Abstract
Intracellular α-synuclein (α-syn)-rich protein aggregates called Lewy pathology (LP) and neuronal death are commonly found in the brains of patients with clinical Parkinson disease (cPD). It is widely believed that LP appears early in the disease and spreads in synaptically coupled brain networks, driving neuronal dysfunction and death. However, post-mortem analysis of human brains and connectome-mapping studies show that the pattern of LP in cPD is not consistent with this simple model, arguing that, if LP propagates in cPD, it must be gated by cell- or region-autonomous mechanisms. Moreover, the correlation between LP and neuronal death is weak. In this Review, we briefly discuss the evidence for and against the spreading LP model, as well as evidence that cell-autonomous factors govern both α-syn pathology and neuronal death.
Parkinson disease (PD) is the most common form of a broad class of movement disorders called parkinsonism and is defined by the appearance of bradykinesia, rigidity or tremor. Clinical PD (cPD) is distinguished by a set of inclusion and exclusion criteria that were recently ratified by the Movement Disorders Society1. The cardinal motor manifestations of cPD are attributable to the progressive loss of dopamine (DA) neurons in the substantia nigra pars compacta (SNc) that innervate the basal ganglia2. However, histological examination of brains of individuals with cPD has revealed that frequently the disease pathology is not limited to neuronal loss in the SNc. Most notably, Lewy pathology (LP) is commonly observed in some brain regions of patients with cPD, particularly within the brainstem3. However, LP also occurs in other ageing-related neurodegenerative diseases, even in the absence of cPD, making it alone an unsuitable diagnostic criterion4.
Comparison of cPD brains (at various times after diagnosis) with the brains of individuals with no neurological or psychiatric symptoms has led to the hypothesis that in preclinical stages of PD, LP first appears in either the olfactory bulb or the dorsal motor nucleus of the vagus (DMV) in the caudal medulla — two brain regions that have axons extending to the body surface. It was proposed that axons at these sites were exposed to a pathogen or infectious agent (for example, a virus) that caused LP and that this pathology then retrogradely spread to neighbouring neurons by passing from the infected cell to the next cell through synaptic connections5. With time, LP was thought to retrogradely spread through the brain connectome, reaching the SNc to initiate the loss of neurons and thus symptom onset, and then spreading to the forebrain by end-stage6.
The recognition that the pathology in cPD is distributed and not restricted to the SNc has fundamentally changed the thinking about PD pathogenesis and its treatment. However, the human data on which the staging hypothesis was based have important limitations. Most importantly, because none of the data was longitudinal in nature, the proposition that LP retrogradely spread sequentially from one structure to another was inferred from comparisons of the severity of the pathology in the brains of patients with symptomatic PD and asymptomatic individuals with LP — the latter of whom may or may not have ever developed PD had they lived long enough. In this regard, it is important to remember that LP is found in several other clinically defined ageing-related neurological diseases that do not have the main features of cPD4.
In this Review, we briefly summarize the relevant evidence that has accumulated since Braak’s proposal that a pathological agent retrogradely spreads through synaptically coupled networks, inducing LP and neuronal death in cPD. Some of this work clearly supports the Braak model. However, there are other observations that are not consistent with this model and argue that cell-autonomous factors play an important part in determining not only the pattern of LP in cPD but, more importantly, the pattern of neuronal loss that is unequivocally linked to symptoms.
The variable patterns of LP in cPD
Our understanding of the development of pathology in cPD is built on brain bank specimens that lack complete medical histories and on a smaller set of brains from well-characterized individuals with or without cPD who have been followed for some time. This latter data set, although sparse, fills much of the gap that is left by the former. It shows that, at the time of diagnosis with cPD, individuals have invariably lost more than 50% of their SNc DA neurons, whereas those individuals without cPD have not. In addition, this data set shows that, although individuals with cPD commonly have LP, this is not always the case. Moreover, LP is also found in the brains of individuals without cPD symptoms1.
Another important piece of information from this latter data set is that LP is not randomly distributed — neither in patients with late-stage cPD nor in asymptomatic individuals4,7. In patients with late-stage cPD, LP appears within a few circumscribed nuclei in the caudal medulla (the DMV and the intermediate reticular zone). LP is also commonly found in the nucleus tractus solitarius, the gigantocellular reticular nucleus (GRN), the raphe magnus and the locus coeruleus (LC) and subcoeruleus. More rostrally, LP is found in the dorsal raphe nucleus (DRN) and median raphe nucleus (MRN), the pedunculopontine nucleus (PPN), the SNc and the ventral tegmental area (VTA). In the diencephalon and telencephalon, LP is commonly found in the magnocellular nuclei of the basal forebrain (BF); the tuberomammillary nucleus of the hypothalamus; the lateral hypothalamus (LH); the intralaminar nuclei of the thalamus (IL); the olfactory and basolateral portions of the amygdala (AM); the anterior olfactory nucleus; the insular, cingulate and prefrontal cortices; and CA2 of the hippocampus.
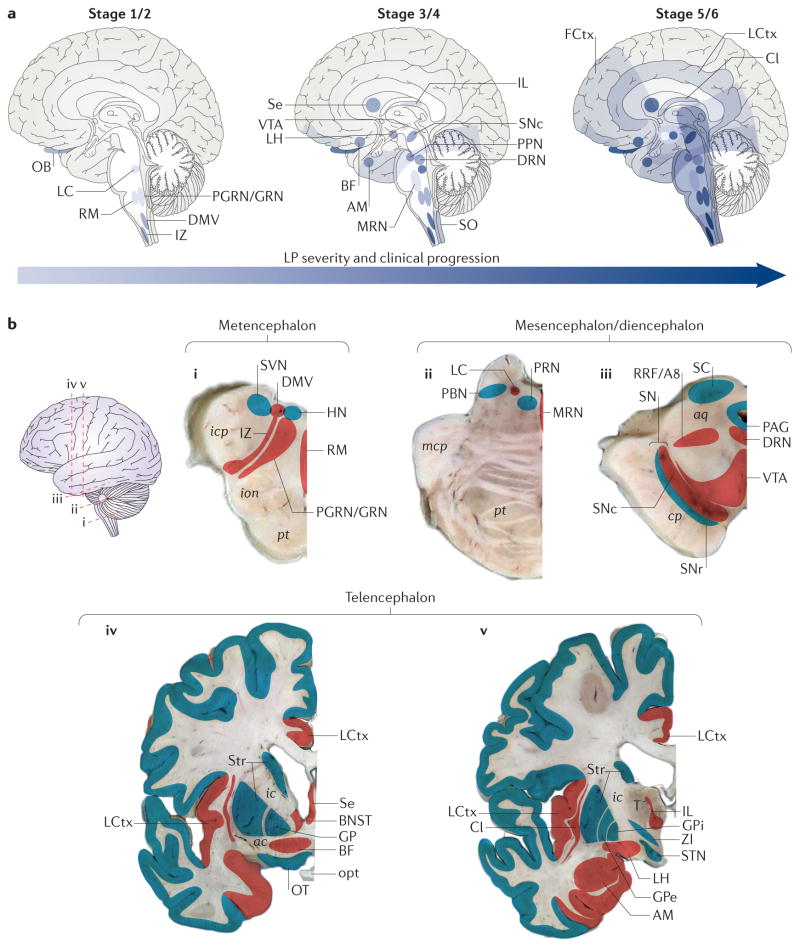
Braak and others have argued that this distribution of LP develops over time (or is staged) from well-defined starting points7–9. In the Braak scheme, the evolution of LP is broken down into six stages that are roughly aligned with cPD symptoms. The distribution of LP in presymptomatic stages (1–2), early symptomatic stages (3–4) and late symptomatic stages (5–6) gives the impression that LP spreads (FIG. 1a). However, without a validated biomarker that enables longitudinal tracking of PD from presymptomatic to symptomatic stages, this spread is not testable. In lieu of this more rigorous test, an attempt has been made to determine whether there is a match between the clinical and pathological stages, as suggested by Braak et al. Only about half of the people with cPD have a distribution of LP in the brain that is consistent with the Braak staging model10,11. Although impressive, this finding argues that, whereas the brain regions that are susceptible to LP are well defined, the sequence in which they manifest it, and the extent to which they do so, is not. Moreover, in patients with cPD with a genetic mutation-associated disease risk, the pattern of LP can be quite distinct from that of idiopathic cPD — for example, there are such cases of cPD that have little or no discernible LP12 (BOX 1).
Figure 1. Staging of Lewy pathology in clinical Parkinson disease.
a | A schematic representation of the spread of Lewy pathology (LP) within different brain structures, based on the study of Braak et al.173, is shown. The anatomical progression of disease through the brain increases over time (from left to right), and the darker the colour the more LP is present in each region at a given stage. b | A lateral external surface of a representative brain identifies the levels of each cross-sectional brain slice (in the figure, i–v). Regions (which are not to scale) that contain LP at any stage are represented in red, whereas those that only rarely show LP, or that only show mild LP, are indicated in blue. ac, anterior commissure; aq, aqueduct; AM, amygdala; BF, magnocellular nuclei of the basal forebrain; BNST, bed nucleus of the stria terminalis; Cl, claustrum; cp, cerebral peduncle; DMV, dorsal motor nucleus of the vagus; DRN, dorsal raphe nucleus; FCtx, frontal cortex; GP, globus pallidus; GPe, GP externa; GPi, GP interna; HN, hypoglossal nucleus; ic, internal capsule; icp, inferior cerebellar peduncle; IL, intralaminar nuclei of the thalamus; ion, inferior olivary nucleus; IZ, intermediate reticular zone; LC, locus coeruleus and subcoeruleus; LCtx, limbic cortex; LH, lateral hypothalamus; mcp, middle cerebellar peduncle; MRN, median raphe nucleus; OB, olfactory bulb; opt, optic tract; OT, olfactory tubercle; PAG, periaqueductal grey; PBN, parabrachial nucleus; PGRN/GRN, paragigantocellular and gigantocellular reticular nucleus; PPN, pedunculopontine nucleus; PRN, pontine reticular nucleus; pt, pyramidal tract; RM, raphe magnus; RRF/A8, retrorubral fields/A8 dopaminergic cell group; SC, superior colliculus; Se, septum; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; SO, solitary tract nuclei; STN, subthalamic nucleus; Str, striatum; SVN, spinal vestibular nucleus; T, thalamus; VTA, ventral tegmental area; ZI, zona incerta.
