Abstract
In order to characterize sleep and the cognitive patterns in patients with acute minor thalamic infarction (AMTI), we enrolled 27 patients with AMTI and 12 matched healthy individuals. Questionnaires about sleep and cognition as well as polysomnography (PSG) were performed on days 14 and 90 post-stroke. Compared to healthy controls, in patients with AMTI, hyposomnia was more prevalent; sleep architecture was disrupted as indicated by decreased sleep efficiency, increased sleep latency, and decreased non-rapid eye movement sleep stages 2 and 3; more sleep-related breathing disorders occurred; and cognitive functions were worse, especially memory. While sleep apnea and long-delay memory recovered to a large extent in the patients, other sleep and cognitive function deficit often persisted. Patients with AMTI are at an increased risk for hyposomnia, sleep structure disturbance, sleep apnea, and memory deficits. Although these abnormalities improved over time, the slow and incomplete improvement suggest that early management should be considered in these patients.
Keywords: Acute minor thalamic infarction, Polysomnography, Cognition
Introduction
Sleep disorders are a common complication in stroke patients: 20%–40% of such patients present with a sleep-wake disorder and 50%–70% present with a sleep-related breathing disorder [1]. The ratio of subcortical stroke patients who suffer attacks of sleep disturbance is greater than that of cerebral infarction patients and cerebellar stroke patients [2]. The more severe sleep disturbances threaten the physical and psychological health of these patients. One study has shown that excessive daytime sleepiness increases the risk of cardiovascular mortality by 33% and the risk of cancer by 23% in a population of elderly people [3].
The thalamus is an important transition nucleus and plays a pivotal role in a number of neuronal activities. Areas of the thalamus are considered to play a role in the formation of new memories partly through their connections with the hippocampus, and partly because the thalamus is important for mental alertness [4]. Normal sleep architecture and arousal also require an intact thalamus. Thalamic injury, particularly in the paramedian region, may cause arousal disturbances, hypersomnolence, and cognitive injury [5–9]. However, the thalamic blood supply is commonly divided into four minor vascular territories, each supplying distinct nuclei: the tuberothalamic, inferolateral, paramedian, and posterior choroid vessels [10]. The stroke syndromes stemming from one of these four vessels are mild or none with nonspecific signs to individual nuclei because focal lesions are minor and seldom confined within nuclear boundaries.
Whether minor thalamic infarction leads to sleep and cognitive abnormalities has not been investigated. Therefore, in this study, we aimed to characterize the sleep patterns and cognitive functions of patients with minor thalamic infarction using full-night nocturnal polysomnography (PSG) and neuropsychological questionnaire evaluation focusing on the following: (1) sleep and cognitive characteristics of patients with minor thalamic infarction; and (2) the long-term consequences of minor thalamic infarction. We then conducted a case-control study to compare the sleep and cognitive aspects in patients with minor thalamic infarction and healthy volunteers.
