Abstract
Regulating soft tissue repair to prevent fibrosis and promote regeneration is central to creating a microenvironment conducive to soft tissue development. Macrophages play an important role in this process. Their response can be modulated using biomaterials, altering cytokine and growth factor secretion to promote regeneration. Electrospun polydioxanone (PDO) fiber scaffolds promoted an M2 phenotype when macrophages were cultured on large diameter, highly porous scaffolds, but an M1 phenotype on smaller diameter fibers. Here we investigated whether incorporation of galectin-1, an immunosuppressive protein that enhances muscle regeneration, could promote the M2 response. Galectin-1 was incorporated into large and small fiber PDO scaffolds during electrospinning. Galectin-1 incorporation increased arginase-1 and reduced iNOS and IL-6 production in mouse bone-marrow derived macrophages compared to PDO alone for both scaffold types. Inhibition of ERK mitogen activated protein kinase did not alter galectin-1 effects on arginase-1 and iNOS expression, but reversed IL-6 suppression, indicating that IL-6 is mediated by a different mechanism. Our results suggest that galectin-1 can be used to modulate macrophage commitment to a pro-regenerative M2 phenotype, which may positively impact tissue regeneration when using small diameter PDO scaffolds.
Keywords: Polydioxanone, Galectin-1, Macrophage, Immunomodulation
1. Introduction
The inflammatory response plays a critical role in tissue repair and regeneration after injury. Acute injuries result in a predictable series of events in which neutrophils and macrophages play important roles to prepare the local microenvironment for regeneration. Within the first two hours, Ly6G/F4/80− neutrophils migrate into the injured tissue. These cells release myeloperoxidase, inducing membrane damage that prompts macrophage phagocytosis and efferocytosis. Following neutrophil invasion, distinct subpopulations of macrophages infiltrate the area to promote regeneration. The first subpopulation are phagocytic CD68+ macrophages (M1), which clear debris and secrete pro-inflammatory cytokines such as interleukin-6 (IL-6). The second subpopulation of macrophages is a non-phagocytic CD163+/CD206+ phenotype (M2), which produces cytokines and growth factors regulating tissue regeneration. This shift in macrophage phenotype is accompanied by increased interleukin-10 (IL-10), vascular endothelial growth factor (VEGF), and transforming growth factor-β1 (TGF-β1) but reduced IL-6, IL-1β, and tumor necrosis factor-α (TNF-α)1–3. When this process is dysregulated, tissue repair and regeneration are impaired.
Soft tissue pathology and abnormal repair and regeneration are closely associated with the immune response and macrophage polarization following injury4. Severe soft tissue injuries caused by lacerations, blast, crush, or tumor resection alter this immune response and create a microenvironment rich in TGF-β1 and IL-6 that permits fibrosis5. In these types of injuries, this environment is mostly mediated by the first subpopulation of phagocytic M1 macrophages, while the second subpopulation of M2 macrophages are suppressed, limiting production of pro-regenerative factors and favoring fibrosis6. Modifying the microenvironment and preventing fibrosis are central to promoting regeneration.
Galectin-1 is a small (14 kDa) non-glycosylated protein encoded by the Lectin, Galactoside-binding, Soluble-1 (LGALS1) gene. Galectin-1 is expressed in many tissues, where it induces cell migration, growth, angiogenesis, and immunomodulation7,8. Several studies collectively show that galectin-1 suppresses inflammation. These include the demonstration that soluble galectin-1 enhances T cell IL-10 production and decreases cytotoxic T cell survival9, reduces macrophage responses to interferon-gamma (IFNγ)10, and promotes an M2-like macrophage phenotype11. Additionally, galectin-1 regulates a wide range of biological functions such as angiogenesis12, neuron development13, cardiovascular disease14, and muscle disease15,16. These findings support the choice of galectin-1 as a potential therapeutic agent for soft tissue injuries using drug delivery strategies to modulate the immune system and promote regeneration.
Previously, we showed that mouse macrophage phenotype can be altered by controlling electrospun polydioxanone (PDO) fiber diameter and porosity. In these previous studies, smaller diameter fibers produced from 60 mg/ml PDO solutions by electrospinning promoted M1 inflammatory macrophage responses, while larger diameter fibers from 140 mg/ml solutions enhanced M2 development 18,19. In the present study, we took advantage of this model to evaluate the effect of recombinant mouse galectin-1 incorporation into these scaffolds.
2. Materials and Methods
2.1 Animals
C57BL/6 male and female mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were approved for use by the Virginia Commonwealth University Institutional Animal Care and Use Committee (IACUC #AM10340). NIH guidelines for the care and use of laboratory animals (NIH Publication #85-23 Rev. 1985) were observed.
2.2. Mouse Macrophage Cultures
Mouse bone marrow-derived macrophages were harvested from femurs and tibias of C57BL/6 mice. The collected bone marrow extract was then cultured in RPMI 1640 (Invitrogen Life Technologies, Carlsbad, CA) containing 10% fetal bovine serum (FBS), 2mM L-glutamine, 100 U/mL penicillin, 100ug/mL streptomycin, 1mM sodium pyruvate, and 1mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (all from Corning, Corning, NY), and supplemented with 30ng/mL macrophage colony stimulating factor (M-CSF, Biolegend, San Diego, CA). Macrophages were used at passage 1 at 90% confluence (approximately 7 days in culture at passage 0).
