Abstract
Mitochondria have various roles in cellular metabolism and homeostasis. Because mitochondrial dysfunction is associated with many acute and chronic degenerative diseases, mitochondrial biogenesis (MB) is a therapeutic target for treating such diseases. Here, we review the role of mitochondrial dysfunction in acute and chronic degenerative diseases and the cellular signaling pathways by which MB is induced. We then review existing work describing the development and application of drugs that induce MB in vitro and in vivo. In particular, we discuss natural products and modulators of transcription factors, kinases, cyclic nucleotides, and G protein-coupled receptors.
Keywords: Mitochondrial biogenesis, mitochondria, PGC-1α, degenerative disease, sirtuin 1, PPARγ, G protein-coupled receptor, cGMP, cAMP, AMPK, ERK1/2, beta-2 adrenergic receptor, 5-hydroxytryptamine
Graphical Abstract
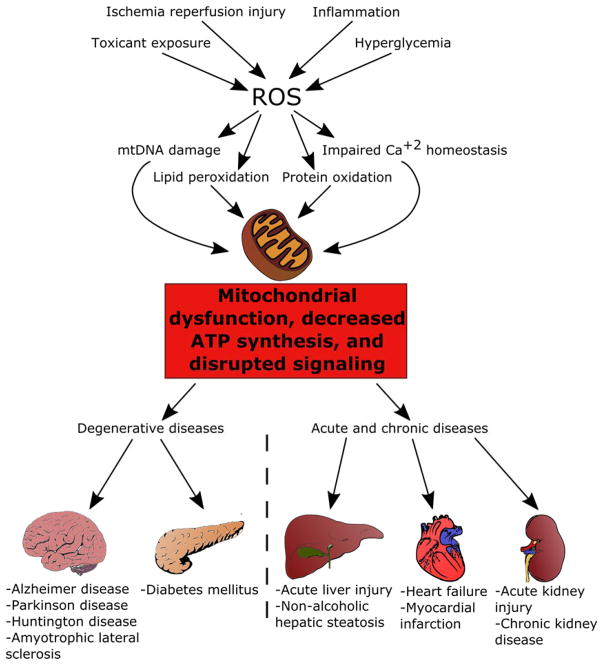
Mitochondria, the metabolic powerhouses of the cell, have diverse functions including ATP production, biomolecule synthesis, ionic homeostasis and antioxidant defense. As cells age and accumulate damage, mitochondria less readily meet ATP demands, thereby diminishing the cells’ functions and regenerative capacity. After toxicant exposure or cell stress, mitochondria can be damaged, and increased free radical production may be followed by persistent mitochondrial dysfunction. Diminished ATP and increased free radicals propagate injury and subsequent tissue and organ dysfunction (Figure 1). Indeed, many acute and chronic degenerative diseases across multiple organ systems are associated with a degree of mitochondrial dysfunction, often with suppression of electron transport chain proteins and activities.1–4
Figure 1.
Multiple insults converge upon the mitochondria, leading to mitochondrial dysfunction and subsequent organ injury and disease.
Because many diseases are associated with mitochondrial dysfunction, research is underway to develop therapeutics that target mitochondria to prevent disease progression. For example, numerous compounds have been studied that prevent cell death by interfering with the formation of the mitochondrial permeability transition pore (MPTP), reducing oxidative stress using mitochondrial-targeted antioxidants, or modulating mitochondrial dynamics by inhibiting mitochondrial fission or promoting mitochondrial networking.5 However, whereas many of these strategies are effective for preventing injury in animal models, they target events that occur early in cellular dysfunction and therefore may be less efficacious for facilitating recovery after an insult. To address this problem, some groups have investigated compounds that induce mitochondrial biogenesis (MB), or the generation of new, functional mitochondria within cells to promote repair and regeneration.1
This perspective will describe the role of the peroxisomal proliferation activated receptor coactivator-1α (PGC-1α) in MB and the role of mitochondrial dysfunction in acute and chronic degenerative diseases. We will also describe existing compounds that induce MB, signaling pathways responsible for their effects, and finally, potential utility of these compounds for treating human acute and chronic degenerative diseases for which there are presently limited therapeutic options.
Regulation of MB
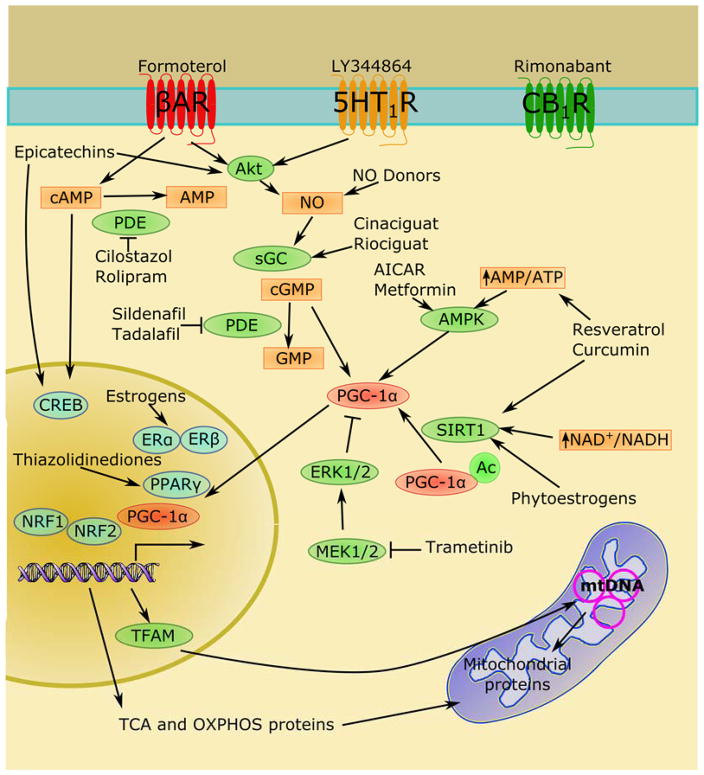
MB requires the activation of a complex transcriptional and translational program integrating both nuclear and mitochondrial genomes.6–7 Nuclear encoded mitochondrial genes, such as the mitochondrial transcription factors and the mitochondrial DNA (mtDNA) replication complex, facilitate transcription, replication, and proofreading of the mitochondrial genome.6 Integrity of mtDNA replication is particularly important in aging and chronic degenerative diseases, where deleterious mtDNA mutations and deletions can lead to dysfunctional mitochondria.8–9 For example, the nuclear transcription factors estrogen receptor (ER) and estrogen related receptor-α (ERRα), nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2), peroxisome proliferator-activated receptor (PPAR) family of transcription factors, thyroid hormone receptor (TR), cAMP-responsive element binding protein (CREB), and yin yang-1 (YY-1)10 increase expression of genes of the electron transport chain, mitochondrial transporters, antioxidant proteins, and other mitochondrial transcription factors. However, these transcription factors are pleotropic with effects on genes unrelated to MB. Selective induction of MB is typically regulated through transcriptional co-activation proteins such as the PGC-1 family (Figure 2). PGC-1 proteins activate transcription and translation of mitochondrial genes and increase energy production in healthy cells, whereas in injured cells PGC-1 activation often normalizes overall mitochondrial function as measured by ATP production, mitochondrial membrane potential, and reactive oxygen species (ROS) generation.11–13
Figure 2.
PGC-1α integrates extracellular and cytosolic signaling inputs to selectively upregulate mitochondrial biogenesis.
The PGC-1 family, composed of PGC-1α, PGC-1β, and PGC-1 related coactivator (PRC), facilitate the formation of complexes capable of activating the transcription of nuclear genes related to MB.14 PRC is thought to play a role in redox-sensitive inflammatory responses and MB during cellular proliferation, whereas PGC-1β appears to contribute more to maintenance of mitochondrial mass. In contrast, PGC-1α has been shown to drive MB in response to various environmental cues. Because PGC-1α tends to be the most inducible and responsive member of the PGC-1 family, its activation has emerged as a key therapeutic strategy for MB induction. However, it is important to note that PGC-1α-independent mechanisms of MB have been reported.15–18 Such mechanisms include compensatory activation of PGC-1β or PRC and direct activation of transcription factors that induce mitochondrial genes.
Through activation of PGC-1α and its associated transcription factors, multiple signaling pathways have been shown to regulate MB. PGC-1α can be directly activated by silent mating type information regulation 2 homolog 1 (SIRT1)-mediated deacetylation,19 methylation by protein arginine methyltransferase 1 (PRMT1),20 or phosphorylation by kinases such as p38,21 protein kinase A (PKA),22 and AMP-dependent kinase (AMPK).23 Additionally, PGC-1α and other transcription factors associated with MB can be activated by NO/cGMP and calcium-dependent signaling.24 In summary, these diverse signaling inputs allow exquisite control of mitochondrial homeostasis to meet cellular energy demands and to maintain proper cellular function.
The Importance of MB in Disease
Because mitochondria regulate many processes within cells, mitochondrial dysfunction or disruptions in mitochondrial homeostasis lead to severe deficits in cellular functions.1–2 Injury to mitochondria following ischemia reperfusion injury, toxicant exposure, or severe inflammatory response leads to deficient ATP and disruption of ion homeostasis. Additionally, mitochondrial stress increases superoxide anion production and which causes damage to proteins and lipid membranes. These mitochondrial derangements disrupt cellular repair, proliferation, and differentiation status and increase cell death.
Mitochondrial dysfunction has been implicated in numerous acute and degenerative disease processes, such as myocardial infarction,25 stroke,26 and acute kidney injury (AKI).27 These disease states may be attributed in part to the role of mitochondria and oxidative metabolism in cellular differentiation as observed in neurons,28 myocytes,29 and immune cells.30 Chronic conditions causally linked to such acute insults (such as chronic kidney disease and heart failure) are also characterized by persistent mitochondrial dysfunction,31–32 suggesting that the lack of mitochondrial recovery after an acute injury can also lead to chronic degenerative disease. For example, deficits in PGC-1 family proteins have been associated with the development heart failure in both animal models and human patients.33–34 Interestingly, mice that overexpress PGC-1 proteins also exhibit abnormal cardiac function,35 indicating that a tight control over mitochondrial content is necessary for normal organ function. Similarly, animal models of chronic kidney disease demonstrate diminished renal mitochondrial function,36 and animal models of mitochondrial dysfunction demonstrate chronic kidney disease.37 Finally, human patients with chronic kidney disease have decreased mtDNA in skeletal muscle and peripheral mononuclear blood cells,36 suggesting that mitochondrial defects in a single organ can lead to global mitochondrial dysfunction.
Other chronic diseases also have been associated with disruption of mitochondrial homeostasis. Type II diabetes mellitus and metabolic syndrome are characterized by mitochondrial dysfunction associated with insulin resistance.38 In metabolic syndrome, pancreatic beta cells exhibit increases in UCP2, decreased ATP synthesis, and increased levels of ROS.39–40 Additionally, reductions in complex IV of the electron transport chain have been associated with the development of diabetes in obese mice and patients.41 Furthermore, epigenetic silencing of electron transport chain genes and mtDNA,42–44 along with genes associated with MB such as PGC-1α and TFAM,45–46 lead to decreased mitochondrial content and a greater proportion of dysfunctional mitochondria, thereby causing sustained deficiencies in cellular respiration.
Multiple neurodegenerative diseases also have been associated with decreased mitochondrial mass, altered mitochondrial dynamics, and dysregulation of MB. Parkinson disease has been linked to a panoply of mutations that lead to mitochondrial dysfunction. Defects in PINK1 and Parkin disrupt clearance of damaged mitochondria, permitting accumulation of oxidative damage in dopaminergic neurons and suppression of PGC-1α and decreased cellular respiration.47–50 Mutations in DJ-1 increase ROS while decreasing anti-oxidant defenses,51 leading to decreases in mitochondrial membrane potential, poor mitochondrial quality control, and altered mitochondrial morphology. Similarly, mutations in mTDNA,52–55 TFAM,56 mortalin,57 and α-synuclein58 lead to increased susceptibility to ROS and subsequent mitochondrial dysfunction. Additionally, huntingtin mutants associated with Huntington’s disease bind to the PGC-1α promoter and prevent its transcription and the transcription of other nuclear transcription factors associated with MB, including CREB.59–60 Huntingtin mutations also cause impaired mitochondrial calcium handling,61 reduced respiration,62–63 and disrupted mitochondrial dynamics.64–65 Finally, genetic and toxicant-induced models of Alzheimer disease and samples from human patients confirm the suppression of mitochondrial proteins and the MB transcriptome in Alzheimer disease,66–67 along with mtDNA damage and disruptions in mitophagy and mitochondrial morphology.68–70 Thus, compounds that induce MB may alleviate cellular dysfunction associated with acute and chronic degenerative diseases and promote organ repair and recovery that leads to improvements in patient health.71
Natural Products
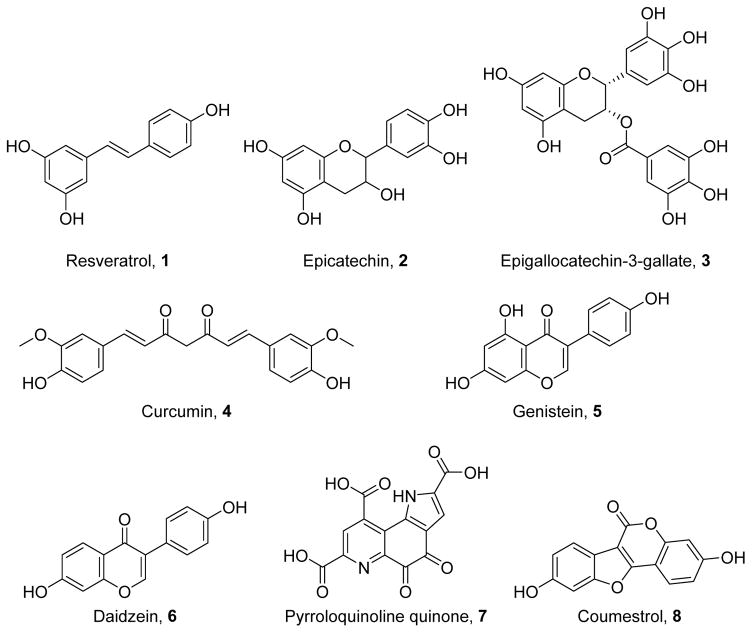
Because mitochondria and oxidative stress are associated with aging, populations with longer lifespans have been studied to identify a potential means for preventing deleterious effects of aging. These studies have identified multiple chemicals capable of inducing MB (Figure 3), and these compounds have shown efficacy in multiple disease models by modulation of multiple signaling axes. Nonetheless, their therapeutic applicability in many cases is limited by poor absorption and low oral bioavailability.
Figure 3.
Naturally occurring polyphenols capable of inducing MB.
Resveratrol
A widely studied nutritional activator of MB is the polyphenol resveratrol (1).72 Compound 1 has been shown to induce MB by activating SIRT1 directly or indirectly through AMPK.73 SIRT1 in turn deacetylates PGC-1α and allows it to exert its transcriptional effects. In particular, 1 activates AMPK by inhibiting components of the electron transport chain such as complex I and F1/F0 ATPase.74–75 Docking studies with complex I suggest that resveratrol binds to the NAD+ binding site of complex I through pi stacking interactions with its aromatic components and by hydrogen bond interactions through its hydroxyl group.75 When binding F1/F0 ATPase, 1 prevents rotation of the ATP synthase complex through a network of hydrophilic and hydrophobic interactions.74 Compound 1 can also directly activate PPARα via interactions with the 4′-hydroxyl group.76 It also activates PPARγ by interactions between R280 and its 4′-hydroxyl group near the opening of the ligand binding pocket as well as Van der Waals interactions with F264, H266, and R288.77 Together, protein-ligand interactions trigger signals that induce MB.
In models of diabetic cardiovascular disease, 1 induces MB and restores vascular reactivity in vitro and in vivo.78 In cellular and animal models of neuronal radiation damage,79 Alzheimer disease,80 Parkinson disease,81 and Huntington’s disease,82 1 normalizes mitochondrial function and rescue cellular viability and function. Compound 1 also attenuates oxidative stress in fibroblasts from patients with Complex I deficiency by increasing SOD2 in a SIRT3-dependent manner.83 Human clinical trials using 1 demonstrated improved lipid profiles, antioxidant defenses, and vascular reactivity in diabetic and obese subjects;84–89 however, there are conflicting data regarding the effect of 1 on insulin sensitivity,84, 88, 90 and 1 had no effect in non-obese subjects.91
Epicatechins
(−)-Epicatechin (2),92 primarily found in cocoa, has been shown to induce MB through multiple signaling pathways, including Akt-dependent nitric oxide (NO) generation,93–94 CREB phosphorylation, and δ-opioid receptor activation.95 The epicatechin epigallocatechin-3-gallate (3),96 promotes cAMP-dependent signaling to increase SIRT1 and PGC-1α.97 Although there are limited data regarding the structural basis for 2 activation of cAMP-dependent signaling, Akt-dependent signaling is mediated by the 3″-, 3′-, and 4′-hydroxyl groups.98 Following oxygen-glucose deprivation, neuronal viability is rescued by 2 via the Akt-eNOS pathway and CREB activation.94 In a mouse model of diabetes, 2 reduces oxidative stress in cardiac tissue by inducing MB.99 Similarly, in mouse models of cardiovascular disease, 2 acts through the δ-opioid receptor to prevent mitochondrial swelling and to increase respiration;95, 100 it can also decrease cardiac ischemia-reperfusion injury through NO and cGMP generation. Even in aged mice, epicatechin increases expression of mitochondrial and antioxidant proteins.101 Through its cAMP-dependent activation of SIRT1 and PGC-1α, 3 enhances MB in Down’s syndrome patient fibroblasts and enhances mitochondrial calcium handling by modulating mitochondrial tethering to the rough endoplasmic reticulum.97 Compound 2 also induces MB in human diabetic patients to improve skeletal muscle metabolism.102
Curcumin
Curcumin (4),103 a diarylheptanoid found in turmeric, has shown promise for promoting MB and improved function in several disease models. By activating multiple signaling molecules, including p38, PKA, AMPK, SIRT1, and NRF2, 4 can induce MB and protect cells against injury.104–106 The o-methoxy group in compound 4 is important for increasing p38-mediated HO-1 expression, which confers cytoprotection in endothelial cells.104 The unsubstituted 5′- and 5″-positions and its olefinic system allow 4 to inhibit NF-κB and activate the NRF2 pathway.107 In cellular models of metabolic syndrome, 4 rescues hepatic mtDNA, NRF1, and TFAM and reduces inflammation and NFκB activity.108 In white adipose tissue, 4 increases browning and markers of MB via increases in norepinephrine and β3 adrenergic receptor expression.109 Pretreatment with 4 improves mitochondrial membrane potential, oxygen consumption rates, and survival in cellular models of Parkinson disease.110 Compound 4 attenuates neuronal death and reduces infarct size following cerebral ischemia-reperfusion injury with concomitant increases in mitochondria and improvements in neurological function.111 In animal models of metabolic syndrome, 4 restores hepatocyte mitochondrial function to reduce hepatosteatosis.112 Following gentamicin-induced nephrotoxicity, 4 can increase PGC-1α and NRF2, thereby elevating mitochondrial protein expression and improving mitochondrial structure.105 In rat skeletal muscle, 4 increases mtDNA content and mitochondrial protein expression following endurance training via PKA-dependent activation of AMPK, SIRT1, and PGC-1α.106
Phytoestrogens
Phytoestrogens, such as genistein (5),113 daidzein (6),114 pyrroloquinoline quinone(7),115 coumestrol (8),116 and equol (9),117 are natural products often found in legumes such as soybeans. They have been shown to exert their effects in part by modulation of estrogen receptors and partly via activation of SIRT1.118–120 5-hydroxyl groups prevent SIRT1 activation, whereas 7-hydroxyl groups are necessary for SIRT1 activation. Similarly, a 3-phenyl group appears to drive increased SIRT1 expression.120 Compounds 5–8 have been shown to induce MB in vitro.120–122 Additionally, through their biogenic effects, 5 and 6 rescued cultured renal proximal tubule cells from oxidant injury.120 In vivo, 5 and 9 induce MB to improve bioenergetics in ovariectomized mice.123–124 Both 5 and 6 increase mitochondrial markers with associated improvements in insulin sensitivity and glucose metabolism in diabetic mice.125–126 Compound 5 also reduces the size of a myocardial infarct in mice by rescuing mitochondrial function.118 Finally, 7 stimulates MB in both wild type mice and transgenic models of Alzheimer disease;127–128 in the latter model, improvements in synaptosomal bioenergetics are correlated with cognitive improvement.
Transcription Factor Modulators
Although natural products have been useful in identifying biological targets for MB, their poor pharmacokinetic parameters limit their therapeutic potential. Modulators of the transcriptional machinery responsible for MB can potently and efficaciously induce MB; however, because they activate transcriptional programs other than MB, these compounds can have severe side effects that limit their clinical utility. Thiazolidinediones
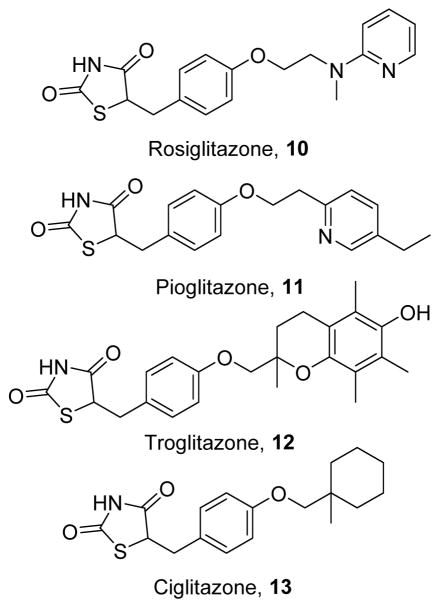
The thiazolidinediones (TZDs) are a class of hypoglycemic drugs used to treat diabetes mellitus that includes rosiglitazone (10),129 pioglitazone (11),130 troglitazone (12),131 and ciglitazone (13) (Figure 4).132 Classically, they act as agonists of the transcription factor peroxisome PPARγ, leading to increased insulin sensitivity. These effects are primarily mediated by the acidic head group, which engages in necessary hydrogen bonding interactions with PPARγ to stabilize its active conformation.133–134 More recently, acute PPARγ-independent effects of TZDs have been discovered, including inhibition of the electron transport chain, which reduces the ATP/AMP ratio, leading to AMPK activation and subsequent MB.135–137 TZDs have also been shown to exert anti-inflammatory effects and to upregulate the mitochondrial stress-response, leading to increased anti-oxidant defenses.135 Although they upregulate multiple signaling pathways, the capacity of TZDs to sensitize tissues to the effects of insulin has been shown to correlate with increased expression of mitochondrial proteins, suggesting that induction of MB may be central to the clinical efficacy of these drugs.138
Figure 4.
