Significance
Nuclear receptors (NRs) are ligand-sensing transcription factors that are crucial for the proper regulation of mammalian development, physiology, and metabolism. Their ligand-binding capability makes NRs attractive drug targets, but can also lead to the adverse side effects of prescription drugs. Our research contributes to a better understanding of how NRs are regulated in a time-of-day–dependent manner by a component of the circadian clock, cryptochrome, and is foundational to further research aiming to make drug administration routines more effective and safer.
Keywords: nuclear hormone receptor, cryptochrome, circadian, corepressor, xenobiotic metabolism
Abstract
Nuclear hormone receptors (NRs) regulate physiology by sensing lipophilic ligands and adapting cellular transcription appropriately. A growing understanding of the impact of circadian clocks on mammalian transcription has sparked interest in the interregulation of transcriptional programs. Mammalian clocks are based on a transcriptional feedback loop featuring the transcriptional activators circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like 1 (BMAL1), and transcriptional repressors cryptochrome (CRY) and period (PER). CRY1 and CRY2 bind independently of other core clock factors to many genomic sites, which are enriched for NR recognition motifs. Here we report that CRY1/2 serve as corepressors for many NRs, indicating a new facet of circadian control of NR-mediated regulation of metabolism and physiology, and specifically contribute to diurnal modulation of drug metabolism.
Nuclear receptors (NRs) regulate transcription in response to lipophilic ligands (1). Most NRs contain four domains: The N-terminal activation function 1 (AF1) is followed by a highly conserved DNA binding domain (DBD), which is connected to the ligand binding domain (LBD) via a flexible hinge region. The LBD contains the hydrophobic ligand binding pocket and activation function 2 (AF2) located in helix 12 (H12). Structural rearrangement of H12 is crucial for ligand-dependent transactivation. Regulation of transcription by NRs depends on interaction with corepressors, such as nuclear receptor corepressor (NCoR) or silencing mediator for retinoid or thyroid-hormone receptors (SMRT), and coactivators, such as peroxisome proliferator-activated receptor-γ coactivator 1⍺ (PGC1⍺) or steroid receptor coactivators. Steroid hormone receptors are enriched in the cytoplasm in the absence of ligand. Upon agonist ligand binding, they translocate to the nucleus and bind DNA. Recruitment of coactivators then allows transcription to occur. Conversely, nonsteroid-binding NRs are bound to DNA and corepressors in the absence of ligand. Binding of agonist ligand displaces corepressors in favor of coactivators, allowing transcription initiation.
Mammalian circadian clocks synchronize physiology and metabolism with daily environmental changes. The mammalian clock is based on a transcription–translation feedback loop featuring the transcription factors circadian locomotor output cycles kaput (CLOCK), brain and muscle ARNT-like 1 (BMAL1), cryptochrome (CRY), and period (PER) (2). Forming a heterodimer, CLOCK and BMAL1 drive transcription of target genes, including those encoding their own repressors period (PER1, PER2, PER3) and cryptochrome (CRY1, CRY2). PER and CRY dimerize and repress CLOCK and BMAL1, allowing the cycle to repeat. This core clock directly or indirectly drives oscillating transcription of ≈43% of all protein coding genes (3).
NRs are intimately connected to the circadian clock and its function in adapting daily metabolic outputs to the 24-h day/night cycle. The NRs REV-ERBα/β and RORα/γ are key regulators of core clock function (4), and many NRs are rhythmically expressed (5). PER, CRY, and CLOCK can regulate NRs by diverse mechanisms (6–9). These and indirect effects, like rhythmic abundance of endogenous ligands and the rhythmic transcription of coactivators and corepressors, convey time-of-day information to NR-regulated pathways, such as lipid, glucose, and xenobiotic metabolism (4).
Upon binding of a xenobiotic ligand, the NRs pregnane X receptor (PXR) and constitutive androstane receptor (CAR) induce expression of proteins required for xenobiotic detoxification. Xenobiotic metabolism is subject to time-of-day–dependent regulation: in humans, the half-life of CYP3A substrates is shortest in the afternoon (10); in rodents, the lethal toxicity of a fixed dose of a drug depends on the time of administration (11). The circadian transcriptome (6, 7) and proteome (8) are enriched for components of xenobiotic detoxification pathways. Here we report a comprehensive survey of CRY–NR interactions and enhanced metabolism of the anesthetic ketamine in CRY-deficient mice.
Results
CRY1 Interacts with Many NRs.
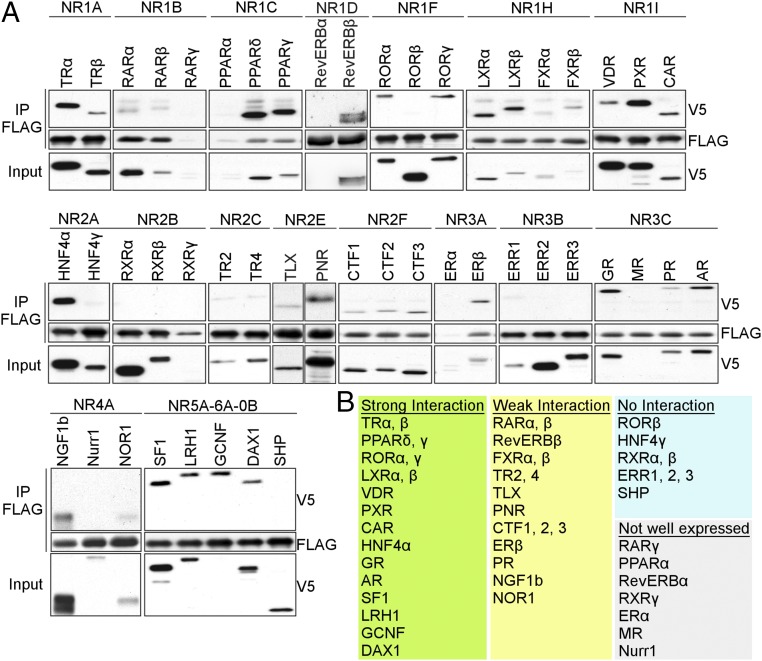
We examined the interaction of all mouse NRs with mouse CRY1 using coimmunoprecipitation (co-IP) (Fig. 1A). Approximately one-third of mouse NRs consistently interact with CRY1, whereas another third is weakly or variably associated with CRY1 (Fig. 1B). The strongest interactors include steroid hormone receptors, lipid-sensing peroxisome proliferator-activated receptors (PPARs), vitamin D receptor (VDR), and the xenobiotic receptors PXR and CAR.
Fig. 1.
CRY1 interacts with a subset of nuclear receptors. (A) Co-IP of FLAG-CRY1 with V5-NRs transiently expressed in HEK293T cells. (B) Table listing strongly or weakly interacting NRs, and NRs that were not or only poorly expressed.
