Abstract
Forkhead box M1 (FOXM1) is a late cell cycle gene that plays a crucial role in carcinogenesis and chemotherapeutic drug resistance. In this study, the impact of FOXM1 expression on patient outcome was investigated for the first time in formalin fixed and paraffin embedded (FFPE) samples of chemotherapy naïve muscle-invasive bladder cancer (MIBC) patients. Expression analyses were performed on the Mannheim cohort (n=84) and validated on the independent Chungbuk cohort (n=61). In a Cox’ proportional hazards model, a distinct FOXM1 expression cut-off dividing both cohorts in a ‘high-risk’ and ‘low-risk’ group has been determined. Multivariate analyses showed that FOXM1 is an independent risk factor for outcome prediction superior to the TNM system. The FOXM1 ‘high-risk’ group had a 4- to 7-fold increased risk of death (p<0.03) and presented further an overexpression of MKI67. Recent studies showed that MIBCs can be subclassified in breast cancer-like subtypes: basal, luminal and p53-like. Here we demonstrated that FOXM1 was differentially expressed between MIBC subtypes concordant to its subtype specific expression in breast cancer. Since the proto-oncogene FOXM1 is known to play an important role in cisplatin resistance and to be a promising drug target, this study supports FOXM1 as a crucial biomarker in the personalization of MIBC therapy and urges prospective translational studies.
Keywords: FOXM1, muscle invasive bladder cancer, KI67, subclassification, survival prediction
INTRODUCTION
Bladder cancer is one of the 10 most common malignancies worldwide with nearly 386,000 new cases and nearly 150,200 deaths per year [1]. While, muscle-invasive bladder carcinoma (MIBC) account only for about 30-40% of the cases, they present the poorest outcome [2]. Patients with metastatic carcinoma have a 5-year overall survival rate of approximately 20% [3]. Additionally, due to the high costs of transurethral resections, cystectomies, chemotherapies and the often necessary lifelong surveillance, bladder cancer is one of the most expensive cancer entities averaged over all US citizens [4].
The current standard of care in MIBC is radical cystectomy. High risk patients are frequently treated with a perioperative platin-based chemotherapy. At the moment, the diagnostic and therapy of MIBC suffers from at least two major problems. First, the therapy selection is heavily influenced by an insufficient clinicopathologic staging system for survival and therapy response prediction, as clinical understaging occurs in 46% of the cases [5]. Second, unlike in other tumor entities, there are no prognostic biomarkers established in the clinical routine. Yet, the search for biomarkers may improve prognostication and personalization of bladder cancer therapy and may pave the way to new targeted neoadjuvant therapy options [6]. In this context, several studies subclassified MIBC through gene expression profiling [7–9]. Those studies identified distinct molecular subtypes which correlated well with outcome and therapy response and were similar to the molecular phenotypes of breast cancer.
The forkhead box M1 gene is known to be involved in many of the hallmarks of carcinogenesis, e.g. cell cycle progression, angiogenesis, epithelial-mesenchymal transition, cell migration, genomic instability and formation of tumor metastasis [10–15]. Its expression is negatively regulated by the tumor suppressor gene TP53 and its downstream gene signature seems to be enriched in the SCC-like and Genomically Unstable bladder cancer subtype as defined by Sjödahl et al. [16–18]. FOXM1 is also associated with resistance development against numerous anti-cancer drugs (e.g. cisplatin, paclitaxel, docetaxel, gefitinib, epirubicin and trastuzumab) [19–25].
Meta-analyses showed that overexpression of FOXM1 was associated with poor prognosis in many solid cancers such as breast, gastric, non-small-cell lung, pancreatic and hepatocellular carcinoma [26–32]. FOXM1 has been shown to be overexpressed on mRNA level as well as on protein level in bladder cancer cells in comparison with normal urothelial cells [15].
This study investigates for the first time the impact of FOXM1 expression on outcome prediction and risk stratification in two independent MIBC cohorts. The Mannheim cohort includes patients treated exclusively by cystectomy in order to investigate the genuine cours of disease for patients with high versus low FOXM1 expression. The Chungbuk cohort includes patients with adjuvant cisplatin therapy in order to investigate the role of FOXM1 expression in the context of cisplatin resistance. Recent transcriptome expression studies showed the existence of molecular subtypes (basal, luminal and p53-like) similar to breast cancer molecular phenotypes.
In this context, FOXM1 was tested for differential expression in the luminal, basal and p53-like bladder cancer subtypes [7, 8]. In this study, the latter has been renamed as, non-luminal non-basal’ (NLNB) given the lack of validation concerning this MIBC subtype [33]. As FOXM1 is a druggable proto-oncogene, the elucidation of its impact on bladder cancer survival may contribute to a further personalization of future MIBC therapy [34].
RESULTS
Cohort characteristics
Clinicopathologic characteristics of the Mannheim and the Chungbuk cohort are listed in Table 1 and Table 2. The 10-year overall survival (OS), disease specific survival (DSS) and progression-free survival (PFS) of 84 patients with MIBC enrolled in the Mannheim cohort were 35%, 50% and 41% respectively. From the Chungbuk cohort (n=165) only patients with muscle-invasive carcinoma were selected, resulting in a cohort of 61 patients with a 10-year OS, DSS and PFS of 40%, 45% and 49% respectively. In the Mannheim cohort, patients were exclusively treated with radical cystectomy and lymphadenectomy in contrast to the Chungbuk cohort, where 43% of the patients received adjuvant systemic chemotherapy after radical cystectomy or transurethral resection (TUR). The risk stratification according to a high or low expression of FOXM1 resulted in an equal distribution of patient characteristics in both cohorts between risk groups, except for low grade tumors in the Chungbuk cohort, which correlated significantly with low FOXM1 expression (p=0.023). Comparing tumor stage and grade, the Mannheim cohort included more advanced disease features.