Materials and Methods
Study Population
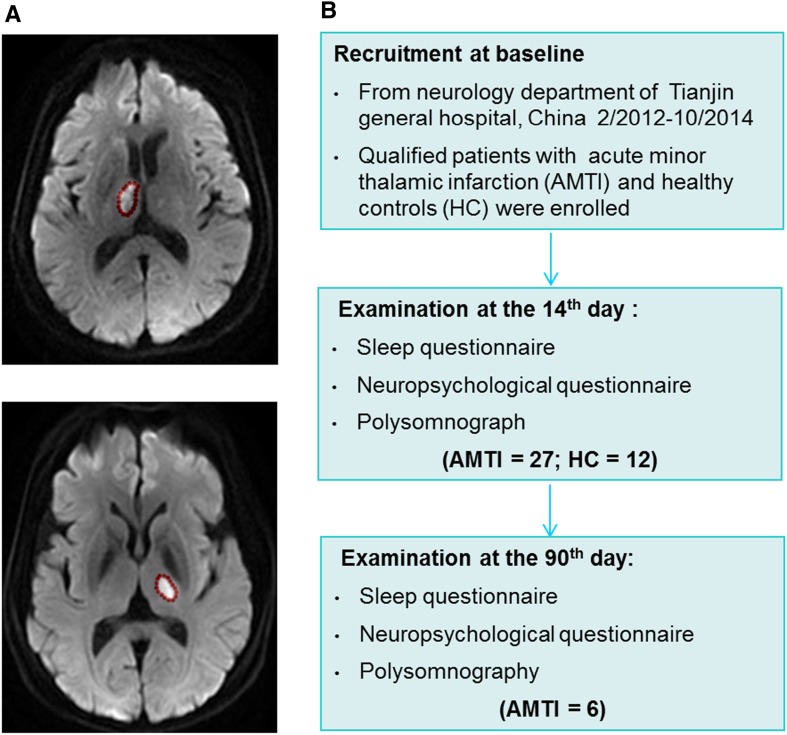
This was a prospective single-center pilot observational study. Participants were recruited from the Neurology Department of Tianjin Medical University General Hospital from February 2012 to October 2014. The patients were enrolled based on these criteria: (1) right or left minor thalamic infarction diagnosed by magnetic resonance imaging; (2) < 14 days from symptom onset; (3) first stroke; (4) > 18 years of age. Exclusion criteria were patients with: (1) language comprehension disorders (MRI); (2) history of sleep disorder, serious head trauma, or neuropsychiatric disease; or (3) dependence on psychoactive substances, such as alcohol; (4) intake of hypnotic or sedative drugs during the preceding 2 weeks. Healthy controls were recruited to match the patients for age, gender, education level, and body mass index (BMI). None had a history of neurological and/or psychiatric disease or other significant medical conditions. Twenty-seven patients with acute minor thalamic infarction (AMTI) and 12 matched healthy controls were enrolled. Informed consent was given by all participants, and the study was approved by the Tianjin Medical University General Hospital Review Board and Ethics Committee.
Clinical assessments were performed upon patient enrollment, then on days 14 and 90 after stroke in an evaluator-blinded fashion (Fig. 1). The extent of neurologic deficit was evaluated using the National Institutes of Health Stroke Scale (NIHSS). A detailed medical history and cardiovascular risk factors were assessed and recorded.
Fig. 1.
Representative MRs of patients with acute right or left minor thalamic infarction (A) and flow-chart for this study (B).
Polysomnography (PSG) Examination
All PSG sessions was monitored by a trained technician, who scored the results visually according to standardized criteria [11]. PSG examination was performed on days 14 and 90 from the onset of symptoms. The following parameters were recorded and systematically evaluated: (1) total in-bed time (TIB); (2) total sleep time (TST); (3) sleep efficiency (SE, the TST/TIB ratio, expressed as a percentage); (4) sleep onset latency (SL, the time to sleep onset); (5) wake after sleep onset (WASO, the number of minutes of wakefulness after the onset of persistent sleep to the end of the PSG recording); (6) arousal index; (7) non-rapid eye movement (non-REM) sleep stages 1, 2, and 3 and REM sleep stage (N1, N2, N3, and REM); (8) sleep respiratory disturbance index (RDI); (9) periodic leg movements per hour of sleep (PLMI); and (10) nadir SpO2. These parameters were analyzed by a specialist in a blinded fashion before comparisons were made between groups. The International Classification of Sleep Disorders-2 was used to diagnose and classify sleep abnormalities [12].
Sleep and Cognitive Assessment
The Pittsburgh Sleep Quality Index (PSQI) was included to measure nocturnal sleep patterns and identify related complaints during the month. The PSQI provides a global score of sleep on a scale from 1 to 21, with higher scores indicating more sleep complaints. Excessive daytime sleepiness was registered using the Epworth sleepiness scale (ESS), which measures the predisposition to sleeping or dozing in eight specific situations. An ESS score > 10 is regarded as an indicator of excessive sleepiness.