Thiazolidinedione inducers of MB.
In vitro, 10–13 increase cell viability and improve neuronal function in models of ischemic injury,139 Alzheimer disease,140 Huntington’s disease,141–142 and multiple sclerosis.143 Similarly, in animal models of neurodegenerative diseases, 10 and 11 improve both cellular and behavioral markers of neurological function.144–145 In animal models of cardiac disease, 10 can rescue cardiac mitochondrial function following septic injury;146 however, other studies indicate that 10 increases cardiac ROS and can be arrhythmogenic.147–148 In models of metabolic syndrome, 10–13 induce MB in adipose tissue,15, 149 pancreatic beta cells,150 and skeletal muscle137, 151 to enhance insulin sensitivity. In humans, 11 induces MB in subcutaneous adipose tissue,152 and 10 can do so in skeletal muscle.153
Estrogens
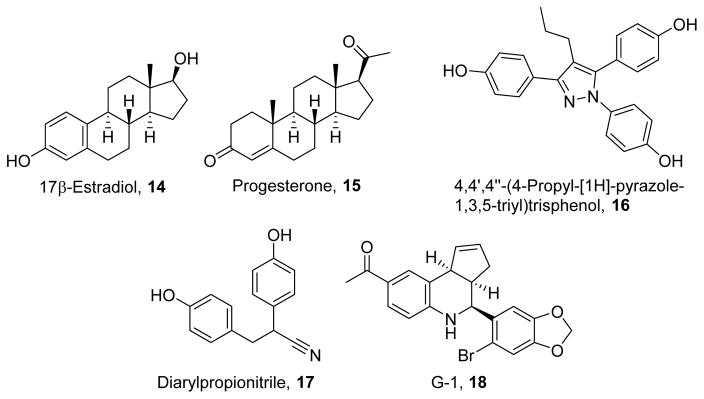
To understand the underlying processes responsible for sex-dependent differences in lifespan and oxidative stress, multiple groups reported that estrogens can be protective in various tissues. Furthermore, reduced levels of estrogens, such as in ovariectomized mice, lead to increased ROS production.154 Estrogens (Figure 5) can bind to the transcription factors estrogen receptor α (ERα) and estrogen receptor β (ERβ) to directly influence gene expression. 17β-Estradiol (14)155 and progesterone (15)156 are the principle biologically active estrogens. 14 and 15 interact with nuclear estrogen receptors by hydrogen bonding interactions between the ligands’ hydroxyl groups and the receptors’ polar residues and by hydrophobic interactions with the receptors’ binding pockets.157 ERα-selectivity, such as by the selective ligand 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (16),158 is mediated by steric bulk to interact with a residue found in ERα but not ERβ.158 Selectivity for ERβ by diarylpropionitrile (17)159 is mediated by phenolic groups, while its efficacy is improved by its nitrile group.159 Recently, it has also been shown that estrogens activate plasma membrane-bound estrogen receptors such as the G protein-coupled estrogen receptor (GPER). The GPER-selective ligand G-1(18)160 is structurally similar to 14 but is unable to form hydrogen bonds in the nuclear estrogen receptors;160 however, 18’s acetyl group and pseudosymmetry allows engagement of specific residues of the GPER to stabilize the active conformation.161–162
Figure 5.
Estrogen inducers of MB.
Compound 14 has been shown to induce MB in immortalized cell lines and in a cellular model of Leber hereditary optic neuropathy, a mitochondrial disease. 163–164 In animal models, 14 normalizes ROS production, increases antioxidant defenses, and enhances respiratory capacity in the heart and brain.154, 165 Furthermore, 15 and synthetic estrogen receptor agonists such as 16 and 17 have been shown to enhance respiratory capacity in the brain and promote clearance of lipid peroxidation products.166 Of note, the use of receptor subtype selective agonists suggests that ERα and ERβ differentially regulate the expression of electron transport chain proteins. Additionally, at least a portion of the cardioprotective effects of estrogen are mediated through the GPER, as shown by stimulation with the GPER-selective agonist 18.154 Despite the clear protective potential of estrogens, their proliferative and endocrine effects limit their use as a long-term therapy for chronic degenerative diseases. However, the development of selective ER and GPER ligands that drive specific signaling and transcriptional programs may improve the utility of such therapeutics.
SIRT1 activators
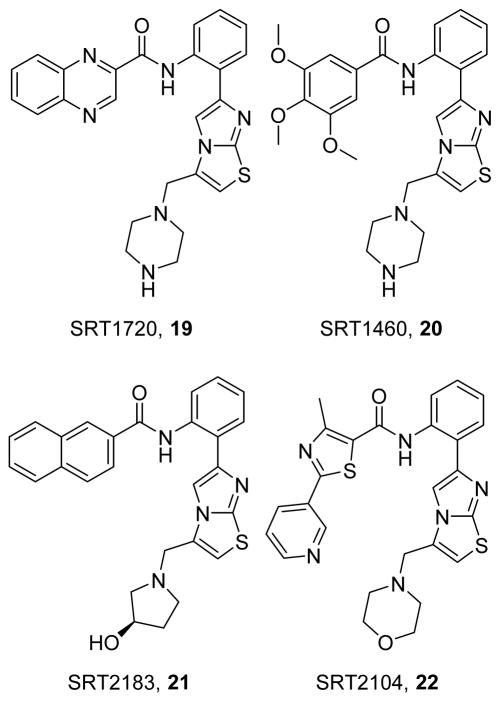
The identification of SIRT1 as a common target of natural product-induced increases in PGC-1α led to the development of multiple SIRT1 activators, such as SRT1720 (19),167 SRT1460 (20),167 SRT2183 (21),167 and SRT2104 (22) (Figure 6).168 In the initial synthesis of SIRT1 activators,169 the basic methylamino ring at C-3 of the imidazothiazole ring of 19 and 20 enhanced water solubility, while derivatization of the amide group (such as with the 2-quinoxaline group of 19) improved potency and efficacy. Interestingly, both 19 and 20 share a methylamino ring and have greater efficacy, whereas 19 and 21 have a 2-quinoxaline group and more potency,167 suggesting that the two groups may play distinct roles in the pharmacodynamic qualities of these compounds. The direct mechanisms of action for the sirtuin class have been controversial. Assays with isolated fluorescent peptides were used for optimization, but direct proteomic assays indicate that 19–21 do not directly activate SIRT1 and, rather, act promiscuously to activate or inhibit numerous targets;170 however, other work has shown that these compounds directly activate SIRT1 by binding to amino acid E230.171
Figure 6.
Activators of SIRT1 that induce MB.
Due to numerous SIRT1 targets, these activators can affect various cellular processes, including inflammation, lysosomal trafficking, and metabolism. Among its targets, SIRT1 deacetylates PGC-1α, facilitating nuclear import of and transcriptional regulation by PGC-1α, leading to MB. In models of type II diabetes mellitus, SIRT1 activators have been shown to improve lifespan, normalize pancreatic morphology, improve insulin, glucose, and fatty acid metabolism and increase mitochondrial markers;167, 172–174 however, other studies have shown a lack of efficacy in diabetic mice, calling into question the beneficial effects of these compounds.170 With respect to neurodegenerative diseases, SIRT1 activators prevent neurodegeneration and restore MB in animal models of Huntington’s disease and multiple sclerosis.175–176 SIRT1 activation has shown promise in renal disease, restoring renal function after AKI and preventing renal medullary damage in obstructive nephropathy.177–179 In models of cardiovascular disease, 19 reduces the size of myocardial infarction and preserves contractility,180 as well as reducing ROS and improving contractility in mice with enhanced ALDH2 activity.181 Compound 19 also preserves endothelial function in aged mice.182 Even in healthy animals, 19 and other SIRT1 activators have been shown to extend lifespan and “healthspan” by preventing the development of age-associated diseases in multiple organ systems.183 In human trials, 22 improved lipid profiles in diabetic patients but did not affect plasma glucose or insulin, likely due to large pharmacokinetic variability.184 Additionally, 22 reduces cholesterol, LDL, and triglycerides in otherwise healthy smokers,185 suggesting that SIRT1 activation is important to the human healthspan.
Kinase Modulators
Kinases either phosphorylate target proteins or function as scaffolds to co-localize other kinases and targets to regulate cellular signaling. Phosphorylation of specific targets can either activate or inhibit cellular signaling pathways in response to environmental cues. Because they are central signaling molecules, kinases are attractive therapeutic targets. In particular, activators of kinases that induce MB, such as AMPK, can be useful in multiple diseases. Unfortunately, inhibitors are easier to develop, and most kinase modulators are inhibitors. However, inhibitors of kinases that negatively regulate MB, such as extracellular signal-regulated kinases 1/2 (ERK1/2), also provide promise as therapeutics.
AMPK
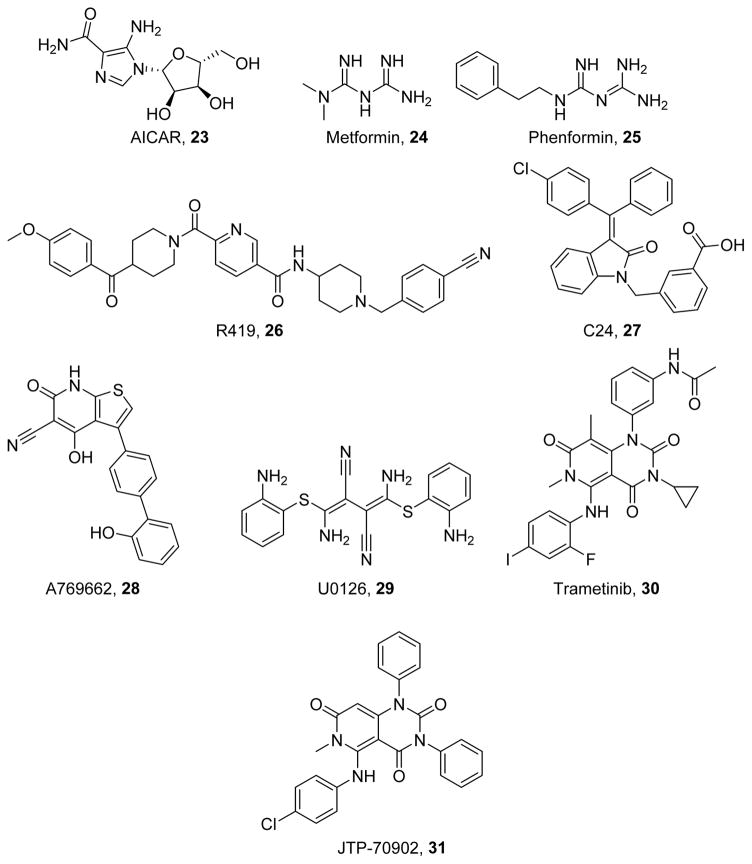
AMPK is an energy sensing kinase involved in the modulation of metabolism through the cellular AMP/ATP ratio. AMPK activation is increased during exercise and induces MB, and it is decreased with aging and during multiple chronic degenerative diseases.186 AMPK activation has been shown to be an upstream regulator of sirtuins and therefore PGC-1α.187 Furthermore, pharmacologic activation of AMPK has been observed with multiple natural products that induce MB. Activators of AMPK (Figure 7), including the indirect activators AICAR (23),188 metformin (24),189 phenformin (25),190 R419 (26),191 and C24 (27),192 and the direct activator A769662 (28),193 have been developed and induce MB in multiple cell lines. Additionally, 23 has been shown to enhance proliferation and increase ATP in models of complex I deficiency and MELAS.194–195 Compound 23 is biotransformed via phosphorylation within the cell and acts as an AMP mimetic to activate AMPK and other AMP-dependent processes.188 The biguanides 24 and 25 activate AMPK in a LKB1-dependent manner and through inhibition of complex I;191, 196 by inhibiting the electron transport chain, the AMP/ATP ratio is increased, leading to AMPK activation. Compound 26 also indirectly activates AMPK via complex I inhibition,191 and 28 activates AMPK by binding to an allosteric site between the alpha and beta subunits of AMPK. 28 both allosterically activates and prevents Thr172 dephosphorylation.197
Figure 7.
Kinase modulators that induce MB.
In models of diabetes and metabolic syndrome, 23 mimics high intensity exercise in skeletal muscle with accompanying increases in SIRT1 activation and PGC-1α activity. These improvements in MB decrease oxidative stress in both renal and endothelial cells,198–200 preventing common comorbidities such as diabetic nephropathy and poor wound healing. Compound 23 can also improve pancreatic morphology via AMPK activation to enhance insulin sensitivity and GLUT4 expression,201 thereby decreasing plasma glucose. In hepatic cells, 27 reduces lipid biosynthesis to prevent lipid accumulation and preserve hepatic function.192 In humans with gestational or type II diabetes, 23 and 25 prevents insulin resistance in multiple tissues.202–204 In the heart, 23 reduces oxidative stress and improves contractility,181 and it is associated with improvements in insulin sensitivity in diabetic mice as well as reductions in cold ischemic injury in mouse models of heart transplant.205
AMPK activators have also shown promise for treating neurodegenerative diseases. Neuronal activity has been shown to drive PGC-1α and NRF-1 expression in an AMPK-dependent manner,206 leading to MB, and pharmacologic activation of AMPK has been shown to mimic these effects. Compound 23 has also been shown to impact neuronal development by promoting mitochondrial accumulation at axonal branch points, thereby facilitating branch formation and retention.207 In models of Alzheimer disease, 23 ameliorated mitochondrial dysfunction and prevented neurotoxicity and tau hyperphosphorylation.208–209 Compound 23 decreased amyloid beta, a protein implicated in Alzheimer disease, in a PPARγ dependent manner.210 Compound 23 has also been shown to decrease inflammation in models of multiple sclerosis, attenuating pathological and behavioral changes. Furthermore, in models of ischemic brain injury, 23 diminishes ischemic neuronal damage.211
ERK1/2
Another means of inducing MB is the inhibition of negative regulators of MB, such as ERK1/2. Following its activation by MEK1/2, ERK1/2 regulates a variety of cellular processes, including differentiation, apoptosis, survival, proliferation, and motility.212 Inhibition of MEK by U0126 (29)213 or trametinib (30)214 leads to a rapid suppression of ERK1/2 phosphorylation (Figure 7). Compound 29 can exist in the (Z,Z) or (Z,E) isomer; however, the (Z,Z) isomer provides better MEK inhibition, as does the presence of electron donating amino groups at o-positions of its phenyl groups.213 The iodo- and cyclopropyl groups of Compound 30 improve potency for cancer cell growth inhibitory activity over its lead compound JTP-70902 (31)214, while its methyl groups improve stability and its acetamide group improves solubility.214 ERK1/2 has been shown to suppress PGC-1α in melanoma cells.215 Additionally, in models of Parkinson disease ERK1/2 activation leads to phosphorylation of TFAM, impairing its ability to bind to mitochondrial DNA.216 MEK1/2 inhibitors, such as 29 and 30, have been developed for cancer chemotherapy. In vitro models of renal oxidative stress indicate that ERK1/2 is a mediator of oxidative damage in proximal tubule cells, and that its inhibition by 29 prevents oxidative damage.217 Our laboratory has shown that ERK1/2 activation increases after AKI and that pre-treatment with the MEK1/2 inhibitor 30 rescues mitochondrial function and restores renal function in a mouse model of AKI.218 These data indicate that inhibition of suppressors of MB can induce MB and restore organ function following injury.
Cyclic Nucleotide Modulators
The cyclic nucleotides cGMP and cAMP are cellular second messengers that are generated in response to extracellular signals. They activate downstream kinases or are hydrolyzed by phosphodiesterases (PDE). NO increases cGMP synthesis by binding to a heme group on soluble guanylate cyclase (sGC), while cAMP is increased through activation of adenylate cyclase by the stimulatory G-protein Gαs. Because cyclic nucleotide generation is disrupted in multiple pathological states, cyclic nucleotide modulators are attractive targeted therapies for the induction of MB in various diseases.
NO-cGMP-PKG Axis
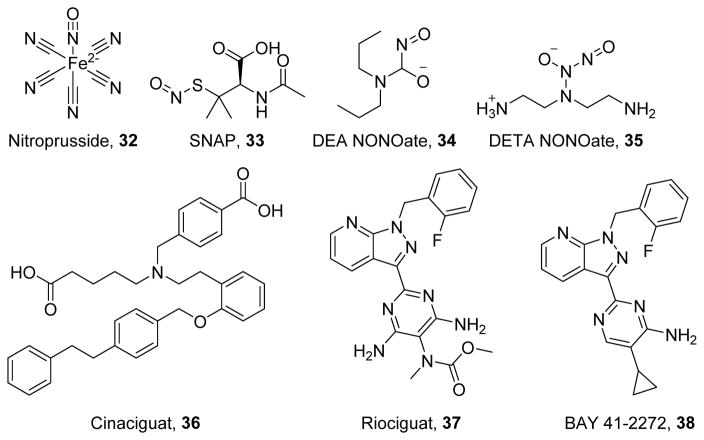
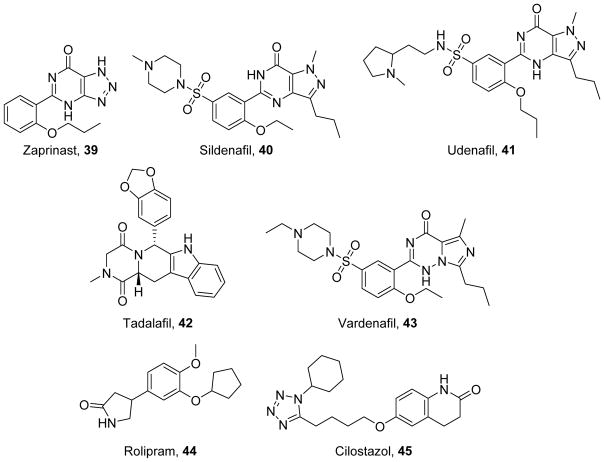
The NO-cGMP-PKG pathway can be modulated by: 1) nitric oxide (NO) donors, such as sodium nitroprusside (32), (±)S-nitroso-N-acetylpenicillamine (SNAP, 33),219 diethylamine NONOate (DEA-NONOate, 34),220 and diethylenetriamine-NONOate (DETA-NONOate, 35)220 which increase cellular NO (Figure 8); 2) sGC stimulators and activators, such as cinaciguat (36),221 riociguat (37),222 and BAY 41-2272 (38)223 which directly increase cGMP production (Figure 8); and 3) phosphodiesterase (PDE) inhibitors, such as zaprinast (39),224 sildenafil (40),225 udenafil (41),226 tadalafil (42),227 and vardenafil (43)228 which increase cGMP by preventing its hydrolysis (Figure 9). Clinically, these compounds are used to induce vasodilation to treat hypertension or erectile dysfunction. Activation of this pathway has been shown to increase PGC-1α and stimulate MB both through the activation of PKG and nitrosylation of transcription factors to increase their binding to the PGC-1α promoter.229–230
Figure 8.
Activators and stimulators of the NO/cGMP pathway.
Figure 9.
Phosphodiesterase (PDE) inhibitors associated with MB.
As their name implies, all NO donors have a group, usually a nitrate or a furoxan group, that can be liberated to form NO. Because the NO donating group is small, NO donors can be “fine-tuned” for multiple clinical uses and to slow the rate of NO release.231–232 However, because NO generation causes such a dramatic drop in blood pressure, NO donors are of limited clinical use. However, these compounds readily confirm the importance of NO for preventing metabolic derangements and cell death, particularly in skeletal muscle. In hypoxia, dietary nitrate (a natural NO donor) prevents PGC-1α suppression, leading to increases in fatty acid oxidation and respiration. Even under normoxic conditions, nitrate stimulates MB in a cGMP/PKG-dependent manner.233 Compound 33 has also been shown to induce MB in myoblasts and reduce the effects of caspase-dependent and –independent apoptotic molecules,234 and 34 also improves synaptic conduction in models of Alzheimer disease in a cGMP-dependent manner.235
sGC activators and stimulators increase the activity of sGC in the absence of NO. Stimulators such as 37 and 38 increase sGC activity with a non-oxidized heme group, whereas activators increase sGC activity even if the heme prosthetic group is oxidized. Both classes of compounds have been approved for clinical use to treat pulmonary hypertension. Compound 38 was optimized for vasorelaxation through the addition of a 2-fluoro-phenyl group, a pyrazolo[3,4-b]pyridine ring, and a cyclopropyl group.223 Compound 37 was optimized to increase oral bioavailability and half-life, and to reduce clearance via amino and N-methylcarbamate substitutions on the pyrimidine group.222 On the other hand, sGC activators have shown greater utility beyond blood pressure control, likely due to their capacity to activate sGC even under high oxidative stress. Compound 36 was identified using a high-throughput screen and was confirmed to displace the heme of sGC by interacting with its YXSXR motif through carboxylic acid moieties.236 In pre-clinical studies, compounds 36–38 improve cardiac, renal, and neurological function across multiple disease models including ischemia reperfusion injury, sepsis, diabetes, and Alzheimer disease.237–241 However, despite the efficacy of cGMP in promoting MB, few studies have examined the role of MB in these functional improvements. Compound 36 protects against myocardial infarction by increasing H2S, a known inducer of MB,242 suggesting that further investigation is warranted into the role of MB in these compounds’ protective effects.
Inhibition of cGMP-selective PDEs prevents cGMP hydrolysis, promoting its accumulation in the cell and facilitating stimulation of MB. Compound 40 was designed from 39 by mimicking the guanosine dipole moment, adding an ethoxy group to improve potency, and adding a piperazine sulfonamide to improve solubility, selectivity, and potency.243 However, both 40 and 41 discriminate poorly between PDE5 and PDE6, leading to visual side effects.226 Compound 42 has better selectivity for PDE5 over PDE6 with the addition of more electron donating groups; however, relative to 40 and 43, 42 is less selective for PDE11.227, 244–245 Although these compounds have been extensively developed for treating pulmonary hypertension and erectile dysfunction, they also have been tested for treating other diseases.
