Abstract
Cadherin adhesion molecules are key determinants of morphogenesis and tissue architecture. Nevertheless, the molecular mechanisms responsible for the morphogenetic contributions of cadherins remain poorly understood in vivo. Besides supporting cell–cell adhesion, cadherins can affect a wide range of cellular functions that include activation of cell signalling pathways, regulation of the cytoskeleton and control of cell polarity. To determine the role of E-cadherin in stratified epithelium of the epidermis, we have conditionally inactivated its gene in mice. Here we show that loss of E-cadherin in the epidermis in vivo results in perinatal death of mice due to the inability to retain a functional epidermal water barrier. Absence of E-cadherin leads to improper localization of key tight junctional proteins, resulting in permeable tight junctions and thus altered epidermal resistance. In addition, both Rac and activated atypical PKC, crucial for tight junction formation, are mislocalized. Surprisingly, our results indicate that E-cadherin is specifically required for tight junction, but not desmosome, formation and this appears to involve signalling rather than cell contact formation.
Keywords: E-cadherin, epidermal barrier, gene targeting, polarity, tight junctions
Introduction
E-cadherin is important for tissue morphogenesis and polarity (Larue et al, 1994). Upon establishing cell–cell adhesion, cadherins cluster in specialized cell junctions, known as adherens junctions, which associate with the actin cytoskeleton (Gottardi et al, 2002). By thus coupling cell adhesion to the cytoskeleton, E-cadherin can create a transcellular network that enables groups of cells or tissues to coordinate their behaviour.
The association of E-cadherin with the actin cytoskeleton is mediated by α-catenin that is linked to the cadherin via β-catenin (Rimm et al, 1995). The latter molecule is also a key player in the Wnt signalling pathway (Gottardi et al, 2002). Another catenin, p120ctn, also binds directly to the cadherin and regulates the stability of the cadherin complex at the cell surface (Reynolds and Roczniak-Ferguson, 2004). The catenin plakoglobin can functionally replace β-catenin in the adherens junctions, but is also a structural component of desmosomes. These cell structures contain nonclassical cadherins and associate with the intermediate filament cytoskeleton. Desmosomes provide tissues with stable and strong cell–cell adhesion (Getsios et al, 2004).
Next to this core cadherin–catenin complex, many other scaffolding, signalling and cytoskeletal molecules are associated with the adherens junctions (Perez-Moreno et al, 2003). For example, the tight junctional marker ZO-1 binds directly to α-catenin and this is considered an intermediate step in the formation of tight junctions (Itoh et al, 1997). Tight junctions act as selective permeability barriers but also form a fence that physically separates the apical membrane domain from the basolateral domain in simple epithelial cells (Anderson et al, 2004). The size and ion selectivity of tight junctions is variable within different tissues and depends on the type and levels of expression of claudins, four span transmembrane molecules, which constitute the tight junctional paired strands (Inai et al, 1999; Colegio et al, 2003).
In vitro studies have shown that E-cadherin is not only necessary for adherens junction formation but its adhesive activity is also crucial for the assembly of other junctional complexes such as desmosomes, gap junctions and tight junctions (Gumbiner et al, 1988; Musil et al, 1990; Watabe et al, 1994). This junctional complex assembly coincides with the establishment of cell polarity and enrichment at the cell junctions of several protein complexes known to be essential for polarity, suggesting an intimate relationship between junction formation and polarity (Nelson, 2003). Indeed, experiments in Drosophila have shown that mutations in junctional proteins not only affect junction formation but also disturb epithelial polarity, whereas polarity mutants have a profound effect on junction formation as well (Cox et al, 1996; Muller and Wieschaus, 1996; Tanentzapf et al, 2000). Together, these data have led to a model in which initial cell–cell adhesion mediated by the cadherin complex is a key step in setting up other cell junctions and epithelial polarity. One of these polarity complexes consists of the Par3/Par6/atypicalPKC (aPKC) complex, which localizes to the apical junctional complex in Drosophila and to tight junctions in mammalian epithelial cells (Nelson, 2003). The activity of the Par3/Par6/aPKC polarity protein complex has been implicated in the formation of tight junctions in simple epithelia (Macara, 2004). Its activation depends on the formation of cell–cell contacts (Yamanaka et al, 2001) and Armadillo, the fly β-catenin homologue, is important for proper localization of this complex in Drosophila (Bilder et al, 2003; Tanentzapf and Tepass, 2003). Activation of the Par3/Par6/aPKC complex is achieved by binding to the small GTPase Cdc42, resulting in the phosphorylation and activation of aPKC (Joberty et al, 2000; Lin et al, 2000).
The epidermis is a stratifying, squamous differentiating multilayered epithelium that serves as the first barrier with the outside environment. Not only does it protect against outside challenges such as microbes or toxic substances, it also prevents the unnecessary loss of water from the organism. Cell–cell adhesion within the epidermis is mediated by both adherens junctions and desmosomes. Two classical cadherins are expressed within the epidermis: P-cadherin, which is confined to the basal layer, and E-cadherin found in all epidermal cell layers (Jensen et al, 1997).
The epidermis is not a classical polarized epithelium, such as intestinal epithelium, which has a clear apical and basolateral membrane domain separated by the apical junctional complex. Nevertheless, the epidermis shows polarization in a broader sense in that the specific layers of the epidermis express a unique set of differentiation and junctional markers (Watt, 2001). For example, hemidesmosomes are restricted to the basal layer, whereas desmosomes are found in all layers but their number and protein composition vary between the different layers (Getsios et al, 2004). Tight junctions, previously thought to be absent in the epidermis, are exclusively found in the granular layer (Morita et al, 1998; Pummi et al, 2001; Brandner et al, 2002). Their importance in maintaining the epidermal barrier was underscored by the observations that in vivo inactivation of the tight junctional membrane protein claudin-1 results in epidermal water loss and ultimately in neonatal death of the mice (Furuse et al, 2002). In addition, claudin-6 overexpression in the skin also disturbs barrier function (Turksen and Troy, 2002).
To study the role of E-cadherin in the morphogenesis and function of stratifying epithelium, we inactivated its gene specifically in the developing epidermis of mice using the Cre-loxP system. We show that E-cadherin is essential for epidermal barrier function. Mice lacking E-cadherin in the epidermis die shortly after birth because of dehydration. Closer molecular examination revealed that key tight junctional components are improperly localized, resulting in altered tight junctional architecture. Indeed, biotin penetrance of the granular layer and altered resistance using impedance measurements of the E-cadherin-negative epidermis revealed impaired tight junction function. Surprisingly, desmosomes are still normally formed, indicating that, unlike previously thought, E-cadherin is specifically required for tight junction formation. Since cell contacts are not majorly altered, E-cadherin may influence tight junction formation via signalling molecules. Potential candidates are the small GTPase Rac and atypical PKC, which both show an altered distribution upon loss of E-cadherin in the epidermis.
