Abstract
We show that the Golgi complex can directly stimulate microtubule nucleation in vivo and in vitro and thus behaves as a potent microtubule-organizing organelle in interphase cells. With the use of nocodazole wash-out experiments in hepatic cells, we found that the occurrence of noncentrosomal, early stabilized microtubules is highly correlated with the subcellular localization of Golgi membranes. With the use of in vitro reconstituted microtubule assembly systems with or without cytosol, we also found that, in contrast to centrosomally attached microtubules, the distal ends of Golgi-attached microtubules are remotely stabilized in a way that requires additional cytosolic component(s). Finally, we demonstrate that Golgi-based microtubule nucleation is direct and involves a subset of γ-tubulin bound to the cytoplasmic face of the organelle.
INTRODUCTION
In interphase cells, the microtubule (MT) network plays a major role in membrane dynamics and organelle localization. In this context, the interaction between the Golgi complex and the MT network has been extensively studied. The Golgi apparatus colocalizes with the minus ends of MTs, which are usually associated with the centrosome (for review see Kreis et al., 1997; Thyberg and Moskalewski, 1999). This localization actually results from an equilibrium between two contradictory movements that have been unraveled with the use of MT-depolymerizing drugs and probably occur on MT subpopulations with different dynamics. The dispersal of Golgi elements occurs along stable MTs after depolymerization of the most labile MT population (Minin, 1997), whereas their reclustering involves newly assembled MTs (Ho et al., 1989). These contradictory movements of Golgi elements are mediated by distinct molecular motors. Consistent with earlier findings by Feiguin et al. (1994), who found that the expression of kinesin antisense oligonucleotide rendered the Golgi apparatus more compact, microinjection of antikinesin antibodies inhibited Golgi dispersion along stable, nocodazole-resistant MTs (Minin, 1997). Conversely, the central localization of the Golgi apparatus involves cytoplasmic dynein (Corthésy-Theulaz et al., 1992; Fath et al., 1994; Burkhardt et al., 1997; Harada et al., 1998). It is also worth noting that, in addition to the kinesin-mediated disruption of the central Golgi complex during MT depolymerization, the dispersion of Golgi elements also relies on the reconstitution of mini Golgi stacks at endoplasmic reticulum (ER) exit sites (Cole et al., 1996; Storrie et al., 1998). Such data on the scattering and the reclustering of the Golgi complex have led to the view that Golgi membranes undergo an intimate relationship with MTs and especially with a population of nocodazole-resistant, stable MTs. Stable MTs are characterized by the occurrence of posttranslationally modified tubulin—especially detyrosinated and acetylated tubulin (for review, see MacRae, 1997), which is thought to accumulate in stable MTs because of their longer half-lives, but does not influence MT stability in vivo (Schulze et al., 1987; Khawaja et al., 1988; Webster et al., 1990). Interestingly, not only MT stability influences Golgi localization, but Golgi membranes may reciprocally influence MT stabilization. Such an idea has been suggested by the spatial and temporal colocalization of detyrosinated or acetylated MTs and Golgi or trans-Golgi network (TGN) membranes that occurs in nocodazole wash-out experiments (Skoufias et al., 1990; Burgess et al., 1991; Thyberg and Moskalewski, 1993). After nocodazole removal, tubulin acetylation not only occurs radially from the centrosome (just like detyrosination does), but it is also found in discrete cytoplasmic locations (Bulinski et al., 1988). The occurrence of scattered acetylated MTs close to Golgi elements during the MT repolymerization process (Thyberg and Moskalewski, 1993) was also reminiscent of the occurrence of stable, noncentrosomal MTs during MT repolymerization in MDCK cells (Bréet al., 1987). Altogether, these data suggested that Golgi membranes could be involved in the assembly and in the early stabilization of a subset of noncentrosomal MTs. Consistently, it has been shown that other biological membranes such as fish melanophore pigment granules and fish fibroblast membranes can readily nucleate MTs in vivo (Rodionov and Borisy, 1997; Kaverina et al., 1998) or that the apical membrane of polarized epithelial cells participate in the organization of the interphase MT network (Meads and Schroer, 1995). Regarding MT stabilization, the recent identification of the cis-Golgi network (CGN)-associated MT-associated protein GMAP-210, and its association with a hyperstable population of detyrosinated MTs are also in favor of Golgi-mediated MT stabilization (Infante et al., 1999).
In this study, we show that in addition to the centrosome, the Golgi apparatus is a potent MT-organizing organelle. In vivo, scattered Golgi elements stimulate the assembly of noncentrosomal MTs upon recovery from nocodazole treatment. We further show that, in contrast to the high level of dynamics found in centrosomal MTs, Golgi-based MTs are stabilized early. The in vitro reconstitution of MT assembly by purified Golgi membranes also showed that MT stabilization required additional cytosolic factor(s) and that MT nucleation directly involved Golgi-bound γ-tubulin.
MATERIALS AND METHODS
Antibodies and Chemicals
Native (clone DM1A) and acetylated (clone 6-11B-1) anti–α-tubulin, anti–γ-tubulin (clone GTU-88), rabbit anti-mouse IgG, anti-rabbit and anti-mouse IgG FITC, and TRITC conjugates, nocodazole, saponin, and protein A Sepharose were purchased from Sigma Chemical Co. (St. Louis, MO). Anti–β-tubulin mAb (clone DM1B) was purchased from Amersham-Pharmacia Biotech (Uppsala, Sweden). Various markers of the Golgi were used including an mAb to rat mannosidase II (clone 53FC3) from Berkeley Antibody Co. (Richmond, CA), antialbumin immunserum raised in rabbits as previously described (Biou et al. 1984), anti-GM130 Golgi matrix protein (Transduction Laboratories, Lexington, KY), and anti-TGN38, kindly provided by Dr. George Banting, Department of Biochemistry, School of Medical Sciences, University of Bristol, UK (Reaves and Banting, 1992). For the detection of centriolar proteins, we used the mAb (clone GT335) to polyglutamylated tubulin (Eddéet al., 1990), kindly provided by Pr. Philippe Denoulet (Centre National de la Recherche Scientifique, FRE2219 Université Paris VI) and a polyclonal antibody to centrin-3 (Middendorp et al., 1997), kindly provided by Dr. Michel Bornens (Centre National de la Recherche Scientifique, Unité Mixte de Recherche 144, Institut Curie, Paris), who also provided the nucleo-centrosomal extract used as a positive control. FITC-conjugated anti-mouse Fab fragments were purchased from Jackson ImmunoResearch (West Grove, PA). Okadaic acid was from Life Technologies (Rockville, MD). Phosphocellulose-purified porcine brain tubulin was prepared as described by Walker et al. (1988).
Cell Culture and Nocodazole Treatments
WIF-B cells were cultured in F12 Coon's-modified medium (Sigma Chemical Co.) supplemented with 5% FCS (Dutscher, Rungis, France) and HAT mixture (10−5 M hypoxantine, 4 ×10−7 M aminopterine and 1.5 × 10−5 M thymidine; Polylabo, Strasbourg, France). Cells were confluent and normally polarized 8–10 d after plating at a initial density of 7000 cells/cm2. Fao cells were cultured in the same medium as WIF-B cells without the HAT mixture. NIH-3T3 cells were cultured on glass coverslips in DMEM medium supplemented with 10% FCS.
Nocodazole and brefeldin A (BFA) were diluted to 10 μM in culture medium, starting from a 10 mM stock solution in dimethyl sulfoxide or in methanol, respectively. To achieve total MT depolymerization, cells were treated with nocodazole for 10 h. The effectiveness of such treatment has been verified previously in WIF-B cells (Poüs et al., 1998) and in Fao cells (our unpublished results).
Immunofluorescence
WIF-B cells were cultured on glass coverslips, and after appropriate treatments were rinsed three times with 0.1 M PBS, pH 7.4, at room temperature and then fixed and permeabilized with methanol at −20°C for 5 min. All antibody incubations and washes were performed in PBS. Cells were incubated with primary antibodies for 1 h at 37°C, washed three times, and incubated (1 h, 37°C) with FITC-conjugated antibodies or a mixture of FITC- and TRITC-conjugated antibodies for single- or double-labeling experiments, respectively. Because secretory albumin is a convenient marker of the Golgi apparatus in untreated WIF-B cells (Cassio et al., 1991; Shanks et al., 1994; Poüs et al., 1998), we verified by double immunofluorescence labeling that during Golgi dispersal and nocodazole wash-out, albumin and mannosidase II, a resident enzyme of the medial Golgi, remained colocalized in WIF-B and Fao cells (our unpublished results). To perform double-labelings with two mouse monoclonal antibodies, cells were first incubated with the first antibody (1 h, 37°C), washed three times, and incubated (2 h, 37°C) with FITC-conjugated anti-mouse Fab fragments. After three washes, immune complexes were fixed with methanol (−20°C, 5 min), and then cells were incubated with the second antibody (always the anti–α-tubulin) for 1 h at 37°C, washed three times, and incubated (1 h, 37°C) with anti-mouse TRITC conjugate. After three washes, the coverslips were mounted in Slow Fade (Molecular Probes, Eugene, OR).
