Abstract
Sperm competition games investigate how males partition limited resources between pre- and post-copulatory competition. Although extensive research has explored how various aspects of mating systems affect this allocation, male allocation between mating, fertilization and parental effort has not previously been considered. Yet, paternal care can be energetically expensive and males are generally predicted to adjust their parental effort in response to expected paternity. Here, we incorporate parental effort into sperm competition games, particularly exploring how the relationship between paternal care and offspring survival affects sperm competition and the relationship between paternity and paternal care. Our results support existing expectations that (i) fertilization effort should increase with female promiscuity and (ii) paternal care should increase with expected paternity. However, our analyses also reveal that the cost of male care can drive the strength of these patterns. When paternal behaviour is energetically costly, increased allocation to parental effort constrains allocation to fertilization effort. As paternal care becomes less costly, the association between paternity and paternal care weakens and may even be absent. By explicitly considering variation in sperm competition and the cost of male care, our model provides an integrative framework for predicting the interaction between paternal care and patterns of paternity.
Keywords: offspring survival, parental investment, paternity, polyandry, sexual selection
1. Introduction
Over the last decades, evolutionary biology has witnessed an explosion of studies of sperm competition in several taxonomic groups, enhancing our understanding of how various ecological and evolutionary conditions affect male reproductive strategies [1–3]. Contrary to what was traditionally assumed, ejaculate production can be energetically demanding, imposing high energetic costs on males [4–6]. Therefore, males are likely to experience a trade-off between investing in traits that increase ejaculate production and traits that increase their mating success [7–10]. Evolutionary models, in particular, sperm competition games, represent a powerful approach to predict how such trade-offs affect the evolution of male reproductive allocation strategies under sperm competition (defined as sperm of rival males competing to fertilize the same clutch of eggs [1,2]). Since sperm competition games were first introduced [11], several extensions have been formulated in the field, exploring, for example, the additional effects of male alternative reproductive tactics [12], sperm displacement by newcomer ejaculates [13], and different forms of pre-copulatory male competition [14]. These models assume that males partition their limited energetic reserves solely between pre- and post-copulatory competition. Sperm competition games have, therefore, largely ignored how paternal care will affect and be affected by male ejaculate allocation.
Paternal care is expressed in a great variety of forms in nature. Males may carry eggs, provision the young, build and defend nests, or just stay around [15–22]. Such behaviours greatly increase offspring survival due to their roles in protection against natural enemies and providing resources necessary for proper offspring development and growth [20–23]. Parental care may also impose a high fitness cost on adults, in terms of increased mortality risks and energetic expenditures and decreased fertility [20–22,24,25]. However, the extent to which offspring performance is improved by additional paternal effort and the consequent cost imposed on males are likely to vary among species as the result of historical and physiological processes, and across ecological conditions. In fact, males from several species are known to change the intensity of their parental behaviour in response to, for example, variation in oxygen availability [26,27], food availability [28,29], adult predation risk [30,31] and egg predation risk [29,32]. Therefore, variation in the level of paternal effort necessary for adequate offspring survival and maintenance affects the associated cost imposed on males and, consequently, is likely to intensify or weaken the trade-off between male allocation to other components of fitness.
Paternal effort is predicted to not only depend on offspring needs but also respond to variation in expected paternity, with males reducing care when they are less likely to be related to the brood (e.g. [33–36], but see [37,38]). Within species, behavioural adjustments in paternal effort are expected when cuckoldry is common, paternity varies between reproductive events and the cost of paternal care is high [23,37]. The positive association between paternity and paternal care among species, however, does not arise from such fine adjustments, but instead as the result of male life-history trade-offs affecting species-specific levels of paternal effort over evolutionary time. Although empirical evidence shows some support for a positive relationship between paternity and paternal effort, both within [23,35] and among species [17,39–42], extensive unexplained variation remains [15,23,43–46].
As widely acknowledged, however, paternity is not a static trait but is instead the outcome of social interactions within and between the sexes [35,37,47]. It is, therefore, essential to consider explicitly how these interactions simultaneously affect patterns of mating, fertilization and parental care, and thus drive the relationship between paternity and paternal effort [35,47]. In this context, sperm competition games provide a powerful framework within which to explore intra- and inter-sexual interactions, as they determine the level of sperm competition, which in turn selects on male differential allocation to ejaculates at the expense of other components of fitness [1,2]. Here, we ask whether simultaneous allocation between mating, fertilization and parental effort in the presence of variation in the cost of male care can contribute to our understanding of some of the unexplained variation observed in the association between paternal care and paternity.
In this paper, we extend sperm competition games by modelling male allocation between traits that affect mating success, ejaculate production and parental care. Building on existing theory [1,2], we consider how the effect of male allocation to parental effort on offspring survival is predicted to affect the relationship between mating rate, fertilization success and paternal care. In particular, we explore between-species variation in the cost of male care, from scenarios where parental behaviour is energetically inexpensive (i.e. low allocation to parental effort results in relatively high offspring survival) to situations where care is very costly (i.e. equivalent offspring survival requires high male allocation to care; see electronic supplementary material, S1). The main goals of this study are to evaluate how variation among species in female promiscuity and the cost of male care affect (i) male allocation between obtaining matings, producing ejaculates and providing parental care and (ii) the association between paternal care and paternity that emerges as the result of male allocation between these three different components of fitness. Our findings reveal that the cost of paternal care can drive how males respond to sperm competition and whether a detectable empirical association is predicted to exist between paternity and paternal care.
