Abstract
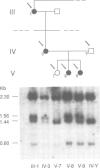
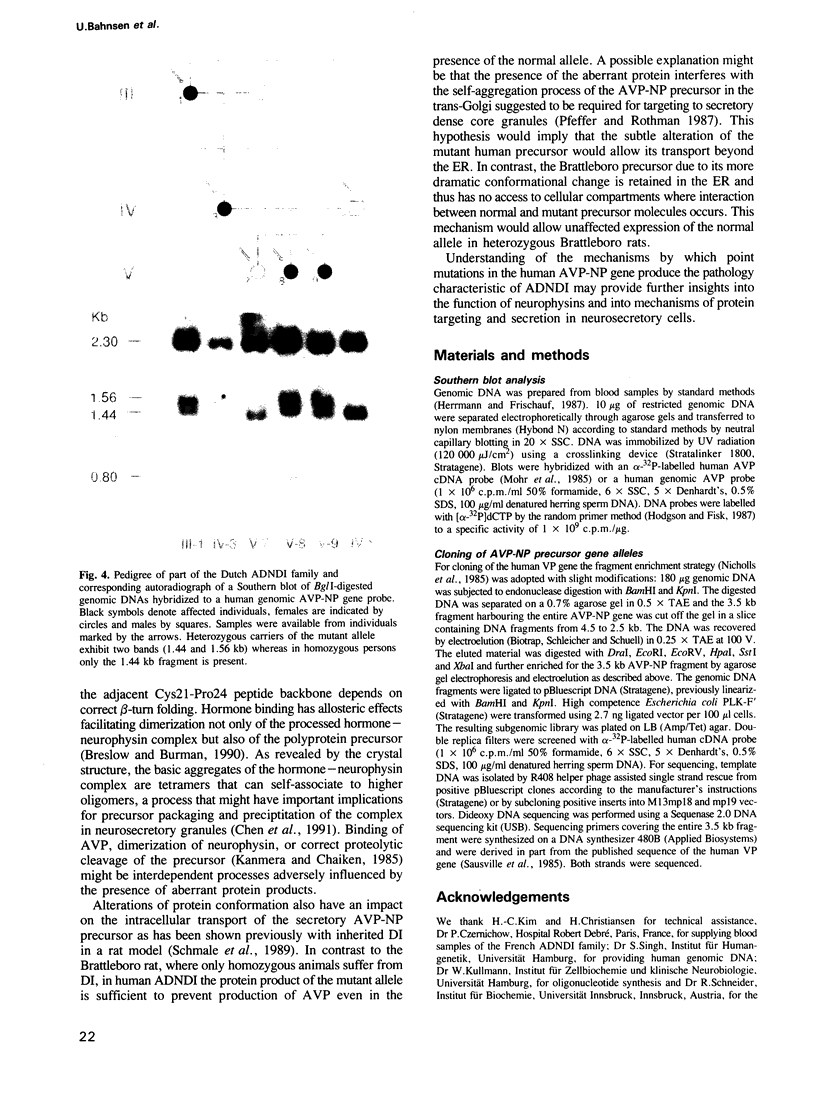
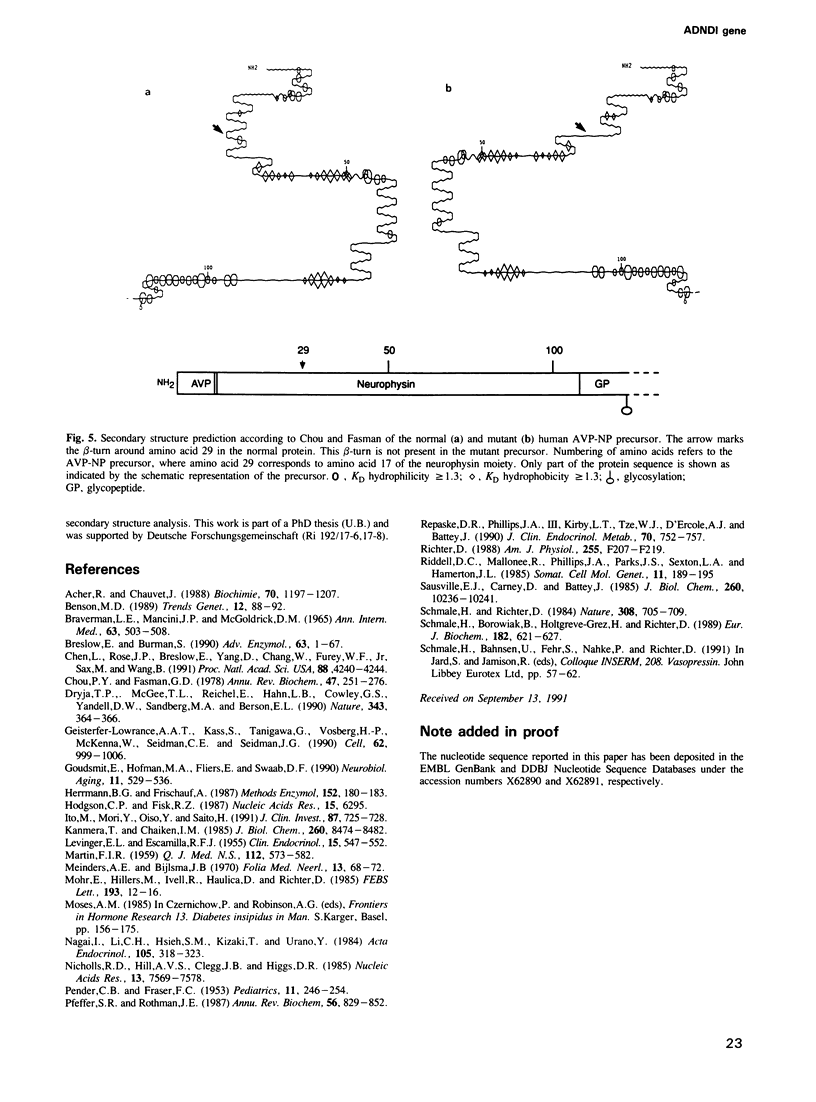
Familial neurohypophyseal diabetes insipidus in humans is a rare disease transmitted as an autosomal dominant trait. Affected individuals have very low or undetectable levels of circulating vasopressin and suffer from polydipsia and polyuria. An obvious candidate gene for the disease is the vasopressin-neurophysin (AVP-NP) precursor gene on human chromosome 20. The 2 kb gene with three exons encodes a composite precursor protein consisting of the neuropeptide vasopressin and two associated proteins, neurophysin and a glycopeptide. Cloning and nucleotide sequence analysis of both alleles of the AVP-NP gene present in a Dutch ADNDI family reveals a point mutation in one allele of the affected family members. Comparison of the nucleotide sequences shows a G----T transversion within the neurophysin-encoding exon B. This missense mutation converts a highly conserved glycine (Gly17 of neurophysin) to a valine residue. RFLP analysis of six related family members indicates cosegregation of the mutant allele with the DI phenotype. The mutation is not present in 96 chromosomes of an unrelated control group. These data suggest that a single amino acid exchange within a highly conserved domain of the human vasopressin-associated neurophysin is the primary cause of one form of ADNDI.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acher R., Chauvet J. Structure, processing and evolution of the neurohypophysial hormone-neurophysin precursors. Biochimie. 1988 Sep;70(9):1197–1207. doi: 10.1016/0300-9084(88)90185-x. [DOI] [PubMed] [Google Scholar]
- BRAVERMAN L. E., MANCINI J. P., MCGOLDRICK D. M. HEREDITARY IDIOPATHIC DIABETES INSIPIDUS. A CASE REPORT WITH AUTOPSY FINDINGS. Ann Intern Med. 1965 Sep;63:503–508. doi: 10.7326/0003-4819-63-3-503. [DOI] [PubMed] [Google Scholar]
- Benson M. D. Familial amyloidotic polyneuropathy. Trends Neurosci. 1989 Mar;12(3):88–92. doi: 10.1016/0166-2236(89)90162-8. [DOI] [PubMed] [Google Scholar]
- Breslow E., Burman S. Molecular, thermodynamic, and biological aspects of recognition and function in neurophysin-hormone systems: a model system for the analysis of protein-peptide interactions. Adv Enzymol Relat Areas Mol Biol. 1990;63:1–67. doi: 10.1002/9780470123096.ch1. [DOI] [PubMed] [Google Scholar]