Table 1. Clinicopathologic characteristics of the Mannheim cohort (n=84): High risk versus low risk MIBCs according to FOXM1 expression.
| Total | % | High Risk | % | Low Risk | % | p-values | |
|---|---|---|---|---|---|---|---|
| Cohort Characteristics | |||||||
| Cohort size | 84 | 54 | (64) | 30 | (36) | ||
| median age | 66 | 66 | 64 | 0.685 | |||
| female | 20 | (24) | 14 | (26) | 6 | (20) | 0.603 |
| male | 64 | (76) | 40 | (74) | 24 | (80) | |
| Progress (n=76) | 43 | (57) | 29 | (62) | 14 | (33) | 0.182 |
| TNM Staging | |||||||
| pT1 | 1 | (1) | 0 | (0) | 1 | (3) | 0.34 |
| pT2 | 21 | (25) | 16 | (30) | 5 | (17) | |
| pT3 | 47 | (56) | 29 | (54) | 18 | (60) | |
| pT4 | 15 | (18) | 9 | (17) | 6 | (20) | |
| pN+ (n=82) | 31 | (38) | 18 | (35) | 13 | (43) | 0.483 |
| cM+ (n=70) | 7 | (10) | 6 | (14) | 1 | (4) | 0.236 |
| Grading | |||||||
| G2 | 17 | (20) | 8 | (15) | 9 | (30) | 0.086 |
| G3 | 67 | (80) | 46 | (85) | 21 | (70) | |
| Additional Therapy (n=81) | |||||||
| NAC | 1 | (1) | 1 | (2) | 0 | (0) | 1.0 |
| AC | 11 | (14) | 7 | (14) | 4 | (14) | 0.607 |
Table 2. Clinicopathologic characteristics of the Chungbuk cohort (n=61): High risk versus low risk MIBCs according to FOXM1 expression.
| Total | % | High Risk | % | Low Risk | % | p-values | |
|---|---|---|---|---|---|---|---|
| Cohort Characteristics | |||||||
| Cohort size | 61 | 10 | (16) | 51 | (84) | ||
| Median age | 66 | 73 | 66 | 0.051 | |||
| Female | 13 | (21) | 4 | (40) | 9 | (18) | 0.198 |
| Male | 48 | (79) | 6 | (60) | 42 | (82) | |
| Progress | 20 | (33) | 3 | (30) | 17 | (33) | 1.000 |
| TNM Staging | |||||||
| pT2 | 31 | (51) | 4 | (40) | 27 | (53) | 0.740 |
| pT3 | 19 | (31) | 4 | (40) | 15 | (29) | |
| pT4 | 11 | (18) | 2 | (20) | 9 | (18) | |
| pN+ | 14 | (23) | 4 | (40) | 10 | (20) | 0.222 |
| cM+ | 6 | (10) | 0 | (0) | 6 | (12) | 0.577 |
| Grading | |||||||
| Low grade | 19 | (31) | 0 | (0) | 19 | (37) | 0.023 |
| High grade | 42 | (69) | 10 | (100) | 32 | (63) | |
| Additional Therapy | |||||||
| Systemic chemotherapy | 26 | (43) | 3 | (30) | 23 | (45) | 0.494 |
FOXM1 is an independent risk factor for survival prediction
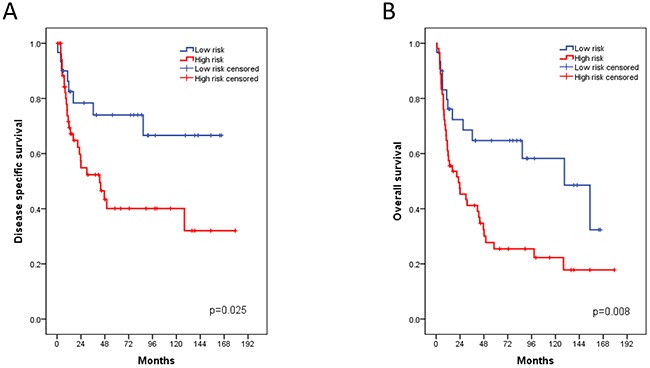
The qRT-PCR analyses of the Mannheim cohort yielded a FOXM1 expression range from 30.7 to 35.5 (40-dCT) (median: 33.5) after normalization to the housekeeping gene CALM2. The FOXM1 cut-off value of 33.1 (40-dCt) showed best discrimination between a high risk and a low risk group. In order to determine the impact of FOXM1 expression on OS, DSS and PFS, we performed Cox's regression analysis, adjusted for grade, gender, TNM, therapy and low versus high FOXM1 expression (Table 3). The risk stratification between low and high FOXM1 expression allowed a concise discrimination between a high risk and low risk group. Multivariate analysis showed that patients with high FOXM1 expression have a 4- to 7-fold higher risk of death, by means of OS and DSS respectively (p<0.003). This risk stratification displayed higher HR than lymph node metastasis and tumor stage in the context of OS and DSS. Thus, FOXM1 presented an independent risk factor for MIBC survival prediction. The Kaplan-Meier estimates showed a 10-year DSS of high-risk versus low-risk patients according to FOXM1 expression of 40% versus 67% (p=0.017) and a 10-year OS of 22% versus 58% (p=0.04, Figure 1). However, this FOXM1 risk stratification gave no additional information concerning PFS (log rank test: p=0.09).
Table 3. Multivariate Cox regression analysis of DSS and OS for the Mannheim cohort after adjustment for standard clinicopathologic characteristics.
| DSS | OS | |||||
|---|---|---|---|---|---|---|
| Cox regression analysis | HR | 95% CI | p | HR | 95% CI | p |
| FOXM1 high vs. low | 6.76 | 2.36-19,35 | <0.003 | 4.18 | 1.96-8.88 | <0.003 |
| pN+ | 5.89 | 2.38-14,57 | <0.003 | 3.57 | 1.83-6.96 | <0.003 |
| pT1-2 vs. pT3-4 | 3.00 | 0.98-9,12 | 0.054 | n.s. | ||
Figure 1. Kaplan-Meier plots of the Mannheim cohort for disease specific (A) and overall survival (B) associated with the FOXM1 risk stratification.