2. Model description
In order to incorporate paternal care into sperm competition games and make direct comparisons between our model and earlier theory, we adopt a few key assumptions made by previous models. For example, we assume that individuals reproduce in a large population (with 1 : 1 sex ratio and negligible chances of double mating between any two individuals), where females determine their own mating rate and, consequently, the intensity of sperm competition (see reviews in [1,2]). Moreover, males have the same total energy budget available for reproduction that is partitioned between n reproductive events, such that a male allocates C units to obtaining each mating and s units to the one ejaculate transferred during each insemination event. A key difference in our model is that males must also allocate P units of their total energy budget to paternal care towards one clutch produced by any particular female during the breeding season (see table 1 for the full list of variables). The general reproductive dynamic represented by this modelling structure is that males and females may mate multiply during the breeding season, but that females lay a single clutch of eggs with one male after this period of mating (fertilized by the sperm of their f mates) and that males only care for one clutch of eggs per reproductive bout. While this does not capture all species, this mating pattern is consistent with species with paternal care (i) in which males and females exhibit social monogamy but may mate multiply (as is common in many birds [17] and mammals [18]), (ii) in which females mate with multiple males but lay their clutch in the nest or territory of their final mate (as observed in some arthropods [23]) or (iii) in which fertilization is external and multiple males release their gametes simultaneously over one clutch of eggs (as is common in many fish [15,48] and amphibians [22]).
Table 1.
Definition of parameters and variables used in the model.
| symbol | definition |
|---|---|
| f | female mating rate |
| C | energetic expenditure to obtain each mating event |
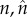 |
number of mating events, respectively, of the mutant and the wild-type males |
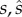 |
allocation strategy adopted, respectively, by the mutant and the wild-type males in terms of the energetic expenditure of the one ejaculate transferred after each mating event |
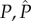 |
allocation strategy adopted, respectively, by the mutant and the wild-type males in terms of the energetic expenditure of the parental activities towards only one clutch produced during the breeding season |
| α | shape parameter of the exponential function relating male allocation to parental care P and the probability of offspring survival |
| B | intercept parameter of the exponential function relating male allocation to parental care P and the probability of offspring survival |
A male that allocates more to parental care increases the survival of his offspring at the cost of reducing his mating opportunities and/or his sperm competitiveness. In this sense, while C represents the energetic cost of obtaining each mating event (involved, for example, in mate searching, territory defence or male–male competition) that is experienced equally by all males within a population, male allocation strategies vary in their expenditure in s and P, and, consequently, their mating rate n. By scaling the total energy budget to 1 (after [1]), the parameters representing male allocation strategies can be readily interpreted as relative allocation to different components of fitness, such that
where a symbol denotes the allocation strategy adopted by wild-type males in the population, and unmodified symbols denote allocation by the mutant male (following standard methods [1]). Each component of male fitness, i.e. mating effort (M = C · n), fertilization effort (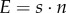 ) and parental effort (P), is expressed as dimensionless proportions of the total energy budget and therefore enables direct comparison among species. The relative gain in mating success n of a mutant male adopting the allocation strategies s and P, in a population of males adopting the allocation strategies
) and parental effort (P), is expressed as dimensionless proportions of the total energy budget and therefore enables direct comparison among species. The relative gain in mating success n of a mutant male adopting the allocation strategies s and P, in a population of males adopting the allocation strategies  and
and 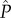 , is given by
, is given by
| 2.1 |
Besides including the additional allocation to parental effort, we also need to explicitly establish the contribution of paternal care to males' fitness in our model. Following previous models of parental investment [33,36,49–51], we model the relationship between parental effort and offspring survival as a nonlinear function with diminishing returns. Here, we assume that offspring survival depends on male allocation to care, so that the probability of offspring survival approaches 1 as P increases. However, offspring survival is also determined by biotic and abiotic conditions that affect offspring development and growth, such as oxygen and temperature levels, food availability, predation risk and maternal care. Although distinguishing the exact source of how offspring survival changes with parental effort might be useful to understand their particular effects, for the purpose of our model, we represent them together as the cost of male care. Therefore, we model offspring survival following the exponential function:
| 2.2 |
where e is the base of the natural logarithm and B and α represent, respectively, the intercept and the shape parameter of the exponential function that captures how offspring survival depends on male allocation to parental effort. Another novelty of our model is that we explicitly incorporate how variation between species in the cost of paternal care affects the pay-offs of male parental allocation strategy in two distinct components: the baseline level B of offspring survival without any paternal care and the difficulty α of providing care. The higher the values for α and/or B, the lower the energetic cost of paternal care and the lower the allocation to care needed to achieve high offspring survival (see electronic supplementary material, S1). Biologically, the cost of paternal care varies among species and is assumed to be determined by historical and ecological processes, physiological constraints or maternal behaviour, instead of being under males’ control.
In our model, the fitness of any male is directly proportional to the number of offspring produced that survives the caring period. As described in greater detail earlier, our model captures the situation where males and females mate multiply during the breeding season and each male provides parental care to one potentially mixed-paternity brood laid by his social or final mate. Therefore, a mutant male's fitness depends on (i) his mating rate 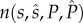 , (ii) his fertilization success after each mating (i.e. proportional to the amount of his ejaculate relative to the amount released by the other f − 1 males that copulate with the focal female—following a ‘fair raffle’ sensu [11]), (iii) the survival of his genetic offspring present in the single clutch left under his care, which depends on his own allocation P to care (i.e. within-pair) and (iv) the survival of his genetic offspring present in the clutches produced by the other
, (ii) his fertilization success after each mating (i.e. proportional to the amount of his ejaculate relative to the amount released by the other f − 1 males that copulate with the focal female—following a ‘fair raffle’ sensu [11]), (iii) the survival of his genetic offspring present in the single clutch left under his care, which depends on his own allocation P to care (i.e. within-pair) and (iv) the survival of his genetic offspring present in the clutches produced by the other 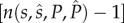 females and left with other males, which depends on their allocation
females and left with other males, which depends on their allocation 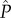 to care (i.e. extra-pair). For simplicity, we do not incorporate variation in the number of eggs per clutch among females or females' reproductive events. Then, the expected fitness of a mutant male adopting the allocation strategies s and P, in a population of males with the mean allocation strategies
to care (i.e. extra-pair). For simplicity, we do not incorporate variation in the number of eggs per clutch among females or females' reproductive events. Then, the expected fitness of a mutant male adopting the allocation strategies s and P, in a population of males with the mean allocation strategies  and
and 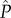 , is given by
, is given by
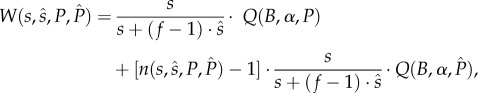 |
2.3 |
where the first term represents the survival of the proportion of his genetic offspring within-pair, while the second term represents the survival of the proportion of the offspring extra-pair.
