Significance
More than 170 mutations in superoxide dismutase 1 (SOD1) are linked to inherited forms of ALS, and aggregates of this protein are a pathological feature associated with this disease. Although it is accepted that SOD1 gains a toxic function in the disease state, a molecular understanding of the toxic species is lacking. Here, we identify a short segment of SOD1 that is both necessary and sufficient for toxicity to motor neurons. The crystal structure of the segment reveals an out-of-register β-sheet oligomer, providing a structural rationale for the toxic effects of mutant SOD1 in ALS.
Keywords: oligomer, SOD1, ALS
Abstract
Fibrils and oligomers are the aggregated protein agents of neuronal dysfunction in ALS diseases. Whereas we now know much about fibril architecture, atomic structures of disease-related oligomers have eluded determination. Here, we determine the corkscrew-like structure of a cytotoxic segment of superoxide dismutase 1 (SOD1) in its oligomeric state. Mutations that prevent formation of this structure eliminate cytotoxicity of the segment in isolation as well as cytotoxicity of the ALS-linked mutants of SOD1 in primary motor neurons and in a Danio rerio (zebrafish) model of ALS. Cytotoxicity assays suggest that toxicity is a property of soluble oligomers, and not large insoluble aggregates. Our work adds to evidence that the toxic oligomeric entities in protein aggregation diseases contain antiparallel, out-of-register β-sheet structures and identifies a target for structure-based therapeutics in ALS.
Since Alzheimer’s pioneering report in 1906 (1), fibrillar protein deposits have been linked to neurodegenerative diseases. More recently, this link has been challenged by findings that transient soluble oligomers formed by these proteins are cytotoxic (2, 3). Whereas atomic-resolution structures of the spines of amyloid fibrils have shown tightly packed β-sheets with interdigitated side chains (4–6), atomic-level details of toxic oligomers remain elusive. Various reports suggest that toxic intermediates formed by amyloid-forming proteins consist of antiparallel β-sheet–rich structures (7–9). These reports used chemical cross-linking, analytical size exclusion, EM, and FTIR, but no atomic structure of toxic amyloid oligomers has been reported.
ALS is a debilitating disease, destroying spinal motor neurons and often leading to death within a few years of symptom onset. More than 170 different mutations in superoxide dismutase 1 (SOD1), a metal-binding, homodimeric protein of 153 residues, are found in familial cases of ALS (10, 11). Most of these SOD1 mutants show little change in enzymatic function, suggesting that toxicity derives not from a loss of native function but from a gain of toxic function (12–14). Transgenic mouse models of the familial mutants show motor neuron degeneration and stain positive for SOD1-containing inclusions, suggesting that protein aggregation is a mode of toxicity (14–16). Enrichment of oligomers has also been observed in cell culture and in transgenic mice (17–19). However, a causal relationship between the appearance of aggregates and neuronal death has not been conclusively supported, and no atomic structure has been described for toxic oligomers of SOD1 or any other neurodegenerative disease-related protein. Here, we propose a structure for toxic oligomers formed by SOD1.
Results
Crystal Structure of SOD1 Residues 28–38 Reveals an Antiparallel β-Sheet Oligomer.
We identified residues 28–38 of SOD1 (with the sequence PVKVWGSIKGL) as having the potential to form a toxic amyloid oligomer based on mutational studies of others (19–23) and our own (discussed below) (SI Appendix, Figs. S1–S3). To increase solubility for crystallization, we engineered a single-residue substitution: P28K. Rod-like crystals measuring 5 μm in the shortest dimension appeared overnight, and upon further optimization, they diffracted X-rays to 2.0-Å resolution. We determined the phases by single isomorphous replacement with anomalous scattering using crystals soaked in potassium iodide (SI Appendix, Table S1).
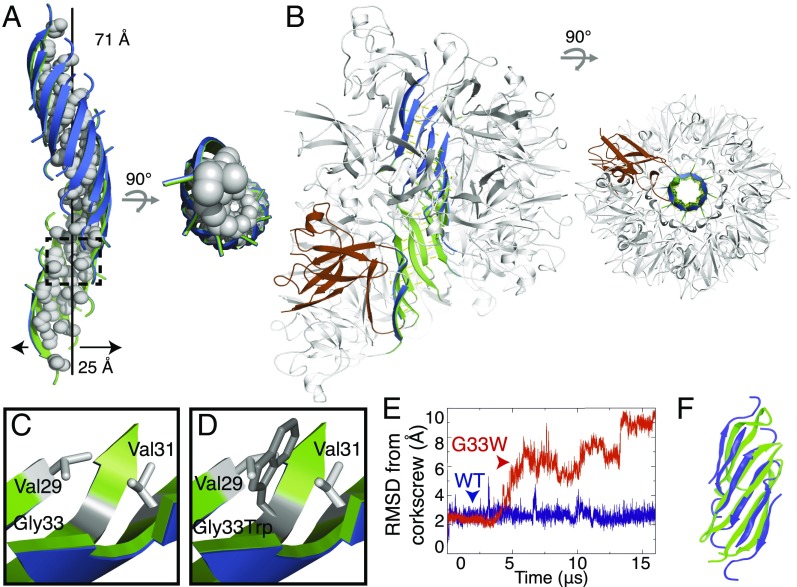
The crystal structure shows a twisted β-sheet built of antiparallel, out-of-register β-strands. Describing its shape, we term it the “corkscrew” (Fig. 1A). Each β-strand in the sheet contains eight residues, from Lys28 to Ile35. The three C-terminal residues, Lys36, Gly37, and Leu38, adopt a β-hairpin conformation, positioning the C-terminal carboxylate to hydrogen-bond with the N-terminal residue of an adjacent strand. The twist of the sheet is left-handed, as is commonly observed for β-sheets. The sheet undergoes a full turn every 16 strands, with a helical pitch of 71 Å corresponding to the unit cell “c” dimension. Unit cell repeats extend the corkscrew throughout the length of the crystal. Examination of Lys28 reveals that the P28K substitution made to facilitate crystallization affects only crystal lattice contacts (SI Appendix, Fig. S4), and suggests that the native sequence would adopt the corkscrew structure seen here. Indeed, SOD1 residues 28–38 assembled preferentially into a corkscrew in our molecular dynamics (MD) simulations (Fig. 1F and SI Appendix, Fig. S5B).