Validation of the FOXM1 risk stratification in the chungbuk cohort
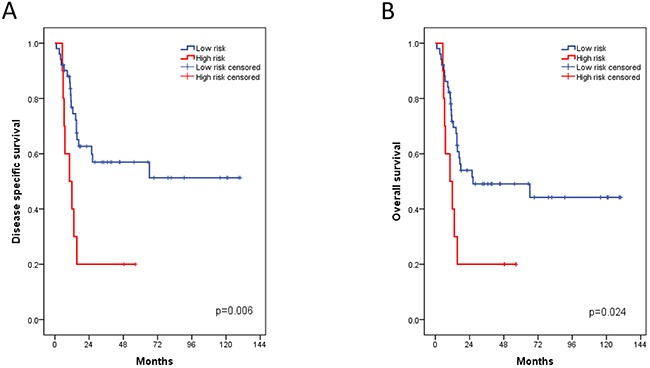
Despite of the differences in MIBC therapy and expression quantification methods, we managed as a first validation to define a clear FOXM1 cut-off in the Chungbuk cohort. Moreover the hazard ratios of the Chungbuk cohort for OS and DSS correlated with the risk stratification of the Mannheim cohort (Table 4). Indeed the FOXM1 log2 cut-off value of 9.7 (range: 7.1-11.6, median: 9.3) allowed a dichotomization of the cohort in a low risk group with low FOXM1 expression and a high risk group with high FOXM1 expression. In the multivariate analysis, adjusted for the same parameters as in the Mannheim cohort, the risk of death is up to 3 times higher in the high risk group for OS and DSS (p<0.03, Table 4). The Kaplan-Meier estimates showed a 4-year DSS of high-risk versus low-risk patients of 20% versus 57% (p=0.006) and a 4-year OS of 20% versus 49% (p=0.024, Figure 2). PFS showed no statistical significance in the log rank test (p=0.4). In both cohorts tumor grade (G2 versus G3) was not an independent risk factor whereas lymph node metastasis and tumor stage were strong survival predictors. FOXM1 was the only parameter that was found as an independent risk factor in the Mannheim and Chungbuk cohort for both OS and DSS (p<0.03).
Table 4. Multivariate Cox regression analysis of DSS and OS for the Chungbuk cohort after adjustment for standard clinicopathologic characteristics.
| DSS | OS | |||||
|---|---|---|---|---|---|---|
| Cox regression analysis | HR | 95% CI | p | HR | 95% CI | p |
| FOXM1 high vs. low | 3.27 | 1.36-7.87 | 0.008 | 2.87 | 1.22-6.75 | 0.016 |
| pN+ | 3.29 | 1.45-7.48 | 0.004 | n.s. | ||
| pT2 vs. pT3-4 | 2.53 | 1.08-5.95 | 0.033 | 2.44 | 1.16-5.15 | 0.019 |
| cM+ | n.s. | 4.65 | 1.81-11.94 | <0.003 | ||
Figure 2. Kaplan-Meier plots of the Chungbuk cohort for disease specific (A) and overall survival (B) associated with the FOXM1 risk stratification.
Subtype specific expression of FOXM1
Recent studies showed that muscle-invasive bladder cancer can be subclassified similarly to the molecular phenotypes of breast cancer into basal, luminal and NLNB subtypes. These subtypes have presented an impact on patient outcome and a differential expression of drug targets (e.g. ERBB2-3, FGFR1-4, ESR1) [7–9]. For MIBC subclassification, subtype specific genes from consensus data tested in silico for subtype enrichment were collected [33, 35, 36]. For both cohorts, we used a 7-gene panel for MIUC subtyping consisting in a curated luminal (KRT20, GATA3), basal (KRT5, KRT6A, CDH3) and NLNB (SORBS1, CNN1) gene signature. These genes formed consistent clusters throughout cohorts, as shown in Figure 3 and 4.
Figure 3. MIBC subclassification of the Mannheim cohort by Nanostring nCounter analysis (n=30).
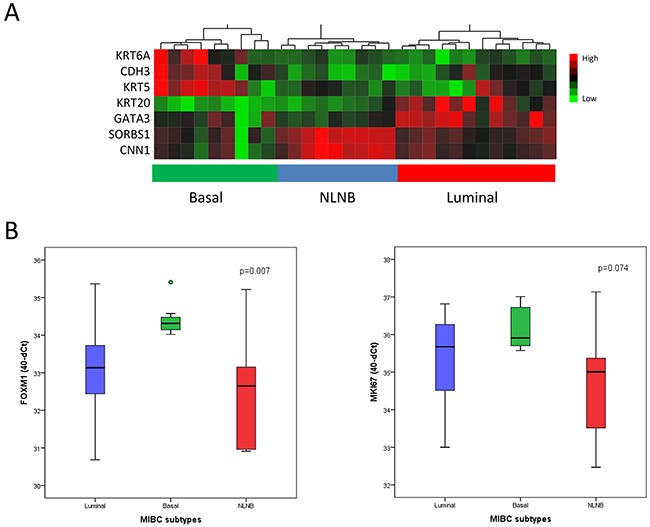
(A) Heatmap generated by unsupervised hierarchical clustering using a 7-gene signature. Luminal: KRT20, GATA3 (blue); basal: KRT6A, CDH3, KRT5 (green); non-luminal non-basal (NLNB): SORBS1, CNN1 (red). (B) Subtype specific expression of FOXM1 and MKI67 analyzed by qRT-PCR. Data are represented as median ±SD.
Figure 4. MIBC subclassification of the Chungbuk cohort by Illumina microarray analysis (n=42).
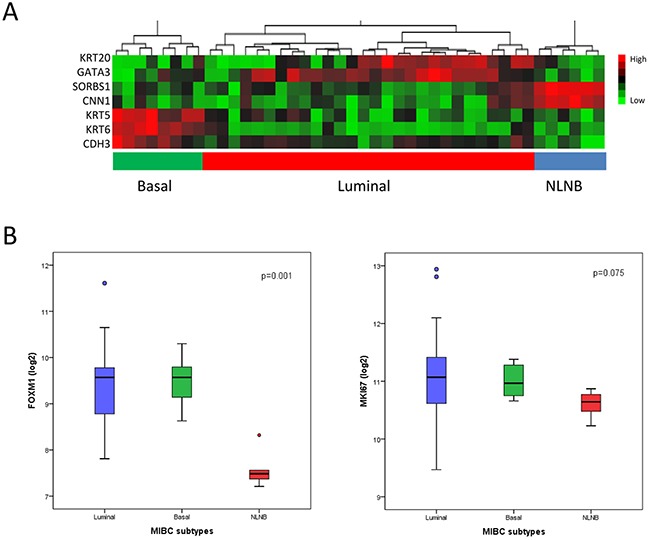
(A) Heatmap generated by unsupervised hierarchical clustering using a 7-gene signature. Luminal: KRT20, GATA3 (blue); basal: KRT6A, CDH3, KRT5 (green); NLNB: SORBS1, CNN1 (red). (B) Subtype specific expression of FOXM1 and MKI67 analyzed by qRT-PCR. Data are represented as median ±SD.