3. Analytical solution
Using standard methods [52], we can find the analytical solution for the evolutionarily stable allocation (ESA) strategies in four steps. First, we obtain the following ESA to ejaculates by solving 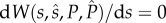 at
at 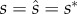 and
and 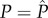 :
:
| 3.1a |
Then, setting  and solving
and solving 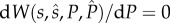 at
at  , we obtain the ESA to paternal care as
, we obtain the ESA to paternal care as
| 3.1b |
The next step regards ensuring not only that the model is internally consistent (regarding its mathematical and logical properties), but also that it is biologically consistent [37,53]. In particular, the model must respect the ‘Fisher condition’: in a diploid species with sexual reproduction, each offspring must necessarily be the result of the fertilization of one female gamete by one male gamete and each mating event must occur between a male and a female [37,53]. Therefore, to ensure self-consistency in a reproducing population with 1 : 1 sex ratio, female and male mating rates must be the same at equilibrium. When females determine their own mating rate, the parameter that ensures such self-consistency is the cost C of males obtaining each mating. To satisfy the ‘Fisher condition’, we solve the equality f = n* for C. Although the cost C may be treated independently from the female mating rate and the cost of male care (parameters α and B), it needs to be a function of all these three parameters such that:
| 3.1c |
where g(x) is the solution z in 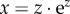 (see electronic supplementary material, S2). Biologically, the ‘Fisher condition’ dictates that, when females determine their own mating rate, low levels of female promiscuity entail high cost to males of obtaining each mating, while high promiscuity is associated with low cost of mating for males.
(see electronic supplementary material, S2). Biologically, the ‘Fisher condition’ dictates that, when females determine their own mating rate, low levels of female promiscuity entail high cost to males of obtaining each mating, while high promiscuity is associated with low cost of mating for males.
The fourth step checks whether the equilibrium solutions (i.e.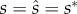 and
and  ) represent fitness maxima. This condition is satisfied for functions with multiple variables if the Hessian matrix has no positive eigenvalues [52]. In our model, equations (3.1a–c) represent fitness maxima when f > 2, B ≥ 0 and α ≥ 10 (see electronic supplementary material, S3). We explore the parametric space delimited by 2 ≤ f ≤ 15 (where females mate multiply, representing an ‘intensity’ sperm competition game [1,2]), 0 ≤ B ≤ 0.8 (which includes scenarios where the lack of male care condemns the entire clutch to death to scenarios where only a small subset of the offspring does not survive; see electronic supplementary material, S1) and 10 ≤ α ≤ 80, and, therefore, our interpretation is restricted to this parameter space. The predicted ESA to mating, fertilization and parental effort are given by
) represent fitness maxima. This condition is satisfied for functions with multiple variables if the Hessian matrix has no positive eigenvalues [52]. In our model, equations (3.1a–c) represent fitness maxima when f > 2, B ≥ 0 and α ≥ 10 (see electronic supplementary material, S3). We explore the parametric space delimited by 2 ≤ f ≤ 15 (where females mate multiply, representing an ‘intensity’ sperm competition game [1,2]), 0 ≤ B ≤ 0.8 (which includes scenarios where the lack of male care condemns the entire clutch to death to scenarios where only a small subset of the offspring does not survive; see electronic supplementary material, S1) and 10 ≤ α ≤ 80, and, therefore, our interpretation is restricted to this parameter space. The predicted ESA to mating, fertilization and parental effort are given by
| 3.2a |
| 3.2b |
| 3.2c |
Finally, because male ESA strategies at equilibrium are predicted to go to fixation in the population over time, the paternity achieved by each male in their clutches (i.e. within-pair paternity) is given by 1/f = 1/n*.
4. Theoretical predictions
(a). Case 1: offspring survival depends entirely on paternal care
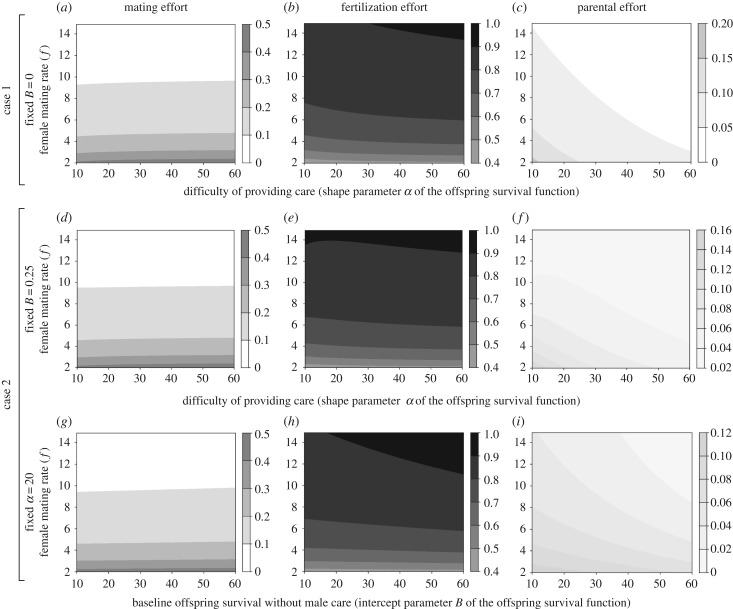
We first consider the case where offspring cannot survive without paternal care (i.e. obligate male care—the baseline offspring survival B equals 0). This may occur in species that experience harsh ecological conditions for offspring performance (survival and/or development) and/or in which maternal care is absent (i.e. paternal allocation P > 0 is necessary for offspring survival; for further details see electronic supplementary material, S1). In this scenario, increasing female promiscuity favours male strategies that increase the allocation of energy to fertilization effort, and, as a consequence, less energy is left for allocation to mating and parental effort (figure 1a–c). This predicted pattern, however, is affected by the extent of the difficulty of providing care. When ecological and physiological conditions do not impose a high energetic cost of paternal care (i.e. high α-values), low allocation to parental effort is sufficient to ensure high offspring survival (see electronic supplementary material, S1 and S4). For this biological scenario, a low cost of care, therefore, favours lower male allocation to parental effort (figure 1c), allowing males to allocate more energy to obtaining matings (figure 1a) and producing ejaculates (figure 1b). As ecological and physiological conditions cause the cost of paternal care to increase (i.e. decreases in α-values), higher allocation to care will be required for offspring to survive (see electronic supplementary material, S1 and S4), which favours increasing allocation to parental effort (figure 1c) and, consequently, reduces allocation to mating and fertilization effort (figure 1a,b).