Because cGMP-selective PDE inhibitors were designed to reduce blood pressure via increased vasodilation, it is reasonable that they have been tested for conditions characterized by endothelial dysfunction, such as diabetes. As expected, in models of diabetes, 40 improves endothelial function as measured by flow-mediated dilation.246–247 In addition to their effects on vascular reactivity, 40, 42, and 43 reduce plasma markers of diabetes, such as lipids, serum glucose, and HbA1c, and are associated with improvements in mitochondrial content.248–251 In adipocytes and hepatocytes, 40 enhances lipid oxidation and increases insulin tolerance and cellular morphology.248 cGMP-selective PDE inhibitors also reduce diabetic complications in other organs, such as the kidney and heart. In models of diabetic nephropathy, 40 reduces microalbuminuria, a predictor of renal and cardiac dysfunction.249 Additionally, in diabetic mice, 42 rescues the expression of cardiac cytoskeletal and redox proteins to improve cardiac morphology and function.251–252
In addition to beneficial reductions in the development of diabetic cardiomyopathy, cGMP-selective PDE inhibitors also ameliorate non-diabetic cardiac dysfunction. In ischemic cardiomyopathy and myocardial infarction, 40, 42, and 43 increase survival and decrease infarct size by reducing cell death and preserving mitochondrial function.253–255 Compound 42 also prevents cardiac remodeling and hypertrophy, stabilizing contractility rather than allowing progression to heart failure and pulmonary edema.256 Similarly, in models of mitral regurgitation and doxorubicin toxicity, 40 inhibits cell death and preserves mitochondrial function by upregulating anti-apoptotic proteins and maintaining the mitochondrial membrane potential.257–258
cAMP-PKA-CREB axis
CREB regulates PGC-1α activity and expression to promote MB and is down-regulated in multiple disease states characterized by mitochondrial dysfunction. In Alzheimer disease, CREB phosphorylation is diminished due to impaired activation by PKA. This loss of activity leads to a downregulation of PGC-1α and an imbalance in tau protein, a driver of Alzheimer disease.259 A similar decrease in CREB activity has been observed in Huntington’s disease.260 Additionally, ethanol decreases cellular cAMP, thereby reducing CREB activity to suppress PGC-1α and thereby exert its toxic effects.261 Taken together, these data indicate that activation of the cAMP-PKA-CREB signaling pathway can promote MB and protect against neurodegenerative diseases.
The primary therapeutic approach for activating this signaling axis is with phosphodiesterase (PDE) inhibitors such as rolipram (44)262 and cilostazol (45)263 (Figure 9). Compound 44 inhibits PDE4, a cAMP-selective PDE, whereas 45 inhibits PDE3, a PDE capable of hydrolyzing both cAMP and cGMP; however, PDE3’s Vmax for cAMP is substantially higher than that of cGMP. Compound 44’s selectivity arises in part from its optimized potency for PDE4 and the unfavorable orientation of a conserved glutamate residue in other PDEs.264 In contrast, the lactam group of 45 engages in hydrogen bonding interactions with multiple receptor residues to promote PDE3 selectivity.265 Both 44 and 45 can increase PGC-1α in vitro, indicating that they induce MB,266 and both have shown potential for therapeutic use in pre-clinical disease models. However, in humans, 44’s narrow therapeutic window limits its application, whereas 45 is approved for clinical use in the treatment of diabetic vascular complications.
Restoration of the cAMP-PKA-CREB pathway substantially reduces the effects of neurodegenerative diseases. In animal models of Huntington’s disease, 44 improves neuronal function, morphology, and survival and decreases neurological impairment.260, 267 Compound 44 also reduces synaptic conduction abnormalities associated with Alzheimer disease, improving cognition.268–269 Interestingly, these effects and increased CREB phosphorylation lasted beyond the cessation of treatment. In ischemic brain injury, 45 reduces neuroinflammation, reducing infarction size and decreasing apoptosis and free radical production.270–271 In models of Alzheimer disease, 45 increases SIRT1 expression, reducing symptoms and improving cognitition.272 Furthermore, in a retrospective study, 45 improved cognition in human patients,273 suggesting that PDE3 inhibition holds promise for treating Alzheimer disease.
Used clinically to treat claudication, the beneficial effects of 45 in models of diabetic cardiovascular disease are well studied. In models of limb ischemia, 45 increases angiogenesis by rescuing PPARγ, increasing angiogenic factors vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF);274–275 this normalization of PPARγ also occurs in other tissues, such as the retina and the kidney.276 Compound 45 also prevents endothelial cell senescence by increasing cAMP, leading to SIRT1 activation. In the heart, 45 reduces oxidant-induced mitochondrial dysfunction and significantly reduces myocardial infarction size.277–279 Furthermore, 45 improves insulin sensitivity and reduces blood glucose and HbA1c in diabetic mice and human subjects,280–282 as well as reducing the urinary excretion of albumin and renal inflammation, indicating that 45 improves diabetic nephropathy.
Despite these promising data, controversy exists regarding use of cAMP-selective PDEs in chronic degenerative diseases of the liver and kidney. On the one hand, 45 improves hepatic function after ischemic insult by inducing MB;283 however, in models of lipotoxicity, increased cAMP acts synergistically to induce cell death despite concurrent stimulation of MB.284 Additionally, despite the promising work in diabetic nephropathy described previously, we found that cAMP-selective PDE inhibitors do not induce MB in proximal tubule cells,285 suggesting they are poor therapeutic options for treating AKI.
GPCR Ligands
G protein-coupled receptors (GPCRs) are well characterized plasma membrane receptors that are the target of a substantial portion of currently available drugs. By coupling to G proteins, GPCRs can modulate cAMP, calcium, and NO and activate various kinases and signaling pathways. Additionally, different ligands of the same receptor can cause activation of distinct signaling programs, a phenomenon known as “functional selectivity” or “biased agonism.”286 By stabilizing different receptor conformations, different ligands can alter receptor interactions with G proteins, G protein-coupled receptor kinases (GRKs), and scaffolding proteins such as arrestins. One such scaffolding protein, GRK interacting protein 1 (GIT1), regulates MB in the heart, likely in an eNOS-dependent manner.287–288 Biased agonism allows for the development of ligands that selectively stimulate signaling pathways that lead to MB while inhibiting negative regulators of MB. Many GPCRs are modulated by endogenous molecules, a fact which has facilitated the development of potent and selective agonists and antagonists for various receptors. Despite the potential of GPCRs to activate pathways known to induce MB and the availability of clinically approved GPCR ligands, little investigation has occurred to explore the potential of such compounds to induce MB.
Cannabinoid-1 receptor
Cannabinoid-1 receptor (CB1R) antagonists such as taranabant (46)289 and rimonobant (47)290 were studied for anorectic effects (Figure 10). Despite the lack of a cyclic linker, 46 binds in a similar mode to 47; however, the amide group on 46 is able to engage in an extra hydrogen bonding interaction, leading to its enhanced affinity for the CB1R.289, 291 By inhibiting CB1R activity in the brain, these compounds can suppress appetite and cause weight loss with concomitant improvements in plasma lipid profiles. Both 46 and 47 were efficacious for inducing weight loss in wild type mice, mice fed a high fat diet, and ob/ob mice.292–293 Inhibition of CB1R by 47 or by genetic ablation induces MB in adipose tissue and MB in a cAMP- and eNOS-dependent manner, leading to decreases in body weight and fat content.292 Interestingly, 47 increased mitochondrial energy consumption did not increase mitochondrial mass in rat livers, indicating improved mitochondrial efficiency.294 Although both 46 and 47 were efficacious in animal models, investigation of 46 was halted in Phase III trials, and 46 was withdrawn from the market in the U.S. after initial approval as an anti-obesity drug. In humans, 47 reduced food intake and increased energy consumption to promote weight loss but caused serious side effects such as suicidal ideation and severe depression.295,296
Figure 10.
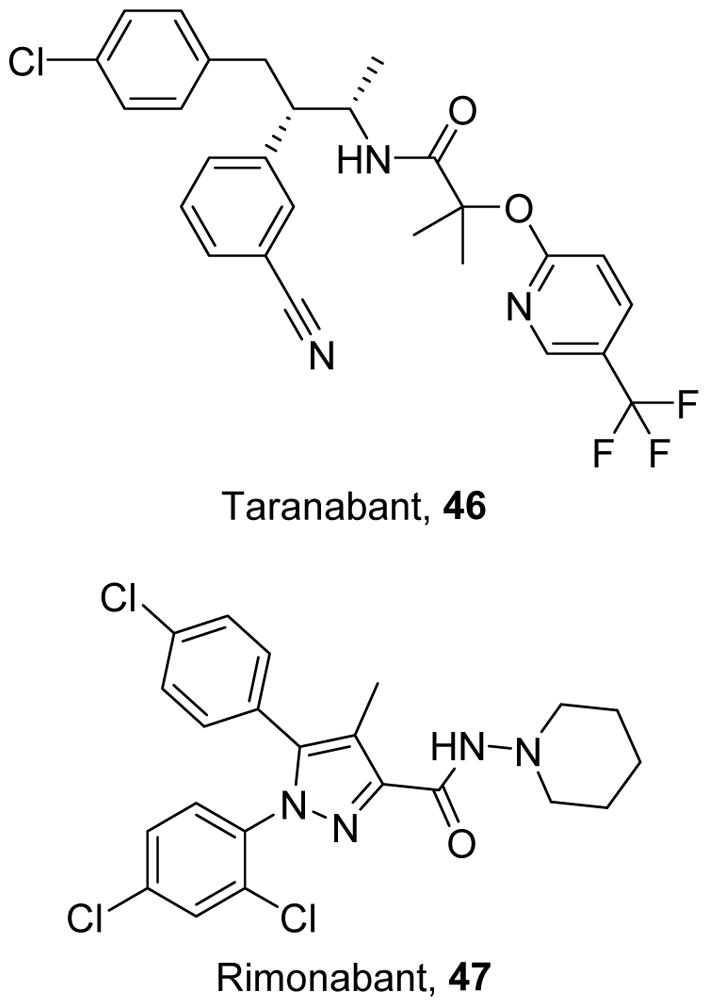
Cannabinoid-1 Receptor antagonists.
5-Hydroxytryptamine receptors
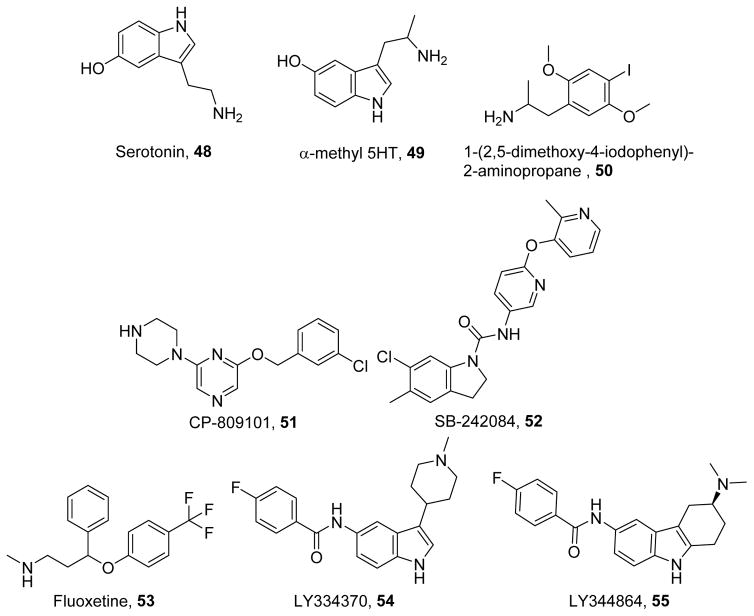
Endogenous serotonin binds to the 5-hydroxytryptamine (5-HT) class of receptors (48, Figure 11)297. 5-HT receptors are primarily GPCRs that have been identified as therapeutic targets for neuropsychiatric, neurologic, and cardiac diseases. The synthetic ligand alpha-methyl-5-hydroxytryptamine (49)298 possesses an extra methyl group that prevents its metabolism by monoamine oxidase.299 The 5-HT2 receptor agonist DOI (50)300 has enhanced selectivity due to its primary amine, with the iodo-group adding to its potency.301 Much work has been done to identify and characterize the pharmacophore of 5-HT2C receptor agonists (e.g., CP809101, 51)302 and antagonists (e.g. SB242084, 52)303 and optimize their selectivity.301–303 5-HT2C receptor agonists stabilize the TM6 domain of the receptor through its aromatic group, whereas antagonists interact with Asn331, Val354, and Ser334 through a positively ionizable group.304
Figure 11.
5-Hydroxytryptamine receptor modulators that induce MB.
In addition to direct 5-HT receptor antagonists, serotonin reuptake inhibitors such as fluoxetine (53)305 prevent the uptake and degradation of 48 and prolong its actions at its receptors. The p-trifluoromethyl group of 53 confers selectivity for the serotonin reuptake transporter by binding to I172 in its transmembrane domain.306–307 Treating rat pups with 53 improves mitochondrial membrane potential, respiratory capacity, and antioxidant defense in the heart, implicating 48 in mitochondrial health during development.308
Our laboratory identified multiple ligands that induce MB through various 5-HT receptors. In renal proximal tubule cells, we have shown that the non-selective 5-HT receptor agonist 49 induces MB.309 The 5-HT2 receptor agonist 50 increased cellular respiration in vitro and improved recovery from oxidant injury by tert-butyl hydrogen peroxide (TBHP); interestingly, induction of MB did not reduce initial injury by TBHP.310 The 5-HT2C selective ligands 51 and 52 induce MB in vitro and in naïve mice; interestingly, siRNA studies and work in knockout mice indicate that the ligands exert these effects through the 5-HT2A receptor.311
In contrast to 5-HT2 receptors, the 5-HT1F receptor has few selective ligands-namely, LY334370 (54) and LY344864 (55) and limited data regarding its pharmacophore. Nevertheless, the selective 5-HT1F agonists 54 and 55 induced MB in vitro, and 55 also improved recovery from ischemia-reperfusion-induced AKI in vivo.309 Additionally, preliminary data suggest that 55 stimulates MB through the Gβγ-dependent activation of Akt and eNOS (Gibbs, W.; Beeson, C.C.; Schnellmann, R.G., unpublished results). These data indicate that the induction of MB by 5-HT agonists could be clinically useful for treating AKI and other acute organ injuries as they effectively promote recovery and regeneration even after initial injury.
Beta adrenergic receptors
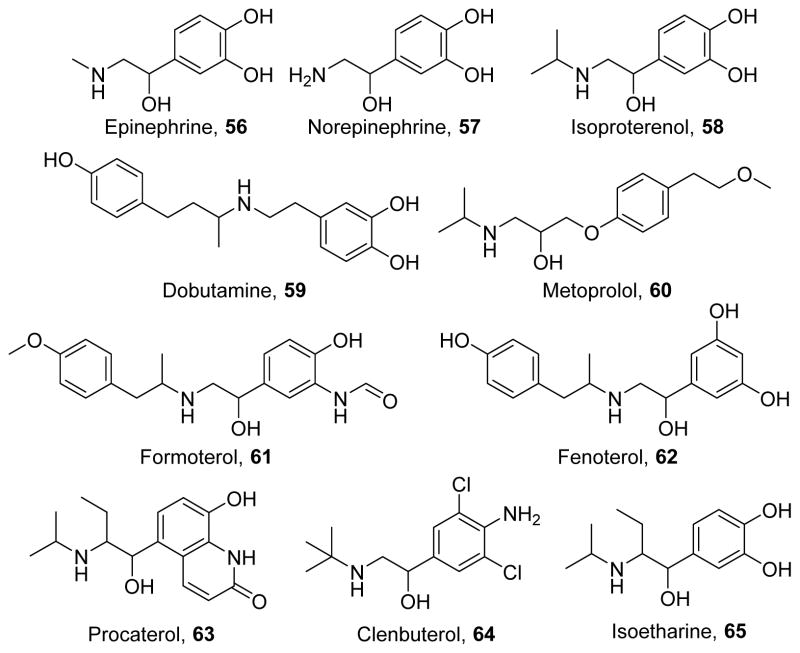
The beta adrenergic receptor family is activated by endogenous stress hormones epinephrine (56)312 and norepinephrine (57, Figure 12)312 and the family comprises three receptors. First, the beta-1 adrenergic receptor, primarily expressed in the heart, is targeted by drugs that affect cardiac contractility and heart rate. The beta-2 adrenergic receptor, which is ubiquitously expressed, is a target of bronchodilators to treat asthma and COPD. The beta-3 adrenergic receptor, which is primarily expressed in adipose tissue and the urinary bladder and is targeted to treat overactive bladder.313
Figure 12.
Beta adrenergic receptor modulators tested for the induction of MB.
Beta-adrenergic agonists contain distinct structural features, specifically a catechol or phenethanolamine core, whereas antagonists have a 3-aminophenoxypropan-2-ol core. However, while beta-adrenergic agonists have been extensively studied to optimize pharmacodynamics and pharmacokinetic parameters, there are few studies relating structural features to the induction of MB. Compounds 56, 57, and the non-selective beta adrenergic receptor agonist isoproterenol (58)314 increase PGC-1α in brown adipose of naïve mice and in models of obesity in a cAMP- and p62-dependent manner.315 Interestingly, in models of cardiac dysfunction, beta-1 adrenergic receptor stimulation by dobutamine (59)316 increases cell death and inflammation,317 but its blockade by the beta-1 selective antagonist metoprolol (60)318 enhances PGC-1α activation and improves cardiac metabolism and function.319–320 Our laboratory has studied beta-2 adrenergic receptor selective agonists in renal MB. In particular, formoterol (61),321 fenoterol (62),322 and procaterol (63)323 induced MB in vitro at pharmacologically relevant doses.324–325 Compound 61 has been confirmed to induce MB in vivo in naïve mice as well as in mice subjected to AKI,326 and this was associated with improvements in renal function, indicating that formoterol has therapeutic promise for treating AKI. However, other beta-2 adrenergic receptor agonists such as clenbuterol (64)327 and isoetharine (65)328 did not induce MB in vitro,324 suggesting that biased agonism can be exploited to develop more effective mitochondrial biogenic beta-2 adrenergic receptor agonists. Because both MB-inducing and non-MB-inducing beta-2 adrenergic receptor agonists increase cAMP, we suggest that the classical Gαs-signaling pathway is not responsible for beta-2 adrenergic receptor-induced MB in the kidney. Preliminary data suggests that 61 but not 64 activates the Akt-eNOS pathway in a Gβγ-dependent manner (Cameron, R.B.; Beeson, C.C.; Schnellmann, R.G., unpublished results). In addition to its renal effects, 61 induces MB in multiple other tissues, including the heart and skeletal muscle.325, 329 Together, these data indicate that certain beta-2 adrenergic receptor agonists such as 61 can be used to treat multiple diseases and improve mitochondrial function and ameliorate symptoms.
Perspectives
Because MB can arise from diverse signaling pathways, a number of drug classes have been identified to induce MB. The earliest identified inducers of MB are natural products, such as 1–9, which are efficacious,73, 97, 111, 120 but MB induction often occurs through multiple signaling pathways and these compounds may activate signaling programs unrelated to MB. Such promiscuity means that these compounds are poor therapeutic agents, particularly for chronic degenerative diseases for which a more targeted approach may be required.
Transcription factor activators such as TZDs (10–13), estrogens (14–18), and SIRT1 activators (19–22) induce MB by activating transcription factors that act on mitochondrial genes.139, 163, 173 This selectivity facilitates the induction of relatively small gene sets. Furthermore, transcription factor modulation can drive the recruitment of a select set of transcriptional machinery, increasing the specificity of the resulting transcriptome. However, ligands with that degree of specificity, particularly for MB, have not yet been designed. Thus, currently, activation of these transcription factors upregulates unwanted genes and causes detrimental neurological and hyperproliferative effects.
Similar to transcription factor modulators, kinase modulators such as 23 and 30 have been developed with a high activity for their targets. Although some kinase activators are available, many kinase inhibitors have been developed and are utilized clinically. These inhibitors will be of particular use as more negative regulators of MB, such as ERK1/2, are identified. Kinase signaling is fairly well-characterized, so acute downstream effects of such modulators are usually predictable. Nonetheless, because kinases have central roles in cellular processes, predicting longer-term effects of such drugs is not straightforward.
Cyclic nucleotide modulators such as sGC stimulators and activators and PDE inhibitors have recently been shown to be efficacious inducers of MB.242, 266, 285, 330 However, as with kinase modulators, these drugs influence central signaling processes, often in a manner that prevents physiological feedback loops to prevent pathological signaling. Additionally, cyclic nucleotides can have tissue-specific effects that can give rise to either injurious or curative effects to different organ systems.
GPCR modulators are the most widely developed and prescribed drug class. Although few of these compounds have been tested for MB induction, several promising classes, have been identified to induce MB, such as cannabinoid, serotonergic, and adrenergic ligands.292, 309–311, 324 These compounds can act through a single target and activate a particular signaling program. 286 Unlike the above-mentioned compound classes, GPCR ligands act at surface receptors and can retain cellular feedback mechanisms to limit signaling if necessary, so GPCR ligands represent promising chemical space for the induction of MB.
In vitro, in vivo, and human studies indicate that induction of MB promotes recovery from disease states among many organ systems due to myriad roles played by mitochondria in both physiological and pathophysiological states. However, relatively few drugs have been identified to induce MB, and much chemical space remains untested for MB. One domain of chemical space that may be promising for phenotypic screens to identify lead compounds is the so-called “dark chemical space,” as compounds derived from this space tend to have high specificity for a given target.331 As more chemical space is investigated for MB, we will gain a better understanding of the role of mitochondria in health and disease and will provide researchers and clinicians with better tools for treating debilitating acute and chronic degenerative diseases.
Acknowledgments
We thank Dr. Jennifer Schnellmann (Medical University of South Carolina) for her thoughtful comments in editing the manuscript. RBC is funded by F30 DK104550 and T32 GM008716 (National Institutes of Health). CCB is funded by P20 GM103542 (National Institutes of Health). RGS is funded by R01 GM084147 (National Institutes of Health) and 1BX000851 (Department of Veterans Affairs).
Funding Sources
RBC is funded by F30 DK104550 and T32 GM008716 (National Institutes of Health). CCB is funded by P20 GM103542 (National Institutes of Health). RGS is funded by R01 GM084147 (National Institutes of Health) and 1BX000851 (Department of Veterans Affairs).