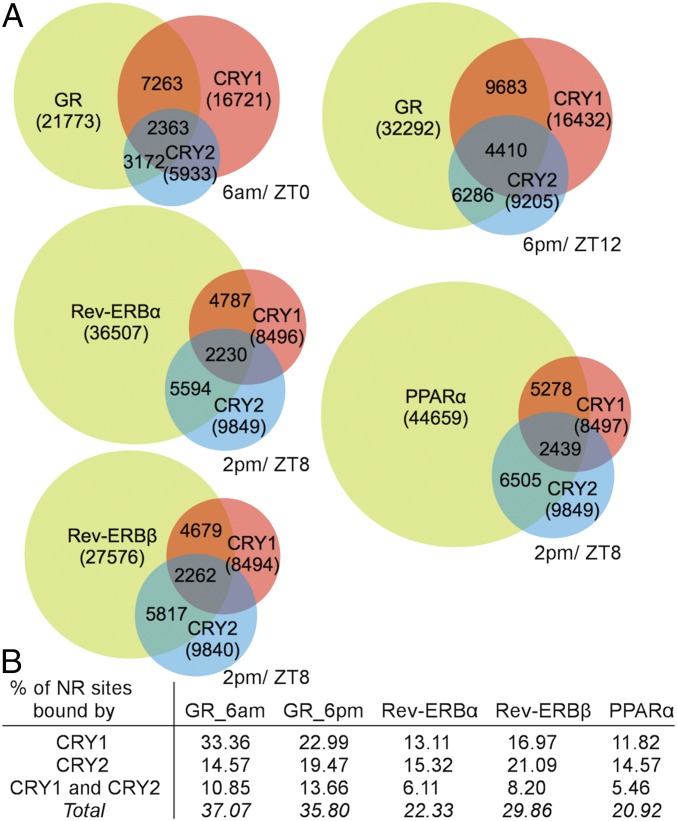
CRY1 and CRY2 bind many genomic sites independent of other clock proteins, and these unique sites were enriched for NR-binding motifs (9). Using these previously reported datasets to perform motif analysis for CRY1 and CRY2 genomic binding sites, we detected consensus sites of liver-expressed NRs, including hepatocyte nuclear factor 4-α (HNF4α), PPAR, Rev-ERB, farnesoid X receptor (FXR), and retinoic acid receptor (RAR) (Datasets S1 and S2). Comparing CRY1 and CRY2 genomic binding sites with those published for glucocorticoid receptor (GR) (12), Rev-ERBα, Rev-ERBβ (13), and PPARα (14) (Fig. 2A), we detected overlap of CRY1 and CRY2 binding with up to 37% of NR binding sites (Fig. 2B). These sites include Pck1 (GR) (Datasets S3 and S4), confirming previous reports (15), Pdk4 (PPARα) (Dataset S5), consistent with our finding that CRYs regulate PPARδ and Pdk4 in muscle (16), and Bmal1 (Rev-ERBα, Rev-ERBβ) (Datasets S6 and S7), suggesting that CRYs could contribute to Bmal1 transcriptional regulation.
Fig. 2.
CRYs and NRs co-occupy many sites in the genome. (A) Overlap of DNA binding sites for indicated proteins in mouse liver at matched ZT. Numbers in parentheses represent total number of binding sites for each protein. Numbers in overlapping areas indicate: (Top) total overlap between NR and CRY1; (Middle) total overlap between CRY1, CRY2, and NR; (Bottom) total overlap between CRY2 and NR. (B) Table depicting percent NR sites bound by CRY1 and CRY2.
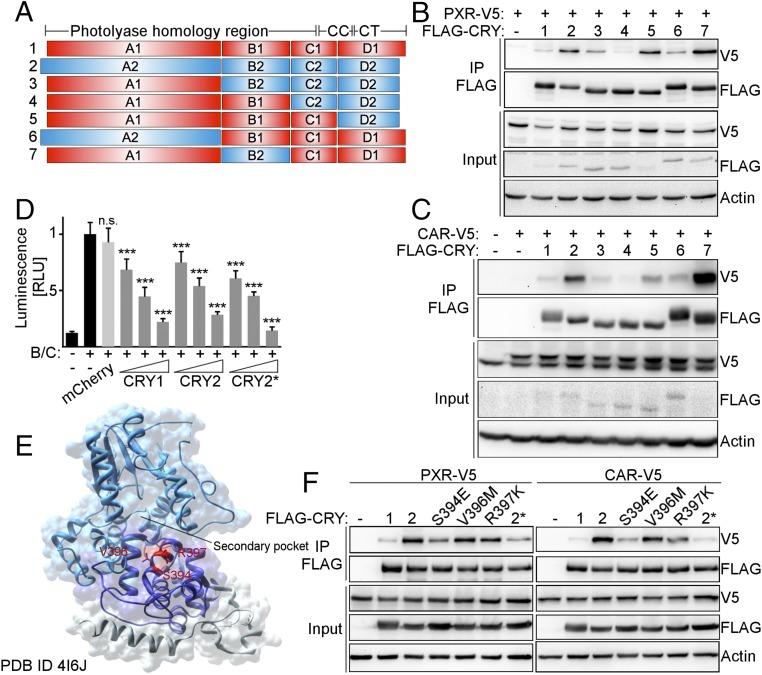
Many NRs display increased affinity for CRY2 compared with CRY1, allowing us to use CRY1/2 hybrid constructs (17) (Fig. 3A) to identify the domains required for preferential interaction. Co-IP of CRY hybrids with PXR and CAR revealed that the A and B domains, which correspond to the photolyase homology region (PHR), as well as the D domain, which mostly consists of the divergent C-terminal tail, contribute to the interactions (Fig. 3 B and C). We identified a helix on the surface of CRY2 in which three exposed amino acids differ from CRY1. Serine 394, valine 396, and arginine 397 are located near the rim of the secondary pocket of CRY2 (Fig. 3E). CRY2S394E, V396M, R397K (amino acids as in CRY1, hereafter denoted CRY2*) represses BMAL1:CLOCK-driven luciferase expression (Fig. 3D), indicating that these mutations did not prevent proper protein folding. Each of these mutations decreases the interaction of CRY2 with PXR or CAR, and CRY2* interacts with them like CRY1 (Fig. 3E), suggesting that this region is important for interaction.
Fig. 3.
The CRY2-NR interaction is disrupted by mutations near the secondary pocket of CRY2. (A) CRY1/2 hybrid constructs (red: CRY1; blue: CRY2). CC, coiled coil; CT, C-terminal tail. (B and C) Co-IP of FLAG-CRY hybrids with V5-PXR (B) and V5-CAR (C) from transfected cells. (D) Repression of BMAL1:CLOCK by CRY1, CRY2, and CRY2*. U2OS cells transiently expressed Per2-luciferase, Bmal1, Clock, and Cry1, Cry2, or Cry2*. mCherry was used as a negative control. Luminescence was normalized to β-Galactosidase activity. (E) CRY2 (PDB ID code 4I6J). A, B, and C domains are light blue, dark blue, and gray, respectively. Amino acids mutated in CRY2* are red. (F) Co-IP of FLAG-CRY1, -CRY2, and -CRY2* with V5-PXR and -CAR from transfected cells. In D, data represent the mean + SD for five to six replicates per condition from one of three experiments. n.s., not significant; ***P < 0.005 vs. BMAL1:CLOCK by t test.
CRYs Exhibit Many Characteristics of NR Corepressors.
CRY1 and CRY2 are transcriptional repressors within the core molecular clock. Our results and those of others (9, 15) suggest that CRYs may function independently of other core clock proteins to regulate NR-driven transcription. NR corepressors are recruited to the LBD, through a conserved hydrophobic motif, the corepressor NR box (CoRNR box), comprising I/L-X-X-I/V-I sequences (18, 19). Corepressors dissociate following a conformational change of H12 caused by agonist ligand binding (20). PXR, because of its crucial role in drug metabolism, has been extensively studied structurally. Potent and specific synthetic agonist ligands are available, making PXR a prime candidate to explore the biochemical features of the interaction with CRY.