To assess MT stability after 15-min nocodazole wash-out, two protocols were used: first, a dilution-induced depolymerization assay in which cells were incubated (5 times, 1 min, 37°C) in HEPES-buffered medium (100 mM HEPES, 2 mM MgCl2, 1 mM EGTA, pH 6.9) containing 200 μg/ml saponin and then fixed in methanol and processed for the immunofluorescence labelings of acetylated tubulin and secretory albumin; and second, after the 15-min wash-out period after nocodazole treatment, cells were subjected to a second nocodazole treatment (20 min, 10 μM, 37°C), rinsed, and then fixed in cold methanol and processed for the immunofluorescence labelings of tubulin and secretory albumin.
Confocal microscopy was performed with the use of a TCS 4D laser scanning microscope (Leica, Heidelberg, Germany). Unless otherwise specified, the images shown are the superimposition of consecutive optical sections taken over the whole cell height, the distance between two consecutive optical sections being less than the z resolution of the microscope (delta z < 0.5 μm). To make sure that no artifactual juxtapositions resulted from the projection of the organelles imaged in distant confocal planes, each optical section was first examined separately to observe the spatial associations between MTs and Golgi fragments. If MTs were found not to be in contact with Golgi fragments, the adjacent upper and lower optical sections were also examined to detect juxtaposed Golgi fragments.
Experiments in Permeabilized Cells
Morphologically intact Golgi membranes from rat liver were prepared according to Hui et al. (1998). This method routinely gave a 80- to 120-fold enrichment of the galactosyl transferase activity compared with the homogenate. Microtubule dynamics was reconstituted in detergent-extracted NIH 3T3 fibroblasts according to Saoudi et al. (1998) with the after modifications. Subconfluent NIH-3T3 cells cultured on glass coverslips (22 × 22 mm) were permeabilized in PEM buffer (100 mM PIPES, 1 mM EGTA, 1 mM MgCl2, pH 6.9) supplemented with 0.05% Triton X-100 (3 times, 1 min, 37°C). Cells were then kept at 4°C for 3 d to achieve cold-induced MT depolymerization. Cytosol from interphase NIH-3T3 cells was prepared as described except that cells were sonicated instead of being permeabilized with Triton. Permeabilized cells were incubated for 30 min at 37°C with 35 μl cytosol, 5 μM okadaic acid, 2.5 μM phosphocellulose-purified porcine brain tubulin, and an ATP-regenerating system. When appropriate, 20 μM nocodazole was added for 30 min before cells were rinsed twice with 1 ml of warm PEM buffer and fixed with methanol (−20°C, 3 min). MTs and Golgi membranes were immunolabeled with antibodies to α-tubulin and rat serum albumin, respectively. Samples were examined in a Leica DMLB microscope with a 100× objective.
In Vitro Experiments
Golgi membranes were incubated with 10 μM purified porcine brain tubulin in the presence of 1 mM GTP for 15–30 min at 37°C. After rapid fixation with 0.25% glutaraldehyde, Golgi membranes were centrifuged (20,000 × g, 30 min) through a 15% sucrose cushion on glass coverslips, postfixed in methanol (−20°C, 4 min), and subjected to immunofluorescence labeling.
Salt-wash experiments were conducted as follows: Golgi membranes were first incubated in 2 M KCl for 1 h on ice. After a rapid spin down of Golgi membranes, the pellet was resuspended in PEM buffer, extensively washed with PEM, and put on the top of a sucrose gradient comprised of a 1.3 M sucrose cushion and a 0.25 M sucrose overlayer, both buffered with 100 mM, pH 6.7, phosphate, and supplemented with 5 mM MgCl2. When appropriate, salt-washed Golgi membranes were gently resuspended and agitated for 1 h at room temperature in WIF-B cytosol. After centrifugation, the Golgi was washed twice with PEM buffer, then resuspended in PEM, and used in the MT-assembly assay as described above. WIF-B cytosol was obtained by sonication of trypsinized cells resuspended 1:1 in PEM buffer. The lysate was ultracentrifuged (30 min, 200,000 × g, 4°C), and the supernatant was frozen in liquid nitrogen and kept at −80°C until use. Immunodepletion of γ-tubulin was performed by adding 15 μl of the GTU-88 antibody to 35 μl WIF-B cytosol diluted 20 times with PEM buffer. After gentle rotation (2 h, room temperature), the immune complexes were harvested by three consecutive incubations with 25 μl of protein A-Sepharose beads precoated with rabbit anti-mouse IgGs (2 h, room temperature). After concentration with the use of Ultrafree centrifugal filter devices (Millipore Corp., Bedford, MA), the cytosolic extract was controlled for the absence of detectable γ-tubulin by Western blotting before use.
RESULTS
Stable Acetylated MTs Occur in the Close Vicinity of Golgi Fragments Early after Nocodazole Wash-out
As a prerequisite to the study of the assembly of new MTs during nocodazole wash-out experiments, we first validated the experimental conditions in which all the MTs from interphase cells were completely depolymerized. Total depolymerization is essential because, depending on the cell type, stable MTs exhibit such a slow turnover that they may remain intact during a whole interphase (Webster et al., 1987). It was achieved in both WIF-B and Fao cells by a 10-h treatment with 10 μM nocodazole. We have shown previously that tubulin acetylation is the only posttranslational modification of stable MTs in WIF-B cells (Poüs et al., 1998). The same was true in Fao cells, except for very rare cases in which detyrosinated tubulin was observed in isolated cells. We thus labeled acetylated tubulin to follow stable MT disassembly and repolymerization during nocodazole treatment and wash-out, respectively.
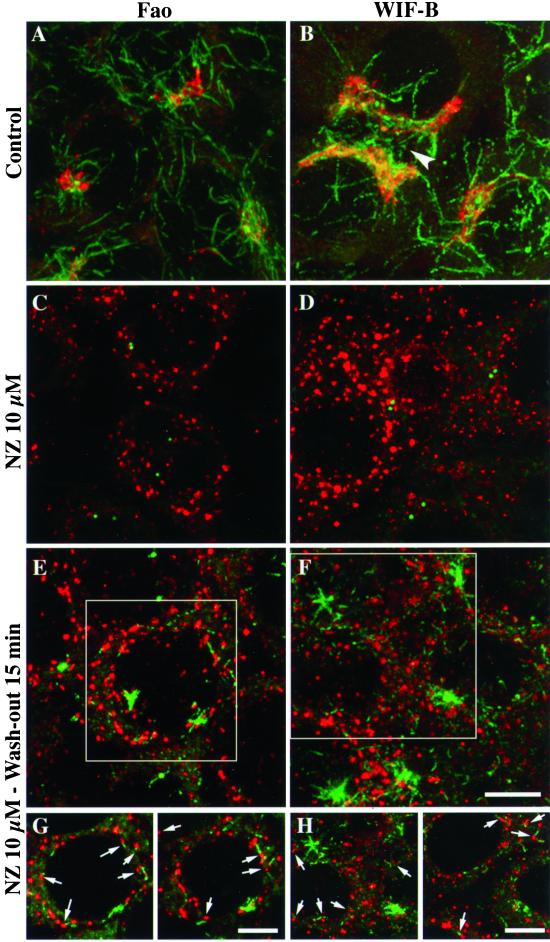
First, as described previously in fibroblasts (Thyberg and Moskalewski, 1993), we observed that in Fao and WIF-B hepatic cells scattered acetylated MTs reformed in the vicinity of Golgi elements. In untreated Fao (Figure 1A) and WIF-B cells (Figure 1B), the Golgi complex was observed as a compact, juxtanuclear organelle, in an acetylated MT-rich region. In WIF-B cells, the Golgi was close to the apical membrane, which delimitates bile canaliculi (Figure 1B, arrowhead). After nocodazole treatment, the Golgi complex was disrupted into numerous elements scattered in the cytoplasm (Figure 1, C and D). In the same conditions, acetylated MTs could not be detected, except for two centriolar spots (Figure 1, C and D), which colocalized with γ-tubulin (our unpublished results). Fifteen minutes after nocodazole removal, two populations of acetylated MTs were found. Some acetylated MTs were radially organized, most likely around the centrosomes, whereas another population appeared to be scattered in the cytoplasm (Figure 1, E and F). Interestingly, these small MT segments were juxtaposed to Golgi elements (Figure 1, E–H). These MT segments did not follow a particular direction in the cytoplasm, nor did cell polarity seem to influence their subcellular localization because their distribution appeared relatively uniform in both cell lines. Additionally, WIF-B cells did not exhibit a higher density of such MTs near their apical membranes. To quantify the juxtaposition of MT segments to Golgi elements, we measured the distribution of the distances between the two organelles on images of individual confocal slices taken at shorter wash-out times than shown in Figure 1, so that proximity could be interpreted as clearly as possible. When an MT was found to be isolated, the two adjacent slices were also taken in consideration to determine the distance of the closer Golgi element. Table 1 shows that the distances measured after 5- or 10-min wash-outs were predominantly shorter than 0.2 μm. Because we measured a 1.5-μm mean distance between scattered Golgi elements, this data confirms that the proximity between Golgi and MTs was not due to random juxtapositions.