ABBREVIATIONS
- ATP
adenosine triphosphate
- AMP
adenosine monophosphate
- MPTP
mitochondrial permeability transition pore
- MB
mitochondrial biogenesis
- PGC-1α
peroxisomal proliferation activated receptor coactivator-1α
- mtDNA
mitochondrial DNA
- ER
estrogen receptor
- ERRα
estrogen related receptor-α
- NRF-1
nuclear respiratory factor 1
- NRF-2
nuclear respiratory factor 2
- PPAR
peroxisome proliferator-activated receptor
- TR
thyroid hormone
- CREB
cAMP-responsive element binding protein
- YY-1
yin yang-1
- ROS
reactive oxygen species
- PGC-1
peroxisomal proliferation activated receptor coactivator-1
- PGC-1β
peroxisomal proliferation activated receptor coactivator-1β
- PRC
PGC-1 related coactivator
- PKA
protein kinase A
- NO
nitric oxide
- AMPK
growth stimulatory AMP-dependent kinase
- SIRT1
silent mating type information regulation 2 homolog 1
- AKI
acute kidney injury
- Tfam
mitochondrial transcription factor A
- UCP2
uncoupling protein 2
- PINK1
PTEN-induced putative kinase 1
- NAD+
nicotinamide adenine dinucleotide
- SOD2
superoxide dismutase 2
- SIRT3
silent mating type information regulation 2 homolog 3
- eNOS
endothelial nitric oxide synthase
- TZD
thiazolidinedione
- PPARγ
peroxisomal proliferation activated receptor-γ
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- GPER
G protein-coupled estrogen receptor
- ALDH2
aldehyde dehydrogenase 2
- LDL
low density lipoprotein
- ERK 1/2
extracellular signal-related kinases 1/2
- MEK 1/2
mitogen-activated protein kinase kinase 1/2
- sGC
soluble guanylate cyclase
- PDE
phosphodiesterase
- PKG
Protein kinase G
- VEGF
vascular endothelial growth factor
- HGF
hepatocyte growth factor
- HbA1c
glycated hemoglobin
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- GIT1
GRK interacting protein 1
- CB1R
cannabinoid-1 receptor
- 5-HT
5-hydroxytryptamine
- TBHP
tert-butyl hydrogen peroxide
Biographies
Robert B. Cameron obtained his B.S. from Davidson College where he performed research on the synthesis and characterization of light-harvesting molecules under the direction of Dr. Durwin R. Striplin. He is currently an M.D./Ph.D. candidate at the Medical University of South Carolina studying the signaling mechanisms by which mitochondrial biogenesis occurs following G protein-coupled receptor stimulation under the direction of Rick G. Schnellmann at the University of Arizona.
Craig C. Beeson obtained his organic chemistry B.S. degree from CSU, Northridge, M.S. degree from San Diego State University and Ph.D. degree from U.C. Irvine. After studying the biophysics of T-cell activation under the direction of Harden M. McConnell at Stanford University, he started his academic career in the Chemistry Department at the University of Washington, Seattle and he is now a Professor in the Drug Discovery and Biomedical Sciences Department at the Medical University of South Carolina.
Rick G. Schnellmann obtained his B.S. in Pharmacy degree from the St. Louis College of Pharmacy, St. Louis, MO and his Ph.D. degree in pharmacology and toxicology from the University of Arizona, Tucson, AZ. After a postdoctoral fellowship at Duke University in mitochondrial biology and renal toxicity, he rose through the ranks at the University of Georgia and University of Arkansas for Medical Sciences, and became Eminent Scholar, Distinguished University Professor, and Chair in the Department of Drug Discovery and Biomedical Sciences at the Medical University of South Carolina. He currently serves as dean of the College of Pharmacy for the University of Arizona.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Whitaker RM, Corum D, Beeson CC, Schnellmann RG. Mitochondrial Biogenesis as a Pharmacological Target: A New Approach to Acute and Chronic Diseases. Annu Rev Pharmacol Toxicol. 2016;56:229–249. doi: 10.1146/annurev-pharmtox-010715-103155. [DOI] [PubMed] [Google Scholar]
- 2.Nunnari J, Suomalainen A. Mitochondria: In Sickness and in Health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane RK, Hilsabeck T, Rea SL. The Role of Mitochondrial Dysfunction in Age-Related Diseases. Biochim Biophys Acta. 2015;1847(11):1387–1400. doi: 10.1016/j.bbabio.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hafizi Abu Bakar M, Kian Kai C, Wan Hassan WN, Sarmidi MR, Yaakob H, Zaman Huri H. Mitochondrial Dysfunction as a Central Event for Mechanisms Underlying Insulin Resistance: The Roles of Long Chain Fatty Acids. Diabetes Metab Res Rev. 2015;31(5):453–475. doi: 10.1002/dmrr.2601. [DOI] [PubMed] [Google Scholar]
- 5.Walters JW, Amos D, Ray K, Santanam N. Mitochondrial Redox Status as a Target for Cardiovascular Disease. Curr Opin Pharmacol. 2016;27:50–55. doi: 10.1016/j.coph.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonawitz ND, Clayton DA, Shadel GS. Initiation and Beyond: Multiple Functions of the Human Mitochondrial Transcription Machinery. Mol Cell. 2006;24(6):813–825. doi: 10.1016/j.molcel.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Scarpulla RC. Transcriptional Paradigms in Mammalian Mitochondrial Biogenesis and Function. Physiol Rev. 2008;88(2):611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 8.Copeland WC, Longley MJ. Mitochondrial Genome Maintenance in Health and Disease. DNA Repair. 2014;19:190–198. doi: 10.1016/j.dnarep.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milenkovic D, Matic S, Kuhl I, Ruzzenente B, Freyer C, Jemt E, Park CB, Falkenberg M, Larsson NG. TWINKLE Is an Essential Mitochondrial Helicase Required for Synthesis of Nascent D-Loop Strands and Complete mtDNA Replication. Hum Mol Genet. 2013;22(10):1983–1993. doi: 10.1093/hmg/ddt051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villena JA. New Insights into PGC-1 Coactivators: Redefining Their Role in the Regulation of Mitochondrial Function and Beyond. FEBS J. 2015;282(4):647–672. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 11.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1alpha Regulates the Mitochondrial Antioxidant Defense System in Vascular Endothelial Cells. Cardiovasc Res. 2005;66(3):562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Soriano FX, Liesa M, Bach D, Chan DC, Palacin M, Zorzano A. Evidence for a Mitochondrial Regulatory Pathway Defined by Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1 Alpha, Estrogen-Related Receptor-Alpha, and Mitofusin 2. Diabetes. 2006;55(6):1783–1791. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- 13.Kotiadis VN, Duchen MR, Osellame LD. Mitochondrial Quality Control and Communications with the Nucleus Are Important in Maintaining Mitochondrial Function and Cell Health. Biochim Biophys Acta. 2014;1840(4):1254–1265. doi: 10.1016/j.bbagen.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarpulla RC. Metabolic Control of Mitochondrial Biogenesis through the PGC-1 Family Regulatory Network. Biochim Biophys Acta. 2011;1813(7):1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardo R, Enguix N, Lasheras J, Feliu JE, Kralli A, Villena JA. Rosiglitazone-Induced Mitochondrial Biogenesis in White Adipose Tissue is Independent of Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1alpha. PLoS ONE. 2011;6(11):e26989. doi: 10.1371/journal.pone.0026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peeters A, Shinde AB, Dirkx R, Smet J, De Bock K, Espeel M, Vanhorebeek I, Vanlander A, Van Coster R, Carmeliet P, Fransen M, Van Veldhoven PP, Baes M. Mitochondria in Peroxisome-Deficient Hepatocytes Exhibit Impaired Respiration, Depleted DNA, and PGC-1alpha Independent Proliferation. Biochim Biophys Acta. 2015;1853(2):285–298. doi: 10.1016/j.bbamcr.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Rowe GC, Patten IS, Zsengeller ZK, El-Khoury R, Okutsu M, Bampoh S, Koulisis N, Farrell C, Hirshman MF, Yan Z, Goodyear LJ, Rustin P, Arany Z. Disconnecting Mitochondrial Content from Respiratory Chain Capacity in PGC-1-Deficient Skeletal Muscle. Cell Rep. 2013;3(5):1449–1456. doi: 10.1016/j.celrep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson L, Yang Q, Szustakowski JD, Gullicksen PS, Halse R. Pyruvate Induces Mitochondrial Biogenesis by a PGC-1 Alpha-Independent Mechanism. Am J Physiol Cell Physiol. 2007;292(5):C1599–1605. doi: 10.1152/ajpcell.00428.2006. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient Control of Glucose Homeostasis through a Complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 20.Teyssier C, Ma H, Emter R, Kralli A, Stallcup MR. Activation of Nuclear Receptor Coactivator PGC-1alpha by Arginine Methylation. Genes Dev. 2005;19(12):1466–1473. doi: 10.1101/gad.1295005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine Stimulation of Energy Expenditure through p38 MAP Kinase Activation of PPARgamma Coactivator-1. Mol Cell. 2001;8(5):971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 22.Chang JS, Huypens P, Zhang Y, Black C, Kralli A, Gettys TW. Regulation of NT-PGC-1alpha Subcellular Localization and Function by Protein Kinase A-Dependent Modulation of Nuclear Export by CRM1. J Biol Chem. 2010;285(23):18039–18050. doi: 10.1074/jbc.M109.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-Activated Protein Kinase (AMPK) Action in Skeletal Muscle via Direct Phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional Control of Mitochondrial Biogenesis: The Central Role of PGC-1alpha. Cardiovasc Res. 2008;79(2):208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 25.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, Hoppel CL. Cardiac Mitochondria in Heart Failure: Decrease in Respirasomes and Oxidative Phosphorylation. Cardiovasc Res. 2008;80(1):30–39. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canevari L, Kuroda S, Bates TE, Clark JB, Siesjo BK. Activity of Mitochondrial Respiratory Chain Enzymes after Transient Focal Ischemia in the Rat. J Cereb Blood Flow Metab. 1997;17(11):1166–1169. doi: 10.1097/00004647-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Funk JA, Schnellmann RG. Persistent Disruption of Mitochondrial Homeostasis after Acute Kidney Injury. Am J Physiol Renal Physiol. 2012;302(7):F853–864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien LC, Keeney PM, Bennett JP., Jr Differentiation of Human Neural Stem Cells into Motor Neurons Stimulates Mitochondrial Biogenesis and Decreases Glycolytic Flux. Stem Cells Dev. 2015;24(17):1984–1994. doi: 10.1089/scd.2015.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortini P, Ferretti C, Iorio E, Cagnin M, Garribba L, Pietraforte D, Falchi M, Pascucci B, Baccarini S, Morani F, Phadngam S, De Luca G, Isidoro C, Dogliotti E. The Fine Tuning of Metabolism, Autophagy and Differentiation during In Vitro Myogenesis. Cell Death Dis. 2016;7:e2168. doi: 10.1038/cddis.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Windt GJ, Pearce EL. Metabolic Switching and Fuel Choice during T-Cell Differentiation and Memory Development. Immunol Rev. 2012;249(1):27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granata S, Zaza G, Simone S, Villani G, Latorre D, Pontrelli P, Carella M, Schena FP, Grandaliano G, Pertosa G. Mitochondrial Dysregulation and Oxidative Stress in Patients with Chronic Kidney Disease. BMC Genomics. 2009;10:388. doi: 10.1186/1471-2164-10-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, Little SE, Neubauer S, Clarke K. Increased Mitochondrial Uncoupling Proteins, Respiratory Uncoupling and Decreased Efficiency in the Chronically Infarcted Rat Heart. J Mol Cell Cardiol. 2008;44(4):694–700. doi: 10.1016/j.yjmcc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed Mitochondrial Transcription Factors and Oxidative Capacity in Rat Failing Cardiac and Skeletal Muscles. J Physiol. 2003;551(Pt 2):491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sebastiani M, Giordano C, Nediani C, Travaglini C, Borchi E, Zani M, Feccia M, Mancini M, Petrozza V, Cossarizza A, Gallo P, Taylor RW, d’Amati G. Induction of Mitochondrial Biogenesis Is a Maladaptive Mechanism in Mitochondrial Cardiomyopathies. J Am Coll Cardiol. 2007;50(14):1362–1369. doi: 10.1016/j.jacc.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 35.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. Cardiac-Specific Induction of the Transcriptional Coactivator Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1alpha Promotes Mitochondrial Biogenesis and Reversible Cardiomyopathy in a Developmental Stage-Dependent Manner. Circ Res. 2004;94(4):525–533. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- 36.Gamboa JL, Billings FTt, Bojanowski MT, Gilliam LA, Yu C, Roshanravan B, Roberts LJ, 2nd, Himmelfarb J, Ikizler TA, Brown NJ. Mitochondrial Dysfunction and Oxidative Stress in Patients with Chronic Kidney Disease. Physiol Rep. 2016;4(9):e12780. doi: 10.14814/phy2.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coughlan MT, Higgins GC, Nguyen TV, Penfold SA, Thallas-Bonke V, Tan SM, Ramm G, Van Bergen NJ, Henstridge DC, Sourris KC, Harcourt BE, Trounce IA, Robb PM, Laskowski A, McGee SL, Genders AJ, Walder K, Drew BG, Gregorevic P, Qian H, Thomas MC, Jerums G, Macisaac RJ, Skene A, Power DA, Ekinci EI, Wijeyeratne XW, Gallo LA, Herman-Edelstein M, Ryan MT, Cooper ME, Thorburn DR, Forbes JM. Deficiency in Apoptosis-Inducing Factor Recapitulates Chronic Kidney Disease via Aberrant Mitochondrial Homeostasis. Diabetes. 2016;65(4):1085–1098. doi: 10.2337/db15-0864. [DOI] [PubMed] [Google Scholar]
- 38.Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA. Impaired Oxidation of Plasma-Derived Fatty Acids in Type 2 Diabetic Subjects during Moderate-Intensity Exercise. Diabetes. 2000;49(12):2102–2107. doi: 10.2337/diabetes.49.12.2102. [DOI] [PubMed] [Google Scholar]
- 39.Joseph JW, Koshkin V, Saleh MC, Sivitz WI, Zhang CY, Lowell BB, Chan CB, Wheeler MB. Free Fatty Acid-Induced Beta-Cell Defects Are Dependent on Uncoupling Protein 2 Expression. J Biol Chem. 2004;279(49):51049–51056. doi: 10.1074/jbc.M409189200. [DOI] [PubMed] [Google Scholar]
- 40.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling Protein-2 Negatively Regulates Insulin Secretion and Is a Major Link between Obesity, Beta Cell Dysfunction, and Type 2 Diabetes. Cell. 2001;105(6):745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 41.Van der Schueren B, Vangoitsenhoven R, Geeraert B, De Keyzer D, Hulsmans M, Lannoo M, Huber HJ, Mathieu C, Holvoet P. Low Cytochrome Oxidase 4I1 Links Mitochondrial Dysfunction to Obesity and Type 2 Diabetes in Humans and Mice. Int J Obes. 2015;39(8):1254–1263. doi: 10.1038/ijo.2015.58. [DOI] [PubMed] [Google Scholar]
- 42.Ling C, Poulsen P, Simonsson S, Ronn T, Holmkvist J, Almgren P, Hagert P, Nilsson E, Mabey AG, Nilsson P, Vaag A, Groop L. Genetic and Epigenetic Factors Are Associated with Expression of Respiratory Chain Component NDUFB6 in Human Skeletal Muscle. J Clin Invest. 2007;117(11):3427–3435. doi: 10.1172/JCI30938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronn T, Poulsen P, Hansson O, Holmkvist J, Almgren P, Nilsson P, Tuomi T, Isomaa B, Groop L, Vaag A, Ling C. Age Influences DNA Methylation and Gene Expression of COX7A1 in Human Skeletal Muscle. Diabetologia. 2008;51(7):1159–1168. doi: 10.1007/s00125-008-1018-8. [DOI] [PubMed] [Google Scholar]
- 44.Zheng LD, Linarelli LE, Brooke J, Smith C, Wall SS, Greenawald MH, Seidel RW, Estabrooks PA, Almeida FA, Cheng Z. Mitochondrial Epigenetic Changes Link to Increased Diabetes Risk and Early-Stage Prediabetes Indicator. Oxid Med Cell Longev. 2016;2016:5290638. doi: 10.1155/2016/5290638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ling C, Del Guerra S, Lupi R, Ronn T, Granhall C, Luthman H, Masiello P, Marchetti P, Groop L, Del Prato S. Epigenetic Regulation of PPARGC1A in Human Type 2 Diabetic Islets and Effect on Insulin Secretion. Diabetologia. 2008;51(4):615–622. doi: 10.1007/s00125-007-0916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi YS, Kim S, Pak YK. Mitochondrial Transcription Factor A (mtTFA) and Diabetes. Diabetes Res Clin Pract. 2001;54(Suppl 2):S3–9. doi: 10.1016/s0168-8227(01)00330-8. [DOI] [PubMed] [Google Scholar]
- 47.Zuo L, Motherwell MS. The Impact of Reactive Oxygen Species and Genetic Mitochondrial Mutations in Parkinson’s Disease. Gene. 2013;532(1):18–23. doi: 10.1016/j.gene.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 48.Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial Dysfunction and Oxidative Damage in Parkin-Deficient Mice. J Biol Chem. 2004;279(18):18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 49.Liu W, Vives-Bauza C, Acin-Perez R, Yamamoto A, Tan Y, Li Y, Magrane J, Stavarache MA, Shaffer S, Chang S, Kaplitt MG, Huang XY, Beal MF, Manfredi G, Li C. PINK1 Defect Causes Mitochondrial Dysfunction, Proteasomal Deficit and Alpha-Synuclein Aggregation in Cell Culture Models of Parkinson’s Disease. PLoS ONE. 2009;4(2):e4597. doi: 10.1371/journal.pone.0004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautier CA, Kitada T, Shen J. Loss of PINK1 Causes Mitochondrial Functional Defects and Increased Sensitivity to Oxidative Stress. Proc Natl Acad Sci U S A. 2008;105(32):11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krebiehl G, Ruckerbauer S, Burbulla LF, Kieper N, Maurer B, Waak J, Wolburg H, Gizatullina Z, Gellerich FN, Woitalla D, Riess O, Kahle PJ, Proikas-Cezanne T, Kruger R. Reduced Basal Autophagy and Impaired Mitochondrial Dynamics due to Loss of Parkinson’s Disease-Associated Protein DJ-1. PLoS ONE. 2010;5(2):e9367. doi: 10.1371/journal.pone.0009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu G, Reyes PE, Golden GT, Woltjer RL, Hulette C, Montine TJ, Zhang J. Mitochondrial DNA Deletions/Rearrangements in Parkinson Disease and Related Neurodegenerative Disorders. J Neuropathol Exp Neurol. 2002;61(7):634–639. doi: 10.1093/jnen/61.7.634. [DOI] [PubMed] [Google Scholar]
- 53.Ikebe S, Tanaka M, Ozawa T. Point Mutations of Mitochondrial Genome in Parkinson’s Disease. Brain Res Mol Brain Res. 1995;28(2):281–295. doi: 10.1016/0169-328x(94)00209-w. [DOI] [PubMed] [Google Scholar]
- 54.Simon DK, Lin MT, Zheng L, Liu GJ, Ahn CH, Kim LM, Mauck WM, Twu F, Beal MF, Johns DR. Somatic Mitochondrial DNA Mutations in Cortex and Substantia Nigra in Aging and Parkinson’s Disease. Neurobiol Aging. 2004;25(1):71–81. doi: 10.1016/s0197-4580(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 55.Simon DK, Pulst SM, Sutton JP, Browne SE, Beal MF, Johns DR. Familial Multisystem Degeneration with Parkinsonism Associated with the 11778 Mitochondrial DNA Mutation. Neurology. 1999;53(8):1787–1793. doi: 10.1212/wnl.53.8.1787. [DOI] [PubMed] [Google Scholar]
- 56.Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG. Progressive Parkinsonism in Mice with Respiratory-Chain-Deficient Dopamine Neurons. Proc Natl Acad Sci U S A. 2007;104(4):1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burbulla LF, Schelling C, Kato H, Rapaport D, Woitalla D, Schiesling C, Schulte C, Sharma M, Illig T, Bauer P, Jung S, Nordheim A, Schols L, Riess O, Kruger R. Dissecting the Role of the Mitochondrial Chaperone Mortalin in Parkinson’s Disease: Functional Impact of Disease-Related Variants on Mitochondrial Homeostasis. Hum Mol Genet. 2010;19(22):4437–4452. doi: 10.1093/hmg/ddq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial Import and Accumulation of Alpha-Synuclein Impair Complex I in Human Dopaminergic Neuronal Cultures and Parkinson Disease Brain. J Biol Chem. 2008;283(14):9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional Repression of PGC-1alpha by Mutant Huntingtin Leads to Mitochondrial Dysfunction and Neurodegeneration. Cell. 2006;127(1):59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Chaturvedi RK, Hennessey T, Johri A, Tiwari SK, Mishra D, Agarwal S, Kim YS, Beal MF. Transducer of Regulated CREB-Binding Proteins (TORCs) Transcription and Function Is Impaired in Huntington’s Disease. Hum Mol Genet. 2012;21(15):3474–3488. doi: 10.1093/hmg/dds178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early Mitochondrial Calcium Defects in Huntington’s Disease Are a Direct Effect of Polyglutamines. Nat Neurosci. 2002;5(8):731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 62.Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH. Mitochondrial Defect in Huntington’s Disease Caudate Nucleus. Ann Neurol. 1996;39(3):385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 63.Milakovic T, Johnson GV. Mitochondrial Respiration and ATP Production are Significantly Impaired in Striatal Cells Expressing Mutant Huntingtin. J Biol Chem. 2005;280(35):30773–30782. doi: 10.1074/jbc.M504749200. [DOI] [PubMed] [Google Scholar]
