Abstract
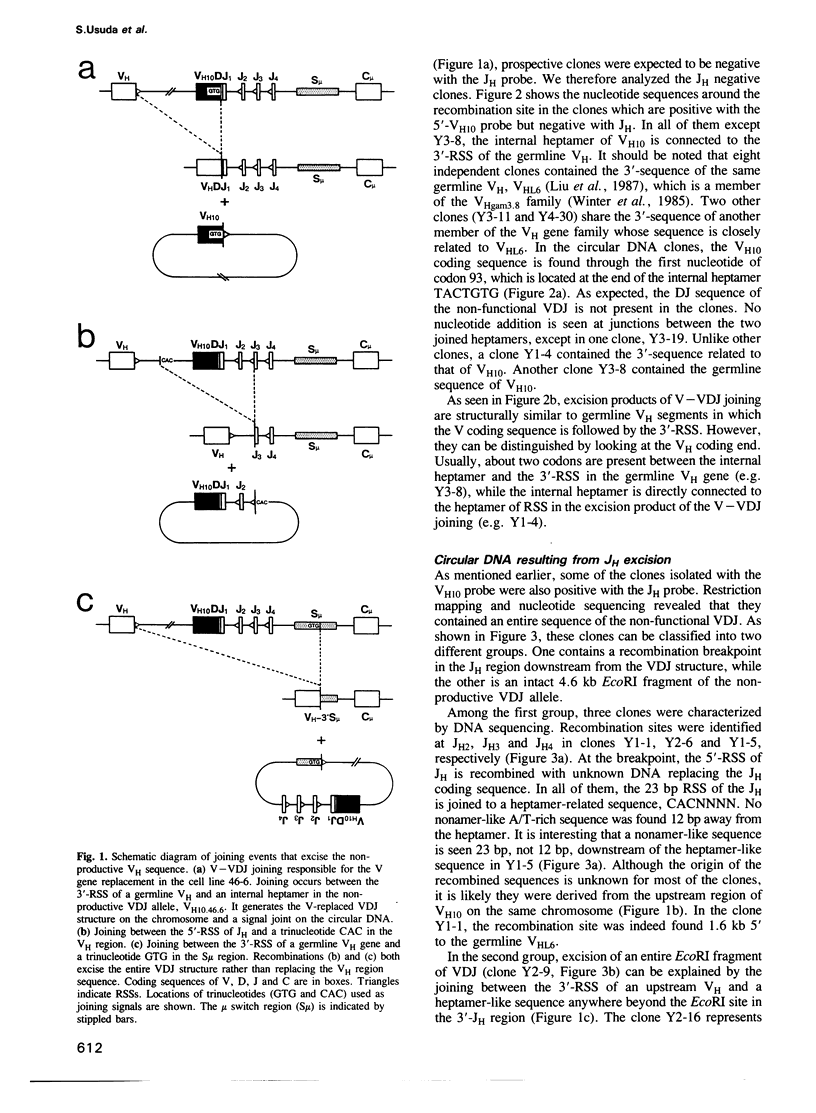
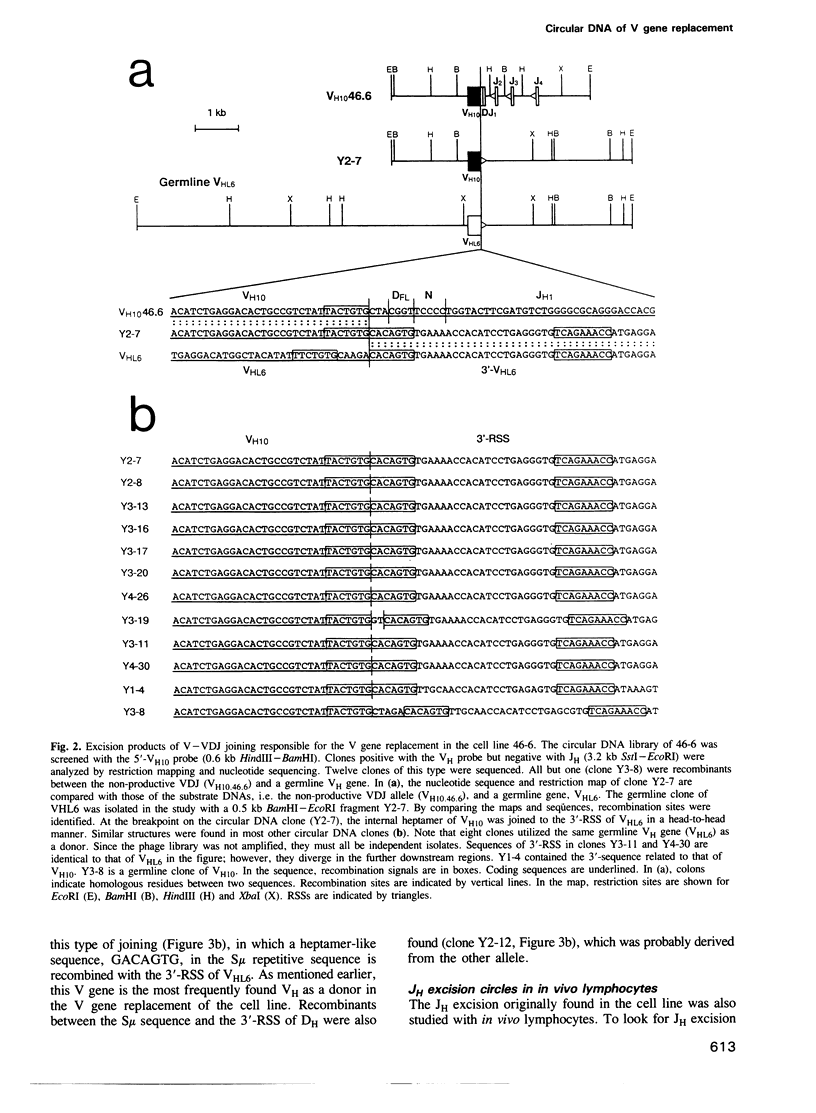
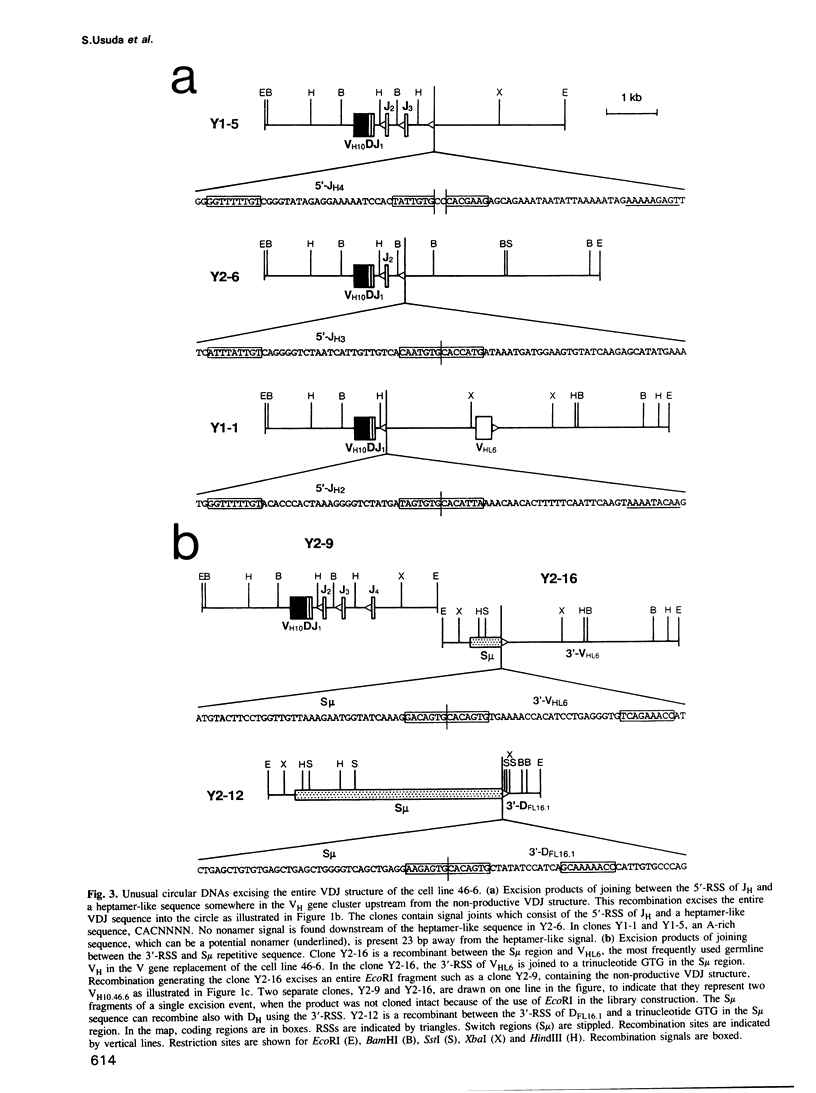
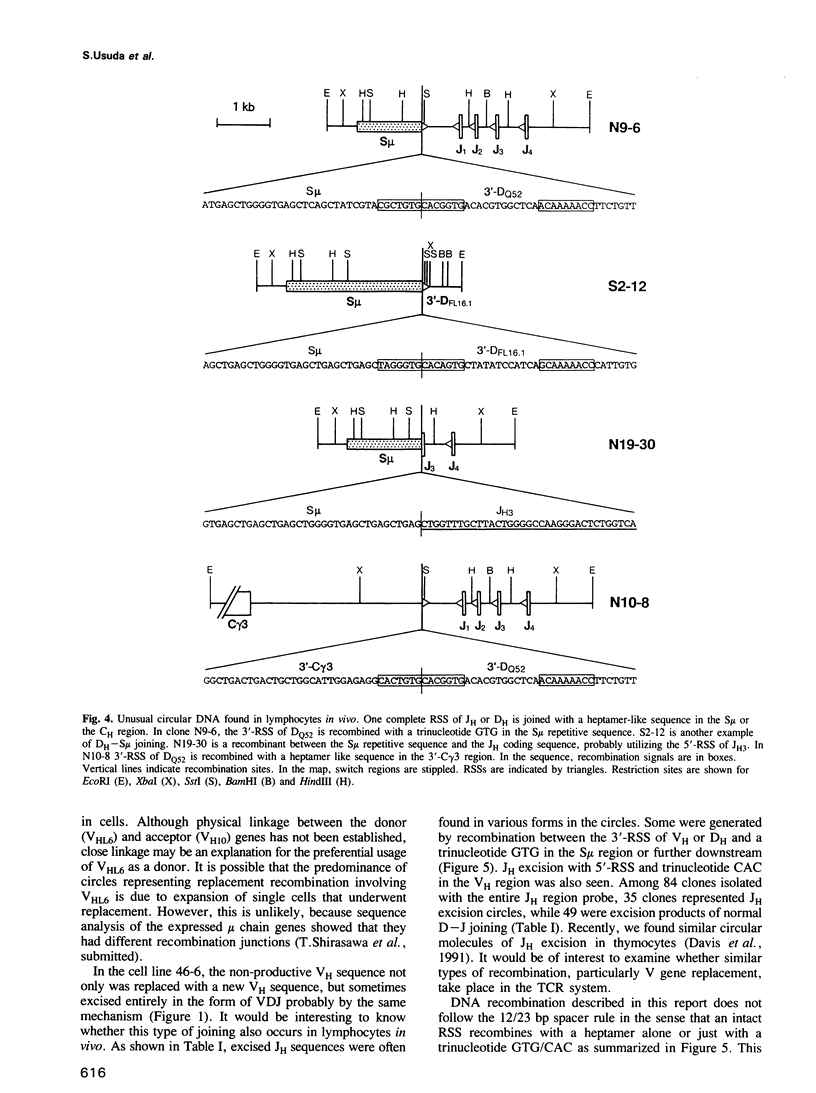
Circular DNA resulting from V gene replacement was studied with an A-MuLV transformed cell line containing ablts. This cell line undergoes V gene replacement at elevated temperatures in the immunoglobulin (Ig) heavy chain (H) gene. Examination of circular DNA revealed that a heptamer-related sequence (TACTGTG) within the coding region of VDJ was joined to the recombination signal sequence (RSS) of a germline VH segment. This provides direct evidence for a intramolecular DNA deletion mechanism for V gene replacement. In the pre-B cell line as well as in in vivo lymphocytes, unusual circular DNAs were found which were structurally similar to the V gene replacement circles. They represented excision products of the deletion type recombination between one complete RSS and a heptamer-like sequence in the Ig H region.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Davey M. P., Nakahara K., Tchorz K., Kurtzberg J., Hershfield M. S., Haynes B. F., Cohen D. I., Waldmann T. A. Chromosomal translocation in a human leukemic stem-cell line disrupts the T-cell antigen receptor delta-chain diversity region and results in a previously unreported fusion transcript. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2031–2035. doi: 10.1073/pnas.86.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Boehm T., Rabbitts T. H. A chromosomal basis of lymphoid malignancy in man. Eur J Biochem. 1989 Oct 20;185(1):1–17. doi: 10.1111/j.1432-1033.1989.tb15074.x. [DOI] [PubMed] [Google Scholar]
