Abstract
Bradykinesia is the most important feature contributing to motor difficulties in Parkinson’s disease (PD). However, the pathophysiology underlying bradykinesia is not fully understood. One important aspect is that PD patients have difficulty in performing learned motor skills automatically, but this problem has been generally overlooked. Here we review motor automaticity associated motor deficits in PD, such as reduced arm swing, decreased stride length, freezing of gait, micrographia and reduced facial expression. Recent neuroimaging studies have revealed some neural mechanisms underlying impaired motor automaticity in PD, including less efficient neural coding of movement, failure to shift automated motor skills to the sensorimotor striatum, instability of the automatic mode within the striatum, and use of attentional control and/or compensatory efforts to execute movements usually performed automatically in healthy people. PD patients lose previously acquired automatic skills due to their impaired sensorimotor striatum, and have difficulty in acquiring new automatic skills or restoring lost motor skills. More investigations on the pathophysiology of motor automaticity, the effect of L-dopa or surgical treatments on automaticity, and the potential role of using measures of automaticity in early diagnosis of PD would be valuable.
Keywords: Parkinson’s disease, Automatic control, Attentional control, Bradykinesia, Basal ganglia
Introduction
Parkinson’s disease (PD) is one of the most common progressive neurological degenerative disorders. The primary manifestations of PD are motor dysfunction, including slowness of movements (bradykinesia), resting tremor, rigidity, gait disturbance and postural instability. In this review, bradykinesia is used to encompass the terms hypokinesia (smallness of movement, as in micrographia) and akinesia (lack of movement, as in freezing or lack of facial expression).
Bradykinesia is the most important reason for motor difficulties in PD, which can affect almost all activities in daily life (Hallett, 2011). Several pathophysiology mechanisms have been proposed to explain bradykinesia, like a failure of basal ganglia output to reinforce the cortical mechanisms that prepare and execute the command to move (Berardelli et al., 2001), loss of motor energy, difficulty with internal driving of movement and the concomitant excess influence of external sensory control of movement, and sequence effect (Hallett, 2011). However, these mechanisms can only provide partial explanation, and, as such, our understanding of the pathophysiology underlying bradykinesia remains incomplete.
Since the 1980s, it was already recognized that PD patients have difficulty in “automatic execution of learned motor plans”, and this problem is likely due to impairment of basal ganglia function (Marsden, 1982). It has been shown that PD patients have a greater abnormality of automatic associated movement than intended voluntary movement, which may be one of the bases of clinical symptoms in the early stage of the disease (Hoshiyama et al., 1994). However, even though impaired motor automaticity is likely an important reason underlying bradykinesia in PD, it has been generally overlooked and much less investigated compared to other motor deficits. In this review we discuss the contributions of defective motor automaticity to bradykinesia in PD, and its neural mechanisms.
What is motor automaticity
Automaticity is the ability to perform movements without attention directed toward the details of the movement, particularly for movements that require low levels of precision or for movements that are commonly made (Bernstein, 1967). After a period of training, and passing through several distinct phases, including fast (early), slow (later), and consolidation stages (Doyon and Benali, 2005), even some complex motor skills can be well established and achieve the automatic phase (Fitts, 1964). At this stage, motor skills can be performed requiring minimal cognitive resources and are resistant to interference and the effects of time. In fact, most of our daily behaviors are carried out automatically. From getting up in the morning, brushing teeth, eating breakfast with spoon, fork or chopstick, to walking or driving to work, people usually pay little attention to these motor behaviors. For example, people can talk on cell phone while walking; even when focusing on talking, they still can maintain walking smoothly without difficulty.
Because improvement on a task after extensive practice does not guarantee that it is automatic (Lang and Bastian, 2002), motor automaticity is commonly evaluated by a dual-task paradigm (Wu et al., 2004), which requires to perform a secondary task at the same time as a first motor task. The secondary task is usually a cognitive task, e.g., a counting task, but also can be another motor task. If both tasks are performed correctly without obvious interference, the first motor task can be said to be automatic (Passingham, 1996; Wu et al., 2004). If the performance of one or both tasks deteriorates under dual-task conditions, then the motor task is not automatic.
Neural mechanisms of automaticity in healthy people
Several studies have investigated neural correlates during motor automatic processing with dual-task to prove automaticity (Wu et al., 2004, 2008; Wu and Hallett 2005b; Lehéricy et al., 2005; Poldrack et al., 2005; Balsters and Ramnani, 2011; Wu et al., in press) (Table 1), a common finding is decreased activity in extensive brain regions, e.g., the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), rostral supplementary motor area (pre-SMA), premotor cortex (PMC), parietal cortex, and cerebellum, accompanied by enhanced effective connectivity between motor areas, like the cerebellum, cingulate motor area, supplementary motor area (SMA), and putamen during automatic processing. The stronger connectivity might be a reflection of enhanced synaptic strength during the automatic process (Hebb, 1949). The reduction of brain activity and enhancement of network connectivity together suggests a more efficient neural code for controlling a motor task (Wu et al., 2008; Mazzoni, 2008). In addition, there was weakened connectivity from the DLPFC to motor areas. The DLPFC and ACC are critical in the attentional networks (Jueptner et al., 1997; Petersen et al., 1988; Isomura et al., 2003). The diminished activity and connectivity in these two regions indicates that when a movement achieves automaticity, the attentional networks become less necessary. A recent study investigated changes in the architecture of functional connectivity patterns that promote learning from initial training through mastery of a simple motor skill. The results of this study show that motor learning induces an autonomy of sensorimotor systems and that the release of cognitive control hubs in frontal and cingulate cortices predicts individual differences in the rate of learning (Bassett et al., 2015).
Table 1.
Neural correlates of automaticity in healthy people.
| Reference | Task paradigm | Neural changes during automatic process |
|---|---|---|
| Wu et al. (2004) | Self-initiated, self-paced, sequential finger movements | Decreased activity in the cerebellum, pre-SMA, CMA, caudate nucleus, PMC, parietal cortex, and prefrontal cortex |
| Lehéricy et al. (2005) | Explicit learning of a sequence of finger movements over a month of training | Decreased activity in the anterior putamen, thalamus, STN, globus pallidus, cerebellum, pons, ACC, rostral premotor and prefrontal areas; Increased activity in the posterior putamen |
| Poldrack et al. (2005) | A serial reaction time task | Decreased activation in the ventral PMC, inferior prefrontal gyrus, DLPFC, SMA, caudate, putamen and globus pallidus |
| Wu and Hallett (2005b) | Self-initiated, self-paced, sequential finger movements | Decreased activation in the pre-SMA, PMC, and parietal cortex |
| Wu et al. (2008) | Self-initiated, self-paced, sequential finger movements | The cerebellum, CMA, pre-SMA and putamen had stronger connectivity with the motor networks; The precuneus had less connectivity with the motor networks |
| Balsters and Ramnani (2011) | A conditional learning task | Decreased activation in Crus I of cerebellar cortical lobule HVIIA, a target of the prefrontal cortex |
| Wu et al. (in press) | A visuomotor association task | Decreased connectivity from the DLPFC and anterior putamen to the motor regions; Strengthened connectivity from the posterior putamen to the motor cortex; Re-attending to automatic movement induced more activation in the DLPFC, anterior cingulate cortex, and rostral supplementary motor area, but did not change the activity and connectivity in the striatum |
Only results from the studies with dual-task to prove automaticity are summarized here.