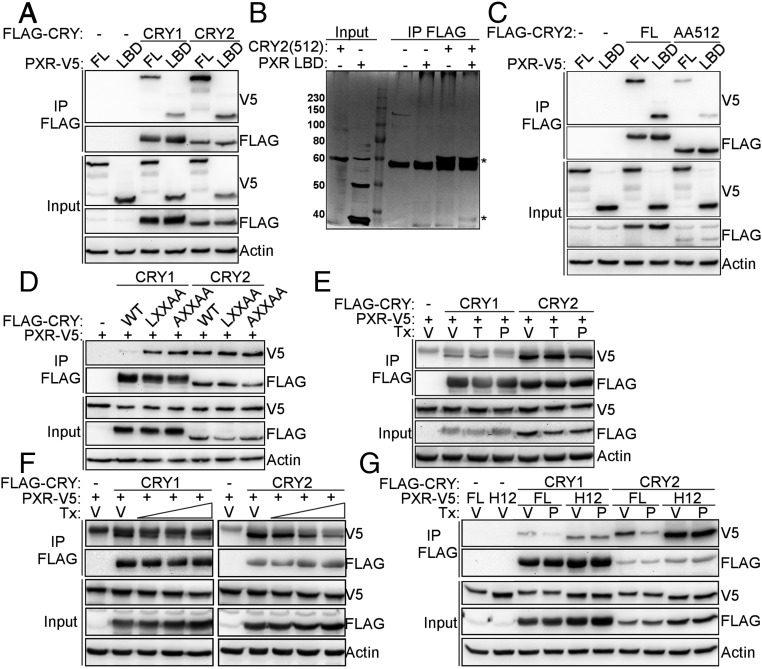
To determine whether the LBD of PXR is sufficient for the interaction with CRYs, we performed co-IP of full-length CRY1 and CRY2 with full-length (FL) PXR or the PXR LBD, and observed that the LBD is sufficient for interaction (Fig. 4A). Furthermore, recombinant CRY2 (amino acids 1–512) interacts directly with recombinant PXR LBD (Fig. 4B). However, the affinity of the interaction is weak, possibly as a result of CRY2 lacking the C-terminal tail, which we and others have thus far been unable to express and purify (21, 22) (Fig. 4C). CRYs contain an amino acid sequence that resembles a CoRNR box, in the PHR domain. However, mutating this motif does not disrupt interaction with PXR as similar mutations in NCOR and SMRT do (18, 19, 23), suggesting that it is not required for interaction of CRY with NRs (Fig. 4D). This finding is further supported by the 3D structures of CRY1 and CRY2 (21, 24) in which the LXXII helix is unusually short and hydrophobic residues face the core of the protein.
Fig. 4.
CRYs exhibit many characteristics of NR corepressors. (A and C–G) Proteins detected by Western blot in IPs from 293T cells expressing the indicated plasmids. (B) Silver stain of recombinant proteins after co-IP performed in vitro. An asterisk (*) indicates CRY2(512) and PXR LBD. In D, CRY1/2 CoRNR box-like sequences were mutated as indicated. In E–G cells were treated overnight with (E) DMSO (V, vehicle), 250 nM TCPOBOP (T, CAR agonist ligand) or 10 µM PCN (P, PXR agonist ligand), or (F) DMSO (V, vehicle), or 2, 10, and 50 µM PCN (P, PXR agonist ligand), or (G) DMSO (V, vehicle) or 10 µM PCN (P, PXR agonist ligand). Tx, treatment.
NR corepressors interact with unliganded or antagonist-bound receptors. Upon binding of agonist ligand, they dissociate, enabling recruitment of coactivators. Consistent with our hypothesis that CRYs are corepressors for PXR, the interaction between CRY1 or CRY2 and PXR is decreased in the presence of the PXR agonist ligand pregnenolone-16α-carbonitrile (PCN), but not CAR agonist ligand TCPOBOP (Fig. 4E). This occurs in a dose-dependent manner (Fig. 4F). Extensive structural characterization of NR LBDs suggests dynamic conformational changes upon ligand binding (25). In the apo form, the C-terminal H12 is extended away from the body of the LBD allowing corepressor binding. In the ligand bound (holo) form, H12 folds back onto the LBD facilitating coactivator binding. Truncation of H12 leads to constitutive corepressor binding in the presence of ligand (26). Truncation of PXR H12 prevents ligand-induced dissociation of CRY (Fig. 4G). Based on this characterization, CRYs strongly resemble established NR corepressors.
The Features of the PXR Interaction with CRY Are Reproduced in Those of Many NRs.
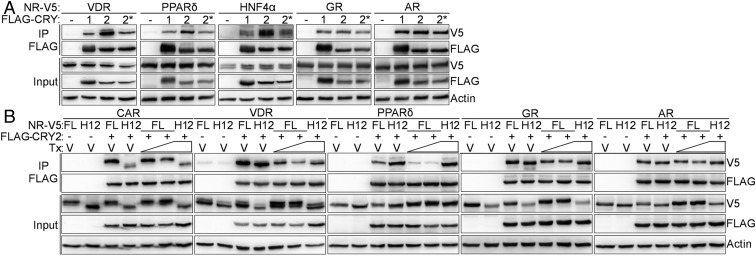
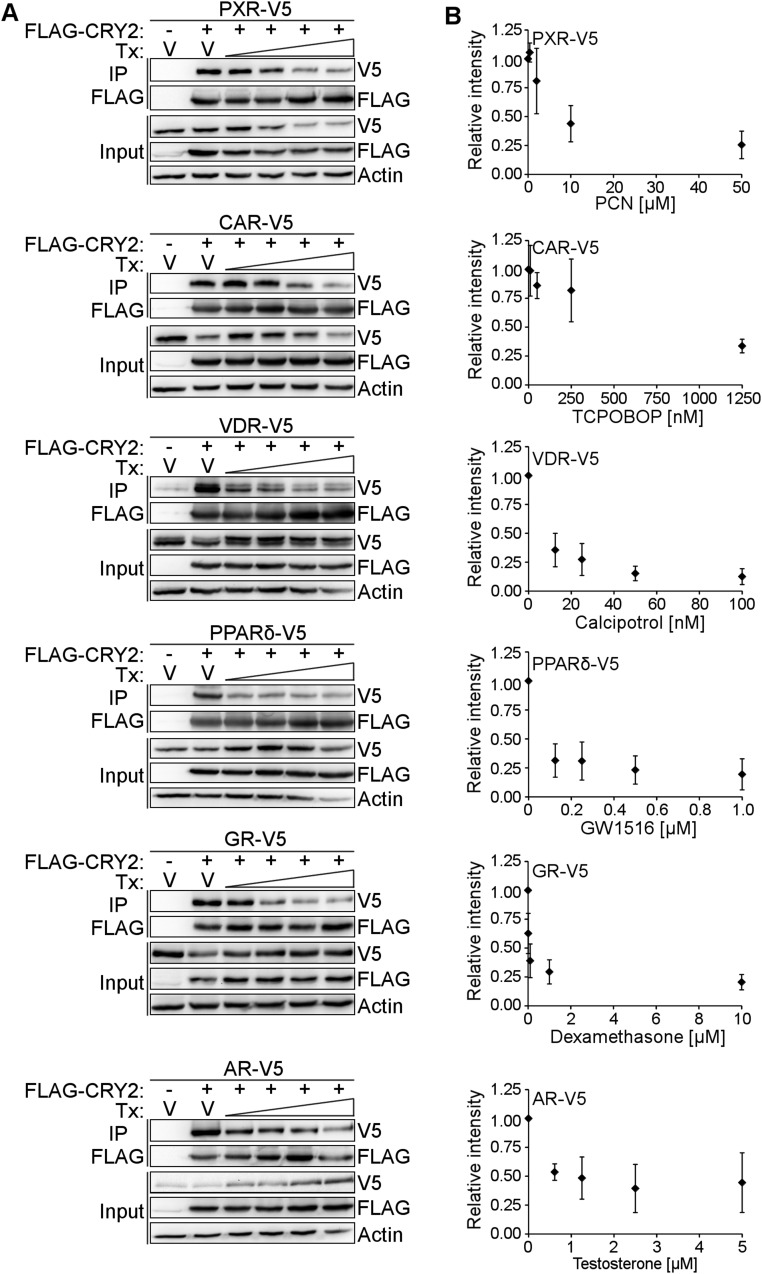
We tested the impact of agonist ligands, truncation of H12, and the CRY2* mutations on the interaction of CRY2 with a subset of the most strongly interacting NRs from our screen (Fig. 1A). VDR, PPARδ, and HNF4α each interact preferentially with CRY2 compared with CRY1, and the CRY2* mutant recapitulates their reduced binding to CRY1 (Fig. 5A). GR and androgen receptor (AR), which show a lesser preference for binding CRY1 or CRY2, are less affected by the CRY2* mutation. Together, these data indicate that this region near the secondary pocket of CRYs plays an important role in the interaction between CRY1/2 and most NRs. Truncation of H12 of CAR, PPARδ, GR, and AR abolishes ligand-dependent dissociation of CRY (Fig. 5B). Dissociation of the interaction between VDR and CRY2 is attenuated in the absence of H12. We quantified ligand-dependent dissociation of CRY2 from PXR, CAR, VDR, PPARδ, GR, and AR for a range of doses (Fig. S1A). At the highest dose, the signal intensity is decreased by at least half (44% of V5-AR remains bound to CRY2). The maximum disruption is almost 90% (13% of V5-tagged VDR was detected in precipitated complexes) (Fig. S1B). In previous studies, we observed that the presence of the agonist GR ligand dexamethasone increased the amount of GR pulled down by CRY1 (15). Because steroid hormone receptors (like GR and AR) translocate into the nucleus upon ligand binding, altered spatial proximity confounds interpretation of the effect of ligand on their interactions with CRYs.