- Chen Q., Cheng J. T., Tasi L. H., Schneider N., Buchanan G., Carroll A., Crist W., Ozanne B., Siciliano M. J., Baer R. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J. 1990 Feb;9(2):415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey L. R., Ferrier P., Alt F. W. VH to VHDJH rearrangement is mediated by the internal VH heptamer. Int Immunol. 1990;2(6):579–583. doi: 10.1093/intimm/2.6.579. [DOI] [PubMed] [Google Scholar]
- Davis D. D., Yoshida K., Kingsbury L., Sakano H. Circular DNA resulting from recombination between V-(D)-J joining signals and switch repetitive sequences in mouse thymocytes. J Exp Med. 1991 Mar 1;173(3):743–746. doi: 10.1084/jem.173.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Finger L. R., Harvey R. C., Moore R. C., Showe L. C., Croce C. M. A common mechanism of chromosomal translocation in T- and B-cell neoplasia. Science. 1986 Nov 21;234(4779):982–985. doi: 10.1126/science.3490692. [DOI] [PubMed] [Google Scholar]
- Finger L. R., Kagan J., Christopher G., Kurtzberg J., Hershfield M. S., Nowell P. C., Croce C. M. Involvement of the TCL5 gene on human chromosome 1 in T-cell leukemia and melanoma. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5039–5043. doi: 10.1073/pnas.86.13.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Yamagishi H. Isolation of an excision product of T-cell receptor alpha-chain gene rearrangements. Nature. 1987 May 21;327(6119):242–243. doi: 10.1038/327242a0. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Björck E., Bjursell G., Lindahl T. Sequence complexity of circular Epstein-Bar virus DNA in transformed cells. J Virol. 