The Chinese versions of the mini mental state examination (MMSE) [13] and the Montreal cognitive assessment (MoCA) [14] were used to evaluate global cognitive performance. The auditory verbal learning test (AVLT) was used to test auditory or verbal episodic memory. According to the previous study, the AVLT short-delay recall (5 min) and long-delayed free recall (20 min after with a full score of 12) were measured to assess memory [15]. All the questionnaires used were validated.
Statistical Analysis
SPSS for Windows version 17.0 (SPSS, Inc., Chicago, IL) was used for analyses. Two-sample t tests (for normal continuous data) and χ 2 tests (for dichotomized data) were used to compare baseline characteristics between patients with AMTI and healthy controls. Questionnaire scores and PSG parameters of interest (TST, SL, SE, WASO, N1%, N2%, N3%, REM%, and SpO2) were tested by two-sample t tests or the Mann-Whitney test (arousal index, RDI and PLMI, MMSE, MoCA, and AVLT). Statistical significance was defined as P < 0.05.
Results
Baseline Data for the Patients with Acute Minor Thalamic Infarction
The baseline characteristics of patients with AMTI and matched healthy controls are shown in Table 1. Twenty-two male and 5 female patients with a mean age of 61.6 years (range, 32 to 79 years) were prospectively included. Among those with AMTI, MRI analyses showed that 15 had lesions on the right and 12 on the left. All patients had an NIHSS score of 0 and no patients had evident disability at the time of enrollment. The most common focal neurological findings were short-lasting sensory deficits of the arms with or without sensory deficits of the legs.
Table 1.
Clinical characteristics.
| Healthy controls (n = 12) | Minor thalamic infarction (n = 27) | P | |
|---|---|---|---|
| Age (years) | 61.9 ± 2.9 | 61.4 ± 2.5 | 0.8975 |
| Female, number (%) | 5 (41.6) | 8 (29.6) | 0.4859 |
| Education (years) | 11.0 ± 0.3 | 12.0 ± 0.3 | 0.1490 |
| BMI (kg/m2) | 24.1 ± 0.8 | 25.0 ± 0.6 | 0.9132 |
| Smoking, number (%) | 0 (0) | 12 (44.4) | 0.0068 |
| Drinking, number (%) | 0 (0) | 11 (40.7) | 0.0087 |
| Hypertension, number (%) | 1 (8.3) | 17 (63.0) | 0.0019 |
| Diabetes, number (%) | 1 (8.3) | 11 (40.7) | 0.0632 |
| Hyperlipidemia, number (%) | 0 (0) | 10 (37.0) | 0.0172 |
Plus-minus values are means ± SEM.
BMI, body mass index; hypertension, systolic pressure ≥ 140 mmHg and/or diastolic pressure ≥ 90 mmHg; diabetes, fasting blood-glucose ≥ 7.0 mmol/L and/or oral glucose tolerance test 2 h blood glucose ≥ 11.1 mmol/L; hyperlipidemia, total cholesterol > 5.72 mmol/L and/ or triglyceride > 1.70 mmol/L.
The 27 patients underwent PSG and cognitive evaluation at 14 days and only 6 patients completed the follow-up evaluation at 90 days. No significant differences with regard to age, gender, BMI, and education were noted between the patients with AMTI and healthy controls (Table 1).
