Abstract
MCAK, a member of the kinesin-13 family, is a microtubule (MT) depolymerase that is necessary to ensure proper kinetochore MT attachment during spindle formation. Regulation of MCAK activity and localization is controlled in part by Aurora B kinase at the centromere. Here we analyzed human cells depleted of the ubiquitous Ca2+/calmodulin-dependent protein kinase IIγ isoform (CaMKIIγ) by RNA interference and found that CaMKIIγ was necessary to suppress MCAK depolymerase activity in vivo. A functional overlap with TOGp, a MT regulator known to counteract MCAK, was suggested by similar CaMKIIγ- and TOGp-depletion phenotypes, namely disorganized multipolar spindles. A replicating vector system, which permits inducible overexpression in cells that simultaneously synthesize interfering short hairpin RNAs, was used to dissect the functional interplay between CaMKIIγ, TOGp, and MCAK. Our results revealed two distinct but functionally overlapping mechanisms for negative regulation of the cytosolic/centrosomal pool of MCAK. These two mechanisms, involving CaMKIIγ and TOGp, respectively, are both essential for spindle bipolarity in a normal physiological context, but not in MCAK-depleted cells.
Keywords: cell cycle, mitosis, monastral, RNA interference, XKCM1
Introduction
The bipolar mitotic spindle segregates duplicated chromosomes during cell division. Generation of a functional spindle requires both the inherent dynamic instability of microtubules (MTs), which are polar polymers comprised of α/β tubulin heterodimers, and regulatory proteins that alter MT dynamics (reviewed in Desai and Mitchison, 1997). Spindle MTs attach to sister chromatids at kinetochores, which are protein complexes that assemble onto centromeres (reviewed in Biggins and Walczak, 2003). Chromosome movement is powered by a combination of motor proteins at the kinetochore and balanced MT dynamics in the spindle. Kinetochores lacking attached MTs also serve as the signal generators for the mitotic checkpoint, which arrests mitosis until all kinetochores are correctly attached. Defects in the mitotic checkpoint results in aneuploidy, which is one of the hallmarks for cancer cells.
Entry into mitosis triggers global changes in MT dynamics including an increased catastrophe frequency, that is, the transition from MT growth to shrinkage (reviewed in Kline-Smith and Walczak, 2004). In Xenopus egg extracts, the kinesin-13 family member MCAK mediates increased catastrophe frequency at the onset of mitosis (Tournebize et al, 2000; Lawrence et al, 2004). The mechanism of MCAK-mediated catastrophe promotion involves binding to both ends of the MT polymer and catalytically depolymerizing protofilament ends (Desai et al, 1999; Hunter et al, 2003). In Xenopus egg extracts (Tournebize et al, 2000) and in an in vitro MT assembly system consisting of purified components (Kinoshita et al, 2001), the potent catastrophe-promoting activity of MCAK is counteracted by the XMAP215 protein.
Human cells differ from Xenopus egg extracts in that the counteractive activities of MCAK and the human homolog of XMAP215, TOGp, are not clearly manifested at the level of stability of MT plus ends. Instead, the phenotype of cells depleted of TOGp by RNA interference has highlighted an essential function of TOGp at the centrosome (Gergely et al, 2003). Bipolar spindles can form in cells depleted of either MCAK alone or doubly depleted of MCAK and TOGp, but not in cells depleted of TOGp alone (Cassimeris and Morabito, 2004; Holmfeldt et al, 2004). Given that endogenous MCAK is required for manifestation of the characteristic TOGp-depletion phenotype of disorganized multipolar spindles, we proposed that centrosomal TOGp has an essential role in protecting spindle MTs from MCAK activity at the centrosome (Holmfeldt et al, 2004).
For accurate chromosome segregation during anaphase, it is essential that each kinetochore of a pair of sister chromatids attaches to spindle MTs from opposing poles, such that all chromosomes are bioriented. MCAK is necessary for proper chromosome segregation (Maney et al, 1998) by mediating proper MT attachments during congression (Walczak et al, 2002; Kline-Smith et al, 2004). Recent studies have shown that the Aurora B kinase and the inner centromere Kin I stimulator ICIS function with MCAK at the centromere to regulate proper MT attachment (Ohi et al, 2003, 2004; Andrews et al, 2004; Lan et al, 2004). The emerging model implies that Aurora B and ICIS regulate MCAK activity at specific regions within the centromere, such that the depolymerizing activity of MCAK is directed towards incorrectly orientated kinetochore MTs.
Spatial and temporal regulation of MCAK by the Aurora B kinase involves phosphorylation on multiple Ser residues, which suppresses and thereby controls the potent MT-destabilizing activity of MCAK at the centromere (Andrews et al, 2004; Lan et al, 2004; Ohi et al, 2004). However, MCAK is also abundant in the cytosol and at the centrosome, and it is unclear how its activity is controlled at these locations. In contrast to what could be expected from the original studies of Xenopus egg extracts (Walczak et al, 1996; Tournebize et al, 2000), it was recently shown that MCAK depletion in human cells by RNA interference has only a minor effect on the level of spindle MT polymer content and no effect on the density of the interphase MT array (Cassimeris and Morabito, 2004; Holmfeldt et al, 2004). This surprisingly modest depletion phenotype suggests that cytosolic/centrosomally located MCAK is also subject to negative regulation, but the mechanism is unknown.
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a multifunctional enzyme that has been implicated in a multitude of Ca2+-regulated biological processes, including synaptic transmission, gene transcription, and growth control (reviewed in Hudmon and Schulman, 2002). There are four isoforms of CaMKII encoded by separate genes; the γ and δ isoforms are ubiquitously expressed, while the α and β isoforms are neural specific. Here we have investigated functions of CaMKIIγ in human cell lines, either by depleting the γ isoform by RNA interference or by overexpressing a constitutively active derivative. These studies revealed an essential function of CaMKIIγ as a negative regulator of MCAK that is required for suppression of cytosolic/centrosomal MCAK activity during spindle assembly. CaMKIIγ depletion resulted in the same type of disorganized multipolar spindles that were previously observed in TOGp-depleted cells. By examining the functional interplay between CaMKIIγ, TOGp, and MCAK, we propose a model in which this interplay is essential for protection of MTs from uncontrolled MCAK activity at the centrosome.
Results
CaMKIIγ is essential for bipolar spindle formation
To explore a potential cell cycle regulatory role of the ubiquitously expressed CaMKIIγ isoform, CaMKIIγ levels were reduced in human K562 leukemia cells by RNA interference by using an short hairpin RNA (shRNA) expression vector. Our approach relies on an EBV-based replicating vector system that confers hygromycin resistance and thus allows selection of cells within 3 days, all of which express the transfected shRNA (Holmfeldt et al, 2004). Graded shRNA expression levels were achieved by premixing the specific shRNA derivative at different ratios with a control vector. As these vectors have a stringent replication control, the relative proportion of transfected DNA is maintained (Melander Gradin et al, 1997). Transfection of a shuttle vector directing expression of CaMKIIγ-specific shRNA (shRNA-CaMKIIγ)-depleted CaMKIIγ, while TOGp and tubulin levels were unaltered (Figure 1A). Quantification of immunoblots by serial dilutions of cell lysates revealed more than 90% specific depletion at all shRNA-CaMKIIγ dilutions. It should be noted that >95% reduction of CaMKIIγ was below the limit of detection.
Figure 1.