2.3 Fabrication and Characterization of Polymer Scaffolds
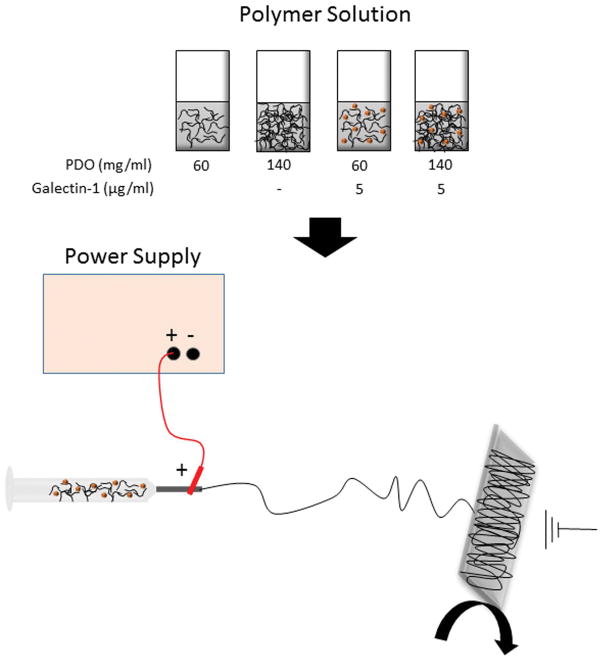
Polymer scaffolds were fabricated by electrospinning as shown in Figure 1. PDO used in this study (RESOMER® X, (C4H6O3)n, Sigma Aldrich, St. Louis, MO) was a 100–120 kDa polymer with a 1.5–2.2 dl/g inherent viscosity, −10°C glass transition temperature, and 110–115°C melting temperature. PDO pellets were dissolved in 1,1,1,3,3,3 hexafluoro-2-propanol (TCI America, Portland, OR) at concentrations of 60 or 140 mg/ml to fabricate small and large diameter fibers, respectively. Recombinant mouse galectin-1 (Gal1, R&D Systems, Minneapolis, MN) was first reconstituted from a lyophilized powder into 1% bovine serum albumin (BSA) and was was added to the PDO polymer solution at 5 μg/mL. Control scaffolds were fabricated using an equal volume of the 1% BSA carrier solution. The polymer solutions were then loaded into 5mL syringes (Becton Dickinson, Franklin Lakes, NJ) with 18 gauge blunt tip needles and electrospun while being dispensed at 6mL/hr. The needle tip was exposed to +25kV and fibers collected on a rectangular mandrel rotating at 200rpm across a 20 cm airgap distance. 140 mg/ml scaffolds were subjected to compression to reduce pore size using a hydraulic press as previously reported18.
Figure 1. Schematic of method used to produce electrospun fibers with galectin-1.
2.4 Cytokines and Reagents
The recombinant murine IL-6 ELISA kit purchased from BioLegend (San Diego, CA). Lipopolysaccharide (LPS, Sigma Aldrich L6529, 100 ng/ml) was purchased from Sigma Aldrich (St. Louis, MO). Mouse vascular endothelial growth factor-A (VEGF-A) ELISA kits were purchased from PeproTech (Rocky Hill, IL-13 was purchased from PeproTech. Anti-galectin-1 (AF1245) was purchased from R&D Systems for immunostaining. Galectin-1 release was assessed using a galectin-1 ELISA kit purchased from R&D Systems (DY1245), NanoOrange (N6666) and the micro BCA assay kit (23235) were purchased from ThermoFisher Scientific (Waltham, MA). Galectin-1 release was quantified at 0, 0.25, 1, 6, 12, and 24 hours using 10mm biopsy punches in 300 μl of 1× PBS at 37°C. Background from protein carrier control scaffolds was subtracted. DRAQ5 dye was purchased from Cell Signaling Technologies (Danvers, MA).
2.5 Effects of Galectin-1
We first determined the effects of galectin-1 (Gal1) on macrophage response prior to incorporating it into PDO fibers. Macrophages were cultured on tissue culture polystyrene (TCPS) to confirm that galectin-1 had similar effects as previously shown10. Macrophages were plated at a density of 10,000 cells/cm2 and allowed to attach for 3 hours. After 3 hours, culture media were aspirated and media containing galectin-1 in concentrations of 50, 100, 500, or 1000 ng/ml were added to the culture wells. As a positive control, IL-13 was diluted to a final concentration of 10 ng/ml20.
2.5.1 Effect of Galectin-1 on Macrophage Phenotype
Western blots were used to determine effects of galectin-1 stimulation. Cells were lysed in Lysis Buffer (Cell Signaling Technology) with 1.5X Protease Arrest (G-Biosciences, Maryland Heights, MO). Lysate protein concentrations were determined using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific). Lysate proteins (40μg of protein per sample) were resolved by SDS-PAGE using 4–20% Mini-Protean TGX Gels (Bio-Rad, Hercules, CA). Samples were transferred onto nitrocellulose membranes and then blocked for 1 hour at room temperature with Blocker Casein in Tris-buffered saline (TBS, ThermoFisher Scientific). Membranes were then rinsed with TBS and incubated overnight at 4°C with primary antibodies at 1:1000 (v:v) in TBS-0.1% Tween (TBS-T) mixed with Blocker Casein at equal volumes. The following primary antibodies were used and purchased from Cell Signaling Technologies: inducible nitric oxide synthase (iNOS), arginase-1, and macrophage chemotactic protein-1 (MCP-1). Following overnight incubation, membranes were washed with TBS-T and then incubated with infrared-labeled secondary antibodies at 1:15,000 (v:v) final dilution. Goat anti-rabbit DyLight800 and goat anti-mouse DyLight680 secondary antibodies were purchased from Cell Signaling Technologies. Membranes were scanned using the LI-COR Odyssey CLx infrared imaging system and analyzed using LI-COR Image Studio 4.0 (Lincoln, NE). Blots were normalized to a single internal reference protein using β-actin. Groups were run in the same gel and bands compared on the same blot. Normalization factors were calculated as a ratio of β-actin signals to scale band intensity values and background was automatically subtracted in Image Studio 4.0 software.
2.5.2 Effect of Galectin-PDO Scaffolds
To first determine optimal concentrations of galectin-1, macrophages were cultured on scaffolds prepared using 1 or 5 μg/ml galectin-1 and arginase-1 levels were determined. Macrophages were seeded onto PDO-Gal1 scaffolds (60 mg/ml PDO alone; 140 mg/ml PDO alone; 60 mg/ml PDO + 5μg Gal1; 140 mg/ml PDO + 5μg Gal1) at a seeding density of 50,000 cells/cm2 18 and cultured for 1 day. Based on the results (Supplemental Figure 1), all remaining scaffold experiments used galectin-1 at 5 μg/ml. Western blots were used to determine effects on iNOS, arginase-1, phosphorylated ERK1/2, total ERK1/2, and β-actin.
2.6 Role of ERK
To determine if the effect of galectin-1 involved extracellular-signal regulated kinase (ERK) mitogen activated protein kinase signaling, we used the selective ERK inhibitor 328006 (CAS No.1043738-54-68, Calbiochem, EMD Millipore, Billerica, MA) at 25 μM in dimethylsulfoxide (DMSO) in cRPMI. Cells were seeded at 50,000 cells/cm2 on PDO scaffolds and cultured for 1 day in medium containing the ERK inhibitor or an equal volume of DMSO. At the end of culture, scaffolds were washed with PBS, and evaluated for arginase-1 and iNOS via western blot and IL-6 via ELISA.
2.7 Statistical Analysis
For all experiments, each variable was tested using N = 6 independent cultures. Experiments were repeated 3 times. Data are presented as mean ± SEM with analysis done using GraphPad Prism 6.0 (GraphPad, La Jolla, CA). Analysis comparing only 2 groups was performed by unpaired Student’s t-test, whereas analysis comparing more than 2 groups used one-way analysis of variance with Tukey’s post-hoc test. All p values <0.05 were considered significant.