Fig. 5.
The features of PXR–CRY interaction are reproduced with other NRs. (A and B) Proteins detected by Western blot in FLAG IPs from 293T cells expressing the indicated plasmids. In B, cells were treated with DMSO (V, vehicle) or 100/500 nM TCPOBOP (CAR agonist ligand), 10/100 nM calcipotrol (VDR agonist ligand), 0.1/1 µM GW1516 (PPARδ agonist ligand), 0.1/1 µM dexamethasone (GR agonist ligand), and 0.1/1 µM testosterone (AR agonist ligand) for 6 h in the presence of 10 µM MG132.
Fig. S1.
Ligand dose dependently decreases the interaction between CRY2 and NRs. (A) One representative experiment of n = 3. FLAG-tagged CRY2 was coexpressed with V5-tagged PXR, CAR, VDR, PPARδ, GR, and AR in HEK293T cells. Cells were treated with either vehicle (DMSO) or 0.4, 2, 10, 50 µM PCN for PXR; 10, 50, 250, 1,250 nM TCPOBOP for CAR; 12.5, 25, 50, 100 nM calcipitrol for VDR; 0.125, 0.25, 0.5, 1 µM GW1516 for PPARδ; 0.01, 0.1, 1, 10 µM dexamethasone for GR; and 0.625, 1.25, 2.5, 5 µM testosterone for AR. Cells were lysed and FLAG-tagged CRY was precipitated using anti-FLAG antibodies. V5-tagged NRs were detected using anti-V5 immunoblot. (B) Quantification of signal intensities for V5 tagged NRs in IP blots corrected for signal intensities for corresponding FLAG-CRY2 in IP blots. n = 3 independent experiments.
CRYs Repress PXR-Driven Transcriptional Activity.
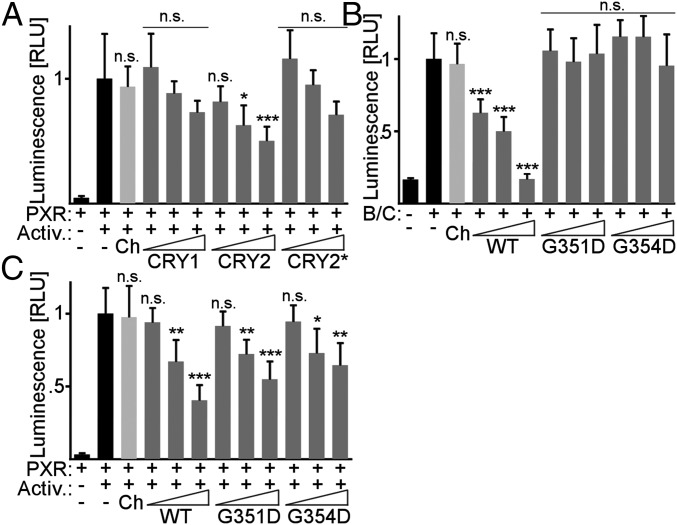
We used a luciferase reporter under the control of a PXR LBD–GAL4 DBD fusion protein. CRY2 significantly represses PXR-driven luciferase expression in a dose-dependent manner (Fig. 6A), whereas CRY1 and CRY2* show a trend toward dose-dependent repression, consistent with the observed preferential interaction of PXR with CRY2 compared with CRY1 and CRY2*. The CRY2 mutants G351D and G354D do not repress BMAL1:CLOCK-driven transcription (27) (Fig. 6B). Interestingly, these mutants retain the ability to repress PXR (Fig. 6C). These data suggest that CRY1, and more potently CRY2, repress NR-mediated transcription through interaction with the LBD by a distinct mechanism from that underlying repression in the core circadian clock.
Fig. 6.
CRYs repress PXR-driven transcriptional activity. (A) Luciferase activity in HepG2 cells expressing Gal4-luciferase, GAL4 DBD–PXR LBD, and 0.5, 1, or 2 ng of CRY1, CRY2, or CRY2*. PXR was activated by PGC1α expression and 1 µM PCN overnight (Activ.). (B) Luciferase activity in U2OS cells expressing Per2-luciferase, Bmal1, Clock, and 10–50 pg of Cry2 (WT), Cry2G351D (G351D), or Cry2G354D (G354D). (C) Luciferase activity as in A with 0.5, 1, or 2 ng of Cry2 (WT), Cry2G351D (G351D), or Cry2G354D (G354D). In A–C mCherry (Ch) was used as a negative control and luminescence was normalized to β-galactosidase activity. Data represent the mean + SD for five to six replicates per condition from a representative of at least three experiments. n.s., not significant; RLU, relative luminescence units; *P < 0.05, **P < 0.01, ***P < 0.005 vs. PXR+activators in A, C, or BMAL1:CLOCK in B by t test.
To study the effect of CRY1/2 on endogenous PXR and CAR, we used HepaRG cells (28) that, unlike most liver-derived cell lines, express crucial xenobiotic metabolism genes. Although we were not able to manipulate CRY expression in a manner that allowed us to determine the effects on PXR- or CAR-mediated gene transcription in HepaRG cells (Fig. S2), these cells exhibit rhythmic expression of xenobiotic genes and could be used to study the impact of circadian rhythm on drug pharmacokinetics in vitro (Fig. S3).
Fig. S2.
HepaRG cells reconstitute xenobiotic metabolism in vitro. (A) HepaRG cell differentiation over 4 wk in culture. Immunofluorescent staining of hepatocyte maker protein albumin in HepaRG cells at low density, in confluent cells (1 wk postseeding), and in differentiated cells (4 wk postseeding) imaged with Axio Imager M1 (Zeiss) 40× objective (total magnification 400×). (B) qPCR analysis of Albumin and xenobiotic genes PXR, CAR, CYP3A4, CYP2B6 mRNA expression in HepaRG cells at low density and differentiated cells. (C) Induction of PXR and CAR target gene expression by agonist ligand. Differentiated HepaRG cells were treated with 10 µM rifampicin (PXR agonist ligand) or 500 µM phenobarbital (CAR agonist ligand) for 8 h. The expression of CYP3A4 and CYP2B6 mRNA was measured using qPCR. (D) HepaRG cells stably expressing shRNAs targeting CRY1 or CRY2. Whole-cell lysate was prepared from HepaRG cells harboring shSCR, shCRY1, or shCRY2. CRY protein expression was detected using anti-CRY1 or anti-CRY2 antibody. (E and F) PXR and CAR expression after CRY protein knock down. PXR and CAR expression was measured using qPCR analysis of mRNA prepared form shSCR, shCRY1, or shCRY2 HepaRG cells. (G and H) PXR and CAR target genes expression after CRY protein knock down. Differentiated shSCR, shCRY1, shCRY2, and shPXR or shCAR HepaRG cells were treated with 50 µM rifampicin (PXR agonist ligand) or 500 µM phenobarbital (CAR agonist ligand) overnight. CYP3A4 or CYP2B6 expression was measured using qPCR. Error bars represent the mean ± SD for three replicates per condition. n.s., not significant; *P ≤ 0.05, **P < 0.01, ***P < 0.001 vs. vehicle treated in C, shSCR in E and F, and vehicle treated shSCR in G and H by t test.