1981 Oct;40(1):11–19. doi: 10.1128/jvi.40.1.11-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiya M., Davis D. D., Takahashi T., Okuda K., Raschke W. C., Sakano H. Two types of immunoglobulin-negative Abelson murine leukemia virus-transformed cells: implications for B-lymphocyte differentiation. Proc Natl Acad Sci U S A. 1986 Jan;83(1):145–149. doi: 10.1073/pnas.83.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Kleinfield R., Hardy R. R., Tarlinton D., Dangl J., Herzenberg L. A., Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. 1986 Aug 28-Sep 3Nature. 322(6082):843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- Liu A. Y., Robinson R. R., Hellström K. E., Murray E. D., Jr, Chang C. P., Hellström I. Chimeric mouse-human IgG1 antibody that can mediate lysis of cancer cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3439–3443. doi: 10.1073/pnas.84.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Yoshida K., Maeda T., Usuda S., Sakano H. Switch circular DNA formed in cytokine-treated mouse splenocytes: evidence for intramolecular DNA deletion in immunoglobulin class switching. Cell. 1990 Jul 13;62(1):135–142. doi: 10.1016/0092-8674(90)90247-c. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Davis D. D., Sakano H. T cell receptor beta gene sequences in the circular DNA of thymocyte nuclei: direct evidence for intramolecular DNA deletion in V-D-J joining. Cell. 1987 May 22;49(4):477–485. doi: 10.1016/0092-8674(87)90450-8. [DOI] [PubMed] [Google Scholar]
- Reth M., Gehrmann P., Petrac E., Wiese P. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. 1986 Aug 28-Sep 3Nature. 322(6082):840–842. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Kurosawa Y., Weigert M., Tonegawa S. Identification and nucleotide sequence of a diversity DNA segment (D) of immunoglobulin heavy-chain genes. Nature. 