Box 1. Do familial cases of clinical Parkinson disease provide some insight into selective vulnerability?
About 10% of clinical Parkinson disease (cPD) cases are associated with known genetic mutations. There are several recent excellent reviews summarizing how these mutations might be linked to PD pathogenesis112,154–156. What these reviews do not do is to establish how PD-linked mutations contribute to the pattern of neuropathology — Lewy pathology (LP) and cell death — in cPD.
The most studied autosomal-dominant mutations are those affecting α-synuclein (α-syn). Some point mutations in the SNCA gene, which encodes α-syn, have been described, along with duplication and triplication variants. What remains unclear is how these genetic variants promote cPD and LP. Cases of cPD with SNCA duplication or triplication are the best characterized from a neuropathological standpoint. They have an early onset of PD motor signs, as well as other non-motor symptoms such as dementia; LP in their brains is more widespread than in non-genetic cPD cases and is found in the cerebral cortex, and disruption of this locus is thought to underlie dementia157,158. These patients have neuronal degeneration in the substantia nigra pars compacta (SNc) but also — unlike patients with non-genetic cPD — commonly have early degeneration in the locus coeruleus and in the hippocampus. Thus, even a modest α-syn overexpression induces considerably greater LP than that observed in non-genetic cPD and is associated with a broader distribution of neuronal death. Precisely how α-syn pathology might cause neuronal death remains to be unequivocally determined, but there are several promising leads that have recently emerged. For example, in cell lines and neurons, the accumulation of misfolded α-syn directly challenges both autophagic and mitochondrial function125,159,160. Pathological forms of α-syn also might alter the association of the endoplasmic reticulum and mitochondria at their junctions, impairing Ca2+-mediated regulation of mitochondrial bioenergetics161–163.
The only other set of autosomal-dominant mutations for which there is a reasonable neuropathological data set comprises mutations in the gene encoding leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2). LRRK2 mutations are the most common autosomal-dominant cause of cPD164. There is very little consensus on how LRRK2 mutations might lead to neuropathology in cPD165,166. Nevertheless, recent work has shown that, although patients with cPD with the most common LRRK2 mutation (G2019S) can manifest LP, not all these individuals do167, and most patients with cPD with other LRRK2 mutations do not have LP167; these observations dissociate SNc degeneration, which is a common feature in cPD associated with LRRK2 mutations, from LP.
The recessive mutations that have been linked to early-onset forms of cPD are loss-of-function mutations in PINK1 (which encodes PTEN-induced putative kinase 1), PARK2 (which encodes parkin) or PARK7 (which encodes protein deglycase DJ1)112,154–156. Loss of function of each of these three genes has been linked to deficits in mitochondrial function. Individuals with mutations in all three genes develop levodopa-responsive cPD, indicating degeneration of SNc dopamine neurons168. Neuropathological analysis of brains of carriers of PARK2 mutations, the most common recessive form of cPD, has found only sparsely distributed LP in older patients, with a pattern that is distinct from that found in non-genetic cPD cases12. Again, this finding challenges the proposition that neuronal degeneration in cPD is caused by a spreading of LP along a neuronal network that is defined by synaptic connectivity. Moreover, it suggests that neuronal degeneration in the SNc does not necessarily depend on α-syn or LP.
It is also worth noting that, even in what are considered the latter stages of the disease, LP does not begin to ‘leak’ to nearest neighbours. For example, even in late stages of PD, LP in the caudal medulla remains restricted to the DMV, intermediate reticular zone and raphe magnus, leaving most other nuclei in this region ostensibly unaffected13 (FIG. 1b). A similar restriction is found in the mesencephalon and diencephalon, where LP remains contained within well-defined group of neurons (FIG. 1b). Moreover, within affected nuclei or regions, only a subset (typically less than 15%) of neurons manifests LP14,15.
Does LP retrogradely spread in cPD?
Although it is currently not possible to directly test the staging hypothesis in humans, several lines of experimental evidence support it. One of the first pieces of evidence for this hypothesis came from histological analyses of fetal tissue grafts that had been transplanted into the striatum of patients with cPD. Most of these studies revealed that, after only a decade or so after implantation, a few of the transplanted DA neurons exhibited proteinaceous inclusions that strongly resembled LP16,17. This was interpreted as spread of LP from the host to the graft. Although open to alternative interpretation18, these observations spurred a flurry of experiments that put this idea on firmer footing.
In the brain, α-synuclein (α-syn) exists in monomeric, oligomeric and more-aggregated forms, such as fibrils19. The factors governing the transition between these forms in vivo are not known, and how these forms might spread is unresolved. For example, viral overexpression of human α-syn in rodent or non-human primate SNc DA neurons can kill them, but the pathology does not spread to neighbouring neurons20–22. By contrast, viral expression of human α-syn in the rodent vagus nerve does lead to spreading of monomeric and oligomeric, but not fibrillar, forms in the brainstem23. The reason why there is a difference in the extent of spreading (and pathology) in these models is unclear.
Nevertheless, there is compelling evidence that α-syn fibrils, when directly injected into the brain, can retrogradely spread. In mice, synthetic, pre-formed α-syn fibrils propagate from the site of stereotaxic injection to synaptically connected, neighbouring structures, creating Lewy-like pathology24–27. Similarly, proteins extracted from human brains with LP (which would contain α-syn fibrils and other LP proteins) and injected into the striatum of monkeys can retrogradely propagate28. Recent work has identified surface proteins (such as lymphocyte activation gene 3 protein and neurexins) that specifically interact with α-syn fibrils and are necessary for spreading29,30. In spite of the methodological and biological issues surrounding these studies (such as dose dependence and cellular specificity31–33), they do demonstrate that extracellular α-syn fibrils can be taken up and retrogradely (and possibly anterogradely) transported, and can induce LP-like pathology.
One of the most interesting features of this phenomenon is the role of endogenous α-syn. In neurons transduced with pre-formed α-syn fibrils, endogenous α-syn is recruited to intracellular aggregates24,26,34,35. In fact, endogenous α-syn seems to be necessary for spreading of fibrillar α-syn LP24 but not for spreading of other forms of α-syn36. This combination of features, which includes the apparent ability of α-syn fibrils to serve as a template for the creation of new fibrils from endogenous α-syn, has led to the proposal that PD is a prion-like disorder37. This is obviously an attractive hypothesis, as it posits a single unified mechanism for the pathology observed in cPD that, taken at face value, explains the development of LP in these patients. It also captures the essence of the original Braak hypothesis that LP staging arises from a pathogen that retrogradely spreads in the brain through synaptic connections.
But is this what happens in cPD? In principle, a prion-like process should follow one of two rules: a ‘nearest neighbour’ rule or a ‘synaptic connectivity’ rule. The nearest neighbour rule — whereby the probability of manifesting LP is directly related to the physical proximity to an initial seeding site — is not consistent with the pattern of LP in patients with cPD (FIG. 1). Does the spread follow a synaptic connectivity rule? Although anterograde propagation of α-syn aggregates is possible, experimental data are largely consistent with the notion of preferential retrograde propagation of α-syn pathology; that is, α-syn fibrils are preferentially taken up by axon terminals and retrogradely transported to the cell body24,28. A rigorous test of the retrograde propagation hypothesis requires a better knowledge of the connectome of well-defined neuronal populations that are thought to seed LP.
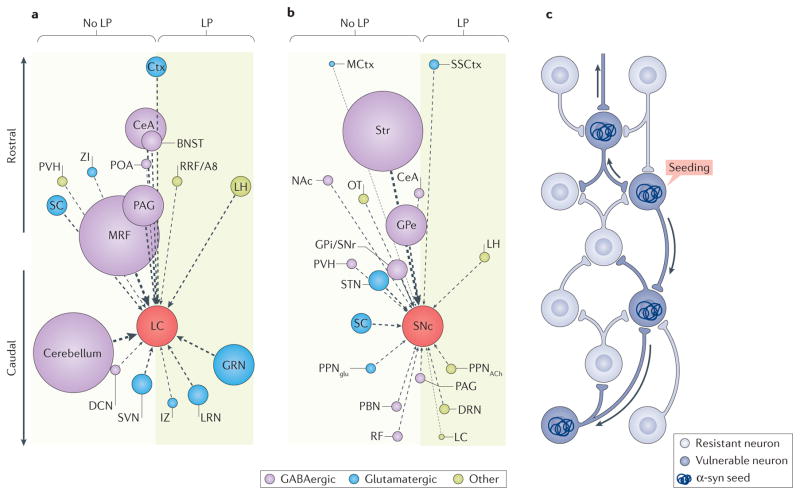
These types of connectomes are beginning to be generated in rodents using retrograde viral mapping strategies38, with the hope that they will faithfully reflect the circuitry of the human brain39. If we assume that the probability of retrograde spread of α-syn is directly proportional to the strength of synaptic connections that are revealed by these mouse maps, and that the connectivity of these structures in mice is similar to that in humans, then these connectomes should largely predict the pattern of LP in patients with cPD. However, these connectomes do not seem to do so. For example, we consider the recent data that mapped the afferent connectome of LC noradrenergic neurons in mice40. In humans, the LC is a ‘hot spot’ of LP in early-stage cPD. If the LC is a node in the spread of LP, then structures that robustly innervate it should have a higher probability of manifesting LP than those that do not. But this is not the case. FIGURE 2 shows this graphically: homologous regions that richly innervate the LC in mice (FIG. 2a), as depicted by the larger circles, are not more likely to display LP in humans; the cerebellum, midbrain reticular nucleus and periaqueductal grey have little or no LP. Maps of the SNc that are based on similar connectome experiments41,42 show a similar pattern: regions with the strongest connectivity with the SNc — the striatum and the globus pallidus externa — do not exhibit substantial LP (although some Lewy neurites, which are probably DA axons, can be observed in these regions early in cPD43) (FIG. 2b).