ACC, anterior cingulate cortex; CMA, cingulate motor area; DLPFC, dorsolateral prefrontal cortex; PMC, premotor cortex, pre-SMA, rostral supplementary motor area; STN, subthalamic nucleus.
Another important feature is the role of the striatum in developing automatic motor skills. It has been shown that during motor automatic processing, the activity in the anterior putamen decreased, while the activation in the posterior putamen increased (Lehéricy et al., 2005). Additionally, the connectivity from the anterior putamen to the primary motor cortex (M1) weakened, while the connectivity from the posterior putamen to the M1 strengthened as motor tasks became automatic (Wu et al., in press). The anterior putamen is considered an association area; while the posterior putamen is a sensorimotor area. Studies with animals have demonstrated that the associative striatum is more involved in acquisition of new motor skills and in regulating goal-directed behaviors; while the sensorimotor striatum is critical in performance of automatic movements (Miyachi et al., 1997, 2002; Yin et al., 2005; Yin and Knowlton, 2006). Therefore, the sensorimotor striatum appears critical for support in running automatic programs.
Once a motor task becomes automatic, it is often difficult to revert to controlled behavior (Schneider and Chein, 2003). We found that re-attending to automated motor task induce more activation in the DLPFC, ACC, and pre-SMA compared to the automatic stage. In contrast, the pattern of the activity and connectivity in the striatum was not modified, which remained at the level of the automatic stage (Wu et al., in press). These results indicate that once automatic stage is achieved, the automatic mode supported in the striatum is relatively stable, and is not further adjusted by the attentional networks even when returning attention to the task.
Given these findings together, in healthy people, when learned motor skills become automatic, (1) the neural system becomes more efficient; (2) the attentional networks are no longer important; (3) the sensorimotor striatum is critical for support; and (4) the automated motor program resists interference. Of course, all the neural processes of motor automaticity are not fully understood and still need further investigations.
Why dual-task paradigm can be used to evaluate automaticity
When people attempt to perform dual-tasks, performance is generally impaired. Such deterioration of performance is defined as dual-task interference (Pashler, 1994). The underlying mechanism of dual-task interference is unclear. One commonly discussed theory is the capacity sharing model, which is based on the assumption that attentional resources are limited. When two tasks are performed in parallel, the limited resources will be divided. According to this model, dual-task interference occurs if the available resource capacity is exceeded, resulting in a decline in performance on one or both of the tasks (Friedman et al., 1982).
As automatic processing is accompanied by increased neural efficiency, performance of automated motor skill only requires minimal attentional demands, and needs little capacity resources. The remaining brain resources are sufficient to perform the secondary task correctly; the limits of the capacity resources will not be exceeded even while performing two tasks simultaneously. As a consequence, people can perform dual-tasking without clear interference. In contrast, if a motor skill is not automatic, it must need considerable attention and neural resources. The limits of the capacity resources will be exceeded, resulting in the deterioration of performance in one or both tasks.
Motor automaticity related clinical presentations in PD
Deterioration of motor automaticity is a general feature in PD patients. In fact, most bradykinesia related clinical presentations can be associated with this problem, as PD patients tend to perform almost all daily behaviors slower or with smaller amplitude. Even in the early stage of PD, the ability to perform automatic behaviors, like arm swing, gait, eye blinking, facial expressions, speech modulation and swallowing, is already impaired. Using external cues or directing attention to these behaviors is always beneficial. One effect of external cues is to draw attention to the movement, thus use more attentionally controlled behavior instead of an automatic pattern. Attentional control is flexible but slow and carries comparatively high computational costs as opposed to the fast but inflexible automatic mode (Schneider and Chein, 2003). In healthy people, while attention is always helpful to improve motor performance at early-learning stage, it can disrupt the performance of well-practiced skills (Wulf and Prinz, 2001). Thus, healthy people tend to use automatic control instead of attentional control to perform daily behaviors. In contrast, as the ability to perform automatic movements is impaired, these motor skills actually revert to the early-learning stage, PD patients have to rely on attentional control helping to perform motor skills that usually executed automatically in healthy people.
There are several features that can help indicate whether a motor deficit is associated with defective automaticity: (1) this motor behavior is usually automatically performed in healthy people; (2) dual-tasking more significantly deteriorates the performance of this motor task in PD patients compared to healthy controls; and (3) external cues or attention always significantly improve the performance of this motor task. In the following, we discuss some typical parkinsonian symptoms that may associate with impaired motor automaticity.
Akinesia
One of the characteristic motor deficits in PD patients is the difficulty in initiating movements (akinesia), particularly for those that are self-initiated. The severity of akinesia correlates with the striatal dopamine terminal integrity in vivo, with maximal involvement in the posterior putamen (Benamer et al., 2003). In performance of tasks requiring motor initiation, PD patients have decreased activation in the SMA (Playford et al., 1992; Haslinger et al., 2001). The functional connectivity between the basal ganglia and SMA in PD is weakened during performing self-initiated movement (Wu et al., 2011). L-dopa administration can relatively normalize the dysfunction of the SMA (Haslinger et al., 2001; Buhmann et al., 2003), while the increased activity in the SMA is highly correlated with improved motor performance. Given the critical role of SMA in motor initiation, the reduced basal ganglia-thalamo-cortical facilitation to the SMA has been suggested to be a critical reason contributing to the akinesia in PD (Grafton, 2004).
Dual-tasking has been proven to exacerbate the difficulty in motor initiation in PD patients (Nocera et al., 2013). In contrast, it is commonly observed that PD patients can overcome their impairment of initiating a movement by external cues (Burleigh-Jacobs et al., 1997; Praamstra et al., 1998; Siegert et al., 2002; Okada et al., 2011). This phenomenon has been called ‘kinesia paradoxica’ (Jankovic, 2008). A study investigated the neurophysiological mechanisms underlying PD patients’ reliance on external cues for hand movement initiation by recording the lateralized movement-related cortical potentials (Praamstra et al., 1998). Results showed that visual stimuli exert an earlier and stronger influence on movement initiation in PD patients than in control subjects. A recent study examined network changes during performing internally and externally cued movement preparation and selection, and showed that preserved performance during external cueing was associated with enhanced connectivity between prefrontal cortex and lateral PMC (Michely et al., 2015).
Slowness of simple repetitive movements
When PD patients attempt to perform a simple repetitive movement, the movement is typically executed slowly and accompanied by a higher timing variability. Clinically, some simple repetitive movements, e.g., finger tapping, opening and closing the hand, or tapping the foot on the ground, are commonly used to assess the bradykinesia. The neurophysiological mechanisms underlying this phenomenon have been investigated for a long time. Inadequate force of the initial impulsive activity in the agonist (Hallett and Khoshbin, 1980), and inappropriate scaling of the dynamic muscle force to the movement parameters (Berardelli et al., 1986) are some likely reasons. It has been suggested that the impaired basal ganglia-thalamo-cortical pathway results in decreased basal ganglia outputs, which in turn produces slowed movements (Contreras-Vidal and Stelmach, 1995; Moroney et al., 2008). An alternative explanation is that the slowness of movement in PD is due to inadequately activated motor cortical and spinal cord centers because of dopamine reduction not only in basal ganglia, but also in cortical and spinal sites (Cutsuridis, 2011).
