Abstract
The operon coding for a respiratory quinol oxidase was cloned from thermoacidophilic archaebacterium Sulfolobus acidocaldarius. It contains three genes, soxA, soxB and soxC. The first two genes code for proteins related to the cytochrome c oxidase subunits II and I, respectively. soxC encodes a protein homologous to cytochrome b, which is a subunit of the mitochondrial and bacterial cytochrome c reductases and the chloroplast cytochrome b6f complex. soxA is preceded by a promoter and the genes are cotranscribed into a 4 kb mRNA. Their protein products form a complex which has been partially purified and has quinol oxidase activity. The reduced minus oxidized absorption spectrum of the complex has two maxima at 586 and 606 nm. The latter is typical of cytochrome c oxidase. The complex contains four haems A. Two haems belong to the 'cytochrome oxidase' part of the complex and two are probably bound to be apocytochrome b (SoxC) and responsible for the 586 nm absorption peak. The homology between the sox gene products and their mitochondrial counterparts suggests that energy conservation coupled to the quinol oxidation catalysed either by the Sulfolobus oxidase or two mitochondrial respiratory enzymes may have a similar mechanism.
Full text
PDF



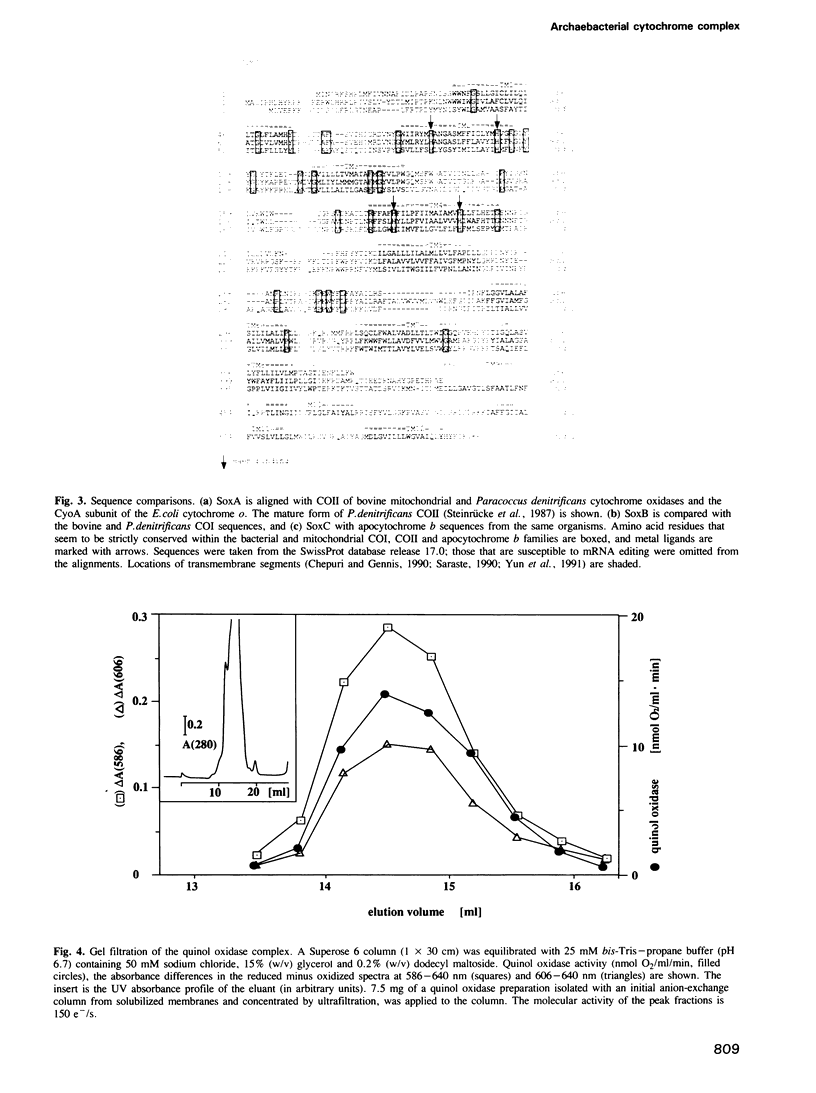
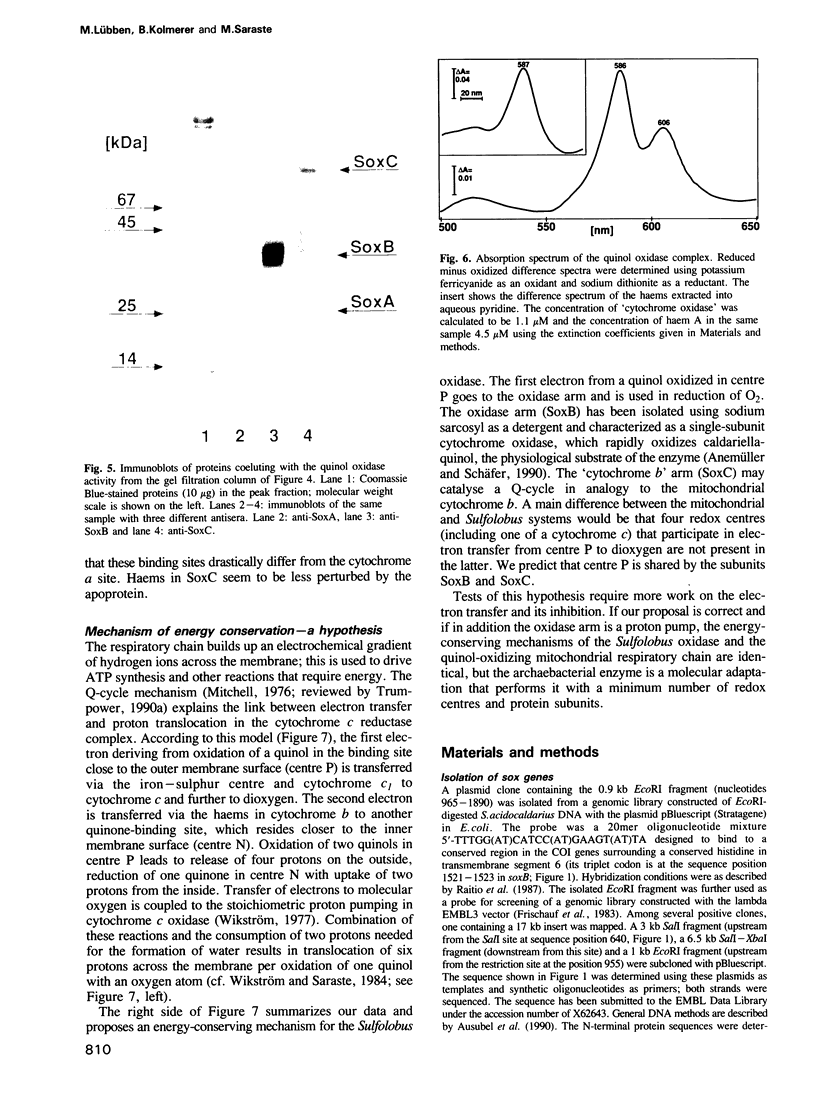

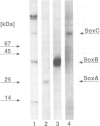
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Anemüller S., Schäfer G. Cytochrome aa3 from Sulfolobus acidocaldarius. A single-subunit, quinol-oxidizing archaebacterial terminal oxidase. Eur J Biochem. 1990 Jul 31;191(2):297–305. doi: 10.1111/j.1432-1033.1990.tb19123.x. [DOI] [PubMed] [Google Scholar]
- Berry E. A., Trumpower B. L. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem. 1987 Feb 15;161(1):1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Callahan P. M., Babcock G. T. Origin of the cytochrome a absorption red shift: a pH-dependent interaction between its heme a formyl and protein in cytochrome oxidase. Biochemistry. 1983 Jan 18;22(2):452–461. doi: 10.1021/bi00271a031. [DOI] [PubMed] [Google Scholar]
- Chan S. I., Li P. M. Cytochrome c oxidase: understanding nature's design of a proton pump. Biochemistry. 1990 Jan 9;29(1):1–12. doi: 10.1021/bi00453a001. [DOI] [PubMed] [Google Scholar]
- Chepuri V., Gennis R. B. The use of gene fusions to determine the topology of all of the subunits of the cytochrome o terminal oxidase complex of Escherichia coli. J Biol Chem. 1990 Aug 5;265(22):12978–12986. [PubMed] [Google Scholar]