Figure 1.
Evolutionary stable allocation strategies to mating, fertilization and parental effort in response to sperm competition intensity (determined by female mating rate) and two components of the cost of male care. (a–c) Predictions for case 1, where the baseline offspring survival without male care B equals zero. (d–f) Predictions for case 2, in particular when B = 0.25. (g–i) Predictions for case 2, in particular, when the shape parameter α of the exponential function of offspring survival in response to paternal effort equals 20. High values of α (a–f) or B (g–i) represent scenarios where male care is energetically inexpensive. The colour code for the predicted relative allocation to each component of fitness is shown in the corresponding bar at the right of each graph.
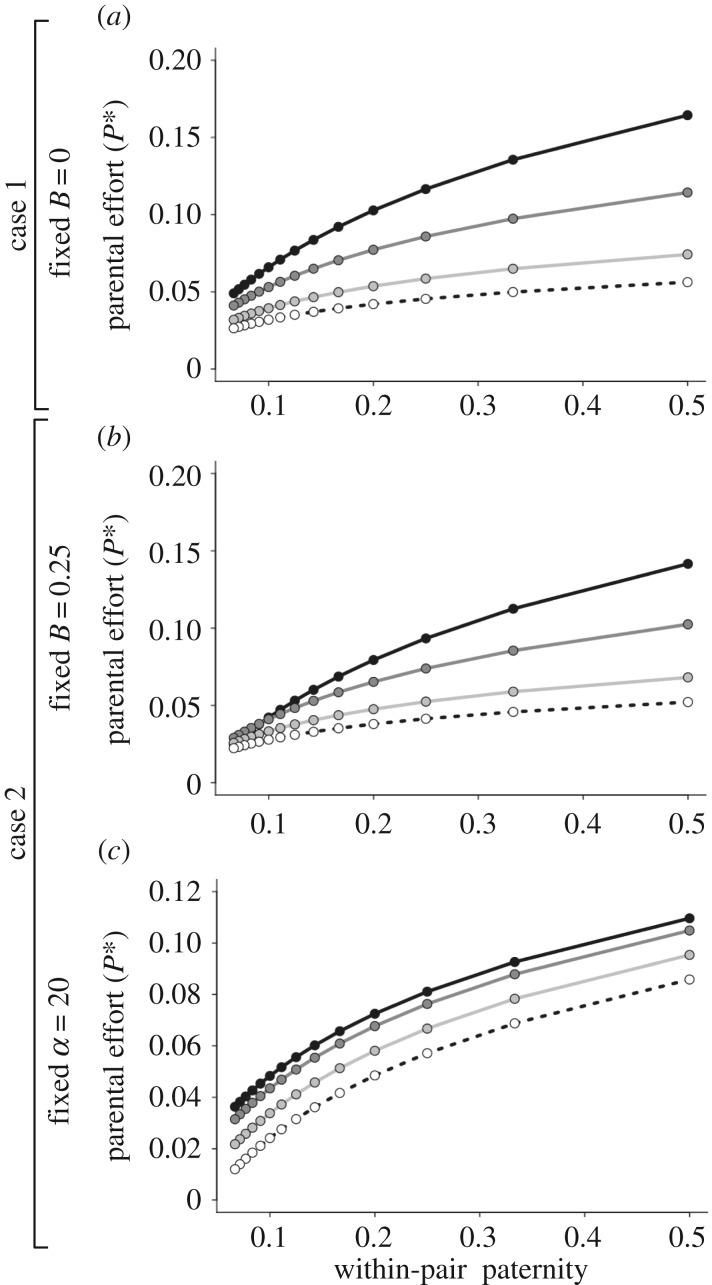
Our model also predicts a positive association between the ESA to parental effort and the expected average within-pair paternity (figure 2a). This pattern, however, is not predicted from an explicit or assumed effect of relatedness on male allocation to care, but instead emerges from how males adaptively allocate to different components of fitness in response to the interactive effect between sperm competition intensity and the cost of male care across species. This theory, therefore, predicts the same positive association between paternity and paternal care, but for a different underlying reason than the effect of relatedness on the benefits of care. In addition, the difference between the ESA to parental effort in species in which females mate just a few times (i.e. high expected average within-pair paternity) and the ESA to parental effort in highly polyandrous species (i.e. low expected average within-pair paternity) is modified by the cost of care (figure 2a). When ecological and physiological conditions impose a high energetic cost of male care (i.e. low α-values), the difference between species with low versus high females' promiscuity is great, with males allocating much more energy to parental effort in species with low than high mating rates (black line in figure 2a). As ecological and physiological conditions impose a decreasing cost of male care (i.e. increases in α-values), the difference in the ESA to parental effort among species diminishes, with males investing similarly to care regardless of female promiscuity, thus weakening the association between paternal care and within-pair paternity (other lines in figure 2a).
Figure 2.
The emergent association between the evolutionary stable allocation to parental effort and the expected average within-pair paternity across species depends on the cost of male care. (a) Case 1, where the baseline offspring survival without male care B equals zero, (b) case 2, in particular when B = 0.25 and (c) case 2, in particular, when the shape parameter α of the exponential function of offspring survival in response to paternal effort equals 20. For (a) and (b), the colour of the points and the lines depict scenarios with different values for the parameter α: black points and solid line when α = 10, dark grey points and solid line when α = 20, light grey points and solid line when α = 40, and white points and dashed line when α = 60. For (c), different colours depict scenarios with different values for the parameter B: black points and solid line when B = 0.10, dark grey points and solid line when B = 0.20, light grey points and solid line when B = 0.40, and white points and dashed line when B = 0.60.