Figure 2. Does connectivity predict Lewy pathology in clinical Parkinson disease?
The connectomes of the mouse locus coeruleus (LC) (part a) and substantia nigra pars compacta (SNc) (part b) do not predict the pattern of postsynaptic, intraneuronal Lewy pathology (LP) that is observed in clinical Parkinson disease (cPD). Because data on the human connectome are not available and because there have been no direct comparisons between the mouse connectome and the spread of LP, a thought experiment was conducted. This thought experiment, as depicted in the figure, assumes that the mouse and human connectomes are largely similar and that retrograde spread of α-synuclein (α-syn) is dictated by the number or strength of synaptic connections. a | A plot of the afferent connectome of mouse LC adrenergic neurons, based on data presented by Schwarz et al.40, is shown. Nuclei projecting to the LC are represented as circles distributed along a rostrocaudal vertical axis. The diameter of the circle represents the strength of the projection as estimated by the number of retrogradely labelled neurons; the dominant transmitter (GABA, glutamate or other) in each nucleus is coded by a given colour. Nuclei with postsynaptic, intraneuronal LP in late-stage cPD are positioned in the shaded box on the right; nuclei with little or no LP are on the left. The plot reveals that the strength of the synaptic connection to the LC is not correlated with LP; most of the larger circles are located on the left of the plot. b | A plot of the connectome of mouse SNc dopamine neurons as in part a, based on the work of Ogawa et al.41 and Watabe-Uchida et al.42, is shown. Again, there is no clear correlation between the strength of the synaptic connection with SNc and the probability of manifesting postsynaptic, intraneuronal LP in humans with cPD. c | The schematic summarizes the conclusion that, if there is spread of α-syn, it is not strictly determined by synaptic connectivity, but rather must be dictated by other factors, such as vulnerability. In the diagram, a synaptic network is depicted with neurons that are resistant to α-syn spread (light blue) and those that are vulnerable (dark blue). An α-syn seeding event will then result in the spread of LP in a retrograde manner only through vulnerable neurons (depicted by the arrows). BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; Ctx, cortex; DCN, deep cerebellar nuclei; DRN, dorsal raphe nucleus; GPe, globus pallidus externa; GPi/SNr, GP interna–SN pars reticulata; GRN, gigantocelluar reticular nucleus; IZ, intermediate reticular zone; LH, lateral hypothalamus; LRN, lateral reticular nucleus; MCtx, motor region of the cerebral cortex; MRF, midbrain reticular nucleus; NAc, nucleus accumbens; OT, olfactory tubercle; PAG, periaqueductal grey; PBN, parabrachial nucleus; POA, preoptic area; PPNglu, glutamatergic neurons of the pedunculopontine nucleus; PPNACh, cholinergic neurons of the pedunculopontine nucleus; PVH, paraventricular hypothalamic nucleus; RF, reticular formation; RRF/A8, retrorubral fields/A8 dopaminergic cell group; SC, superior colliculus; SSCtx, somatosensory cortex; STN, subthalamic nucleus; Str, striatum; SVN, spinal vestibular nucleus; ZI, zona incerta.
While bearing in mind the stated caveats that are associated with this approach, these data argue that, if LP retrogradely spreads in cPD, it does not follow a simple connectivity rule (FIG. 2c). Including the anterograde connectomes in this analysis (to accommodate the possibility of anterograde propagation of α-syn pathology) would only have made the discrepancy more dramatic. Thus, if LP spreads in cPD, then it must have other determinants than just synaptic connectivity.
LP and neuronal death
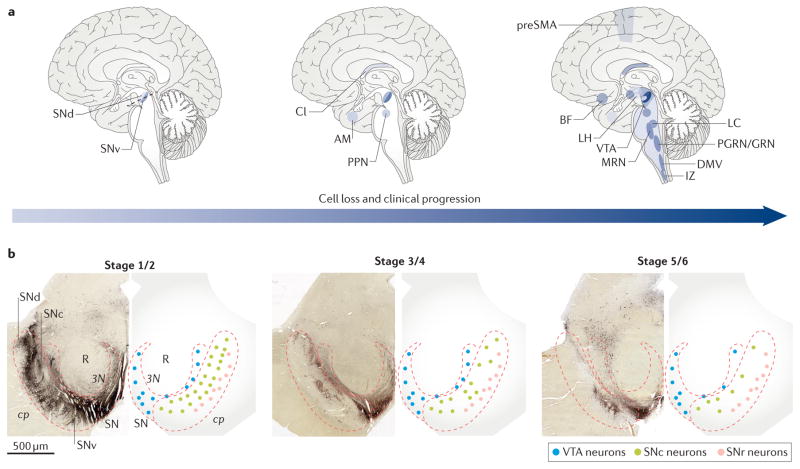
In contrast to LP, there has been relatively little rigorous study of neuronal death in cPD. Because the functional consequences of LP are uncertain, distinguishing between LP and frank cell loss is crucial to understanding the pathogenesis and clinical features of the disease. Recent studies have investigated whether there is neuronal death in the brains of asymptomatic (probably presymptomatic) individuals in whom LP was restricted to the medulla and pons (that is, in Braak stages 1–2). Indeed, in these brains, there was a substantial (10–20%) loss of SNc DA neurons in the ventral tier of the SNc, but not elsewhere, including regions with LP4,44. Later, in the early symptomatic stages of cPD, nearly all DA neurons in the ventral tier of the SNc are dead45,46. At this stage, neurodegeneration is also apparent in a few other regions. For example, there is a considerable loss of cholinergic PPN neurons but not of glutamatergic or GABAergic PPN neurons47. There is also a modest loss of glutamatergic neurons in the IL and in the AM in patients with early-symptomatic cPD without Alzheimer disease48,49. Apart from this small group of nuclei — including those with LP — unbiased studies have failed to detect considerable levels of neuronal death at this early-symptomatic stage of cPD.
With clinical progression, neurons are lost in other regions — particularly those with LP47,50–53. But there are exceptions. For example, there is neuronal death in the supraoptic nucleus, even though LP is not present there; by contrast, there is no discernible neuron loss in the neighbouring, LP-laden tuberomammillary nucleus of the hypothalamus54. In patients who do not manifest dementia, the only cortical region that shows substantial neuronal death is the presupplementary motor cortex, where small intra-telencephalic pyramidal neurons degenerate in the absence of LP55,56. Thus, neuronal loss in cPD (FIG. 3) follows a different pattern than does LP (FIG. 1).
Figure 3. Staging of neurodegeneration in clinical Parkinson disease.
a | The schematics represent the progression of neuronal cell loss following the onset of clinical Parkinson disease (cPD), based on the literature44–56. The anatomical distribution of neuronal loss increases with time, and the darker the colour, the more neuronal loss evident in each region. b | Transverse sections of the midbrain, as indicated in the left brain schematic in part a (dotted line), are shown; the normal distribution of tyrosine hydroylase-immunopositive dopaminergic neurons is shown in the left panels, and the pattern is schematized in the right panels. Heavily pigmented neurons of the substantia nigra pars compacta (SNc) are depicted in green; less pigmented neurons of the ventral tegmental area (VTA) are depicted in blue; neurons of the SN pars reticulata (SNr) are depicted in pink. The initial loss of ventral-tier SNc observed in patients with stage 4 cPD is depicted in the middle panel, with greater cell loss observed over time at later stages, as indicated in the right panel. 3N, third nerve; AM, amygdala; BF, magnocellular nuclei of the basal forebrain; Cl, claustrum; cp, cerebral peduncle; DMV, dorsal motor nucleus of the vagus; IZ, intermediate reticular zone; LC, locus coeruleus and sub-coeruleus; LH, lateral hypothalamus; MRN, median raphe nucleus; PGRN/GRN, paragigantocellular and gigantocellular reticular nucleus; PPN, pedunculopontine nucleus; preSMA, presupplementary motor area; R, red nucleus; SNd, dorsal tier of the SNc; SNv, ventral tier of the SNc.
Cell-autonomous determinants of cPD pathology
If the patterns of neuronal death and LP in cPD are not simply a consequence of the spread of misfolded α-syn through the brain connectome, then what dictates them? The effort to answer this question has focused on the distinguishing features of neurons that die prematurely in cPD or that manifest LP, with the hope that this exercise will provide clues about pathogenesis.
Interestingly, many of the neurons that are vulnerable in cPD (FIGS 1,3) have a loosely connected functional role in the brain. They are principal neurons in neuromodulatory control networks (that is, networks with diffuse axonal projections that regulate other neurons primarily by releasing transmitters, such as DA, that activate slow G protein-coupled receptors), in contrast to neurons in brain networks that are responsible for epicritic sensation and precise motor control, which have topographically restricted synaptic connectivity and rely on classical neurotransmitters that activate fast ionotropic receptors. The SNc, GRN, DRN–MRN, LC, LH, PPN, BF and IL are involved in the arousal or mobilization of sensorimotor networks that are necessary for rapid and effective action, which is crucial for vigilance, escape and attack57–62. The DMV and the nucleus tractus solitarius share a similar role through their control of the autonomic nervous system63,64. The AM is part of a default activity network in the forebrain that regulates fear and affective behaviours, and so again is important for coordinating appropriate action in response to salient events in a threatening environment65.