In the Mannheim cohort, FOXM1 showed a significant differential expression between subtypes, in particular a low expression in the p53-like subtype in comparison to the luminal and basal subtype (p=0.007, Figure 3B). These data were confirmed by the Chungbuk cohort, revealing as well a significant downregulation in the NLNB subtype (p=0.001, Figure 4B). The NLNB subtype is known to show good prognosis and to present immune infiltrative characteristics which may contribute to a dilution of the measured FOXM1 transcript level and thus distort the FOXM1 risk stratification [7, 37]. As a consequence the degree of immune infiltration was tested for the high and low risk group but showed no differential expression (Supplementary Figure 1, p>0.05).
Differential expression of MKI67 between FOXM1 risk groups and MIBC subtypes
As reported in previous studies FOXM1 regulates cell proliferation and invasion. Therefore we further investigated the differential expression of the proliferation marker MKI67 between the risk groups and MIBC subtypes (Figure 3–5). The expression of MKI67, determined by qRT-PCR, positively correlated with high FOXM1 expression in the Mannheim cohort (p=0.001). The same tendency could be observed in the Chungbuk cohort but without significance (p=0.167, Figure 5). The NLNB subtype, known to have a favorable outcome, presented the lowest MKI67 expression (Figure 4B). The Mannheim cohort confirmed this trend but without statistical significance (p=0.074, Figure 3B).
Figure 5. Differential expression of MKI67 between risk groups.
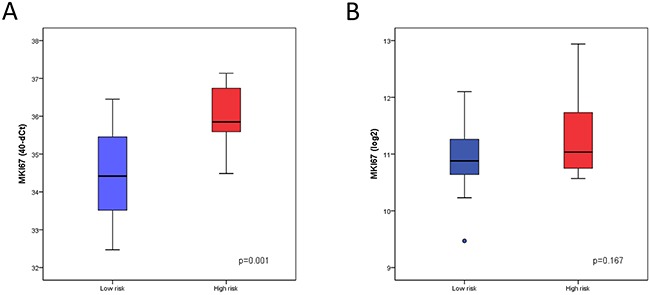
(A) Expression analysis of MKI67 determined by qRT-PCR in the low risk group (n=30) and high risk group (n=54) of the Mannheim cohort. (B) Expression analysis of MKI67 determined by Illumina microarray analysis in the low risk group (n=10) and high risk group (n=51) of the Chungbuk cohort.
DISCUSSION
The impact of the FOXM1 gene expression on survival has been investigated on a large scale of solid tumors and was associated with poor prognosis throughout these studies [26]. In the present study the prognostic value of FOXM1 has been evaluated for the first time in urothelial bladder cancer.
We stratified patients from two different cohorts according to their FOXM1 expression into two risk groups. Patients with a high expression of FOXM1 showed a 4- to 7-fold higher risk of death. The FOXM1 risk stratification even appeared superior to the TNM staging system for the prediction of DSS and OS in the Mannheim cohort. However, FOXM1 expression had no impact on predicting PFS in both cohorts. Nevertheless, FOXM1 allowed a consistent risk stratification and was confirmed to be an independent risk factor in multivariate Cox’ regression analysis. As mentioned above, high expression of FOXM1 had already been confirmed to be related to bad prognosis in many other cancer entities. From a biological point of view this seems plausible given the many roles of this protooncogene in cancerogenesis [26, 38].
In the Mannheim cohort 64% of the patients were ranked in the high risk group in contrast to the 16% of the Chungbuk cohort. Whether this is due to different quantification methods or therapy options between both cohorts remained unclear. However, this may partly be explained by the positive correlation of FOXM1 with cell proliferation, which enhances chemosensitivity [39]. Yet, exceeding a certain transcript level of FOXM1 may constitute a point of no return for resistance development. The Chungbuk cohort showed further 31% low grade tumors despite the selection of muscle invasive (>T2) tumors. As MIBC are rarely low grade, we cannot explain this high percentage.
In both cohorts tumor grade was not an independent risk factor. Tumor grade is known to have lower impact in muscle-invasive bladder cancer, as these tumors are mainly high-grade. Furthermore, radical cystectomy diminishes its predictive power [40–42]. In concordance with previous studies, lymph node metastasis and tumor stage were strong predictors in both cohorts [30]. Large scale studies showed MKI67 to be a valuable biomarker for outcome prediction in localized urothelial carcinoma but provided in advanced bladder cancer no additional information in comparison to tumor stage in advanced bladder cancer [43, 44]. Also in this study, MKI67 has not been retained in multivariate analyses.
In order to further investigate the translational impact of FOXM1 in MIBC, we analyzed its expression in the recent context of MIBC subclassification. We showed that FOXM1 is consistently enriched in the basal and luminal subtypes and suppressed in the NLNB subtype. These findings can be paralleled with data concerning the basal breast cancer subtype (triple negative), which also showed a FOXM1 overexpression and a poorer outcome [45–47]. Interestingly, our findings showed a considerable down regulation of FOXM1 in a MIBC subclass characterized by an activated signature of TP53 downstream genes [7]. Those have already been shown to be influential transcription factors in the downregulation of FOXM1 [16]. Thus, an overexpression of this proto-oncogene may be a surrogate marker for TP53 pathway inactivation, which is often associated with alterations common in aggressive urothelial carcinoma and correlates with the poor outcome of basal and luminal MIBC as described in recent studies [7, 16, 48, 49]. However, Choi et al. showed that the p53-like subtype had the highest rate of cisplatin non-responders. Considering the proliferation marker KI67, the NLNB subtype seemed to present a more quiescent subtype, which might have led to the described cisplatin resistance and thus present a FOXM1 independent resistance mechanism. On the other hand, this hypothesis needs further validation as the FOXM1 expression in the NLNB subtype may be diluted by inflammatory cells, though immune markers showed no enrichment in the FOXM1 low expression group in this study [37].