(b). Case 2: offspring survival depends partially on paternal care
We now consider the situation when offspring can survive without paternal care (i.e. the baseline B > 0, see electronic supplementary material, S1). This may arise in species with male-only care, where, for example, ecological conditions for offspring performance (survival and/or development) are not so restrictive, or in species with biparental care, where females provide care independent of paternal behaviour. The qualitative predictions for this case are similar to those from case 1. First, although increases in female promiscuity also favour male strategies that preferentially allocate energy to fertilization effort (figure 1d–i), this pattern is modified by the extent of the cost of male care, represented in case 2 by the combination of two components: the difficulty of providing care (i.e. by the shape parameter α of the offspring survival function; figure 1d–f) and the baseline offspring survival (i.e. by the intercept parameter B of the offspring survival function; figure 1g–i). When ecological and physiological conditions do not impose a high energetic cost of care on males (i.e. high α- or B-values), less allocation to parental effort is favoured, while increases in the energetic cost of male care (i.e. decreases in either α- or B-values) favour increasing allocation to parental effort.
Second, case 2 also predicts an emergent positive association between parental effort and expected within-pair paternity, with the cost of male care modulating this association (figure 2b,c). The strength of this modification effect, however, depends on which of the two parameters that capture the different aspects of the cost of paternal care one considers. For the shape parameter α, when ecological and physiological conditions lead to care being difficult (i.e. costly) for males to provide (i.e. low α-values), the difference in parental effort between species where females mate just a few times and species where females are highly polyandrous is large (black line in figure 2b). In species where paternal care is less costly to provide (i.e. increases in α-values), the association between paternal care and within-pair paternity is predicted to be present but weaker (other lines in figure 2b) and thus may be harder to detect empirically. Finally, the baseline offspring survival B does not affect how parental effort is predicted to vary among species as a function of whether females mate just a few times or are highly polyandrous (figure 2c). Instead, variation across species in baseline offspring survival in the absence of male care, B, has only a small quantitative effect on parental effort (different lines in figure 2c).
The overall results from both cases explored here show that variation among species in ecological, physiological or maternal conditions that affect the cost of male parental behaviour in terms of the difficulty of providing care (i.e. the parameter α), but not the baseline offspring survival without care (i.e. the parameter B), is predicted to alter the strength of the association between parental effort and expected within-pair paternity. From an empirical perspective, all else being equal, an association between paternity and paternal effort will be more likely detected when focusing on species in which male care is consistently very difficult to provide. In species where the costs of care vary or male care is not difficult to provide, other aspects of the breeding system and the reproductive biology of species are more likely to explain patterns of parental effort across species than variation in female promiscuity and, ultimately, variation in paternity.
5. Discussion
Our model predicts how male allocation between mating, fertilization and parental effort is expected to evolve in response to among-species variation in the intensity of sperm competition and the cost of male care, both of which are likely to be shaped evolutionarily by even small changes in ecological conditions among species [26–32]. Consistent with ubiquitous theoretical predictions [1,2] and patterns observed in nature (e.g. insects: [9,54]; vertebrates: [7,8,10,54–56]), our model predicts that increases in sperm competition intensity favour male allocation to fertilization effort over other components of reproduction. Though sperm competition games have not previously been used to consider the situation where males must also allocate limited energy to parental effort, our results reveal that existing sperm competition expectations are generally robust, even for species in which males benefit from allocating a large amount of energy to paternal care. Moreover, our model predicts the positive association between parental effort and the expected average within-pair paternity that is predicted by parental investment theory [25,33], simply by considering that male allocation to paternity assurance (i.e. ejaculate production) and paternal care trade-off with each other (see similar arguments in [37,38]). Although previous work modelled the benefit function of parental care on offspring survival explicitly [36,50,51], little attention has been given to exploring variation in this function (but see [33,49]), despite the recognition that such variation may drive an association between parental effort and paternity that does not necessarily represent a causal relationship between these two variables [23,34,37,38]. Therefore, our theory not only shows that the core predictions of sperm competition theory also apply to species with paternal care, but also reveals an important role for the cost of male care in modulating both the evolution of male allocation to parental effort, the association between parental effort and paternity and our ability to detect and understand these patterns empirically.
Our model shows that the cost of paternal care can affect the resolution of life-history trade-offs between mating, fertilization and care. Conditions that increase this cost (due to either changes in the baseline offspring survival or in the difficulty of providing care) are predicted to favour strategies that divert energy from fertilization to parental effort, even at moderately high levels of sperm competition. To our knowledge, only a few empirical studies have addressed this question. In the black redstart Phoenicurus ochruros, increases in food availability (which likely represents decreases in the cost of paternal care) are indeed associated with decreases in male attendance and increases in male mating success [28], as expected by our model. In cuckoos with paternal care (which are likely to represent species where the cost of male assistance are stronger than species without care), male fertilization effort represented by testes size is higher (instead of lower) than in cuckoos without parental assistance [57], contrary to our model's prediction. A possible explanation for this latter case could be that the particular biotic or abiotic conditions that affect the cost of male care across species simultaneously affect other aspects of the mating system, such as, female promiscuity. In this sense, future empirical and theoretical work may contribute to our understanding on how and when the cost of male care should be expected to covary with sperm competition intensity. Nevertheless, there is still insufficient empirical data at present on how the cost of paternal care affects male behaviour and the relationship between mating, sperm allocation and care to test whether our predictions generally hold in nature.