Although there has been relatively little ‘deep phenotyping’ of neurons in these neuromodulatory networks, what is known suggests that they share several traits that might make them vulnerable to age, genetic mutations associated with cPD or environmental toxins.
The most notable and best characterized of these traits is a long and highly branched axon with a very large number of transmitter release sites. This diffuse axonal arbor helps neurons to coordinate the activity in spatially distributed networks, such as the basal ganglia or the spinal cord. For example, SNc DA neurons in the rodent have axons that branch profusely in the striatum and possess as many as 200,000 vesicular release sites66. Although less well characterized, neurons in the DMV, GRN, DRN–MRN, LC, LH, PPN, BF and IL exhibit large, diffuse axonal projections (to varying degrees), distinguishing them from most, for example, sensory or motor neurons in the brain, which typically have modestly branched axons that conform to topographic maps59,60,67–73.
Why might a long and highly branched axon increase vulnerability? Several theories have been proposed73,74. One theory involves mitochondrial stress. Indeed, mitochondrial oxidant stress — one of the potential drivers of neurodegeneration — is elevated in the axons of SNc DA neurons, and this stress is reduced by diminishing the size of the arbor (by modulating axon guidance signals)75. That said, not all neurons with long, branched axons are vulnerable in PD. For example, striatal cholinergic interneurons have highly branched axons with as many release sites as SNc DA neurons76, but they do not degenerate or manifest LP in PD, suggesting that this alone is not sufficient for pathogenesis.
Another shared feature of vulnerable neurons in cPD is their distinctive physiology. In vivo, the neuromodulatory neurons that have been examined have slow, tonic activity77. The best-studied member of this class is the SNc DA neuron. These neurons have broad action potentials (spikes) and are autonomous pacemakers; that is, they spike on their own in the absence of any excitatory synaptic input78–80. Their slow, rhythmic (2–10 Hz) spiking is accompanied by large oscillations in intracellular Ca2+ concentration that are driven by the opening of voltage-dependent Cav1 Ca2+ channels (also known as L-type Ca2+ channels)78–82. Once in the cytoplasm, Ca2+ is relatively free to interact with other proteins in these neurons, as the levels of Ca2+-buffering proteins such as calbindin are low83. This particular combination of features — broad spikes, pacemaking, low intrinsic Ca2+ buffering and cytosolic Ca2+ oscillations — (and not any one feature alone) distinguishes SNc DA neurons from neurons that are less vulnerable in cPD. For example, VTA DA neurons, which are considerably less vulnerable than SNc DA neurons (see above), are autonomous pacemakers with broad spikes but have smaller Cav1 channel currents and strong intrinsic Ca2+ buffering (by calbindin)80,82,84,85. Although there have been very few studies that have examined these features in other cPD vulnerable neurons, those that have investigated the LC, DMV and PPN show that this phenotype is largely shared86–88.
The slow Ca2+ oscillations in SNc DA neurons subserve two complementary functions. First, they help to maintain the slow tonic spiking by creating an oscillation in membrane potential78,79,89. Second, they promote Ca2+ entry into the mitochondria86,90,91; this mitochondrial Ca2+ entry stimulates oxidative phosphorylation (OXPHOS) and the production of ATP92. In principle, this feedforward control of OXPHOS helps to ensure that bioenergetic needs are met92,93 and that intracellular ATP levels do not fall into a range that would trigger protective activation of ATP-sensitive K+ channels and cessation or slowing of ongoing activity94. Why is this important? Protective slowing of ongoing spiking of SNc DA neurons would result in a drop in striatal DA levels and disinhibition of indirect pathway striatal neurons that suppress or stop movement95,96 — a potentially disastrous event in a threatening environment in which rapid, effective action is crucial for survival. It is easy to imagine a similar need in other at-risk neuromodulatory networks controlling arousal, wakefulness and reflex circuitry. As a consequence, there should have been a strong evolutionary pressure to maintain this type of feedforward control mechanism.
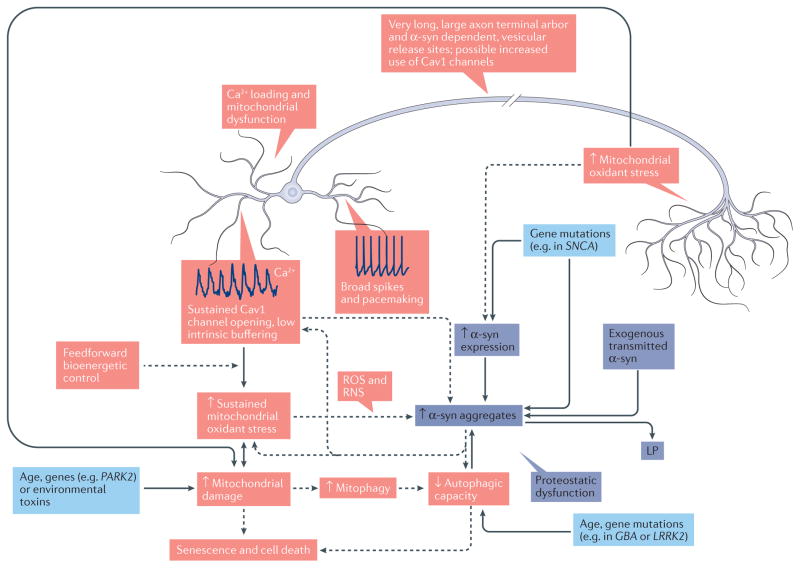
Granted that this type of bioenergetic control mechanism confers a functional advantage, what are the disadvantages? There are two that are apparent. The first downside is that stimulating OXPHOS in the absence of strong ATP demand (which is most of the time) leads to mitochondrial hyperpolarization, slowed electron flux through the electron transport chain and increased production of reactive oxygen species (ROS)90,97. In rodents, SNc, LC and DMV neurons (which are the only ones that have been studied at this level) manifest a basal mitochondrial oxidant and nitrosative stress in the somatodendritic region that is attributable to the feedforward control of OXPHOS86,87,90. The mitochondrial oxidant stress in dopaminergic axons that was mentioned above could stem from the same feedforward mechanism involving Cav1 channels75,98. ROS and reactive nitrogen species (RNS) can damage proteins, lipids and DNA, particularly in mitochondria. Damage stemming from sustained ROS or RNS stress not only will compromise mitochondrial function but also will challenge the machinery that is responsible for mitochondrial quality control and mitophagy99, diminishing the capacity of neurons to deal with other proteostatic challenges (such as aggregated α-syn) that engage autophagy100. ROS and RNS also increase the propensity of α-syn to aggregate in vitro and possibly in vivo101 (FIG. 4).
Figure 4. How cell-autonomous factors might contribute to Lewy pathology and cell death in clinical Parkinson disease.
The schematic summarizes the key features of vulnerable neurons and how these features might contribute to Lewy pathology (LP) and neuronal death in clinical Parkinson disease (cPD). These mechanisms are divided into two classes: Ca2+ loading and/or mitochondrial dysfunction (red), and proteostatic dysfunction (purple). The arrows indicate causality. Ca2+ loading and/or mitochondrial dysfunction: vulnerable neurons are typically autonomous pacemakers (example spiking is shown near the cell body) with large fluctuations in cytosolic Ca2+ (dendritic Ca2+ oscillations are also shown) that are not strongly buffered (reflecting feedforward control of oxidative phosphorylation); this design leads to increased mitochondrial oxidant stress and damage. Mitochondrial damage could be exacerbated by age, environmental toxins and genetic mutations affecting mitochondrial quality control (light blue boxes). In addition, a large axonal arbor leads to elevated mitochondrial oxidant stress and damage. Accumulation of mitochondrial defects and/or damage leads to senescence and/or cell death. Proteostatic dysfunction: in vulnerable neurons, α-synuclein (α-syn; which is encoded by SNCA) aggregation could be increased by elevated α-syn expression due to the long and highly branched axon, uptake of α-syn from synaptically coupled cells or genetic mutations. As described in the text, elevated cytosolic Ca2+, reactive oxygen species (ROS) and reactive nitrogen species (RNS), or uptake of aggregated α-syn could promote formation of intracellular α-syn aggregates, leading to LP. In addition, decreased autophagic capacity resulting from increased mitophagy (due to mitochondrial damage), genetic mutations or ageing could impair clearance of aggregates, increasing their prominence. Decreased autophagy, as reviewed in the text, could also promote cell death. Last, α-syn aggregates could contribute to mitochondrial dysfunction, oxidant stress and elevated cytosolic Ca2+ levels, further promoting pathology. Solid arrows indicate connections between events that are well established in mammalian models; dashed arrows indicate connections between mechanisms for which there is good but not unequivocal support. GBA, glucosylceramidase; LRRK2, leucine-rich repeat serine/threonine-protein kinase 2; PARK2, parkin.
The second downside that is associated with feedforward control of OXPHOS is that it results in high cytosolic and mitochondrial Ca2+ concentrations. Elevated mitochondrial Ca2+ is a well-known trigger of permeability transition pore opening and apoptosis102. Ca2+ also directly promotes α-syn aggregation103,104; it activates the protease calpain, which increases α-syn aggregation105–107; it activates the protein phosphatase calcineurin, increasing α-syn toxicity108; and it impairs lysosomal motility and turnover of misfolded proteins109 (FIG. 4).
Thus, owing to these properties, many vulnerable neurons seem to reside close to bioenergetic and proteostatic ‘tipping points’ (FIG. 4). Flagging mitochondrial and proteasomal or autophagic function with age — the biggest risk factor for cPD (BOX 2) — should undoubtedly push these neurons closer to these tipping points, elevating the probability of neurodegeneration and death102,110. In this cellular context, it also makes sense that genetic mutations that diminish mitochondrial or proteostatic function, either directly or indirectly, will have a larger impact99,111–114. These properties should also increase the probability of developing de novo LP or of a cell being unable to handle the burden that is created by taking up pathological α-syn species from the extracellular space115 (FIG. 4).