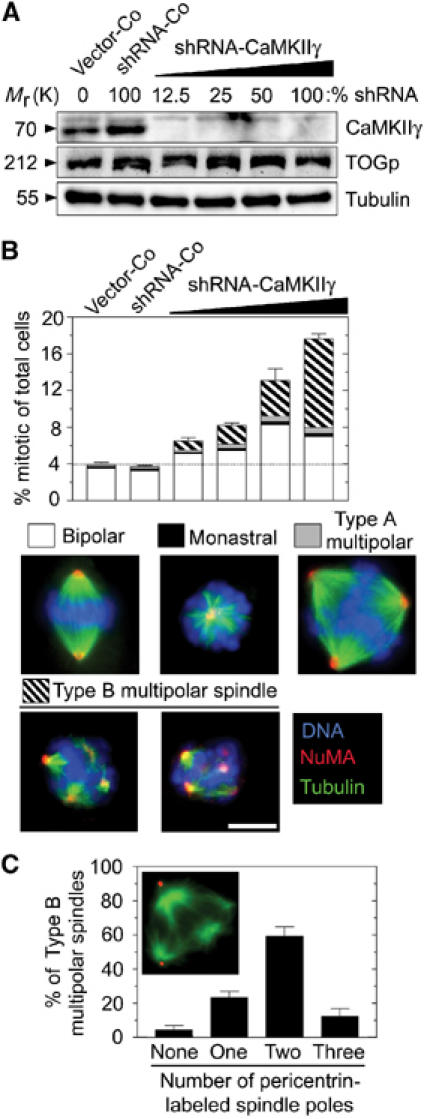
Phenotype of K562 cells expressing CaMKIIγ-specific interfering shRNA. Cells were transfected with a total of 18 μg DNA comprising the indicated percentage of shRNA-scrambled or shRNA-CaMKIIγ made up of Vector-Co. Cells were counter-selected for 5 days with hygromycin to kill off nontransfected cells. (A) Immunoblots of total cellular lysates using the indicated antibody for detection. (B) Cells were triple-stained for DNA, MTs, and the MT minus-end marker NUMA. Representative examples of each spindle type are shown below the histogram. Spindles of mitotic cells were categorized into four groups, namely, bipolar, monastral, and multipolar types A and B, as indicated by the subdivision of bars. The data in this and subsequent figures are presented as percentage of total cells and represent the means of duplicate determinations of coded samples (n=300 mitotic cells). The most notable difference between type A and type B multipolar spindles is that the chromosomes of type B spindles are completely disorganized and appear aggregated (two representative examples shown). Bar: 10 μm. (C) Cells were transfected with 100% shRNA-CaMKIIγ and counter-selected for 5 days with hygromycin. The histogram depicts the distribution of the number of spindle poles in individual cells with type B multipolar spindles, as determined by pericentrin-labeled dots positioned at the spindle pole (n=40). The data represent the means of duplicate determinations. A representative epifluorescence image of a type B multipolar spindle of a shRNA-CaMKIIγ-expressing cell stained with anti-α-tubulin (green) and anti-pericentrin (red) is also shown. The data in this and subsequent figures are representative of at least three independent transfection experiments.
Analysis of CaMKIIγ-depleted cells revealed no detectable phenotype on the level of MT polymer in interphase cells, suggesting that endogenous CaMKIIγ is not important for regulating the density of the interphase MT array (data not shown). However, whereas cells harboring a control vector (Vector-Co) or cells expressing a control shRNA with a scrambled sequence (shRNA-Co) were unaffected, analysis of cell cycle profiles after 5 days of CaMKIIγ depletion revealed an increased fraction of cells with G2/M DNA content (data not shown) and accumulation of mitotic cells with multipolar spindles (see subdivision of bars in Figure 1B). This phenotype became increasingly pronounced at increasing shRNA-CaMKIIγ concentrations. The multipolar spindles observed could be divided into two categories: (1) multipolar spindles with chromosomes that appear well organized between three or more spindle poles (type A), which are also observed among Vector-Co cells, and (2) disorganized multipolar spindles with multiple MT asters of various sizes and with disorganized chromosomes aggregated around each aster (type B) (see images in Figure 1B). The organization of MTs at the minus end in the type B multipolar spindles was not defective, as evidenced by NUMA staining. We found that the type B multipolar spindles were not observed among control cells and are therefore a hallmark of CaMKIIγ-depleted cells.
The shRNA-CaMKIIγ derivative used here depletes its target more efficiently than the shRNA-CaMKIIγ derivative that was used as a negative control in a previous study (Holmfeldt et al, 2004). This observation, as well as a comparison of immunoblot analysis (Figure 1A) and the finding of accumulation of defective mitotic cells (Figure 1B), emphasizes the requirement of efficiently depleting CaMKIIγ in order to detect a phenotype. To ascertain that the phenotype observed was specific for CaMKIIγ depletion, we also analyzed the effect of an independent CaMKIIγ-specific shRNA derivative and the CaMKII inhibitory drug KN93. In both cases, type B multipolar spindles were generated (data not shown). This spindle phenotype was also observed as a major phenotype in the Jurkat T-cell leukemia and the DG 75 Burkitt lymphoma cell lines expressing either of two distinct shRNA-CaMKIIγ derivatives (data not shown). Thus, the ubiquitously expressed CaMKIIγ appears to be necessary for efficient bipolar spindle formation in human cells.
To look more closely at the organization of poles in CaMKIIγ-depleted cells, the type B multipolar spindles of CaMKIIγ-depleted cells were further characterized by dual labeling of tubulin and the centrosomal protein pericentrin. We found that the majority of these abnormal spindles in shRNA-CaMKIIγ-expressing cells contained centrosomes at only two of the multiple spindle poles (Figure 1C), which shows that type B multipolar spindles are not caused by nucleation from multiple centrosomes.
The CaMKIIγ-depletion phenotype is dependent on MCAK
It is notable that the type B multipolar spindles observed among CaMKIIγ-depleted cells appear indistinguishable from the multipolar spindles observed after depletion of TOGp (Gergely et al, 2003). It has previously been shown that the type B multipolar spindles caused by depletion of TOGp are dependent on MCAK (Cassimeris and Morabito, 2004; Holmfeldt et al, 2004). To determine if MCAK is also involved in generating the CaMKIIγ-depletion phenotype, cells were either singly or doubly depleted of CaMKIIγ and MCAK by expressing specific interfering shRNAs. Cell proliferation and mitotic figures were followed over 10 days and compared to cells transfected with Vector-Co or a scrambled control derivative (shRNA-Co). As an additional control for the specificity of phenotypes, we also included shRNA-MAP4 to deplete the MT-associated MAP4 protein, which is not required for spindle assembly (Wang et al, 1996). It is shown in Figure 2A that the present system for RNA interference allows depletion of the specific gene product for prolonged culture periods and, as expected, depletion of MAP4 did not cause interference with cell proliferation (Figure 2B) or spindle assembly (Figure 2C). Moreover, depletion of MCAK caused a modest inhibition of cell proliferation, which is consistent with our previous report (Holmfeldt et al, 2004), whereas depletion of CaMKIIγ caused an almost complete growth arrest. Most importantly, simultaneous depletion of MCAK and CaMKIIγ greatly reduced the cell proliferation defect caused by depletion of CaMKIIγ alone and proliferation of doubly depleted cells was very similar to that observed after depletion of MCAK alone. These results suggest that MCAK is involved in growth inhibition of CaMKIIγ-depleted cells.
Figure 2.