Results
Generation of mice lacking E-cadherin in the epidermis
To study how cadherins contribute to junction formation and morphogenesis in stratifying epithelia, we specifically inactivated E-cadherin in developing mouse epidermis by crossing mice carrying two floxed E-cadherin alleles with mice expressing the Cre-recombinase enzyme under the human Keratin 14 promoter (Hafner et al, 2004) in combination with either one floxed E-cadherin allele or one deleted E-cadherin allele (Boussadia et al, 2002). This resulted in either K14-Cre/EcadFl/Fl or K14-Cre/EcadFl/− mice (Figure 1A) that both died within 1 day after birth. Since we have found no phenotypic difference between the two E-cadherin mutant lines, only data on the K14-Cre/EcadFl/− mice are described.
Figure 1.
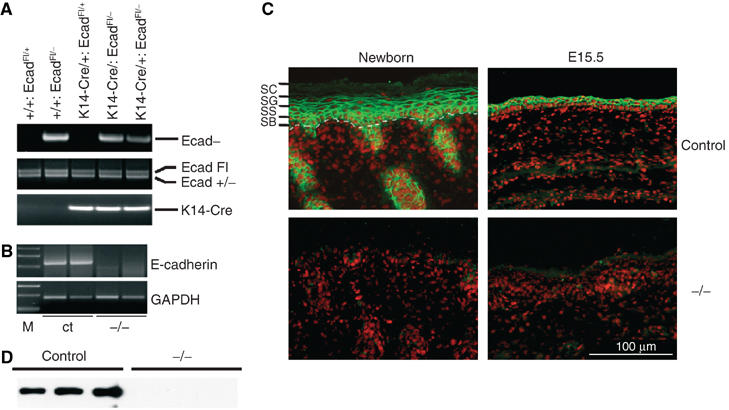
(A) PCR analysis of offspring from E-cadherinFl/Fl mice crossed with a deleter K14-Cre/E-cadherin+/− line. Indicated is the DNA fragment generated by PCR reaction. (B) RT–PCR analysis of RNA isolated from skin biopsies of control (ct) and K14-Cre/EcadFl/− (−/−) mice. M: marker. (C) Immunohistochemical analysis of newborn and embryonic day 15.5 (E15.5) skin sections for E-cadherin (green). Nuclei were counterstained with propidium iodide (red). The different epidermal layers are also indicated: SB: stratum basale or basal layer; SS: stratum spinosum or spinal layer; SG: stratum granulosum or granular layer; SC: stratum corneum or cornified layer. The dotted white line indicates the epidermal–dermal junction. (D) Western blot analysis of total newborn mouse skin for E-cadherin.
The E-cadherin gene was efficiently deleted from the epidermis of K14-Cre/EcadFl/− mice, as no RNA expression was observed (Figure 1B). Indeed, staining for E-cadherin was negative in the newborn epidermis of mutant mice, whereas E-cadherin staining was found as expected in all layers of the epidermis in the skin of newborn control mice (Figure 1C). In addition, E-cadherin was already almost completely absent in the epidermis of 15.5-day-old embryos (E15.5), indicating efficient deletion during development of the epidermis (Figure 1C). Western blot analysis of total skin lysates confirmed the absence of E-cadherin protein in knockout but not control skin (Figure 1D).
Cell–cell contacts are maintained in the absence of E-cadherin
Macroscopic examination of the K14-Cre/EcadFl/− mice did not reveal any blistering of the skin, even after rubbing, suggesting no major changes in cell contact formation. We therefore examined the expression and localization of other adherens junction molecules. P-cadherin expression is upregulated (Figure 2B) but was still mainly found in the basal layer of the epidermis of E-cadherin mutant mice (Figure 2A), suggesting that P-cadherin only compensates for the loss of E-cadherin in the basal layer. Absence of E-cadherin resulted in loss or largely reduced staining in the suprabasal layers for the cadherin-associated molecules α-catenin, β-catenin and p120ctn (Figure 2A). These results suggested that the absence of E-cadherin from the suprabasal layers was not compensated by the expression of other classical cadherins. Catenin protein expression in the skin was not substantially altered (Figure 2B), most likely because of the upregulation of P-cadherin expression in the basal layer.
Figure 2.
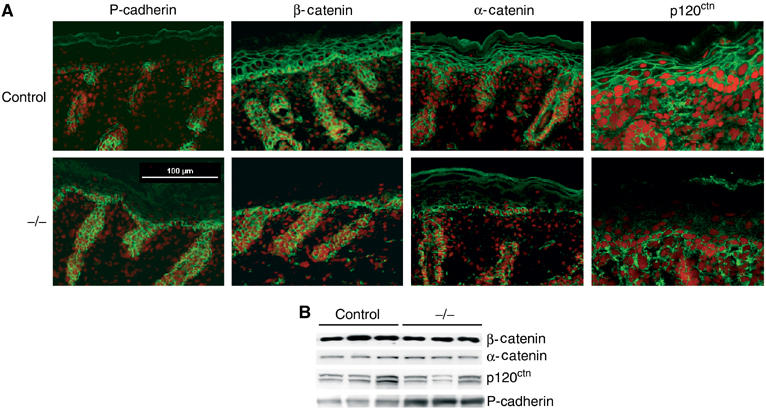
Adherens junctions expression is altered in K14-Cre/EcadFl/− mice. (A) Immunohistochemical analysis of newborn control and K14-Cre/EcadFl/− (−/−) skin sections stained for adherens junction markers. Nuclei are counterstained with propidium iodide. (B) Western blot analysis of total newborn skin lysates for the indicated adherens junction markers.
In vitro studies have shown that E-cadherin is essential for desmosome formation, and we therefore asked the question if in vivo loss of E-cadherin affects desmosomal components. As described previously (Young et al, 2003; Tinkle et al, 2004), no difference in localization of desmosomal markers like plakoglobin, desmocollin 2 and desmoglein 1/2 was observed (Figure 3A). Indeed, ultrastructural analysis showed the presence of well developed desmosomes in the K14-Cre/EcadFl/− mice (Figure 3B). Desmosomal cadherin expression was upregulated (Figure 3C), suggesting compensation for the loss of classical cadherins in the suprabasal layers. Thus, E-cadherin is not essential for desmosome formation in vivo. Importantly, histological and ultrastructural analysis revealed no obvious defects in cell contacts in skin sections of control and K14-Cre/EcadFl/− mice (Figure 4 and Supplementary Figure 1). Differentiation markers and proliferation appeared normal (Supplementary Figure 2). The upregulation of P-cadherin in the basal layer in combination with an increase in desmosomal cadherins may explain why no obvious loss of cell–cell cohesion is observed in the K14-Cre/EcadFl/− mice.
Figure 3.