Box 2. Ageing and clinical Parkinson disease.
Does a better understanding of the vulnerable phenotype help to understand the reason why ageing is the most important risk factor for clinical Parkinson disease (cPD)? Various recent reviews have focused on the potential role of ageing in the selective vulnerability of substantia nigra pars compacta (SNc) dopamine (DA) neurons110,169. It is unclear to what extent ageing diminishes the capacity of SNc DA neurons to successfully cope with stress arising from their phenotype and to what extent this phenotype may accelerate the ageing process. Many, if not all, of the correlates of ageing — genetic mutations, mitochondrial dysfunction, proteostatic dysfunction and telomere shortening170 — could be promoted by the conditions found in vulnerable neurons. Telomere shortening, which is a relatively new addition to this list, has recently been found to be driven by oxidant stress171, making it also relevant to ageing of non-dividing neurons in the brain. In non-human primates, neurons in the ventral tier of the SNc, which is one of the first regions to degenerate in patients with cPD, manifest signs of senescence (for example, downregulation of tyrosine hydroxylase) sooner than do neurons in the dorsal tier or in the ventral tegmental area172. ‘Premature’ cellular ageing should increase the vulnerability to the challenges that are posed by protein aggregation, genetic mutations, environmental toxins or infection, just as ageing increases our vulnerability at the organismal level. Interestingly, rodent models do not recapitulate the ageing-dependent telomere shortening that has been seen in humans. This might explain why mouse genetic models of PD have consistently failed to reproduce the pattern of pathology that is observed in patients with cPD.
But do cell-autonomous factors account for the pattern of LP in cPD? The simple answer is no. If this were the case, LP should appear in the SNc before it does in the DMV. Although there is considerably more variation in the temporal pattern of LP than originally hypothesized by Braak, this prediction is not consistent with our current understanding. Barring the emergence of some other cell-autonomous factors that drive LP, the most parsimonious explanation for the LP pattern in cPD is that there is spreading of α-syn pathology — as posited by Braak et al. and the proponents of the prion model — but that spreading is limited to a subset of neurons with a phenotype that renders them unable to cope with the burden of misfolded α-syn.
What may be better explained by cell-autonomous factors is the sequence of neuronal death in cPD. As outlined above, the temporal pattern of neuronal death in cPD is not correlated with the appearance of LP. Attempts to correlate the severity of LP with cPD symptoms have consistently failed116. From the standpoint of known cell-autonomous risk factors, the neuron that should be lost first is the SNc DA neuron — precisely what the human pathology tells us. These neurons are at one extreme of the phenotypic range, exhibiting the highest basal levels of mitochondrial oxidant stress and free cytosolic Ca2+ of any of the vulnerable neurons studied to date. The reliance on DA as a transmitter undoubtedly adds to their burden117. Even within the SNc, there is a correlation between phenotype and vulnerability. Moving from the ventrolateral SNc to the medial SNc and VTA, there is a gradient in axonal arbor dimension, pacemaking mechanisms, Ca2+ signalling and mitochondrial oxidant stress77,118,119 that mirrors the pattern of cell loss in cPD45,46,120–122. In SNc neurons from aged humans, there are clear signs of sustained oxidant stress, particularly in mitochondria123,124. Unlike LP, mitochondrial dysfunction and intracellular Ca2+ are necessary elements for all three major neuronal death cascades (that is, those involved in apoptosis, autophagic death and necrosis)102. From this perspective, it is not surprising that genetic mutations that compromise mitochondrial oxidant defences, biogenesis or quality control (BOX 1) are associated with a preferential loss of SNc DA neurons and early-onset forms of cPD, commonly without LP.
This scenario does not exclude a role for α-syn pathology in triggering neuronal death117,125. However, given the ability of many neurons to tolerate LP for decades13,126–129, it is apparent that the emergence of α-syn pathology alone does not cause cell death. Cellular context must be important. In those neurons that are already burdened by mitochondrial or autophagic stress, α-syn pathology, which can compromise both systems112,114,130–134, could create a tipping point beyond which cell death occurs. This could help to explain the differential vulnerability of VTA and SNc DA neurons to α-syn overexpression20,22. Along these same lines, a recent study has demonstrated that in mouse SNc DA neurons, but not in neighbouring VTA DA neurons, the oxidant stress that is induced by α-syn overexpression triggered an increase in pacemaking rate135; this effect presumably reflects the ability of pre-existing or baseline oxidant stress in SNc DA neurons to sum with that created by α-syn overexpression.
But do all neurons that are at risk of LP or cell death in cPD exhibit this phenotype? It has only been in the past few years that the necessary tools for ‘deep’ physiological phenotyping (for example, in situ mitochondrial redox measurements) and anatomical phenotyping of genetically defined neuronal populations in mouse models have become available. To date, this kind of in-depth analysis has only been performed in SNc, LC and DMV neurons. Although the available experimental data on other neurons in the brainstem, mesencephalon and diencephalon are consistent with a shared phenotype, more deep phenotyping, including that at the genetic level136,137, needs to be carried out to determine the extent to which these neurons are alike. Nevertheless, it is clear that there are deviations. Although there are a few relevant data on superficial presupplementary motor cortex pyramidal neurons, other cortical pyramidal neurons and AM neurons are not physiological phenocopies of SNc DA neurons, and neither are BF cholinergic neurons138. However, many of the telencephalic regions that are vulnerable to LP or cell death in cPD are part of a ‘default’ network that manifests high resting-state activity, albeit of synaptic (rather than cell-autonomous) origin139. It is possible that, in aged patients with late-stage cPD, network dysfunction140 triggers adaptations in bioenergetic control mechanisms that bring them phenotypically closer to other vulnerable neurons. Cav1 channels, which are key determinants of the SNc phenotype, could be a major factor in this process. Ageing-induced elevation in Ca2+ entry through Cav1 channels in forebrain neurons has long been associated with cognitive decline and Alzheimer disease141. Moreover, the expression of Cav1 Ca2+ channels has been found to be upregulated in limbic and motor cortices of patients with cPD142,143.
Perhaps the most compelling piece of evidence that physiological phenotype is a determinant of pathology in cPD is the observation that dihydropyridine-mediated inhibition of Cav1 channels — which lowers cytosolic Ca2+ levels, mitochondrial oxidant stress and sensitivity to toxins in neurons at risk of LP or cell death in cPD82,86,87,90,144–146 — has consistently been linked by epidemiological studies to reduced risk of developing cPD147–152. The combination of preclinical and clinical data implicating Cav1 channels in PD pathogenesis motivated the US National Institutes of Health to mount a 5-year, phase III, disease-modification clinical trial in patients with early-stage PD with the dihydropyridine isradipine in 2013 (REF. 153).
Conclusions
The prevailing view of PD aetiology is that LP retrogradely spreads in the brain through synaptically coupled networks, driving neuronal death and clinical manifestations. However, the distribution of pathology in the brains of patients with cPD is not consistent with this model. Rather, if LP spreads trans-synaptically in cPD, spreading is likely to be gated by cell- or region-autonomous mechanisms. Many, if not all, of the neurons that are vulnerable to LP in cPD are part of large neuromodulatory networks involved in orchestrating behaviours that are necessary for survival. Although incomplete, the available evidence suggests that these neurons share a common set of anatomical and physiological properties that could explain not only the pattern of LP in cPD but also the pattern of cell death.
As plausible as the combination of neuronal properties and propagated pathology is in explaining the aetiology of cPD, there are many important and unresolved issues. From the standpoint of selective neuronal vulnerability, there are four major gaps in our current knowledge. First, it has not been proved that there is spread of LP in human cPD. To show this convincingly, there needs to be a quantitative assessment of the distribution and severity of LP — and neuronal loss — in clinically well-characterized and staged cPD cases and in age-matched controls without neuronal loss or LP. Better imaging biomarkers, particularly for α-syn LP, will be needed to validate concepts of LP progression. Second, a better understanding of the relationships between intraneuronal LP, neuronal dysfunction and death, and clinical symptoms is needed. Attempts to connect LP assessed post-mortem to symptoms that appeared more than a decade before death are problematic. Resolving these limitations will require mechanistic studies in animal models of cPD, as well as functional brain imaging in combination with LP biomarkers in well-characterized patients with cPD and age-matched controls. Third, there needs to be better animal and cellular models of cPD that allow the hypotheses about disease mechanisms and spreading to be tested. For unknown reasons, the currently available animal models of cPD do not recapitulate the patterns of LP or neuronal loss that are observed in human cases. Last, we need to carry out ‘deep’ molecular and physiological phenotyping of a broader set of neuronal populations that are at risk of, or resistant to, LP or cell death in cPD to identify translational strategies that could broadly decrease neuronal vulnerability.
Acknowledgments
This work was supported by US National Institutes of Health grant NS047085 and grants from the JPB Foundation and the IDP Foundation (to D.J.S.). G.M.H. is a National Health and Medical Research Council of Australia Senior Principal Research Fellow (grant #1079679). J.A.O.’s research is supported by grants SAF2012-40216 and SAF2015-67239-P from the Plan Nacional, Ministerio de Economíy Competitividad, Spain.
Glossary
- Lewy pathology (LP)
Abnormal, proteinaceous aggregates in the neuronal cytoplasm that are rich in α-synuclein.
- Connectome
A detailed map of the synaptic connections that are formed between neurons in the brain.
- Levodopa
The precursor to the neurotransmitter dopamine; it is given to patients with Parkinson disease because, unlike dopamine, it can pass through the blood–brain barrier and elevate brain dopamine concentrations.