In normal subjects, simple repetitive movements are almost always automatically executed. PD patients often have difficulty with internally generated fast repetitive movements, but externally-paced tapping is preserved (Yahalom et al., 2004). Moreover, repetitive finger tapping performance can be improved in presence of external auditory cues (Freeman et al., 1993; Fernández del Olmo et al., 2006). Additionally, it has been long recognized that dual-tasking can exacerbate the difficulty in performing simple repetitive movements in PD (Schwab et al., 1954; Benecke et al., 1986). The basal ganglia, specifically the putamen, have been suggested to be critical in timing control during movement (Rao et al., 1997; Harrington et al., 1998; Garraux et al., 2005). The basal ganglia-thalamo-cortical motor circuits are likely involved in the internal regulation of well-practiced, repetitive movements (Almeida et al., 2003). Thus, the impaired motor automaticity as a consequence of damaged basal ganglia is likely a factor resulting in the slowness and high timing variability in performing simple repetitive movements.
Reduced arm swing
It is generally thought that arm swing is usually automatically performed. It has been speculated that there are central pattern generators in the spinal cord that may be responsible for arm swing during locomotion (Jackson et al., 1983a, 1983b; Dietz, 2011). However, extensive subcortical and cortical regions may be involved in regulation of interlimb coordination (Duysens et al., 2004). Cortical contributions may be present even for the natural swinging of the arms (Barthelemy and Nielsen, 2010).
Decreased arm swing is one of the most frequently reported and early motor signs in PD patients (Nieuwboer et al., 1998; Becker et al., 2002), which is an independent predictor of falling in PD (Wood et al., 2002). It has been shown that reduced arm swing in particular revealed significant differences between early-stage PD, dystonia patients and healthy controls (Schneider et al., 2012). Arm swing is especially reduced on the more affected body side (Wood et al., 2002; Roggendorf et al., 2012), while reduction in bilateral arm coordination may contribute to this asymmetry (Lewek et al., 2010; Huang et al., 2012). This asymmetry of arm swing attenuates with ongoing disease (Roggendorf et al., 2012), and appears linked to the asymmetric process of nigrostriatal dopaminergic denervation (Lewek et al., 2010; Huang et al., 2012).
Both deep brain stimulation of the subthalamic nucleus (STN) and L-dopa can improve arm swing (Crenna et al., 2008), which proves that basal ganglia have a critical role in the control of upper limb locomotor automatism, and the dysfunction of basal ganglia secondary to dopaminergic impairment is an important reason contributing to the damaged arm swing in PD. In addition, external cues (Kaminsky et al., 2007) or attention (Behrman et al., 1998) can improve arm swing; while dual-tasking induces in coordination of arm swing in PD (Chomiak et al., in press).
Decreased stride length
Decreased stride length during walking is a hallmark of PD, which progressively worsens as the disease advances. In addition, PD patients have higher step-to-step variability of stride duration and increased variability of leg muscle activation during walking (Miller et al., 1996; Vieregge et al., 1997; Hausdorff et al., 1998; Baltadjieva et al., 2006). Both L-dopa and deep brain stimulation (DBS) treatment can improve stride length (Ferrandez and Blin, 1991; Bakker et al., 2004; St George et al., 2010). PD patients have particular difficulty with the internal regulation of stride length, even though cadence (steps per minute) remains intact. They have a higher cadence rate than control subjects for any given velocity, which may be a compensation for reduced step size (Morris et al., 1994a, 1994b).
Visual or verbal cues can significantly enhance stride length in PD patients (Morris et al., 1996; Lewis et al., 2000). Attentional strategies such as mentally rehearsing the forthcoming movement sequence, or concentrating on the movement during execution also improve the gait pattern (Yekutiel et al., 1991; Morris et al., 1996; Lewis et al., 2000). However, training with visual cues and attentional strategies only has short-term effect on gait (Morris et al., 1996). In contrast, dual-tasking decreases gait speed and increases stride-to-stride variability (Morris et al., 1996; Lewis et al., 2000; Yogev et al., 2005). These findings confirm that the ability to generate a normal stride length is not lost in PD. Indeed, the ability to generate normal stride length automatically is defective. Both external cues and attentional strategies are using attentional control to bypass automatic control mechanisms, and to help patients to improve their stride length. However, as attentional control is difficult to be long lasting, their strides lengths reduce again in a short period.
Freezing of gait
Freezing of gait (FoG) is a transient inability to initiate or maintain stepping, which is defined as “brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk” (Bloem et al., 2004; Giladi and Nieuwboer, 2008; Nutt et al., 2011). Typically, FoG lasts a couple of seconds, but can exceed 30 s (Schaafsma et al., 2003). The pathogenesis of FoG is not understood. Several hypotheses have been proposed, like abnormal gait generation, abnormal coupling of posture with gait, a perceptual malfunction, and a consequence of frontal executive dysfunction (for a recent review, see Nutt et al., 2011). FoG occurs more often during the performance of a dual-task, such as talking or using a cell phone while walking (Spildooren et al., 2010). In contrast, external cues help to overcome FoG (Rahman et al., 2008). Thus, defective motor automaticity has been suggested as a reason contributing to FoG (Nutt et al., 2011; Hallett, 2008).
Step initiation is usually internally generated. The connectivity of basal ganglia-motor cortex (i.e. SMA, PMC) loop in PD is disrupted in performing self-initiated movement (Wu et al., 2011). It has been suggested that the impaired basal ganglia-SMA pathway may contribute to FoG (Fling et al., 2013, 2014). In addition, there is reduced connectivity between the pedunculopontine nucleus (PPN) and the cerebellar locomotor region, STN, SMA, and prefrontal cortex (Fling et al., 2013), as well as greater functional connectivity between the SMA and the mesencephalic and cerebellar locomotor regions (Fling et al., 2014) in PD patients with FoG. This pattern of altered connectivity may indicate a failed attempt by the central neural system to compensate for the loss of connectivity between the STN and SMA and may reflect a loss of lower-order, automatic control of gait by the basal ganglia in FoG (Fling et al., 2014). A recent model hypothesizes that during FoG, increased firing rate within the STN induces an increase in activity within the globus pallidus internus (GPi), leading to decreased activity within the PPN. The inhibited PPN is unable to inform the motor spinal cord (SCm) properly, which in turn causes FoG. External cues or attention may help to trigger the striatum to inhibit the GPi, effectively releasing the STN-mediated “brake” on the PPN and the SCm (Shine et al., 2013).
Snijders and Bloem (2010) reported a PD patient with severe FOG. Surprisingly, the patient’s ability to ride a bicycle was remarkably preserved. The pathophysiological reason underlying this interesting phenomenon is unclear. The authors speculated that the forces transferred from the rotating pedals to the patient’s feet might provide tactile cues that triggered appropriate rhythmic cycling movements; and cycling could provide external pacing cues that are less prominent during gait. An alternative explanation is that FOG involves the most common gait routines but spares more complex walking programs (Snijders et al., 2011). Actually, this phenomenon is not rare in PD, some severely akinetic PD patients are still able dance, ice-skate, or ski. This case presented some characters of impaired automaticity: the simple daily walking manner was more damaged, while more complex motor behaviors could be executed with external cues or attention. Thus, it is possible that defective motor automaticity is one of the reasons contributing to this phenomenon, which needs to be studied in future.