- Chen L. Q., Rose J. P., Breslow E., Yang D., Chang W. R., Furey W. F., Jr, Sax M., Wang B. C. Crystal structure of a bovine neurophysin II dipeptide complex at 2.8 A determined from the single-wavelength anomalous scattering signal of an incorporated iodine atom. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4240–4244. doi: 10.1073/pnas.88.10.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990 Jan 25;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance A. A., Kass S., Tanigawa G., Vosberg H. P., McKenna W., Seidman C. E., Seidman J. G. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990 Sep 7;62(5):999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- Goudsmit E., Hofman M. A., Fliers E., Swaab D. F. The supraoptic and paraventricular nuclei of the human hypothalamus in relation to sex, age and Alzheimer's disease. Neurobiol Aging. 1990 Sep-Oct;11(5):529–536. doi: 10.1016/0197-4580(90)90114-f. [DOI] [PubMed] [Google Scholar]
- Herrmann B. G., Frischauf A. M. Isolation of genomic DNA. Methods Enzymol. 1987;152:180–183. doi: 10.1016/0076-6879(87)52018-3. [DOI] [PubMed] [Google Scholar]
- Hodgson C. P., Fisk R. Z. Hybridization probe size control: optimized 'oligolabelling'. Nucleic Acids Res. 1987 Aug 11;15(15):6295–6295. doi: 10.1093/nar/15.15.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Mori Y., Oiso Y., Saito H. A single base substitution in the coding region for neurophysin II associated with familial central diabetes insipidus. J Clin Invest. 1991 Feb;87(2):725–728. doi: 10.1172/JCI115052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmera T., Chaiken I. M. Molecular properties of the oxytocin/bovine neurophysin biosynthetic precursor. Studies using a semisynthetic precursor. J Biol Chem. 1985 Jul 15;260(14):8474–8482. [PubMed] [Google Scholar]
- LEVINGER E. L., ESCAMILLA R. F. Hereditary diabetes insipidus: report of 20 cases in seven generations. J Clin Endocrinol Metab. 1955 May;15(5):547–552. doi: 10.1210/jcem-15-5-547. [DOI] [PubMed] [Google Scholar]
- Meinders A. E., Bijlsma J. B. A family with congenital hypothalamic neurohypophyseal diabetes insipidus. Folia Med Neerl. 1970;13(2):68–72. [PubMed] [Google Scholar]