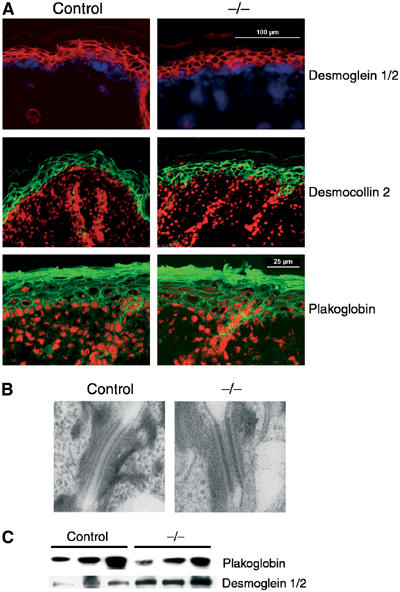
Desmosomes are formed in the absence of E-cadherin. (A) Immunohistochemical analysis of newborn control and K14-Cre/EcadFl/− (−/−) skin sections stained for desmosomal markers. Desmoglein 1/2 is red with nuclei counterstained with dapi (blue) whereas desmocollin 2 and plakoglobin are green with nuclei counterstained with propidium iodide (red). (B) Ultrastructural analysis of desmosomes in newborn control and K14-Cre/EcadFl/− (−/−) skin. (C) Western blot analysis of total newborn skin lysates for plakoglobin and desmoglein 1/2.
Figure 4.
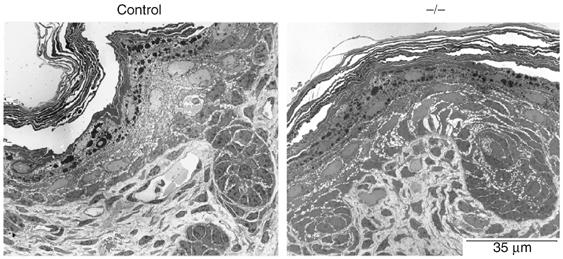
Normal structure and cell–cell contacts in K14-Cre/EcadFl/− mice. Ultrastructural analysis of newborn control and K14-Cre/EcadFl/− (−/−) skin.
Early deletion of E-cadherin in the epidermis results in perinatal death due to water loss
The K14-Cre/EcadFl/− mice die perinatally around 7–12 h after birth. The mutant skin appears more red and shiny than that of their control littermates, and has a parchment-like resemblance. In all K14-Cre/EcadFl/− mice, scaling of the upper ventral side occurs after a few hours (Figure 5A). This macroscopic appearance of the mice suggested a perturbed water barrier. Weighing experiments showed that E-cadherin mutant mice lost around 9% of their body weight during their short lifespan in comparison to an average 3% in control mice (Figure 5B). An increased haematocrit of mutant mice was also observed (Figure 5C), indicating loss of fluids from the K14-Cre/EcadFl/− mice. Indeed, a considerable increase in transepidermal water loss (TEWL) was measured in the K14-Cre/EcadFl/− mice compared to control (Figure 5D). Thus, the lethal phenotype of the E-cadherin mutant mice most likely results from water loss as a result of a disturbance in epidermal barrier function.
Figure 5.
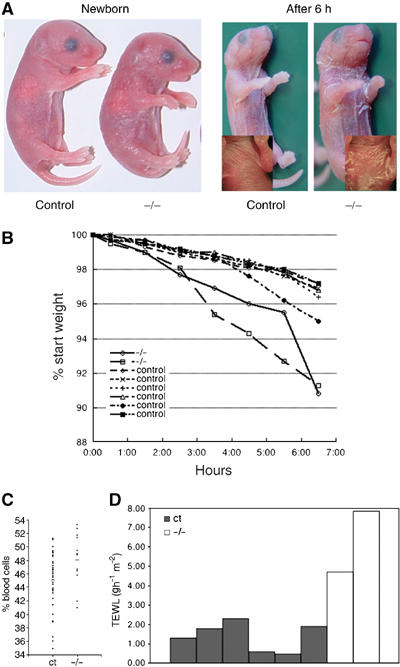
K14-Cre/EcadFl/− mice die of water loss. (A) Macroscopic appearance of newborn control E-cadherin and K14-Cre/EcadFl/− mice. The inset shows close-up of the ventral side. (B) Weight time-course analysis of newborn control and K14-Cre/EcadFl/− mice. (C) Haematocrit analysis of blood isolated from control (n=35) and mutant mice (n=13). (D) TEWL measurements of control and E-cadherin mutant mice.
The outside-in barrier is functional in K14-Cre/EcadFl/− mice
To examine if the observed TEWL was due to a nonfunctional stratum corneum, we assessed the permeability of the newborn epidermal barrier from the outside using the dye lucifer yellow. Only staining of the surface of the cornified layer was observed in both control and K14-Cre/EcadFl/− newborn mice (Figure 6A), indicating the presence of a functional stratum corneum. Barrier formation may be delayed in the K14-Cre/EcadFl/− mice and we therefore performed toluidine blue penetration assays of E18.5 and E16.5 embryos. The skin was impermeable for both control and K14-Cre/EcadFl/− E18.5 embryos (Figure 6B), indicating that the outside-in barrier is functional at this stage in the E-cadherin mutant embryos. Barrier formation starts around E16 in a patterned fashion (Hardman et al, 1998). Indeed, partial penetration of toluidine blue is observed around the paws and the ventral side in control E16.5 embryos and this pattern is similar for K14-Cre/EcadFl/− E16.5 embryos (Figure 6B). We therefore conclude that maturation of the cornified layer developed normal in the absence of E-cadherin, resulting in a properly functioning outside-in barrier.
Figure 6.
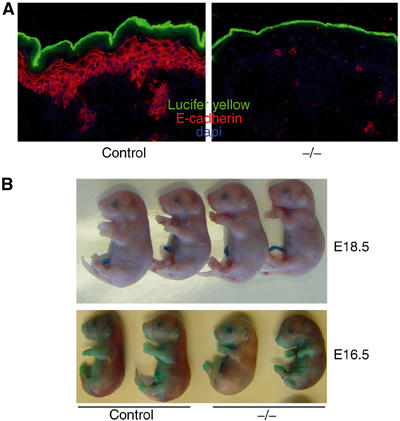
Epidermal loss of E-cadherin does not affect the outside-in barrier. (A) Lucifer yellow dye penetration assay of newborn control and E-cadherin mutant mice. No penetration of lucifer yellow across the stratum corneum is observed when comparing control mice to K14-Cre/EcadFl/− mice. (B) Toluidine blue penetration assay of E18.5- and E16.5-day-old control and K14-Cre/EcadFl/− mice.
Increased permeability of tight junctions in the absence of E-cadherin
Interestingly, mice deficient for the tight junctional protein claudin-1 also show extensive water loss, appear red, shiny and wrinkled, and die within the same time frame as our mutant mice negative for E-cadherin in the epidermis (Furuse et al, 2002). These mice also have an apparently normal cornified envelope. To examine if the observed TEWL is a result of increased inside-out permeability due to leaky tight junctions, which are present in the stratum granulosum (Brandner et al, 2002; Furuse et al, 2002), we injected newborn mice intradermally with biotin and assessed its diffusion into the epidermis. In control skin, biotin moves up to the stratum granulosum, where it is halted due to the presence of tight junctions, marked by occludin staining, between the cells of the granular layer (Furuse et al, 2002). However, in K14-Cre/EcadFl/− epidermis, biotin penetrates the granular layer and is able to reach the stratum corneum (Figure 7A, left panel). This suggested that tight junctions are either absent or are no longer properly functioning in E-cadherin mutant skin.