3. Results
3.1 Soluble galectin-1 increases arginase-1 and reduces pro-inflammatory cytokine production from mouse macrophages
Soluble galectin-1 has been reported to increase arginase, while decreasing nitric oxide (NO), IL-1β, and IL-6 production in rat peritoneal macrophages stimulated with LPS11,21. To establish a comparison for PDO polymers, we first cultured mouse bone marrow-derived macrophages in media +/− soluble galectin-1, and compared the effects to the known M2-inducing cytokine, IL-13. Galectin-1 increased arginase-1 expression, and decreased IL-6 and MCP-1 secretion in a manner similar to IL-13 (Figure 2). In contrast, IL-13 suppressed VEGF production, while galectin-1 had no effect. Galectin-1 also had no effect on iNOS levels (data not shown).
Figure 2. Galectin-1 promotes arginase and reduces IL-6 cytokine production similar to the effects of IL-13.
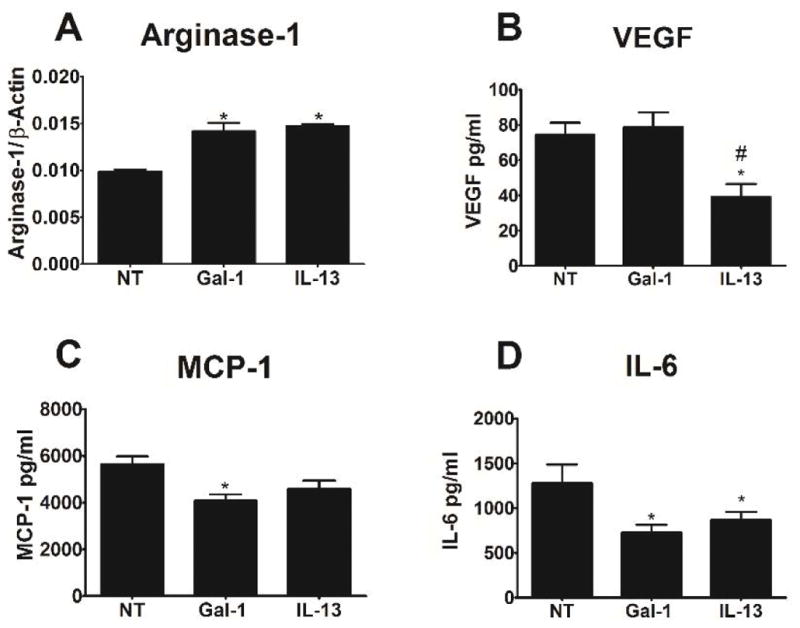
Bone marrow-derived macrophages were cultured in media containing soluble galectin-1 (Gal1), IL-13, or no further treatment (NT) for 1 day. (A) Arginase-1 was measured by western blotting. (B–D) VEGF, MCP-1, and IL-6 were measured by ELISA. * indicates a difference from no treatment (p < 0.05). Data shown are means ± SEM of 6 samples from a representative experiment. Experiments were repeated to ensure validity.
3.2 Galectin-1 incorporation does not alter PDO scaffold characteristics
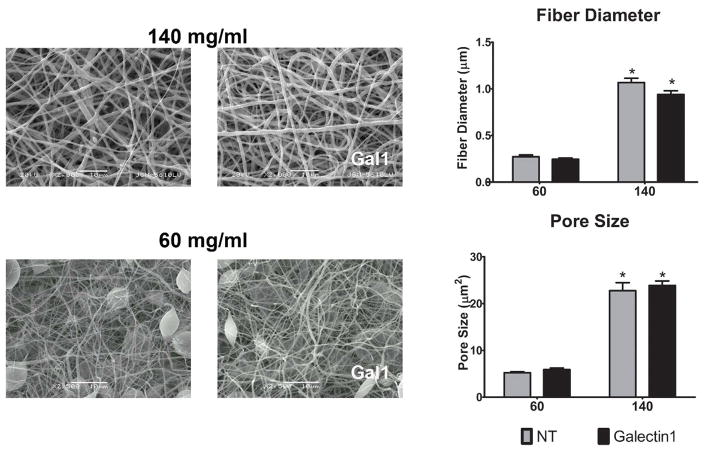
PDO scaffolds produced from 60 mg/ml solutions have fibers of less than 0.5μm diameter, while 140 mg/ml solutions yield fibers of approximately 2.5μm18. 60 mg/ml PDO concentrations resulted in beads with small diameter fibers14. To ensure that galectin-1 incorporation did not change scaffold fiber characteristics, we assessed the effect of galectin-1 incorporation on scaffold structure. Scaffolds were created by electrospinning PDO at concentrations of 60 mg/ml and 140 mg/ml with galectin-1 or protein carrier, as described in Materials and Methods. Fiber diameter and pore size were quantified. Neither of these critical physical characteristics were altered by galectin-1 incorporation (Figure 3).
Figure 3. Fiber diameter and pore size are unaffected by galectin-1 incorporation.
Representative scanning electron micrographs of 140 mg/ml and 60 mg/ml electrospun scaffolds incorporating with 5 μg/ml galectin-1. Bar charts show the diameter and pore size measurements of 60 fibers from 3 samples. * indicates a difference when comparing 140 mg/ml scaffolds to the corresponding 60 mg/ml scaffold (p < 0.05).
3.3 Galectin-1 release varies with fiber diameter
Release of galectin-1 from electrospun PDO fibers varied with the diameter size (Figure 4A). The initial burst release was greater from 140 mg/ml PDO scaffolds than from 60 mg/ml scaffolds. After the initial burst, however, sustained release was comparable. No galectin-1 was detected in releasates of the control scaffolds. We then confirmed galectin-1 specific immunostaining in galectin loaded scaffolds (Figure 4B).
Figure 4. Galectin-1 release.
