is resoundingly clear that nonhuman primate studies fill a vital need in aging research. A high-resolution molecular understanding of aging and delayed aging in rhesus monkeys could be used as a framework against which other interventions might be tested. With this groundwork laid, the possibility exists to conduct effective translational studies, and in the short term, offset the usual high-cost impediment placed on nonhuman primate work. The advantages of conducting translatability studies of longevity agents in primates are manifold: first, acting as a filter to identify the “most-likely to work” candidates; second, to uncover potential requirements for optimization in the move from rodents to primates; and third, to identify biomarkers of efficacy that will ensure progress and productivity in the implementation of human trials. With all this promise, it would seem remiss not to engage in some serious monkey business.
Acknowledgments
This work has been supported by NIH grants AG047358 and AG040178, the Glenn Foundation for Medical Research, and the National Institute on Aging Intramural Research Program at NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Zimin AV, et al. A new rhesus macaque assembly and annotation for next-generation sequencing analyses. Biol Direct. 2014;9:20. doi: 10.1186/1745-6150-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowden DM, Williams DD. Aging. Adv Vet Sci Comp Med. 1984;28:305–341. doi: 10.1016/b978-0-12-039228-5.50015-2. [DOI] [PubMed] [Google Scholar]
- 3.Uno H. Age-related pathology and biosenescent markers in captive rhesus macaques. Age (Omaha) 1997;20:1–13. doi: 10.1007/s11357-997-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colman RJ, Anderson RM. Nonhuman primate calorie restriction. Antioxid Redox Signal. 2011;14:229–239. doi: 10.1089/ars.2010.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King FA, Yarbrough CJ, Anderson DC, Gordon TP, Gould KG. Primates. Science. 1988;240:1475–1482. doi: 10.1126/science.3287624. [DOI] [PubMed] [Google Scholar]
- 6.Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. Am J Primatol. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan KL, Mattison JA. Obesity and Aging in Humans and Nonhuman Primates: A Mini-Review. Gerontology. 2016;62:611–617. doi: 10.1159/000445800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weindruch RH, Walford RL. The retardation of aging and disease by dietary restriction. Charles C Thomas; 1988. [Google Scholar]
- 9.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane MA, et al. Dietary restriction in nonhuman primates: progress report on the NIA study. Ann N Y Acad Sci. 1992;673:36–45. doi: 10.1111/j.1749-6632.1992.tb27434.x. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey JJ, et al. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 12.Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 2003;58:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- 13.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. doi:325/5937/201 [pii] 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. doi:nature11432 [pii] 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colman RJ, et al. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudson JC, Baum ST, Frye DM, Roecker EB, Kemnitz JW. Age and sex differences in body size and composition during rhesus monkey adulthood. Aging (Milano) 1996;8:197–204. doi: 10.1007/BF03339677. [DOI] [PubMed] [Google Scholar]
- 17.Colman RJ, et al. Body fat distribution with long-term dietary restriction in adult male rhesus macaques. J Gerontol A Biol Sci Med Sci. 1999;54:B283–290. doi: 10.1093/gerona/54.7.b283. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey JJ, Laatsch JL, Kemnitz JW. Age and gender differences in body composition, energy expenditure, and glucoregulation of adult rhesus monkeys. J Med Primatol. 2000;29:11–19. doi: 10.1034/j.1600-0684.2000.290102.x. [DOI] [PubMed] [Google Scholar]
- 19.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 20.Lane MA, et al. Diet restriction in rhesus monkeys lowers fasting and glucose-stimulated glucoregulatory end points. Am J Physiol. 1995;268:E941–948. doi: 10.1152/ajpendo.1995.268.5.E941. [DOI] [PubMed] [Google Scholar]
- 21.Kemnitz JW, et al. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266:E540–547. doi: 10.1152/ajpendo.1994.266.4.E540. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37:47–51. doi: 10.1177/0192623308329476. doi:0192623308329476 [pii] 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemnitz JW, Goy RW, Flitsch TJ, Lohmiller JJ, Robinson JA. Obesity in male and female rhesus monkeys: fat distribution, glucoregulation, and serum androgen levels. J Clin Endocrinol Metab. 1989;69:287–293. doi: 10.1210/jcem-69-2-287. [DOI] [PubMed] [Google Scholar]
- 24.Ding SY, Tigno XT, Hansen BC. Nuclear magnetic resonance-determined lipoprotein abnormalities in nonhuman primates with the metabolic syndrome and type 2 diabetes mellitus. Metabolism. 2007;56:838–846. doi: 10.1016/j.metabol.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Winegar DA, et al. Effects of fenofibrate on lipid parameters in obese rhesus monkeys. J Lipid Res. 2001;42:1543–1551. [PubMed] [Google Scholar]