Micrographia
Micrographia is characterized by small handwriting with further progressive reduction in size. It is one of the first and common symptoms in PD, and may be used for early diagnosis (McLennan et al., 1972; Duarte et al., 1995; Jarzebska, 2006; Ling et al., 2012; Wagle Shukla et al., 2012). The neurophysiological mechanisms of micrographia remain unknown. Some studies found that micrographia had a significant correlation with bradykinesia, and micrographia is considered a component of bradykinesia (Wagle Shukla et al., 2012). Inappropriate scaling of the dynamic muscle force to the movement parameters, which has been proposed to contribute to bradykinesia (Berardelli et al., 1986), may be a reason for micrographia (Van Gemmert et al., 1999; Wagle Shukla et al., 2012). However, the relationship between micrographia and bradykinesia remains controversial (McLennan et al., 1972); it is well known that micrographia can present at early stage of PD, even without significant bradykinesia otherwise, rigidity or tremor.
Handwriting is a well-habituated, coordinated motor skill which has been exercised for many years (Gross, 1975). Although writing can be considered a visually controlled motor task, it also consists highly automatically performed features, including writing size (Gross, 1975; Longstaff and Heath, 1997; Nackaerts et al., 2013). Some findings support the assumption that impaired automaticity is a reason underlying micrographia. External visual, auditory or verbal cues or attention could increase the amplitude and diminish the amplitude variability of handwriting in PD (Oliveira et al., 1997; Swinnen et al., 2000; Nieuwboer et al., 2009; Bryant et al., 2010; Ringenbach et al., 2011), while dual-tasking significantly reduces writing amplitude in PD patients, but not in healthy controls (Broeder et al., 2014).
Facial movement deficits
PD patients have some distinctive features of facial movement abnormalities, i.e. reduction of facial expression and eye blink rate. The reduction of automatic and controlled expressive movement of facial musculature creates an appearance of lack of interest in the surrounding environment (masked face). An early study showed that although PD patients fail to make spontaneous emotional facial expressions, they have no difficulty in mimicking facial emotions voluntarily (Rinn, 1984). However, later studies have reported contradictory findings, while both normal (Smith et al., 1996) and impaired (Jacobs et al., 1995; Simons et al., 2004) mimicking facial expressions have been reported in PD patients.
Facial movement abnormalities in PD have often been characterized in neurophysiological studies in terms of decreased frequency of spontaneous blinking rate (Agostino et al., 2008; Korosec et al., 2006; Kimber and Thompson, 2000). The reduced spontaneous blinking in PD seems to be related to central dopamine deficiency and improves following dopaminergic replacement (Karson, 1983) or STN–DBS (Bologna et al., 2012). It has been suggested that the abnormalities of facial movement control in PD largely reflect basal ganglia dysfunction (Agostino et al., 2008; Bologna et al., 2013).
Neural mechanisms underlying deficits of automaticity in PD
Only few imaging studies have investigated motor automaticity in PD (Wu and Hallett, 2005a; Wu et al., 2010a; Wu et al., in press) (Table 2). These studies found that even at a relatively early stage, motor automaticity is already impaired in most PD patients. The patients need greater activity in several regions, i.e., the DLPFC, PMC, parietal cortex, and cerebellum, compared with normal subjects while performing automatic movements (Wu and Hallett, 2005a; Wu et al., in press). The hyperactivation in these regions have been extensively reported in PD while performing various motor tasks, and is possibly a compensation for the dysfunction of basal ganglia (Rascol et al., 1997; Catalan et al., 1999; Haslinger et al., 2001; Wu and Hallett, 2005a; Wu et al., 2010b). Additionally, there was less effective connectivity between motor areas that are associated with the production of automatic movements in PD patients (Wu et al., 2010a). The more activation outside M1 and less connectivity between motor areas indicate a less efficient neural coding of movement in PD patients compared to healthy people.
Table 2.
Neural correlates of automaticity in patients with Parkinson’s disease.
| Reference | Task paradigm | Task Performance | Neural correlates |
|---|---|---|---|
| Wu and Hallett (2005a) | Self-initiated, self-paced, sequential finger movements | Most patients could only perform the simple sequence automatically | Greater activity in the cerebellum, PMC, parietal cortex, precuneus and DLPFC compared with normal subjects while performing automatic movements |
| Wu et al. (2010a) | Self-initiated, self-paced, sequential finger movements | Most patients could only perform the simple sequence automatically | The pre-SMA, cerebellum, and CMA had significantly more increased effective connectivity with several regions in normal subjects than in patients. |
| Wu et al. (in press) | A visuomotor association task | 25 of 30 patients achieved the automatic stage | More activity in the PMC, parietal cortex, cerebellum, anterior putamen, and DLPFC, less activity in the posterior putamen compared with controls; The connectivity from the anterior putamen to the motor cortex was strengthened during automatic processing; Re-attending to automatic movement enhanced activity in the DLPFC, premotor cortex, and cerebellum, but the connectivity from the anterior putamen to the motor cortex decreased. |
CMA, cingulate motor area; DLPFC, dorsolateral prefrontal cortex; PMC, premotor cortex, pre-SMA, rostral supplementary motor area.
In PD, the activity in the posterior putamen was not enhanced during automatic processing, and was decreased compared to the controls in the automatic stage. In addition, the connectivity from the posterior putamen to the cortical motor areas was not increased as movements became automatic (Wu et al., in press). This finding suggests that the significant dopamine depletion in the posterior putamen (Brooks et al., 1990) induces a failure to shift automated motor skills to the sensorimotor striatum, which in turn likely contributes to the difficulty of acquiring/executing automatic program/actions in PD.
In contrast, the activation in the anterior putamen was increased compared to the controls in the automatic stage. The connectivity from the anterior putamen to the M1 was strengthened during automatic processing (Wu et al., in press). Loss of dopaminergic neurons can trigger collateral sprouting of residual neurons (Finkelstein et al., 2000; Song and Haber, 2000), and thus spared dopaminergic fibers in the anterior putamen may compensate for severe dopamine depletion in the posterior putamen (Mounayar et al., 2007) helping to store and execute automatic programs. Alternatively, as the associative striatum is more involved in acquisition of new motor skills and in regulating attentionally controlled behaviors, the increased influence from the anterior putamen to motor cortex might be an indication of using attentional control to overcome impaired automatic control in PD (Redgrave et al., 2010). This idea is supported by the observation that in contrast to healthy people, the effective connectivity from the DLPFC to cortical motor areas remains significant at the automatic stage in PD patients (Wu et al., 2010a, in press). Anatomical studies have demonstrated the connection between the associative striatum and prefrontal cortex (Middleton and Strick, 2000).
In addition, unlike that in the healthy controls, re-attending to automatic movements modified connectivity pattern of the striatum in PD patients. The connectivity from the anterior putamen to the M1 was decreased compared with that in the automatic stage (Wu et al., in press). This finding suggests that in PD patients, the automatic mode within the striatum is not stable, which can be disrupted by interference and has a trend shifting from the automatic mode back to the controlled pattern.