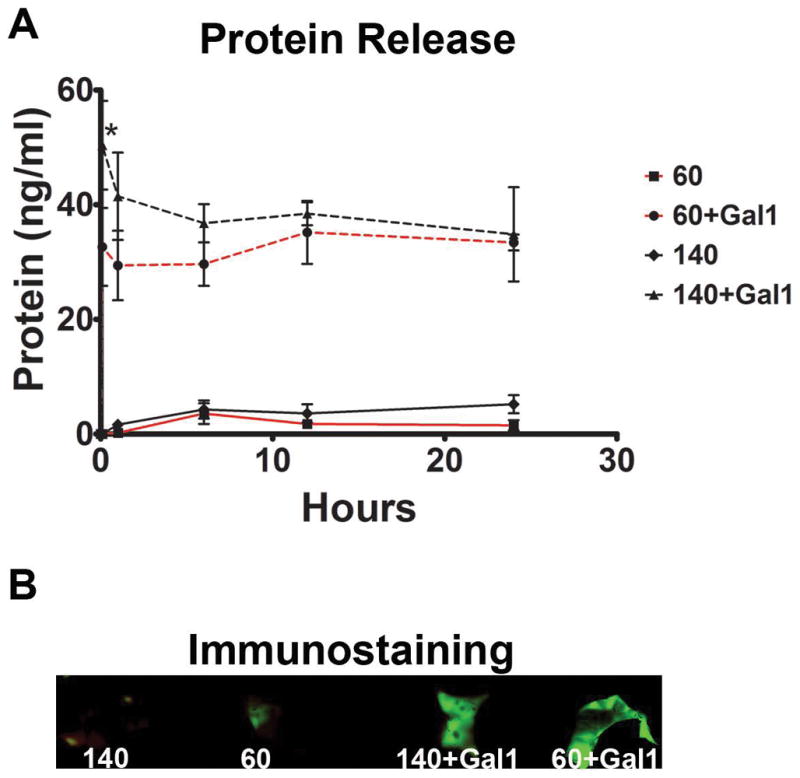
Protein levels were measured at 0, 0.25, 1, 6, 12, and 24 hours using 140 mg/ml and 60 mg/ml electrospun PDO scaffolds with galectin-1 or vehicle (A). * indicates a difference between 60+Gal1 and 140+Gal1 (p < 0.05). Data shown are means of triplicate samples. Images of scaffolds immunostained with anti-galectin-1 antibody demonstrate a larger signal from galectin-1 scaffolds then controls (B).
3.4 Galectin-1 incorporation greatly reduces the impact of pore size on macrophage phenotype
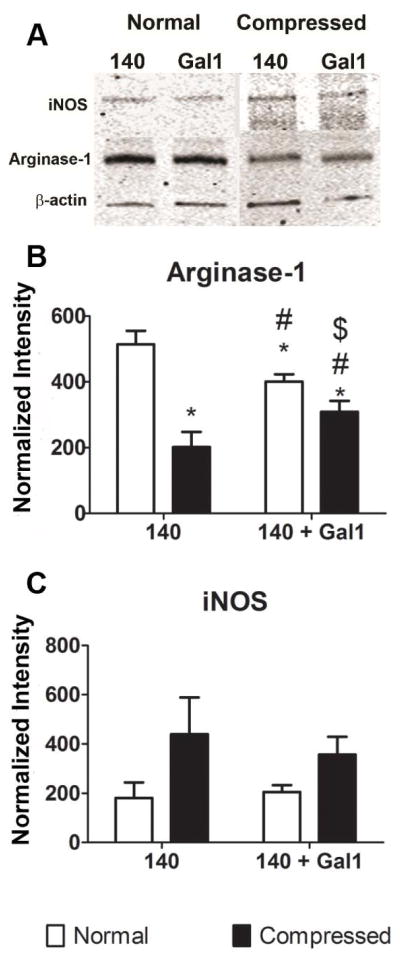
PDO scaffolds generated from 140 mg/ml solutions have pore radii greater than 14μm on average, compared to approximately 1μm pores produced by 60 mg/ml solutions18,22. We previously showed that compressing a 140 mg/ml scaffold reduced pore sizes without significantly changing fiber diameter18. This decreased pore size reduced the M2-polarizing effects of the polymer. To determine if galectin-1 modified the effects of scaffold compression, macrophages were cultured on compressed and normal 140 mg/ml PDO + Gal1 fibers. Arginase-1 was reduced by more than 50% in macrophages cultured on compressed scaffolds compared to normal scaffolds. Addition of galectin-1 to normal scaffolds caused a small reduction in arginase-1, but addition of galectin-1 to compressed scaffolds increased arginase compared to normal scaffolds without galectin-1 (Figure 5A,B). In contrast, iNOS was unaffected by compression or addition of galectin-1 (Figure 5A,C). These results suggest that the M2-promoting abilities of galectin-1 overcome the macrophage response to small pore size on bioresorbable polymers.
Figure 5. Galectin-1 blunts the effect of reduced porosity caused by compression.
Bone marrow-derived macrophages were cultured for 1 day on normal or compressed 140mg/ml scaffolds +/− Gal1, as described in Materials and Methods. Arginase-1 (A) and iNOS (B) protein levels were determined by western blotting. Data shown are means ± SEM of 6 independent cultures from a representative experiment.
3.5 Galectin-1 incorporation into smaller diameter fibers promotes arginase-1 and inhibits iNOS partly through an ERK-independent pathway
In addition to pore radius, fiber diameter affects macrophage phenotype. 140 mg/ml PDO scaffolds promoted significantly more arginase-1 and less iNOS than 60 mg/ml scaffolds. Addition of galectin-1 to the 60 mg/ml PDO scaffolds resulted in similar arginase-1 levels as observed in macrophages cultured on 140mg/ml polymers (Figure 6B). iNOS was reduced when galectin-1 was incorporated into 60 mg/ml fibers to levels comparable to those observed on 140 mg/ml fibers (Figure 6C). IL-6 cytokine secretion was modestly reduced in the presence of galectin-1, but was not affected by fiber size (Figure 6D). These data demonstrated that macrophage sensing of small versus large diameter fibers is greatly blunted by the presence of galectin-1.
Figure 6. Galectin-1 incorporation promotes M2 phenotype on small fiber scaffolds.