Decreased Sleep Quality in Patients with Acute Minor Thalamic Infarction
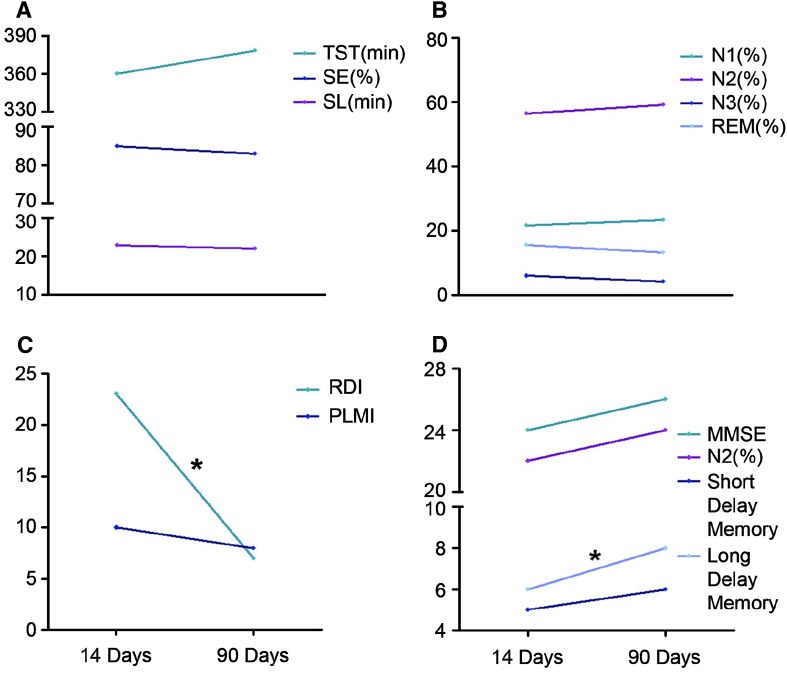
Hyposomnia was more pronounced in patients within the first 14 days, and this was accompanied by decreased self-estimated sleepiness at night and increased day-time sleepiness. Compared to healthy controls, the patients had significantly decreased levels of SE, TST decreased by 42 min, and the SL was 14 min longer according to PSG assessment on day 14 after the stroke (Table 2). Sleep quality and hyposomnia slightly improved within 90 days of the stroke (Fig. 2A) but they were still worse than in healthy controls (Table 2).
Table 2.
Sleep characteristics at days 14 and 90 after minor thalamic infarction.
| Healthy controls (n = 12) | 14 days post-stroke (n = 27) | 90 days post-stroke (n = 6) | P (14 days vs healthy controls) | P (90 days vs healthy controls) | |
|---|---|---|---|---|---|
| TST (min) | 424.8 ± 17.4 | 382.1 ± 14.7 | 378.8 ± 15.2 | 0.0959 | 0.1345 |
| SL (min) | 9.2 ± 1.9 | 23.1 ± 4.7 | 22.5 ± 6.9 | 0.0336 | 0.0427 |
| SE (%) | 88.7 ± 1.9 | 78.8 ± 2.6 | 83.2 ± 2.7 | 0.0202 | 0.0459 |
| WASO | 21.1 ± 2.4 | 21.4 ± 2.6 | 20.3 ± 2.5 | 0.9482 | 0.8336 |
| N1 (%) | 12.3 ± 1.6 | 25.5 ± 2.4 | 23.3 ± 3.1 | <0.0001 | 0.0002 |
| N2 (%) | 61.1 ± 1.9 | 54.1 ± 1.6 | 59.3 ± 3.2 | <0.0001 | 0.0423 |
| N3 (%) | 10.6 ± 1.9 | 6.3 ± 1.0 | 4.2 ± 2.0 | 0.0332 | 0.0297 |
| REM (%) | 16.0 ± 1.5 | 13.7 ± 1.1 | 13.1 ± 2.1 | 0.2261 | 0.3761 |
| Arousal index | 15.6 ± 1.6 | 12.9 ± 1.2 | 14.9 ± 2.3 | 0.2034 | 0.5321 |
| RDI | 4.3 ± 3.0 | 12.0 ± 2.9 | 4.0 ± 1.6 | 0.0453 | 0.5261 |
| RDI > 5, number (%) | 1 (8.3) | 16 (59.2) | 3 (50.0) | 0.0045 | 0.0833 |
| nadir SpO2 (%) | 89.6 ± 1.1 | 86.8 ± 1.1 | 86.7 ± 1.2 | 0.1614 | 0.2456 |
| PLMI | 2.2 ± 0.9 | 13.7 ± 5.1 | 10.0 ± 4.2 | 0.1678 | 0.3145 |
| PLMI > 5, number (%) | 3 (25) | 11 (40.7) | 3 (50.0) | 0.0824 | 0.3441 |
| PSQI | 1.8 ± 0.8 | 6.6 ± 0.6 | 5.1 ± 1.1 | <0.0001 | <0.0001 |
| ESS | 5.3 ± 0.9 | 8.0 ± 1.1 | 8.2 ± 2.0 | <0.0001 | <0.0001 |
Plus-minus values are mean ± SEM.