- Lewy neurites
Lewy pathology found within neuronal processes, including axons and dendrites.
- Mitophagy
A form of autophagy in which damaged mitochondria are degraded.
- Apoptosis
A process of programmed cell death involving activation of caspase signalling cascades that are commonly triggered by release of cytochrome c from mitochondria.
- Necrosis
A form of cell death that is distinct from apoptosis and that is commonly caused by infection, toxins or ischaemia.
- Mitochondrial redox
The state of reduction– oxidation reactions in mitochondria.
- Phenocopies
In this case, neurons with a similar set of traits (for example, activity patterns) resulting from the interaction between their genome and the environment.
- Dihydropyridine
A class of US-approved drugs that are negative allosteric modulators of Ca2+ channels with a Cav1 pore-forming subunit.
Footnotes
Competing interests statement:
The authors declare no competing interests.
References
- 1.Berg D, et al. Time to redefine PD? Introductory statement of the MDS task force on the definition of Parkinson’s disease. Mov Disord. 2014;29:454–462. doi: 10.1002/mds.25844. This article reviews clinical and biological knowledge on PD showing that LP is not essential for cPD and occurs in a large proportion of patients who may not have cPD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hornykiewicz O. Dopamine miracle: from brain homogenate to dopamine replacement. Mov Disord. 2002;17:501–508. doi: 10.1002/mds.10115. [DOI] [PubMed] [Google Scholar]
- 3.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2012;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 4.Dijkstra AA, et al. Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov Disord. 2014;29:1244–1251. doi: 10.1002/mds.25952. This paper describes a negative correlation between neuronal density and local α-syn burden in the SN of patients with PD and shows that the severity of neurodegeneration and local burden of α-syn pathological conditions are closely coupled during disease progression. [DOI] [PubMed] [Google Scholar]
- 5.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. This seminal paper reviews the evidence supporting the notion that LP is distributed and staged in PD. [DOI] [PubMed] [Google Scholar]
- 7.Beach TG, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Tredici K, Rüb U, de Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 9.Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree — a new disease? Clin Neuropathol. 1984;3:185–192. [PubMed] [Google Scholar]
- 10.Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RKB. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease: a critical analysis of α-synuclein staging. Neuropathol Appl Neurobiol. 2008;34:284–295. doi: 10.1111/j.1365-2990.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 11.Halliday G, McCann H, Shepherd C. Evaluation of the Braak hypothesis: how far can it explain the pathogenesis of Parkinson’s disease? Expert Rev Neurother. 2012;12:673–686. doi: 10.1586/ern.12.47. This review evaluates the main elements underpinning the Braak hypothesis of propagation, comparing data for both PD and the similar pathological entity of dementia with Lewy bodies, as well as data using cohorts with potential prodromal features of PD. [DOI] [PubMed] [Google Scholar]
- 12.Doherty KM, et al. Parkin disease: a clinicopathologic entity? JAMA Neurol. 2013;70:571–579. doi: 10.1001/jamaneurol.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingsbury AE, et al. Brain stem pathology in Parkinson’s disease: an evaluation of the Braak staging model. Mov Disord. 2010;25:2508–2515. doi: 10.1002/mds.23305. [DOI] [PubMed] [Google Scholar]
- 14.Dugger BN, Dickson DW. Cell type specific sequestration of choline acetyltransferase and tyrosine hydroxylase within Lewy bodies. Acta Neuropathol. 2010;120:633–639. doi: 10.1007/s00401-010-0739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 16.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 17.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 18.Mendez I, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalia LV, Kalia SK. α-Synuclein and Lewy pathology in Parkinson’s disease. Curr Opin Neurol. 2015;28:375–381. doi: 10.1097/WCO.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 20.Kirik D, et al. Parkinson-like neurodegeneration induced by targeted overexpression of α-synuclein in the nigrostriatal system. J Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirik D, et al. Nigrostriatal α-synucleinopathy induced by viral vector-mediated overexpression of human α-synuclein: a new primate model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100:2884–2889. doi: 10.1073/pnas.0536383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maingay M, Romero-Ramos M, Carta M, Kirik D. Ventral tegmental area dopamine neurons are resistant to human mutant alpha-synuclein overexpression. Neurobiol Dis. 2006;23:522–532. doi: 10.1016/j.nbd.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Ulusoy A, et al. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol Med. 2013;5:1051–1059. doi: 10.1002/emmm.201302475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. This was the first demonstration that pre-formed α-syn fibrils can propagate from one neuron to another and induce cell death in vivo when inoculated into the brain of mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuda-Suzukake M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peelaerts W, et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 27.Rey NL, et al. Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med. 2016;213:1759–1778. doi: 10.1084/jem.20160368. This paper provides the first clear evidence of transneuronal propagation of pre-formed α-syn fibril-induced pathology from the olfactory bulb in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recasens A, et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. This paper used purified pathological α-syn from the SN of patients with PD to show the intracellular and presynaptic accumulation of endogenous α-syn and the progressive axon-initiated neurodegeneration. [DOI] [PubMed] [Google Scholar]
- 29.Mao X, et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353:aah3374. doi: 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrivastava AN, et al. α-Synuclein assemblies sequester neuronal α3-Na+/K+-ATPase and impair Na+ gradient. EMBO J. 2015;34:2408–2423. doi: 10.15252/embj.201591397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchihara T, Giasson BI. Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2016;131:49–73. doi: 10.1007/s00401-015-1485-1. This review systematically evaluates data for and against the hypothesis that α-syn propagates in PD and other neurodegenerative disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh DM, Selkoe DJ. A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nat Rev Neurosci. 2016;17:251–260. doi: 10.1038/nrn.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacino AN, et al. Proteolysis of α-synuclein fibrils in the lysosomal pathway limits induction of inclusion pathology. J Neurochem. 2016 doi: 10.1111/jnc.13743. http://dx.doi.org/10.1111/jnc.13743. [DOI] [PMC free article] [PubMed]
- 34.Volpicelli-Daley LA, Luk KC, Lee VMY. Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc. 2014;9:2135–2146. doi: 10.1038/nprot.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volpicelli-Daley LA, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helwig M, et al. Brain propagation of transduced α-synuclein involves non-fibrillar protein species and is enhanced in α-synuclein null mice. Brain. 2016;139:856–870. doi: 10.1093/brain/awv376. Using viral gene delivery of an α-syn expression construct to the vagus nerve, the authors examined the propagation of different forms of α-syn through the brainstem; they found that endogenous α-syn impeded, rather than promoted, the spread of oligomeric forms of α-syn. [DOI] [PubMed] [Google Scholar]
- 37.Olanow CW, Brundin P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord. 2013;28:31–40. doi: 10.1002/mds.25373. [DOI] [PubMed] [Google Scholar]
- 38.Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc Natl Acad Sci USA. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch C, Reid RC. Neuroscience: observatories of the mind. Nature. 2012;483:397–398. doi: 10.1038/483397a. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz LA, et al. Viral-genetic tracing of the input– output organization of a central noradrenaline circuit. Nature. 2015;524:88–92. doi: 10.1038/nature14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa SK, Cohen JY, Hwang D, Uchida N, Watabe-Uchida M. Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Rep. 2014;8:1105–1118. doi: 10.1016/j.celrep.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Halliday GM, Song YJC, Harding AJ. Striatal β-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J Neural Transm. 2011;118:713–719. doi: 10.1007/s00702-011-0641-6. [DOI] [PubMed] [Google Scholar]
- 44.Milber JM, et al. Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology. 2012;79:2307–2314. doi: 10.1212/WNL.0b013e318278fe32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 46.Halliday GM, et al. Midbrain neuropathology in idiopathic Parkinson’s disease and diffuse Lewy body disease. J Clin Neurosci. 1996;3:52–60. doi: 10.1016/s0967-5868(96)90083-1. [DOI] [PubMed] [Google Scholar]
- 47.Halliday GM, et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol. 1990;27:373–385. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- 48.Harding AJ, Stimson E, Henderson JM, Halliday GM. Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain. 2002;125:2431–2445. doi: 10.1093/brain/awf251. [DOI] [PubMed] [Google Scholar]
- 49.Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centré median– parafascicular complex in Parkinson’s disease. Ann Neurol. 2000;47:345–352. [PubMed] [Google Scholar]
- 50.Thannickal TC, et al. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fronczek R, et al. Hypocretin (orexin) loss and sleep disturbances in Parkinson’s Disease. Brain. 2008;131:e88. doi: 10.1093/brain/awm222. [DOI] [PubMed] [Google Scholar]
- 52.Kremer HPH, Bots GTAM. Lewy bodies in the lateral hypothalamus: do they imply neuronal loss? Mov Disord. 1993;8:315–320. doi: 10.1002/mds.870080310. [DOI] [PubMed] [Google Scholar]
- 53.Jellinger KA. Formation and development of Lewy pathology: a critical update. J Neurol. 2009;256:270–279. doi: 10.1007/s00415-009-5243-y. [DOI] [PubMed] [Google Scholar]
- 54.Ansorge O, Daniel SE, Pearce RK. Neuronal loss and plasticity in the supraoptic nucleus in Parkinson’s disease. Neurology. 1997;49:610–613. doi: 10.1212/wnl.49.2.610. [DOI] [PubMed] [Google Scholar]