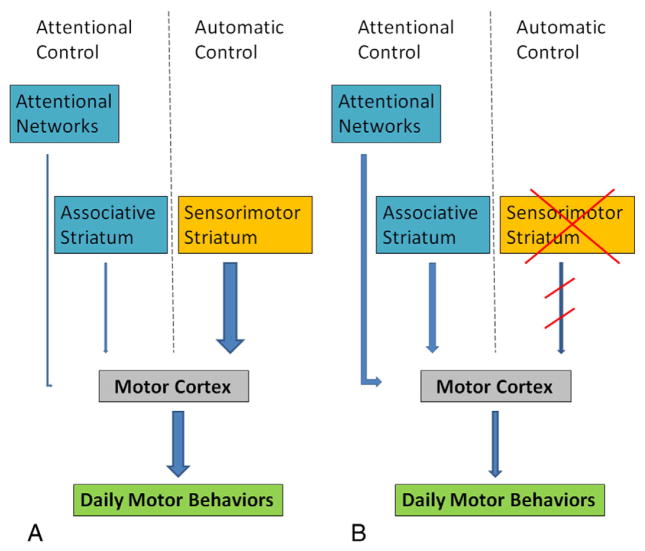
Taken together, neural mechanisms underlying defective motor automaticity in PD include: (1) less efficient neural coding of movement; (2) failure to shift automated motor skills to the sensorimotor striatum; (3) the automatic mode within the striatum is not stable and can be disrupted by interference; and (4) using attentional control and/or compensatory efforts to execute movements usually performed automatically in healthy people. PD patients appear to lose previously stored automatic skills due to the impairment of the sensorimotor striatum, and, simultaneously, they have difficulty to acquire new automatic skills or restore lost motor skills. As a consequence, PD patients cannot perform some daily motor skills automatically; instead, they perform these motor skills as that in the early-learning stage. Attentional strategies can help PD patients to maintain movements. This is in accordance with findings that animals with sensorimotor striatal lesions returned to attentional control and adjusted their behavior accordingly (Yin et al., 2004, 2006). However, as this requires comparatively high computational costs (Schneider and Chein, 2003), it is difficult to sustain attentional control, and patients always fatigue rapidly. PD patients can only improve their movements with external cues or attention for a short period. An illustration of automatic and attentional controls on daily motor behaviors is shown in Fig. 1.
Fig. 1.
An illustration of automatic and attentional controls on daily motor behaviors. In healthy people (A), daily motor behaviors are more controlled by automatic programs facilitated by the sensorimotor striatum. Impairment of the sensorimotor striatum in PD (B) damages the automatic control. The patients need more attentional control to perform daily motor behaviors. Thicker lines indicate stronger influences.
Why PD patients have difficulty performing dual-tasks
PD patients commonly have difficulty in performing two tasks at the same time (Benecke et al., 1986, 1987; Castiello and Bennett, 1997; Wu and Hallett, 2008). Difficulty in performing two tasks simultaneously in patients with PD is probably due to limited attentional resources (Brown and Marsden, 1991), defective central executive function and less automaticity in performing the tasks (Wu and Hallett, 2008). As discussed previously, the impaired basal ganglia, especially the posterior putamen, may result in the deficient motor automaticity in PD. In order to maintain normal movements, PD patients tend to use attentional control strategies to bypass impaired automatic control, and, therefore, more attentional resources are needed. As a consequence, the remaining attentional resources are not enough to support the performance of the secondary task properly. Thus, deteriorated dual-task performance can be a reflection of impaired motor automaticity in PD.
What needs to be further investigated
Several important issues on motor automaticity in PD remain unclear and need further investigation. First, the neural mechanisms underlying defected motor automaticity is not fully understood. Previous studies have focused on the striatum, cortical motor networks and attentional networks; the role of other regions associated with motor learning should be investigated in future. Second, the degree impaired motor automaticity contributes to bradykinesia is unclear. Third, whether L-dopa or surgical treatments improve motor automaticity has not been fully investigated. Fourth, whether motor automaticity has the potential to be used for early diagnosis and a target for treatment of PD also needs further investigation.
Conclusion
Defective motor automaticity is likely an important reason contributing to bradykinesia in PD. The impaired striatum plays a key role in supporting automatized motor programs and helping to acquire new automatic skills and restoring lost motor skills. Further clinical, neuroimaging, and neurophysiological investigations are needed to better understand the role of motor automaticity in bradykinesia in PD patients.
Acknowledgments
This work was supported by grants from the National Science Foundation of China (81071012 and 81271429), and Seed Grant of International Alliance of Translational Neuroscience (PXM2014_014226_000015). Dr. Hallett is supported by the NINDS Intramural Program.
References
- Agostino R, Bologna M, Dinapoli L, Gregori B, Fabbrini G, Accornero N, Berardelli A. Voluntary, spontaneous, and reflex blinking in Parkinson’ disease. Mov Disord. 2008;23:669–675. doi: 10.1002/mds.21887. [DOI] [PubMed] [Google Scholar]
- Almeida QJ, Wishart LR, Lee TD. Disruptive influences of a cued voluntary shift on coordinated movement in Parkinson’s disease. Neuropsychologia. 2003;41:442–452. doi: 10.1016/s0028-3932(02)00155-0. [DOI] [PubMed] [Google Scholar]
- Bakker M, Esselink RA, Munneke M, Limousin-Dowsey P, Speelman HD, Bloem BR. Effects of stereotactic neurosurgery on postural instability and gait in Parkinson’s disease. Mov Disord. 2004;19:1092–1099. doi: 10.1002/mds.20116. [DOI] [PubMed] [Google Scholar]
- Balsters JH, Ramnani N. Cerebellar plasticity and the automation of first-order rules. J Neurosci. 2011;31:2305–2312. doi: 10.1523/JNEUROSCI.4358-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. Eur J Neurosci. 2006;24:1815–1820. doi: 10.1111/j.1460-9568.2006.05033.x. [DOI] [PubMed] [Google Scholar]
- Barthelemy D, Nielsen JB. Corticospinal contribution to arm muscle activity during human walking. J Physiol. 2010;588:967–979. doi: 10.1113/jphysiol.2009.185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Yang M, Wymbs NF, Grafton ST. Learning-induced autonomy of sensorimotor systems. Nat Neurosci. 2015;18:744–751. doi: 10.1038/nn.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G, Muller A, Braune S, Buttner T, Benecke R, Greulich W, Klein W, Mark G, Rieke J, Thümler R. Early diagnosis of parkinson’s disease. J Neurol. 2002;249(Suppl 3):40–48 (III). doi: 10.1007/s00415-002-1309-9. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Teitelbaum P, Cauraugh JH. Verbal instructional sets to normalise the temporal and spatial gait variables in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;65:580–582. doi: 10.1136/jnnp.65.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamer HT, Oertel WH, Patterson J, Hadley DM, Pogarell O, Hoffken H, Gerstner A, Grosset DG. Prospective study of presynaptic dopaminergic imaging in patients with mild parkinsonism and tremor disorders: part 1. Baseline and 3-month observations. Mov Disord. 2003;18:977–984. doi: 10.1002/mds.10482. [DOI] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Performance of simultaneous movements in patients with Parkinson’s disease. Brain. 1986;109:739–757. doi: 10.1093/brain/109.4.739. [DOI] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson’s disease. Brain. 1987;110:361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Dick JP, Rothwell JC, Day BL, Marsden CD. Scaling of the size of the first agonist EMG burst during rapid wrist movements in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1986;49:1273–1279. doi: 10.1136/jnnp.49.11.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124:2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- Bernstein N. The co-ordination and regulation of movements. Pergamon Press Ltd; London: 1967. [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Bologna M, Fasano A, Modugno N, Fabbrini G, Berardelli A. Effects of subthalamic nucleus deep brain stimulation and L-dopa on voluntary, spontaneous and reflex blinking in Parkinson’s disease. Exp Neurol. 2012;235:265–272. doi: 10.1016/j.expneurol.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Bologna M, Fabbrini G, Marsili L, Defazio G, Thompson PD, Berardelli A. Facial bradykinesia. J Neurol Neurosurg Psychiatry. 2013;84:681–685. doi: 10.1136/jnnp-2012-303993. [DOI] [PubMed] [Google Scholar]