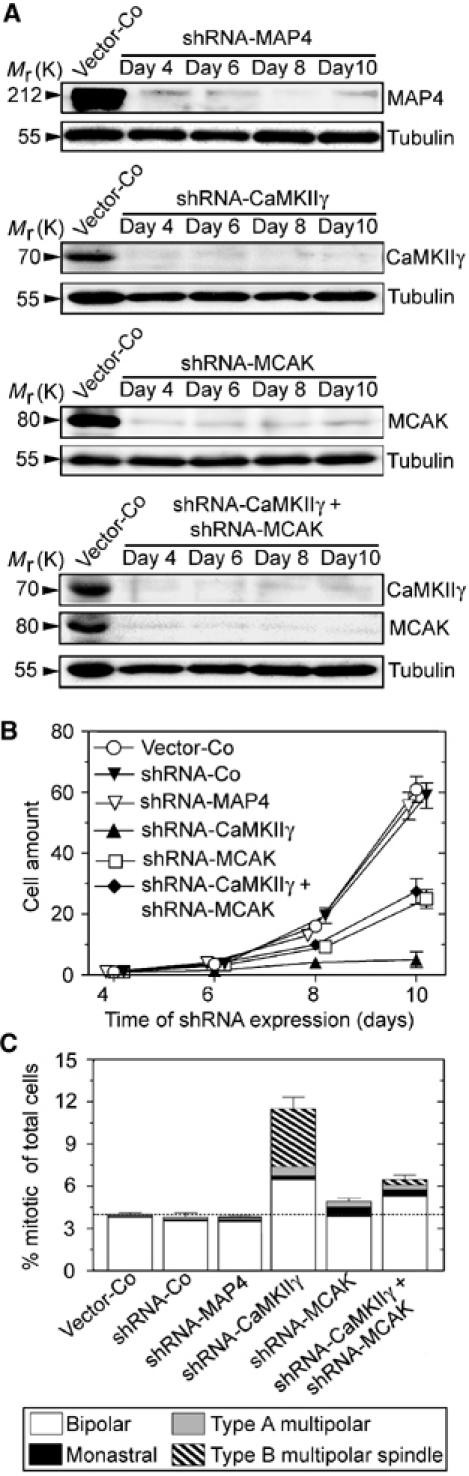
The essential function of CaMKIIγ during cell division is dependent on MCAK. Cells were co-transfected, as in Figure 1, with DNA mixtures containing either 50% of each shRNA, or individually with 50% Vector-Co. Nontransfected cells were counter-selected by culturing in the presence of hygromycin, which killed off most nontransfected cells within 3 days. (A) Total cellular lysates were analyzed by immunoblotting using the indicated antibodies for detection. (B) Viable cells grown in the presence of hygromycin were determined on the days indicated. The graph represents the mean±s.d. of data from three independent transfection experiments, each of which was performed in duplicate. (C) Mitotic figures were categorized after 6 days of depletion, as described under Figure 1B.
Consistent with our previous study (Holmfeldt et al, 2004), analysis of mitotic figures revealed a relatively mild MCAK-depletion phenotype in which there was a slight increase in the number of cells with monastral spindles (Figure 2C). We also observed the expected accumulation of mitotic cells in the CaMKIIγ-depleted population, many of which contained multipolar spindles (Figure 2C). It should be noted that the shRNA-CaMKIIγ has to be diluted with vector control DNA to allow direct comparisons with doubly depleted cells (i.e., 50% of each shRNA in the transfection mix); therefore, the mitotic CaMKIIγ-depletion phenotype is not as dramatic as for maximally depleted cells (i.e., 100% shRNA-CaMKIIγ DNA, Figure 1). Most importantly, double depletion of MCAK and CaMKIIγ significantly reduced the mitotic index and spindle abnormalities caused by depletion of CaMKIIγ alone. In fact, the defects in the doubly depleted cells appeared similar to the relatively mild mitotic phenotype of MCAK depletion alone. Consistent with the cell proliferation assays, these data show that the presence of MCAK is necessary for the mitotic phenotypes caused by depletion of CaMKIIγ, perhaps by a mechanism in which CaMKIIγ downregulates MCAK activity.
The mitotic phenotype of CaMKIIγ depletion includes not only type B multipolar spindles, but also an increased population of bipolar spindles (see subdivision of bars in Figure 2C). Classification of these bipolar spindles revealed an accumulation of prometaphase/metaphase cells, which we found in CaMKIIγ-depleted cells but not in cells depleted of MCAK or doubly depleted of CaMKIIγ and MCAK (Supplementary Figure S1). Many of the prometaphase spindles found in CaMKIIγ-depleted cell populations appeared unusually small, but these features were not distinctive enough to allow a clear-cut classification into a specific subgroup. Nevertheless, given that accumulation of prometaphase/metaphase cells in the CaMKIIγ-depleted population is dependent on MCAK, and that excessive cytosolic MCAK activity is likely to cause accumulation of prometaphase/metaphase cells, these results suggest that CaMKIIγ depletion results in uncontrolled cytosolic MCAK activity.
Excessive MCAK levels cause type B multipolar spindles besides other spindle abnormalities
If uncontrolled MCAK activity is the direct cause of the type B multipolar spindle abnormality, we would expect that increasing the levels of cytosolic and/or centrosomal MCAK will at some point out-titrate the negative regulation by CaMKIIγ, and thereby generate type B multipolar spindles in addition to other mitotic abnormalities. To test this model, we created a pMEP-xMCAK derivate that lacks the N-terminal centromere-targeting domain and, consequently, will only act in the cytosol and at the centrosome (Walczak et al, 2002). In addition, to increase the expression level, the coding region of xMCAK was fused with a 5′ untranslated region that directs high-level protein expression in human cells. The resulting xMCAK(187–732) derivative was expressed four- to eight-fold higher than the intact xMCAK derivative (Figure 3A). At these high levels, interphase MTs were almost completely depolymerized within 8 h (Figure 3B). Consistent with increased expression levels, cells expressing xMCAK(187–732) for 24 h showed a much higher mitotic index than cells expressing xMCAK (Figure 3C).
Figure 3.
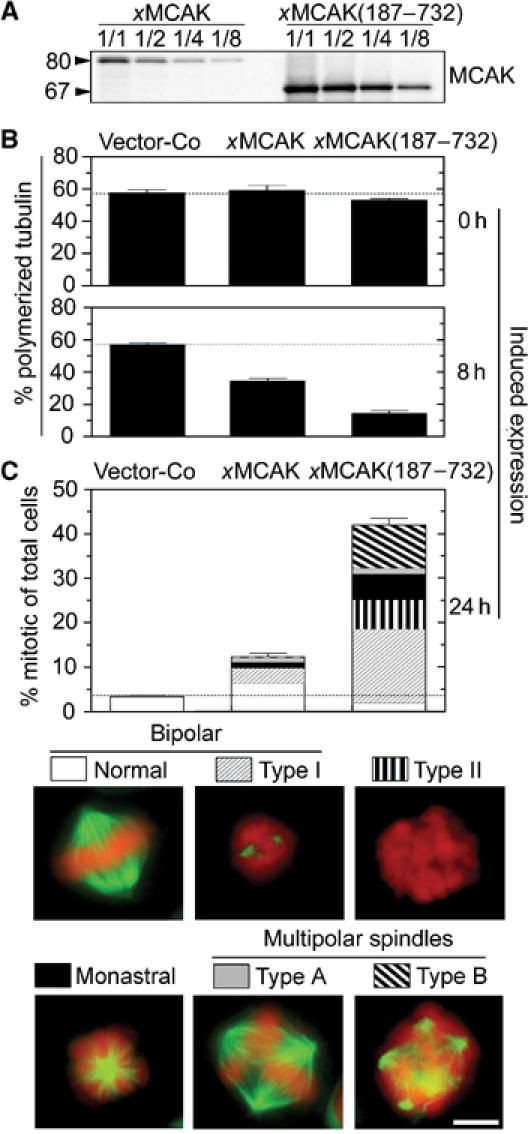
MT depolymerization and mitotic defects caused by high levels of xMCAK active in the cytosol and/or the centrosome. K562 cells were transfected with the pMEP derivative indicated (18 μg) and counter-selected with hygromycin for 5 days. Cd2+ was added to induce ectopic xMCAK from the hMTIIa promoter for the indicated time period. (A) To compare expression of pMEP-xMCAK(187–732), which contains an optimized 5′ untranslated sequence, and pMEP-xMCAK, serially diluted lysates from Cd2+-induced (8 h) transfected cells were analyzed by immunoblotting (1/1: 25 μg/lane; 1/2: 12.5 μg/lane; 1/4: 6.3 μg/lane; 1/8: 3.1 μg/lane). To allow comparison of full-length and N-terminal truncated xMCAK proteins, an antiserum raised against the C-terminus of xMCAK was used for detection. (B) MT content expressed as percentage polymerized tubulin after 0 and 8 h of induced expression. (C) After 24 h of induced expression, mitotic figures were categorized as described in Figure 1B. Representative examples of each spindle type found among xMCAK(187–732)-expressing cells are shown below. Characteristic features of type I bipolar spindles as compared to normal spindles include the observation that most kinetochore MTs are absent and MTs appear as two dense star-like asters. Bar: 10 μm.