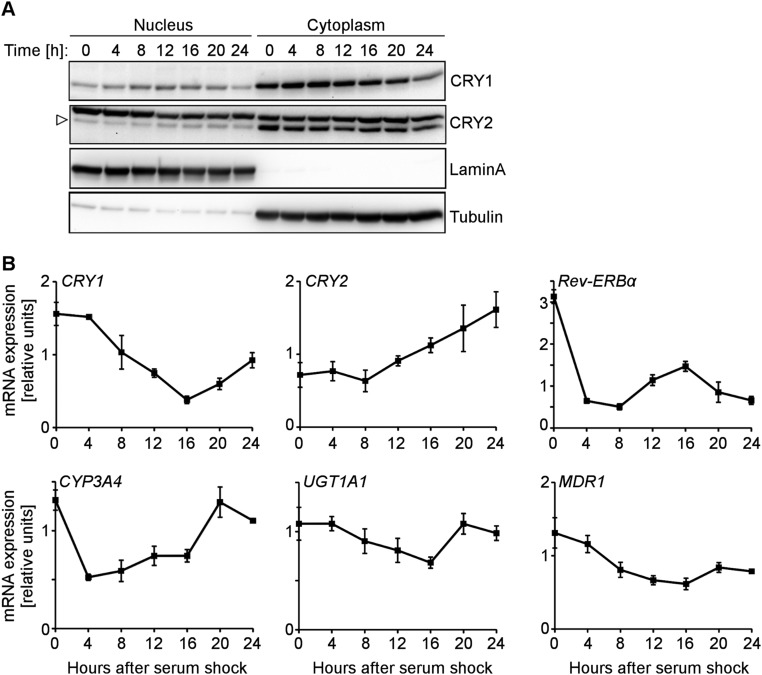
Fig. S3.
HepaRG cell circadian clocks can be synchronized. (A) HepaRG cell circadian rhythms were synchronized using 2-h treatment with 50% horse serum. Nuclear and cytosolic lysates were prepared at indicated times after synchronization. CRY protein expression was detected using CRY1- or CRY2-specific antibodies. For antibody validation, see Fig. S2D. (B) HepaRG cell circadian rhythms were synchronized as described above and mRNA was prepared from cells collected at indicated times after synchronization. Clock and xenobiotic gene expression was measured using qPCR. Error bars represent the mean ± SD for three replicates per condition.
CRY-Deficient Mice Exhibit Reduced Anesthesia Sleep Time.
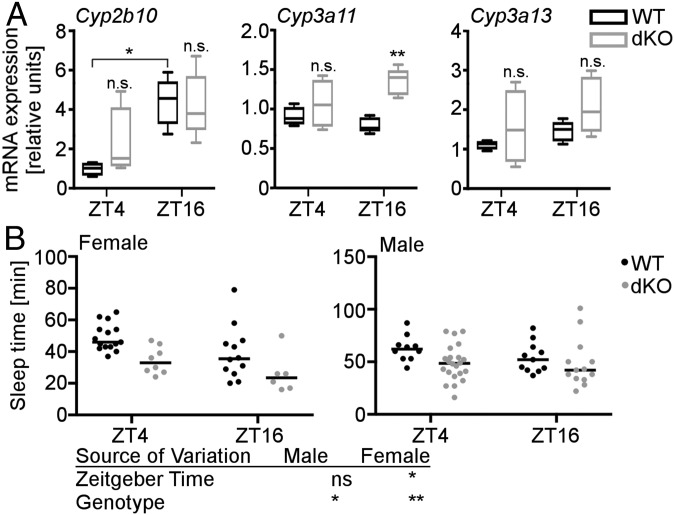
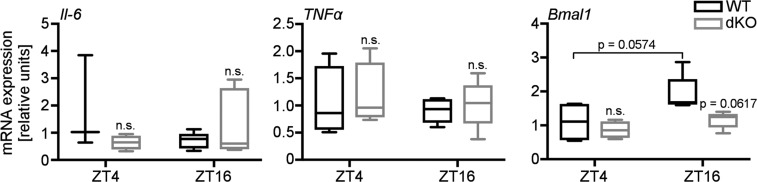
Our findings suggest that CRYs could limit activation of xenobiotic receptors, thus contributing to rhythmicity of xenobiotic metabolism. To investigate whether CRY1/2 regulate PXR and CAR in vivo, we measured xenobiotic gene expression in livers of WT and Cry1−/−;Cry2−/− (dKO) mice at two times during the day. The PXR and CAR target genes Cyp3a11, Cyp3a13, and Cyp2b10 encode drug-metabolizing enzymes (homologs of human CYP3A4 and CYP2B6). Each of these transcripts was elevated in dKO mouse livers; the effect of daytime on their expression was more variable (Fig. 7A). Tnfα and Il-6 were unaffected by the CRY genotype (Fig. S4), suggesting that increased inflammation (29) cannot explain the elevated Cyp expression. Taken together, these data suggest that the time of day, as well as CRY expression, could influence drug metabolism. Ketamine is a widely used anesthetic that is metabolized in human liver by CYP3A4, CYP2B6, and CYP2B9 (30). In mice, increased PXR and CAR activity is expected to increase expression of the CYP3A4 and CYP2B6 orthologs Cyp3a11, Cyp3a13, and Cyp2b10, leading to enhanced ketamine metabolism and reduced ketamine-induced sleep. Female dKO mice anesthetized with ketamine sleep less than their WT littermates (Fig. 7B). Both WT and dKO female mice wake up more quickly at zeitgeber time (ZT; hours after lights on) 16 when Cyp expression was higher. In male mice, we observed a significant effect of genotype but not of time. Taken together, these data support the idea that CRYs limit PXR and CAR activity to suppress xenobiotic metabolism in vivo.
Fig. 7.
CRY-deficient mice exhibit reduced anesthesia sleep time. (A) Cyp2b10, Cyp3a11, and Cyp3a13 gene expression in livers from female dKO mice and WT littermates. n = 4–5 female animals per group. *P < 0.05, **P < 0.01, ***P < 0.005 by t test. (B) Duration of ketamine induced sleep in dKO mice and WT littermates. n (female) = 6–15 per genotype, n (male) = 10–22 per genotype. n.s., not significant; *P < 0.05, **P < 0.01 by two-way ANOVA.
Fig. S4.
Inflammatory gene expression in WT and dKO livers. Il-6, TNFα, and Bmal1 expression in livers from female dKO mice and WT littermates. P values calculated by t test.
Discussion
It is increasingly clear that mammalian circadian clocks coordinate metabolic physiology with predictable daily fluctuations in metabolic demand because of rhythms in the external environment (31). NRs regulate large gene networks that are critical to adjusting metabolic physiology in response to hormones, vitamins, and other lipophilic ligands (1). The notion that clock proteins function as NR coregulators is supported by the finding that CLOCK, PER2, and CRYs modify or physically interact with some NRs to regulate NR-mediated transcription (32–34). Here we demonstrate that CRY1 interacts with more than half of all mouse NRs, suggesting a widespread mechanism of circadian NR regulation. Analysis of genome-wide DNA binding revealed that CRYs and NRs co-occupy many genomic sites.
Many mechanisms contribute to circadian regulation of NR target gene expression. For example, PAR bZip transcription factors promote the rhythmic expression of Car and CAR targets (35). Whereas DBP, HLF, and E4BP4 may contribute to rhythmic expression of CYP3A4 in HepG2 cells (36) and of Mdr1 in mouse intestine (37), mechanisms facilitating rhythmic transcription of PXR targets generally are not well understood. Our data suggest that direct repression by CRY1/2 enables circadian modulation of nonrhythmic NRs like PXR, and pose an additional avenue for circadian clock regulation of rhythmically transcribed NRs, including CAR. The mechanisms by which CRYs regulate NRs remains largely undetermined. Interestingly, we found that CRYs share many characteristic biochemical features with the well-studied NR corepressors NCOR1 and SMRT, although they do not require a CoRNR box (18, 19, 23, 38) to interact with NRs.