- 55.MacDonald V, Halliday GM. Selective loss of pyramidal neurons in the pre-supplementary motor cortex in Parkinson’s disease. Mov Disord. 2002;17:1166–1173. doi: 10.1002/mds.10258. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen KM, Marner L, Pakkenberg H, Pakkenberg B. No global loss of neocortical neurons in Parkinson’s disease: a quantitative stereological study. Mov Disord. 2005;20:164–171. doi: 10.1002/mds.20289. [DOI] [PubMed] [Google Scholar]
- 57.Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 59.Pfaff DW, Martin EM, Faber D. Origins of arousal: roles for medullary reticular neurons. Trends Neurosci. 2012;35:468–476. doi: 10.1016/j.tins.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Aston-Jones G, Waterhouse B. Locus coeruleus: from global projection system to adaptive regulation of behavior. Brain Res. 2016;1645:75–78. doi: 10.1016/j.brainres.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexandre C, Andermann ML, Scammell TE. Control of arousal by the orexin neurons. Curr Opin Neurobiol. 2013;23:752–759. doi: 10.1016/j.conb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmiter RD. Dopamine signaling as a neural correlate of consciousness. Neuroscience. 2011;198:213–220. doi: 10.1016/j.neuroscience.2011.06.089. [DOI] [PubMed] [Google Scholar]
- 63.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2003;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 64.Silvani A, Calandra-Buonaura G, Dampney RAL, Cortelli P. Brain–heart interactions: physiology and clinical implications. Philos Trans A Math Phys Eng Sci. 2016;374:20150181. doi: 10.1098/rsta.2015.0181. [DOI] [PubMed] [Google Scholar]
- 65.Miskovic V, Schmidt LA. Social fearfulness in the human brain. Neurosci Biobehav Rev. 2012;36:459–478. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Matsuda W, et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat. 2011;5:22. doi: 10.3389/fnana.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Ratcliffe EM, Farrar NR, Fox EA. Development of the vagal innervation of the gut: steering the wandering nerve. Neurogastroenterol Motil. 2011;23:898–911. doi: 10.1111/j.1365-2982.2011.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baufreton J, et al. Sparse but selective and potent synaptic transmission from the globus pallidus to the subthalamic nucleus. J Neurophysiol. 2009;102:532–545. doi: 10.1152/jn.00305.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu B, Yang N, Qiao QC, Hu ZA, Zhang J. Roles of the orexin system in central motor control. Neurosci Biobehav Rev. 2015;49:43–54. doi: 10.1016/j.neubiorev.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Liu AKL, Chang RCC, Pearce RKB, Gentleman SM. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015;129:527–540. doi: 10.1007/s00401-015-1392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bolam JP, Pissadaki EK. Living on the edge with too many mouths to feed: why dopamine neurons die. Mov Disord. 2012;27:1478–1483. doi: 10.1002/mds.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hunn BHM, Cragg SJ, Bolam JP, Spillantini MG, Wade-Martins R. Impaired intracellular trafficking defines early Parkinson’s disease. Trends Neurosci. 2015;38:178–188. doi: 10.1016/j.tins.2014.12.009. This review highlights the potential role of intracellular trafficking in PD pathogenesis, which is particularly important in view of the growing importance of RAB proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pacelli C, et al. Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr Biol. 2015;25:2349–2360. doi: 10.1016/j.cub.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 76.Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- 77.Surmeier DJ, Guzman JN, Sanchez J, Schumacker PT. Physiological phenotype and vulnerability in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009290. doi: 10.1101/cshperspect.a009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nedergaard S, Flatman JA, Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J Physiol. 1993;466:727–747. [PMC free article] [PubMed] [Google Scholar]
- 79.Puopolo M, Raviola E, Bean BP. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J Neurosci. 2007;27:645–656. doi: 10.1523/JNEUROSCI.4341-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guzman JN, Sáchez-Padilla J, Chan CS, Surmeier DJ. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci. 2009;29:11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mercuri NB, et al. Effects of dihydropyridine calcium antagonists on rat midbrain dopaminergic neurones. Br J Pharmacol. 1994;113:831–838. doi: 10.1111/j.1476-5381.1994.tb17068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan CS, et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 83.Foehring RC, Zhang XF, Lee JCF, Callaway JC. Endogenous calcium buffering capacity of substantia nigral dopamine neurons. J Neurophysiol. 2009;102:2326–2333. doi: 10.1152/jn.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khaliq ZM, Bean BP. Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J Neurosci. 2010;30:7401–7413. doi: 10.1523/JNEUROSCI.0143-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Philippart F, et al. Differential somatic Ca2+ channel profile in midbrain dopaminergic neurons. J Neurosci. 2016;36:7234–7245. doi: 10.1523/JNEUROSCI.0459-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sáchez-Padilla J, et al. Mitochondrial oxidant stress in locus coeruleus is regulated by activity and nitric oxide synthase. Nat Neurosci. 2014;17:832–840. doi: 10.1038/nn.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldberg JA, et al. Calcium entry induces mitochondrial oxidant stress in vagal neurons at risk in Parkinson’s disease. Nat Neurosci. 2012;15:1414–1421. doi: 10.1038/nn.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang Y, Kitai ST. Electrophysiological properties of pedunculopontine neurons and their postsynaptic responses following stimulation of substantia nigra reticulata. Brain Res. 1990;535:79–95. doi: 10.1016/0006-8993(90)91826-3. [DOI] [PubMed] [Google Scholar]
- 89.Putzier I, Kullmann PHM, Horn JP, Levitan ES. CaV1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J Neurosci. 2009;29:15414–15419. doi: 10.1523/JNEUROSCI.4742-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guzman JN, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. This is the first demonstration that Ca2+ entry through Cav1 channels during pacemaking elevates mitochondrial oxidant stress in SN DA neurons; the authors examined ex vivo brain slices from young adult mice and also showed that this oxidant stress was exacerbated by deletion of the gene encoding protein deglycase DJ1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hayashi T, Rizzuto R, Hajnózky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balaban RS. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nicholls DG. Mitochondria in the life and death of neurons. Essays Biochem. 1998;33:43–52. doi: 10.1042/bse0330043. [DOI] [PubMed] [Google Scholar]
- 94.Dragicevic E, Schiemann J, Liss B. Dopamine midbrain neurons in health and Parkinson’s disease: emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience. 2015;284:798–814. doi: 10.1016/j.neuroscience.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 95.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 96.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Votyakova TV, Reynolds IJ. Δψm-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 98.Brimblecombe KR, Gracie CJ, Platt NJ, Cragg SJ. Gating of dopamine transmission by calcium and axonal N-, Q-, T-and L-type voltage-gated calcium channels differs between striatal domains. J Physiol. 2015;593:929–946. doi: 10.1113/jphysiol.2014.285890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Vries RLA, Przedborski S. Mitophagy and Parkinson’s disease: be eaten to stay healthy. Mol Cell Neurosci. 2013;55:37–43. doi: 10.1016/j.mcn.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 100.Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gupta A, Dawson VL, Dawson TM. What causes cell death in Parkinson’s disease? Ann Neurol. 2009;64:S3–S15. doi: 10.1002/ana.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagley P, Higgins GC, Atkin JD, Beart PM. Multifaceted deaths orchestrated by mitochondria in neurones. Biochim Biophys Acta. 2010;1802:167–185. doi: 10.1016/j.bbadis.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 103.Rcom-H’cheo-Gauthier A, Goodwin J, Pountney DL. Interactions between calcium and alpha-synuclein in neurodegeneration. Biomolecules. 2014;4:795–811. doi: 10.3390/biom4030795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nath S, Goodwin J, Engelborghs Y, Pountney DL. Raised calcium promotes α-synuclein aggregate formation. Mol Cell Neurosci. 2011;46:516–526. doi: 10.1016/j.mcn.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 105.Dufty BM, et al. Calpain-cleavage of α-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007;170:1725–1738. doi: 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Diepenbroek M, et al. Overexpression of the calpain-specific inhibitor calpastatin reduces human alpha-synuclein processing, aggregation and synaptic impairment in [A30P]αSyn transgenic mice. Hum Mol Genet. 2014;23:3975–3989. doi: 10.1093/hmg/ddu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Melachroinou K, et al. Deregulation of calcium homeostasis mediates secreted α-synuclein-induced neurotoxicity. Neurobiol Aging. 2013;34:2853–2865. doi: 10.1016/j.neurobiolaging.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Caraveo G, et al. Calcineurin determines toxic versus beneficial responses to α-synuclein. Proc Natl Acad Sci USA. 2014;111:E3544–E3552. doi: 10.1073/pnas.1413201111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Góez-Sintes R, Ledesma MD, Boya P. Lysosomal cell death mechanisms in aging. Ageing Res Rev. 2016;32:150–168. doi: 10.1016/j.arr.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 110.Reeve A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. This article summarizes and discusses how ageing might make SN neurons especially vulnerable to changes in protein metabolism and mitochondrial function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duda J, Pöschke C, Liss B. Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson’s disease. J Neurochem. 2016;139:156–178. doi: 10.1111/jnc.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beilina A, Cookson MR. Genes associated with Parkinson’s disease: regulation of autophagy and beyond. J Neurochem. 2015;139(Suppl 1):91–107. doi: 10.1111/jnc.13266. The authors review the proposition that many known PD-related genes can be assigned to pathways that affect autophagy and mitochondrial quality control through mitophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cookson MR. α-Synuclein and neuronal cell death. Mol Neurodegener. 2009;4:9. doi: 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gegg ME, Schapira AHV. Mitochondrial dysfunction associated with glucocerebrosidase deficiency. Neurobiol Dis. 2016;90:43–50. doi: 10.1016/j.nbd.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schneider JL, Cuervo AM. Autophagy and human disease: emerging themes. Curr Opin Genet Dev. 2014;26:16–23. doi: 10.1016/j.gde.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jellinger KA, Jellinger KA. A critical evaluation of current staging of α-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2009;1792:730–740. doi: 10.1016/j.bbadis.2008.07.006. This review summarizes the deviation from the Braak staging model and the difficulty of linking LP and disease symptom severity. [DOI] [PubMed] [Google Scholar]