As described above, our model also highlights the importance of the difficulty of providing care to alter the emergent association between paternal care and paternity: the easier paternal care is to provide, the weaker the association between paternity and parental effort. In fact, a recent and very comprehensive study of birds [17] found that, while male share of care decreases with extra-pair paternity in species where nestlings demand extensive care (i.e. altricial species), this association disappears when offspring require little care (i.e. precocial species). One could also test our model's predictions by exploring paternity patterns at different stages of parental care, which are likely to vary in the cost and benefit of care. In birds, provisioning is the most energetically expensive stage [58] and the common attendance by both parents suggests an essential role of male contribution. Conversely, although incubation greatly affects offspring development [59], females are generally able to compensate for decreases in male contribution during this stage [60], suggesting paternal care may not be very difficult for males to provide. Nest construction plays an important role not only in protecting the offspring but also in sexual signalling [61], so female compensation for a male's failure at this stage is unlikely. Therefore, the difficulty of providing paternal care in birds with biparental care seems to be low during incubation, increasing in importance during nest construction (when females cannot compensate for a male's failure to provide care) and is the highest during provisioning. In fact, extra-pair paternity and male share of feeding are strongly and negatively associated across 122 bird species (based on standardized regression coefficients), followed by the association with nest construction and, then, with incubation ([41]; for qualitatively similar results [39], but see [40]). Therefore, although empirical evidence is still scarce, results from the most comprehensive dataset in birds so far are consistent with our predictions for how the difficulty of providing male care affects the association between paternity and paternal care in two scales: among species with different development modes and within species between different stages of care.
In order to compare our results more directly to existing theory, we used a set of assumptions common to most sperm competition games (reviews in [1,2]) and parental investment theory [34–37]. Particularly, we assumed a priori the existence of energetic and temporal trade-offs between paternal care and other components of fitness. In numerous species with male-only care, however, parental males are able to attend several clutches simultaneously [16,22,48], reducing the extent of such trade-offs. Our predictions for situations with little energetic cost of male care may be similar to scenarios with weak trade-offs, although the mechanisms leading to little male allocation to parental effort are substantially different in each case. Moreover, males in some species are more attractive when attending the offspring than when at a non-parental state [22,27,62–64], resulting in a synergistic relationship between paternal care and mating and/or fertilization effort. This situation, however, must dramatically change the outcome of intra- and inter-sexual interactions and, consequently, the expected patterns of paternal care [25,65]. Therefore, the assumptions of existing theory do not capture the entire diversity of forms of paternal care and its relationship with sexual selection. We suggest not only that the extent but also the existence of trade-offs between different components of fitness deserve further attention. Particularly, future theoretical work could explore ‘loading factors’ in sperm competition games (i.e. parameters that scale the relative effect of energy expenditure on male traits and their corresponding component of fitness, which have been used in the context of pre- [14] and post-copulatory [11] male competition). In this sense, our model provides a strong starting point from which to study the evolution of paternal care, using a framework that explicitly considers how allocation strategies and social interactions affect patterns of mating, fertilization and care, where the extent of trade-offs between different components of fitness can easily be explored and evaluated.
Here, we demonstrate the importance of considering explicitly the cost of paternal care on the evolution of male allocation strategies, which in turn mediates expected paternity. Our main results show that the core predictions of sperm competition games [1,2] and parental investment theory [34–37] can be modified by the cost of paternal care. Although existing empirical evidence supports the expected association between paternity and male care across species [17,39–42]), extensive unexplained variation remains [23], particularly in species where males are exclusively responsible for offspring survival and high levels of paternity are expected [15,43–46]. The cost of paternal care varies widely among natural populations, and we argue that investigating how this variation affects patterns of mating, fertilization and care has the potential to improve our understanding of the coevolution between parental investment and reproduction.
Supplementary Material
Acknowledgements
We are grateful for the suggestions and discussion promoted by two anonymous referees that certainly improved the clarity of the manuscript.
Ethics
The authors declare that this research (i) has not been published previously elsewhere, (ii) was not misconducted, (iii) did not involved animal treatment or (iv) involved plagiarism in any form.
Data accessibility
Mathematica code is available at https://github.com/RequenaGS/Sperm_Competition_Paternal_Care.
Authors' contributions
G.S.R. and S.H.A. conceived of the study and discussed the model, G.S.R. implemented and analysed the model and wrote the manuscript, with substantial revisions from S.H.A.
Competing interests
We declare we no competing interests.
Funding