Structurally, CRYs are completely distinct from NCOR1 and SMRT, which are large, disordered scaffolds that recruit histone-modifying enzymes like HDAC3 (39). CRYs are compact and well-ordered, with the exception of the C-terminal tail (21, 24). We identified an α-helix near the secondary pocket of CRY2 that is crucial for interaction with NRs. It was recently discovered that CLOCK interacts directly with the secondary pocket of CRY1 (40) and our results, together with that finding, suggest that this area of the CRY1/2 surface could be an important site of interaction with transcription factor targets of CRY-mediated repression more generally.
The mechanisms by which CRYs repress transcription are not fully understood (41–44). In our hands, CRY transcriptional repression of BMAL1:CLOCK is much more robust than CRY repression of PXR or GR (15), which could reflect technical limitations of this assay. Interestingly, CRY2 mutants G351D and G354D that cannot repress BMAL1:CLOCK (27) repress PXR-dependent transcription. This finding indicates that CRYs repress NRs by a distinct mechanism.
We observed a striking sex difference in ketamine-induced sleep duration. Sex differences in the metabolism of drugs have been described for many species, including humans and mice. Increased expression of drug-metabolizing enzymes (45) and increased sensitivity of PXR and CAR (46–49) in female mice, could contribute to the more pronounced time-of-day–dependent variations in ketamine clearance of female mice.
Appreciation of the daily temporal regulation of physiology and metabolism is only recently emerging. Moving forward, this new dimension of CRY proteins corepressing NRs could be integrated into drug target discovery, as well as the timing and dosage regimen of existing drugs directed at NRs.
Materials and Methods
For details on co-IP, Western blotting, ChIP-seq data analysis, luciferase assays, qPCR, and ketamine sleep-time assay, please see SI Materials and Methods.
All animal care and treatments were in accordance with The Scripps Research Institute guidelines for the care and use of animals and were approved by the The Scripps Research Institute Institutional Animal Care and Use Committee under protocol #10–0019.
SI Materials and Methods
Cell Culture and Transfection.
HEK 293T, U2OS, and HepG2 cells were purchased from the American Type Culture Collection (ATCC). HEK 293T and U2OS cells were grown in complete DMEM (Life Technologies cat. #10569010) supplemented with 10% FBS, and 1% penicillin and streptomycin. HepG2 cells were grown in Eagle’s Minimum Essential Medium (EMEM) (ATCC cat. #30-2003) supplemented with 10% FBS. HepaRG cells were a gift from Gaetan Billioud, Department of Immunology, The Scripps Research Institute, La Jolla, CA. HepaRG cells were grown in William’s Medium E (LifeTech cat. #12551-032). Medium was supplemented with 10% fetal clone II serum (HyClone cat. #SH30066), 1% glutamine (Gibco cat. #35050-061), 5 µg/mL human insulin (Sigma cat. #19278) and, 50 µM hydrocorticosone 21-hemisuccinate (Sigma cat. #H2270). HepaRG cells were differentiated by seeding 20,000 cells/cm2 and maintaining them for 4 wk in culture replacing the medium every 2–3 d. All cells were grown in a 37 °C incubator maintained at 5% CO2 and 20% O2. TCPOBOP (Sigma cat. #T1443), PCN (Sigma cat. #P0543), GW1516 (Enzo cat. #ALX-420-032-M001), dexamethasone (Sigma cat. #4902), testosterone (Sigma cat. #T1500), calcipotrol (Tocris cat. #2700), Rifampicin (Sigma cat. #R3501), and phenobarbitol (Sigma cat. #p1636) were used at indicated concentrations. Transfections were carried out using linear polyethylenimine (PEI; Polysciences cat. #23966-2) by standard protocols.
Generation of Viruses and Stable Cell Lines.
Lentiviral shRNAs were transfected into HEK 293T cells using psPAX and pMD2.G packaging plasmids for virus generation. Viral supernatants were collected 48 h after transfection, filtered through a 0.45-μm filter, supplemented with 6 μg/mL polybrene, and added to parental cell lines. After 4 h, additional media was added to dilute the polybrene to <3 μg/mL 48 h after viral transduction, the infected cells were transferred into selection media containing 2 μg/mL puromycin. Selection media was replaced every 2–3 d until selection was complete (as judged by death of mock-infected cells; typically 1–2 wk).
Plasmids.
pcDNA3-2xFlag-mCRY1 and pcDNA3-2xFlag-mCRY2 are as described previously (50). pcDNA3.2-mNR-V5 plasmids were generated by standard cloning procedures (15, 51). CRY hybrid constructs were a gift from Andrew C. Liu, Department of Biological Sciences, University of Memphis, Memphis, TN (17); the hybrid coding sequences were corrected by site-directed mutagenesis and transferred to pcDNA3-2xFlag using standard protocols. All mutations were generated using the QuikChange II Site-Directed Mutagenesis Kit and protocol (Agilent Technologies cat. #200521) or Q5 Site-Directed Mutagenesis Kit and protocol (New England Biolabs cat. #E0554S). Deletions were generated using the Q5 Site-Directed Mutagenesis kit and protocol. pFastBacHFT and pSV272 were gifts from Ian MacRae, Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA. pcDNA3-Gal4 vectors, pcDNA3-V5-mPGC1α and pGK1 were gifts from Anastasia Kralli, Department of Physiology, Johns Hopkins University School of Medicine, Baltimore (52). pcDNA3-2xFlag-mBMAL1, pcDNA3-2xFlag-CLOCK, and pGL2-Per2-luciferase were gifts from Charles Weitz, Department of Neurobiology, Harvard Medical School, Boston.
shRNAs.
shRNAs against PXR, CAR, hCRY1, and hCRY2 were purchased from Open Biosystems. pLKO.1 shScramble deposited by David Sabatini was purchased from Addgene (Addgene plasmid 1864). psPAX plasmid (Addgene plasmid 12260) and pMD2.G plasmid (Addgene plasmid 12259), deposited by Didier Trono, used for infection, were also purchased from Addgene.
Luciferase Assays.
HepG2 and U2OS cells were seeded at a density of 10,000 cells per 96-well. HepG2 cells were transfected after 24 h with 40 ng pGK1, 5 ng β-galactosidase, 0.2 ng Gal4-PXR LBD or empty vector, 1 ng PGC1α, and 0.5–2 ng CRY1/2/mutants/mCherry for PXR luciferase assays. U2OS cells were transfected after 8 h with 35 ng pGL2-Per2-luciferase, 5 ng β-galactosidase, 0.5 ng BMAL1, 1.5 ng CLOCK, and 0.01–0.05 ng CRY1/2/mutants/mCherry for BMAL1/CLOCK luciferase assays. All low-concentration plasmid dilutions were prepared fresh immediately before transfection. The following day medium was replaced. Ligand treatments were performed overnight with 1 µM PCN in phenol red-free medium supplemented with 10% charcoal-stripped FBS. The following day luciferase activity was measured using the britelite plus Reporter Gene Assay System (Perkin-Elmer cat. #6066761) according to the manual, followed by measuring β-galactosidase activity measurement using 2-Nitrophenyl β-d-galactopyranoside (Sigma cat #N1127) as substrate.
Cell Lysates, IP, and Western Blotting.
HEK 293T whole-cell extracts and were prepared using lysis buffer containing 1% TX-100, phosphatase inhibitors (Sigma cat. #P5266 and cat #P0044), and protease inhibitor (Roche cat. #11697498001) as previously described (53). Then IP was performed using anti-Flag M2 agarose beads (Sigma cat. #A2220). HepaRG whole-cell extracts were prepared from RIPA buffer containing 1% Triton X-100, 147 mM NaCl, 12 mM sodium deoxycolate, 0.1% SDS, 50 mM Tris pH 8.0, 10 mM EDTA, 50 μM PMSF, phosphatase inhibitors, and protease inhibitor. Antibodies for Western blots were anti-Flag polyclonal (Sigma cat. #F7425), anti-V5 polyclonal (Bethyl Labs cat. #A190-120A), and anti–β-actin (Sigma cat. #A1978), anti-LaminA (Sigma cat. #1293), anti–α-Tubulin (Sigma cat. #T5168), antialbumin (Bethyl Labs cat. #A80-129A), and Cry1-CT and Cry2-CT, as described previously (15).