TST, total sleep time; SE, sleep efficiency; SL, latency to sleep onset; WASO, wake time after sleep onset; RDI, respiratory disturbance index; nadir SpO2, minimal oxy-hemoglobin saturation; PLMI, periodic leg movement index; REM, rapid eye movement; ESS, Epworth sleepiness scale; PSQI, Pittsburgh sleep quality index.
Fig. 2.
Sleep (A–C) and cognition (D) characteristics on days 14 and 90 after stroke. A Sleep quality. B Sleep structure. C Sleep disordered breathing and periodic leg movements. D Cognitive function. *P < 0.05.
Disturbed Sleep Structure in Patients with Acute Minor Thalamic Stroke
Compared with healthy controls at 14 days, patients with AMTI had a 7% decrease in N2 sleep, a 4% decrease in N3 sleep, and N1 sleep lengthened by 13% (Table 2). The duration of REM sleep and arousal index were similar in the two groups at 14 days. In the total group of patients, sleep structure disturbance mildly improved from the acute phase to 90 days later (Fig. 2B), but was still worse than in healthy controls (Table 2).
Prevalence of Periodic Leg Movements (PLMs) in Patients with Acute Minor Thalamic Infarction
The PLMI in patients at 14 days was higher than in their healthy counterparts but the difference was not statistically significant due to the wide variability (Table 2). As evaluated by PSG, a value > 5 was used to diagnose PLM, and with that criterion, this increased PLMI was found in 11 of 27 patients as well as in 3 of 12 healthy controls. There was no significant change in the PLMI from days 14 to 90 day after stroke (Fig. 2C).
Disordered Breathing During Sleep in Patients with Acute Minor Thalamic Stroke
Sixteen of 27 (59.2%) patients with thalamic infarction had a respiratory disturbance-hypopnea index > 5 at 14 days post-stroke. Among them, 4 had severe sleep apnea (15 > RDI > 5); 4 had moderate sleep apnea (30 > RDI > 15), and 4 had severe sleep apnea (RDI > 30). The rate and level of disordered breathing during sleep in the patient group exceeded those in healthy controls (Table 2). The nadir SpO2 in these patients was slightly lower than in controls but the difference was statistically insignificant. Most sleep-related breathing parameters such as the number of apneas and hypopneas, including obstructive central and mixed apneas, decreased significantly at day 90 (Fig. 2C) and approached that of healthy controls (Table 2). Despite this improvement, 50% of patients still had an RDI > 5 at 90 days after the stroke.
Cognitive Deficits in Patient with Acute Minor Thalamic Stroke
MMSE and MoCA scores were significantly lower in patients with AMTI than in healthy controls at 14 days post-stroke. Among the impaired cognitive functions, short-delay and long-delay memory were especially profoundly decreased (Table 3). And, sleep abnormalities (N1, N2, N3, and RDI) were associated with cognitive dysfunctions (MMSE, MoCA, short-delay memory, long-delay memory) at day 14, which suggested that AMTI induces sleep and cognitive dysfunctions at the same time (Table 4). Only long-delay memory significantly increased and approached that of healthy controls, while the other cognitive deficits still lingered 90 days later (Fig. 2D; Table 3).
Table 3.
Cognition at days 14 and 90 after minor thalamic infarction.
| Healthy controls (n = 12) | 14 days post-stroke (n = 27) | 90 days post-stroke (n = 6) | P (14 days vs healthy controls) | P (90 days vs healthy controls) | |
|---|---|---|---|---|---|
| MMSE | 29.3 ± 0.2 | 22.5 ± 1.1 | 25.5 ± 2.0 | <0.0001 | 0.0461 |
| MoCA | 28.3 ± 0.3 | 18.2 ± 1.1 | 22.8 ± 2.5 | <0.0001 | 0.0231 |
| AVLT | |||||
| Short-delay memory | 7.2 ± 0.8 | 4.6 ± 0.7 | 6.0 ± 1.6 | 0.0203 | 0.0493 |
| Long-delay memory | 9.1 ± 0.7 | 4.8 ± 0.7 | 7.5 ± 2.0 | 0.0037 | 0.2127 |
| Recognition | 12.9 ± 0.6 | 11.3 ± 0.8 | 12.3 ± 0.9 | 0.8792 | 0.9653 |
Plus-minus values are mean ± SEM.