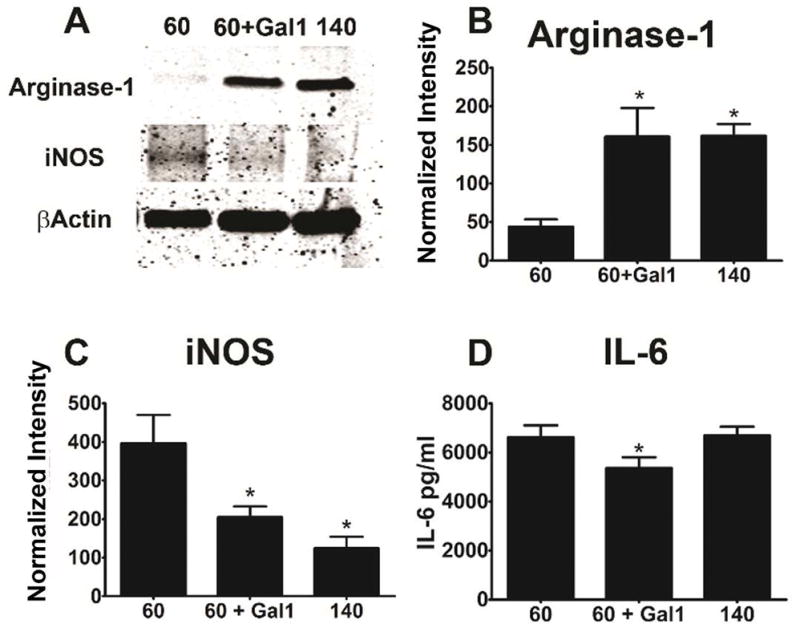
Bone marrow-derived macrophages were cultured as described in Figure 3. The effect of galectin-1 incorporation into 60mg/ml scaffolds was assessed by measuring arginase-1 and iNOS by western blotting, and IL-6 secretion by ELISA. (A) shows a representative blot, while (B–C) are normalized values. Data shown are representative means ± SEM of 6 independent cultures from one of three independent experiments. * indicates a difference when comparing to 60mg/ml scaffolds (p < 0.05).
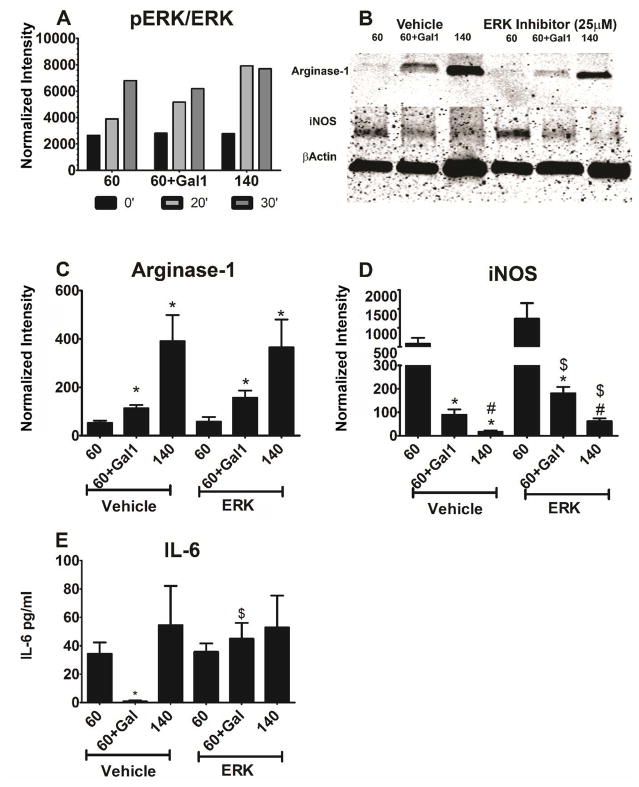
Previous reports have shown that ERK MAPK mediates galectin-1 signaling in macrophages10, and this signaling is different from our previous studies showing a MyD88-mediated pathway on PDO fibers18. We therefore assessed the impact of ERK blockade in our system, focusing on how galectin-1 alters the response to 60 mg/ml PDO fibers. As shown in Figure 7A, ERK phosphorylation was unaffected by galectin-1 on 60 mg/ml fibers. ERK phosphorylation occurred more rapidly on 140 mg/ml PDO fibers than on the small diameter fibers (Figure 7A). Galectin-1 increased arginase-1 in macrophages grown on 60 mg/ml fibers, but not to the same extent as seen on 140 mg/ml scaffolds in the absence of galectin-1 (Figure 7A,B). Inhibition of ERK MAPK appeared to reduce the effect of galectin-1 on arginase-1 on western blots (Figure 7B), but quantification of multiple gels indicated that ERK inhibition had no effect (Figure 7C). Addition of galectin-1 to 60 mg/ml scaffolds resulted in a marked reduction in iNOS but not to the same extent as was seen in macrophages were grown on 140 mg/ml scaffolds (Figure 7B,D). Inhibition of ERK reduced the effect of fiber diameter and of galectin-1 on iNOS (Figure 7B,D). Addition of galectin-1 to 60 mg/ml scaffolds blocked IL-6 produced by macrophages on 60 mg/ml fibers. IL-6 levels were unaffected by fiber dimaeter (Figure 7D). Inhibition of ERK blocked the inhibitory effect of galectin-1 on the 60 mg/ml scaffolds.
Figure 7. Galectin-1 effects on arginase-1 and iNOS are ERK-independent, while IL-6 inhibition is ERK-dependent.
Bone marrow-derived macrophages were cultured on the indicated scaffolds as described in Figure 3, and Western blotting was used to measure ERK phosphorylation (A) and levels of arginase-1 and iNOS protein (B–D). (B) shows a representative blot, while (C–D) are normalized values. In (E) IL-6 secretion was measured by ELISA. Data shown are means ± SEM of 3 independent cultures from one of two experiments. * indicates a difference when comparing 60 mg/ml within each group (p < 0.05). # represents a difference compared to 60 mg/ml+Gal1 within each group (p < 0.05). $ represents a difference between Vehicle and ERK inhibitor (p < 0.05).
4. Discussion
Bioresorbable fibers show promise to enhance wound healing, and provide flexibility during their fabrication that offers an ability to tailor scaffolds to specific needs. By altering the concentration of PDO solutions, fiber diameter and pore size are greatly varied. These changes in topography alter how immune cells respond to scaffolds, influencing the healing progress. Further, bioactive molecules can be incorporated during synthesis, providing a variety of stimuli to promote angiogenesis and diminish inflammation 23–26.
Galectin-1 has been shown to promote a pro-regenerative M2 macrophage response in other systems 2,10,27. This prompted our investigation of galectin-1’s effects on macrophage polarization when incorporated into polymeric scaffolds. Consistent with prior publications, soluble galectin-1 was equal to IL-13, a known M2 inducer, in regulating arginase-1 and cytokine production by macrophages. Galectin-1 incorporation into PDO fibers shifted the macrophage response, generally promoting the M2 phenotype, indicated by increased arginase-1 and decreased iNOS. Moreover, galectin-1 shifted macrophage commitment from the M1 phenotype noted previously on small diameter PDO fiber scaffolds, to an M2 phenotype typical of large diameter PDO scaffolds. These pro-M2 effects persisted when galectin-1 was embedded in PDO fibers and overcame the importance of pore size and fiber diameter in regulating macrophage phenotype18.