The in vitro knockdown of FOXM1 in a bladder cancer cell line showed a decrease of cell migration and proliferation [15, 45]. The same has been shown for the triple negative basal breast cancer cells [45]. In accordance with this, MKI67 has been positively correlated with FOXM1 expression in the Mannheim cohort (p=0.01), indicating a higher proliferation in MIBCs of the high risk group (Figure 5). Also of note is the presence of different FOXM1 isoforms, with FOXM1b exclusively expressed in cancer cells and FOXM1c with enhanced transforming potential [50]. As in this study all isoforms were covered by the different quantification methods, further investigations are warranted.
MIBC patients of our high risk group may profit from various direct FOXM1 inhibitors like siomycin A, thiostrepton and bortezomib [51, 52]. As luminal and basal bladder cancer subtypes are suspected to present a subtype specific overexpression of other well known drug targets like the FGFR, EGFR and ERBB gene families, the number of promising personalized therapy options rises for FOXM1 enriched MIBC [36]. The potential role in chemotherapy resistance against cisplatin, argues for further translational investigations on potential interactions of FOXM1 with current treatment options. As this biomarker improved survival prediction, FOXM1 may be integrated in future biomarker panels for molecular characterization of bladder cancer. Given the different quantification platforms between cohorts in this study, further prospective validation is needed. It has further been shown that elevated FOXM1 transcript levels in bladder cancer correlated with its protein expression. Thus, immunohistochemistry may also be a valuable tool in the detection of patients a risk [15]. The molecular phenotype may provide a superior tool for survival prediction than the TNM staging, given the heterogeneity of bladder cancer biology and the need for therapy personalization [53–56]. Since FOXM1 itself is praised to be a promising drug target in many solid tumor entities [21, 34, 38], translational studies are needed in order to implement FOXM1 in the race for MIBC therapy personalization.
MATERIALS AND METHODS
Patient population and specimen collection
Formalin fixed paraffin embedded (FFPE) tumor tissue samples were obtained from cystectomy of 84 muscle-invasive urothelial carcinoma patients (pT2-4, N0/1), who were treated exclusively with radical cystectomy in conjunction with bilateral lymphadenectomy (only 14% received a platin based combination therapy) at the University Medical Center Mannheim between July 1998 and January 2006. All patients gave informed consent. The retrospective analysis was approved by the relevant institutional review board under number 2016-814R-MA. The samples were evaluated for pathological stage according to the 2002 TNM classification of the American Joint Committee on Cancer. Histopathological parameters of cases were assessed by a pathologist specialized in uropathology (AH).
In order to validate our results in silico, we studied array expression data of 61 MIBC patients of the Chungbuck cohort (GSE13507). Tissue and histopathologic staging were obtained by cystectomy in muscle-invasive bladder cancer. MIBC patients were treated with at least 4 cycles of cisplatin-based chemotherapy as described before [57]. Patients with squamous cell carcinoma were excluded as they may distort clustering and deflect from the genuine pathophysiology of muscle-invasive transitional cell carcinoma. Grading of this cohort was assessed according to WHO grading classification 2004.
Expression analysis of FOXM1 and MKI67 in the Mannheim cohort
RNA was extracted from 81 FFPE samples of MIBC patients according to a fully automated, high-throughput extraction workflow which runs on an Xtract XL liquid-handling robot (STRATIFYER Molecular Pathology GmbH, Cologne, Germany). One-step qRT-PCR was applied for the relative quantification of FOXM1 and MKI67 mRNA by using TaqMan quantitative RT-PCR. Calmodulin 2 (CALM2) was used as reference gene [58–60]. Gene expression has been assessed in duplicates by qRT-PCR using the SuperScript III PLATINUM One-Step quantitative RT-PCR System (Invitrogen, Karlsruhe, Germany) on a Stratagene Mx3005p (Agilent Technologies, Böblingen, Germany). FOXM1 expression analysis was performed with the following primers covering all isoforms (forward 5’-GACCACCTGGAGCCCTTTG-3’, reverse 5’-GATGTTGGATAGGCTATTGTTGATAGTG-3’, Tamra probe 5’- AGAAACGGGAGACCTGTGCAGATG-3’). MKI67 expression analysis was performed with the following primers (forward 5’-CGAGACGCCTGGTTACTATCAA-3’, reverse 5’-GGATACGGATGTCACATTCAATACC-3’, Tamra probe 5’-ACGGTCCCCACTTTCCCCTGAGC-3’). Ct values were normalized by subtracting the Cq value of the endogenous reference gene CALM2 from the Ct value of the target genes (ΔCt) [60]. Expression results were then reported as 40-ΔCq values which correlate proportionally with the mRNA expression level of the target genes.
Statistical analysis
Clinico-demographic characteristics were compared with Fishers exact test, the Mann-Whitney U-test and the Kruskal-Wallis test. A distinct FOXM1 cut-off for risk stratification by means of survival prediction was determined by comparing iteratively the HR for different cut-off levels with the Cox proportional hazards model. The cut-off with highest HR was considered as appropriate for discrimination between a high risk and a low risk group. Kaplan-Meier estimates together with the log-rank test were used for survival analysis. The level of significance was <0.05. The primary endpoints were disease specific survival (DSS) and overall survival (OS) defined as death for any reason. Also progression-free survival (PFS) was recorded as time interval between cystectomy with lymphadenectomy and local or metastatic progression. Statistical analyses were performed with SPSS software version 20 (IBM, Armonk, NY, USA).