MMSE, mini-mental state examination; MoCA, Montreal cognitive assessment; AVLT, Auditory verbal learning test.
Table 4.
Correlation between sleep and cognitive dysfunctions at day 14 (Spearman r).
| MMSE | MoCA | Short-delay memory | Long-delay memory | |
|---|---|---|---|---|
| SL (min) | −0.1330 | −0.1538 | −0.0602 | −0.0602 |
| SE (%) | 0.2789 | 0.2015 | 0.2150 | 0.0847 |
| N1 (%) | −0.3429 | −0.5768** | −0.3261 | −0.3940* |
| N2 (%) | 0.4195* | 0.5291** | 0.3380 | 0.1489 |
| N3 (%) | 0.3488 | 0.4161* | 0.3095 | 0.3487 |
| RDI | −0.4555* | −0.4393* | −0.4636* | −0.5001** |
| PSQI | −0.1392 | −0.0763 | −0.2559 | −0.4141 |
| ESS | −0.2172 | −0.1037 | −0.1989 | −0.1989* |
SE, sleep efficiency; SL, latency to sleep onset; RDI, respiratory disturbance index; ESS, Epworth sleepiness scale; PSQI, Pittsburgh sleep quality index; MMSE, mini-mental state examination; MoCA, Montreal cognitive assessment; AVLT, auditory verbal learning test.
* P < 0.05; ** P < 0.01.
Discussion
Our results offer a comprehensive analysis of sleep and cognitive disturbances after AMTI. Analysis of all relevant tests revealed the following five novel results: (1) hyposomnia was more pronounced in these patients, as indicated by PSQI and ESS analysis; (2) sleep architecture was disrupted in these patients, as indicated by decreased TST and SE, increased SL, and decreased N3 and N2; (3) during the acute phase after the stroke, these patients had significantly more episodes of sleep-related breathing disorders than healthy controls; (4) the patients had worse cognitive function, especially memory function, than healthy controls; and (5) while sleep apnea and long-delay memory recovered to a large extent in the patients, other deficits in sleep and cognitive function persisted.
In this study, patients were included consecutively. As a typical university Department of Neurology diagnoses ~10 minor thalamic strokes each year, patients in this study were collected over >2 years. During this time, we systematically investigated the neurological, cognitive, and sleep–wake deficits wherever possible in a routine clinical setting. We are therefore confident that our data set is representative of this stroke syndrome. However, in our study, not all patients received both kinds of investigation (i.e., cognitive function and sleep assessment), which was impossible for logistical reasons related to patient care. We compensated for this lack of a uniform patient cohort by sampling high patient numbers in both data subsets providing the statistical power necessary to draw possible conclusions on the recovery of cognitive and sleep deficits.
Hyposomnia was more pronounced in patients with AMTI and there was mild improvement within 3 months post-stroke. Sleep architecture was also worse and did not show any improvement over time. In fact, increases of stage 1 sleep as well as decreases of stages 2 and 3 in the patients, which was independent of the hemisphere affected by the stroke, remained abnormal, representing a “sleep EEG signature” of thalamic infarcts. Our data are in line with previous observations in which these persistent disturbances in sleep patterns have been reported [16]. In addition, during the acute phase, our patients had significantly more episodes of sleep-related breathing disorder, both central and obstructive, than healthy controls. This difference largely disappeared in the chronic phase, mainly in the form of a marked decrease of hypopneas. Even so, more patients with thalamic infarction (50%) remained at pathological levels (RDI > 5) 3 months after the stroke compared with healthy controls (8%), in agreement with previous studies [17–21]. Together with our results, this suggests that monitoring of sleep-related breathing might also be considered in the chronic phase to identify patients with significant sleep-related breathing disorders.