1981 Apr 16;290(5807):562–565. doi: 10.1038/290562a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Schiff C., Milili M., Hue I., Rudikoff S., Fougereau M. Genetic basis for expression of the idiotypic network. One unique Ig VH germline gene accounts for the major family of Ab1 and Ab3 (Ab1') antibodies of the GAT system. J Exp Med. 1986 Mar 1;163(3):573–587. doi: 10.1084/jem.163.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe L. C., Croce C. M. The role of chromosomal translocations in B- and T-cell neoplasia. Annu Rev Immunol. 1987;5:253–277. doi: 10.1146/annurev.iy.05.040187.001345. [DOI] [PubMed] [Google Scholar]
- Takemori T., Miyazoe I., Shirasawa T., Taniguchi M., Graf T. A temperature-sensitive mutant of Abelson murine leukemia virus confers inducibility of IgM expression to transformed lymphoid cells. EMBO J. 1987 Apr;6(4):951–956. doi: 10.1002/j.1460-2075.1987.tb04844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemer D., Enquist L., Leder P. Improved derivative of a phage lambda EK2 vector for cloning recombinant DNA. Nature. 1976 Oct 7;263(5577):526–527. doi: 10.1038/263526a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Winter E., Radbruch A., Krawinkel U. Members of novel VH gene families are found in VDJ regions of polyclonally activated B-lymphocytes. EMBO J. 1985 Nov;4(11):2861–2867. doi: 10.1002/j.1460-2075.1985.tb04015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Matsuoka M., Usuda S., Mori A., Ishizaka K., Sakano H. Immunoglobulin switch circular DNA in the mouse infected with Nippostrongylus brasiliensis: evidence for successive class switching from mu to epsilon via gamma 1. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7829–7833. doi: 10.1073/pnas.87.20.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]