This material is based upon work supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) under a ‘Ciência sem Fronteiras’ postdoctoral fellowship and the Fundação de Amparo à Pesquisa do Estado de São Paulo under a postdoctoral fellowship (no. 2014/21790-2) to G.S.R., and funding from the National Science Foundation (under grant no. IOS-0950472), Yale University and the University of California, Santa Cruz to S.H.A.
References
- 1.Parker GA. 1998. Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead TR, Møller AP), pp. 3–54. New York, NY: Academic Press. [Google Scholar]
- 2.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. ( 10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 3.Simmons LW. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Olsson M, Madsen T, Shine R. 1997. Is sperm really so cheap? Costs of reproduction in male adders, Vipera berus. Proc. R. Soc. Lond. B 264, 455–459. ( 10.1098/rspb.1997.0065) [DOI] [Google Scholar]
- 5.Dewsbury DA. 1982. Ejaculate cost and male choice. Am. Nat. 119, 601–610. ( 10.1086/283938) [DOI] [Google Scholar]
- 6.Hayward A, Gillooly JF. 2011. The cost of sex: quantifying energetic investment in gamete production by males and females. PLoS ONE 6, e16557 ( 10.1371/journal.pone.0016557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stockley P, Gage MJG, Parker GA, Møller AP. 1997. Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am. Nat. 149, 933–954. ( 10.1086/286031) [DOI] [PubMed] [Google Scholar]
- 8.Gomendio M, Harcourt AH, Rold ERS. 1998. Sperm competition in mammals. In Sperm competition and sexual selection (eds Birkhead TR, Møller AP), pp. 667–755. New York, NY: Academic Press. [Google Scholar]
- 9.Simmons LW, García-González F. 2008. Evolutionary reduction in testes size and competitive fertilization success in response to the experimental removal of sexual selection in dung beetles. Evolution 62, 2580–2591. ( 10.1111/j.1558-5646.2008.00479.x) [DOI] [PubMed] [Google Scholar]
- 10.Dziminski MA, Roberts JD, Beveridge M, Simmons LW. 2010. Among-population covariation between sperm competition and ejaculate expenditure in frogs. Behav. Ecol. 21, 322–328. ( 10.1093/beheco/arp191) [DOI] [Google Scholar]
- 11.Parker GA. 1990. Sperm competition games: raffles and roles. Proc. R. Soc. Lond. B 242, 120–126. ( 10.1098/rspb.1990.0114) [DOI] [Google Scholar]
- 12.Parker GA. 1990. Sperm competition games: sneaks and extra pair copulations. Proc. R. Soc. Lond. B 242, 127–133. ( 10.1098/rspb.1990.0115) [DOI] [Google Scholar]
- 13.Parker GA, Immler S, Pitnick S, Birkhead TR. 2010. Sperm competition games: sperm size (mass) and number under raffle and displacement, and the evolution of P2. J. Theor. Biol. 264, 1003–1023. ( 10.1016/j.jtbi.2010.03.003) [DOI] [PubMed] [Google Scholar]
- 14.Parker GA, Lessells CM, Simmons LW. 2013. Sperm competition games: a general model for precopulatory male–male competition. Evolution 67, 95–109. ( 10.1111/j.1558-5646.2012.01741.x) [DOI] [PubMed] [Google Scholar]
- 15.Coleman SW, Jones AG. 2011. Patterns of multiple paternity and maternity in fishes. Biol. J. Linn. Soc. 103, 735–760. ( 10.1111/j.1095-8312.2011.01673.x) [DOI] [Google Scholar]
- 16.Wells KD. 2007. The ecology and behaviour of amphibians. Chicago, IL: University of Chicago Press. [Google Scholar]
- 17.Liker A, Freckleton RP, Remeš V, Székely T. 2015. Sex differences in parental care: gametic investment, sexual selection, and social environment. Evolution 69, 2862–2875. ( 10.1111/evo.12786) [DOI] [PubMed] [Google Scholar]
- 18.Lukas D, Clutton-Brock TH. 2013. The evolution of social monogamy in mammals. Science 341, 526–530. ( 10.1126/science.1238677) [DOI] [PubMed] [Google Scholar]
- 19.West HE, Capellini I. 2016. Male care and life history traits in mammals. Nat. Commun 7, 11854 ( 10.1038/ncomms11854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- 21.Royle NJ, Smiseth PT, Kölliker M. 2012. The evolution of parental care. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Requena GS, Munguía-Steyer R, Machado G. 2013. Paternal care and sexual selection in arthropods. In Sexual selection: perspectives and models from the neotropics (eds Macedo RH, Machado G), pp. 201–234. New York, NY: Academic Press. [Google Scholar]
- 23.Griffin AS, Alonzo SH, Cornwallis CK. 2013. Why do cuckolded males provide paternal care? PLoS Biol. 11, e1001520 ( 10.1371/journal.pbio.1001520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross MR, Sargent RC. 1985. The evolution of male and female parental care in fishes. Am. Zool. 25, 807–822. ( 10.1093/icb/25.3.807) [DOI] [Google Scholar]
- 25.Stiver KA, Alonzo SH. 2009. Parental and mating effort: is there necessarily a trade-off? Ethology 115, 1101–1126. ( 10.1111/j.1439-0310.2009.01707.x) [DOI] [Google Scholar]
- 26.Jones JC, Reynolds JD. 1999. Costs of egg ventilation for male common gobies breeding in conditions of low dissolved oxygen. Anim. Behav. 57, 181–188. ( 10.1006/anbe.1998.0939) [DOI] [PubMed] [Google Scholar]
- 27.Lindström K, Mary CMS, Pampoulie C. 2006. Sexual selection for male parental care in the sand goby, Pomatoschistus minutus. Behav. Ecol. Sociobiol. 60, 46–51. ( 10.1007/s00265-005-0138-0) [DOI] [Google Scholar]
- 28.Cucco M, Malacarne G. 1997. The effect of supplemental food on time budget and body condition in the black redstart Phoenicurus ochruros. Ardea 85, 211–221. [Google Scholar]
- 29.Komdeur J, Kats RKH. 1999. Predation risk affects trade-off between nest guarding and foraging in Seychelles warblers. Behav. Ecol. 10, 648–658. ( 10.1093/beheco/10.6.648) [DOI] [Google Scholar]
- 30.Gravel MA, Cooke SJ. 2009. Influence of inter-lake variation in natural nest predation pressure on the parental care behaviour of smallmouth bass (Micropterus dolomieu). Ethology 115, 608–616. ( 10.1111/j.1439-0310.2009.01641.x) [DOI] [Google Scholar]
- 31.Magnhagen C. 1991. Predation as a cost of reproduction. Trends. Ecol. Evol. 6, 183–186. ( 10.1016/0169-5347(91)90210-O) [DOI] [PubMed] [Google Scholar]
- 32.Steinhart GB, Sandrene ME, Weaver S, Stein RA, Marschall EA. 2005. Increased parental care cost for nest-guarding fish in a lake with hyperabundant nest predators. Behav. Ecol. 16, 427–434. ( 10.1093/beheco/ari006) [DOI] [Google Scholar]
- 33.Westneat DF, Sherman PW. 1993. Parentage and the evolution of parental behaviour. Behav. Ecol. 4, 66–77. ( 10.1093/beheco/4.1.66) [DOI] [Google Scholar]