Subclassification of MIBC patients and data validation
For MIBC subclassification, subtype specific genes from consensus data tested insilico for subtype enrichment were collected [33, 35, 36]. For both cohorts, we used a 7-gene panel for MIUC subtyping consisting in a curated luminal (KRT20, GATA3), basal (KRT5, KRT6A, CDH3) and p53-like (SORBS1, CNN1) gene signature. Patients were assigned to the different subtypes by the Ward unsupervised hierarchical clustering method using the JMP software version 12 (SAS Institute, Cary, NC, USA). The subtype specific expression of FOXM1 and MKI67 was verified by the Kruskal-Wallis test.
The MIBC subclassification was performed by the nCounter technology (Nanostring, Seattle, WA, USA) on a subgroup of the Mannheim cohort [61]. An amount of 100ng total RNA was used as input after quality control with qPCR and Nanodrop 1000 (Thermo Scientific, Wilmington, DE, USA). Preprocessing was performed by the nSolver Software 2.5 (Nanostring). The nCounter assay was normalized using the geometric mean of 6 reference genes and 6 positive controls. Reference genes were selected in order to cover low expression (G6PD, TUBB) as well as high expression genes (B2M, CALM2, GAPDH and RPL37A) with a Spearman correlation of at least 0.40 and showed a mean probe normalization factor of 1.3. Negative background substraction was performed by 8 negative controls.
Validation of MIBC risk stratification and subclassification was performed in silico on the Chungbuk cohort (GSE13507) based on Illumina human-6 v2.0 beadchip data of 61 MIBC patients. Preprocessing of array data was realized by the Illumina BeadStudio software using quantile normalization and log2 transformation. Only high grade tumors were selected for MIBC subclassification given the heterogeneity of tumor grade in the Chungbuk cohort. Indeed, it has been shown that molecular subclasses vary strongly between tumor grade [62].
The other authors declare to have no conflicts of interest.
SUPPLEMENTARY MATERIALS FIGURES
Acknowledgments
We thank Annette Steidler, Elke Veltrup and Silke Claas for excellent technical assistance.
Footnotes
CONFLICTS OF INTEREST
RMW is an employee and founder of STRATIFYER Molecular Pathology GmbH.
FUNDING
TW was supported by the German Association of Urology (DGU; Eisenberger Stipendium).
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Fleshner NE, Herr HW, Stewart AK, Murphy GP, Mettlin C, Menck HR, The National Cancer Data Base report on bladder carcinoma The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1996;78:1505–13. doi: 10.1002/(sici)1097-0142(19961001)78:7<1505::aid-cncr19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 4.Svatek RS, Hollenbeck BK, Holmäng S, Lee R, Kim SP, Stenzl A, Lotan Y. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014;66:253–62. doi: 10.1016/j.eururo.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Svatek RS, Shariat SF, Novara G, Skinner EC, Fradet Y, Bastian PJ, Kamat AM, Kassouf W, Karakiewicz PI, Fritsche HM, Izawa JI, Tilki D, Ficarra V, et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011;107:898–904. doi: 10.1111/j.1464-410X.2010.09628.x. [DOI] [PubMed] [Google Scholar]
- 6.Shah JB, McConkey DJ, Dinney CPN. New strategies in muscle-invasive bladder cancer: on the road to personalized medicine. Clin Cancer Res. 2011;17:2608–12. doi: 10.1158/1078-0432.CCR-10-2770. [DOI] [PubMed] [Google Scholar]
- 7.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, Melquist J, Bondaruk J, Majewski T, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–65. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damrauer JS, Hoadley KA, Chism DD, Fan C, Tiganelli CJ, Wobker SE, Yeh JJ, Milowsky MI, Iyer G, Parker JS, Kim WY. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111:3110–5. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi W, Czerniak B, Ochoa A, Su X, Siefker-Radtke A, Dinney C, McConkey DJ. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat Rev Urol. 2014;11:400–10. doi: 10.1038/nrurol.2014.129. [DOI] [PubMed] [Google Scholar]
- 10.Laoukili J, Kooistra MRH, Brás A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–36. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 11.Kalin TV, Ustiyan V, Kalinichenko VV. Multiple faces of FoxM1 transcription factor: lessons from transgenic mouse models. Cell Cycle Georget Tex. 2011;10:396–405. doi: 10.4161/cc.10.3.14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HJ, Gusarova G, Wang Z, Carr JR, Li J, Kim KH, Qiu J, Park YD, Williamson PR, Hay N, Tyner AL, Lau LF, Costa RH, et al. Deregulation of FoxM1b leads to tumour metastasis. EMBO Mol Med. 2011;3:21–34. doi: 10.1002/emmm.201000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan X, Fu Y, Chen L, Lee W, Lai Y, Rezaei K, Tabbara S, Latham P, Teal CB, Man YG, Siegel RS, Brem RF, Fu SW. miR-671-5p inhibits epithelial-to-mesenchymal transition by downregulating FOXM1 expression in breast cancer. Oncotarget. 2016;7:293–307. doi: 10.18632/oncotarget.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67:8293–300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Zhang Z, Kong C. High FOXM1 expression was associated with bladder carcinogenesis. Tumour Biol. 2013;34:1131–8. doi: 10.1007/s13277-013-0654-x. [DOI] [PubMed] [Google Scholar]
- 16.Pandit B, Halasi M, Gartel AL. p53 negatively regulates expression of FoxM1. Cell Cycle. 2009;8:3425–7. doi: 10.4161/cc.8.20.9628. [DOI] [PubMed] [Google Scholar]