Figure 1.
Acetylated MTs reform in the close vicinity of Golgi fragments. Fao rat hepatoma cells (A, C, E, and G) and WIF-B polarized hepatic cells (B, D, F, and H) were treated without (A and B) or with (C–F) 10 μM nocodazole for 10 h, washed twice with cold culture medium, and incubated at 37°C in nocodazole-free medium for 15 min (E, F, G, and H). Cells were then fixed and processed for double immunofluorescence labeling with the use of antibodies to acetylated tubulin (green) and secretory albumin (red). All cells were examined by confocal microscopy. The images shown in A–F are the superimposition of serial optical sections acquired over the whole cell height (delta z = 0.5 μm). In G and H, two images with a shorter depth of field (made by superimposing 3 consecutive optical sections) were extracted from the indicated regions in E and F, respectively. The arrowhead indicates a bile canaliculus between adjacent WIF-B cells. Scale bar, 10 μm.
Table 1.
Frequency distribution of the distances between Golgi elements and MT segments
| Distance interval (μm) | Frequency (%)
|
|
|---|---|---|
| 5-min wash-out (n = 243) | 10-min wash-out (n = 352) | |
| 0–0.2 | 91.0 | 84.7 |
| 0.2–0.4 | 7.0 | 10.7 |
| 0.4–0.6 | 0.8 | 2.3 |
| >0.6 | 1.2 | 2.3 |
WIF-B cells were treated with 10 μM nocodazole for 10 h, then washed twice in cold medium, and incubated for 5 or 10 min in nocodazole-free medium to allow MT repolymerization. After fixation and double-labeling of secretory albumin and acetylated tubulin, individual confocal slices were analyzed for the distances between MT segments and Golgi elements. The frequency distributions of the measured distances are indicated for each wash-out time.
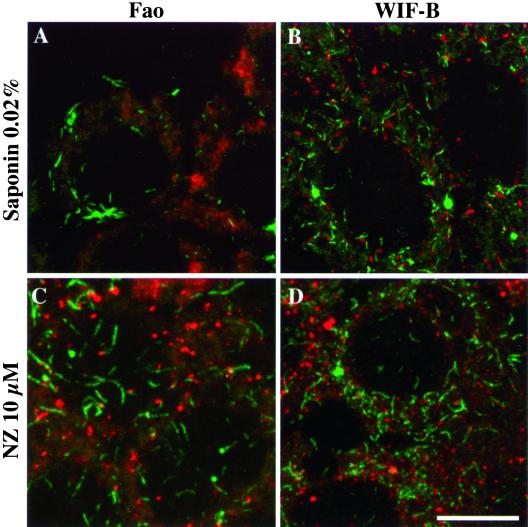
To test if these newly assembled acetylated MTs were actually stable, two approaches were used after recovery from nocodazole treatment. We first triggered the depolymerization of dynamically unstable MTs in Fao (Figure 2A) and WIF-B (Figure 2B) cells by perforating the plasma membrane with saponin. The second approach consisted in treating cells a second time with nocodazole to depolymerize highly dynamic MTs (Figure 2, C and D). Although centrosomal MTs were acetylated, they did not resist the second nocodazole treatment in both cell lines (Figure 2, C and D) or dilution in WIF-B cells (Figure 2B). In contrast, and in both conditions, the scattered acetylated MT population was still present, indicating that most of the early regrown MTs juxtaposed to Golgi fragments were more stable than centrosomally assembled acetylated MTs.
Figure 2.
Newly formed acetylated MTs associated with Golgi elements are stable. Nocodazole wash-out experiments were conducted as described in Figure 1 on Fao (A and C) and WIF-B cells (B and D). Cells were either permeabilized with HEPES-buffered medium containing 0.2 mg/ml saponin (A, B) or incubated again with 10 μM nocodazole for 20 min at 37°C (C and D). After fixation, cells were subjected to double immunofluorescence labeling of acetylated tubulin (green) and secretory albumin (red). All cells were examined by confocal microscopy. The images are the superimposition of consecutive optical sections taken over the whole cell height (delta z = 0.5 μm). Scale bar, 10 μm.
The Occurrence of Noncentrosomal Acetylated MTs Is Golgi Dependent
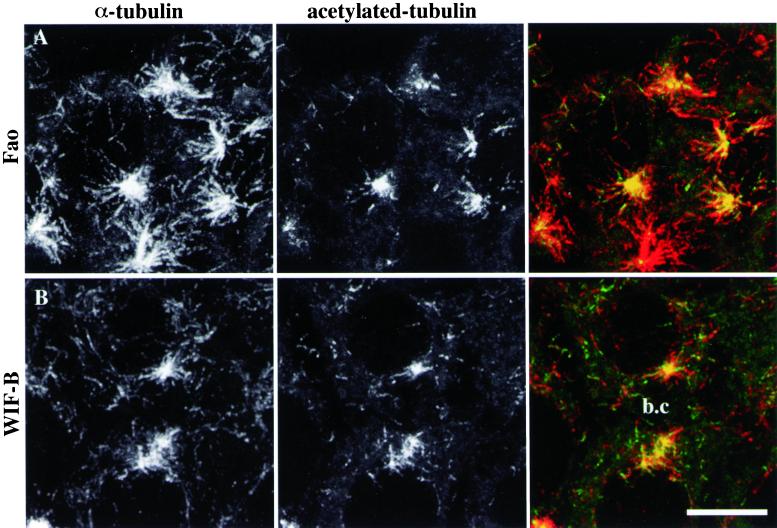
Because they were not radially organized, the scattered acetylated MT segments that occurred close to Golgi fragments were likely to be noncentrosomal. However, it was still possible that these MTs would be centrosomally attached and that they would be stabilized and acetylated on their distal domains because of contact with Golgi elements. To discriminate between these two hypotheses, we repeated nocodazole wash-out experiments, visualizing both nonmodified and acetylated tubulin. The centrosomal MT population was composed of a mixture of acetylated (as observed in Figure 1, E and F) and unmodified MTs, which were longer and more abundant in Fao (Figure 3A) than in WIF-B cells (Figure 3B). As for discrete MT acetylation, the superimposition of consecutive confocal slices clearly shows that the scattered acetylated MT segments belonged to longer MTs, which were not connected with the centrosomes. These noncentrosomal MTs were randomly oriented in the cytoplasm and were acetylated at only one end (Figure 3, A and B). The fact that noncentrosomal MT repolymerization did not occur more frequently below the apical membrane than in the rest of the cytoplasm of WIF-B cells also indicates that cell polarity did not influence the early building of the MT network in hepatic cells.
Figure 3.
Scattered acetylated MTs belong to noncentrosomal microtubules. Fao rat hepatoma cells (A) and WIF-B polarized hepatic cells (B) were subjected to the same nocodazole wash-out experiments as described in Figure 1. After fixation, acetylated tubulin (center panels) and α-tubulin (left panels) were visualized by immunofluorescence on a confocal microscope. The images shown are the superimposition of consecutive optical sections acquired over the whole cell height (delta z = 0.5 μm). A bile canaliculus (b.c) is indicated between two adjacent WIF-B cells. Scale bar, 10 μm.