Figure 7.
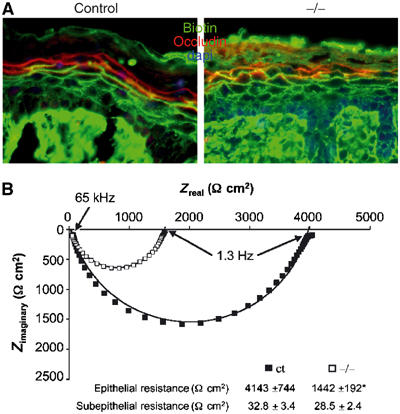
Tight junctions are functionally impaired in K14-Cre/EcadFl/− mice. (A) Inside-out permeability assay. Skin sections of control and mutant newborn mice intradermally injected with biotin were stained with streptavidin to follow the penetration of biotin (green) and counterstained with occludin to mark the tight junctions present in the granular layer. (B) Impedance measurements of ventral skin biopsies isolated from newborn control and E-cadherin mutant mice. Typical experiments are depicted in Nyquist plots; numerical values represent mean±s.e.m. *P-value=0.03. N=8 for control and N=4 for K14-Cre/EcadFl/− (−/−) mice.
Tight junctions restrict paracellular diffusion of ions, and this function can be determined by measuring transepithelial resistance. We performed impedance analysis on skin biopsies from control and K14-Cre/EcadFl/− mice in order to measure simultaneously the resistance of epidermal and dermal tissues (Fromm et al, 1985). A three-fold lower epidermal resistance was observed in mutant skin compared to control skin, whereas the dermal resistance was not significantly affected (Figure 7B). Together, these results strongly indicate that the water loss seen in K14-Cre/EcadFl/− mice is directly related to increased tight junctional permeability.
Altered tight junction formation in K14-Cre/EcadFl/− mice
Since the permeability of tight junction is affected in K14-Cre/EcadFl/− mice, we examined the localization of different tight junctional components. Occludin is specifically concentrated on the membrane in the granular layer of control mice (Morita et al, 1998; Furuse et al, 2002). Occludin is still enriched at the membrane in the granular layer of K14-Cre/EcadFl/− mice (Figure 8A), implying that tight junctions are not completely disturbed in the absence of E-cadherin. This is also suggested by the observation of tight junction-like structures in the granular layer of K14-Cre/EcadFl/− mice by conventional electron microscopy (Supplementary Figure 3).
Figure 8.
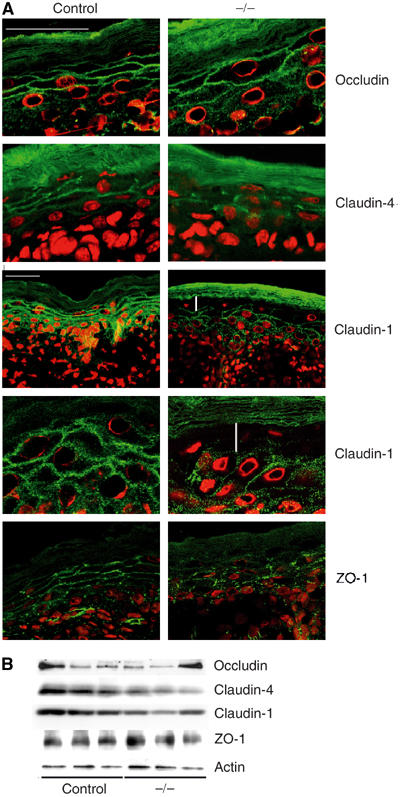
Improper formation of tight junctions in K14-Cre/EcadFl/− mice. (A) Immunohistochemical analysis of skin sections for the tight junctional markers (green) occludin, claudin-4, claudin-1 and ZO-1. Nuclei were counterstained with propidium iodide (red). For claudin-1, both × 400 and × 1000 images are shown. White vertical lines indicate absence of claudin-1 staining from granular layer. White bars=25 μM. (B) Western blot of total skin lysates isolated from newborn control and K14-Cre/EcadFl/− (−/−) mice using antibodies against the indicated tight junctional proteins.
Since claudins are essential for the size and ion specificity of the tight junctional barrier, we next addressed the localization of these proteins. Like occludin, claudin-4 is found at the membrane in the granular layer of the epidermis in control mice (Furuse et al, 2002). Although its localization is still confined to the granular layer in K14-Cre/EcadFl/− mice, its distribution on the membrane is altered, showing a more punctate staining pattern. This appears to correlate with less protein expression in the K14-Cre/EcadFl/− mice (Figure 8B).
Claudin-1 is not a specific marker for tight junctions in the epidermis but, as described previously (Furuse et al, 2002), is found at the membrane of all epidermal layers in control mice (Figure 8A). Interestingly, in K14-Cre/EcadFl/− mice, claudin-1 staining was absent from cells of the granular layer of the epidermis (Figure 8A), which form the functional tight junctions. A slight decrease of total claudin-1 protein was also observed (Figure 8B).
How does loss of E-cadherin affect proper formation of tight junctions? The most direct link between adherens and tight junctions is ZO-1, which can directly interact with the cadherin complex via α-catenin (Itoh et al, 1997). This is suggested to be an intermediate step in tight junction formation. To examine if loss of E-cadherin affects recruitment of ZO-1 to the membrane, we stained control and mutant skin for this protein. ZO-1 membrane staining was still observed, indicating that the improper formation of epidermal tight junctions is not due to the inability to recruit ZO-1 to the membrane. However, we did see altered distribution of ZO-1 at the cell surface, showing a more punctate pattern in the epidermis of K14-Cre/EcadFl/− mice (Figure 8A). Inappropriate localization of ZO-1 may thus contribute to altered tight junction function. Overall, our results show that even though tight junction formation is not completely disturbed, key components are not properly incorporated, which likely underlies the functional impairment of tight junctions.
Altered localization of atypical PKC and its activated forms in K14-Cre/EcadFl/− mice
The activity of the Par3/Par6/aPKC polarity protein complex has been implicated in the formation of tight junctions in simple epithelia (Hirose et al, 2002; Macara, 2004). To examine if E-cadherin is important for the proper distribution of this complex in epidermis, we stained control and mutant skin for its different components. Par3 is found at cell–cell contacts in all suprabasal layers of the epidermis, and its distribution (Figure 9A) and amount of protein (Figure 9B) were not significantly altered in K14-Cre/EcadFl/− mice.
Figure 9.