Analysis of mitotic figures revealed that essentially all xMCAK(187–732)-expressing mitotic cells had some type of distinctive abnormality, which were categorized into five distinct types (Figure 3C). We found that expression of either xMCAK derivative caused a major increase in the percentage of the previously described type I bipolar spindle (see subdivision of bars in Figure 3C), which has characteristic small MT asters that are indicative of excess catastrophe promotion (Holmfeldt et al, 2004). Moreover, in cells expressing high levels of xMCAK(187–732), many mitotic cells were completely devoid of detectable MTs, which indicates complete depolymerization (termed type II). Importantly, while analysis of mitotic figures revealed very few type B multipolar spindles among cells expressing the full-length xMCAK derivative, essentially all multipolar spindles in cells expressing xMCAK(187–732) were the disorganized type B, and this spindle type comprised ∼20 % of the accumulated mitotic cells. Thus, MCAK has, at sufficiently high levels, the potential to generate a fraction of type B multipolar spindles. This is consistent with a model in which unregulated MCAK activity is the cause of the multipolar spindle phenotype seen when CaMKIIγ is inhibited.
Ectopic CaMKIIγ suppresses the MT-depolymerizing activity of ectopic xMCAK
If CaMKIIγ is responsible for downregulating MCAK activity, then we would also expect that overexpressing active CaMKIIγ would suppress the defects seen upon overexpression of xMCAK. To test this idea, we coexpressed xMCAK with a constitutively active CaMKIIγB derivative (CaMKIIγB(c)). To allow selection of cell populations that coexpress two potentially growth-inhibitory gene products, we used a previously described system for inducible coexpression of genes encoding a substrate and a cognate kinase carried on pMEP vectors under the control of the tightly regulatable hMTIIa promotor (Gradin et al, 1998). We found that stimulation of the hMTIIa promotor for 8 h resulted in a six- to 10-fold overexpression of CaMKIIγB(c) and xMCAK proteins as compared to the cognate endogenous gene products (Figure 4A).
Figure 4.
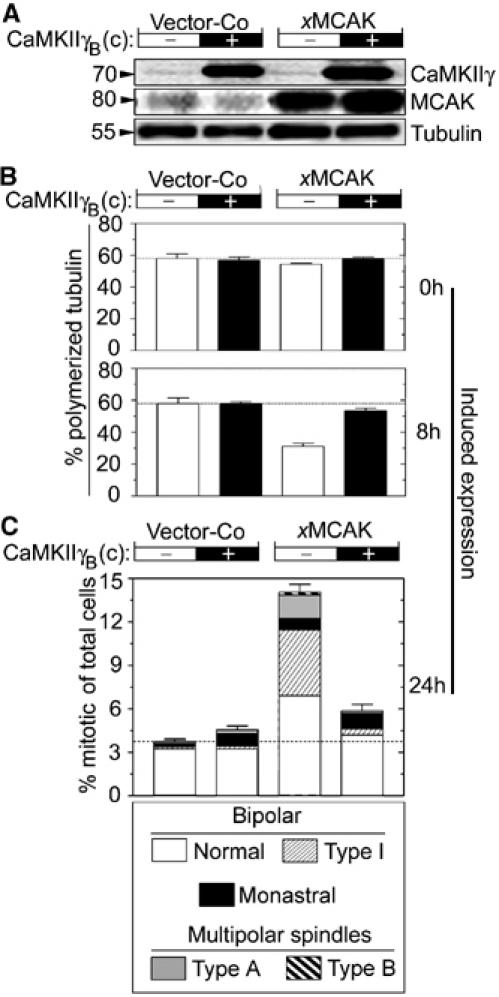
Constitutively active CaMKIIγB(c) suppresses the activity of ectopic xMCAK during both interphase and mitosis. K562 cells were co-transfected (18 μg in total, DNA ratio 1:1) as indicated, with either Vector-Co or pMEP-xMCAK combined with either Vector-Co (−) or pMEP-CaMKIIγB(c) (+). After 5 days of counter-selection with hygromycin, Cd2+ was added to induce ectopic expression from the hMTIIa promoter of the pMEP vector. (A) Immunoblots of total cellular lysates from Cd2+-induced cells (8 h) using the indicated antibody for detection. (B) MT content in interphase cells expressed as percentage of polymerized tubulin prior to (0 h) or after Cd2+-induced expression (8 h). (C) After 24 h of induced expression, mitotic cells were categorized as described in Figure 1B, into the six groups depicted in Figure 3C.
Analysis of the total MT content in interphase cells prior to induced expression (0 h) revealed similar tubulin polymer levels in all four transfected cell populations (Figure 4B), which reflects the tight regulation from the hMTIIa promoter. Expression of CaMKIIγB(c) alone caused no changes in the MT polymer level after 8 h, while expression of xMCAK caused ∼2-fold reduction in the MT content. We found that the MT depolymerization caused by xMCAK overexpression was efficiently suppressed by coexpressed CaMKIIγB(c), which is consistent with our model wherein MCAK activity is downregulated by CaMKIIγ. To explore the functional interactions between ectopic CaMKIIγB(c) and xMCAK during spindle formation, the mitotic index and the appearance of mitotic cells were analyzed after 24 h of induced expression. CaMKIIγB(c) overexpression caused only a very subtle phenotype (Figure 4C). In contrast, high levels of xMCAK expression caused the expected accumulation of mitotic cells, which frequently displayed the bipolar type I abnormality described above (Figure 3). Most significantly, if xMCAK was coexpressed with CaMKIIγB(c), the frequency of mitotic cells was close to normal, and the percentage of cells with type I abnormal spindles was reduced to control levels. These data show that, similar to the observed suppression of MT depolymerization by ectopic xMCAK activity in interphase cells shown in panel B, CaMKIIγB(c) efficiently suppresses the spindle disruptive effect of excessive xMCAK levels during mitosis. It should be noted that we aimed for an expression level of CaMKIIγB(c) that by itself causes a minimal phenotype, which was restricted to a limited increase in monastral spindles. Under these conditions, the observed suppression of MCAK-dependent abnormalities appears remarkably specific.
To evaluate the specificity of CaMKIIγB(c)-mediated suppression further, we analyzed mechanistically distinct types of MT destabilization. We found that ectopic CaMKIIγB(c) neither modulated the potency by which the drug nocodazole destabilizes interphase MTs (Supplementary Figure S2), nor suppressed the activity of the MT-destabilizing protein Op18/Stathmin during interphase and mitosis (Supplementary Figure S3). These results show that CaMKIIγB(c) does not support MT stability against any depolymerizer, and they are consistent with a model in which CaMKIIγB(c) suppresses MT destabilization through MCAK suppression. This implies that ectopic CaMKIIγB(c) should have the same effect on the MT system as MCAK depletion, which indeed appears to be the case since either ectopic CaMKIIγB(c) or MCAK depletion caused a small accumulation of monastral spindles without having an effect on the MT density of interphase cells (Figure 4; Holmfeldt et al, 2004).