- 17.Barsotti AM, Prives C. Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene. 2009;28:4295–305. doi: 10.1038/onc.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksson P, Aine M, Veerla S, Liedberg F, Sjödahl G, Höglund M. Molecular subtypes of urothelial carcinoma are defined by specific gene regulatory systems. BMC Med Genomics. 2015;8:25. doi: 10.1186/s12920-015-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok JM, Peck B, Monteiro LJ, Schwenen HD, Millour J, Coombes RC, Myatt SS, Lam EW. FOXM1 confers acquired cisplatin resistance in breast cancer cells. Mol Cancer Res. 2010;8:24–34. doi: 10.1158/1541-7786.MCR-09-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halasi M, Gartel AL. FOX(M1) news--it is cancer. Mol Cancer Ther. 2013;12:245–54. doi: 10.1158/1535-7163.MCT-12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saba R, Alsayed A, Zacny JP, Dudek AZ. The Role of Forkhead Box Protein M1 in Breast Cancer Progression and Resistance to Therapy. Int J Breast Cancer. 2016;2016:9768183. doi: 10.1155/2016/9768183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khongkow P, Gomes AR, Gong C, Man EP, Tsang JW, Zhao F, Monteiro LJ, Coombes RC, Medema RH, Khoo US, Lam EW. Paclitaxel targets FOXM1 to regulate KIF20A in mitotic catastrophe and breast cancer paclitaxel resistance. Oncogene. 2016;35:990–1002. doi: 10.1038/onc.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carr JR, Park HJ, Wang Z, Kiefer MM, Raychaudhuri P. FoxM1 mediates resistance to herceptin and paclitaxel. Cancer Res. 2010;70:5054–63. doi: 10.1158/0008-5472.CAN-10-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millour J, de Olano N, Horimoto Y, Monteiro LJ, Langer JK, Aligue R, Hajji N, Lam EW. ATM and p53 regulate FOXM1 expression via E2F in breast cancer epirubicin treatment and resistance. Mol Cancer Ther. 2011;10:1046–58. doi: 10.1158/1535-7163.MCT-11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Qiu W, Liu B, Yao R, Liu S, Yao Y, Liang J. Forkhead box transcription factor 1 expression in gastric cancer: FOXM1 is a poor prognostic factor and mediates resistance to docetaxel. J Transl Med. 2013;11:204. doi: 10.1186/1479-5876-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai J, Yang L, Wang J, Xiao Y, Ruan Q. Prognostic Value of FOXM1 in Patients with Malignant Solid Tumor: A Meta-Analysis and System Review. Dis Markers. 2015;2015:352478. doi: 10.1155/2015/352478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders DA, Ross-Innes CS, Beraldi D, Carroll JS, Balasubramanian S. Genome-wide mapping of FOXM1 binding reveals co-binding with estrogen receptor alpha in breast cancer cells. Genome Biol. 2013;14:R6. doi: 10.1186/gb-2013-14-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia L, Huang W, Tian D, Zhu H, Zhang Y, Hu H, Fan D, Nie Y, Wu K. Upregulated FoxM1 expression induced by hepatitis B virus X protein promotes tumor metastasis and indicates poor prognosis in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012;57:600–12. doi: 10.1016/j.jhep.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Okada K, Fujiwara Y, Takahashi T, Nakamura Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Mori M, Doki Y. Overexpression of forkhead box M1 transcription factor (FOXM1) is a potential prognostic marker and enhances chemoresistance for docetaxel in gastric cancer. Ann Surg Oncol. 2013;20:1035–43. doi: 10.1245/s10434-012-2680-0. [DOI] [PubMed] [Google Scholar]
- 30.Xu N, Jia D, Chen W, Wang H, Liu F, Ge H, Zhu X, Song Y, Zhang X, Zhang D, Ge D, Bai C. FoxM1 is associated with poor prognosis of non-small cell lung cancer patients through promoting tumor metastasis. PloS One. 2013;8:e59412. doi: 10.1371/journal.pone.0059412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ning Z, Wang A, Liang J, Xie Y, Liu J, Feng L, Yan Q, Wang Z. USP22 promotes the G1/S phase transition by upregulating FoxM1 expression via β-catenin nuclear localization and is associated with poor prognosis in stage II pancreatic ductal adenocarcinoma. Int J Oncol. 2014;45:1594–608. doi: 10.3892/ijo.2014.2531. [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Teng M, Liu J, Jin D, Wu J, Yan D, Fan J, Qin X, Tang H, Peng Z. FOXM1 expression predicts the prognosis in hepatocellular carcinoma patients after orthotopic liver transplantation combined with the Milan criteria. Cancer Lett. 2011;306:214–22. doi: 10.1016/j.canlet.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Lerner SP, McConkey DJ, Hoadley KA, Chan KS, Kim WY, Radvanyi F, Höglund M, Real FX. Bladder Cancer Molecular Taxonomy: Summary from a Consensus Meeting. Bladder Cancer Amst Neth. 2016;2:37–47. doi: 10.3233/BLC-150037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halasi M, Gartel AL. Targeting FOXM1 in cancer. Biochem Pharmacol. 2013;85:644–52. doi: 10.1016/j.bcp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Aine M, Eriksson P, Liedberg F, Sjödahl G, Höglund M. Biological determinants of bladder cancer gene expression subtypes. Sci Rep. 2015;5:10957. doi: 10.1038/srep10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aine M, Eriksson P, Liedberg F, Höglund M, Sjödahl G. On Molecular Classification of Bladder Cancer: Out of One, Many. Eur Urol. 2015;68:921–3. doi: 10.1016/j.eururo.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Dadhania V, Zhang M, Zhang L, Bondaruk J, Majewski T, Siefker-Radtke A, Guo CC, Dinney C, Cogdell DE, Zhang S, Lee S, Lee JG, Weinstein JN, et al. Meta-Analysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use. EBioMedicine. 2016;12:105–17. doi: 10.1016/j.ebiom.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu XS, Miao RC, Wan Y, Zhang LQ, Qu K, Liu C. FoxM1 as a novel therapeutic target for cancer drug therapy. Asian Pac J Cancer Prev. 2015;16:23–9. doi: 10.7314/apjcp.2015.16.1.23. [DOI] [PubMed] [Google Scholar]
- 39.Mellor HR, Ferguson DJ, Callaghan R. A model of quiescent tumour microregions for evaluating multicellular resistance to chemotherapeutic drugs. Br J Cancer. 2005;93:302–9. doi: 10.1038/sj.bjc.6602710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 41.Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ, Sagalowsky AI, Schoenberg MP, Lerner SP. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol. 2006;176:2414–2422. doi: 10.1016/j.juro.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM, Sesterhenn IA, Tachibana M, Weider J. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 43.Margulis V, Lotan Y, Karakiewicz PI, Fradet Y, Ashfaq R, Capitanio U, Montorsi F, Bastian PJ, Nielsen ME, Müller SC, Rigaud J, Heukamp LC, Netto G, et al. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst. 2009;101:114–9. doi: 10.1093/jnci/djn451. [DOI] [PubMed] [Google Scholar]