- Mohr E., Hillers M., Ivell R., Haulica I. D., Richter D. Expression of the vasopressin and oxytocin genes in human hypothalami. FEBS Lett. 1985 Nov 25;193(1):12–16. doi: 10.1016/0014-5793(85)80069-7. [DOI] [PubMed] [Google Scholar]
- Nagai I., Li C. H., Hsieh S. M., Kizaki T., Urano Y. Two cases of hereditary diabetes insipidus, with an autopsy finding in one. Acta Endocrinol (Copenh) 1984 Mar;105(3):318–323. doi: 10.1530/acta.0.1050318. [DOI] [PubMed] [Google Scholar]
- Nicholls R. D., Hill A. V., Clegg J. B., Higgs D. R. Direct cloning of specific genomic DNA sequences in plasmid libraries following fragment enrichment. Nucleic Acids Res. 1985 Nov 11;13(21):7569–7578. doi: 10.1093/nar/13.21.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENDER C. B., FRASER F. C. Dominant inheritance of diabetes insipidus; a family study. Pediatrics. 1953 Mar;11(3):246–254. [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Repaske D. R., Phillips J. A., 3rd, Kirby L. T., Tze W. J., D'Ercole A. J., Battey J. Molecular analysis of autosomal dominant neurohypophyseal diabetes insipidus. J Clin Endocrinol Metab. 1990 Mar;70(3):752–757. doi: 10.1210/jcem-70-3-752. [DOI] [PubMed] [Google Scholar]
- Richter D. Molecular events in expression of vasopressin and oxytocin and their cognate receptors. Am J Physiol. 1988 Aug;255(2 Pt 2):F207–F219. doi: 10.1152/ajprenal.1988.255.2.F207. [DOI] [PubMed] [Google Scholar]
- Riddell D. C., Mallonee R., Phillips J. A., Parks J. S., Sexton L. A., Hamerton J. L. Chromosomal assignment of human sequences encoding arginine vasopressin-neurophysin II and growth hormone releasing factor. Somat Cell Mol Genet. 1985 Mar;11(2):189–195. doi: 10.1007/BF01534707. [DOI] [PubMed] [Google Scholar]
- Sausville E., Carney D., Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem. 1985 Aug 25;260(18):10236–10241. [PubMed] [Google Scholar]
- Schmale H., Borowiak B., Holtgreve-Grez H., Richter D. Impact of altered protein structures on the intracellular traffic of a mutated vasopressin precursor from Brattleboro rats. Eur J Biochem. 1989 Jul 1;182(3):621–627. doi: 10.1111/j.1432-1033.1989.tb14871.x. [DOI] [PubMed] [Google Scholar]
- Schmale H., Richter D. Single base deletion in the vasopressin gene is the cause of diabetes insipidus in Brattleboro rats. Nature. 1984 Apr 19;308(5961):705–709. doi: 10.1038/308705a0. [DOI] [PubMed] [Google Scholar]