- 26.Edwards IJ, et al. Caloric restriction in rhesus monkeys reduces low density lipoprotein interaction with arterial proteoglycans. J Gerontol A Biol Sci Med Sci. 1998;53:B443–448. doi: 10.1093/gerona/53a.6.b443. [DOI] [PubMed] [Google Scholar]
- 27.Rezzi S. Metabolic shifts due to long-term caloric restriction revealed in nonhuman primates. Exp Gerontol. 2009;44:356–362. doi: 10.1016/j.exger.2009.02.008. doi:S0531-5565(09)00030-8 [pii] 10.1016/j.exger.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdery RB, Ingram DK, Roth GS, Lane MA. Caloric restriction increases HDL2 levels in rhesus monkeys (Macaca mulatta) Am J Physiol. 1997;273:E714–719. doi: 10.1152/ajpendo.1997.273.4.E714. [DOI] [PubMed] [Google Scholar]
- 29.Colman RJ, McKiernan SH, Aiken JM, Weindruch R. Muscle mass loss in Rhesus monkeys: age of onset. Exp Gerontol. 2005;40:573–581. doi: 10.1016/j.exger.2005.05.001. doi:S0531-5565(05)00089-6 [pii] 10.1016/j.exger.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 30.McKiernan SH. Longitudinal analysis of early stage sarcopenia in aging rhesus monkeys. Exp Gerontol. 2009;44:170–176. doi: 10.1016/j.exger.2008.09.014. doi: S0531-5565(08)00321-5 [pii] 10.1016/j.exger.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–559. doi: 10.1093/gerona/63.6.556. doi: 63/6/556 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKiernan SH. Cellular adaptation contributes to calorie restriction-induced preservation of skeletal muscle in aged rhesus monkeys. Exp Gerontol. 2012;47:229–236. doi: 10.1016/j.exger.2011.12.009. doi:S0531-5565(11)00351-2 [pii] 10.1016/j.exger.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKiernan SH. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp Gerontol. 2011;46:23–29. doi: 10.1016/j.exger.2010.09.011. doi:S0531-5565(10)00303-7 [pii] 10.1016/j.exger.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugh TD. A shift in energy metabolism anticipates the onset of sarcopenia in rhesus monkeys. Aging Cell. 2013;12:672–681. doi: 10.1111/acel.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada Y. Long-term calorie restriction decreases metabolic cost of movement and prevents decrease of physical activity during aging in rhesus monkeys. Exp Gerontol. 2013;48:1226–1235. doi: 10.1016/j.exger.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radic Biol Med. 2000;29:946–968. doi: 10.1016/s0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 37.Sridharan A. Brain volumetric and microstructural correlates of executive and motor performance in aged rhesus monkeys. Front Aging Neurosci. 2012;4:31. doi: 10.3389/fnagi.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bendlin BB. Effects of aging and calorie restriction on white matter in rhesus macaques. Neurobiol Aging. 2011;32:2319 e2311–2311. doi: 10.1016/j.neurobiolaging.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matochik JA, et al. Age-related decline in striatal volume in rhesus monkeys: assessment of long-term calorie restriction. Neurobiol Aging. 2004;25:193–200. doi: 10.1016/s0197-4580(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 40.Kastman EK, et al. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J Neurosci. 2012;32:11897–11904. doi: 10.1523/JNEUROSCI.2553-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willette AA. Calorie restriction reduces the influence of glucoregulatory dysfunction on regional brain volume in aged rhesus monkeys. Diabetes. 2012;61:1036–1042. doi: 10.2337/db11-1187. doi:db11-1187 [pii] 10.2337/db11-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang TH, Ables GP. Dietary restrictions, bone density, and bone quality. Ann N Y Acad Sci. 2016;1363:26–39. doi: 10.1111/nyas.13004. [DOI] [PubMed] [Google Scholar]
- 43.Colman RJ, Beasley TM, Allison DB, Weindruch R. Skeletal effects of long-term caloric restriction in rhesus monkeys. Age (Dordr) 2012;34:1133–1143. doi: 10.1007/s11357-011-9354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black A, et al. Calorie restriction and skeletal mass in rhesus monkeys (Macaca mulatta): evidence for an effect mediated through changes in body size. J Gerontol A Biol Sci Med Sci. 2001;56:B98–107. doi: 10.1093/gerona/56.3.b98. [DOI] [PubMed] [Google Scholar]
- 45.Messaoudi I. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nikolich-Zugich J, Messaoudi I. Mice and flies and monkeys too: caloric restriction rejuvenates the aging immune system of non-human primates. Exp Gerontol. 2005;40:884–893. doi: 10.1016/j.exger.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Sell DR, et al. The effect of caloric restriction on glycation and glycoxidation in skin collagen of nonhuman primates. J Gerontol A Biol Sci Med Sci. 2003;58:508–516. doi: 10.1093/gerona/58.6.b508. [DOI] [PubMed] [Google Scholar]