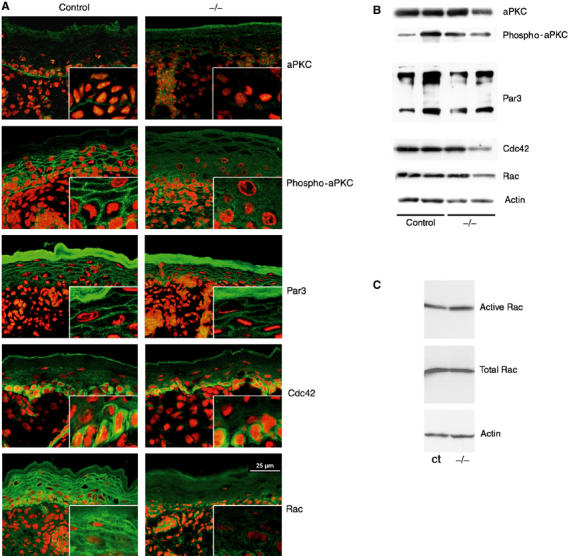
Altered localization of activated atypical PKC and the small GTPase Rac in K14-Cre/EcadFl/− mice. (A) Immunohistochemical analysis of skin sections for the indicated polarity complex proteins and the small GTPases Rac and Cdc42 (green). Nuclei were counterstained with propidium iodide (red). (B) Western blot analysis of the indicated proteins in total skin lysates isolated from newborn control and K14-Cre/EcadFl/− (−/−) mice. (C) Rac activation assay on total skin lysates of control and K14-Cre/EcadFl/− mice.
Although aPKC forms a complex with Par3, its overall distribution was not similar to that of Par3 but, instead, was highly concentrated at the epidermal–dermal junction in basal keratinocytes of control mice. Surprisingly, this basal staining pattern was disturbed upon loss of E-cadherin, resulting in a patchy pattern instead of the relatively regular lining at the basal side of these cells (Figure 9A). This phenotype was observed in 50% of the K14-Cre/EcadFl/− mice. No major difference in protein amounts was found (Figure 9B).
To examine if E-cadherin also regulates epidermal aPKC activation, necessary for tight junction formation in simple epithelia (Suzuki et al, 2002), we studied the distribution of phosphorylated forms of aPKC, indicative of activated aPKC. In control mice, phospho-aPKC antibodies stained cell–cell contacts, next to the epidermal–dermal junction, showing that the predominant total protein localization does not necessarily reflect the distribution of active forms. More importantly, we found that phospho-aPKC was absent or largely diminished at cell–cell borders of the suprabasal layers in K14-Cre/EcadFl/− mice (Figure 9A). Thus, E-cadherin is essential for activation of aPKC at the appropriate localization or for recruitment of activated forms to sites of cell–cell contact. It is not necessary for phosphorylation of aPKC per se because phospho-aPKC was detected in total skin lysates of control and K14-Cre/EcadFl/− mice (Figure 9B). The absence of activated aPKC from sites of epidermal tight junction formation offers a likely explanation for the inability of K14-Cre/EcadFl/− mice to form functional tight junctions.
The small GTPase Rac is lost from the membrane in K14-Cre/EcadFl/− mice
How does E-cadherin affect activation of aPKC at cell–cell contacts in the epidermis? A potential candidate is Cdc42, which is activated upon E-cadherin ligation (Kim et al, 2000; Braga, 2002) and regulates Par6/aPKC complex activation (Joberty et al, 2000; Lin et al, 2000). We examined if E-cadherin is necessary for the recruitment of Cdc42 to sites of cell–cell adhesion in the epidermis. Cdc42 is predominantly found in the cytoplasm of basal keratinocytes of control and mutant epidermis (Figure 9A), suggesting that E-cadherin is not required for Cdc42 function.
The small GTPase Rac can bind Par6 (Johansson et al, 2000; Lin et al, 2000), is also activated by E-cadherin (Noren et al, 2001; Braga, 2002) and its activity has been implicated in tight junction formation (Jou et al, 1998). Rac was found at the membrane in control mice, consistent with its function in cell–cell contact formation (Ehrlich et al, 2002). Interestingly, this cell–cell membrane pattern was absent or largely diminished in suprabasal layers of K14-Cre/EcadFl/− mice (Figure 9A). However, no differences in the levels of activated Rac were found between skin lysates of control and K14-Cre/EcadFl/− mice (Figure 9C), suggesting that E-cadherin is important for the recruitment of activated Rac to sites of cell–cell contact but not for its activation per se.
Discussion
Epidermal E-cadherin is essential for water barrier formation
The skin forms an important barrier with the outside environment. Not only does it protect us from outside challenges such as microbes or toxic substances, but it also protects the organism from dehydration by regulating the amount of water evaporating through the skin. In the present paper we show that E-cadherin contributes to the formation of the epidermal barrier of skin: early loss of E-cadherin from the developing epidermis results in TEWL leading to perinatal death. Surprisingly, this effect is not due to a loss of cell contact formation or altered epidermal differentiation. Instead, we find that essential tight junctional components are not properly targeted to tight junctions, resulting in malfunctioning of these structures. This is associated with inappropriate localization of activated aPKC and Rac.
A previous paper did not reveal a lethal phenotype around birth in mice in which E-cadherin was deleted in the epidermis using K14-Cre (Tinkle et al, 2004). Nevertheless, these authors did observe a slight disturbance in the epidermal barrier but have not examined tight junctional markers and function. Since the floxed E-cadherin mice in both studies were the same, the most likely difference is the K14-Cre mice used. In our K14-cre/EcadFl/− mice, E-cadherin is already almost completely absent at E15.5 (Figure 1C) shortly before the onset of barrier formation (Hardman et al, 1998), perhaps explaining why loss of E-cadherin has more dramatic consequences in our mice. This paper, and another study, in which E-cadherin was inactivated in the epidermis after birth, showed a gradual loss of hair in the E-cadherin mutant mice (Young et al, 2003; Tinkle et al, 2004). This fits with the observation that our mice lack whiskers despite the presence of hair follicles (not shown).
Loss of E-cadherin does not result in loss or impairment of cell contact formation
Inactivation of α-catenin in the epidermis resulted in severe blistering and overgrowth (Vasioukhin et al, 2001). Both phenotypes are not observed in K14-Cre/EcadFl/− mice (Young et al, 2003; Tinkle et al, 2004; this paper). Loss of E-cadherin is partially compensated by P-cadherin in the basal layer (Figure 2). In control mice, P-cadherin is most abundantly expressed around the hair follicles and only weakly in the interfollicular epidermis. However, in the E-cadherin-deficient epidermis, P-cadherin expression is strongly increased in the basal cells of this area. In addition, protein expression of desmosomal cadherins is also upregulated (Figure 3C). The increased expression of these two adhesive systems may explain why no blistering occurs and why cell contact formation appears normal in newborn epidermis (Figure 4). The upregulation of P-cadherin may also be sufficient to confer normal growth and differentiation properties to the basal epidermal keratinocytes in the absence of E-cadherin. Alternatively, growth regulation may be a cadherin-independent function of α-catenin (Vasioukhin et al, 2001).