- 44.Yurakh AO, Ramos D, Calabuig-Fariñas S, López-Guerrero JA, Rubio J, Solsona E, Romanenko AM, Vozianov AF, Pellin A, Llombart-Bosch A. Molecular and immunohistochemical analysis of the prognostic value of cell-cycle regulators in urothelial neoplasms of the bladder. Eur Urol. 2006;50:506–515. doi: 10.1016/j.eururo.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Hamurcu Z, Ashour A, Kahraman N, Ozpolat B. FOXM1 regulates expression of eukaryotic elongation factor 2 kinase and promotes proliferation, invasion and tumorgenesis of human triple negative breast cancer cells. Oncotarget. 2016;7:16619–35. doi: 10.18632/oncotarget.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JJ, Lee HJ, Son BH, Kim SB, Ahn JH, Ahn SD, Cho EY, Gong G. Expression of FOXM1 and related proteins in breast cancer molecular subtypes. Int J Exp Pathol. 2016;97:170–7. doi: 10.1111/iep.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craig DW, O’Shaughnessy JA, Kiefer JA, Aldrich J, Sinari S, Moses TM, Wong S, Dinh J, Christoforides A, Blum JL, Aitelli CL, Osborne CR, Izatt T, et al. Genome and transcriptome sequencing in prospective metastatic triple-negative breast cancer uncovers therapeutic vulnerabilities. Mol Cancer Ther. 2013;12:104–16. doi: 10.1158/1535-7163.MCT-12-0781. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Cheng L, Minn K, Madan R, Godwin AK, Shridhar V, Chien J. Targeting of mutant p53-induced FoxM1 with thiostrepton induces cytotoxicity and enhances carboplatin sensitivity in cancer cells. Oncotarget. 2014;5:11365–80. doi: 10.18632/oncotarget.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He F, Mo L, Zheng XY, Hu C, Lepor H, Lee EY, Sun TT, Wu XR. Deficiency of pRb family proteins and p53 in invasive urothelial tumorigenesis. Cancer Res. 2009;69:9413–21. doi: 10.1158/0008-5472.CAN-09-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam AK, Ngan AW, Leung MH, Kwok DC, Liu VW, Chan DW, Leung WY, Yao KM. FOXM1b, which is present at elevated levels in cancer cells, has a greater transforming potential than FOXM1c. Front Oncol. 2013;3:11. doi: 10.3389/fonc.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhat UG, Halasi M, Gartel AL. Thiazole antibiotics target FoxM1 and induce apoptosis in human cancer cells. PloS One. 2009;4:e5592. doi: 10.1371/journal.pone.0005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhat UG, Halasi M, Gartel AL. FoxM1 is a general target for proteasome inhibitors. PloS One. 2009;4:e6593. doi: 10.1371/journal.pone.0006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karam JA, Lotan Y, Karakiewicz PI, Ashfaq R, Sagalowsky AI, Roehrborn CG, Shariat SF. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007;8:128–36. doi: 10.1016/S1470-2045(07)70002-5. [DOI] [PubMed] [Google Scholar]
- 54.Youssef RF, Lotan Y. Predictors of outcome of non-muscle-invasive and muscle-invasive bladder cancer. ScientificWorldJournal. 2011;11:369–81. doi: 10.1100/tsw.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen T, Wallden B, Schaper C, Ferree S, Liu S, Gao D, Barry G, Dowidar N, Maysuria M, Storhoff J. Analytical validation of the PAM50-based Prosigna Breast Cancer Prognostic Gene Signature Assay and nCounter Analysis System using formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer. 2014;14:177. doi: 10.1186/1471-2407-14-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wirtz RM, Sihto H, Isola J, Heikkilä P, Kellokumpu-Lehtinen PL, Auvinen P, Turpeenniemi-Hujanen T, Jyrkkiö S, Lakis S, Schlombs K, Laible M, Weber S, Eidt S, et al. Biological subtyping of early breast cancer: a study comparing RT-qPCR with immunohistochemistry. Breast Cancer Res Treat. 2016;157:437–46. doi: 10.1007/s10549-016-3835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS, Jeong P, Kim MJ, Yun SJ, Lee KM, Moon SK, Lee SC, Cha EJ, Bae SC. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer. 2010;9:3. doi: 10.1186/1476-4598-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sikic D, Breyer J, Hartmann A, Burger M, Erben P, Denzinger S, Eckstein M, Stöhr R, Wach S, Wullich B, Keck B, Wirtz RM, Otto W. High Androgen Receptor mRNA Expression Is Independently Associated with Prolonged Cancer-Specific and Recurrence-Free Survival in Stage T1 Bladder Cancer. Transl Oncol. 2017;10:340–5. doi: 10.1016/j.tranon.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breyer J, Wirtz RM, Otto W, Erben P, Kriegmair MC, Stoehr R, Eckstein M, Eidt S, Denzinger S, Burger M, Hartmann A, BRIDGE Consortium In stage pT1 non-muscle-invasive bladder cancer (NMIBC), high KRT20 and low KRT5 mRNA expression identify the luminal subtype and predict recurrence and survival. Virchows Arch Int J Pathol. 2017;470:267–74. doi: 10.1007/s00428-017-2064-8. [DOI] [PubMed] [Google Scholar]
- 60.Tramm T, Sørensen BS, Overgaard J, Alsner J. Optimal reference genes for normalization of qRT-PCR data from archival formalin-fixed, paraffin-embedded breast tumors controlling for tumor cell content and decay of mRNA. Diagn Mol Pathol. 2013;22:181–7. doi: 10.1097/PDM.0b013e318285651e. [DOI] [PubMed] [Google Scholar]
- 61.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–25. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 62.Sjödahl G, Lauss M, Lövgren K, Chebil G, Gudjonsson S, Veerla S, Patschan O, Aine M, Fernö M, Ringnér M, Månsson W, Liedberg F, Lindgren D, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18:3377–86. doi: 10.1158/1078-0432.CCR-12-0077-T. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