- 64.Bayram-Weston Z, Torres EM, Jones L, Dunnett SB, Brooks SP. Light and Electron Microscopic Characterization of the Evolution of Cellular Pathology in the Hdh(CAG)150 Huntington’s Disease Knock-In Mouse. Brain Res Bull. 2012;88(2–3):189–198. doi: 10.1016/j.brainresbull.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 65.Squitieri F, Falleni A, Cannella M, Orobello S, Fulceri F, Lenzi P, Fornai F. Abnormal Morphology of Peripheral Cell Tissues from Patients with Huntington Disease. J Neural Transm. 2010;117(1):77–83. doi: 10.1007/s00702-009-0328-4. [DOI] [PubMed] [Google Scholar]
- 66.Rice AC, Keeney PM, Algarzae NK, Ladd AC, Thomas RR, Bennett JP., Jr Mitochondrial DNA Copy Numbers in Pyramidal Neurons Are Decreased and Mitochondrial Biogenesis Transcriptome Signaling Is Disrupted in Alzheimer’s Disease Hippocampi. J Alzheimers Dis. 2014;40(2):319–330. doi: 10.3233/JAD-131715. [DOI] [PubMed] [Google Scholar]
- 67.Ojaimi J, Masters CL, McLean C, Opeskin K, McKelvie P, Byrne E. Irregular Distribution of Cytochrome C Oxidase Protein Subunits in Aging and Alzheimer’s Disease. Ann Neurol. 1999;46(4):656–660. [PubMed] [Google Scholar]
- 68.Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased Oxidative Damage in Nuclear and Mitochondrial DNA in Alzheimer’s Disease. J Neurochem. 2005;93(4):953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 69.Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, Zhu X, Perry G. Autophagocytosis of Mitochondria Is Prominent in Alzheimer Disease. J Neuropathol Exp Neurol. 2007;66(6):525–532. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Su B, Fujioka H, Zhu X. Dynamin-Like Protein 1 Reduction Underlies Mitochondrial Morphology and Distribution Abnormalities in Fibroblasts from Sporadic Alzheimer’s Disease Patients. Am J Pathol. 2008;173(2):470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Funk JA, Schnellmann RG. Accelerated Recovery of Renal Mitochondrial and Tubule Homeostasis with SIRT1/PGC-1alpha Activation Following Ischemia-Reperfusion Injury. Toxicol Appl Pharmacol. 2013;273(2):345–354. doi: 10.1016/j.taap.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takaoka M. Resveratrol, a New Phenolic Compound, from Veratrum Grandiflorum. Nippon Kagaku Kaishi. 1939;60:1090–1100. [Google Scholar]
- 73.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Mechanism of Inhibition of Bovine F1-ATPase by Resveratrol and Related Polyphenols. Proc Natl Acad Sci U S A. 2007;104(34):13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gueguen N, Desquiret-Dumas V, Leman G, Chupin S, Baron S, Nivet-Antoine V, Vessieres E, Ayer A, Henrion D, Lenaers G, Reynier P, Procaccio V. Resveratrol Directly Binds to Mitochondrial Complex I and Increases Oxidative Stress in Brain Mitochondria of Aged Mice. PLoS ONE. 2015;10(12):e0144290. doi: 10.1371/journal.pone.0144290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takizawa Y, Nakata R, Fukuhara K, Yamashita H, Kubodera H, Inoue H. The 4′-Hydroxyl Group of Resveratrol Is Functionally Important for Direct Activation of PPARalpha. PLoS ONE. 2015;10(3):e0120865. doi: 10.1371/journal.pone.0120865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calleri E, Pochetti G, Dossou KS, Laghezza A, Montanari R, Capelli D, Prada E, Loiodice F, Massolini G, Bernier M, Moaddel R. Resveratrol and Its Metabolites Bind to PPARs. Chembiochem. 2014;15(8):1154–1160. doi: 10.1002/cbic.201300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joshi MS, Williams D, Horlock D, Samarasinghe T, Andrews KL, Jefferis AM, Berger PJ, Chin-Dusting JP, Kaye DM. Role of Mitochondrial Dysfunction in Hyperglycaemia-Induced Coronary Microvascular Dysfunction: Protective Role of Resveratrol. Diab Vasc Dis Res. 2015;12(3):208–216. doi: 10.1177/1479164114565629. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Feng L, Xing Y, Wang Y, Du L, Xu C, Cao J, Wang Q, Fan S, Liu Q, Fan F. Radioprotective and Antioxidant Effect of Resveratrol in Hippocampus by Activating Sirt1. Int J Mol Sci. 2014;15(4):5928–5939. doi: 10.3390/ijms15045928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Porquet D, Grinan-Ferre C, Ferrer I, Camins A, Sanfeliu C, Del Valle J, Pallas M. Neuroprotective Role of trans-Resveratrol in a Murine Model of Familial Alzheimer’s Disease. J Alzheimers Dis. 2014;42(4):1209–1220. doi: 10.3233/JAD-140444. [DOI] [PubMed] [Google Scholar]
- 81.Ferretta A, Gaballo A, Tanzarella P, Piccoli C, Capitanio N, Nico B, Annese T, Di Paola M, Dell’aquila C, De Mari M, Ferranini E, Bonifati V, Pacelli C, Cocco T. Effect of Resveratrol on Mitochondrial Function: Implications in Parkin-Associated Familiar Parkinson’s Disease. Biochim Biophys Acta. 2014;1842(7):902–915. doi: 10.1016/j.bbadis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 82.Solans A, Zambrano A, Rodriguez M, Barrientos A. Cytotoxicity of a Mutant Huntingtin Fragment in Yeast Involves Early Alterations in Mitochondrial OXPHOS Complexes II and III. Hum Mol Genet. 2006;15(20):3063–3081. doi: 10.1093/hmg/ddl248. [DOI] [PubMed] [Google Scholar]
- 83.Mathieu L, Costa AL, Le Bachelier C, Slama A, Lebre AS, Taylor RW, Bastin J, Djouadi F. Resveratrol Attenuates Oxidative Stress in Mitochondrial Complex I Deficiency: Involvement of SIRT3. Free Radic Biol Med. 2016;96:190–198. doi: 10.1016/j.freeradbiomed.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 84.Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, Meszaros LG, Sumegi B, Wittmann I. Resveratrol Improves Insulin Sensitivity, Reduces Oxidative Stress and Activates the Akt Pathway in Type 2 Diabetic Patients. Br J Nutr. 2011;106(3):383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 85.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie Restriction-Like Effects of 30 days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011;14(5):612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute Resveratrol Supplementation Improves Flow-Mediated Dilatation in Overweight/Obese Individuals with Mildly Elevated Blood Pressure. Nutr Metab Cardiovasc Dis. 2011;21(11):851–856. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Bhatt JK, Thomas S, Nanjan MJ. Resveratrol Supplementation Improves Glycemic Control in Type 2 Diabetes Mellitus. Nutr Res. 2012;32(7):537–541. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 88.Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot Study of Resveratrol in Older Adults with Impaired Glucose Tolerance. J Gerontol A Biol Sci Med Sci. 2012;67(12):1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Groote D, Van Belleghem K, Deviere J, Van Brussel W, Mukaneza A, Amininejad L. Effect of the Intake of Resveratrol, Resveratrol Phosphate, and Catechin-Rich Grape Seed Extract on Markers of Oxidative Stress and Gene Expression in Adult Obese Subjects. Ann Nutr Metab. 2012;61(1):15–24. doi: 10.1159/000338634. [DOI] [PubMed] [Google Scholar]
- 90.Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, Moller N, Jessen N, Pedersen SB, Jorgensen JO. High-Dose Resveratrol Supplementation in Obese Men: An Investigator-Initiated, Randomized, Placebo-Controlled Clinical Trial of Substrate Metabolism, Insulin Sensitivity, and Body Composition. Diabetes. 2013;62(4):1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, Klein S. Resveratrol Supplementation Does Not Improve Metabolic Function in Nonobese Women with Normal Glucose Tolerance. Cell Metab. 2012;16(5):658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Freudenberg K, Cox RFB, Braun E. The Catechin of the Cacao Bean. J Am Chem Soc. 1932;54(5):1913–1917. [Google Scholar]
- 93.Moreno-Ulloa A, Cid A, Rubio-Gayosso I, Ceballos G, Villarreal F, Ramirez-Sanchez I. Effects of (−)-Epicatechin and Derivatives on Nitric Oxide Mediated Induction of Mitochondrial Proteins. Bioorg Med Chem Lett. 2013;23(15):4441–4446. doi: 10.1016/j.bmcl.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nichols M, Zhang J, Polster BM, Elustondo PA, Thirumaran A, Pavlov EV, Robertson GS. Synergistic Neuroprotection by Epicatechin and Quercetin: Activation of Convergent Mitochondrial Signaling Pathways. Neuroscience. 2015;308:75–94. doi: 10.1016/j.neuroscience.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 95.Panneerselvam M, Ali SS, Finley JC, Kellerhals SE, Migita MY, Head BP, Patel PM, Roth DM, Patel HH. Epicatechin Regulation of Mitochondrial Structure and Function Is Opioid Receptor Dependent. Mol Nutr Food Res. 2013;57(6):1007–1014. doi: 10.1002/mnfr.201300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bondarovich HA, Giammarino AS, Renner JA, Shephard FW, Shingler AJ, Gianturco MA. Volatiles in Tea: Some Aspects of the Chemistry of Tea. A Contribution to the Knowledge of the Volatile Constituents. J Agric Food Chem. 1967;15(1):36–47. [Google Scholar]
- 97.Valenti D, De Rasmo D, Signorile A, Rossi L, de Bari L, Scala I, Granese B, Papa S, Vacca RA. Epigallocatechin-3-Gallate Prevents Oxidative Phosphorylation Deficit and Promotes Mitochondrial Biogenesis in Human Cells from Subjects with Down’s Syndrome. Biochim Biophys Acta. 2013;1832(4):542–552. doi: 10.1016/j.bbadis.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 98.Kurita I, Kim JH, Auger C, Kinoshita Y, Miyase T, Ito T, Schini-Kerth VB. Hydroxylation of (−)-Epigallocatechin-3-O-Gallate at 3″, but Not 4″, Is Essential for the PI3-Kinase/Akt-Dependent Phosphorylation of Endothelial NO Synthase in Endothelial Cells and Relaxation of Coronary Artery Rings. Food Funct. 2013;4(2):249–257. doi: 10.1039/c2fo30087g. [DOI] [PubMed] [Google Scholar]
- 99.Ramirez-Sanchez I, Rodriguez A, Moreno-Ulloa A, Ceballos G, Villarreal; F. (−)-Epicatechin-Induced Recovery of Mitochondria from Simulated Diabetes: Potential Role of Endothelial Nitric Oxide Synthase. Diab Vasc Dis Res. 2016 doi: 10.1177/1479164115620982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamazaki KG, Andreyev AY, Ortiz-Vilchis P, Petrosyan S, Divakaruni AS, Wiley SE, De La Fuente C, Perkins G, Ceballos G, Villarreal F, Murphy AN. Intravenous (−)-Epicatechin Reduces Myocardial Ischemic Injury by Protecting Mitochondrial Function. Int J Cardiol. 2014;175(2):297–306. doi: 10.1016/j.ijcard.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moreno-Ulloa A, Nogueira L, Rodriguez A, Barboza J, Hogan MC, Ceballos G, Villarreal F, Ramirez-Sanchez I. Recovery of Indicators of Mitochondrial Biogenesis, Oxidative Stress, and Aging With (−)-Epicatechin in Senile Mice. J Gerontol A Biol Sci Med Sci. 2015;70(11):1370–1378. doi: 10.1093/gerona/glu131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taub PR, Ramirez-Sanchez I, Ciaraldi TP, Perkins G, Murphy AN, Naviaux R, Hogan M, Maisel AS, Henry RR, Ceballos G, Villarreal F. Alterations in Skeletal Muscle Indicators of Mitochondrial Structure and Biogenesis in Patients with Type 2 Diabetes and Heart Failure: Effects of Epicatechin Rich Cocoa. Clin Transl Sci. 2012;5(1):43–47. doi: 10.1111/j.1752-8062.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of Curcumin, a Component of Golden Spice, and Its Miraculous Biological Activities. Clin Exp Pharmacol Physiol. 2012;39(3):283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jeong GS, Oh GS, Pae HO, Jeong SO, Kim YC, Shin MK, Seo BY, Han SY, Lee HS, Jeong JG, Koh JS, Chung HT. Comparative Effects of Curcuminoids on Endothelial Heme Oxygenase-1 Expression: ortho-Methoxy Groups Are Essential to Enhance Heme Oxygenase Activity and Protection. Exp Mol Med. 2006;38(4):393–400. doi: 10.1038/emm.2006.46. [DOI] [PubMed] [Google Scholar]
- 105.Negrette-Guzman M, Garcia-Nino WR, Tapia E, Zazueta C, Huerta-Yepez S, Leon-Contreras JC, Hernandez-Pando R, Aparicio-Trejo OE, Madero M, Pedraza-Chaverri J. Curcumin Attenuates Gentamicin-Induced Kidney Mitochondrial Alterations: Possible Role of a Mitochondrial Biogenesis Mechanism. Evid Based Complement Alternat Med. 2015;2015:917435. doi: 10.1155/2015/917435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ray Hamidie RD, Yamada T, Ishizawa R, Saito Y, Masuda K. Curcumin Treatment Enhances the Effect of Exercise on Mitochondrial Biogenesis in Skeletal Muscle by Increasing cAMP Levels. Metabolism. 2015;64(10):1334–1347. doi: 10.1016/j.metabol.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 107.Minassi A, Sanchez-Duffhues G, Collado JA, Munoz E, Appendino G. Dissecting the Pharmacophore of Curcumin. Which Structural Element is Critical for Which Action? J Nat Prod. 2013;76(6):1105–1112. doi: 10.1021/np400148e. [DOI] [PubMed] [Google Scholar]
- 108.Kuo JJ, Chang HH, Tsai TH, Lee TY. Curcumin Ameliorates Mitochondrial Dysfunction Associated with Inhibition of Gluconeogenesis in Free Fatty Acid-Mediated Hepatic Lipoapoptosis. Int J Mol Med. 2012;30(3):643–649. doi: 10.3892/ijmm.2012.1020. [DOI] [PubMed] [Google Scholar]
- 109.Wang S, Wang X, Ye Z, Xu C, Zhang M, Ruan B, Wei M, Jiang Y, Zhang Y, Wang L, Lei X, Lu Z. Curcumin Promotes Browning of White Adipose Tissue in a Norepinephrine-Dependent Way. Biochem Biophys Res Commun. 2015;466(2):247–253. doi: 10.1016/j.bbrc.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 110.van der Merwe C, van Dyk HC, Engelbrecht L, van der Westhuizen FH, Kinnear C, Loos B, Bardien; S. Curcumin Rescues a PINK1 Knock Down SH-SY5Y Cellular Model of Parkinson’s Disease from Mitochondrial Dysfunction and Cell Death. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9843-0. [DOI] [PubMed] [Google Scholar]
- 111.Liu L, Zhang W, Wang L, Li Y, Tan B, Lu X, Deng Y, Zhang Y, Guo X, Mu J, Yu G. Curcumin Prevents Cerebral Ischemia Reperfusion Injury via Increase of Mitochondrial Biogenesis. Neurochem Res. 2014;39(7):1322–1331. doi: 10.1007/s11064-014-1315-1. [DOI] [PubMed] [Google Scholar]
- 112.Kuo JJ, Chang HH, Tsai TH, Lee TY. Positive Effect of Curcumin on Inflammation and Mitochondrial Dysfunction in Obese Mice with Liver Steatosis. Int J Mol Med. 2012;30(3):673–679. doi: 10.3892/ijmm.2012.1049. [DOI] [PubMed] [Google Scholar]
- 113.Walter ED. Genistin (an Isoflavone Glucoside) and Its Aglucone, Genistein, from Soybeans. J Am Chem Soc. 1941;63(12):3273–3276. [Google Scholar]
- 114.Bickoff EM, Livingston AL, Hendrickson AP, Booth AN. Forage Estrogens, Relative Potencies of Several Estrogenlike Compounds Found in Forages. J Agric Food Chem. 1962;10(5):410–412. [Google Scholar]
- 115.Hauge JG. Glucose Dehydrogenase of Bacterium Anitratum: An Enzyme with a Novel Prosthetic Group. J Biol Chem. 1964;239:3630–3639. [PubMed] [Google Scholar]
- 116.Bickoff EM, Booth AN, Lyman RL, Livingston AL, Thompson CR, Deeds F. Coumestrol, a New Estrogen Isolated from Forage Crops. Science. 1957;126(3280):969–970. doi: 10.1126/science.126.3280.969-a. [DOI] [PubMed] [Google Scholar]
- 117.Marrian GF, Haslewood GA. Equol, a New Inactive Phenol Isolated from the Ketohydroxyoestrin Fraction of Mares’ Urine. Biochem J. 1932;26(4):1227–1232. doi: 10.1042/bj0261227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tissier R, Waintraub X, Couvreur N, Gervais M, Bruneval P, Mandet C, Zini R, Enriquez B, Berdeaux A, Ghaleh B. Pharmacological Postconditioning with the Phytoestrogen Genistein. J Mol Cell Cardiol. 2007;42(1):79–87. doi: 10.1016/j.yjmcc.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 119.Nadal-Serrano M, Pons DG, Sastre-Serra J, del Blanquer-Rossello MM, Roca P, Oliver J. Genistein Modulates Oxidative Stress in Breast Cancer Cell Lines According to ERalpha/ERbeta Ratio: Effects on Mitochondrial Functionality, Sirtuins, Uncoupling Protein 2 and Antioxidant Enzymes. Int J Biochem Cell Biol. 2013;45(9):2045–2051. doi: 10.1016/j.biocel.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 120.Rasbach KA, Schnellmann RG. Isoflavones Promote Mitochondrial Biogenesis. J Pharmacol Exp Ther. 2008;325(2):536–543. doi: 10.1124/jpet.107.134882. [DOI] [PubMed] [Google Scholar]
- 121.Yoshino M, Naka A, Sakamoto Y, Shibasaki A, Toh M, Tsukamoto S, Kondo K, Iida K. Dietary Isoflavone Daidzein Promotes Tfam Expression That Increases Mitochondrial Biogenesis in C2C12 Muscle Cells. J Nutr Biochem. 2015;26(11):1193–1199. doi: 10.1016/j.jnutbio.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 122.Pons DG, Nadal-Serrano M, Blanquer-Rossello MM, Sastre-Serra J, Oliver J, Roca P. Genistein Modulates Proliferation and Mitochondrial Functionality in Breast Cancer Cells Depending on ERalpha/ERbeta Ratio. J Cell Biochem. 2014;115(5):949–958. doi: 10.1002/jcb.24737. [DOI] [PubMed] [Google Scholar]
- 123.Xu XW, Shi C, He ZQ, Ma CM, Chen WH, Shen YP, Guo Q, Shen CJ, Xu J. Effects of Phytoestrogen on Mitochondrial Structure and Function of Hippocampal CA1 Region of Ovariectomized Rats. Cell Mol Neurobiol. 2008;28(6):875–886. doi: 10.1007/s10571-008-9265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yao J, Zhao L, Mao Z, Chen S, Wong KC, To J, Brinton RD. Potentiation of Brain Mitochondrial Function by S-Equol and R/S-Equol Estrogen Receptor Beta-Selective PhytoSERM Treatments. Brain Res. 2013;1514:128–141. doi: 10.1016/j.brainres.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee YM, Choi JS, Kim MH, Jung MH, Lee YS, Song J. Effects of Dietary Genistein on Hepatic Lipid Metabolism and Mitochondrial Function in Mice Fed High-Fat Diets. Nutrition. 2006;22(9):956–964. doi: 10.1016/j.nut.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 126.Cederroth CR, Vinciguerra M, Gjinovci A, Kuhne F, Klein M, Cederroth M, Caille D, Suter M, Neumann D, James RW, Doerge DR, Wallimann T, Meda P, Foti M, Rohner-Jeanrenaud F, Vassalli JD, Nef S. Dietary Phytoestrogens Activate AMP-Activated Protein Kinase with Improvement in Lipid and Glucose Metabolism. Diabetes. 2008;57(5):1176–1185. doi: 10.2337/db07-0630. [DOI] [PubMed] [Google Scholar]
- 127.Stites T, Storms D, Bauerly K, Mah J, Harris C, Fascetti A, Rogers Q, Tchaparian E, Satre M, Rucker RB. Pyrroloquinoline Quinone Modulates Mitochondrial Quantity and Function in Mice. J Nutr. 2006;136(2):390–396. doi: 10.1093/jn/136.2.390. [DOI] [PubMed] [Google Scholar]
- 128.Martino Adami PV, Quijano C, Magnani N, Galeano P, Evelson P, Cassina A, Do Carmo S, Leal MC, Castano EM, Cuello AC, Morelli; L. Synaptosomal Bioenergetic Defects Are Associated with Cognitive Impairment in a Transgenic Rat Model of Early Alzheimer’s Disease. J Cereb Blood Flow Metab. 2015 doi: 10.1177/0271678X15615132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oakes ND, Kennedy CJ, Jenkins AB, Laybutt DR, Chisholm DJ, Kraegen EW. A New Antidiabetic Agent, BRL 49653, Reduces Lipid Availability and Improves Insulin Action and Glucoregulation in the Rat. Diabetes. 1994;43(10):1203–1210. doi: 10.2337/diab.43.10.1203. [DOI] [PubMed] [Google Scholar]
- 130.Sohda T, Momose Y, Meguro K, Kawamatsu Y, Sugiyama Y, Ikeda H. Studies on Antidiabetic Agents. Synthesis and Hypoglycemic Activity of 5-[4-(Pyridylalkoxy)Benzyl]-2,4-Thiazolidinediones. Arzneimittelforschung. 1990;40(1):37–42. [PubMed] [Google Scholar]
- 131.Fujiwara T, Yoshioka S, Yoshioka T, Ushiyama I, Horikoshi H. Characterization of New Oral Antidiabetic Agent CS-045. Studies in KK and ob/ob Mice and Zucker Fatty Rats. Diabetes. 1988;37(11):1549–1558. doi: 10.2337/diab.37.11.1549. [DOI] [PubMed] [Google Scholar]