- 34.Sheldon BC. 2002. Relating paternity to paternal care. Phil. Trans. R. Soc. Lond. B 357, 341–350. ( 10.1098/rstb.2001.0931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonzo SH, Klug H. 2012. Paternity, maternity, and parental care. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kölliker M), pp. 189–205. Oxford, UK: Oxford University Press. [Google Scholar]
- 36.Fromhage L, Jennions MD. 2016. Coevolution of parental investment and sexually selected traits drives sex-role divergence. Nat. Commun. 7, 12517 ( 10.1038/ncomms12517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houston AI, McNamara JM. 2002. A self-consistent approach to paternity and parental effort. Phil. Trans. R. Soc. Lond. B 357, 351–362. ( 10.1098/rstb.2001.0925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kempenaers B, Sheldon BC. 1997. Studying paternity and parental care: pitfalls and problems. Anim. Behav. 53, 423–427. ( 10.1006/anbe.1996.0377) [DOI] [Google Scholar]
- 39.Møller AP, Birkhead TR. 1993. Certainty of paternity covaries with paternal care in birds. Behav. Ecol. Sociobiol. 33, 261–268. ( 10.1007/BF02027123) [DOI] [Google Scholar]
- 40.Schwagmeyer PL, St. Clair RC, Moodie JD, Lamey TC, Schnell GD, Moodie MN. 1999. Species differences in male parental care in birds: a reexamination of correlates with paternity. Auk 116, 487–503. ( 10.2307/4089381) [DOI] [Google Scholar]
- 41.Møller AP, Cuervo JJ. 2000. The evolution of paternity and paternal care in birds. Behav. Ecol. 11, 472–485. ( 10.1093/beheco/11.5.472) [DOI] [Google Scholar]
- 42.Matysioková B, Remeš V. 2013. Faithful females receive more help: the extent of male parental care during incubation in relation to extra-pair paternity in songbirds. J. Evol. Biol. 26, 155–162. ( 10.1111/jeb.12039) [DOI] [PubMed] [Google Scholar]
- 43.Birks SM. 1997. Paternity in the Australian brush-turkey, Alectura lathami, a megapode bird with uniparental male care. Behav. Ecol. 8, 560–568. ( 10.1093/beheco/8.5.560) [DOI] [Google Scholar]
- 44.Emlen ST, Wrege PH, Webster MS. 1998. Cuckoldry as a cost of polyandry in the sex-role-reversed wattled jacana, Jacana jacana. Proc. R. Soc. Lond. B 265, 2359–2364. ( 10.1098/rspb.1998.0584) [DOI] [Google Scholar]
- 45.Brennan PL. 2012. Mixed paternity despite high male parental care in great tinamous and other Palaeognathes. Anim. Behav. 84, 693–699. ( 10.1016/j.anbehav.2012.06.026) [DOI] [Google Scholar]
- 46.Kamel SJ, Grosberg RK. 2012. Exclusive male care despite extreme female promiscuity and low paternity in a marine snail. Ecol. Lett. 15, 1167–1173. ( 10.1111/j.1461-0248.2012.01841.x) [DOI] [PubMed] [Google Scholar]
- 47.Alonzo SH. 2010. Social and coevolutionary feedbacks between mating and parental investment. Trends Ecol. Evol. 25, 99–108. ( 10.1016/j.tree.2009.07.012) [DOI] [PubMed] [Google Scholar]
- 48.Ah-King M, Kvarnemo C, Tullberg BS. 2005. The influence of territoriality and mating system on the evolution of male care: a phylogenetic study on fish. J. Evol. Biol. 18, 371–382. ( 10.1111/j.1420-9101.2004.00823.x) [DOI] [PubMed] [Google Scholar]
- 49.Whittingham LA, Taylor PD, Robertson RJ. 1992. Confidence of paternity and male parental care. Am. Nat. 139, 1115–1125. ( 10.1086/285376) [DOI] [Google Scholar]
- 50.Klug H, Bonsall MB. 2010. Life history and the evolution of parental care. Evolution 64, 823–835. ( 10.1111/j.1558-5646.2009.00854.x) [DOI] [PubMed] [Google Scholar]
- 51.Klug H, Bonsall MB, Alonzo SH. 2013. Sex differences in life history drive evolutionary transitions among maternal, paternal, and bi-parental care. Ecol. Evol. 3, 792–806. ( 10.1002/ece3.494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otto SP, Day T. 2007. A biologist's guide to mathematical modeling in ecology and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 53.Houston AI, McNamara JM. 2005. John Maynard Smith and the importance of consistency in evolutionary game theory. Biol. Philos. 20, 933–950. ( 10.1007/s10539-005-9016-4) [DOI] [Google Scholar]
- 54.Hosken DJ, Ward PI. 2001. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4, 10–13. ( 10.1046/j.1461-0248.2001.00198.x) [DOI] [Google Scholar]
- 55.Pitcher TE, Dunn PO, Whittingham LA. 2005. Sperm competition and the evolution of testes size in birds. J. Evol. Biol. 18, 557–567. ( 10.1111/j.1420-9101.2004.00874.x) [DOI] [PubMed] [Google Scholar]
- 56.Soulsbury CD. 2010. Genetic patterns of paternity and testes size in mammals. PLoS ONE 5, e9581 ( 10.1371/journal.pone.0009581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maurer G, Blomberg S. 2009. Does testis size in cuckoos vary with paternal care? Auk 126, 24–30. ( 10.1525/auk.2009.07185) [DOI] [Google Scholar]
- 58.Walsberg GE. 1983. Avian ecological energetics. In Avian biology (eds Farner DS, King JR, Parkes KC), vol. VII, pp. 161–214. New York, NY: Academic Press. [Google Scholar]
- 59.Deeming DC, Ferguson MW. 1991. Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 60.Harrison F, Barta Z, Cuthill I, Székely T. 2009. How is sexual conflict over parental care resolved? A meta-analysis. J. Evol. Biol. 22, 1800–1812. ( 10.1111/j.1420-9101.2009.01792.x) [DOI] [PubMed] [Google Scholar]
- 61.Mainwaring MC, Hartley IR, Lambrechts MM, Deeming DC. 2014. The design and function of birds’ nests. Ecol. Evol. 4, 3909–3928. ( 10.1002/ece3.1054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kraak SBM, Groothuis TGG. 1994. Female preference for nests with eggs is based on the presence of the eggs themselves. Behaviour 131, 189–206. ( 10.1163/156853994X00433) [DOI] [Google Scholar]
- 63.Requena GS, Machado G. 2015. Effects of egg attendance on male mating success in a harvestman with exclusive paternal care. Behav. Ecol. 26, 926–935. ( 10.1093/beheco/arv035) [DOI] [Google Scholar]
- 64.Ohba S, Okuda N, Kudo S. 2016. Sexual selection of male parental care in giant water bugs. R. Soc. open sci. 3, 150720 ( 10.10.1098/rsos.150720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alonzo SH. 2012. Sexual selection favours male parental care, when females can choose. Proc. R. Soc. B 279, 1784–1790. ( 10.1098/rspb.2011.2237) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mathematica code is available at https://github.com/RequenaGS/Sperm_Competition_Paternal_Care.