Our results indicate that the mechanisms by which galectin-1 alters macrophage response to scaffold topography is partly ERK-independent. Inhibition of ERK1/2 MAPK did not affect arginase-1 and had a minor effect on iNOS. In contrast, inhibition of ERK1/2 MAPK completely blocked the reduction in IL-6 caused by addition of galectin-1 to small diameter scaffolds. This indicates that this effect of galectin-1 is ERK-dependent.
Macrophage participation in soft tissue regeneration following injury is highly regulated. Successful regeneration relies on a delicate balance of M1 macrophages to clear the wound site and M2 macrophages to secrete factors into the local microenvironment that promote regeneration. Elevated numbers of M1 or M2 macrophages can impair regeneration, leading to fibrosis. This is a central problem in several soft tissue disorders, including skin, nerve, muscle, ligament, and tendon28–31. For example, depletion of phagocytic myeloid cells prior to cardiotoxin-induced muscle injury blocked the removal of cell debris and impaired normal muscle regeneration32, demonstrating the importance of phagocytic M1 macrophages in the initial stages of muscle regeneration. Controlling the ratio of M1 and M2 macrophages is critical; and modulating these events using galectin-1 could improve healing in muscle and other soft tissues.
To begin developing a drug delivery system that could alter the immune response and aid in regeneration using recombinant galectin-1, we needed to establish its effect on bone marrow derived mouse macrophages cultured on TCPS. We first tested the dose-dependent response to galectin-1 to determine if arginase-1 increased and iNOS decreased as previously reported10,11. We confirmed increased arginase-1, decreased iNOS, and IL-6 suppression11.
The advantage to electrospun fibers is its flexibility to incorporate molecules and simultaneously tailor fiber morphology to regulate M1 and M2 phenotypes. Studies have incorporated immunosuppressive proteins into electrospun fibers using IL-1033, nuclear factor kappa-light-chain-enhancer of activated B cell inhibitors34, and cyclosporine A35 and successfully altered immune cell phenotype. We opted to use immunosuppressive galectin-1 as opposed to another M2 inducing biologic because of its direct involvement in muscle regeneration15,36, a focus of our group. In this study, immunosuppressive galectin-1 was incorporated into differently sized PDO fibers to affect macrophage phenotype, where we demonstrated enhanced M2 macrophage characteristics.
We tested the ability of galectin-1 to recover M2 macrophage qualities in electrospun scaffolds by incorporating it into compressed large fiber scaffolds with reduced pore size and normal small fiber scaffolds with small fibers and small pores, which were both shown to promote M1 phenotypes18. While compressed large fiber scaffolds lost much of their M2-promoting capacity, incorporating galectin-1 recovered these effects. More, incorporating galectin-1 into small fibers, generated a polymer with M2-inducing effects indistinguishable from 140mg/ml fibers. Because galectin-1 incorporation did not affect porosity or fiber diameter, these effects were ascribed to galectin-1 signaling. These data suggest that galectin-1 alters how macrophages interpret small diameter pores and fibers.
Aside from intracellular iNOS and arginase-1 production, extracellular IL-6 secretion was also measured based on its importance in regeneration and fibrosis. IL-6 is an inflammatory mediator that regulates M1-like macrophage phenotype and when IL-6 is secreted at low levels it facilitates regeneration37–39. Conversely, severe soft tissue injuries elevate IL-6 production beyond normal levels, prolonging an M1 response40,41 and promoting fibrosis42,43. Here, galectin-1 was able to suppress IL-6 in macrophages cultured on small diameter PDO fibers that normally enhance IL-6 synthesis, suggesting therapeutic potential.
Several studies have emphasized the importance of ERK in galectin-1-mediated effects. For example, Barrionuevo and colleagues showed that soluble galectin-1 stimulated ERK-1/2 phosphorylation in human monocytes, and that inhibiting the upstream kinase MEK could reverse the effects of galectin-1 on macrophage major histocompatibility complex II and fragment crystallizable gamma receptor I expression10. Galectin-1 stimulation has further been shown to activate ERK in airway epithelium44 and correlates with ERK activity in allergic conjunctivitis45, osteosarcoma growth46, and lung cancer progression47.
This ERK-mediated signaling is distinct from the myeloid differentiation primary response gene 88-dependent signaling we have noted on PDO fibers18. Using an ERK inhibitor, we examined the role of ERK signaling in our galectin-1 scaffolds. Our results showed that galectin-1 effects on arginase-1 were ERK-independent, while iNOS and IL-6 inhibition required ERK function. Signal transducer and activator of transcription (STAT) pathways facilitate IL-6 signaling in monocytes48,49, and ERK has been shown to antagonize STAT-mediated gene activation and cell differentiation50,51. This may explain our observed IL-6 results. However, more study is needed to identify signaling intermediates.
5. Conclusion
Galectin-1 modulated macrophage polarization and protein secretion using electrospun drug delivery strategies. These modified PDO scaffolds did not display altered fiber morphology or pore size when galectin-1 was incorporated, and were able to stimulate arginase-1 production while suppressing the pro-inflammatory markers, iNOS and IL-6. The macrophage response was further characterized by investigating the role of ERK MAPK. Results showed that arginase-1 regulation was ERK-independent, while iNOS and IL-6 suppression was ERK-dependent. Future studies should determine how galectin-1 delivery materials affect signaling pathways, and address how these materials can be used clinically.
Supplementary Material
Acknowledgments
This work was supported by the Department of Veterans Affairs Interprofessional Polytrauma and Traumatic Brain Injury Rehabilitation Research Fellowship and the National Institutes for Health R01 AI101153/AI/NIAID and R01 AI059638/AI/NIAD.