- Broeder S, Nackaerts E, Nieuwboer A, Smits-Engelsman BC, Swinnen SP, Heremans E. The effects of dual tasking on handwriting in patients with Parkinson’s disease. Neuroscience. 2014;263:193–202. doi: 10.1016/j.neuroscience.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, Bannister R, Marsden CD, Frackowiak RS. Differing patterns of striatal 18 F-dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. 1990;28:547–555. doi: 10.1002/ana.410280412. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Dual task performance and processing resources in normal subjects and patients with Parkinson’s disease. Brain. 1991;114:215–231. [PubMed] [Google Scholar]
- Bryant MS, Rintala DH, Lai EC, Protas EJ. An investigation of two interventions for micrographia in individuals with Parkinson’s disease. Clin Rehabil. 2010;24:1021–1026. doi: 10.1177/0269215510371420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. Pharmacologically modulated fMRI - cortical responsiveness to levodopa in drug-naïve hemiparkinsonian patients. Brain. 2003;126:451–461. doi: 10.1093/brain/awg033. [DOI] [PubMed] [Google Scholar]
- Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- Castiello U, Bennett KM. The bilateral reach-to-grasp movement of Parkinson’s disease subjects. Brain. 1997;120:593–604. doi: 10.1093/brain/120.4.593. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson’s disease. Brain. 1999;122:483–495. doi: 10.1093/brain/122.3.483. [DOI] [PubMed] [Google Scholar]
- Chomiak T, Pereira FV, Clark TW, Cihal A, Hu B. Concurrent arm swing-stepping (CASS) can reveal gait start hesitation in Parkinson’s patients with low self-efficacy and fear of falling. Aging Clin Exp Res. 2015 doi: 10.1007/s40520-014-0313-0. (in press) [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Stelmach G. A neural model of basal ganglia-thalamocortical relations in normal and Parkinsonian movements. Biol Cybern. 1995;73:467–476. doi: 10.1007/BF00201481. [DOI] [PubMed] [Google Scholar]
- Crenna P, Carpinella I, Lopiano L, Marzegan A, Rabuffetti M, Rizzone M, Lanotte M, Ferrarin M. Influence of basal ganglia on upper limb locomotor synergies. Evidence from deep brain stimulation and L-DOPA treatment in Parkinson’s disease. Brain. 2008;131:3410–3420. doi: 10.1093/brain/awn272. [DOI] [PubMed] [Google Scholar]
- Cutsuridis V. Origins of a repetitive and co-contractive pattern of muscle activation in Parkinson’s disease. Neural Netw. 2011;24:592–601. doi: 10.1016/j.neunet.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Dietz V. Quadrupedal coordination of bipedal gait: implications for movement disorders. J Neurol. 2011;258:1406–1412. doi: 10.1007/s00415-011-6063-4. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Duarte J, Claveria LE, de Pedro-Cuesta J, Sempere AP, Coria F, Calne DB. Screening Parkinson’s disease: a validated questionnaire of high specificity and sensitivity. Mov Disord. 1995;10:643–649. doi: 10.1002/mds.870100518. [DOI] [PubMed] [Google Scholar]
- Duysens J, Donker SF, Verschueren SMP, Smits-Engelsman BCM, Swinnen SP. Sensory influences on interlimb coordination during gait. In: Swinnen P, Duysens J, editors. Neurobehavioural determinants of interlimb coordination. A multidisciplinary approach. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2004. pp. 3–33. [Google Scholar]
- Fernández del Olmo M, Arias P, Furio MC, Pozo MA, Cudeiro J. Evaluation of the effect of training using auditory stimulation on rhythmic movement in Parkinsonian patients - a combined motor and [18 F]-FDG PET study. Parkinsonism Relat Disord. 2006;12:155–164. doi: 10.1016/j.parkreldis.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Ferrandez AM, Blin O. A comparison between the effect of intentional modulations and the action of L-dopa on gait in Parkinson’s disease. Behav Brain Res. 1991;45:177–183. doi: 10.1016/s0166-4328(05)80083-x. [DOI] [PubMed] [Google Scholar]
- Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience. 2000;97:99–112. doi: 10.1016/s0306-4522(00)00009-9. [DOI] [PubMed] [Google Scholar]
- Fitts PM. Perceptual-motor skills learning. In: Melton AW, editor. Categories of human learning. Academic Press; London: 1964. pp. 243–285. [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013;136:2405–2418. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, Horak FB. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One. 2014;9(6):e100291. doi: 10.1371/journal.pone.0100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JS, Cody FW, Schady W. The influence of external timing cues upon the rhythmic of voluntary movements in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1993;56:1078–1084. doi: 10.1136/jnnp.56.10.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Polson MC, Dafoe DG, Gaskill S. Dividing attention within and between hemispheres: testing a multiple resources approach to limited-capacity information processing. J Exp Psychol Hum Percept Perform. 1982;8:625–650. doi: 10.1037//0096-1523.8.5.625. [DOI] [PubMed] [Google Scholar]
- Garraux G, McKinney C, Wu T, Kansaku K, Nolte G, Hallett M. Shared brain areas but not functional connections controlling movement timing and order. J Neurosci. 2005;25:5290–5297. doi: 10.1523/JNEUROSCI.0340-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. 2008;23(Suppl 2):S423–S425. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- Grafton ST. Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol. 2004;14:715–719. doi: 10.1016/j.conb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Gross LJ. Drug-induced handwriting changes: An empirical review. Tex Rep Biol Med. 1975;33:371–390. [PubMed] [Google Scholar]
- Hallett M. The intrinsic and extrinsic aspects of freezing of gait. Mov Disord. 2008;23(Suppl 2):S439–S443. doi: 10.1002/mds.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Bradykinesia: Why do Parkinson’s patients have it and what trouble does it cause? Mov. Disord. 2011;26:1579–1581. doi: 10.1002/mds.23730. [DOI] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12:3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain. 2001;124:558–570. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in Parkinson’s and Huntington’s disease. Mov Disord. 1998;13:428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior: A neuropsychological theory. JohnWiley; New York: 1949. [Google Scholar]
- Hoshiyama M, Kaneoke Y, Koike Y, Takahashi A, Watanabe S. Hypokinesia of associated movement in Parkinson’s disease: a symptom in early stages of the disease. J Neurol. 1994;241:517–521. doi: 10.1007/BF00873512. [DOI] [PubMed] [Google Scholar]
- Huang X, Mahoney JM, Lewis MM, Du G, Piazza SJ, Cusumano JP. Both coordination and symmetry of arm swing are reduced in Parkinson’s disease. Gait Posture. 2012;35(3):373–377. doi: 10.1016/j.gaitpost.2011.10.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura Y, Ito Y, Akazawa T, Nambu A, Takada M. Neural coding of “attention for action” and “response selection” in primate anterior cingulate cortex. J Neurosci. 2003;23:8002–8012. doi: 10.1523/JNEUROSCI.23-22-08002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Joseph J, Wyard SJ. The upper limbs during human walking. Part I: sagittal movement. Electromyogr Clin Neurophysiol. 1983a;23:425–434. [PubMed] [Google Scholar]