- 117.Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinsons disease. Mov Disord. 2013;28:715–724. doi: 10.1002/mds.25187. [DOI] [PubMed] [Google Scholar]
- 118.Pissadaki EK, Bolam JP. The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson’s disease. Front Comput Neurosci. 2013;7:13. doi: 10.3389/fncom.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liss B, Roeper J. Individual dopamine midbrain neurons: functional diversity and flexibility in health and disease. Brain Res Rev. 2008;58:314–321. doi: 10.1016/j.brainresrev.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 120.German DC, Manaye KF, Sonsalla PK, Brooks BA. Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann NY Acad Sci. 1992;648:42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- 121.Dopeso-Reyes IG, et al. Calbindin content and differential vulnerability of midbrain efferent dopaminergic neurons in macaques. Front Neuroanat. 2014;8:146. doi: 10.3389/fnana.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCormack AL, Atienza JG, Langston JW, Di Monte DA. Decreased susceptibility to oxidative stress underlies the resistance of specific dopaminergic cell populations to paraquat-induced degeneration. Neuroscience. 2006;141:929–937. doi: 10.1016/j.neuroscience.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 123.Bender A, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 124.Bender A, et al. Dopaminergic midbrain neurons are the prime target for mitochondrial DNA deletions. J Neurol. 2008;255:1231–1235. doi: 10.1007/s00415-008-0892-9. [DOI] [PubMed] [Google Scholar]
- 125.Roberts HL, Brown DR. Seeking a mechanism for the toxicity of oligomeric α-synuclein. Biomolecules. 2015;5:282–305. doi: 10.3390/biom5020282. This review nicely summarizes the literature linking α-syn and neuronal toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol. 2008;115:409–415. doi: 10.1007/s00401-008-0344-8. [DOI] [PubMed] [Google Scholar]
- 127.Petit GH, Olsson TT, Brundin P. The future of cell therapies and brain repair: Parkinson’s disease leads the way. Neuropathol Appl Neurobiol. 2014;40:60–70. doi: 10.1111/nan.12110. [DOI] [PubMed] [Google Scholar]
- 128.Brundin P, Kordower JH. In: Functional Neural Transplantation III — Primary and Stem Cell Therapies for Brain Repair, Part I. Dunnett SB, Bjorklund A, editors. Elsevier; 2012. pp. 221–241. [DOI] [PubMed] [Google Scholar]
- 129.Li JY, et al. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord. 2010;25:1091–1096. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- 130.Brini M, Calì T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guardia-Laguarta C, Area-Gomez E, Schon EA, Przedborski S. A new role for α-synuclein in Parkinson’s disease: alteration of ER-mitochondrial communication. Mov Disord. 2015;30:1026–1033. doi: 10.1002/mds.26239. This review advances the novel concept that α-syn affects neuronal function by modulating endoplasmic reticulum–mitochondrial crosstalk. [DOI] [PubMed] [Google Scholar]
- 132.McCoy MK, Cookson MR. Mitochondrial quality control and dynamics in Parkinson’s disease. Antioxid Redox Signal. 2012;16:869–882. doi: 10.1089/ars.2011.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dryanovski DI, et al. Calcium entry and α-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J Neurosci. 2013;33:10154–10164. doi: 10.1523/JNEUROSCI.5311-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mosharov EV, et al. Interplay between cytosolic dopamine, calcium, and α-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Subramaniam M, et al. Mutant α-synuclein enhances firing frequencies in dopamine substantia nigra neurons by oxidative impairment of A-type potassium channels. J Neurosci. 2014;34:13586–13599. doi: 10.1523/JNEUROSCI.5069-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brichta L, et al. Identification of neurodegenerative factors using translatome-regulatory network analysis. Nat Neurosci. 2015;18:1325–1333. doi: 10.1038/nn.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Poulin JF, et al. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 2014;9:930–943. doi: 10.1016/j.celrep.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murchison D, Griffith WH. Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging Cell. 2007;6:297–305. doi: 10.1111/j.1474-9726.2007.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hafkemeijer A, van der Grond J, Rombouts SARB. Imaging the default mode network in aging and dementia. Biochim Biophys Acta. 2012;1822:431–441. doi: 10.1016/j.bbadis.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 140.Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 141.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hurley MJ, Gentleman SM, Dexter DT. Calcium CaV1 channel subtype mRNA expression in Parkinson’s disease examined by in situ hybridization. J Mol Neurosci. 2014;55:1–10. doi: 10.1007/s12031-014-0410-8. [DOI] [PubMed] [Google Scholar]
- 143.Hurley MJ, Brandon B, Gentleman SM, Dexter DT. Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain. 2013;136:2077–2097. doi: 10.1093/brain/awt134. [DOI] [PubMed] [Google Scholar]
- 144.Ilijic E, Guzman JN, Surmeier DJ. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis. 2011;43:364–371. doi: 10.1016/j.nbd.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kupsch A, et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydr opyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain Res. 1996;741:185–196. doi: 10.1016/s0006-8993(96)00917-1. [DOI] [PubMed] [Google Scholar]
- 146.Singh A, Verma P, Balaji G, Samantaray S, Mohanakumar KP. Nimodipine, an L-type calcium channel blocker attenuates mitochondrial dysfunctions to protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neurochem Int. 2016;99:221–232. doi: 10.1016/j.neuint.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 147.Lee YC, et al. Antihypertensive agents and risk of Parkinson’s disease: a nationwide cohort study. PLoS ONE. 2014;9:e98961. doi: 10.1371/journal.pone.0098961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pasternak B, et al. Use of calcium channel blockers and Parkinson’s disease. Am J Epidemiol. 2012;175:627–635. doi: 10.1093/aje/kwr362. [DOI] [PubMed] [Google Scholar]
- 149.Marras C, et al. Dihydropyridine calcium channel blockers and the progression of parkinsonism. Ann Neurol. 2012;71:362–369. doi: 10.1002/ana.22616. [DOI] [PubMed] [Google Scholar]
- 150.Ritz B, et al. L-type calcium channel blockers and Parkinson disease in Denmark. Ann Neurol. 2010;67:600–606. doi: 10.1002/ana.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Becker C, Jick SS, Meier CR. Use of antihypertensives and the risk of Parkinson disease. Neurology. 2008;70:1438–1444. doi: 10.1212/01.wnl.0000303818.38960.44. [DOI] [PubMed] [Google Scholar]
- 152.Gudala K, Kanukula R, Bansal D. Reduced risk of Parkinson’s disease in users of calcium channel blockers: a meta-analysis. Int J Chron Dis. 2015;2015:697404. doi: 10.1155/2015/697404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.US National Library of Medicine. ClinicalTrialsgov. 2016 https://clinicaltrials.gov/ct2/show/record/NCT02168842.
- 154.Mullin S, Schapira A. The genetics of Parkinson’s disease. Br Med Bull. 2015;114:39–52. doi: 10.1093/bmb/ldv022. This article reviews the role of genome-wide association studies in deciphering inter-related genetic mechanisms and biochemical pathways. [DOI] [PubMed] [Google Scholar]
- 155.Lin MK, Farrer MJ. Genetics and genomics of Parkinson’s disease. Genome Med. 2014;6:48. doi: 10.1186/gm566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kumaran R, Cookson MR. Pathways to parkinsonism redux: convergent pathobiological mechanisms in genetics of Parkinson’s disease. Hum Mol Genet. 2015;24:R32–R44. doi: 10.1093/hmg/ddv236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Poulopoulos M, Levy OA, Alcalay RN. The neuropathology of genetic Parkinson’s disease. Mov Disord. 2012;27:831–842. doi: 10.1002/mds.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Konno T, Ross OA, Puschmann A, Dickson DW, Wszolek ZK. Autosomal dominant Parkinson’s disease caused by SNCA duplications. Parkinsonism Relat Disord. 2016;22(Suppl 1):1–6. doi: 10.1016/j.parkreldis.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40:200–210. doi: 10.1016/j.tibs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 161.Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ. Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem. 2014;289:21490–21507. doi: 10.1074/jbc.M113.545749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Calì T, Ottolini D, Negro A, Brini M. α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J Biol Chem. 2012;287:17914–17929. doi: 10.1074/jbc.M111.302794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guardia-Laguarta C, Area-Gomez E, Schon EA, Przedborski S. Novel subcellular localization for α-synuclein: possible functional consequences. Front Neuroanat. 2015;9:17. doi: 10.3389/fnana.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cookson MR. LRRK2 pathways leading to neurodegeneration. Curr Neurol Neurosci Rep. 2015;15:564–510. doi: 10.1007/s11910-015-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Esteves AR, Swerdlow RH, Cardoso SM. LRRK2, a puzzling protein: insights into Parkinson’s disease pathogenesis. Exp Neurol. 2014;261:206–216. doi: 10.1016/j.expneurol.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Martin I, Kim JW, Dawson VL, Dawson TM. LRRK2 pathobiology in Parkinson’s disease. J Neurochem. 2014;131:554–565. doi: 10.1111/jnc.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kalia LV, et al. Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 2015;72:100–105. doi: 10.1001/jamaneurol.2014.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bonifati V. Autosomal recessive parkinsonism. Parkinsonism Relat Disord. 2012;18(Suppl 1):S4–S6. doi: 10.1016/S1353-8020(11)70004-9. [DOI] [PubMed] [Google Scholar]
- 169.Zucca FA, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol. 2015 doi: 10.1016/j.pneurobio.2015.09.012. http://dx.doi.org/10.1016/j.pneurobio.2015.09.012. [DOI] [PMC free article] [PubMed]
- 170.Kirkwood TBL. Global aging and the brain. Nutr Rev. 2010;68(Suppl 2):65–69. doi: 10.1111/j.1753-4887.2010.00343.x. [DOI] [PubMed] [Google Scholar]
- 171.Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012;13:397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human \primates. Nat Rev Neurosci. 2011;12:359–366. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]