Nuclear and Cytoplasmic Fractionation of Cultured Cells.
Cells were washed once with ice-cold PBS, fresh cold PBS was added, and the cells were transferred to a 5-mL tube and centrifuged 5 min at 400 × g. The resulting pellets were washed with cold PBS and transferred to 1.5-mL Eppendorf tubes and centrifuged 5 min at 2,000 rpm. The resulting pellets were resuspended in Solution A (10 mM Hepes pH 8, 1.5 mM MgCl2, 10 mM KCl, plus protease and phosphatase inhibitors), and incubated for 15 min at 4 °C. An equal volume of Solution B (solution A + 1% Nonidet P-40) was added and the samples were further incubated for 5 min at 4 °C and centrifuged 5 min at 800 × g. Supernatants from this step represent the cytoplasmic fraction. The remaining nuclear pellets were washed twice with cold PBS and lysed in RIPA buffer, as described above.
Purification of Recombinant Proteins.
Protein purification was performed as described previously (54). Briefly, pFastBacHTF-mCRY2(512) and pSV272-mPXR LBD were expressed in Sf-9 cells and BL21(DE3) cells, respectively. Proteins were expressed with an N-terminal hexa-histidine tag upstream of a tobacco etch virus protease cleavage site. Proteins were purified from clarified lysate using standard nickel-affinity purification with an agarose Ni-NTA column. The tag was removed using tobacco etch virus protease after elution from the Ni-NTA resin. The protein was then passed over a second Ni-NTA column and the unbound protein was collected and further purified using size-exclusion chromatography. Purified protein was concentrated to 3 mg/mL in 10 mM Tris (pH 8.0), 0.1 M NaCl, and 0.5 mM Tris(2- carboxyethyl) phosphine hydrochloride (TCEP).
Immunofluorescence Staining.
For immunofluorescence, HepaRG cells were differentiated on glass coverslips and washed twice with PBS before fixation with 4% (wt/vol) paraformaldehyde in PBS for 30 min at room temperature. Cells were blocked and permeabilized with blocking solution containing 0.3% (vol/vol) Triton X-100, 3% (wt/vol) BSA, and 10% (vol/vol) FBS in PBS for 30 min at room temperature. Then, coverslips were washed three times with PBS before incubation with primary antibody antialbumin (Bethyl Labs cat. #A80-129A) diluted 1:200 in binding buffer (3% BSA, 0.3% Triton X-100 in PBS). Cells were incubated with primary antibody for 1 h at room temperature. Cells were washed three times for 5 min with PBS and incubated for 30 min with secondary antibody Alexa Fluor 594 donkey anti-goat IgG (Life Technologies cat. #A11058) diluted 1:500 in binding buffer. Cells were then washed three times for 5 min with PBS. DAPI (0.4 μg/mL) was added to PBS during the second wash step to visualize DNA. The coverslips were mounted onto glass slides with Fluoromount G (Electron Microscopy Science cat. #17984-25).
Quantitative RT-PCR.
RNA was extracted from powderized tissue with Qiazol reagent using standard protocols (Qiagen cat. #79306). cDNA was prepared using QScript cDNA Supermix (Quanta Biosciences cat. #95048) and analyzed for gene expression using quantitative real-time PCR with iQ SYBR Green Supermix (Bio-Rad cat. #1708885).
Quantitative RT-PCR Primer Sequences.
The quantitative RT-PCR primer sequences are as follows:
| Target gene | Forward primer | Reverse primer |
| Hprt | TGCTCGAGATGTCATGAAGG | TATGTCCCCCGTTGACTGAT |
| Cyp2b10 | AAAGTCCCGTGGCAACTTCC | TTGGCTCAACGACAGCAACT |
| Cyp3a11 | ACAAGCAGGGATGGACCTGGTTT | CCCATATCGGTAGAGGAGCACCAAG |
| Cyp3a13 | GACGATTCTTGCTTACCAGAAGG | CCGGTTTGTGAAGGTAGAGTAAC |
| Tnfa | CCTATGTCTCAGCCTCTTCTC | GGCCATTTGGGAACTTCTCATC |
| Il-6 | CATAGCTACCTGGAGTACATGAAG | GGAAATTGGGGTAGGAAGGACTA |
| Bmal1 | TCAAGACGACATAGGACACCT | GGACATTGGCTAAAACAACAGTG |
| U36B4 | AGATGCAGCAGATCCGCA | GTTCTTGCCCATCAGCACC |
| ALBUMIN | TGCTTGAATGTGCTGATGACAGG | AAGGCAAGTCAGCAGGCATCTCATC |
| PXR | CCCAGCCTGCTCATAGGTTC | GGGTGTGCTGAGCATTGATG |
| CAR | TTCATGGTACTGCAAGTCATCAA | TTGAGAAGGGAGATCTGGTCTTC |
| CYP3A4 | ACAAAAGCACCGAGTGGATTT CCTT | GGCCACGAGCTCCAGATCGGA |
| CYP2B6 | TCTGGCCGGGGAAAAATCG | GGTCACAGAGAATCGCCGAAG |
| UGT1A1 | GCTATGGCAATTGCTGATGCT TTGGGC | CCATGGGAACCAGCATGGGTGATA AAG |
| MDR1 | CATGAATCTGGAGGAAGACAT GACCAG | CATCGTGCACATCAAACCAGCCTATC |
| Rev-ERBα | TGACCAAGTCACCCTGCTTAAG | AAGCAAAGCGCACCATCA |
Ketamine Sleep Time Assay.
Cry1−/−; Cry2−/− mice were from Aziz Sancar, School of Medicine, University of North Carolina, Chapel Hill, NC (55). Mice were entrained to a 12:12 light:dark cycle for at least 2 wk before experiments and all experiments were performed at 13–14 wk of age. All animal care and treatments were in accordance with The Scripps Research Institute guidelines for the care and use of animals and were approved by the The Scripps Research Institute Institutional Animal Care and Use Committee under protocol no. 10–0019.
Mice were fasted with access to water in multihoused cages for 14–16 h before the experiment. At the indicated ZT, mice were single-housed in clean cages (without food) and were injected intraperitoneally with ketamine hydrochloride (200 mg/kg of body weight dissolved in saline at a concentration of 20 mg/mL; Hospira). Following unconsciousness (reflex to paw pinch still present, indicating deep sedation), mice were gently placed on their backs in their cage. Sleep time was calculated as time from injection to regaining the righting reflex (i.e., the time the animal could right itself three times within 1 min). Mice were not returned to their multihoused cages until fully recovered from sedation, as indicated by maintaining an upright posture and walking normally. For tissue harvest, mice were killed by cervical dislocation while under deep sedation, 10 min after injection with ketamine.
ChIP-Seq Data Analysis.
Enriched motifs in ChIP-Seq peaks were identified using Homer (56), using default parameters with findMotifsGenome.pl. Overlapping peaks were found with the Homer “mergePeaks” function using “-d” of 200 bp (homer.ucsd.edu/homer/ngs/). The GSE accession numbers are indicated as follows:
Supplementary Material
Acknowledgments
We thank Drs. Ian MacRae, Nicole Schirle-Oakdale, Siobhan Hughes, and Jessica Sheu-Gruttadauria (The Scripps Research Institute) for help with recombinant protein expression and purification; and Dr. Anastasia Kralli (Department of Physiology, Johns Hopkins University School of Medicine) for help with luciferase assays and helpful discussions. This work was supported by NIH Grants DK090188 and DK097164 (to K.A.L.); a Searle scholars award from the Kinship Foundation (to K.A.L.); a fellowship from the Deutsche Forschungsgemeinschaft (to S.D.J.); American Heart Association Fellowships 15POST22510020 (to S.D.J.) and 16PRE3041001 (to S.J.P.); a fellowship from the Swedish Research Council (to E.H.); and a fellowship from the National Science Foundation (to C.R.S.). R.M.E. is a Howard Hughes Medical Institute Investigator at the Salk Institute and March of Dimes Chair in Developmental and Molecular Biology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704955114/-/DCSupplemental.