- Chepuri V., Lemieux L., Au D. C., Gennis R. B. The sequence of the cyo operon indicates substantial structural similarities between the cytochrome o ubiquinol oxidase of Escherichia coli and the aa3-type family of cytochrome c oxidases. J Biol Chem. 1990 Jul 5;265(19):11185–11192. [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Holm L., Saraste M., Wikström M. Structural models of the redox centres in cytochrome oxidase. EMBO J. 1987 Sep;6(9):2819–2823. doi: 10.1002/j.1460-2075.1987.tb02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka M., Machida K., Shimada S., Mogi A., Tsuchiya T., Ohmori T., Souma Y., Gonda M., Sone N. Nucleotide sequence of the gene coding for four subunits of cytochrome c oxidase from the thermophilic bacterium PS3. J Biochem. 1990 Nov;108(5):866–873. doi: 10.1093/oxfordjournals.jbchem.a123294. [DOI] [PubMed] [Google Scholar]
- Knaff D. B. The cytochrome bc1 complex of photosynthetic bacteria. Trends Biochem Sci. 1990 Aug;15(8):289–291. doi: 10.1016/0968-0004(90)90013-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemieux L. J., Calhoun M. W., Thomas J. W., Ingledew W. J., Gennis R. B. Determination of the ligands of the low spin heme of the cytochrome o ubiquinol oxidase complex using site-directed mutagenesis. J Biol Chem. 1992 Jan 25;267(3):2105–2113. [PubMed] [Google Scholar]
- Lübben M., Schäfer G. A plasma-membrane associated ATPase from the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Eur J Biochem. 1987 May 4;164(3):533–540. doi: 10.1111/j.1432-1033.1987.tb11159.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol. 1976 Oct 21;62(2):327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Schäfer G. Purification and characterisation of an archaebacterial succinate dehydrogenase complex from the plasma membrane of the thermoacidophile Sulfolobus acidocaldarius. Eur J Biochem. 1991 Nov 1;201(3):593–600. doi: 10.1111/j.1432-1033.1991.tb16319.x. [DOI] [PubMed] [Google Scholar]
- Müller M., Schlapfer B., Azzi A. Preparation of a one-subunit cytochrome oxidase from Paracoccus denitrificans: spectral analysis and enzymatic activity. Biochemistry. 1988 Sep 20;27(19):7546–7551. doi: 10.1021/bi00419a055. [DOI] [PubMed] [Google Scholar]
- Olsen G. J., Pace N. R., Nuell M., Kaine B. P., Gupta R., Woese C. R. Sequence of the 16S rRNA gene from the thermoacidophilic archaebacterium Sulfolobus solfataricus and its evolutionary implications. J Mol Evol. 1985;22(4):301–307. doi: 10.1007/BF02115685. [DOI] [PubMed] [Google Scholar]
- Puustinen A., Finel M., Haltia T., Gennis R. B., Wikström M. Properties of the two terminal oxidases of Escherichia coli. Biochemistry. 1991 Apr 23;30(16):3936–3942. doi: 10.1021/bi00230a019. [DOI] [PubMed] [Google Scholar]