Compared with the healthy group, all neuropsychological test scores (MMSE, MoCA, and AVLT) were worse in the patient group, short- and long-delay memory problems being more evident, suggesting that thalamic lesions result in a general decrease in cognitive function and memory. Both the dorsomedial and the anterior nuclei of the thalamus are involved in memory function [22] and Papez circuit disruption by thalamic damage also causes memory and emotional impairment [23], which may provide an anatomical explanation for the results that the memory deficit was more prominent and recovered little in patients with minor thalamic stroke in our study.
Our study has some limitations which have to be taken into consideration. For one, the sample size was relatively small, resulting in low statistical power for detecting significant differences between groups. Since the number of participants in this study was not sufficient to allow multivariate analyses to produce more accurate and general conclusions, more careful evaluation in a large number of participants will be required in future. Another limitation is the low stroke severity in our sample, but these may be features specific for minor thalamic stroke. A further caveat concerns the PSG recordings: sleep was initially recorded in the Stroke Unit during the acute phase and subsequently in the Sleep Laboratory 3 months later. The possibility, therefore, remains that the change in testing location contributed to the sleep characteristics that we recorded. Brain rhythm abnormalities also should be monitored during wakefulness in the future, as there is a report that thalamic lesions are sometimes associated with a pathologic delta rhythm in overlying cortical areas during wakefulness [24].
In summary, we found that patients with minor thalamic lesions are at increased risk for sleep disturbance, sleep-related breathing disorders, and memory deficits which are frequent in this group. Although the sleep disturbance, sleep-related breathing disorders, and memory improved over time, the slow and incomplete improvement and the poor recovery of patients with minor thalamic infarction suggest that early treatment interventions should be considered for these patients.
Acknowledgments
This work was supported by the Health Industry Key Research Project of Tianjin Municipality, China (12KG132), and the Science and Technology Plan Project of Tianjin Municipality, China (13ZCZDSY01900).
Footnotes
Wei Wu and Linyang Cui have contributed equally to this work.
References
- 1.Pasic Z, Smajlovic D, Dostovic Z, Kojic B, Selmanovic S. Incidence and types of sleep disorders in patients with stroke. Med Arh. 2011;65:225–227. doi: 10.5455/medarh.2011.65.225-227. [DOI] [PubMed] [Google Scholar]
- 2.Terzoudi A, Vorvolakos T, Heliopoulos I, Livaditis M, Vadikolias K, Piperidou H. Sleep architecture in stroke and relation to outcome. Eur Neurol. 2009;61:16–22. doi: 10.1159/000165344. [DOI] [PubMed] [Google Scholar]
- 3.Empana JP, Dauvilliers Y, Dartigues JF, Ritchie K, Gariepy J, Jouven X, et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke: J Cereb Circ 2009, 40: 1219–1224. [DOI] [PubMed]
- 4.Fama R, Sullivan EV. Thalamic structures and associated cognitive functions: Relations with age and aging. Neurosci Biobehav Rev. 2015;54:29–37. doi: 10.1016/j.neubiorev.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassetti C, Mathis J, Gugger M, Lovblad KO, Hess CW. Hypersomnia following paramedian thalamic stroke: a report of 12 patients. Ann Neurol. 1996;39:471–480. doi: 10.1002/ana.410390409. [DOI] [PubMed] [Google Scholar]
- 6.Hermann DM, Siccoli M, Brugger P, Wachter K, Mathis J, Achermann P, et al. Evolution of neurological, neuropsychological and sleep-wake disturbances after paramedian thalamic stroke. Stroke: J Cereb Circ 2008, 39: 62–68. [DOI] [PubMed]