- 132.Fujita T, Sugiyama Y, Taketomi S, Sohda T, Kawamatsu Y, Iwatsuka H, Suzuoki Z. Reduction of Insulin Resistance in Obese and/or Diabetic Animals by 5-[4-(1-Methylcyclohexylmethoxy)Benzyl]-Thiazolidine-2,4-dione (ADD-3878, U-63,287, Ciglitazone), a New Antidiabetic Agent. Diabetes. 1983;32(9):804–810. doi: 10.2337/diab.32.9.804. [DOI] [PubMed] [Google Scholar]
- 133.Yamagishi K, Yamamoto K, Mochizuki Y, Nakano T, Yamada S, Tokiwa H. Flexible Ligand Recognition of Peroxisome Proliferator-Activated Receptor-Gamma (PPARgamma) Bioorg Med Chem Lett. 2010;20(11):3344–3347. doi: 10.1016/j.bmcl.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 134.Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR, Beck KD, Moore LB, Kliewer SA, Lehmann JM. The Structure-Activity Relationship between Peroxisome Proliferator-Activated Receptor Gamma Agonism and the Antihyperglycemic Activity of Thiazolidinediones. J Med Chem. 1996;39(3):665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 135.Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello Russo C. Receptor-Independent Actions of PPAR Thiazolidinedione Agonists: Is Mitochondrial Function the Key? Biochem Pharmacol. 2005;70(2):177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 136.LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E. Thiazolidinediones can Rapidly Activate AMP-Activated Protein Kinase in Mammalian Tissues. Am J Physiol Endocrinol Metab. 2006;291(1):E175–181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- 137.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, Furnsinn C. Thiazolidinediones, Like Metformin, Inhibit Respiratory Complex I: A Common Mechanism Contributing to Their Antidiabetic Actions? Diabetes. 2004;53(4):1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 138.Bolten CW, Blanner PM, McDonald WG, Staten NR, Mazzarella RA, Arhancet GB, Meier MF, Weiss DJ, Sullivan PM, Hromockyj AE, Kletzien RF, Colca JR. Insulin Sensitizing Pharmacology of Thiazolidinediones Correlates with Mitochondrial Gene expression Rather Than Activation of PPAR Gamma. Gene Regul Syst Bio. 2007;1:73–82. [PMC free article] [PubMed] [Google Scholar]
- 139.Miglio G, Rosa AC, Rattazzi L, Collino M, Lombardi G, Fantozzi R. PPARgamma Stimulation Promotes Mitochondrial Biogenesis and Prevents Glucose Deprivation-Induced Neuronal Cell Loss. Neurochem Int. 2009;55(7):496–504. doi: 10.1016/j.neuint.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 140.Zolezzi JM, Silva-Alvarez C, Ordenes D, Godoy JA, Carvajal FJ, Santos MJ, Inestrosa NC. Peroxisome Proliferator-Activated Receptor (PPAR) Gamma and PPARalpha Agonists Modulate Mitochondrial Fusion-Fission Dynamics: Relevance to Reactive Oxygen Species (ROS)-Related Neurodegenerative Disorders? PLoS ONE. 2013;8(5):e64019. doi: 10.1371/journal.pone.0064019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Quintanilla RA, Jin YN, Fuenzalida K, Bronfman M, Johnson GV. Rosiglitazone Treatment Prevents Mitochondrial Dysfunction in Mutant Huntingtin-Expressing Cells: Possible Role of Peroxisome Proliferator-Activated Receptor-Gamma (PPARgamma) in the Pathogenesis of Huntington Disease. J Biol Chem. 2008;283(37):25628–25637. doi: 10.1074/jbc.M804291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chiang MC, Cheng YC, Nicol CJ, Lin KH, Yen CH, Chen SJ, Huang RN. Rosiglitazone Activation of PPARgamma-Dependent Signaling is Neuroprotective in Mutant Huntingtin Expressing Cells. Exp Cell Res. 2015;338(2):183–193. doi: 10.1016/j.yexcr.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 143.De Nuccio C, Bernardo A, Cruciani C, De Simone R, Visentin S, Minghetti L. Peroxisome Proliferator Activated Receptor-Gamma Agonists Protect Oligodendrocyte Progenitors against Tumor Necrosis Factor-Alpha-Induced Damage: Effects on Mitochondrial Functions and Differentiation. Exp Neurol. 2015;271:506–514. doi: 10.1016/j.expneurol.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 144.Strum JC, Shehee R, Virley D, Richardson J, Mattie M, Selley P, Ghosh S, Nock C, Saunders A, Roses A. Rosiglitazone Induces Mitochondrial Biogenesis in Mouse Brain. J Alzheimers Dis. 2007;11(1):45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- 145.Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG. Pioglitazone Attenuates Mitochondrial Dysfunction, Cognitive Impairment, Cortical Tissue Loss, and Inflammation Following Traumatic Brain Injury. Exp Neurol. 2011;227(1):128–135. doi: 10.1016/j.expneurol.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Drosatos K, Khan RS, Trent CM, Jiang H, Son NH, Blaner WS, Homma S, Schulze PC, Goldberg IJ. Peroxisome Proliferator-Activated Receptor-Gamma Activation Prevents Sepsis-Related Cardiac Dysfunction and Mortality in Mice. Circ Heart Fail. 2013;6(3):550–562. doi: 10.1161/CIRCHEARTFAILURE.112.000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He H, Tao H, Xiong H, Duan SZ, McGowan FX, Jr, Mortensen RM, Balschi JA. Rosiglitazone Causes Cardiotoxicity via Peroxisome Proliferator-Activated Receptor Gamma-Independent Mitochondrial Oxidative Stress in Mouse Hearts. Toxicol Sci. 2014;138(2):468–481. doi: 10.1093/toxsci/kfu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Palee S, Weerateerangkul P, Surinkeaw S, Chattipakorn S, Chattipakorn N. Effect of Rosiglitazone on Cardiac Electrophysiology, Infarct Size and Mitochondrial Function in Ischaemia and Reperfusion of Swine and Rat Heart. Exp Physiol. 2011;96(8):778–789. doi: 10.1113/expphysiol.2011.057885. [DOI] [PubMed] [Google Scholar]
- 149.Mercer SW, Trayhurn P. Effects of Ciglitazone on Insulin Resistance and Thermogenic Responsiveness to Acute Cold in Brown Adipose Tissue of Genetically Obese (ob/ob) Mice. FEBS Lett. 1986;195(1–2):12–16. doi: 10.1016/0014-5793(86)80120-x. [DOI] [PubMed] [Google Scholar]
- 150.Bruin JE, Petrik JJ, Hyslop JR, Raha S, Tarnopolsky MA, Gerstein HC, Holloway AC. Rosiglitazone Improves Pancreatic Mitochondrial Function in an Animal Model of Dysglycemia: Role of the Insulin-Like Growth Factor Axis. Endocrine. 2010;37(2):303–311. doi: 10.1007/s12020-009-9294-8. [DOI] [PubMed] [Google Scholar]
- 151.Takada S, Hirabayashi K, Kinugawa S, Yokota T, Matsushima S, Suga T, Kadoguchi T, Fukushima A, Homma T, Mizushima W, Masaki Y, Furihata T, Katsuyama R, Okita K, Tsutsui H. Pioglitazone Ameliorates the Lowered Exercise Capacity and Impaired Mitochondrial Function of the Skeletal Muscle in Type 2 Diabetic Mice. Eur J Pharmacol. 2014;740:690–696. doi: 10.1016/j.ejphar.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 152.Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone Induces Mitochondrial Biogenesis in Human Subcutaneous Adipose Tissue In Vivo. Diabetes. 2005;54(5):1392–1399. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- 153.Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P. Improved Skeletal Muscle Oxidative Enzyme Activity and Restoration of PGC-1 Alpha and PPAR Beta/Delta Gene Expression upon Rosiglitazone Treatment in Obese Patients with Type 2 Diabetes Mellitus. Int J Obes. 2007;31(8):1302–1310. doi: 10.1038/sj.ijo.0803567. [DOI] [PubMed] [Google Scholar]
- 154.Sbert-Roig M, Bauza-Thorbrugge M, Galmes-Pascual BM, Capllonch-Amer G, Garcia-Palmer FJ, Llado I, Proenza AM, Gianotti M. GPER Mediates the Effects of 17beta-Estradiol in Cardiac Mitochondrial Biogenesis and Function. Mol Cell Endocrinol. 2016;420:116–124. doi: 10.1016/j.mce.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 155.Thayer SA, Doisy EA, Doisy EA. The Bio-Assay of Beta-Estradiol. Yale J Biol Med. 1944;17(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- 156.Johnson WS, Gravestock MB, McCarry BE. Acetylenic Bond Participation in Biogenetic-Like Olefinic Cyclizations. II. Synthesis of dl-Progesterone. J Am Chem Soc. 1971;93(17):4332–4334. doi: 10.1021/ja00746a062. [DOI] [PubMed] [Google Scholar]
- 157.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular Basis of Agonism and Antagonism in the Oestrogen Receptor. Nature. 1997;389(6652):753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 158.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole Ligands: Structure-Affinity/Activity Relationships and Estrogen Receptor-Alpha-Selective Agonists. J Med Chem. 2000;43(26):4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 159.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen Receptor-Beta Potency-Selective Ligands: Structure-Activity Relationship Studies of Diarylpropionitriles and Their Acetylene and Polar Analogues. J Med Chem. 2001;44(24):4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 160.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and Biomolecular Screening Converge on a Selective Agonist for GPR30. Nat Chem Biol. 2006;2(4):207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 161.Arnatt CK, Zhang Y. G Protein-Coupled Estrogen Receptor (GPER) Agonist Dual Binding Mode Analyses toward Understanding of Its Activation Mechanism: A Comparative Homology Modeling Approach. Mol Inform. 2013;32(7):647–658. doi: 10.1002/minf.201200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mendez-Luna D, Martinez-Archundia M, Maroun RC, Ceballos-Reyes G, Fragoso-Vazquez MJ, Gonzalez-Juarez DE, Correa-Basurto J. Deciphering the GPER/GPR30-Agonist and Antagonists Interactions Using Molecular Modeling Studies, Molecular Dynamics, and Docking Simulations. J Biomol Struct Dyn. 2015;33(10):2161–2172. doi: 10.1080/07391102.2014.994102. [DOI] [PubMed] [Google Scholar]
- 163.Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM. Estradiol Stimulates Transcription of Nuclear Respiratory Factor-1 and Increases Mitochondrial Biogenesis. Mol Endocrinol. 2008;22(3):609–622. doi: 10.1210/me.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giordano C, Montopoli M, Perli E, Orlandi M, Fantin M, Ross-Cisneros FN, Caparrotta L, Martinuzzi A, Ragazzi E, Ghelli A, Sadun AA, d’Amati G, Carelli V. Oestrogens Ameliorate Mitochondrial Dysfunction in Leber’s Hereditary Optic Neuropathy. Brain. 2011;134(Pt 1):220–234. doi: 10.1093/brain/awq276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and Estrogen Regulate Oxidative Metabolism in Brain Mitochondria. Endocrinology. 2008;149(6):3167–3175. doi: 10.1210/en.2007-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Irwin RW, Yao J, To J, Hamilton RT, Cadenas E, Brinton RD. Selective Oestrogen Receptor Modulators Differentially Potentiate Brain Mitochondrial Function. J Neuroendocrinol. 2012;24(1):236–248. doi: 10.1111/j.1365-2826.2011.02251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small Molecule Activators of SIRT1 as Therapeutics for the Treatment of Type 2 Diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hoffmann E, Wald J, Lavu S, Roberts J, Beaumont C, Haddad J, Elliott P, Westphal C, Jacobson E. Pharmacokinetics and Tolerability of SRT2104, a First-In-Class Small Molecule Activator of SIRT1, after Single and Repeated Oral Administration in Man. Br J Clin Pharmacol. 2013;75(1):186–196. doi: 10.1111/j.1365-2125.2012.04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vu CB, Bemis JE, Disch JS, Ng PY, Nunes JJ, Milne JC, Carney DP, Lynch AV, Smith JJ, Lavu S, Lambert PD, Gagne DJ, Jirousek MR, Schenk S, Olefsky JM, Perni RB. Discovery of Imidazo[1,2-b]Thiazole Derivatives as Novel SIRT1 Activators. J Med Chem. 2009;52(5):1275–1283. doi: 10.1021/jm8012954. [DOI] [PubMed] [Google Scholar]
- 170.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and Resveratrol Are Not Direct Activators of SIRT1. J Biol Chem. 2010;285(11):8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, ESY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA. Evidence for a Common Mechanism of SIRT1 Regulation by Allosteric Activators. Science. 2013;339(6124):1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martin-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R. SRT1720 Improves Survival and Healthspan of Obese Mice. Sci Rep. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 Activation Mimics Low Energy Levels and Protects against Diet-Induced Metabolic Disorders by Enhancing Fat Oxidation. Cell Metab. 2008;8(5):347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 174.Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang Y, Becker KG, Khraiwesh H, Gonzalez-Reyes JA, Villalba JM, Baur JA, Elliott P, Westphal C, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M, de Cabo R. SRT2104 Extends Survival of Male Mice on a Standard Diet and Preserves Bone and Muscle Mass. Aging Cell. 2014;13(5):787–796. doi: 10.1111/acel.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xiang Z, Krainc D. Pharmacological Upregulation of PGC1alpha in Oligodendrocytes: Implications for Huntington’s Disease. J Huntingtons Dis. 2013;2(1):101–105. doi: 10.3233/JHD-130046. [DOI] [PubMed] [Google Scholar]
- 176.Jiang M, Zheng J, Peng Q, Hou Z, Zhang J, Mori S, Ellis JL, Vlasuk GP, Fries H, Suri V, Duan W. Sirtuin 1 Activator SRT2104 Protects Huntington’s Disease Mice. Ann Clin Transl Neurol. 2014;1(12):1047–1052. doi: 10.1002/acn3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Funk JA, Odejinmi S, Schnellmann RG. SRT1720 Induces Mitochondrial Biogenesis and Rescues Mitochondrial Function after Oxidant Injury in Renal Proximal Tubule Cells. J Pharmacol Exp Ther. 2010;333(2):593–601. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Khader A, Yang WL, Kuncewitch M, Jacob A, Prince JM, Asirvatham JR, Nicastro J, Coppa GF, Wang P. Sirtuin 1 Activation Stimulates Mitochondrial Biogenesis and Attenuates Renal Injury after Ischemia-Reperfusion. Transplantation. 2014;98(2):148–156. doi: 10.1097/TP.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 179.He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM. Sirt1 Activation Protects the Mouse Renal Medulla from Oxidative Injury. J Clin Invest. 2010;120(4):1056–1068. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, Wang J, Thomas DP, Li J. Impaired SIRT1 Nucleocytoplasmic Shuttling in the Senescent Heart during Ischemic Stress. FASEB J. 2013;27(11):4332–4342. doi: 10.1096/fj.12-216473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang Y, Mi SL, Hu N, Doser TA, Sun A, Ge J, Ren J. Mitochondrial Aldehyde Dehydrogenase 2 Accentuates Aging-Induced Cardiac Remodeling and Contractile Dysfunction: Role of AMPK, Sirt1, and Mitochondrial Function. Free Radic Biol Med. 2014;71:208–220. doi: 10.1016/j.freeradbiomed.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gano LB, Donato AJ, Pasha HM, Hearon CM, Jr, Sindler AL, Seals DR. The SIRT1 Activator SRT1720 Reverses Vascular Endothelial Dysfunction, Excessive Superoxide Production, and Inflammation with Aging in Mice. Am J Physiol Heart Circ Physiol. 2014;307(12):H1754–1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M, de Cabo R. The SIRT1 Activator SRT1720 Extends Lifespan and Improves Health of Mice Fed a Standard Diet. Cell Rep. 2014;6(5):836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Baksi A, Kraydashenko O, Zalevkaya A, Stets R, Elliott P, Haddad J, Hoffmann E, Vlasuk GP, Jacobson EW. A phase II, Randomized, Placebo-Controlled, Double-Blind, Multi-Dose Study of SRT2104, a SIRT1 Activator, in Subjects with Type 2 Diabetes. Br J Clin Pharmacol. 2014;78(1):69–77. doi: 10.1111/bcp.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Venkatasubramanian S, Noh RM, Daga S, Langrish JP, Joshi NV, Mills NL, Hoffmann E, Jacobson EW, Vlasuk GP, Waterhouse BR, Lang NN, Newby DE. Cardiovascular Effects of a Novel SIRT1 Activator, SRT2104, in Otherwise Healthy Cigarette Smokers. J Am Heart Assoc. 2013;2(3):e000042. doi: 10.1161/JAHA.113.000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-Associated Reductions in AMP-Activated Protein Kinase Activity and Mitochondrial Biogenesis. Cell Metab. 2007;5(2):151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Suwa M, Nakano H, Radak Z, Kumagai S. Short-Term Adenosine Monophosphate-Activated Protein Kinase Activator 5-Aminoimidazole-4-Carboxamide-1-Beta-D-Ribofuranoside Treatment Increases the Sirtuin 1 Protein Expression in Skeletal Muscle. Metabolism. 2011;60(3):394–403. doi: 10.1016/j.metabol.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 188.Zhang L, Frederich M, He H, Balschi JA. Relationship Between 5-Aminoimidazole-4-Carboxamide-Ribotide and AMP-Activated Protein Kinase Activity in the Perfused Mouse Heart. Am J Physiol Heart Circ Physiol. 2006;290(3):H1235–1243. doi: 10.1152/ajpheart.00906.2005. [DOI] [PubMed] [Google Scholar]
- 189.Sterne J. Treatment of Diabetes Mellitus with N, N-Dimethylguanylguanidine (LA. 6023 Glucophage) Therapie. 1959;14:625–630. [PubMed] [Google Scholar]
- 190.Ungar G, Freedman L, Shapiro SL. Pharmacological Studies of a New Oral Hypoglycemic Drug. Proc Soc Exp Biol Med. 1957;95(1):190–192. doi: 10.3181/00379727-95-23163. [DOI] [PubMed] [Google Scholar]
- 191.Jenkins Y, Sun TQ, Markovtsov V, Foretz M, Li W, Nguyen H, Li Y, Pan A, Uy G, Gross L, Baltgalvis K, Yung SL, Gururaja T, Kinoshita T, Owyang A, Smith IJ, McCaughey K, White K, Godinez G, Alcantara R, Choy C, Ren H, Basile R, Sweeny DJ, Xu X, Issakani SD, Carroll DC, Goff DA, Shaw SJ, Singh R, Boros LG, Laplante MA, Marcotte B, Kohen R, Viollet B, Marette A, Payan DG, Kinsella TM, Hitoshi Y. AMPK Activation through Mitochondrial Regulation Results in Increased Substrate Oxidation and Improved Metabolic Parameters in Models of Diabetes. PLoS ONE. 2013;8(12):e81870. doi: 10.1371/journal.pone.0081870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li YY, Yu LF, Zhang LN, Qiu BY, Su MB, Wu F, Chen DK, Pang T, Gu M, Zhang W, Ma WP, Jiang HW, Li JY, Nan FJ, Li J. Novel Small-Molecule AMPK Activator Orally Exerts Beneficial Effects on Diabetic db/db Mice. Toxicol Appl Pharmacol. 2013;273(2):325–334. doi: 10.1016/j.taap.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 193.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and Characterization of a Small Molecule AMPK Activator That Treats Key Components of Type 2 Diabetes and the Metabolic Syndrome. Cell Metab. 2006;3(6):403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 194.Golubitzky A, Dan P, Weissman S, Link G, Wikstrom JD, Saada A. Screening for Active Small Molecules in Mitochondrial Complex I Deficient Patient’s Fibroblasts, Reveals AICAR as the Most Beneficial Compound. PLoS ONE. 2011;6(10):e26883. doi: 10.1371/journal.pone.0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Garrido-Maraver J, Paz MV, Cordero MD, Bautista-Lorite J, Oropesa-Avila M, de la Mata M, Pavon AD, de Lavera I, Alcocer-Gomez E, Galan F, Ybot Gonzalez P, Cotan D, Jackson S, Sanchez-Alcazar JA. Critical Role of AMP-Activated Protein Kinase in the Balance Between Mitophagy and Mitochondrial Biogenesis in MELAS Disease. Biochim Biophys Acta. 2015;1852(11):2535–2553. doi: 10.1016/j.bbadis.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 196.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The Kinase LKB1 Mediates Glucose Homeostasis in Liver and Therapeutic Effects of Metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the Mechanism of Activation of AMP-Activated Protein Kinase by the Small Molecule A-769662, a Member of the Thienopyridone family. J Biol Chem. 2007;282(45):32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 198.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W, Chepetan A, Le TP, Wang L, Xu M, Paik KP, Fogo A, Viollet B, Murphy A, Brosius F, Naviaux RK, Sharma K. AMPK Dysregulation Promotes Diabetes-Related Reduction of Superoxide and Mitochondrial Function. J Clin Invest. 2013;123(11):4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang XR, Zhang MW, Chen DD, Zhang Y, Chen AF. AMP-Activated Protein Kinase Rescues the Angiogenic Functions of Endothelial Progenitor Cells via Manganese Superoxide Dismutase Induction in Type 1 Diabetes. Am J Physiol Endocrinol Metab. 2011;300(6):E1135–1145. doi: 10.1152/ajpendo.00001.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of Mitochondrial Uncoupling Protein-2 by the AMP-Activated Protein Kinase in Endothelial Cells Attenuates Oxidative Stress in Diabetes. Diabetes. 2008;57(12):3222–3230. doi: 10.2337/db08-0610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 201.Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S. Long-Term AICAR Administration and Exercise Prevents Diabetes in ZDF Rats. Diabetes. 2005;54(4):928–934. doi: 10.2337/diabetes.54.4.928. [DOI] [PubMed] [Google Scholar]