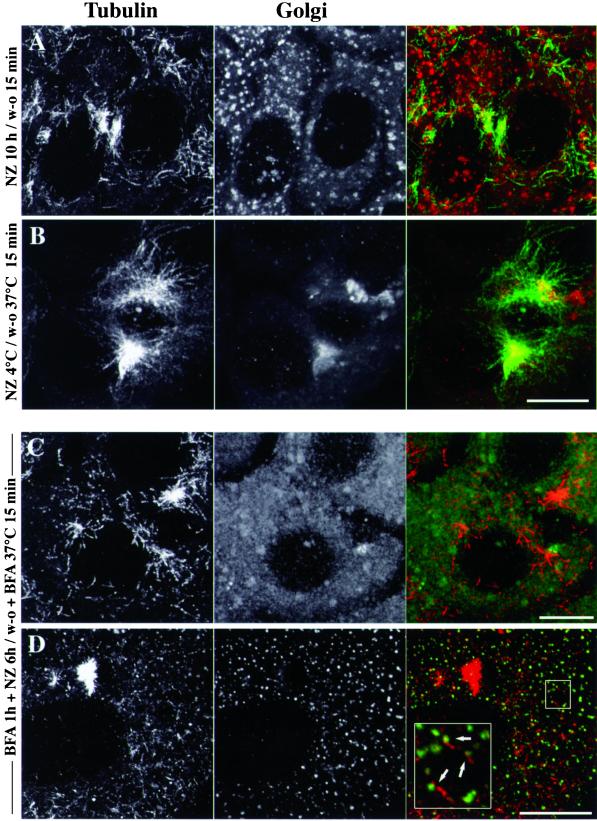
To evaluate further whether the subcellular localization of noncentrosomal MTs correlates with that of Golgi membranes, MTs were depolymerized by the combined actions of cold and nocodazole to prevent the dispersion of the Golgi complex (Turner and Tartakoff, 1989), and then the temperature was shifted back to 37°C for 15 min after nocodazole removal to allow MT assembly. As shown for WIF-B cells (Figure 4B), the Golgi remained essentially in a centrosomal location, although very small Golgi fragments also occurred throughout the cytoplasm and were probably rebuilt from the ER (Cole et al., 1996; Storrie et al., 1998). In these conditions, there was a dramatic drop in the amount of noncentrosomal MTs (Figure 4B) compared with cells in which nocodazole treatment was performed at 37°C (Figure 4A). The sites of noncentrosomal MT assembly thus appeared to follow the position of Golgi elements, strongly suggesting that Golgi elements are actually involved in the assembly of the noncentrosomal MT population. The fact that newly assembled MTs gathered around the centrosome/Golgi location in these conditions ruled out the possibility that other organelles distributed throughout the cytoplasm such as the ER or mitochondria could be involved in the occurrence of noncentrosomal MTs. Regarding early endosomes that are localized in the same area as the Golgi, we also checked that upon nocodazole wash-out, previously internalized fluorescent transferrin did not colocalize with Golgi and TGN markers (our unpublished results). To further study the dependence of noncentrosomal MT assembly on the localization of Golgi membranes, we tested whether the nucleating capacity of Golgi elements can be redistributed to the ER upon BFA treatment. Additional experiments were conducted in which cells were treated with BFA before MT depolymerization and nocodazole wash-out. In Figure 4C where cells were subjected to tubulin and albumin labeling, noncentrosomal MTs still occurred, whereas albumin was redistributed back into the ER. As shown in Figure 4D, however, the Golgi matrix protein GM130, which redistributes to the CGN and/or to the intermediate compartment upon BFA treatment (Nakamura et al., 1995), occurred as discrete elements that were closely associated with noncentrosomal MTs. Consistent with this observation, the distribution of the distance measurements between MTs and GM130 locations was very similar to that described in Table 1 (our unpublished results), suggesting that in these conditions, MTs might regrow off the intermediate compartment that contained Golgi matrix proteins.
Figure 4.
The subcellular localization of newly assembled microtubules is correlated with Golgi membrane location. WIF-B polarized hepatic cells were pretreated (C, D) or not with 10 μM BFA for 1 h at 37°C and then subjected to MT depolymerization by 10 μM nocodazole at 37°C for 6 (C and D) or 10 h (A). In B, MT depolymerization was conducted at 4°C for 2 h. Cells were then allowed to recover from nocodazole treatment at 37°C for 15 min. After fixation, cells were either subjected to the double-immunofluorescence labeling of α-tubulin (left panels) and secretory albumin (middle panels of A–C) or to that of α-tubulin and GM130 (middle panel of D). The images are the superimposition of equidistant optical sections acquired by confocal microscopy over the whole cell height (delta z = 0.5 μm). Scale bars, 10 μm.
Purified Golgi Stimulate MT Assembly and Stabilization In Vitro
The previous experiments gave highly correlative results, suggesting that Golgi stacks could promote the assembly of noncentrosomal MTs and stabilize them in vivo, but there were two issues with this approach. First, we could not formally exclude the hypothesis that these peripheral stable MTs could be due to the severing of centrosomal MTs followed by treadmilling (Rodionov and Borisy, 1997) before capture and stabilization by Golgi elements. Second, it was impossible to determine by in vivo experiments whether MT stabilization was direct or not and whether MT stabilization and assembly resulted from the same mechanism.
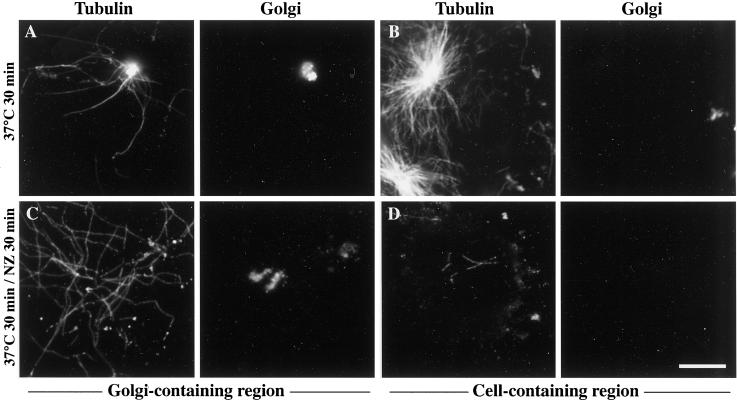
We thus conducted MT assembly and stabilization experiments in a system slightly modified from that described by Saoudi et al. (1998), in which interphase-like MT dynamics was reconstituted in detergent-extracted cells. In this system, MT dynamics were reestablished in permeabilized NIH 3T3 cells with the use of a mixture of detergent-free fibroblastic cytosol, an ATP-regenerating system, and a protein phosphatase inhibitor. Because detergent extraction in PEM buffer temporarily preserved cellular MTs from depolymerization, we further modified this protocol to completely depolymerize the MT network in the cold before testing MT reassembly and stability in the presence of purified Golgi. When incubated for 30 min with purified Golgi, this system allowed both the formation of Golgi-based MTs (Figure 5A) and that of centrosomal MT asters (Figure 5B). When the MT assembly period was followed by a 30-min treatment with 20 μM nocodazole, centrosomal asters, as expected, completely depolymerized (Figure 5D). In contrast, MTs surrounding Golgi elements resisted nocodazole treatment (Figure 5C) in a very similar way as MTs attached to Golgi elements did in vivo. This indicates that, in conditions that mimic as much as possible what happens in living cells, including the high level of MT dynamics found in centrosomal asters, exogenous Golgi was still capable of stimulating MT assembly and actually stabilized MTs.
Figure 5.
Golgi membranes nucleate and stabilize microtubules when added to a permeabilized cell system in which interphase microtubule dynamics were reconstituted. NIH-3T3 fibroblasts cultured on glass coverslips were detergent-extracted with 0.05% Triton X-100 in PEM buffer, rinsed extensively, and kept at 4°C for 3 d. Permeabilized cells were then incubated for 30 min at 37°C with detergent-free, autologous cytosol containing 2.5 μM tubulin, 5 μM okadaic acid, an ATP-regenerating system, and purified Golgi. In C–D, 20 μM nocodazole was added for 30 more min at 37°C. After two rinses with warm PEM buffer, samples were fixed with methanol (−20°C, 4 min) and processed for the double-immunofluorescence labeling of tubulin (left panels) and albumin (right panels). Depending on the distribution of Golgi elements and cells on the coverslip, images of Golgi-containing or control (containing extracted cells) regions are shown as indicated. Scale bar, 10 μm.