References
- 1.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55(3):410–22. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 2.Rostoker R, Yaseen H, Schif-Zuck S, Lichtenstein RG, Rabinovich GA, Ariel A. Galectin-1 induces 12/15-lipoxygenase expression in murine macrophages and favors their conversion toward a pro-resolving phenotype. Prostaglandins Other Lipid Mediat. 2013;107:85–94. doi: 10.1016/j.prostaglandins.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Rao KM. MAP kinase activation in macrophages. J Leukoc Biol. 2001;69(1):3–10. [PubMed] [Google Scholar]
- 4.Wang H, Melton DW, Porter L, Sarwar ZU, McManus LM, Shireman PK. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol. 2014;184(4):1167–84. doi: 10.1016/j.ajpath.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klingler W, Jurkat-Rott K, Lehmann-Horn F, Schleip R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol. 2012;31(3):184–95. [PMC free article] [PubMed] [Google Scholar]
- 6.Hurtgen BJ, Ward CL, Garg K, Pollot BE, Goldman SM, McKinley TO, Wenke JC, Corona BT. Severe muscle trauma triggers heightened and prolonged local musculoskeletal inflammation and impairs adjacent tibia fracture healing. J Musculoskelet Neuronal Interact. 2016;16(2):122–34. [PMC free article] [PubMed] [Google Scholar]
- 7.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16(11):137r–157r. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 8.Rabinovich GA, Vidal M. Galectins and microenvironmental niches during hematopoiesis. Current Opin Hematol. 2011;18(6):443–451. doi: 10.1097/MOH.0b013e32834bab18. [DOI] [PubMed] [Google Scholar]
- 9.Cedeno-Laurent F, Opperman M, Barthel SR, Kuchroo VK, Dimitroff CJ. Galectin-1 triggers an immunoregulatory signature in Th cells functionally defined by IL-10 expression. J Immunol. 2012;188(7):3127–37. doi: 10.4049/jimmunol.1103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrionuevo P, Beigier-Bompadre M, Ilarregui JM, Toscano MA, Bianco GA, Isturiz MA, Rabinovich GA. A novel function for galectin-1 at the crossroad of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J Immunol. 2007;178(1):436–45. doi: 10.4049/jimmunol.178.1.436. [DOI] [PubMed] [Google Scholar]
- 11.Correa SG, Sotomayor CE, Aoki MP, Maldonado CA, Rabinovich GA. Opposite effects of galectin-1 on alternative metabolic pathways of L-arginine in resident, inflammatory, and activated macrophages. Glycobiology. 2003;13(2):119–128. doi: 10.1093/glycob/cwg010. [DOI] [PubMed] [Google Scholar]
- 12.Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, Mayo KH, Poirier F, Griffioen AW. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci. 2006;103(43):15975–80. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi M, Arruda-Carvalho M, Kang NH, Imaizumi Y, Poirier F, Okano H, Frankland PW. Impaired spatial and contextual memory formation in galectin-1 deficient mice. Mol Brain. 2011;4:33. doi: 10.1186/1756-6606-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Salam S, Hashmi S. Galectin-1 in early acute myocardial infarction. PLoS One. 2014;9(1):e86994. doi: 10.1371/journal.pone.0086994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watt DJ, Jones GE, Goldring K. The involvement of galectin-1 in skeletal muscle determination, differentiation and regeneration. Glycoconj J. 2002;19(7–9):615–619. doi: 10.1023/B:GLYC.0000014093.23509.92. [DOI] [PubMed] [Google Scholar]
- 16.Van Ry PM, Wuebbles RD, Key M, Burkin DJ. Galectin-1 Protein Therapy Prevents Pathology and Improves Muscle Function in the mdx Mouse Model of Duchenne Muscular Dystrophy. Mol Ther. 2015;23(8):1285–97. doi: 10.1038/mt.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lecourt S, Lepelletier Y, Vanneaux V, Jarray R, Domet T, Raynaud F, Hadj-Slimane R, Cras A, Hermine O, Marolleau JP, Larghero J. Human muscle progenitor cells displayed immunosuppressive effect through galectin-1 and semaphorin-3A. Stem Cells Int. 2012;2012:412610. doi: 10.1155/2012/412610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg K, Pullen NA, Oskeritzian CA, Ryan JJ, Bowlin GL. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials. 2013;34(18):4439–4451. doi: 10.1016/j.biomaterials.2013.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg K, Sell SA, Madurantakam P, Bowlin GL. Angiogenic Potential of Human macrophages on electrospun bioresorbable vascular grafts. Biomed Mater. 2009;4(3):031001. doi: 10.1088/1748-6041/4/3/031001. [DOI] [PubMed] [Google Scholar]
- 20.Kittan NA, Allen RM, Dhaliwal A, Cavassani KA, Schaller M, Gallagher KA, Carson WFt, Mukherjee S, Grembecka J, Cierpicki T, Jarai G, Westwick J, Kunkel SL, Hogaboam CM. Cytokine induced phenotypic and epigenetic signatures are key to establishing specific macrophage phenotypes. PLoS One. 2013;8(10):e78045. doi: 10.1371/journal.pone.0078045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kogawa Y, Nakajima K, Sasaguri K, Hamada N, Kawasaki H, Sato S, Kadoya T, Horie H. Oxidized galectin-1 reduces lipopolysaccharide-induced increase of proinflammatory cytokine mRNA in cultured macrophages. Clin Cosmet Investig Dent. 2011;3:1–8. doi: 10.2147/CCIDEN.S16066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boersema GS, Grotenhuis N, Bayon Y, Lange JF, Bastiaansen-Jenniskens YM. The effect of biomaterials used for tissue regeneration purposes on polarization of macrophages. Biores Open Access. 2016;5(1):6–14. doi: 10.1089/biores.2015.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He CL, Huang ZM, Han XJ. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J Biomed Mater Res A. 2009;89A(1):80–95. doi: 10.1002/jbm.a.32004. [DOI] [PubMed] [Google Scholar]
- 24.Lakshmanan R, Kumaraswamy P, Krishnan UM, Sethuraman S. Engineering a growth factor embedded nanofiber matrix niche to promote vascularization for functional cardiac regeneration. Biomaterials. 2016;97:176–s95. doi: 10.1016/j.biomaterials.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 25.Wang K, Zheng W, Pan Y, Ma S, Guan Y, Liu R, Zhu M, Zhou X, Zhang J, Zhao Q, Zhu Y, Wang L, Kong D. Three-layered PCL grafts promoted vascular regeneration in a rabbit carotid artery model. Macromol Biosci. 2016;16(4):608–18. doi: 10.1002/mabi.201500355. [DOI] [PubMed] [Google Scholar]
- 26.Kai D, Prabhakaran MP, Jin G, Tian L, Ramakrishna S. Potential of VEGF-encapsulated electrospun nanofibers for in vitro cardiomyogenic differentiation of human mesenchymal stem cells. J Tissue Eng Regen Med. 2017;11(4):1002–10. doi: 10.1002/term.1999. [DOI] [PubMed] [Google Scholar]