It was shown previously that MCAK is phosphorylated by Aurora B, which inhibits the depolymerization activity of MCAK (Andrews et al, 2004; Lan et al, 2004; Ohi et al, 2004). To determine if the previously identified Aurora B kinase phosphorylation sites were the regulatory sites used by CaMKII, CaMKIIγB(c) was coexpressed with an N-terminal truncated xMCAK(187–732) derivative with or without Ala substitutions at Ser-196, which is the only major negative regulatory Aurora B phosphorylation site that remains after N-terminal truncation (Lan et al, 2004). We found that CaMKIIγB(c) suppressed the potent destabilization of the interphase MT array caused by either xMCAK(187–732) or xMCAK(187–732)-S196A overexpression (Figure 5B). Moreover, analysis of mitotic figures revealed that CaMKIIγB(c) also mediated suppression of the potent mitotic block and accumulation of all characteristic spindle aberrancies (Figure 5C). Consistent with our data on interphase cells, suppression of overexpressed MCAK was unaltered by Ala substitution at Ser-196. These data show that suppression of MCAK activity by CaMKIIγ does not require phosphorylation of any of the previously identified Aurora B sites.
Figure 5.
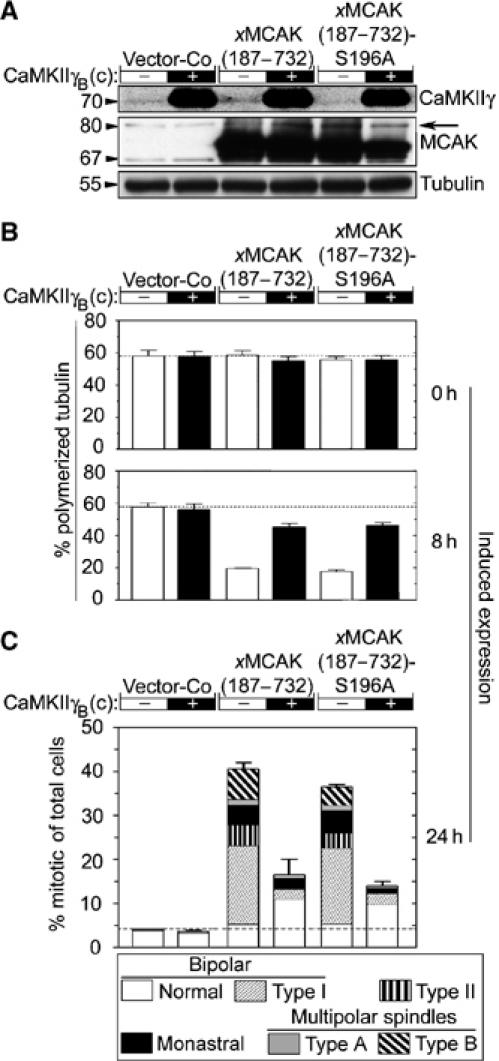
CaMKIIγB(c) suppresses MCAK-dependent generation of defective spindles independently of the major negative Aurora B phosphorylation site. K562 cells were co-transfected as in Figure 4 with either Vector-Co or the indicated pMEP-xMCAK(187–732) derivative combined with either Vector-Co (−) or pMEP- CaMKIIγB(c) (+). After 5 days of counter-selection with hygromycin, Cd2+ was added to induce ectopic expression from the hMTIIa promoter of the pMEP vector. (A) Immunoblots of total cellular lysates from Cd2+-induced cells (8 h) using the indicated antibody for detection. The migration of endogenous MCAK is indicated by an arrow. (B) MT content in interphase cells expressed as percentage polymerized tubulin prior to (0 h) or after Cd2+-induced expression (8 h). (C) After 24 h of induced expression, mitotic cells were categorized as described under Figure 1B, into the six groups depicted in Figure 3C.
To determine whether the effects of CaMKII suppression of MCAK activity were direct, we pre-phosphorylated xMCAKin vitro with recombinant CaMKII and then assayed MT depolymerization activity. We found that MCAK was readily phosphorylated by CaMKII, but phosphorylation by this kinase did not inhibit the MT depolymerization activity of MCAK under any of the conditions tested (Supplementary Figure S4). Thus, it seems that CaMKIIγB(c) suppresses xMCAK activity in intact cells by an indirect mechanism. As removal of the Aurora B phosphorylation sites on MCAK also did not inhibit the functional relationship between MCAK and CaMKII, our data suggest that the intermediate player is not Aurora B kinase.
Functional interplay of CaMKIIγ, MCAK, and TOGp during spindle formation
XMAP215, which is the Xenopus ortholog of TOGp, and xMCAK act in an antagonistic manner during spindle assembly in Xenopus egg extracts and in an MT assembly system consisting of purified components (Tournebize et al, 2000; Kinoshita et al, 2001). It follows that the observed phenotypes of CaMKIIγ depletion/overexpression can in principle be explained by differential modulation of the activity of either of these two MT regulators. It was therefore important to establish whether constitutively active CaMKIIγ suppresses the type B multipolar spindle phenotype in TOGp-depleted cells, as would be predicted by suppression of cytosolic/centrosomal MCAK activity. Replicating vectors that direct constitutive synthesis of shRNA-TOGp and inducible expression of CaMKIIγB(c) were co-transfected, and a TOGp-depleted cell line was generated by counter-selection for 4 days with hygromycin. This system allowed regulated ectopic CaMKIIγB(c) expression in a TOGp-depleted cell line (Figure 6A). Prior to induced CaMKIIγB(c) expression (0 h), the mitotic index was high and type B multipolar spindles were the most common mitotic abnormality among TOGp-depleted cells. However, 24 h of induced CaMKIIγB(c) expression decreased the mitotic index, and the total frequency of type B spindles was suppressed ∼5-fold (Figure 6A). In addition, CaMKIIγB(c) expression not only suppressed generation of type B spindles but also facilitated cell division in TOGp-depleted cells (Figure 6B). Given the previously demonstrated requirement of MCAK for manifestation of the type B multipolar phenotype in TOGp-depleted cells (Holmfeldt et al, 2004), the present data are consistent with a model in which CaMKIIγ suppresses the activity of centrosomal and/or cytosolic MCAK as depicted in Figure 7.
Figure 6.