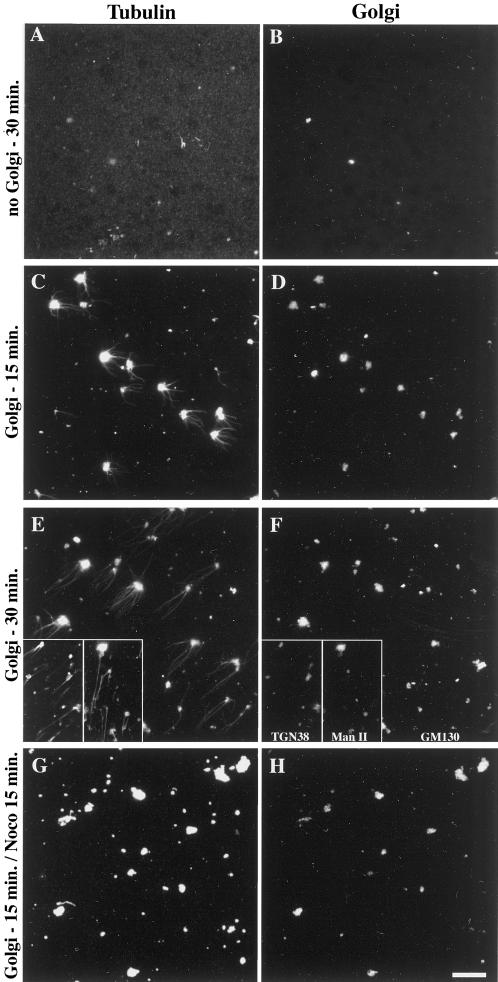
To test whether Golgi-mediated MT assembly and stabilization are direct or not, we further simplified the above system to finally keep purified rat liver Golgi, purified tubulin, and GTP. After assembly periods of 15 or 30 min, samples were fixed, centrifuged onto glass coverslips, and subjected to the double-immunofluorescence labeling of Golgi markers and α-tubulin. As expected, no spontaneous MT assembly occurred in the absence of Golgi membranes (Figure 6, A and B). When the system was supplemented with purified Golgi, MTs assembled, increasing in length as a function of time (Figure 6, C–F). It is worth noting that Golgi elements exhibited significant tubulin labeling over the whole membrane (Figure 6, C and E), even when they were incubated without tubulin (our unpublished results). As measured from six independent experiments (overall n = 300), only 65 ± 8% of the labeled Golgi elements actually allowed MT assembly. By performing labelings of TGN38 or mannosidase II instead of GM130 (Figure 6, E and F, insets), we checked that both markers were generally detected whatever the effectiveness of Golgi elements in nucleating MTs, suggesting that this effectiveness did not result from the enrichment in a Golgi subcompartment. To know further whether the MTs assembling around Golgi elements were also stabilized by the organelle, 10 μM nocodazole was added for 15 min after a 15-min assembly period. In these conditions, all the MTs were depolymerized (Figure 6, G and H), indicating that these MTs were highly dynamic in contrast with the in vivo situation and with what happened in the presence of cytosol and ATP in the permeabilized cell system. This experiment demonstrated that Golgi elements are directly capable of stimulating MT assembly, and, together with the data shown in Figure 5, it indicated that additional factor(s) are required to stabilize them. Adding the ATP-regenerating system and the protein phosphatase inhibitor to the minimal in vitro assembly system did not allow Golgi-based MTs to further resist nocodazole treatment (our unpublished results), indicating that cytosolic component(s) are required to specifically stabilize Golgi-based MTs.
Figure 6.
Purified Golgi membranes promote the assembly of microtubules in vitro but have no microtubule-stabilizing effect. Purified Golgi membranes were added (C–H) or not (A and B) to 0.1% purified porcine brain tubulin and 1 mM GTP in PEM buffer. After 15- or 30-min incubations at 37°C, followed (G and H) or not (A–F) by the addition of 10 μM nocodazole for 15 more min as indicated, samples were fixed by a 20-fold dilution with 0.25% glutaraldehyde in PEM buffer and sedimented although a 15% sucrose cushion onto glass coverslips. After postfixation in methanol (−20°C, 4 min), samples were processed for the immunofluorescence labeling of α-tubulin (left panels) and GM130 (right panels, B–H), TGN38 or Mannosidase II (F, insets) as indicated. Scale bar, 10 μm
γ-Tubulin Is Involved in the Nucleation of Golgi-based MTs
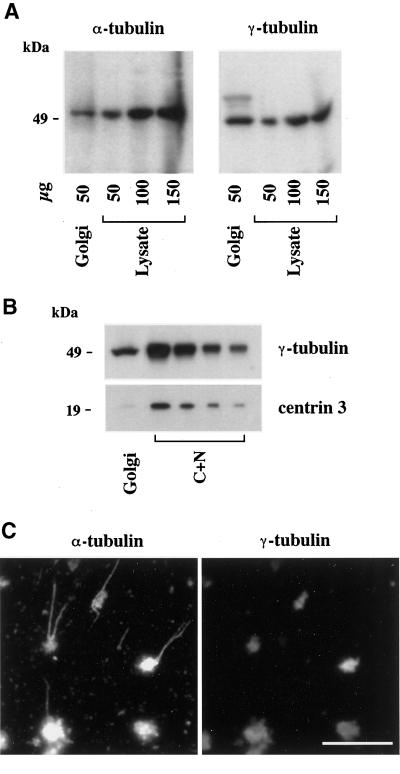
To further understand the mechanism of Golgi-based MT assembly, we first tested the specificity of our minimal in vitro assembly assay, considering the possibility that Golgi preparations might be systematically contaminated with centrosomes. To this end, purified Golgi membranes were analyzed by Western blotting for the presence of centrosomal markers, as well as for that of tubulins to confirm their presence on the Golgi from a biochemical point of view. As expected, both α- (Figure 7A) and β-tubulin (our unpublished results) were detected in Golgi samples. The Golgi preparation also contained significant amounts of γ-tubulin (Figure 7A). From a quantitative point of view, although the specific concentration of α-tubulin in the Golgi was identical to that measured in a hepatocyte lysate, γ-tubulin was slightly enriched (∼2 times) in the Golgi preparation. As shown in Figure 7B, the centriolar marker centrin-3 (Middendorp et al., 1997) was not detected in significant amounts in the Golgi compared with a nucleo-centrosomal extract. Consistently, neither was polyglutamylated tubulin (Eddéet al., 1990) detected in Golgi samples (our unpublished results). To evaluate from Figure 7B the specificity of γ-tubulin binding to Golgi membranes, a γ-tubulin to centrin ratio was measured in the Golgi and in the nucleo-centrosomal extract. This ratio was ∼25 times higher in the Golgi than in the nucleo-centrosomal extract, thus confirming that the presence of γ-tubulin on Golgi membranes was not due to a high level of contamination by centrosomes.
Figure 7.
γ-Tubulin is peripherally associated with Golgi membranes. (A) Purified rat liver Golgi membranes (50 μg of total proteins) and increasing amounts of rat hepatocyte lysate were analyzed by 9% SDS-PAGE followed by Western blotting for the presence of α- and γ-tubulin. Band intensities were analyzed by densitometry to estimate the enrichment of Golgi membranes in both markers relative to the lysate (see the text). (B) The amount of centriolar material was measured in Golgi samples compared with a nucleo-centrosomal extract (C+N) by SDS-PAGE (9 and 15% for γ-tubulin and centrin-3, respectively), followed by electrotransfer onto polyvinyl difluoride membranes, postfixation with 0.2% glutaraldehyde (only for centrin), and immunorevelation. Densitometric measurements of band intensities were used to evaluate the ratios of γ-tubulin to centrin signals and to allow a comparison between the Golgi and the C+N extract (see the text). (C) Purified Golgi was incubated for 30 min at 37°C with 0.1% tubulin and 1 mM GTP. After fixation with 0.25% glutaraldehyde, samples were centrifuged onto glass coverslips and postfixed with methanol before the double-labeling of α- and γ-tubulins as indicated. Scale bar, 10 μm.
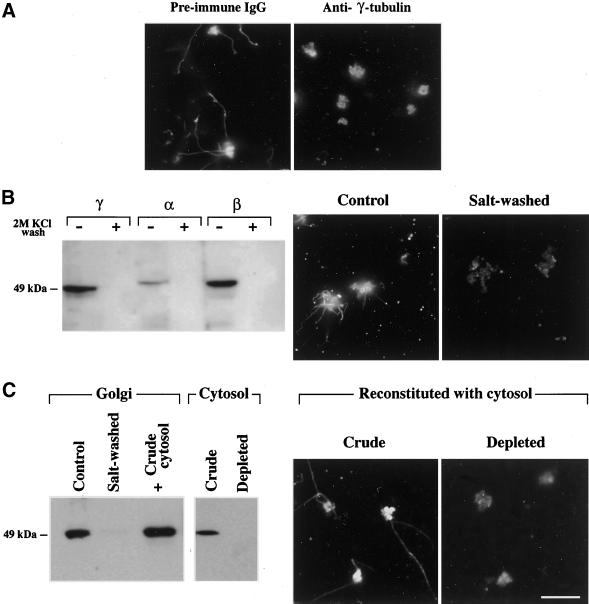
Even though it has been shown to be largely distributed in the cytosol (Moudjou et al., 1996), γ-tubulin was only known to play a role at the level of the MTOC. The binding of γ-tubulin to Golgi membranes was quite unexpected, but it also suggested a mechanism for Golgi-based MT nucleation. We thus checked that γ-tubulin was actually present at the surface of those Golgi elements that nucleate MTs. As shown above for other Golgi markers (see Figure 6), γ-tubulin was readily detected on both nucleating and nonnucleating Golgi elements (Figure 7C). We further tried to address the function of Golgi-bound γ-tubulin in two ways. First, we used a monoclonal anti–γ-tubulin (clone GTU-88) directed against the N-terminal domain of the protein (amino acids 38–53), which is involved in the MT-nucleating activity of γ-tubulin in centrosomes (Joshi et al., 1992). Figure 8A shows that the preincubation of Golgi membranes with GTU-88 completely inhibited in vitro MT nucleation, whereas preimmune mouse IgGs did not affect MT assembly. Second, as GTU-88 binding at the surface of the Golgi might have also sterically blocked any other neighboring proteins involved in MT assembly, we tried to overcome this limitation in the following way. We initially found that a 2 M KCl wash caused the complete loss of α-, β-, and γ-tubulin from purified Golgi, confirming that they were peripherally associated with the organelle. Accordingly, its MT-nucleating property was completely abolished (Figure 8B). Interestingly, all the attempts to reconstitute γ-tubulin binding failed when we used the proteins that had been stripped off the Golgi membranes. We then reconstituted the interaction of γ-tubulin with salt-washed Golgi membranes by incubating them with a WIF-B cytosolic extract similar to that used in the semiintact cells experiments (see Figure 5). In these conditions, γ-tubulin did rebind to the Golgi (Figure 8C) and that binding resulted in the recovery of the MT-nucleating property of Golgi membranes. When a γ-tubulin–depleted extract was used instead of crude cytosol, no MT nucleation could be observed (Figure 8C). It is also worth noting that γ-tubulin immunodepletion had to be quantitative as low residual concentrations of γ-tubulin were still able to restore the MT-nucleating capacity. Altogether these data indicate that peripherally bound γ-tubulin is actually involved in nucleating MTs on Golgi membranes.