- 27.Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG. Evidence of a role for galectin-1 in acute inflammation. Eur J Immunol. 2000;30(5):1331–9. doi: 10.1002/(SICI)1521-4141(200005)30:5<1331::AID-IMMU1331>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 28.Brown BN, Sicari BM, Badylak SF. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol. 2014;5:510. doi: 10.3389/fimmu.2014.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castellano D, Sanchis A, Blanes M, Perez Del Caz MD, Ruiz-Sauri A, Piquer M, Pelacho B, Marco B, Garcia N, Ontoria-Oviedo I, Cambra V, Prosper F, Sepulveda P. Electrospun poly(hydroxybutyrate) scaffolds promote engraftment of human skin equivalents via macrophage M2 polarization and angiogenesis. J Tissue Eng Regen Med. 2017 doi: 10.1002/term.2420. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Chong BF, Tseng LC, Hosler GA, Teske NM, Zhang S, Karp DR, Olsen NJ, Mohan C. A subset of CD163+ macrophages displays mixed polarizations in discoid lupus skin. Arthritis Res Ther. 2015;17:324. doi: 10.1186/s13075-015-0839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aktas E, Chamberlain CS, Saether EE, Duenwald-Kuehl SE, Kondratko-Mittnacht J, Stitgen M, Lee JS, Clements AE, Murphy WL, Vanderby R. Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J Orthop Res. 2017;35(2):269–280. doi: 10.1002/jor.23258. [DOI] [PubMed] [Google Scholar]
- 32.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potas JR, Haque F, Maclean FL, Nisbet DR. Interleukin-10 conjugated electrospun polycaprolactone (PCL) nanofibre scaffolds for promoting alternatively activated (M2) macrophages around the peripheral nerve in vivo. J Immunol Methods. 2015;420:38–49. doi: 10.1016/j.jim.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Kim C, Shores L, Guo Q, Aly A, Jeon OH, Kim do H, Bernstein N, Bhattacharya R, Chae JJ, Yarema KJ, Elisseeff JH. Electrospun microfiber scaffolds with anti-inflammatory tributanoylated n-acetyl-d-glucosamine promote cartilage regeneration. Tissue Eng Part A. 2016;22(7–8):689–97. doi: 10.1089/ten.tea.2015.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holan V, Chudickova M, Trosan P, Svobodova E, Krulova M, Kubinova S, Sykova E, Sirc J, Michalek J, Juklickova M, Munzarova M, Zajicova A. Cyclosporine A-loaded and stem cell-seeded electrospun nanofibers for cell-based therapy and local immunosuppression. J Control Release. 2011;156(3):406–12. doi: 10.1016/j.jconrel.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Chan J, O’Donoghue K, Gavina M, Torrente Y, Kennea N, Mehmet H, Stewart H, Watt DJ, Morgan JE, Fisk NM. Galectin-1 induces skeletal muscle differentiation in human fetal mesenchymal stem cells and increases muscle regeneration. Stem Cells. 2006;24(8):1879–91. doi: 10.1634/stemcells.2005-0564. [DOI] [PubMed] [Google Scholar]
- 37.Peake JM, Della Gatta P, Suzuki K, Nieman DC. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev. 2015;21:8–25. [PubMed] [Google Scholar]
- 38.Tagliaferri C, Wittrant Y, Davicco MJ, Walrand S, Coxam V. Muscle and bone, two interconnected tissues. Ageing Res Rev. 2015;21:55–70. doi: 10.1016/j.arr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2006;39(1):61–9. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Jackson WM, Aragon AB, Onodera J, Koehler SM, Ji Y, Bulken-Hoover JD, Vogler JA, Tuan RS, Nesti LJ. Cytokine expression in muscle following traumatic injury. J Orthop Res. 29(10):1613–20. doi: 10.1002/jor.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beyer C, Huang J, Beer J, Zhang Y, Palumbo-Zerr K, Zerr P, Distler A, Dees C, Maier C, Munoz L, Kronke G, Uderhardt S, Distler O, Jones S, Rose-John S, Oravecz T, Schett G, Distler JH. Activation of liver X receptors inhibits experimental fibrosis by interfering with interleukin-6 release from macrophages. Ann Rheum Dis. 2015;74(6):1317–24. doi: 10.1136/annrheumdis-2013-204401. [DOI] [PubMed] [Google Scholar]
- 42.Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1(1):21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelosi L, Berardinelli MG, Forcina L, Spelta E, Rizzuto E, Nicoletti C, Camilli C, Testa E, Catizone A, De Benedetti F, Musaro A. Increased levels of interleukin-6 exacerbate the dystrophic phenotype in mdx mice. Hum Mol Genet. 2015;24(21):6041–53. doi: 10.1093/hmg/ddv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nita-Lazar M, Banerjee A, Feng C, Vasta GR. Galectins regulate the inflammatory response in airway epithelial cells exposed to microbial neuraminidase by modulating the expression of SOCS1 and RIG1. Mol Immunol. 2015;68(2 Pt A):194–202. doi: 10.1016/j.molimm.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mello CB, Ramos L, Gimenes AD, Andrade TR, Oliani SM, Gil CD. Immunomodulatory effects of galectin-1 on an IgE-mediated allergic conjunctivitis model. Invest Ophthalmol Vis Sci. 2015;56(2):693–704. doi: 10.1167/iovs.14-15100. [DOI] [PubMed] [Google Scholar]
- 46.Miao JH, Wang SQ, Zhang MH, Yu FB, Zhang L, Yu ZX, Kuang Y. Knockdown of galectin-1 suppresses the growth and invasion of osteosarcoma cells through inhibition of the MAPK/ERK pathway. Oncol Rep. 2014;32(4):1497–504. doi: 10.3892/or.2014.3358. [DOI] [PubMed] [Google Scholar]
- 47.Chung LY, Tang SJ, Sun GH, Chou TY, Yeh TS, Yu SL, Sun KH. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res. 2012;18(15):4037–47. doi: 10.1158/1078-0432.CCR-11-3348. [DOI] [PubMed] [Google Scholar]
- 48.Miranda MB, Xu H, Torchia JA, Johnson DE. Cytokine-induced myeloid differentiation is dependent on activation of the MEK/ERK pathway. Leuk Res. 2005;29(11):1293–306. doi: 10.1016/j.leukres.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Sengupta TK, Talbot ES, Scherle PA, Ivashkiv LB. Rapid inhibition of interleukin-6 signaling and Stat3 activation mediated by mitogen-activated protein kinases. Proc Natl Acad Sci U S A. 1998;95(19):11107–12. doi: 10.1073/pnas.95.19.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sengupta TK, Schmitt EM, Ivashkiv LB. Inhibition of cytokines and JAK-STAT activation by distinct signaling pathways. Proc Natl Acad Sci U S A. 1996;93(18):9499–504. doi: 10.1073/pnas.93.18.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhat GJ, Abraham ST, Baker KM. Angiotensin II interferes with interleukin 6-induced Stat3 signaling by a pathway involving mitogen-activated protein kinase kinase 1. J Biol Chem. 1996;271(37):22447–52. doi: 10.1074/jbc.271.37.22447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.