References
- 1.Sonoda J, Pei L, Evans RM. Nuclear receptors: Decoding metabolic disease. FEBS Lett. 2008;582:2–9. doi: 10.1016/j.febslet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustafson CL, Partch CL. Emerging models for the molecular basis of mammalian circadian timing. Biochemistry. 2015;54:134–149. doi: 10.1021/bi500731f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao X, et al. Nuclear receptors rock around the clock. EMBO Rep. 2014;15:518–528. doi: 10.1002/embr.201338271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 6.Panda S, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 7.Storch K-F, et al. 2002. Extensive and divergent circadian gene expression in liver and heart. Nature 417:78–83; erratum in Nature (2002) 418:665.
- 8.Robles MS, Cox J, Mann M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014;10:e1004047. doi: 10.1371/journal.pgen.1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomalik-Scharte D, et al. Population pharmacokinetic analysis of circadian rhythms in hepatic CYP3A activity using midazolam. J Clin Pharmacol. 2014;54:1162–1169. doi: 10.1002/jcph.318. [DOI] [PubMed] [Google Scholar]
- 11.Chassard D, Duflo F, de Queiroz Siqueira M, Allaouchiche B, Boselli E. Chronobiology and anaesthesia. Curr Opin Anaesthesiol. 2007;20:186–190. doi: 10.1097/ACO.0b013e328136c55e. [DOI] [PubMed] [Google Scholar]
- 12.Lim H-W, et al. Genomic redistribution of GR monomers and dimers mediates transcriptional response to exogenous glucocorticoid in vivo. Genome Res. 2015;25:836–844. doi: 10.1101/gr.188581.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho H, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JM, et al. Nutrient-sensing nuclear receptors coordinate autophagy. Nature. 2014;516:112–115. doi: 10.1038/nature13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamia KA, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan SD, et al. CRY1/2 selectively repress PPARδ and limit exercise capacity. Cell Metab. 2017;26:243–255. doi: 10.1016/j.cmet.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan SK, et al. Identification of a novel cryptochrome differentiating domain required for feedback repression in circadian clock function. J Biol Chem. 2012;287:25917–25926. doi: 10.1074/jbc.M112.368001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Lazar MA. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402:93–96. doi: 10.1038/47069. [DOI] [PubMed] [Google Scholar]
- 19.Perissi V, et al. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999;13:3198–3208. doi: 10.1101/gad.13.24.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 21.Xing W, et al. SCF(FBXL3) ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature. 2013;496:64–68. doi: 10.1038/nature11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papp SJ, et al. DNA damage shifts circadian clock time via Hausp-dependent Cry1 stabilization. eLife. 2015;4:e04883. doi: 10.7554/eLife.04883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy L, et al. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 1999;13:3209–3216. doi: 10.1101/gad.13.24.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czarna A, et al. Structures of Drosophila cryptochrome and mouse cryptochrome1 provide insight into circadian function. Cell. 2013;153:1394–1405. doi: 10.1016/j.cell.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Moras D, Gronemeyer H. The nuclear receptor ligand-binding domain: Structure and function. Curr Opin Cell Biol. 1998;10:384–391. doi: 10.1016/s0955-0674(98)80015-x. [DOI] [PubMed] [Google Scholar]
- 26.Lin BC, Hong SH, Krig S, Yoh SM, Privalsky ML. A conformational switch in nuclear hormone receptors is involved in coupling hormone binding to corepressor release. Mol Cell Biol. 1997;17:6131–6138. doi: 10.1128/mcb.17.10.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy EV, Baggs JE, Geskes JM, Hogenesch JB, Green CB. Generation of a novel allelic series of cryptochrome mutants via mutagenesis reveals residues involved in protein-protein interaction and CRY2-specific repression. Mol Cell Biol. 2009;29:5465–5476. doi: 10.1128/MCB.00641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gripon P, et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA. 2002;99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narasimamurthy R, et al. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hijazi Y, Boulieu R. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2002;30:853–858. doi: 10.1124/dmd.30.7.853. [DOI] [PubMed] [Google Scholar]
- 31.Marcheva B, et al. Circadian clocks and metabolism. Handb Exp Pharmacol. 2013;217:127–155. doi: 10.1007/978-3-642-25950-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: Potential physiological implications. FASEB J. 2009;23:1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimaldi B, et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev. 2010;24:345–357. doi: 10.1101/gad.564110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006;4:25–36. doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Takiguchi T, et al. Molecular basis for rhythmic expression of CYP3A4 in serum-shocked HepG2 cells. Pharmacogenet Genomics. 2007;17:1047–1056. doi: 10.1097/FPC.0b013e3282f12a61. [DOI] [PubMed] [Google Scholar]
- 37.Murakami Y, Higashi Y, Matsunaga N, Koyanagi S, Ohdo S. Circadian clock-controlled intestinal expression of the multidrug-resistance gene mdr1a in mice. Gastroenterology. 2008;135:1636–1644.e3. doi: 10.1053/j.gastro.2008.07.073. [DOI] [PubMed] [Google Scholar]
- 38.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 39.Ishizuka T, Lazar MA. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol. 2003;23:5122–5131. doi: 10.1128/MCB.23.15.5122-5131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michael AK, et al. Formation of a repressive complex in the mammalian circadian clock is mediated by the secondary pocket of CRY1. Proc Natl Acad Sci USA. 2017;114:1560–1565. doi: 10.1073/pnas.1615310114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng B, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 42.Zheng B, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 43.Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science. 2011;332:1436–1439. doi: 10.1126/science.1196766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu H, et al. Cryptochrome 1 regulates the circadian clock through dynamic interactions with the BMAL1 C terminus. Nat Struct Mol Biol. 2015;22:476–484. doi: 10.1038/nsmb.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–228. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurley BJ, et al. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 47.Ledda-Columbano GM, et al. Sex difference in the proliferative response of mouse hepatocytes to treatment with the CAR ligand, TCPOBOP. Carcinogenesis. 2003;24:1059–1065. doi: 10.1093/carcin/bgg063. [DOI] [PubMed] [Google Scholar]
- 48.Felmlee MA, Lon H-K, Gonzalez FJ, Yu A-M. 2008. Cytochrome P450 expression and regulation in CYP3A4/CYP2D6 double transgenic humanized mice. Drug Metab Dispos 36:435–441; erratum in Drug Metab Dispos (2009) 37:1563. [DOI] [PubMed]
- 49.Hernandez JP, Mota LC, Huang W, Moore DD, Baldwin WS. Sexually dimorphic regulation and induction of P450s by the constitutive androstane receptor (CAR) Toxicology. 2009;256:53–64. doi: 10.1016/j.tox.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamia KA, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao X, et al. Circadian amplitude regulation via FBXW7-targeted REV-ERBα degradation. Cell. 2016;165:1644–1657. doi: 10.1016/j.cell.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knutti D, Kressler D, Kralli A. Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc Natl Acad Sci USA. 2001;98:9713–9718. doi: 10.1073/pnas.171184698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamia KA, et al. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase beta−/− mice. Mol Cell Biol. 2004;24:5080–5087. doi: 10.1128/MCB.24.11.5080-5087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schirle NT, MacRae IJ. The crystal structure of human Argonaute2. Science. 2012;336:1037–1040. doi: 10.1126/science.1221551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thresher RJ, et al. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science. 1998;282:1490–1494. doi: 10.1126/science.282.5393.1490. [DOI] [PubMed] [Google Scholar]
- 56.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.