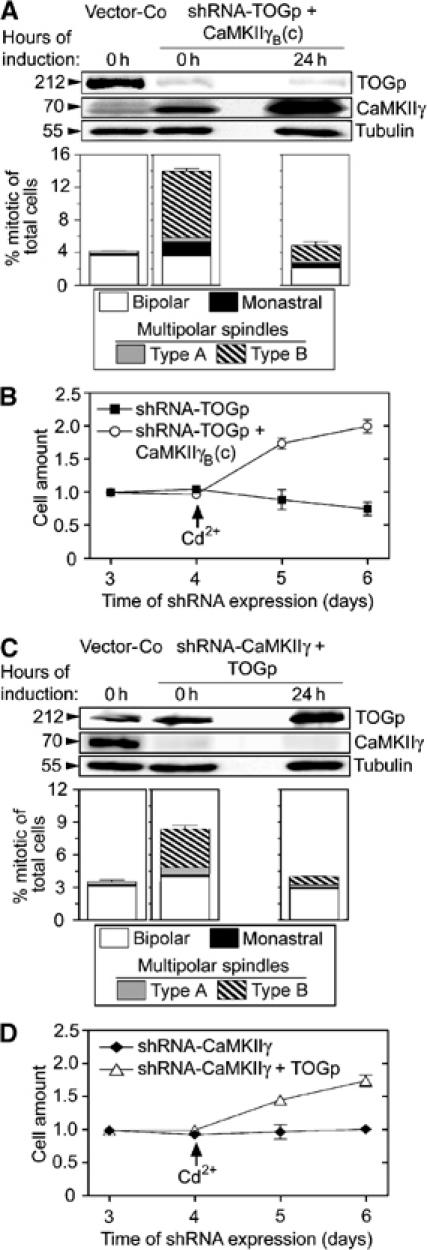
A reciprocal functional link between CaMKIIγ and TOGp as revealed by inducible ectopic expression in cells depleted of specific products via the indicated shRNAs. Cells were transfected either with pMEP vector alone or co-transfected with a mixture of replicating shuttle vectors that direct constitutive synthesis of shRNA-TOGp and inducible expression of CaMKIIγB(c) (18 μg total DNA comprising 20% shRNA-TOG and 80% pMEP-CaMKIIγB(c)). After 4 days of culture, Cd2+ was added for 24 h to specifically induce ectopic CaMKIIγB(c) from the hMTIIa promoter of the pMEP vector. (A) Upper panels show immunoblots of total cellular lysates using the indicated antibody for detection, prior to or after induced CaMKIIγB(c) expression. Lower panels show the distribution of the indicated types of mitotic figures, which were categorized as described under Figure 1B after 0 and 24 h of induced CaMKIIγB(c) expression. (B) The transfected cells described in panel A were counted on the indicated day after transfection. Cells were cultured for the first 4 days in media designed to suppress the hMTIIa promoter. As indicated by an arrow, the media was changed to Cd2+-containing media to induce a high level of CaMKIIγB(c) expression at day 4. The data represent the means of duplicate determinations. (C) Cells were transfected, cultured and Cd2+-induced as in panel A after transfection with the indicated mixture of replicating shuttle vectors (18 μg total DNA comprising 50% shRNA-CaMKIIγ and 50% pMEP-TOGp). Analyses of protein expression and mitotic figures was performed as in panel A. (D) Transfected cells described in panel C were cultured in the absence and presence of Cd2+, as described under panel B.
Figure 7.
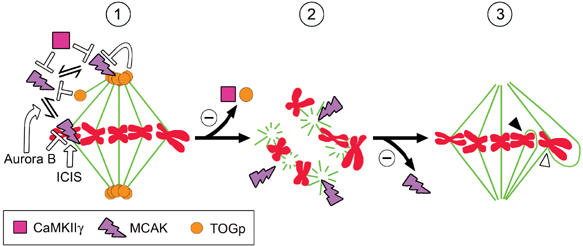
Model for MCAK involvement in the generation of type B multipolar spindles. (1) Depiction of negative regulation of MCAK by TOGp and CaMKIIγ at both free MT plus ends and at the centrosome. The previously described regulation of centromere/kinetochore-associated MCAK is also depicted, that is, centromere targeting and phosphorylation inactivation by Aurora B and stimulation of MCAK activity by ICIS. (2) Depletion of either CaMKIIγ or TOGp, or excessive levels of ectopic cytosolic/centrosomal MCAK, generates type B multipolar spindles. It is assumed that uncontrolled MCAK activity generates this defective spindle type by acting on spindle MT minus ends. (3) Co-depletion of MCAK suppresses the type B multipolar spindle phenotype, and most spindles in co-depleted cells become bipolar and at least partially functional. The appearance of multipolar spindles can also be suppressed by MCAK inhibition through ectopic CaMKIIγB(c), as demonstrated in either TOGp-depleted cells or cells expressing excessive levels of xMCAK(187–732). It follows that CaMKIIγ and TOGp are only strictly required for spindle bipolarity, in as much as they are both essential to control cytoplasmic/centrosomal MCAK activity. As indicated in the model, however, many of the bipolar spindles formed in cells lacking active MCAK at the kinetochores can be defective with respect to kinetochore attachment, as shown by previous reports. This may lead to syntelic (closed arrowheads) or merotelic (open arrowheads) attachments.
We have previously proposed that TOGp is essential at the centrosome for protection of the minus end of spindle MTs from MCAK (see Figure 7; Holmfeldt et al, 2004). The present study suggests an essential role of CaMKIIγ to control MCAK activity at the centrosome and that failure to do so results in the same apparent phenotype as TOGp depletion. This implies that the endogenous level of TOGp is not sufficient for protection of MT minus ends from uncontrolled MCAK activity under conditions of CaMKIIγ depletion. It was therefore of interest to evaluate if induced TOGp overexpression counteracts the apparent uncontrolled MCAK activity that results from CaMKIIγ depletion and thereby suppresses the generation of type B multipolar spindles in CaMKIIγ-depleted cells. By using the same principle experimental design as in Figure 6A and B, TOGp overexpression was induced in cells that had expressed shRNA-CaMKIIγ for 4 days (Figure 6C). We found that overexpression of TOGp in CaMKIIγ-depleted cells decreased the frequency of type B multipolar spindles (Figure 6C) and facilitated cell division (Figure 6D). Thus, given the assumption that uncontrolled MCAK activity is the cause of type B multipolar spindles in CaMKIIγ-depleted cells, these data show that increased TOGp expression provides increased protection against uncontrolled spindle disruptive MCAK activity. This is consistent with the counteractive activities of TOGp and MCAK at the centrosome as depicted in Figure 7.
Discussion
We found that the ubiquitous γ isoform of CaMKII was essential for bipolar spindle formation in all three human cell lines of hematopoetic origin analyzed. It is presently unknown if the other CaMKII family members share this essential function in some non-neural or neural cell types. It is notable that even low CaMKIIγ levels are sufficient to maintain the essential mitotic function of this kinase and a substantial phenotype requires more than 90% depletion (Figure 1). Based on the effect of inhibitory peptides and drugs, in many reports CaMKII has been implicated as the effector of Ca2+-mediated signaling during mitosis (reviewed in Hudmon and Schulman, 2002).
The present study reveals important functional links between CaMKIIγ, TOGp,and MCAK. By combining shRNA-mediated depletion and inducible ectopic expression, we have recently provided evidence for three distinct levels of interdependencies between TOGp and MCAK in human cells (Holmfeldt et al, 2004). The first level involves a role of TOGp in protecting spindle MTs from cytosolic/centrosomal MCAK activity, which prevents formation of disorganized multipolar spindles. The second level involves TOGp-dependent counteraction of excessive MCAK activity during mitosis, which recapitulates the previously established plus-end specific counteractive activities in Xenopus egg extracts and in vitro. The third level involves an unexpected destabilization of the interphase MT array by overexpressed TOGp, which was dependent on endogenous MCAK.
Overexpression of CaMKIIγ alone does not alter the MT content in interphase cells, which illustrates a distinction between the overexpression phenotypes of CaMKIIγ and TOGp. However, there are striking similarities in the depletion and overexpression phenotypes of CaMKIIγ and TOGp in mitotic cells. First, depletion of CaMKIIγ as well as TOGp results in MCAK-dependent generation of type B multipolar spindles. Second, overexpression of CaMKIIγ as well as TOGp suppresses the spindle disrupting effect of excessive MCAK levels (Figures 2, 4, 5, and 6; Holmfeldt et al, 2004). The type B multipolar spindles observed in TOGp- and CaMKIIγ-depleted cells appear closely related by morphological criteria. Direct evidence for a close relationship was provided by our finding that ectopic expression of both TOGp and CaMKIIγ reciprocally suppresses the generation of type B multipolar spindles and facilitates cell division in cells depleted of CaMKIIγ or TOGp (Figure 6). Another level of similarity between CaMKIIγ and TOGp is that high (>10-fold) overexpression of these two proteins alone does not cause a substantial mitotic phenotype, while overexpression of either is sufficient for suppression of the spindle disrupting activity of overexpressed xMCAK (Figures 4 and 5; Holmfeldt et al, 2004). Thus, bipolar spindle assembly in human cells is not critically dependent on excessive TOGp or CaMKIIγ levels being balanced by MCAK. In the case of TOGp, this finding contrasts the reciprocal counteractions of XMAP215/TOGp and MCAK described in embryonic Xenopus systems (Tournebize et al, 2000).