- 7.Guilleminault C, Quera-Salva MA, Goldberg MP. Pseudo-hypersomnia and pre-sleep behaviour with bilateral paramedian thalamic lesions. Brain. 1993;116:1549–1563. doi: 10.1093/brain/116.6.1549. [DOI] [PubMed] [Google Scholar]
- 8.Bjornstad B, Goodman SH, Sirven JI, Dodick DW. Paroxysmal sleep as a presenting symptom of bilateral paramedian thalamic infarctions. Mayo Clin Proc. 2003;78:347–349. doi: 10.4065/78.3.347. [DOI] [PubMed] [Google Scholar]
- 9.Woerner J, Friolet R, Ventura F, Kardan R, Vuadens P, Arnold P. Acute bilateral paramedian thalamic infarction presenting on EEG as stage 2 non-REM sleep. Cerebrovasc Dis. 2005;19:407–409. doi: 10.1159/000086102. [DOI] [PubMed] [Google Scholar]
- 10.Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hakkinen V, Hirvonen K, Hasan J, Kataja M, Varri A, Loula P, et al. The effect of small differences in electrode position on EOG signals: application to vigilance studies. Electroencephalogr Clin Neurophysiol. 1993;86:294–300. doi: 10.1016/0013-4694(93)90111-8. [DOI] [PubMed] [Google Scholar]
- 12.Roth T, Coulouvrat C, Hajak G, Lakoma MD, Sampson NA, Shahly V, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Cui GH, Yao YH, Xu RF, Tang HD, Jiang GX, Wang Y, et al. Cognitive impairment using education-based cutoff points for CMMSE scores in elderly Chinese people of agricultural and rural Shanghai China. Acta Neurol Scand. 2011;124:361–367. doi: 10.1111/j.1600-0404.2010.01484.x. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry. 2012;12:156. doi: 10.1186/1471-244X-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Fang R, Liu LH, Chen SD, Tang HD. Clinical Characteristics for the Relationship between Type-2 Diabetes Mellitus and Cognitive Impairment: A Cross-Sectional Study. Aging Dis. 2015;6:236–244. doi: 10.14336/AD.2014.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris AL, Elder J, Schiff ND, Victor JD, Goldfine AM. Post-stroke apathy and hypersomnia lead to worse outcomes from acute rehabilitation. Transl Stroke Res. 2014;5:292–300. doi: 10.1007/s12975-013-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parra O, Arboix A, Bechich S, Garcia-Eroles L, Montserrat JM, Lopez JA, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161:375–380. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 18.Harbison J, Ford GA, James OF, Gibson GJ. Sleep-disordered breathing following acute stroke. QJM : monthly journal of the Association of Physicians. 2002;95:741–747. doi: 10.1093/qjmed/95.11.741. [DOI] [PubMed] [Google Scholar]
- 19.Broadley SA, Jorgensen L, Cheek A, Salonikis S, Taylor J, Thompson PD, et al. Early investigation and treatment of obstructive sleep apnoea after acute stroke. J Clin Neurosci. 2007;14:328–333. doi: 10.1016/j.jocn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Hui DS, Choy DK, Wong LK, Ko FW, Li TS, Woo J, et al. Prevalence of sleep-disordered breathing and continuous positive airway pressure compliance: results in chinese patients with first-ever ischemic stroke. Chest. 2002;122:852–860. doi: 10.1378/chest.122.3.852. [DOI] [PubMed] [Google Scholar]
- 21.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke: J Cereb Circ 2006, 37: 967–972. [DOI] [PubMed]
- 22.Exner C, Weniger G, Irle E. Implicit and explicit memory after focal thalamic lesions. Neurology. 2001;57:2054–2063. doi: 10.1212/WNL.57.11.2054. [DOI] [PubMed] [Google Scholar]
- 23.Nishio Y, Hashimoto M, Ishii K, Ito D, Mugikura S, Takahashi S, et al. Multiple thalamo-cortical disconnections in anterior thalamic infarction: implications for thalamic mechanisms of memory and language. Neuropsychologia. 2014;53:264–273. doi: 10.1016/j.neuropsychologia.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Gloor P, Ball G, Schaul N. Brain lesions that produce delta waves in the EEG. Neurology. 1977;27:326–333. doi: 10.1212/WNL.27.4.326. [DOI] [PubMed] [Google Scholar]