- Jackson KM, Joseph J, Wyard SJ. The upper limbs during human walking. Part 2: function. Electromyogr Clin Neurophysiol. 1983b;23:435–446. [PubMed] [Google Scholar]
- Jacobs DH, Shuren J, Bowers D, Heilman KM. Emotional facial imagery, perception, and expression in Parkinson’s disease. Neurology. 1995;45:1696–1702. doi: 10.1212/wnl.45.9.1696. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jarzebska E. Evaluation of effectiveness of the micrographia’s therapy in Parkinson’s disease patients. Pol Merkur Lekarski. 2006;20:688–690. [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RSJ. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol. 1997;77:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- Kaminsky TA, Dudgeon BJ, Billingsley FF, Mitchell PH, Weghorst SJ. Virtual cues and functional mobility of people with Parkinson’s disease: a single-subject pilot study. J Rehabil Res Dev. 2007;44:437–448. doi: 10.1682/jrrd.2006.09.0109. [DOI] [PubMed] [Google Scholar]
- Karson CN. Spontaneous eye-blink rates and dopaminergic systems. Brain. 1983;106:643–653. doi: 10.1093/brain/106.3.643. [DOI] [PubMed] [Google Scholar]
- Kimber TE, Thompson PD. Increased blink rate in advanced Parkinson’s disease: a form of ‘off’-period dystonia? Mov. Disord. 2000;15:982–985. doi: 10.1002/1531-8257(200009)15:5<982::aid-mds1033>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Korosec M, Zidar I, Reits D, Evinger C, Vanderwerf F. Eyelid movements during blinking in patients with Parkinson’s disease. Mov Disord. 2006;21:1248–1251. doi: 10.1002/mds.20930. [DOI] [PubMed] [Google Scholar]
- Lang CE, Bastian AJ. Cerebellar damage impairs automaticity of a recently practiced movement. J Neurophysiol. 2002;87:1336–1347. doi: 10.1152/jn.00368.2001. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele P, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewek MD, Poole R, Johnson J, Halawa O, Huang X. Arm swing magnitude and asymmetry during gait in the early stages of Parkinson’s disease. Gait Posture. 2010;31:256–260. doi: 10.1016/j.gaitpost.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD, Walt SE. Stride length regulation in Parkinson’s disease: The use of extrinsic, visual cues. Brain. 2000;123:2077–2090. doi: 10.1093/brain/123.10.2077. [DOI] [PubMed] [Google Scholar]
- Ling H, Massey LA, Lees AJ, Brown P, Day BL. Hypokinesia without decrement distinguishes progressive supranuclear palsy from Parkinson’s disease. Brain. 2012;135:1141–1153. doi: 10.1093/brain/aws038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstaff MG, Heath RA. Space-time invariance in adult handwriting. Acta Psychol. 1997;97:201–214. [Google Scholar]
- Marsden CD. The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology. 1982;32:514–539. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- Mazzoni P. Efficient motor control: how can less be more? J Physiol. 2008;586:4031. doi: 10.1113/jphysiol.2008.160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan JE, Nakano K, Tyler HR, Schwab RS. Micrographia in Parkinson’s disease. J Neurol Sci. 1972;15:141–152. doi: 10.1016/0022-510x(72)90002-0. [DOI] [PubMed] [Google Scholar]
- Michely J, Volz LJ, Barbe MT, Hoffstaedter F, Viswanathan S, Timmermann L, Eickhoff SB, Fink GR, Grefkes C. Dopaminergic modulation of motor network dynamics in Parkinson’s disease. Brain. 2015;138:664–678. doi: 10.1093/brain/awu381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Miller RA, Thaut MH, McIntosh GC, Rice RR. Components of EMG symmetry and variability in parkinsonian and healthy elderly gait. Electroencephalogr Clin Neurophysiol. 1996;101:1–7. doi: 10.1016/0013-4694(95)00209-x. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. 1997;115:1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu XF. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Moroney R, Heida C, Geelen J. Increased bradykinesia in Parkinson’s disease with increased movement complexity: elbow flexion-extension movements. J Comput Neurosci. 2008;25:501–519. doi: 10.1007/s10827-008-0091-9. [DOI] [PubMed] [Google Scholar]
- Morris ME, Iansek R, Matyas TA, Summers JJ. Ability to modulate walking cadence remains intact in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1994a;57:1532–1534. doi: 10.1136/jnnp.57.12.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Lansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain. 1994b;117:1169–1181. doi: 10.1093/brain/117.5.1169. [DOI] [PubMed] [Google Scholar]
- Morris ME, Lansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996;119:551–568. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- Mounayar S, Boulet S, Tande D, Jan C, Pessiglione M, Hirsch EC, Féger J, Savasta M, François C, Tremblay L. A new model to study compensatory mechanisms in MPTP-treated monkeys exhibiting recovery. Brain. 2007;130:2898–2914. doi: 10.1093/brain/awm208. [DOI] [PubMed] [Google Scholar]
- Nackaerts E, Vervoort G, Heremans E, Smits-Engelsman BC, Swinnen SP, Nieuwboer A. Relearning of writing skills in Parkinson’s disease: a literature review on influential factors and optimal strategies. Neurosci Biobehav Rev. 2013;37:349–357. doi: 10.1016/j.neubiorev.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, De Weerdt W, Dom R, Lesaffre E. A frequency and correlation analysis of motor deficits in parkinson patients. Disabil Rehabil. 1998;20:142–150. doi: 10.3109/09638289809166074. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Vercruysse S, Feys P, Levin O, Spildooren J, Swinnen S. Upper limb movement interruptions are correlated to freezing of gait in Parkinson’s disease. Eur J Neurosci. 2009;29:1422–1430. doi: 10.1111/j.1460-9568.2009.06681.x. [DOI] [PubMed] [Google Scholar]
- Nocera JR, Roemmich R, Elrod J, Altmann LJ, Hass CJ. Effects of cognitive task on gait initiation in Parkinson disease: evidence of motor prioritization? J Rehabil Res Dev. 2013;50:699–708. doi: 10.1682/jrrd.2012.06.0114. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Fukumoto T, Takatori K, Nagino K, Hiraoka K. Variable initial swing side and prolonged double limb support represent abnormalities of the first three steps of gait initiation in patients with Parkinson’s disease with freezing of gait. Front Neurol. 2011;2:85 (29). doi: 10.3389/fneur.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RM, Gurd JM, Nixon P, Marshall JC, Passingham RE. Micrographia in Parkinson’s disease: the effect of providing external cues. J Neurol Neurosurg Psychiatry. 1997;63:429–433. doi: 10.1136/jnnp.63.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Dual-task in simple tasks: data and theory. Psychol Bull. 1994;2:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Attention to action. Philos Trans R Soc Lond B Biol Sci. 1996;351:1473–1479. doi: 10.1098/rstb.1996.0132. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Poaner MI, Mintum M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of singleword process. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RSJ, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. The neural correlates of motor skill automaticity. J Neurosci. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praamstra P, Stegeman DF, Cools AR, Horstink MW. Reliance on external cues for movement initiation in Parkinson’s disease. Evidence from movement-related potentials. Brain. 1998;121:167–177. doi: 10.1093/brain/121.1.167. [DOI] [PubMed] [Google Scholar]
- Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. The factors that induce or overcome freezing of gait in Parkinson’s disease. Behav Neurol. 2008;19:127–136. doi: 10.1155/2008/456298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed Neural Systems Underlying the Timing of Movements. J Neurosci. 1997;17:5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, Senard JM, Montastruc JL, Chollet F. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 1997;120:103–110. doi: 10.1093/brain/120.1.103. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringenbach SD, van Gemmert AW, Shill HA, Stelmach GE. Auditory instructional cues benefit unimanual and bimanual drawing in Parkinson’s disease patients. Hum Mov Sci. 2011;30:770–782. doi: 10.1016/j.humov.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn WE. The neuropsychology of facial expression: a review of the neurological and psychological mechanisms for producing facial expressions. Psychol Bull. 1984;95:52–77. [PubMed] [Google Scholar]
- Roggendorf J, Chen S, Baudrexel S, van de Loo S, Seifried C, Hilker R. Arm swing asymmetry in Parkinson’s disease measured with ultrasound based motion analysis during treadmill gait. Gait Posture. 2012;35:116–120. doi: 10.1016/j.gaitpost.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur J Neurol. 2003;10:391–398. doi: 10.1046/j.1468-1331.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- Schneider W, Chein JM. Controlled & automatic processing: behavior, theory, and biological mechanisms. Cogn Sci. 2003;27:525–559. [Google Scholar]
- Schneider SA, Drude L, Kasten M, Klein C, Hagenah J. A study of subtle motor signs in early Parkinson’s disease. Mov Disord. 2012;27:1563–1566. doi: 10.1002/mds.25161. [DOI] [PubMed] [Google Scholar]
- Schwab RS, Chafetz ME, Walker S. Control of two simultaneous voluntary motor acts in parkinsonism. Arch Neurol Psychiatr. 1954;72:591–598. doi: 10.1001/archneurpsyc.1954.02330050061010. [DOI] [PubMed] [Google Scholar]
- Shine JM, Moustafa AA, Matar E, Frank MJ, Lewis SJG. The role of frontostriatal impairment in freezing of gait in Parkinson’s disease. Front Syst Neurosci. 2013;7:61. doi: 10.3389/fnsys.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert RJ, Harper DN, Cameron FB, Abernethy D. Self-initiated versus externally cued reaction times in Parkinson’s disease. J Clin Exp Neuropsychol. 2002;24:146–153. doi: 10.1076/jcen.24.2.146.991. [DOI] [PubMed] [Google Scholar]
- Simons G, Pasqualini MC, Reddy V, Wood J. Emotional and nonemotional facial expressions in people with Parkinson’s disease. J Int Neuropsychol Soc. 2004;10:521–535. doi: 10.1017/S135561770410413X. [DOI] [PubMed] [Google Scholar]
- Smith MC, Smith MK, Ellgring H. Spontaneous and posed facial expression in Parkinson’s disease. J Int Neuropsychol Soc. 1996;2:383–391. doi: 10.1017/s1355617700001454. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Bloem BR. Images in clinical medicine. Cycling for freezing of gait. N Engl J Med. 2010;362:e46. doi: 10.1056/NEJMicm0810287. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Toni I, Ružička E, Bloem BR. Bicycling breaks the ice for freezers of gait. Mov Disord. 2011;26:367–371. doi: 10.1002/mds.23530. [DOI] [PubMed] [Google Scholar]
- Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci. 2000;20:5102–5114. doi: 10.1523/JNEUROSCI.20-13-05102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A. Freezing of gait in Parkinson’s disease: The impact of dual-tasking and turning. Mov Disord. 2010;25:2563–2570. doi: 10.1002/mds.23327. [DOI] [PubMed] [Google Scholar]
- St George RJ, Nutt JG, Burchiel KJ, Horak FB. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. 2010;75:1291–1299. doi: 10.1212/WNL.0b013e3181f61329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen SP, Steyvers M, Van Den Bergh L, Stelmach GE. Motor learning and Parkinson’s disease: refinement of within-limb and between-limb coordination as a result of practice. Behav Brain Res. 2000;111:45–59. doi: 10.1016/s0166-4328(00)00144-3. [DOI] [PubMed] [Google Scholar]
- Van Gemmert AW, Teulings HL, Contreras-Vidal JL, Stelmach GE. Parkinson’s disease and the control of size and speed in handwriting. Neuropsychologia. 1999;37:685–694. doi: 10.1016/s0028-3932(98)00122-5. [DOI] [PubMed] [Google Scholar]
- Vieregge P, Stolze H, Klein C, Heberlein I. Gait quantitation in Parkinson’s disease: Locomotor disability and correlation to clinical rating scales. J Neural Transm. 1997;104:237–248. doi: 10.1007/BF01273184. [DOI] [PubMed] [Google Scholar]
- Wagle Shukla A, Ounpraseuth S, Okun MS, Gray V, Schwankhaus J, Metzer WS. Micrographia and related deficits in Parkinson’s disease: a cross-sectional study. BMJ. 2012;2(3) doi: 10.1136/bmjopen-2011-000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002;72:721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005a;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. The influence of normal human ageing on automatic movements. J Physiol. 2005b;562:605–615. doi: 10.1113/jphysiol.2004.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79:760–766. doi: 10.1136/jnnp.2007.126599. [DOI] [PubMed] [Google Scholar]
- Wu T, Kansaku K, Hallett M. How self-initiated memorized movements become automatic: a fMRI study. J Neurophysiol. 2004;91:1690–1698. doi: 10.1152/jn.01052.2003. [DOI] [PubMed] [Google Scholar]
- Wu T, Chan P, Hallett M. Modifications of the interactions in the motor network when a movement becomes automatic. J Physiol. 2008;586:4295–4304. doi: 10.1113/jphysiol.2008.153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson’s disease. NeuroImage. 2010a;49:2581–2587. doi: 10.1016/j.neuroimage.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain. 2010b;133:2394–2409. doi: 10.1093/brain/awq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. NeuroImage. 2011;55:204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
- Wu T, Liu J, Zhang H, Hallett M, Zheng Z, Chan P. Attention to automatic movements in Parkinson’s disease: modified automatic mode in the striatum. Cereb Cortex. 2015 doi: 10.1093/cercor/bhu135. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf G, Prinz W. Directing attention to movement effects enhances learning: a review. Psychon Bull Rev. 2001;8:648–660. doi: 10.3758/bf03196201. [DOI] [PubMed] [Google Scholar]
- Yahalom G, Simon ES, Thorne R, Peretz C, Giladi N. Hand rhythmic tapping and timing in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:143–148. doi: 10.1016/j.parkreldis.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Yekutiel MP, Pinhasov A, Shahar G, Sroka H. A clinical trial of the re-education of movement in patients with Parkinson’s disease. Clin Rehabil. 1991;5:207–214. [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Blockade of NMDA receptors in the dorsomedial striatum prevents action–outcome learning in instrumental conditioning. Eur J Neurosci. 2005;22:505–512. doi: 10.1111/j.1460-9568.2005.04219.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action–outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: Which aspects of gait is attention demanding? Eur. J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]