- 202.Boon H, Bosselaar M, Praet SF, Blaak EE, Saris WH, Wagenmakers AJ, McGee SL, Tack CJ, Smits P, Hargreaves M, van Loon LJ. Intravenous AICAR Administration Reduces Hepatic Glucose Output and Inhibits Whole Body Lipolysis in Type 2 Diabetic Patients. Diabetologia. 2008;51(10):1893–1900. doi: 10.1007/s00125-008-1108-7. [DOI] [PubMed] [Google Scholar]
- 203.Liong S, Lappas M. Activation of AMPK Improves Inflammation and Insulin Resistance in Adipose Tissue and Skeletal Muscle from Pregnant Women. J Physiol Biochem. 2015;71(4):703–717. doi: 10.1007/s13105-015-0435-7. [DOI] [PubMed] [Google Scholar]
- 204.Bikman BT, Zheng D, Reed MA, Hickner RC, Houmard JA, Dohm GL. Lipid-Induced Insulin Resistance Is Prevented in Lean and Obese Myotubes by AICAR Treatment. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1692–1699. doi: 10.1152/ajpregu.00190.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang C, Xu H, Cai L, Du X, Jiang Y, Zhang Y, Zhou H, Chen ZK. Donor Pretreatment with Adenosine Monophosphate-Activated Protein Kinase Activator Protects Cardiac Grafts from Cold Ischaemia/Reperfusion Injury. Eur J Cardiothorac Surg. 2016;49(5):1354–1360. doi: 10.1093/ejcts/ezv413. [DOI] [PubMed] [Google Scholar]
- 206.Yu L, Yang SJ. AMP-Activated Protein Kinase Mediates Activity-Dependent Regulation of Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1alpha and Nuclear Respiratory Factor 1 Expression in Rat Visual Cortical Neurons. Neuroscience. 2010;169(1):23–38. doi: 10.1016/j.neuroscience.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 207.Tao K, Matsuki N, Koyama R. AMP-Activated Protein Kinase Mediates Activity-Dependent Axon Branching by Recruiting Mitochondria to Axon. Dev Neurobiol. 2014;74(6):557–573. doi: 10.1002/dneu.22149. [DOI] [PubMed] [Google Scholar]
- 208.Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, Liu LB, Wu K, Liu R, Wang JZ, Zhou XW. AMPK Activation Ameliorates Alzheimer’s Disease-Like Pathology and Spatial Memory Impairment in a Streptozotocin-Induced Alzheimer’s Disease Model in Rats. J Alzheimers Dis. 2015;43(3):775–784. doi: 10.3233/JAD-140564. [DOI] [PubMed] [Google Scholar]
- 209.Kim J, Park YJ, Jang Y, Kwon YH. AMPK Activation Inhibits Apoptosis and Tau Hyperphosphorylation Mediated by Palmitate in SH-SY5Y Cells. Brain Res. 2011;1418:42–51. doi: 10.1016/j.brainres.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 210.Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin Regulates Tau Phosphorylation and Amyloid through AMPK in Neuronal Cells. Biochem Biophys Res Commun. 2009;380(1):98–104. doi: 10.1016/j.bbrc.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Clough-Helfman C, Phillis JW. 5-Aminoimidazole-4-Carboxamide Riboside (AICAR) Administration Reduces Cerebral Ischemic Damage in the Mongolian Gerbil. Brain Res Bull. 1990;25(1):203–206. doi: 10.1016/0361-9230(90)90277-7. [DOI] [PubMed] [Google Scholar]
- 212.Roskoski R., Jr ERK1/2 MAP Kinases: Structure, Function, and Regulation. Pharmacol Res. 2012;66(2):105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 213.Duncia JV, Santella JB, 3rd, Higley CA, Pitts WJ, Wityak J, Frietze WE, Rankin FW, Sun JH, Earl RA, Tabaka AC, Teleha CA, Blom KF, Favata MF, Manos EJ, Daulerio AJ, Stradley DA, Horiuchi K, Copeland RA, Scherle PA, Trzaskos JM, Magolda RL, Trainor GL, Wexler RR, Hobbs FW, Olson RE. MEK Inhibitors: The Chemistry and Biological Activity of U0126, Its Analogs, and Cyclization Products. Bioorg Med Chem Lett. 1998;8(20):2839–2844. doi: 10.1016/s0960-894x(98)00522-8. [DOI] [PubMed] [Google Scholar]
- 214.Abe H, Kikuchi S, Hayakawa K, Iida T, Nagahashi N, Maeda K, Sakamoto J, Matsumoto N, Miura T, Matsumura K, Seki N, Inaba T, Kawasaki H, Yamaguchi T, Kakefuda R, Nanayama T, Kurachi H, Hori Y, Yoshida T, Kakegawa J, Watanabe Y, Gilmartin AG, Richter MC, Moss KG, Laquerre SG. Discovery of a Highly Potent and Selective MEK Inhibitor: GSK1120212 (JTP-74057 DMSO Solvate) ACS Med Chem Lett. 2011;2(4):320–324. doi: 10.1021/ml200004g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Haq R, Shoag J, Andreu-Perez P, Yokoyama S, Edelman H, Rowe GC, Frederick DT, Hurley AD, Nellore A, Kung AL, Wargo JA, Song JS, Fisher DE, Arany Z, Widlund HR. Oncogenic BRAF Regulates Oxidative Metabolism via PGC1alpha and MITF. Cancer Cell. 2013;23(3):302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang KZ, Zhu J, Dagda RK, Uechi G, Cherra SJ, 3rd, Gusdon AM, Balasubramani M, Chu CT. ERK-Mediated Phosphorylation of TFAM Downregulates Mitochondrial Transcription: Implications for Parkinson’s Disease. Mitochondrion. 2014;17:132–140. doi: 10.1016/j.mito.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of ERK1/2 Pathway Mediates Oxidant-Induced Decreases in Mitochondrial Function in Renal Cells. Am J Physiol Renal Physiol. 2006;291(4):F840–855. doi: 10.1152/ajprenal.00219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Smith JA, Stallons LJ, Collier JB, Chavin KD, Schnellmann RG. Suppression of Mitochondrial Biogenesis through Toll-Like Receptor 4-Dependent Mitogen-Activated Protein Kinase Kinase/Extracellular Signal-Regulated Kinase Signaling in Endotoxin-Induced Acute Kidney Injury. J Pharmacol Exp Ther. 2015;352(2):346–357. doi: 10.1124/jpet.114.221085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Field L, Dilts RV, Ravichandran R, Lenhert PG, Carnahan GE. An Unusually Stable Thionitrite from N-Acetyl-D,L-Penicillamine; X-Ray Crystal and Molecular Structure of 2-(Acetylamino)-2-Carboxy-1,1-Dimethylethyl Thionitrite. J Chem Soc, Chem Commun. 1978;(6):249–250. doi: 10.1039/C39780000249. [DOI] [Google Scholar]
- 220.Hrabie JA, Klose JR, Wink DA, Keefer LK. New Nitric Oxide-Releasing Zwitterions Derived from Polyamines. J Org Chem. 1993;58(6):1472–1476. [Google Scholar]
- 221.Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, Schramm M, Schroeder W, Schroder H, Stahl E, Steinke W, Wunder F. NO- and Haem-Independent Activation of Soluble Guanylyl Cyclase: Molecular Basis and Cardiovascular Implications of a New Pharmacological Principle. Br J Pharmacol. 2002;136(5):773–783. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mittendorf J, Weigand S, Alonso-Alija C, Bischoff E, Feurer A, Gerisch M, Kern A, Knorr A, Lang D, Muenter K, Radtke M, Schirok H, Schlemmer KH, Stahl E, Straub A, Wunder F, Stasch JP. Discovery of Riociguat (BAY 63-2521): A Potent, Oral Stimulator of Soluble Guanylate Cyclase for the Treatment of Pulmonary Hypertension. ChemMedChem. 2009;4(5):853–865. doi: 10.1002/cmdc.200900014. [DOI] [PubMed] [Google Scholar]
- 223.Straub A, Stasch JP, Alonso-Alija C, Benet-Buchholz J, Ducke B, Feurer A, Furstner C. NO-Independent Stimulators of Soluble Guanylate Cyclase. Bioorg Med Chem Lett. 2001;11(6):781–784. doi: 10.1016/s0960-894x(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 224.Bergstrand H, Kristoffersson J, Lundquist B, Schurmann A. Effects of Antiallergic Agents, Compound 48/80, and Some Reference Inhibitors on the Activity of Partially Purified Human Lung Tissue Adenosine Cyclic 3′,5′-Monophosphate and Guanosine Cyclic 3′,5′-Monophosphate Phosphodiesterases. Mol Pharmacol. 1977;13(1):38–43. [PubMed] [Google Scholar]
- 225.Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingell C. Sildenafil: An Orally Active Type 5 Cyclic GMP-Specific Phosphodiesterase Inhibitor for the Treatment of Penile Erectile Dysfunction. Int J Impot Res. 1996;8(2):47–52. [PubMed] [Google Scholar]
- 226.Doh H, Shin CY, Son M, Ko JI, Yoo M, Kim SH, Kim WB. Mechanism of Erectogenic Effect of the Selective Phosphodiesterase Type 5 Inhibitor, DA-8159. Arch Pharm Res. 2002;25(6):873–878. doi: 10.1007/BF02977007. [DOI] [PubMed] [Google Scholar]
- 227.Daugan A, Grondin P, Ruault C, Le Monnier de Gouville AC, Coste H, Kirilovsky J, Hyafil F, Labaudiniere R. The Discovery of Tadalafil: A Novel and Highly Selective PDE5 Inhibitor. 1: 5,6,11,11a-Tetrahydro-1H-Imidazo[1′,5′:1,6]Pyrido[3,4-b]Indole-1,3(2H)-dione Analogues. J Med Chem. 2003;46(21):4525–4532. doi: 10.1021/jm030056e. [DOI] [PubMed] [Google Scholar]
- 228.Haning H, Niewohner U, Schenke T, Es-Sayed M, Schmidt G, Lampe T, Bischoff E. Imidazo[5,1-f]Triazin-4(3H)-ones, a New Class of Potent PDE 5 Inhibitors. Bioorg Med Chem Lett. 2002;12(6):865–868. doi: 10.1016/s0960-894x(02)00030-6. [DOI] [PubMed] [Google Scholar]
- 229.Aquilano K, Baldelli S, Ciriolo MR. Nuclear Recruitment of Neuronal Nitric-Oxide Synthase by Alpha-Syntrophin Is Crucial for the Induction of Mitochondrial Biogenesis. J Biol Chem. 2014;289(1):365–378. doi: 10.1074/jbc.M113.506733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Haas B, Mayer P, Jennissen K, Scholz D, Berriel Diaz M, Bloch W, Herzig S, Fassler R, Pfeifer A. Protein Kinase G Controls Brown Fat Cell Differentiation and Mitochondrial Biogenesis. Sci Signal. 2009;2(99):ra78. doi: 10.1126/scisignal.2000511. [DOI] [PubMed] [Google Scholar]
- 231.Kerwin JF, Jr, Lancaster JR, Jr, Feldman PL. Nitric Oxide: A New Paradigm for Second Messengers. J Med Chem. 1995;38(22):4343–4362. doi: 10.1021/jm00022a001. [DOI] [PubMed] [Google Scholar]
- 232.Miller MR, Megson IL. Recent Developments in Nitric Oxide Donor Drugs. Br J Pharmacol. 2007;151(3):305–321. doi: 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ashmore T, Roberts LD, Morash AJ, Kotwica AO, Finnerty J, West JA, Murfitt SA, Fernandez BO, Branco C, Cowburn AS, Clarke K, Johnson RS, Feelisch M, Griffin JL, Murray AJ. Nitrate Enhances Skeletal Muscle Fatty Acid Oxidation via a Nitric Oxide-cGMP-PPAR-Mediated Mechanism. BMC Biol. 2015;13:110. doi: 10.1186/s12915-015-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dam AD, Mitchell AS, Quadrilatero J. Induction of Mitochondrial Biogenesis Protects against Caspase-Dependent and Caspase-Independent Apoptosis in L6 Myoblasts. Biochim Biophys Acta. 2013;1833(12):3426–3435. doi: 10.1016/j.bbamcr.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 235.Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, Arancio O. Amyloid-Beta Peptide Inhibits Activation of the Nitric Oxide/cGMP/cAMP-Responsive Element-Binding Protein Pathway during Hippocampal Synaptic Plasticity. J Neurosci. 2005;25(29):6887–6897. doi: 10.1523/JNEUROSCI.5291-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Schmidt PM, Schramm M, Schroder H, Wunder F, Stasch JP. Identification of Residues Crucially Involved in the Binding of the Heme Moiety of Soluble Guanylate Cyclase. J Biol Chem. 2004;279(4):3025–3032. doi: 10.1074/jbc.M310141200. [DOI] [PubMed] [Google Scholar]
- 237.Methner C, Buonincontri G, Hu CH, Vujic A, Kretschmer A, Sawiak S, Carpenter A, Stasch JP, Krieg T. Riociguat Reduces Infarct Size and Post-Infarct Heart Failure in Mouse Hearts: Insights from MRI/PET Imaging. PLoS ONE. 2013;8(12):e83910. doi: 10.1371/journal.pone.0083910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Vandendriessche B, Rogge E, Goossens V, Vandenabeele P, Stasch JP, Brouckaert P, Cauwels A. The Soluble Guanylate Cyclase Activator BAY 58-2667 Protects against Morbidity and Mortality in Endotoxic Shock by Recoupling Organ Systems. PLoS ONE. 2013;8(8):e72155. doi: 10.1371/journal.pone.0072155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Geschka S, Kretschmer A, Sharkovska Y, Evgenov OV, Lawrenz B, Hucke A, Hocher B, Stasch JP. Soluble Guanylate Cyclase Stimulation Prevents Fibrotic Tissue Remodeling and Improves Survival in Salt-Sensitive Dahl Rats. PLoS ONE. 2011;6(7):e21853. doi: 10.1371/journal.pone.0021853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kalk P, Godes M, Relle K, Rothkegel C, Hucke A, Stasch JP, Hocher B. NO-Independent Activation of Soluble Guanylate Cyclase Prevents Disease Progression in Rats with 5/6 Nephrectomy. Br J Pharmacol. 2006;148(6):853–859. doi: 10.1038/sj.bjp.0706792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bonkale WL, Winblad B, Ravid R, Cowburn RF. Reduced Nitric Oxide Responsive Soluble Guanylyl Cyclase Activity in the Superior Temporal Cortex of Patients with Alzheimer’s Disease. Neurosci Lett. 1995;187(1):5–8. doi: 10.1016/0304-3940(95)11323-o. [DOI] [PubMed] [Google Scholar]
- 242.Salloum FN, Das A, Samidurai A, Hoke NN, Chau VQ, Ockaili RA, Stasch JP, Kukreja RC. Cinaciguat, a Novel Activator of Soluble Guanylate Cyclase, Protects against Ischemia/Reperfusion Injury: Role of Hydrogen Sulfide. Am J Physiol Heart Circ Physiol. 2012;302(6):H1347–1354. doi: 10.1152/ajpheart.00544.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Terrett NK, Bell AS, Brown D, Ellis P. Sildenafil (VIAGRA(TM)), a Potent and Selective Inhibitor of Type 5 cGMP Phosphodiesterase with Utility for the Treatment of Male Erectile Dysfunction. Bioorg Med Chem Lett. 1996;6(15):1819–1824. [Google Scholar]
- 244.Daugan A, Grondin P, Ruault C, Le Monnier de Gouville AC, Coste H, Linget JM, Kirilovsky J, Hyafil F, Labaudiniere R. The Discovery of Tadalafil: a Novel and Highly Selective PDE5 Inhibitor. 2: 2,3,6,7,12,12a-Hexahydropyrazino[1′,2′:1,6]Pyrido[3,4-b]Indole-1,4-dione Analogues. J Med Chem. 2003;46(21):4533–4542. doi: 10.1021/jm0300577. [DOI] [PubMed] [Google Scholar]
- 245.Weeks JL, Zoraghi R, Beasley A, Sekhar KR, Francis SH, Corbin JD. High Biochemical Selectivity of Tadalafil, Sildenafil and Vardenafil for Human Phosphodiesterase 5A1 (PDE5) over PDE11A4 Suggests the Absence of PDE11A4 Cross-Reaction in Patients. Int J Impot Res. 2005;17(1):5–9. doi: 10.1038/sj.ijir.3901283. [DOI] [PubMed] [Google Scholar]
- 246.Desouza C, Parulkar A, Lumpkin D, Akers D, Fonseca VA. Acute and Prolonged Effects of Sildenafil on Brachial Artery Flow-Mediated Dilatation in Type 2 Diabetes. Diabetes Care. 2002;25(8):1336–1339. doi: 10.2337/diacare.25.8.1336. [DOI] [PubMed] [Google Scholar]
- 247.Aversa A, Vitale C, Volterrani M, Fabbri A, Spera G, Fini M, Rosano GM. Chronic Administration of Sildenafil Improves Markers of Endothelial Function in Men with Type 2 Diabetes. Diabet Med. 2008;25(1):37–44. doi: 10.1111/j.1464-5491.2007.02298.x. [DOI] [PubMed] [Google Scholar]
- 248.Fu L, Li F, Bruckbauer A, Cao Q, Cui X, Wu R, Shi H, Xue B, Zemel MB. Interaction Between Leucine and Phosphodiesterase 5 Inhibition in Modulating Insulin Sensitivity and Lipid Metabolism. Diabetes Metab Syndr Obes. 2015;8:227–239. doi: 10.2147/DMSO.S82338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Grover-Paez F, Villegas Rivera G, Guillen Ortiz R. Sildenafil Citrate Diminishes Microalbuminuria and the Percentage of A1c in Male Patients with Type 2 Diabetes. Diabetes Res Clin Pract. 2007;78(1):136–140. doi: 10.1016/j.diabres.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 250.De Toni L, Strapazzon G, Gianesello L, Caretta N, Pilon C, Bruttocao A, Foresta C. Effects of Type 5-Phosphodiesterase Inhibition on Energy Metabolism and Mitochondrial Biogenesis in Human Adipose Tissue Ex Vivo. J Endocrinol Invest. 2011;34(10):738–741. doi: 10.1007/BF03346724. [DOI] [PubMed] [Google Scholar]
- 251.Koka S, Aluri HS, Xi L, Lesnefsky EJ, Kukreja RC. Chronic Inhibition of Phosphodiesterase 5 with Tadalafil Attenuates Mitochondrial Dysfunction in Type 2 Diabetic Hearts: Potential Role of NO/SIRT1/PGC-1alpha Signaling. Am J Physiol Heart Circ Physiol. 2014;306(11):H1558–1568. doi: 10.1152/ajpheart.00865.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Koka S, Xi L, Kukreja RC. Chronic Treatment with Long Acting Phosphodiesterase-5 Inhibitor Tadalafil Alters Proteomic Changes Associated with Cytoskeletal Rearrangement and Redox Regulation in Type 2 Diabetic Hearts. Basic Res Cardiol. 2012;107(2):249. doi: 10.1007/s00395-012-0249-5. [DOI] [PubMed] [Google Scholar]
- 253.Sesti C, Florio V, Johnson EG, Kloner RA. The Phosphodiesterase-5 Inhibitor Tadalafil Reduces Myocardial Infarct Size. Int J Impot Res. 2007;19(1):55–61. doi: 10.1038/sj.ijir.3901497. [DOI] [PubMed] [Google Scholar]
- 254.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 Inhibitor Sildenafil Preconditions Adult Cardiac Myocytes against Necrosis and Apoptosis. Essential Role of Nitric Oxide Signaling. J Biol Chem. 2005;280(13):12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 255.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) Induces Powerful Cardioprotective Effect via Opening of Mitochondrial K(ATP) Channels in Rabbits. Am J Physiol Heart Circ Physiol. 2002;283(3):H1263–1269. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- 256.Salloum FN, Chau VQ, Hoke NN, Kukreja RC. Tadalafil Prevents Acute Heart Failure with Reduced Ejection Fraction in Mice. Cardiovasc Drugs Ther. 2014;28(6):493–500. doi: 10.1007/s10557-014-6559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 Inhibition with Sildenafil Attenuates Cardiomyocyte Apoptosis and Left Ventricular Dysfunction in a Chronic Model of Doxorubicin Cardiotoxicity. Circulation. 2005;111(13):1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 258.Kim KH, Kim YJ, Ohn JH, Yang J, Lee SE, Lee SW, Kim HK, Seo JW, Sohn DW. Long-Term Effects of Sildenafil in a Rat Model of Chronic Mitral Regurgitation: Benefits of Ventricular Remodeling and Exercise Capacity. Circulation. 2012;125(11):1390–1401. doi: 10.1161/CIRCULATIONAHA.111.065300. [DOI] [PubMed] [Google Scholar]
- 259.Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X. Impaired Mitochondrial Biogenesis Contributes to Mitochondrial Dysfunction in Alzheimer’s Disease. J Neurochem. 2012;120(3):419–429. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.DeMarch Z, Giampa C, Patassini S, Martorana A, Bernardi G, Fusco FR. Beneficial Effects of Rolipram in a Quinolinic Acid Model of Striatal Excitotoxicity. Neurobiol Dis. 2007;25(2):266–273. doi: 10.1016/j.nbd.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 261.Liu Z, Liu Y, Gao R, Li H, Dunn T, Wu P, Smith RG, Sarkar PS, Fang X. Ethanol Suppresses PGC-1alpha Expression by Interfering with the cAMP-CREB Pathway in Neuronal Cells. PLoS ONE. 2014;9(8):e104247. doi: 10.1371/journal.pone.0104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Schwabe U, Miyake M, Ohga Y, Daly JW. 4-(3-Cyclopentyloxy-4-Methoxyphenyl)-2-Pyrrolidone (ZK 62711): A Potent Inhibitor of Adenosine Cyclic 3′,5′-Monophosphate Phosphodiesterases in Homogenates and Tissue Slices from Rat Brain. Mol Pharmacol. 1976;12(6):900–910. [PubMed] [Google Scholar]
- 263.Dawson DL, Cutler BS, Meissner MH, Strandness DE., Jr Cilostazol Has Beneficial Effects in Treatment of Intermittent Claudication: Results from a Multicenter, Randomized, Prospective, Double-Blind Trial. Circulation. 1998;98(7):678–686. doi: 10.1161/01.cir.98.7.678. [DOI] [PubMed] [Google Scholar]
- 264.Kodimuthali A, Jabaris SS, Pal M. Recent Advances on Phosphodiesterase 4 Inhibitors for the Treatment of Asthma and Chronic Obstructive Pulmonary Disease. J Med Chem. 2008;51(18):5471–5489. doi: 10.1021/jm800582j. [DOI] [PubMed] [Google Scholar]
- 265.Roma G, Di Braccio M, Grossi G, Piras D, Leoncini G, Bruzzese D, Signorello MG, Fossa P, Mosti L. Synthesis and In Vitro Antiplatelet Activity of New 4-(1-Piperazinyl)Coumarin Derivatives. Human Platelet Phosphodiesterase 3 Inhibitory Properties of the Two Most Effective Compounds Described and Molecular Modeling Study on their Interactions with Phosphodiesterase 3A Catalytic Site. J Med Chem. 2007;50(12):2886–2895. doi: 10.1021/jm0611511. [DOI] [PubMed] [Google Scholar]