- Raitio M., Jalli T., Saraste M. Isolation and analysis of the genes for cytochrome c oxidase in Paracoccus denitrificans. EMBO J. 1987 Sep;6(9):2825–2833. doi: 10.1002/j.1460-2075.1987.tb02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W. D., Hüdepohl U., Zillig W. Mutational analysis of an archaebacterial promoter: essential role of a TATA box for transcription efficiency and start-site selection in vitro. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9509–9513. doi: 10.1073/pnas.87.24.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W. D., Palm P., Zillig W. Transcription termination in the archaebacterium Sulfolobus: signal structures and linkage to transcription initiation. Nucleic Acids Res. 1988 Mar 25;16(6):2445–2459. doi: 10.1093/nar/16.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Holm L., Lemieux L., Lübben M., van der Oost J. The happy family of cytochrome oxidases. Biochem Soc Trans. 1991 Aug;19(3):608–612. doi: 10.1042/bst0190608. [DOI] [PubMed] [Google Scholar]
- Saraste M., Metso T., Nakari T., Jalli T., Lauraeus M., Van der Oost J. The Bacillus subtilis cytochrome-c oxidase. Variations on a conserved protein theme. Eur J Biochem. 1991 Jan 30;195(2):517–525. doi: 10.1111/j.1432-1033.1991.tb15732.x. [DOI] [PubMed] [Google Scholar]
- Saraste M. Structural features of cytochrome oxidase. Q Rev Biophys. 1990 Nov;23(4):331–366. doi: 10.1017/s0033583500005588. [DOI] [PubMed] [Google Scholar]
- Steinrücke P., Steffens G. C., Panskus G., Buse G., Ludwig B. Subunit II of cytochrome c oxidase from Paracoccus denitrificans. DNA sequence, gene expression and the protein. Eur J Biochem. 1987 Sep 15;167(3):431–439. doi: 10.1111/j.1432-1033.1987.tb13356.x. [DOI] [PubMed] [Google Scholar]
- Trumpower B. L. Cytochrome bc1 complexes of microorganisms. Microbiol Rev. 1990 Jun;54(2):101–129. doi: 10.1128/mr.54.2.101-129.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpower B. L. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990 Jul 15;265(20):11409–11412. [PubMed] [Google Scholar]
- Wakagi T., Yamauchi T., Oshima T., Müller M., Azzi A., Sone N. A novel a-type terminal oxidase from Sulfolobus acidocaldarius with cytochrome c oxidase activity. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1110–1114. doi: 10.1016/0006-291x(89)92717-4. [DOI] [PubMed] [Google Scholar]
- Wakao H., Wakagi T., Oshima T. Purification and properties of NADH dehydrogenase from a thermoacidophilic archaebacterium, Sulfolobus acidocaldarius. J Biochem. 1987 Aug;102(2):255–262. doi: 10.1093/oxfordjournals.jbchem.a122049. [DOI] [PubMed] [Google Scholar]
- Wikstrom M. K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977 Mar 17;266(5599):271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- Yun C. H., Van Doren S. R., Crofts A. R., Gennis R. B. The use of gene fusions to examine the membrane topology of the L-subunit of the photosynthetic reaction center and of the cytochrome b subunit of the bc1 complex from Rhodobacter sphaeroides. J Biol Chem. 1991 Jun 15;266(17):10967–10973. [PubMed] [Google Scholar]