Live analysis of TOGp-depleted cells indicates that type B multipolar spindles evolve gradually from bipolarity to multipolarity during prometaphase (Gergely et al, 2003). Such a gradual evolution is consistent with the idea that the type B multipolar spindles of CaMKIIγ- or TOGp-depleted cells do not involve nucleation from multiple centrosomes, as evidenced by co-staining of pericentrin and tubulin (Figure 1D; Holmfeldt et al, 2004). Based on the evolution and MCAK dependence of type B multipolar spindles, we have previously proposed that TOGp, from its centrosomal localization, has an essential role in protecting spindle MTs from cytosolic/centrosomal MCAK activity at the centrosome (Holmfeldt et al, 2004). An important prediction from this model is that excessive centrosomal and/or cytosolic MCAK activity should generate type B multipolar spindles, which was indeed shown to be the case in the present study (Figures 3 and 5). Based on the demonstrated interplay of CaMKIIγ, TOGp, and MCAK during mitosis, we have extended our previous model to the model depicted in Figure 7. In this model, the type B multipolar spindle is viewed as a phenotype diagnostic for deficient control of cytosolic/centrosomal MCAK activity.
The present study suggests two independent levels of MCAK regulation involving CaMKIIγ and TOGp, in which CaMKIIγ and TOGp appear only strictly required for spindle bipolarity in so much as they are both essential to control cytosolic/centrosomal MCAK activity. Thus, co-depletion of MCAK restores formation of bipolar spindles and to an appreciative extent also cell proliferation in CaMKIIγ- or TOGp-depleted cells (Figure 2; Holmfeldt et al, 2004). Given that overexpression of either CaMKIIγ(c) or TOGp reciprocally suppresses generation of type B multipolar spindles in cells depleted of one of these two proteins (Figure 6), our data reveal a significant functional overlap between TOGp and CaMKIIγ, which can be linked to negative regulation of cytosolic/centrosomal MCAK activity. We also propose that the essential function of TOGp and CaMKIIγ for bipolar spindle formation is exerted from a centrosomal location, which in the case of TOGp is consistent with a prominent centrosomal localization in animal cells (Charrasse et al, 1998; Gergely et al, 2003). Interestingly, and consistent with our model, CaMKII has also been described as a spindle component that is enriched at the centrosome (Ohta et al, 1990).
Analysis of K562 leukemia cells reveals that the major part of overexpressed xMCAK protein resides in the cytosol and at spindle poles (Supplementary Figure S5). Our results demonstrate suppression of ectopic xMCAK activity by ectopic CaMKIIγB(c) on three distinct levels, namely (1) generation of type B multipolar spindles, (2) destabilization of the interphase MT array, and (3) generation of the mitotic type I bipolar spindle phenotype (Figures 4 and 5). As CaMKIIγB(c) does not suppress MT destabilization by Op18/stathmin or nocodazole (Supplementary Figures S2 and S3), phenotypic suppression cannot be attributed to nonspecific stabilization of MTs. While we propose that the first level reflects an action at the centrosome, it seems reasonable to assume that the second and third levels reflect suppression of MT plus-end-directed MCAK activity. We suggest that, similar to TOGp, CaMKIIγ has the potential to suppress both the MT plus-end- and minus-end-directed destabilizing activity of the cytosolic/centrosomal pool of MCAK. This interpretation is consistent with the observation that CaMKIIγ depletion is also associated with an increased frequency of prometaphase cells (Supplementary Figure S1), similar to what occurs upon overexpression of MCAK (Kline-Smith and Walczak, 2002).
The antagonistic activities of MCAK and XMAP215/TOGp at MT plus ends have been well established in the Xenopus system (Tournebize et al, 2000; Kinoshita et al, 2001). However, these studies do not provide any clear-cut mechanistic clue as to how centrosomal TOGp may protect the minus ends of centrosomal MTs from MCAK activity. On the other hand, in the case of CaMKIIγ-mediated MCAK suppression, it seems relevant that MCAK has been shown to be phosphorylated and inactivated by the Aurora B kinase (Andrews et al, 2004; Lan et al, 2004; Ohi et al, 2004). Thus, one simple model is that CaMKIIγ may activate Aurora B, or a related kinase, or by itself phosphorylate MCAK, and thereby inactivate MCAK. However, our present experimental data seem to exclude these possibilities since (1) coexpressed CaMKIIγ(c) still suppresses an MCAK derivative lacking the important negative regulatory Aurora B phosphorylation sites (Figure 5) and (2) MCAK phosphorylated in vitro by CaMKII still retains full activity (Supplementary Figure S4). Moreover, we have also analyzed MCAK localization in cells that either overexpress or are depleted of CaMKIIγ(c), or that have been treated with the CaMKII inhibitor KN-93. These analyses did not indicate that CaMKIIγ-mediated control of MCAK activity involves alterations in the localization of centrosomal MCAK (Supplementary Figure S5; data not shown). Finally, our finding that CaMKIIγ(c) suppresses the mitotic phenotype of TOGp-depleted cells excludes the idea that CaMKIIγ operates through stimulation of the MCAK counteractive activity of TOGp. Thus, the mechanism behind CaMKIIγ-dependent suppression of cytosolic/centrosomal MCAK activity remains to be elucidated, but likely involves an intermediate in the pathway.
Depletion of MCAK alone or in combination with CaMKIIγ or TOGp in cultured human cells has no detectable effect on the density or appearance of the interphase MT array (Holmfeldt et al, 2004; data not shown), which suggest that the essential MT regulatory role of these proteins is restricted to the mitotic phases of the cell cycle. While centromeric MCAK is necessary for correction of chromosome attachment errors on spindles (Kline-Smith et al, 2004), there is presently no evidence that the cytosolic/centrosomal pool of MCAK exerts an essential function in mammalian cells. However, Kif2a, which is another kinesin-13 family member, has recently been shown to be necessary for bipolar spindle formation and depletion of Kif2a results in cells with monopolar spindles (Ganem and Compton, 2004). Based on subcellular localization and Si-RNA knockdown in human cells, the authors derived a model in which the activities of Kif2a and MCAK are spatially restricted such that Kif2a regulates MT minus ends at centrosomes while MCAK regulates MT plus ends at kinetochores. This model is indeed reminiscent of how the two kinesin-13 family members, Klp59C and Klp10A, function in chromosome segregation in Drosophila embryos (Goshima and Vale, 2003; Rogers et al, 2004). One apparent problem with the model is that a large fraction of all MCAK protein resides in both the cytosol and at the centrosome in cultured cells. Our current demonstration of TOGp and CaMKIIγ-dependent negative control of cytosolic/centrosomal MCAK provides a mechanism to keep MCAK inactive at those places, but leaves open the question of whether MCAK in those locations is activated at certain times during mitosis to act in spindle assembly.
Materials and methods
DNA constructs, transfection, and cell culture
The pMEP4 shuttle vector derivative directing inducible expression of the Xenopus ortholog of MCAK (xMCAK), originally termed XKCM1 (Walczak et al, 1996), human TOGp, human Op18/Stathmin, and the human constitutively active mutant of CaMKIIγB (CaMKIIγB(c)) have been described previously (Melander Gradin et al, 1997; Holmfeldt et al, 2002). CaMKIIγB(c) was generated by substituting Thr-286 with Asp, which mimics autophosphorylation and results in a kinase that is substantially Ca2+ independent (Hudmon and Schulman, 2002). To construct the N-terminally truncated xMCAK(187–732) derivative, a PCR approach was used with a 5′ primer containing 21 nucleotides (nt) from the 5′ untranslated region of Op18 (primer sequence: 5′-GAC CGG ATC CGG TAC CGT CGC TTG TCT TCT ATT CAC CAT GGA GCG ACT AGT GGC AAC TCG G-3′) and the T3 primer of pBluescript. The purpose of fusing the initiator Met-codon of xMCAK(187–732) with the untranslated 5′ Op18 sequence was to increase protein expression in human cells. The PCR fragment was digested with KpnI and XhoI and used to replace the corresponding fragment of the pMEP4 vector.