Figure 8.
γ-Tubulin is involved in the nucleation of Golgi-based MTs. (A) As indicated, purified Golgi was preincubated either with anti–γ-tubulin or with preimmune mouse IgGs for 1 h at 37°C and then used in the in vitro MT assembly assay. Samples were processed for the immunofluorescence labeling of α-tubulin. (B) Purified rat liver Golgi was washed as indicated with 2 M KCl and then rinsed three times in PEM buffer before being assayed for the presence of tubulins or to be used in the in vitro MT assembly assay. Samples were analyzed for the presence of tubulins by Western blotting, as described in Figure 7. After the in vitro MT assembly assays, samples were subjected to the immunofluorescence labeling of α-tubulin. (C) Salt-washed Golgi membranes were incubated (1 h, room temperature) with crude WIF-B cytosol or cytosol that was immunodepleted of γ-tubulin and then rinsed twice in PEM buffer and either analyzed by Western blot for the presence of peripherally bound γ-tubulin or used in the in vitro MT assembly assay, followed by the immunofluorescence labeling of α-tubulin. Scale bar, 10 μm.
DISCUSSION
We found that the Golgi complex is involved in organizing a subset of stable MTs independently from the centrosome in interphase cells. Upon nocodazole removal after complete MT depolymerization and Golgi breakdown, a population of acetylated MTs occurred in noncentrosomal cytoplasmic locations that were highly correlated with those of Golgi elements. By contrast with the labile population of centrosomal acetylated MTs, this noncentrosomal MT subset was stabilized early. We further showed that purified Golgi membranes exhibited an intrinsic MT nucleating property, whereas they needed additional cytosolic component(s) to achieve MT stabilization. Having found that α-, β-, and γ-tubulins are bound to Golgi membranes, we showed that peripherally associated proteins were required for performing Golgi-based MT nucleation and more specifically that γ-tubulin itself was involved in the nucleating process.
We showed in vitro that Golgi membranes are directly responsible for the assembly of noncentrosomal MTs. This means that our in vivo observations were likely not to result from the severing and release of centrosomal MTs (Baas and Joshi, 1992; Yu et al., 1993; Keating et al., 1997; Vorobjev et al., 1997) or even spontaneous cytoplasmic assembly, followed by treadmilling or migration en bloc (Rodionov and Borisy, 1997; Vorobjev et al., 1997; Yvon and Wadsworth, 1997; Tucker et al., 1998) until they were captured and stabilized by scattered Golgi elements. Because the reconstitution of mini-Golgi stacks at ER exit sites actively participates in the dispersion of the Golgi complex after nocodazole-mediated MT depolymerization (Cole et al., 1996; Storrie et al., 1998), the ER would also have been a likely candidate for stimulating noncentrosomal MT assembly. This possibility was clearly ruled out because the vast majority of newly assembled MTs followed the Golgi when the organelle was kept in a central location during MT depolymerization. Interestingly, the results we obtained upon BFA treatment suggested that the MT-assembling properties of the Golgi are not linked to glycosyltransferases or to other proteins that are redistributed back to the ER but rather to proteins like GM130, which are retained in the intermediate compartment/CGN in these conditions. Our data thus identify the Golgi as a central organelle involved in the assembly of a subset of stable MTs. Other membrane organelles such as fish melanophore pigment granules (Rodionov and Borisy, 1997) might be involved in organizing MTs in the absence of centrosome. These data, together with the fact that pinocytic vesicles organize actin bursts upon internalization (Merrifield et al., 1999), support the view that some membrane organelles are not solely organized in a passive way by the cytoskeleton but that they actively participate in the organization of cytoskeletal structures.
We found that significant amounts of α- and β-tubulins are bound to the Golgi. A direct anchoring of palmitoylated tubulin in membranes has been demonstrated in platelets (Caron, 1997; Ozols and Caron, 1997), but such an anchoring was unlikely to be involved in the case of Golgi membranes because tubulins were all lost after salt-wash. The binding of tubulin to Golgi membranes might also be due to an interaction with Golgi-resident proteins such as β-1,4-galactosyltransferase (Yamaguchi and Fukuda, 1995). Another possibility would be that very short tubulin polymers might interact with molecular motors constitutively bound to Golgi membranes (Vaisberg et al., 1996; for review, Hirokawa et al., 1998) or with γ-tubulin, which we identified as a key molecule involved in nucleating Golgi-based MTs. γ-Tubulin could be recruited from the cytosolic pool to promote MT nucleation, but, by analogy with what happens in the centrosomes, it should be organized in a nucleating complex such as a ring complex (Zheng et al., 1995) that may cap MT minus ends (Wiese and Zheng, 2000). The molecules that actually recruit and/or organize γ-tubulin on Golgi membranes still remain to be identified. The assembly of γ-tubulin onto centrosomes being mediated by cytoplasmic dynein in a MT-dependent manner (Young et al., 2000), one might even speculate that cytoplasmic dynein, which was also identified on Golgi membranes (Vaisberg et al., 1996; Roghi and Allan, 1999), might be needed to some extent for assembling and/or docking γ-tubulin on the Golgi.
At the level of the centrosome, a sequential model has emerged in which MTs are first nucleated on γ-tubulin–containing complexes and then released and anchored on distinct sites (Quintyne et al., 1999; Mogensen et al., 2000). A similar model might also apply for the Golgi complex in which the Golgi-associated MAP GMAP-210 that was shown to bind both the minus end of MTs and CGN membranes (Infante et al., 1999) would be a good docking candidate. Additionally, the MT-stabilizing effect of Golgi membranes and the fact that GMAP associates with a subset of stable MTs in vivo (Infante et al., 1999) might also fit well with this hypothesis. In this respect, the in vitro system we used to reconstitute the MT-stabilizing effect only reproduced plus-end dynamics, which is necessary and sufficient to restore the sensitivity of MTs to nocodazole (Saoudi et al., 1998). This also is consistent with the fact that MTs might be anchored to Golgi membranes by their minus ends, with their plus ends being free to grow and shrink. A puzzling question would remain, however: how can MT plus ends be remotely stabilized when the minus ends binds to Golgi membranes? Insoluble cytoplasmic structures such as intermediate filaments might be involved, even though they are not indispensable for the reclustering of Golgi elements around the centrosome (Ho et al., 1989). More likely, soluble cytosolic factor(s) might specifically recognize Golgi-bound MTs and participate directly in their early stabilization or be needed to activate a Golgi-bound molecular motor that would slide toward the plus end and contribute to its stabilization. Such a hypothesis would be supported by the recent finding that detyrosinated MTs are stabilized by an ATP-sensitive, plus-end cap that resembles kinesins (Infante et al., 2000).
Although our data provide many evidence for the capability of Golgi membranes to assemble MTs and stabilize them, the physiological role of such properties is still unclear.