E-cadherin contributes to epidermal barrier formation by regulating the incorporation of claudins into tight junctions
Until a few years ago, the epidermal barrier was thought to solely consist of the most outer layer of the skin, the stratum corneum. This layer consists of dead keratin-filled cells embedded in a lipid matrix, thus providing a tough insoluble layer that separates the outside from the inside (Nemes and Steinert, 1999). The discovery of tight junctions and their importance in the epidermal water barrier therefore came as a surprise (Brandner et al, 2002; Furuse et al, 2002). We were unable to detect any obvious functional differences in the barrier function of the stratum corneum between control and K14-Cre/EcadFl/− mice, indicating that the cornified layer is not disturbed and still constitutes a functional barrier. Although the cornified layer may contribute to the water loss seen in the K14-Cre/EcadFl/− mice, this is most likely not the primary cause.
Our results clearly show that loss of E-cadherin in the epidermis alters the permeability of tight junctions (Figure 7A) resulting in decreased epithelial resistance (Figure 7B). The molecular basis of the leaky tight junctional barrier is most likely the aberrant or lack of incorporation of key tight junctional proteins, like claudin-1 and -4, and ZO-1, into junctions. Claudins determine size and ion specificity of tight junctional barriers. For example, overexpression of claudin-1 in MDCK cells results in a four-fold increase in transepidermal resistance, a measure of ion permeability (Inai et al, 1999). This is consistent with our finding that loss of claudin-1 from the epidermal tight junctions, in combination with mislocalization of claudin-4, decreases epithelial resistance. In vivo, claudin-1 is essential for the epidermal water barrier (Furuse et al, 2002). The observed TEWL in K14-Cre/EcadFl/− mice might thus be due to a failure to incorporate claudin-1 into tight junctions.
Does aPKC play a role in E-cadherin-mediated epidermal tight junction formation?
One possibility how E-cadherin could regulate the incorporation of claudins into tight junctions is via ZO-1, the most direct link between adherens junctions and tight junctions (Itoh et al, 1997). Although loss of E-cadherin does not result in absence of ZO-1 from the membrane, its distribution is dramatically altered, suggesting that it is no longer incorporated into tight junctions. Since ZO-1 binds directly to claudin-1, this might contribute to the absence of claudin-1 in the stratum granulosum. However, such a model does not explain why specifically claudin-1, but not claudin-4 or occludin, is lacking from the granular layer whereas ZO-1 interacts with the C-terminus of all claudins and occludin (Fanning et al, 1998; Itoh et al, 1999). In addition, epithelial cells lacking ZO-1 can still incorporate claudins and occludin in tight junctions (Umeda et al, 2004).
Instead, our results suggest that E-cadherin regulates claudin-1, claudin-4 and ZO-1 localization by activating aPKC, implicated in tight junction formation in simple epithelial cells (Suzuki et al, 2002). We find that the inability to form functional tight junctions in the absence of E-cadherin coincides with loss or largely reduced presence of activated aPKC from sites of cell–cell contacts (Figure 9). The latter observation seems to be a direct effect of E-cadherin and not dependent on its ability to initiate and maintain cell–cell contacts, as previous results in Drosophila suggested (Bilder et al, 2003; Tanentzapf and Tepass, 2003).
A candidate molecule that could link aPKC to tight junctions is Par3, which can interact with the tight junctional protein JAM-1, thus connecting signalling with structural components at the tight junctions (Ebnet et al, 2004). However, E-cadherin loss did not alter the distribution of Par3, which is still found at sites of cell–cell contacts in the epidermis.
The small GTPase Rac may mediate E-cadherin-dependent localization of activated aPKC
It still needs to be clarified if loss of E-cadherin results in the impairment to recruit activated aPKC to the membrane or that aPKC is first recruited to adherens junctions where it subsequently gets activated. A suggestion for the latter possibility comes from the observation that cell–cell membrane association of the small GTPase Rac is greatly reduced in the absence of E-cadherin (Figure 9). Most data point to a role for Cdc42 as the small GTPase that activates the aPKC/Par3/Par6 complex (Macara, 2004). However, this is perhaps unlikely in the upper layers of the epidermis, since Cdc42 expression appears to be confined to the basal layer and is not altered by the loss of E-cadherin. One should keep in mind, though, that alterations in activated Cdc42 may not necessarily be reflected by changes in total Cdc42 and, similar to aPKC, activated forms may show a different distribution.
It has been shown that Rac can bind to Par6 and thus potentially activate aPKC (Johansson et al, 2000; Lin et al, 2000). In addition, Rac activity has been implicated in the regulation of tight junction gate and fence function (Jou et al, 1998). Although cadherin engagement has been shown to directly activate Rac (Noren et al, 2001), we find that loss of E-cadherin did not substantially alter the amount of activated Rac in total skin lysates (Figure 9C), suggesting that E-cadherin is important for the recruitment of activated Rac to the membrane. We propose that Rac may mediate E-cadherin-induced recruitment or activation of aPKC at cell–cell contacts in the epidermis.
E-cadherin is specifically required for correct tight junction formation
In vitro studies using mostly simple epithelial cells have indicated that E-cadherin-mediated adhesion is a necessary prerequisite for the formation of other cell junctions, such as desmosomes, gap junctions and tight junctions (Gumbiner et al, 1988; Musil et al, 1990; Watabe et al, 1994). The ability to initiate and maintain cell–cell contacts is considered key to this E-cadherin function (Nelson, 2003). The data show that at least in stratifying epithelia, E-cadherin is not generally required for junction formation. Desmosomes are abundantly present in the absence of E-cadherin, and desmosomal markers appear to have their normal differentiation pattern within the epidermis. Even tight junction formation is not completely disturbed since occludin is still normally expressed and tight junction-like structures are observed, indicating that E-cadherin is not generally required for proper positioning of all tight junctional components.
Our results do show for the first time that E-cadherin is essential for appropriate tight junction formation in vivo. The results suggest that P-cadherin, although quite similar to E-cadherin, is unable to substitute for E-cadherin in regulating tight junction formation. However, P-cadherin is only substantially upregulated in the basal layer in the absence of E-cadherin and it may require cadherin expression in the suprabasal layers to support tight junctions. Overall, an important implication of our work is that this effect of E-cadherin on tight junction formation is not simply the result of cell adhesion but rather seems to arise from signalling, most likely by recruitment of the small GTPase Rac and atypical PKC to sites of cell–cell contact.
Materials and methods
Generation of transgenic mice
Transgenic E-cadherin flox/flox mice, E-cadherin+/− mice and K14-Cre mice and their genotyping were described previously (Boussadia et al, 2002; Hafner et al, 2004). RT–PCR analysis was carried out as described (Hafner et al, 2004).