- 266.Zuo L, Li Q, Sun B, Xu Z, Ge Z. Cilostazol Promotes Mitochondrial Biogenesis in Human Umbilical Vein Endothelial Cells through Activating the Expression of PGC-1alpha. Biochem Biophys Res Commun. 2013;433(1):52–57. doi: 10.1016/j.bbrc.2013.02.068. [DOI] [PubMed] [Google Scholar]
- 267.DeMarch Z, Giampa C, Patassini S, Bernardi G, Fusco FR. Beneficial Effects of Rolipram in the R6/2 Mouse Model of Huntington’s Disease. Neurobiol Dis. 2008;30(3):375–387. doi: 10.1016/j.nbd.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 268.Dragicevic N, Delic V, Cao C, Copes N, Lin X, Mamcarz M, Wang L, Arendash GW, Bradshaw PC. Caffeine Increases Mitochondrial Function and Blocks Melatonin Signaling to Mitochondria in Alzheimer’s Mice and Cells. Neuropharmacology. 2012;63(8):1368–1379. doi: 10.1016/j.neuropharm.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 269.Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent Improvement in Synaptic and Cognitive Functions in an Alzheimer Mouse Model after Rolipram Treatment. J Clin Invest. 2004;114(11):1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Choi JM, Shin HK, Kim KY, Lee JH, Hong KW. Neuroprotective Effect of Cilostazol against Focal Cerebral Ischemia via Antiapoptotic Action in Rats. J Pharmacol Exp Ther. 2002;300(3):787–793. doi: 10.1124/jpet.300.3.787. [DOI] [PubMed] [Google Scholar]
- 271.Lee JH, Park SY, Shin YW, Hong KW, Kim CD, Sung SM, Kim KY, Lee WS. Neuroprotection by Cilostazol, a Phosphodiesterase type 3 Inhibitor, against Apoptotic White Matter Changes in Rat after Chronic Cerebral Hypoperfusion. Brain Res. 2006;1082(1):182–191. doi: 10.1016/j.brainres.2006.01.088. [DOI] [PubMed] [Google Scholar]
- 272.Lee HR, Shin HK, Park SY, Kim HY, Lee WS, Rhim BY, Hong KW, Kim CD. Cilostazol Suppresses Beta-Amyloid Production by Activating a Disintegrin and Metalloproteinase 10 via the Upregulation of SIRT1-Coupled Retinoic Acid Receptor-Beta. J Neurosci Res. 2014;92(11):1581–1590. doi: 10.1002/jnr.23421. [DOI] [PubMed] [Google Scholar]
- 273.Taguchi A, Takata Y, Ihara M, Kasahara Y, Tsuji M, Nishino M, Stern D, Okada M. Cilostazol Improves Cognitive Function in Patients with Mild Cognitive Impairment: A Retrospective Analysis. Psychogeriatrics. 2013;13(3):164–169. doi: 10.1111/psyg.12021. [DOI] [PubMed] [Google Scholar]
- 274.Sanada F, Kanbara Y, Taniyama Y, Otsu R, Carracedo M, Ikeda-Iwabu Y, Muratsu J, Sugimoto K, Yamamoto K, Rakugi H, Morishita R. Induction of Angiogenesis by a Type III Phosphodiesterase Inhibitor, Cilostazol, through Activation of Peroxisome Proliferator-Activated Receptor-Gamma and cAMP Pathways in Vascular Cells. Arterioscler Thromb Vasc Biol. 2016;36(3):545–552. doi: 10.1161/ATVBAHA.115.307011. [DOI] [PubMed] [Google Scholar]
- 275.Biscetti F, Pecorini G, Arena V, Stigliano E, Angelini F, Ghirlanda G, Ferraccioli G, Flex A. Cilostazol Improves the Response to Ischemia in Diabetic Mice by a Mechanism Dependent on PPARgamma. Mol Cell Endocrinol. 2013;381(1–2):80–87. doi: 10.1016/j.mce.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 276.Wang F, Gao L, Gong B, Hu J, Li M, Guan Q, Zhao J. Tissue-Specific Expression of PPAR mRNAs in Diabetic Rats and Divergent Effects of Cilostazol. Can J Physiol Pharmacol. 2008;86(7):465–471. doi: 10.1139/y08-043. [DOI] [PubMed] [Google Scholar]
- 277.Chattipakorn SC, Thummasorn S, Sanit J, Chattipakorn N. Phosphodiesterase-3 Inhibitor (Cilostazol) Attenuates Oxidative Stress-Induced Mitochondrial Dysfunction in the Heart. J Geriatr Cardiol. 2014;11(2):151–157. doi: 10.3969/j.issn.1671-5411.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ye Y, Qian J, Castillo AC, Ling S, Ye H, Perez-Polo JR, Bajaj M, Birnbaum Y. Phosphodiesterase-3 Inhibition Augments the Myocardial Infarct Size-Limiting Effects of Exenatide in Mice with Type 2 Diabetes. Am J Physiol Heart Circ Physiol. 2013;304(1):H131–141. doi: 10.1152/ajpheart.00609.2012. [DOI] [PubMed] [Google Scholar]
- 279.Birnbaum Y, Castillo AC, Qian J, Ling S, Ye H, Perez-Polo JR, Bajaj M, Ye Y. Phosphodiesterase III Inhibition Increases cAMP Levels and Augments the Infarct Size Limiting Effect of a DPP-4 Inhibitor in Mice with Type-2 Diabetes Mellitus. Cardiovasc Drugs Ther. 2012;26(6):445–456. doi: 10.1007/s10557-012-6409-x. [DOI] [PubMed] [Google Scholar]
- 280.Lee WC, Chen HC, Wang CY, Lin PY, Ou TT, Chen CC, Wen MC, Wang J, Lee HJ. Cilostazol Ameliorates Nephropathy in Type 1 Diabetic Rats Involving Improvement in Oxidative Stress and Regulation of TGF-Beta and NF-kappaB. Biosci Biotechnol Biochem. 2010;74(7):1355–1361. doi: 10.1271/bbb.90938. [DOI] [PubMed] [Google Scholar]
- 281.Jiao XM, Jiao XJ, Zhang XG, Xu XP, Wu JX, Yao L, Zhao J, Lu XF. Cilostazol Reduces Microalbuminuria in Type 2 Diabetic Nephropathy. Chin Med J (Engl) 2013;126(22):4395–4396. [PubMed] [Google Scholar]
- 282.Shinoda-Tagawa T, Yamasaki Y, Yoshida S, Kajimoto Y, Tsujino T, Hakui N, Matsumoto M, Hori M. A Phosphodiesterase Inhibitor, Cilostazol, Prevents the Onset of Silent Brain Infarction in Japanese Subjects with Type II Diabetes. Diabetologia. 2002;45(2):188–194. doi: 10.1007/s00125-001-0740-2. [DOI] [PubMed] [Google Scholar]
- 283.Joe Y, Zheng M, Kim HJ, Uddin MJ, Kim SK, Chen Y, Park J, Cho GJ, Ryter SW, Chung HT. Cilostazol Attenuates Murine Hepatic Ischemia and Reperfusion Injury via Heme Oxygenase-Dependent Activation of Mitochondrial Biogenesis. Am J Physiol Gastrointest Liver Physiol. 2015;309(1):G21–29. doi: 10.1152/ajpgi.00307.2014. [DOI] [PubMed] [Google Scholar]
- 284.Zhang L, Seitz LC, Abramczyk AM, Chan C. Synergistic Effect of cAMP and Palmitate in Promoting Altered Mitochondrial Function and Cell Death in HepG2 Cells. Exp Cell Res. 2010;316(5):716–727. doi: 10.1016/j.yexcr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Whitaker RM, Wills LP, Stallons LJ, Schnellmann RG. cGMP-Selective Phosphodiesterase Inhibitors Stimulate Mitochondrial Biogenesis and Promote Recovery from Acute Kidney Injury. J Pharmacol Exp Ther. 2013;347(3):626–634. doi: 10.1124/jpet.113.208017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Rankovic Z, Brust TF, Bohn LM. Biased Agonism: An Emerging Paradigm in GPCR Drug Discovery. Bioorg Med Chem Lett. 2016;26(2):241–250. doi: 10.1016/j.bmcl.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pang J, Xu X, Getman MR, Shi X, Belmonte SL, Michaloski H, Mohan A, Blaxall BC, Berk BC. G Protein Coupled Receptor Kinase 2 Interacting Protein 1 (GIT1) Is a Novel Regulator of Mitochondrial Biogenesis in Heart. J Mol Cell Cardiol. 2011;51(5):769–776. doi: 10.1016/j.yjmcc.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Liu S, Premont RT, Rockey DC. G-Protein-Coupled Receptor Kinase Interactor-1 (GIT1) Is a New Endothelial Nitric-Oxide Synthase (eNOS) Interactor with Functional Effects on Vascular Homeostasis. J Biol Chem. 2012;287(15):12309–12320. doi: 10.1074/jbc.M111.320465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lin LS, Lanza TJ, Jr, Jewell JP, Liu P, Shah SK, Qi H, Tong X, Wang J, Xu SS, Fong TM, Shen CP, Lao J, Xiao JC, Shearman LP, Stribling DS, Rosko K, Strack A, Marsh DJ, Feng Y, Kumar S, Samuel K, Yin W, Van der Ploeg LH, Goulet MT, Hagmann WK. Discovery of N-[(1S,2S)-3-(4-Chlorophenyl)-2-(3-Cyanophenyl)-1-Methylpropyl]-2-Methyl-2-{[5-(Trifluoromethyl)Pyridin-2-yl]oxy}Propanamide (MK-0364), a Novel, Acyclic Cannabinoid-1 Receptor Inverse Agonist for the Treatment of Obesity. J Med Chem. 2006;49(26):7584–7587. doi: 10.1021/jm060996+. [DOI] [PubMed] [Google Scholar]
- 290.Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara P, Soubrié P, Brelière JC, Le Fur G. SR141716A, a Potent and Selective Antagonist of the Brain Cannabinoid Receptor. FEBS Lett. 1994;350(2–3):240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 291.Lin LS, Ha S, Ball RG, Tsou NN, Castonguay LA, Doss GA, Fong TM, Shen CP, Xiao JC, Goulet MT, Hagmann WK. Conformational Analysis and Receptor Docking of N-[(1S,2S)-3-(4-Chlorophenyl)-2-(3-Cyanophenyl)-1-Methylpropyl]-2-Methyl-2-{[5-(Trifluoromethyl)Pyridin-2-yl]oxy}Propanamide (Taranabant, MK-0364), a Novel, Acyclic Cannabinoid-1 Receptor Inverse Agonist. J Med Chem. 2008;51(7):2108–2114. doi: 10.1021/jm7014974. [DOI] [PubMed] [Google Scholar]
- 292.Tedesco L, Valerio A, Cervino C, Cardile A, Pagano C, Vettor R, Pasquali R, Carruba MO, Marsicano G, Lutz B, Pagotto U, Nisoli E. Cannabinoid Type 1 Receptor Blockade Promotes Mitochondrial Biogenesis through Endothelial Nitric Oxide Synthase Expression in White Adipocytes. Diabetes. 2008;57(8):2028–2036. doi: 10.2337/db07-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Liu YL, Connoley IP, Wilson CA, Stock MJ. Effects of the Cannabinoid CB1 Receptor Antagonist SR141716 on Oxygen Consumption and Soleus Muscle Glucose Uptake in Lep(ob)/Lep(ob) Mice. Int J Obes. 2005;29(2):183–187. doi: 10.1038/sj.ijo.0802847. [DOI] [PubMed] [Google Scholar]
- 294.Flamment M, Gueguen N, Wetterwald C, Simard G, Malthiery Y, Ducluzeau PH. Effects of the Cannabinoid CB1 Antagonist Rimonabant on Hepatic Mitochondrial Function in Rats Fed a High-Fat Diet. Am J Physiol Endocrinol Metab. 2009;297(5):E1162–1170. doi: 10.1152/ajpendo.00169.2009. [DOI] [PubMed] [Google Scholar]
- 295.Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, Hamm CW, Montalescot G, Steg PG, Pearson TA, Cohen E, Gaudin C, Job B, Murphy JH, Bhatt DL Investigators C. Rimonabant for Prevention of Cardiovascular Events (CRESCENDO): A Randomised, Multicentre, Placebo-Controlled Trial. Lancet. 2010;376(9740):517–523. doi: 10.1016/S0140-6736(10)60935-X. [DOI] [PubMed] [Google Scholar]
- 296.Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohorquez SM, Stoch A, Stevens C, Fong TM, De Lepeleire I, Cilissen C, Cote J, Rosko K, Gendrano IN, 3rd, Nguyen AM, Gumbiner B, Rothenberg P, de Hoon J, Bormans G, Depre M, Eng WS, Ravussin E, Klein S, Blundell J, Herman GA, Burns HD, Hargreaves RJ, Wagner J, Gottesdiener K, Amatruda JM, Heymsfield SB. The Acyclic CB1R Inverse Agonist Taranabant Mediates Weight Loss by Increasing Energy Expenditure and Decreasing Caloric Intake. Cell Metab. 2008;7(1):68–78. doi: 10.1016/j.cmet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 297.Rapport MM, Green AA, Page IH. Serum Vasoconstrictor, Serotonin; Isolation and Characterization. J Biol Chem. 1948;176(3):1243–1251. [PubMed] [Google Scholar]
- 298.Ismaiel AM, Titeler M, Miller KJ, Smith TS, Glennon RA. 5-HT1 and 5-HT2 Binding Profiles of the Serotonergic Agents Alpha-Methylserotonin and 2-Methylserotonin. J Med Chem. 1990;33(2):755–758. doi: 10.1021/jm00164a046. [DOI] [PubMed] [Google Scholar]
- 299.Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL. A New Method to Measure Brain Serotonin Synthesis In Vivo. I. Theory and Basic Data for a Biological Model. J Cereb Blood Flow Metab. 1990;10(1):1–12. doi: 10.1038/jcbfm.1990.1. [DOI] [PubMed] [Google Scholar]
- 300.Glennon RA. Discriminative Stimulus Properties of the Serotonergic Agent 1-(2,5-Dimethoxy-4-Iodophenyl)-2-Aminopropane (DOI) Life Sci. 1986;39(9):825–830. doi: 10.1016/0024-3205(86)90461-3. [DOI] [PubMed] [Google Scholar]
- 301.Glennon RA, McKenney JD, Lyon RA, Titeler M. 5-HT1 and 5-HT2 Binding Characteristics of 1-(2,5-Dimethoxy-4-Bromophenyl)-2-Aminopropane Analogues. J Med Chem. 1986;29(2):194–199. doi: 10.1021/jm00152a005. [DOI] [PubMed] [Google Scholar]
- 302.Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, Iredale PA. CP-809,101, a Selective 5-HT2C Agonist, Shows Activity in Animal Models of Antipsychotic Activity. Neuropharmacology. 2007;52(2):279–290. doi: 10.1016/j.neuropharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 303.Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP. SB 242084, a Selective and Brain Penetrant 5-HT2C Receptor Antagonist. Neuropharmacology. 1997;36(4–5):609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- 304.Heifetz A, Storer RI, McMurray G, James T, Morao I, Aldeghi M, Bodkin MJ, Biggin; PC. Application of an Integrated GPCR SAR-Modeling Platform to Explain the Activation Selectivity of Human 5-HT2C over 5-HT2B. ACS Chem Biol. 2016 doi: 10.1021/acschembio.5b01045. [DOI] [PubMed] [Google Scholar]
- 305.Wong DT, Horng JS, Bymaster FP, Hauser KL, Molloy BB. A Selective Inhibitor of Serotonin Uptake: Lilly 110140, 3-(p-Trifluoromethylphenoxy)-N-Methyl-3-Phenylpropylamine. Life Sci. 1974;15(3):471–479. doi: 10.1016/0024-3205(74)90345-2. [DOI] [PubMed] [Google Scholar]
- 306.Andersen J, Kristensen AS, Bang-Andersen B, Stromgaard K. Recent Advances in the Understanding of the Interaction of Antidepressant Drugs with Serotonin and Norepinephrine Transporters. Chem Commun (Camb) 2009;(25):3677–3692. doi: 10.1039/b903035m. [DOI] [PubMed] [Google Scholar]
- 307.Henry LK, Field JR, Adkins EM, Parnas ML, Vaughan RA, Zou MF, Newman AH, Blakely RD. Tyr-95 and Ile-172 in Transmembrane Segments 1 and 3 of Human Serotonin Transporters Interact to Establish High Affinity Recognition of Antidepressants. J Biol Chem. 2006;281(4):2012–2023. doi: 10.1074/jbc.M505055200. [DOI] [PubMed] [Google Scholar]
- 308.Braz GR, Freitas CM, Nascimento L, Pedroza AA, da Silva AI, Lagranha C. Neonatal SSRI Exposure Improves Mitochondrial Function and Antioxidant Defense in Rat Heart. Appl Physiol Nutr Metab. 2016;41(4):362–369. doi: 10.1139/apnm-2015-0494. [DOI] [PubMed] [Google Scholar]
- 309.Garrett SM, Whitaker RM, Beeson CC, Schnellmann RG. Agonism of the 5-Hydroxytryptamine 1F Receptor Promotes Mitochondrial Biogenesis and Recovery from Acute Kidney Injury. J Pharmacol Exp Ther. 2014;350(2):257–264. doi: 10.1124/jpet.114.214700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rasbach KA, Funk JA, Jayavelu T, Green PT, Schnellmann RG. 5-Hydroxytryptamine Receptor Stimulation of Mitochondrial Biogenesis. J Pharmacol Exp Ther. 2010;332(2):632–639. doi: 10.1124/jpet.109.159947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Harmon JL, Wills LP, McOmish CE, Demireva EY, Gingrich JA, Beeson CC, Schnellmann RG. 5-HT2 Receptor Regulation of Mitochondrial Genes: Unexpected Pharmacological Effects of Agonists and Antagonists. J Pharmacol Exp Ther. 2016;357(1):1–9. doi: 10.1124/jpet.115.228395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sneader W. The Discovery and Synthesis of Epinephrine. Drug News Perspect. 2001;14(8):491–494. doi: 10.1358/dnp.2001.14.8.858417. [DOI] [PubMed] [Google Scholar]
- 313.Chapple C, Khullar V, Nitti VW, Frankel J, Herschorn S, Kaper M, Blauwet MB, Siddiqui E. Efficacy of the Beta3-Adrenoceptor Agonist Mirabegron for the Treatment of Overactive Bladder by Severity of Incontinence at Baseline: A Post Hoc Analysis of Pooled Data from Three Randomised Phase 3 Trials. Eur Urol. 2015;67(1):11–14. doi: 10.1016/j.eururo.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 314.Konzett H, Rössler R. Versuchsanordnung zu Untersuchungen an der Bronchiàlmuskulatur. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1940;195(1):71–74. [Google Scholar]
- 315.Muller TD, Lee SJ, Jastroch M, Kabra D, Stemmer K, Aichler M, Abplanalp B, Ananthakrishnan G, Bhardwaj N, Collins S, Divanovic S, Endele M, Finan B, Gao Y, Habegger KM, Hembree J, Heppner KM, Hofmann S, Holland J, Kuchler D, Kutschke M, Krishna R, Lehti M, Oelkrug R, Ottaway N, Perez-Tilve D, Raver C, Walch AK, Schriever SC, Speakman J, Tseng YH, Diaz-Meco M, Pfluger PT, Moscat J, Tschop MH. p62 Links Beta-Adrenergic Input to Mitochondrial Function and Thermogenesis. J Clin Invest. 2013;123(1):469–478. doi: 10.1172/JCI64209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Tuttle RR, Mills J. Dobutamine: Development of a New Catecholamine to Selectively Increase Cardiac Contractility. Circ Res. 1975;36(1):185–196. doi: 10.1161/01.res.36.1.185. [DOI] [PubMed] [Google Scholar]
- 317.Wang Y, Wang Y, Yang D, Yu X, Li H, Lv X, Lu D, Wang H. Beta(1)-Adrenoceptor Stimulation Promotes LPS-Induced Cardiomyocyte Apoptosis through Activating PKA and Enhancing CaMKII and IkappaBalpha Phosphorylation. Crit Care. 2015;19:76. doi: 10.1186/s13054-015-0820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Regardh CG, Borg KO, Johansson R, Johnsson G, Palmer L. Pharmacokinetic Studies on the Selective Beta1-Receptor Antagonist Metoprolol in Man. J Pharmacokinet Biopharm. 1974;2(4):347–364. doi: 10.1007/BF01061407. [DOI] [PubMed] [Google Scholar]
- 319.Sharma V, Dhillon P, Parsons H, Allard MF, McNeill JH. Metoprolol Represses PGC1alpha-Mediated Carnitine Palmitoyltransferase-1B Expression in the Diabetic Heart. Eur J Pharmacol. 2009;607(1–3):156–166. doi: 10.1016/j.ejphar.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 320.Sharma V, Dhillon P, Wambolt R, Parsons H, Brownsey R, Allard MF, McNeill JH. Metoprolol Improves Cardiac Function and Modulates Cardiac Metabolism in the Streptozotocin-Diabetic Rat. Am J Physiol Heart Circ Physiol. 2008;294(4):H1609–1620. doi: 10.1152/ajpheart.00949.2007. [DOI] [PubMed] [Google Scholar]
- 321.Ida H. General Pharmacology of (Alpha RS)-3-Formamido-4-Hydroxy-Alpha-[[[(Alpha RS)-p-Methoxy-Alpha-Methylphenethyl]Amino]Methyl] Benzyl Alcohol Fumarate Dihydrate (BD 40A), a New Bronchodilator Agent (Author’s Transl) Nippon Yakurigaku Zasshi. 1980;76(7):633–654. [PubMed] [Google Scholar]
- 322.Rominger KL, Pollmann W. Comparative Pharmacokinetic Studies on Fenoterol-Hydrobromide in Rat, Dog and Man. Arzneimittelforschung. 1972;22(7):1190–1196. [PubMed] [Google Scholar]
- 323.Yoshizaki S, Manabe Y, Tamada S, Nakagawa K, Tei S. Isomers of Erythro-5-(1-Hydroxy-2-Isopropylaminobutyl)-8-Hydroxycarbostyril, a New Bronchodilator. J Med Chem. 1977;20(8):1103–1104. doi: 10.1021/jm00218a024. [DOI] [PubMed] [Google Scholar]
- 324.Peterson YK, Cameron RB, Wills LP, Trager RE, Lindsey CC, Beeson CC, Schnellmann RG. Beta2-Adrenoceptor Agonists in the Regulation of Mitochondrial Biogenesis. Bioorg Med Chem Lett. 2013;23(19):5376–5381. doi: 10.1016/j.bmcl.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, Schnellmann RG. The Beta2-Adrenoceptor Agonist Formoterol Stimulates Mitochondrial Biogenesis. J Pharmacol Exp Ther. 2012;342(1):106–118. doi: 10.1124/jpet.112.191528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, Schnellmann RG. Formoterol Restores Mitochondrial and Renal Function after Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2014;25(6):1157–1162. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Nolte D, Laumen F. Pulmonary Function Tests Using the Broncholytic Agent NAB 365. Med Monatsschr. 1972;26(7):325–328. [PubMed] [Google Scholar]
- 328.Lands AM, Luduena FP, Hoppe JO, Oyen IH. The Pharmacologic Actions of the Bronchodilator Drug, Isoetharine. J Am Pharm Assoc Am Pharm Assoc. 1958;47(10):744–748. [PubMed] [Google Scholar]
- 329.Jesinkey SR, Korrapati MC, Rasbach KA, Beeson CC, Schnellmann RG. Atomoxetine Prevents Dexamethasone-Induced Skeletal Muscle Atrophy in Mice. J Pharmacol Exp Ther. 2014;351(3):663–673. doi: 10.1124/jpet.114.217380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ott IM, Alter ML, von Websky K, Kretschmer A, Tsuprykov O, Sharkovska Y, Krause-Relle K, Raila J, Henze A, Stasch JP, Hocher B. Effects of Stimulation of Soluble Guanylate Cyclase on Diabetic Nephropathy in Diabetic eNOS Knockout Mice on Top of Angiotensin II Receptor Blockade. PLoS ONE. 2012;7(8):e42623. doi: 10.1371/journal.pone.0042623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wassermann AM, Lounkine E, Hoepfner D, Le Goff G, King FJ, Studer C, Peltier JM, Grippo ML, Prindle V, Tao J, Schuffenhauer A, Wallace IM, Chen S, Krastel P, Cobos-Correa A, Parker CN, Davies JW, Glick M. Dark Chemical Matter as a Promising Starting Point for Drug Lead Discovery. Nat Chem Biol. 2015;11(12):958–966. doi: 10.1038/nchembio.1936. [DOI] [PubMed] [Google Scholar]