Replicating EBV-based shuttle vectors for constitutive expression of shRNA that targets TOGp- and MCAK-specific RNA has been described (Holmfeldt et al, 2004). The targeting sequences (AA(19 nt)TT) used in the present study were as follows: CaMKIIγ (accession: L07044): (shRNA-CaMKIIγ) CCT GCT GCT GGC GAG TAA A; scrambled version of shRNA-CaMKIIγ (shRNA-Co): GGA GGC TGA ACA TTC CGT C; MAP4 (accession: U19727): (shRNA-MAP4) GATAGTCCCAGCCAAGGAT. A BLAST search of the NCBI database ensured specific targeting of the cognate mRNA.
Single and double co-transfection using pMEP4 and shRNA derivatives, and subsequent selection of hygromycin-resistant cell lines over 4–6 days, were performed as described (Holmfeldt et al, 2004). Conditional expression/coexpression was induced from the hMTIIa promoter, which can be suppressed by cultivation in a specifically formulated medium and subsequently induced by 0.5 μM Cd2+ (Melander Gradin et al, 1997). The data from transfection cell lines shown in this report have been reproduced in at least three independent transfection experiments.
Immunoblotting and immunofluorescence
Quantification by immunoblotting, analysis of cellular MT content by flow cytometry, immunofluorescence analysis of spindles, and immunolocalization of centrosomes by pericentrin staining were performed as described previously (Holmfeldt et al, 2002, 2004). The NuMA antibody (clone A73-B/D12) and Alexa Fluor 488-conjugated anti-α-tubulin, was used for triple staining of NuMA, MTs and DNA using methanol fixed cells. Epifluorescence images were acquired on an Olympus CellR imaging station (Olympus-Biosystems) equipped with an inverted microscope (IX81; Olympus), an × 100, 1.4 NA Planapochromat objective, and a cooled CCD camera (Orca ER; Hamamatsu Photonics).
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Data
Acknowledgments
We thank Lynne Cassimeris for anti-TOGp antibodies, Christian Larroque for TOGp cDNA, and Sofia Edin and Thomas Grundström for shRNA-CaMKIIγ. This work was supported by the Swedish Research Council (MG) and by the NIH (CEW). XZ is supported by an American Heart Association predoctoral fellowship. CEW is a Scholar of the Leukemia and Lymphoma Society.
References
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR (2004) Aurora B regulates MCAK at the mitotic centromere. Dev Cell 6: 253–268 [DOI] [PubMed] [Google Scholar]
- Biggins S, Walczak CE (2003) Captivating capture: how microtubules attach to kinetochores. Curr Biol 13: R449–R460 [DOI] [PubMed] [Google Scholar]
- Cassimeris L, Morabito J (2004) TOGp, the human homolog of XMAP215/Dis1, is required for centrosome integrity, spindle pole organization, and bipolar spindle assembly. Mol Biol Cell 15: 1580–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S, Schroeder M, Gauthier-Rouviere C, Ango F, Cassimeris L, Gard DL, Larroque C (1998) The TOGp protein is a new human microtubule-associated protein homologous to the Xenopus XMAP215. J Cell Sci 111: 1371–1383 [DOI] [PubMed] [Google Scholar]
- Desai A, Mitchison TJ (1997) Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 13: 83–117 [DOI] [PubMed] [Google Scholar]
- Desai A, Verma S, Mitchison TJ, Walczak CE (1999) Kin I kinesins are microtubule-destabilizing enzymes. Cell 96: 69–78 [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Compton DA (2004) The KinI kinesin Kif2a is required for bipolar spindle assembly through a functional relationship with MCAK. J Cell Biol 166: 473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely F, Draviam VM, Raff JW (2003) The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev 17: 336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Vale RD (2003) The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J Cell Biol 162: 1003–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin HM, Larsson N, Marklund U, Gullberg M (1998) Regulation of microtubule dynamics by extracellular signals: cAMP-dependent protein kinase switches off the activity of oncoprotein 18 in intact cells. J Cell Biol 140: 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmfeldt P, Brattsand G, Gullberg M (2002) MAP4 counteracts microtubule catastrophe promotion but not tubulin-sequestering activity in intact cells. Curr Biol 12: 1034–1039 [DOI] [PubMed] [Google Scholar]
- Holmfeldt P, Stenmark S, Gullberg M (2004) Differential functional interplay of TOGp/XMAP215 and the KinI kinesin MCAK during interphase and mitosis. EMBO J 23: 627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H (2002) Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem 71: 473–510 [DOI] [PubMed] [Google Scholar]
- Hunter AW, Caplow M, Coy DL, Hancock WO, Diez S, Wordeman L, Howard J (2003) The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Mol Cell 11: 445–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K, Arnal I, Desai A, Drechsel DN, Hyman AA (2001) Reconstitution of physiological microtubule dynamics using purified components. Science 294: 1340–1343 [DOI] [PubMed] [Google Scholar]
- Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE (2004) Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol Biol Cell 15: 1146–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Smith SL, Walczak CE (2002) The microtubule-destabilizing kinesin XKCM1 regulates microtubule dynamic instability in cells. Mol Biol Cell 13: 2718–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Smith SL, Walczak CE (2004) Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol Cell 15: 317–327 [DOI] [PubMed] [Google Scholar]
- Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT (2004) Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol 14: 273–286 [DOI] [PubMed] [Google Scholar]
- Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy AS, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L (2004) A standardized kinesin nomenclature. J Cell Biol 167: 19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney T, Hunter AW, Wagenbach M, Wordeman L (1998) Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J Cell Biol 142: 787–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander Gradin H, Marklund U, Larsson N, Chatila TA, Gullberg M (1997) Regulation of microtubule dynamics by Ca2+/calmodulin-dependent kinase IV/Gr-dependent phosphorylation of oncoprotein 18. Mol Cell Biol 17: 3459–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi R, Coughlin ML, Lane WS, Mitchison TJ (2003) An inner centromere protein that stimulates the microtubule depolymerizing activity of a KinI kinesin. Dev Cell 5: 309–321 [DOI] [PubMed] [Google Scholar]
- Ohi R, Sapra T, Howard J, Mitchison TJ (2004) Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell 15: 2895–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Ohba T, Miyamoto E (1990) Ca2+/calmodulin-dependent protein kinase II: localization in the interphase nucleus and the mitotic apparatus of mammalian cells. Proc Natl Acad Sci USA 87: 5341–5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GC, Rogers SL, Schwimmer TA, Ems-McClung SC, Walczak CE, Vale RD, Scholey JM, Sharp DJ (2004) Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature 427: 364–370 [DOI] [PubMed] [Google Scholar]
- Tournebize R, Popov A, Kinoshita K, Ashford AJ, Rybina S, Pozniakovsky A, Mayer TU, Walczak CE, Karsenti E, Hyman AA (2000) Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat Cell Biol 2: 13–19 [DOI] [PubMed] [Google Scholar]
- Walczak CE, Gan EC, Desai A, Mitchison TJ, Kline-Smith SL (2002) The microtubule-destabilizing kinesin XKCM1 is required for chromosome positioning during spindle assembly. Curr Biol 12: 1885–1889 [DOI] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ, Desai A (1996) XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell 84: 37–47 [DOI] [PubMed] [Google Scholar]
- Wang XM, Peloquin JG, Zhai Y, Bulinski JC, Borisy GG (1996) Removal of MAP4 from microtubules in vivo produces no observable phenotype at the cellular level. J Cell Biol 132: 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Data