- 48.Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fontana L. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 50.Heilbronn LK. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lefevre M. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Racette SB. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. doi:61/9/943 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Redman LM. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–872. doi: 10.1210/jc.2006-2184. doi:jc.2006-2184 [pii] 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss EP. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. doi:84/5/1033 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ravussin E. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci. 2015;70:1097–1104. doi: 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romashkan SV. Safety of two-year caloric restriction in non-obese healthy individuals. Oncotarget. 2016;7:19124–19133. doi: 10.18632/oncotarget.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin CK. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Nonobese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA Intern Med. 2016;176:743–752. doi: 10.1001/jamainternmed.2016.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sparks LM. EFFECTS OF 12 MONTHS OF CALORIC RESTRICTION ON MUSCLE MITOCHONDRIAL FUNCTION IN HEALTHY INDIVIDUALS. J Clin Endocrinol Metab. 2016:jc20163211. doi: 10.1210/jc.2016-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ingram DK, Roth GS. Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res Rev. 2015;20:46–62. doi: 10.1016/j.arr.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Warner HR, Ingram D, Miller RA, Nadon L, Richardson AG. Mech Ageing Dev. 2000;115:199–207. doi: 10.1016/s0047-6374(00)00118-4. [DOI] [PubMed] [Google Scholar]
- 61.Harrison DE. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller RA. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strong R. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhullar KS, Hubbard BP. Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta. 2015;1852:1209–1218. doi: 10.1016/j.bbadis.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Kulkarni SS, Cantó C. The molecular targets of resveratrol. Biochim Biophys Acta. 2015;1852:1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Jimenez-Gomez Y. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18:533–545. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fiori JL. Resveratrol prevents β-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes. 2013;62:3500–3513. doi: 10.2337/db13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mattison JA. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hyatt JP. Muscle-Specific Myosin Heavy Chain Shifts in Response to a Long-Term High Fat/High Sugar Diet and Resveratrol Treatment in Nonhuman Primates. Front Physiol. 2016;7:77. doi: 10.3389/fphys.2016.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernier M. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 2016;8:899–916. doi: 10.18632/aging.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Timmers S. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. doi:S1550-4131(11)00386-X [pii] 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kennedy BK, Lamming DW. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016;23:990–1003. doi: 10.1016/j.cmet.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang GR. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol. 2009;105:188–198. doi: 10.1111/j.1742-7843.2009.00427.x. doi:TO427 [pii] 10.1111/j.1742-7843.2009.00427.x. [DOI] [PubMed] [Google Scholar]
- 74.Houde VP. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi: 10.2337/db09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fraenkel M. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945–957. doi: 10.2337/db07-0922. [DOI] [PubMed] [Google Scholar]
- 76.Fang Y. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. doi: 10.1016/j.cmet.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bitto A. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife. 2016;5 doi: 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arriola Apelo SI, Pumper CP, Baar EL, Cummings NE, Lamming DW. Intermittent Administration of Rapamycin Extends the Life Span of Female C57BL/6J Mice. J Gerontol A Biol Sci Med Sci. 2016;71:876–881. doi: 10.1093/gerona/glw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ross C. Metabolic consequences of long-term rapamycin exposure on common marmoset monkeys (Callithrix jacchus) Aging (Albany NY) 2015;7:964–973. doi: 10.18632/aging.100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lelegren M, Liu Y, Ross C, Tardif S, Salmon AB. Pharmaceutical inhibition of mTOR in the common marmoset: effect of rapamycin on regulators of proteostasis in a non-human primate. Pathobiol Aging Age Relat Dis. 2016;6:31793. doi: 10.3402/pba.v6.31793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutr J. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gee MK, et al. Rosiglitazone treatment improves insulin regulation and dyslipidemia in type 2 diabetic cynomolgus monkeys. Metabolism. 2004;53:1121–1125. doi: 10.1016/j.metabol.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 83.Tozzo E, Bhat G, Cheon K, Camacho RC. Pioglitazone increases whole body insulin sensitivity in obese, insulin-resistant rhesus monkeys. PLoS One. 2015;10:e0126642. doi: 10.1371/journal.pone.0126642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ortmeyer HK. Insulin signaling and insulin sensitizing in muscle and liver of obese monkeys: peroxisome proliferator-activated receptor gamma agonist improves defective activation of atypical protein kinase C. Antioxid Redox Signal. 2011;14:207–219. doi: 10.1089/ars.2010.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kemnitz JW, et al. Pioglitazone increases insulin sensitivity, reduces blood glucose, insulin, and lipid levels, and lowers blood pressure, in obese, insulin-resistant rhesus monkeys. Diabetes. 1994;43:204–211. doi: 10.2337/diab.43.2.204. [DOI] [PubMed] [Google Scholar]