The simplest situation in which cell physiology resembles nocodazole wash-out conditions is the postmitotic reclustering of Golgi elements in the pericentrosomal area. Astral MTs seem indispensable for the reclustering of the organelle in a central location (Shima et al., 1998), but intermediate steps in this process might involve the capture of Golgi-attached MTs in the centrosomal area to facilitate the reclustering process. Such a model would require Golgi MTs to exhibit reverse polarity or would be quite complicated in terms of molecular motor regulation, because Golgi reclustering is dynein dependent (Corthésy-Theulaz et al., 1992; Fath et al., 1994, Burkhardt et al., 1997; Harada et al., 1998). This would be in a way similar to the behavior of chromosomes during mitosis because they have the possibility to directly nucleate MTs in addition to their capture by centrosomal MTs (Carazo-Salas et al., 1999). Another possibility would be that Golgi-attached MTs participate in the local merger of postmitotic Golgi elements before their final reclustering. It is known from nocodazole wash-out experiments that each Golgi fragment is not directed to the centrosomal region but that local merger occurs before final reclustering (Ho et al., 1989). The role of Golgi-based MTs would be to organize locally the neighboring mini-stacks into bigger structures that would finally be targeted to the centrosomal area. Because Golgi membranes can actually stabilize MTs, one could propose that the Golgi complex plays a key role in the differentiation of the MT network by specifically stabilizing a MT subset on exit from mitosis. Indeed, Golgi elements also codistribute with a subset of early acetylated MTs during telophase, before the occurrence of detyrosinated MTs (Thyberg and Moskalewski, 1993). After the growing evidence for the functional specialization of distinct classes of MTs, depending on their stability (Mizuno and Singer, 1994; Nagasaki et al., 1994;Gurland and Gundersen, 1995; Tanaka et al., 1995; Minin, 1997; Poüs et al., 1998), our understanding of the molecular events that lead to MT stabilization has also grown regularly (for review see Gundersen and Cook, 1999). However, even though some biochemical data show that the small GTPase RhoA stabilizes only a subset of MTs (Cook et al., 1998), virtually nothing is known about how a specific subset of MTs might be selectively recognized before stabilization. Because postmitotic Golgi fragments are reclustered around the centrosome along astral MTs (Shima et al., 1998), Golgi elements might serve as a differentiation device to identify the future subset of stable MTs in interphase cells.
During the interphase, MTs are required for performing effective protein trafficking toward the cell surface or back to the endoplasmic reticulum (for review see Cole and Lippincott-Schwartz, 1995; Bloom and Goldstein, 1998). Dynamic and stable MTs are specialized in mediating specific transport steps (Mizuno and Singer, 1994; Poüs et al., 1998), which may involve various molecular motors, often localized on Golgi membranes (Vaisberg et al., 1996; for review see Hirokawa, 1998). The way such specialized motor proteins specifically interact with the appropriate target membranes is still an open question but may be related to the regulation of membrane traffic, as suggested by the example of rabkinesin-6, which binds the small GTPase Rab6 involved in Golgi-to-ER retrograde transport (Echard et al., 1998). This example illustrates the probable need for specific GTPases, specific molecular motors and appropriate MTs to operate a given membrane transport step in vivo. From this perspective, our results also suggest that upon the activation of the transport machinery, Golgi membranes might assemble and perhaps stabilize the appropriate MTs instead of depending on centrosomally attached MTs for the vectorization of membrane carriers. This would allow the cell to define priority tracks to use for efficient vesicular transport. Because the Golgi complex does behave as a potent organizer and a differentiating device of a stable subset of MTs, it will be interesting now to address the corresponding molecular mechanisms in more detail. In this perspective, the in vitro systems that we developed will be valuable.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Doris Cassio for her enthusiastic encouragements; to Drs. Thu Phung-Koskas, Germain Trugnan, Michèle Maurice, and Tounsia Ait Slimane for critical advice in discussing the experimental work; and to Drs. George Banting, Michel Bornens, and Philippe Denoulet for providing cell extracts and antibodies. The authors are also grateful to the Institut Federatif de Recherche 02 “Cellules épithéliales” from the Institut National de la Santé et de la Recherche Medicale, Faculté de Médecine Xavier Bichat, 75018 Paris, for the availability of the confocal microscope. This work was supported by a grant from the “Institut de Recherches Internationales Servier.”
Abbreviations used:
- BFA
brefeldin A
- CGN
cis-Golgi network
- ER
endoplasmic reticulum
- MT
microtubule
- TGN
trans-Golgi network
REFERENCES
- Baas PW, Joshi HC. γ-Tubulin distribution in the neuron: implications for the origins of neuritic microtubules. J Cell Biol. 1992;119:171–178. doi: 10.1083/jcb.119.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biou D, Monnet D, Miller D, Feger J, Durand G. An immunochemical procedure to evaluate the degree of desialylation of α1-acid glycoprotein in rat serum. J Immunol Methods. 1984;74:267–271. doi: 10.1016/0022-1759(84)90293-x. [DOI] [PubMed] [Google Scholar]
- Bloom GS, Goldstein LS. Cruising along microtubule highways: how membranes move through the secretory pathway. J Cell Biol. 1998;140:1277–1280. doi: 10.1083/jcb.140.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bré MH, Kreis TE, Karsenti E. Control of microtubule nucleation and stability in Madin-Darby canine kidney cells: the occurrence of noncentrosomal, stable detyrosinated microtubules. J Cell Biol. 1987;105:1283–1296. doi: 10.1083/jcb.105.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski JC, Richards JE, Piperno G. Posttranslational modifications of α-tubulin: detyrosination and acetylation differentiate populations of interphase microtubules in cultured cells. J Cell Biol. 1988;106:1213–1220. doi: 10.1083/jcb.106.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TL, Skoufias DA, Wilson L. Disruption of the Golgi apparatus with brefeldin A does not destabilize the associated detyrosinated microtubule network. Cell Motil Cytoskeleton. 1991;20:289–300. doi: 10.1002/cm.970200405. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas RE, Guargualini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 1999;400:178–181. doi: 10.1038/22133. [DOI] [PubMed] [Google Scholar]
- Caron JM. Posttranslational modification of tubulin by palmitoylation: I. In vivo and cell-free studies. Mol Biol Cell. 1997;8:621–636. doi: 10.1091/mbc.8.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassio D, Hamon-Benais C, Guerin M, Lecoq O. Hybrid cell lines constitute a potential reservoir of polarized cells: isolation and study of highly differentiated hepatoma-derived hybrid cells able to form functional bile canaliculi in vitro. J Cell Biol. 1991;115:1397–1408. doi: 10.1083/jcb.115.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Lippincott-Schwartz J. Organization of organelles and membrane traffic by microtubules. Curr Opin Cell Biol. 1995;7:55–64. doi: 10.1016/0955-0674(95)80045-x. [DOI] [PubMed] [Google Scholar]
- Cole NB, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol Biol Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook TA, Nagasaki T, Gundersen GG. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J Cell Biol. 1998;141:175–185. doi: 10.1083/jcb.141.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthésy-Theulaz I, Pauloin A, Pfeffer S. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992;118:1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echard A, Jollivet F, Martinez O, Lacapère JJ, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- Eddé B, Rossier J, Le Caer JP, Desbruyères E, Gros F, Denoulet P. Posttranslational glutamylation of α-tubulin. Science. 1990;247:83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Fath KR, Trimbur GM, Burgess TR. Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. J Cell Biol. 1994;126:661–675. doi: 10.1083/jcb.126.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiguin F, Ferreira A, Kosik KS, Caceres A. Kinesin-mediated organelle translocation revealed by specific cellular manipulation. J Cell Biol. 1994;127:1021–1039. doi: 10.1083/jcb.127.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Cook TA. Microtubules and signal transduction. Curr Opin Cell Biol. 1999;11:81–94. doi: 10.1016/s0955-0674(99)80010-6. [DOI] [PubMed] [Google Scholar]
- Gurland G, Gundersen GG. Stable detyrosinated microtubules function to localize vimentin intermediate filaments in fibroblasts. J Cell Biol. 1995;131:1275–1290. doi: 10.1083/jcb.131.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Takei Y, Kanai Y, Tanaka Y, Nonaka S, Hirokawa N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol. 1998;141:51–59. doi: 10.1083/jcb.141.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y, Okada Y. Kinesin and dynein superfamily proteins in organelle transport and cell division. Curr Opin Cell Biol. 1998;10:60–73. doi: 10.1016/s0955-0674(98)80087-2. [DOI] [PubMed] [Google Scholar]