Histology and immunohistochemistry
Immunohistochemistry was performed on paraffin or cryostat sections using polyclonal antibodies against E-cadherin (Boussadia et al, 2002), β-catenin, α-catenin (Sigma), claudin-1, ZO-1 and occludin (Zymed), desmocollin 2 (a gift of Dr Werner Franke), aPKC and Cdc42 (Santa-Cruz), Par3 (Upstate), phospho-aPKC (NEB) and monoclonal antibodies to Rac (Sigma), P-cadherin (Zymed), p120ctn, Plakoglobin and desmoglein 1/2 (BD). Secondary antibodies were coupled to Alexa 488 (Molecular Probes) or Cy3 (Jackson Laboratories). Nuclei were counterstained using either dapi or propidium iodide. Fluorescent staining was photographed using either a Nikon Eclipse 800 microscope equipped with a DXM1200 digital camera or by a Leica TCS confocal laser microscope. Paraffin sections were stained with haematoxylin/eosin (Shandon).
Ultrastructural analysis
Decapitated newborn mice were immersed in 4% formaldehyde in 200 mM HEPES pH 7.5. After 10 min at room temperature, back skin samples were taken and incubated in 8% formaldehyde in 200 mM HEPES on ice. For ultrastructural analysis, samples were postfixed for 1 h with 1% osmium tetroxide in 100 mM phosphate buffer pH 7.2 on ice, washed with H2O, stained for 1 h with 1% aqueous uranyl acetate, dehydrated in a graded series of ethanol and finally embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and viewed in Philips CM10 electron microscope.
Western blot analysis
Total skin biopsies were snap-frozen in liquid nitrogen, grinded and lysed in lysis buffer containing 1% NP-40, 0.5% deoxycholate, 0.2% SDS, 2 mM EDTA, 150 mM NaCl, 50 mM Tris, pH 7.4, and a cocktail of protease inhibitors (Sigma). Total protein (30 μg) was separated by SDS–PAGE, transferred to nitrocellulose and probed with the appropriate antibodies also used in immunohistochemistry.
Rac activation assays
Skin biopsies were taken from mice, suspended in lysis buffer containing 50 mM Tris–HCl pH 7.4, 100 mM NaCl, 10 mM MgCl2, protease inhibitor cocktail (Sigma) and 4 μg biotin-CRIB-Pak peptide (Price et al, 2003). After grinding of the tissue in a mixer mill 300 (Qiagen), 1% NP-40 was added to the lysate and rotated for 1 h at 4°C. After centrifugation for 15 min at 4°C, an aliquot of total lysate was taken after which supernatants were rotated for 1 h at 4°C. Lysates were incubated with streptavidin-agarose beads for another hour at 4°C. Beads were washed three times with lysis buffer and bead samples and equal amounts of total lysate were separated by SDS–PAGE, transferred to nitrocellulose and probed with either a Rac mAb (Pierce) or actin mAb (ICN). Experiments were repeated three times.
Water loss assays
The weight of newborn mice was monitored every hour without feeding the mice until the time of death of the E-cadherin mutant mice. Results are displayed as percentage of initial weight. Haematocrit was determined by decapitating newborn mice and collecting blood in HEPES-coated capillary tubes. Percentage of blood cells was determined after centrifugation. TEWL was measured using a Tewameter (Courage and Khazaka) equipped with a small probe for newborn mice.
Barrier function assays
For penetration assays, backs of newborn mice were immersed in 1 mM lucifer yellow solution for 1 h after which the mice were killed (Koch et al, 2000). Frozen sections were counterstained for E-cadherin, and penetration of the dye was assessed by immunofluorescence microscopy. Toluidine blue dye staining of embryos was carried out as described (Koch et al, 2000). Developmental stages of mice were determined by assuming that fertilization occurred in the middle of the dark cycle the day before plugs were identified. Inside-out barrier assays were performed by dermal injection of biotin in newborn mice (Furuse et al, 2002). After 30 min, mice were killed and skin biopsies embedded in tissue-tec. Cryostat sections were stained with streptavidin labelled with Alexa488 and counterstained using polyclonal anti-occludin followed by Cy3-labelled secondary antibodies (Jackson Laboratories) to mark the tight junctions.
Impedance measurements
Impedance analysis was performed to distinguish between epithelial (i.e. tight junction-bearing epidermal) and subepithelial (i.e. dermal) portions of the total skin resistance based on a model describing the epithelium as a resistor and a capacitor in parallel and the subepithelium as a resistor in series (Fromm et al, 1985; Gitter et al, 1997). Changes in tissue voltage were detected by phase-sensitive amplifiers (402 frequency response analyser, Beran Instruments; 1286 electrochemical interface; Solartron Schlumberger) after application of an alternating current (35 μA cm−2, frequency range 1.3 Hz to 65 kHz). Calculated complex impedance values (Zreal, Zimaginary) were plotted in Nyquist diagrams. Because of the capacitive property of the epithelium, total resistance is obtained at low frequencies and subepithelial resistance at high frequencies, with epithelial resistance being total minus subepithelial resistance. The composition of the bath solution (mM) was as follows: NaCl 113.6, KCl 5.4, CaCl2 1.2, MgCl2 1.2, Na2HPO4 2.4, NaH2PO4 0.6, NaHCO3 21, D(+)-glucose 10, β-OH-butyrate 0.5, glutamine 2.5, D(+)-mannose 10, piperacillin 50 mg l−1, imipenem 10 mg l−1, equilibrated with 5% CO2 in O2.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Acknowledgments
We thank Angelika Arora for excellent technical assistance and Dr Schindler for help with the haematocrit assays. We thank Dr John Collard (Netherlands Cancer Institute, Amsterdam) for kindly providing us with the biotin-Pak peptide. We also thank Drs Alan Fanning and Christina Van Itallie (University of North Carolina, Chapel Hill), Werner W Franke (DKFZ, Heidelberg), Ingo Haase and Neil Smyth (University of Cologne) for generous gifts of antibodies and valuable suggestions and Dr Cara Gottardi (Northwestern University, Chicago) and Karla Billion for valuable discussions and critical reading of the manuscript. This research was supported by the Deutsche Forschungsgemeinschaft through SFB589 at the University of Cologne.