- 86.Zhang X. Rhesus macaques develop metabolic syndrome with reversible vascular dysfunction responsive to pioglitazone. Circulation. 2011;124:77–86. doi: 10.1161/CIRCULATIONAHA.110.990333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ortmeyer HK. Skeletal muscle glycogen synthase subcellular localization: effects of insulin and PPAR-alpha agonist (K-111) administration in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1509–1517. doi: 10.1152/ajpregu.00692.2004. [DOI] [PubMed] [Google Scholar]
- 88.Bodkin NL, Pill J, Meyer K, Hansen BC. The effects of K-111, a new insulin-sensitizer, on metabolic syndrome in obese prediabetic rhesus monkeys. Horm Metab Res. 2003;35:617–624. doi: 10.1055/s-2003-43510. [DOI] [PubMed] [Google Scholar]
- 89.Hansen BC. Effects of aleglitazar, a balanced dual peroxisome proliferator-activated receptor α/γ agonist on glycemic and lipid parameters in a primate model of the metabolic syndrome. Cardiovasc Diabetol. 2011;10:7. doi: 10.1186/1475-2840-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ding SY, Tigno XT, Braileanu GT, Ito K, Hansen BC. A novel peroxisome proliferator-activated receptor alpha/gamma dual agonist ameliorates dyslipidemia and insulin resistance in prediabetic rhesus monkeys. Metabolism. 2007;56:1334–1339. doi: 10.1016/j.metabol.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 91.Edwards IJ. Caloric restriction lowers plasma lipoprotein (a) in male but not female rhesus monkeys. Exp Gerontol. 2001;36:1413–1418. doi: 10.1016/s0531-5565(01)00107-3. doi:S0531-5565(01)00107-3 [pii] [DOI] [PubMed] [Google Scholar]
- 92.Gresl TA, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281:E757–765. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- 93.Salminen A, Kauppinen A, Kaarniranta K. FGF21 activates AMPK signaling: impact on metabolic regulation and the aging process. J Mol Med (Berl) 2016 doi: 10.1007/s00109-016-1477-1. [DOI] [PubMed] [Google Scholar]
- 94.Holland WL. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin Z. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci U S A. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kharitonenkov A. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 99.Adams AC. LY2405319, an Engineered FGF21 Variant, Improves the Metabolic Status of Diabetic Monkeys. PLoS One. 2013;8:e65763. doi: 10.1371/journal.pone.0065763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ding Q, Ash C, Mracek T, Merry B, Bing C. Caloric restriction increases adiponectin expression by adipose tissue and prevents the inhibitory effect of insulin on circulating adiponectin in rats. J Nutr Biochem. 2012;23:867–874. doi: 10.1016/j.jnutbio.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 101.Kondo M. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–1724. doi: 10.1074/jbc.M805301200. doi:M805301200 [pii] 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shinmura K. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. doi:CIRCULATIONAHA.107.725697 [pii] 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 103.Zhu M. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol. 2004;39:1049–1059. doi: 10.1016/j.exger.2004.03.024. S0531556504001287 [pii] [DOI] [PubMed] [Google Scholar]
- 104.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 105.Iwabu M. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 106.Kadowaki T. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/jci29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Véniant MM, et al. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology. 2012;153:4192–4203. doi: 10.1210/en.2012-1211. [DOI] [PubMed] [Google Scholar]
- 108.Foltz IN. Treating diabetes and obesity with an FGF21-mimetic antibody activating the β Klotho/FGFR1c receptor complex. Sci Transl Med. 2012;4:162ra153. doi: 10.1126/scitranslmed.3004690. [DOI] [PubMed] [Google Scholar]
- 109.Smith R. FGF21 can be mimicked in vitro and in vivo by a novel anti-FGFR1c/β-Klotho bispecific protein. PLoS One. 2013;8:e61432. doi: 10.1371/journal.pone.0061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fisher FM. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burd CE. Barriers to the Preclinical Development of Therapeutics that Target Aging Mechanisms. J Gerontol A Biol Sci Med Sci. 2016;71:1388–1394. doi: 10.1093/gerona/glw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Justice J. Frameworks for Proof-of-Concept Clinical Trials of Interventions That Target Fundamental Aging Processes. J Gerontol A Biol Sci Med Sci. 2016;71:1415–1423. doi: 10.1093/gerona/glw126. [DOI] [PMC free article] [PubMed] [Google Scholar]