- Ho WC, Allan VJ, van Meer G, Berger EG, Kreis TE. Reclustering of scattered Golgi elements occurs along microtubules. Eur J Cell Biol. 1989;48:250–263. [PubMed] [Google Scholar]
- Hui N, Nakamura N, Sluzarewicz P, Warren G. Purification of rat liver Golgi stacks. In: Celis J E, editor. Cell biology, a laboratory handbook. 2nd edition. New York: Academic Press; 1998. pp. 46–55. [Google Scholar]
- Infante C, Ramos-Morales F, Fedriani C, Bornens M, Rios R. GMAP-210, a cis-Golgi network-associated protein, is a minus-end microtubule-binding protein. J Cell Biol. 1999;145:83–98. doi: 10.1083/jcb.145.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante AS, Stein MS, Zhai Y, Borisy GG, Gundersen GG. Detyrosinated (Glu) microtubules are stabilized by an ATP-sensitive plus-end cap. J Cell Sci. 2000;113:3907–3919. doi: 10.1242/jcs.113.22.3907. [DOI] [PubMed] [Google Scholar]
- Joshi HC, Palacios MJ, McNamara L, Cleveland DW. γ-Tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992;356:80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- Kaverina I, Rottner K, Small JV. Targeting, capture and stabilization of microtubules at early focal adhesions. J Cell Biol. 1998;142:181–190. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D, Borisy GG. Microtubule release from the centrosome. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988;106:141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE, Goodson HV, Perez F, Rönnholm R. Golgi apparatus-cytoskeleton interactions. In: Berger EG, Roth J, editors. The Golgi apparatus. Basel: Birkhäuser Verlag/Switzerland; 1997. pp. 179–193. [Google Scholar]
- MacRae TH. Tubulin post-translational modification. Enzymes and their mechanisms of action. Eur J Biochem. 1997;244:265–278. doi: 10.1111/j.1432-1033.1997.00265.x. [DOI] [PubMed] [Google Scholar]
- Meads T, Schroer TA. Polarity and nucleation of microtubules in polarized epithelial cells. Cell Motil Cytoskeleton. 1995;32:273–288. doi: 10.1002/cm.970320404. [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Moss SE, Ballestrem C, Imhoff BA, Giese G, Wunderlich I, Almers W. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat Cell Biol. 1999;1:72–74. doi: 10.1038/9048. [DOI] [PubMed] [Google Scholar]
- Middendorp S, Paoletti A, Schiebel E, Bornens M. Identification of a new mammalian centrin gene, more closely related to Saccharomyces cerevisiae CDC31 gene. Proc Natl Acad Sci USA. 1997;94:9141–9146. doi: 10.1073/pnas.94.17.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minin AA. Dispersal of Golgi apparatus in nocodazole-treated fibroblasts is a kinesin-driven process. J Cell Sci. 1997;110:2495–2505. doi: 10.1242/jcs.110.19.2495. [DOI] [PubMed] [Google Scholar]
- Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J Cell Sci. 2000;113:3013–3023. doi: 10.1242/jcs.113.17.3013. [DOI] [PubMed] [Google Scholar]
- Moudjou M, Bordes N, Paintrand M, Bornens M. γ-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J Cell Sci. 1996;109:875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Singer SJ. A possible role for stable microtubules in intracellular transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Sci. 1994;107:1321–1331. doi: 10.1242/jcs.107.5.1321. [DOI] [PubMed] [Google Scholar]
- Nagasaki T, Liao G, Gundersen GG. Isolated plasma membranes induce the loss of oriented detyrosinated microtubules and other contact inhibition-like responses in migrating NRK cells. J Cell Sci. 1994;107:3413–3423. doi: 10.1242/jcs.107.12.3413. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J, Caron JM. Posttranslational modification of tubulin by palmitoylation: II. Identification of sites of palmitoylation. Mol Biol Cell. 1997;8:637–645. doi: 10.1091/mbc.8.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poüs C, Chabin K, Drechou A, Barbot L, Phung-Koskas T, Settegrana C, Bourguet-Kondracki ML, Maurice M, Cassio D, Guyot M, Durand G. Functional specialization of stable and dynamic microtubules in protein traffic in WIF-B cells. J Cell Biol. 1998;142:153–165. doi: 10.1083/jcb.142.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA. Dynactin is required for microtubule anchoring at centrosomes. J Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B, Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN 38. J Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov VI, Borisy GG. Microtubule treadmilling in vivo. Science. 1997;275:215–218. doi: 10.1126/science.275.5297.215. [DOI] [PubMed] [Google Scholar]
- Roghi C, Allan VJ. Dynamic association of cytoplasmic dynein heavy chain 1a with the Golgi apparatus and intermediate compartment. J Cell Sci. 1999;112:4673–4685. doi: 10.1242/jcs.112.24.4673. [DOI] [PubMed] [Google Scholar]
- Saoudi Y, Fotedar R, Abrieu A, Dorée M, Wheland J, Margolis RL, Job D. Stepwise reconstitution of interphase microtubule dynamics in permeabilized cells and comparison to dynamic mechanisms in intact cells. J Cell Biol. 1998;142:1519–1532. doi: 10.1083/jcb.142.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E, Asai DJ, Bulinski JC, Kirschner M. Posttranslational modifications and microtubule stability. J Cell Biol. 1987;105:2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks MR, Cassio D, Lecoq O, Hubbard AL. An improved polarized rat hepatoma hybrid cell line: generation and comparison to its hepatoma relatives and hepatocytes in vivo. J Cell Sci. 1994;107:813–824. doi: 10.1242/jcs.107.4.813. [DOI] [PubMed] [Google Scholar]
- Shima DT, Cabrera-Poch N, Pepperkok R, Warren G. An ordered inheritance strategy for the Golgi apparatus: visualization of mitotic disassembly reveals a role for the mitotic spindle. J Cell Biol. 1998;141:955–966. doi: 10.1083/jcb.141.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias DA, Burgess TL, Wilson L. Spatial and temporal colocalization of the Golgi apparatus and microtubules rich in detyrosinated tubulin. J Cell Biol. 1990;111:1929–1937. doi: 10.1083/jcb.111.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, White J, Röttger S, Stelzer EHK, Suganuma T, Nilsson T. Recycling of Golgi-resident glycosyltransferases through the endoplasmic reticulum reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Ho T, Kirschner MW. The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol. 1995;128:139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg J, Moskalewski S. Relationship between the Golgi complex and microtubules enriched in detyrosinated or acetylated α-tubulin: studies on cells recovering from nocodazole and cells in the terminal phase of cytokinesis. Cell Tissue Res. 1993;273:457–466. doi: 10.1007/BF00333700. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Moskalewski S. Role of microtubules in the organization of the Golgi complex. Exp Cell Res. 1999;246:263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- Tucker JB, Mogensen MM, Henderson CG, Doxsey SJ, Wright M, Stearns T. Nucleation and capture of large cell surface-associated microtubule arrays that are not located near centrosomes in certain cochlear epithelial cells. J Anat. 1998;192:119–130. doi: 10.1046/j.1469-7580.1998.19210119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Tartakoff AM. The response of the Golgi complex to microtubule alterations: the roles of metabolic energy and membrane traffic in Golgi complex organization. J Cell Biol. 1989;109:2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg EA, Grissom PM, McIntosh JR. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J Cell Biol. 1996;133:831–842. doi: 10.1083/jcb.133.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev IA, Svitkina TM, Borisy GG. Cytoplasmic assembly of microtubules in cultured cells. J Cell Sci. 1997;110:2635–2645. doi: 10.1242/jcs.110.21.2635. [DOI] [PubMed] [Google Scholar]
- Walker RA, Inoué S, Salmon ED. Asymmetric behavior of severed microtubule ends following ultraviolet-microbeam irradiation of individual microtubules in vitro. J Cell Biol. 1988;108:931–937. doi: 10.1083/jcb.108.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci USA. 1987;84:9040–9044. doi: 10.1073/pnas.84.24.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DR, Wehland J, Weber K, Borisy GG. Detyrosination of α tubulin does not stabilize microtubules in vivo. J Cell Biol. 1990;111:113–122. doi: 10.1083/jcb.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C, Zheng Y. A new function for the γ-tubulin ring complex as a microtubule minus-end cap. Nat Cell Biol. 2000;2:358–364. doi: 10.1038/35014051. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Fukuda MN. Golgi retention mechanism of β-1,4-galactosyltransferase. Membrane-spanning domain-dependent homodimerization and association with α- and β-tubulins. J Biol Chem. 1995;270:12170–12176. doi: 10.1074/jbc.270.20.12170. [DOI] [PubMed] [Google Scholar]
- Young A, Dictenberg JB, Purohit A, Tuft R, Doxsey SJ. Cytoplasmic dynein-mediated assembly of pericentrin and γ-tubulin onto centrosomes. Mol Biol Cell. 2000;11:2047–2056. doi: 10.1091/mbc.11.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon AM, Wadsworth P. Non-centrosomal microtubule formation and measurement of minus end microtubule dynamics in A498 cells. J Cell Sci. 1997;110:2391–2401. doi: 10.1242/jcs.110.19.2391. [DOI] [PubMed] [Google Scholar]
- Yu W, Centonze VE, Ahmad FJ, Baas PW. Microtubule nucleation and release from the neuronal centrosome. J Cell Biol. 1993;122:349–359. doi: 10.1083/jcb.122.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]