References
- Anderson JM, Van Itallie CM, Fanning AS (2004) Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol 16: 140–145 [DOI] [PubMed] [Google Scholar]
- Bilder D, Schober M, Perrimon N (2003) Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol 5: 53–58 [DOI] [PubMed] [Google Scholar]
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R (2002) E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev 115: 53–62 [DOI] [PubMed] [Google Scholar]
- Braga VM (2002) Cell–cell adhesion and signalling. Curr Opin Cell Biol 14: 546–556 [DOI] [PubMed] [Google Scholar]
- Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, Tschachler E, Franke WW, Moll I (2002) Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol 81: 253–263 [DOI] [PubMed] [Google Scholar]
- Colegio OR, Van Itallie C, Rahner C, Anderson JM (2003) Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 284: C1346–C1354 [DOI] [PubMed] [Google Scholar]
- Cox RT, Kirkpatrick C, Peifer M (1996) Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J Cell Biol 134: 133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Ohno S, Vestweber D (2004) Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci 117: 19–29 [DOI] [PubMed] [Google Scholar]
- Ehrlich JS, Hansen MD, Nelson WJ (2002) Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell–cell adhesion. Dev Cell 3: 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273: 29745–29753 [DOI] [PubMed] [Google Scholar]
- Fromm M, Schulzke JD, Hegel U (1985) Epithelial and subepithelial contributions to transmural electrical resistance of intact rat jejunum, in vitro. Pflugers Arch 405: 400–402 [DOI] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S (2002) Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156: 1099–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsios S, Huen AC, Green KJ (2004) Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol 5: 271–281 [DOI] [PubMed] [Google Scholar]
- Gitter AH, Schulzke JD, Sorgenfrei D, Fromm M (1997) Ussing chamber for high-frequency transmural impedance analysis of epithelial tissues. J Biochem Biophys Methods 35: 81–88 [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Niessen CM, Gumbiner BM (2002) The adherens junction. In Cell Adhesion, The Frontiers in Molecular Biology Series, Hames BD, Glover DM, Beckerle M (eds). chap 8, Oxford University Press [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A (1988) The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol 107: 1575–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Wenk J, Nenci A, Pasparakis M, Scharffetter-Kochanek K, Smyth N, Peters T, Kess D, Holtkotter O, Shephard P, Kudlow JE, Smola H, Haase I, Schippers A, Krieg T, Muller W (2004) Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis 38: 176–181 [DOI] [PubMed] [Google Scholar]
- Hardman MJ, Sisi P, Banbury DN, Byrne C (1998) Patterned acquisition of skin barrier function during development. Development 125: 1541–1552 [DOI] [PubMed] [Google Scholar]
- Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, Sakai T, Suzuki Y, Yamanaka T, Suzuki A, Mizuno K, Ohno S (2002) Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci 115: 2485–2495 [DOI] [PubMed] [Google Scholar]
- Inai T, Kobayashi J, Shibata Y (1999) Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol 78: 849–855 [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S (1999) Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147: 1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S (1997) Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol 138: 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PJ, Telegan B, Lavker RM, Wheelock MJ (1997) E-cadherin and P-cadherin have partially redundant roles in human epidermal stratification. Cell Tissue Res 288: 307–316 [DOI] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, Macara IG (2000) The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2: 531–539 [DOI] [PubMed] [Google Scholar]
- Johansson A, Driessens M, Aspenstrom P (2000) The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases Cdc42 and Rac1. J Cell Sci 113: 3267–3275 [DOI] [PubMed] [Google Scholar]
- Jou TS, Schneeberger EE, Nelson WJ (1998) Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol 142: 101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Li Z, Sacks DB (2000) E-cadherin-mediated cell–cell attachment activates Cdc42. J Biol Chem 275: 36999–37005 [DOI] [PubMed] [Google Scholar]
- Koch PJ, de Viragh PA, Scharer E, Bundman D, Longley MA, Bickenbach J, Kawachi Y, Suga Y, Zhou Z, Huber M, Hohl D, Kartasova T, Jarnik M, Steven AC, Roop DR (2000) Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J Cell Biol 151: 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R (1994) E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA 91: 8263–8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T (2000) A mammalian PAR-3–PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2: 540–547 [DOI] [PubMed] [Google Scholar]
- Macara IG (2004) Parsing the polarity code. Nat Rev Mol Cell Biol 5: 220–231 [DOI] [PubMed] [Google Scholar]
- Morita K, Itoh M, Saitou M, Ando-Akatsuka Y, Furuse M, Yoneda K, Imamura S, Fujimoto K, Tsukita S (1998) Subcellular distribution of tight junction-associated proteins (occludin, ZO-1, ZO-2) in rodent skin. J Invest Dermatol 110: 862–866 [DOI] [PubMed] [Google Scholar]
- Muller HA, Wieschaus E (1996) armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol 134: 149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Cunningham BA, Edelman GM, Goodenough DA (1990) Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol 111: 2077–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ (2003) Adaptation of core mechanisms to generate cell polarity. Nature 422: 766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes Z, Steinert PM (1999) Bricks and mortar of the epidermal barrier. Exp Mol Med 31: 5–19 [DOI] [PubMed] [Google Scholar]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K (2001) Cadherin engagement regulates Rho family GTPases. J Biol Chem 276: 33305–33308 [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Jamora C, Fuchs E (2003) Sticky business: orchestrating cellular signals at adherens junctions. Cell 112: 535–548 [DOI] [PubMed] [Google Scholar]
- Price LS, Langeslag M, ten Klooster JP, Hordijk PL, Jalink K, Collard JG (2003) Calcium signaling regulates translocation and activation of Rac. J Biol Chem 278: 39413–39421 [DOI] [PubMed] [Google Scholar]
- Pummi K, Malminen M, Karvonene SL, Peltonen J, Peltonen S (2001) Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J Invest Dermatol 117: 1050–1057 [DOI] [PubMed] [Google Scholar]
- Reynolds AB, Roczniak-Ferguson A (2004) Emerging roles for p120–catenin in cell adhesion and cancer. Oncogene 23: 7947–7956 [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS (1995) Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA 92: 8813–8817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S (2002) aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci 115: 3565–3573 [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Smith C, McGlade J, Tepass U (2000) Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J Cell Biol 151: 891–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G, Tepass U (2003) Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol 5: 46–52 [DOI] [PubMed] [Google Scholar]
- Tinkle CL, Lechler T, Pasolli HA, Fuchs E (2004) Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc Natl Acad Sci USA 101: 552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K, Troy TC (2002) Permeability barrier dysfunction in transgenic mice overexpressing claudin 6. Development 129: 1775–1784 [DOI] [PubMed] [Google Scholar]
- Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S (2004) Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem 279: 44785–44794 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E (2001) Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell 104: 605–617 [DOI] [PubMed] [Google Scholar]
- Watabe M, Nagafuchi A, Tsukita S, Takeichi M (1994) Induction of polarized cell–cell association and retardation of growth by activation of the E-cadherin–catenin adhesion system in a dispersed carcinoma line. J Cell Biol 127: 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM (2001) Stem cell fate and patterning in mammalian epidermis. Curr Opin Genet Dev 11: 410–417 [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, Ohno S (2001) PAR-6 regulates aPKC activity in a novel way and mediates cell–cell contact-induced formation of the epithelial junctional complex. Genes Cells 6: 721–731 [DOI] [PubMed] [Google Scholar]
- Young P, Boussadia O, Halfter H, Grose R, Berger P, Leone DP, Robenek H, Charnay P, Kemler R, Suter U (2003) E-cadherin controls adherens junctions in the epidermis and the renewal of hair follicles. EMBO J 22: 5723–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3