Fig. 1.
Structure of an 11-residue segment derived from SOD1 in its oligomeric state. (A) Crystal structure of the SOD1 segment with the sequence KVKVWGSIKGL at 2.0-Å resolution shows antiparallel, out-of-register β-strands forming a continuous left-handed helix. Sixteen strands form one complete turn of the helix, with a 25-Å outer diameter and 71-Å pitch. The hydrophobic interior lined with valine, isoleucine, and leucine side chains (shown in spheres) excludes water molecules, as shown in side and top views. (B) Model of a full-length SOD1 toxic oligomer. This model contains 16 protomers. Strands 2 and 3 of each protomer detach from the native fold to form the corkscrew spine (green interior, blue exterior) as observed in the crystal structure of residues 28–38. The remaining protein decorates the exterior of corkscrew, retaining much of the SOD1 native structure. The model is illustrated in the same two orientations as A. One monomer is colored brown for clarity. (C) Gly33 is essential for creating the concave inner surface of the corkscrew. The lack of a side chain on Gly33 permits the close approach of hydrophobic side chains of Val29 and Val31 located on β-strands bordering opposite sides of Gly33. (D) Unavoidable steric clash results from mutating Gly33 to tryptophan. (E) All-atom MD simulations of the corkscrew-forming segment suggest that introduction of the G33W substitution destabilizes the structure. Blue and red curves correspond to Cα rmsd from the corkscrew crystal structure in MD simulations of eight chains of the corkscrew segment (KVKVWGSIKGL) and G33W mutant segment (KVKVWWSIKGL), respectively. The structure of the corkscrew segment remained stable throughout the length of the simulation, whereas the G33W mutant deviated from the corkscrew structure. (F) SOD1 segment 28–38 preferentially assembled into a corkscrew-like structure in an MD simulation. MD simulations of weakly restrained monomers of SOD1 spontaneously assembled into a corkscrew-like structure. A snapshot of an assembled corkscrew-like structure from the MD simulations (green) is overlaid onto the crystal structure (blue). As a control, we found that monomers of the cylindrin-forming segment of αB-crystallin spontaneously assembled into a cylindrin structure using the same simulation protocol (additional simulation details are provided in SI Appendix, Fig. S5B).
The corkscrew architecture differs markedly from amyloid fibrils. Sheets from adjacent corkscrews do not mate together tightly as sheets do in amyloid fibrils, but instead contact weakly through polar and charged side chains scattered over the exterior of the corkscrew (Lys28, Lys30, Ser34, and Lys36), Trp32, and water-mediated contacts. Hence, unlike amyloid fibrils, the corkscrew has no dry interface between sheets to stabilize its assembly. Instead, the corkscrew assembly is stabilized by weaker hydrophobic forces arising from the concave interior filled with aliphatic side chains of Val29, Val31, Ile35, and Leu38 (Fig. 1A and SI Appendix, Fig. S3).
The absence of a stable, amyloid-like, dry sheet–sheet interface suggests that fragmentation of the corkscrew could be relatively facile and that its subsets of various sizes could fit the definition of soluble oligomers. Indeed, ion mobility MS experiments confirm that this SOD1 segment 28–38 forms low-molecular-weight oligomers in solution similar in cross-section to the crystal structure of the corkscrew (SI Appendix, Fig. S6), supporting our hypothesis that the corkscrew represents the structure of a soluble oligomer. Furthermore, the β-sheet–rich nature of the corkscrew is a property shared in common with other amyloid-related oligomers, such as α-synuclein, amyloid-β, and HET-s (8, 9, 24). In fact, several amyloid oligomers have been reported to share antiparallel, out-of-register β-strand architecture (24–26).
The corkscrew shares several structural features with another soluble oligomer, cylindrin, from the nonpathogenic amyloid-forming protein, αB-crystallin (27). Both oligomers are composed of antiparallel β-strands, shifted out-of-register by two residues. Both sheets are highly curved, and opposite faces of the sheet display different sets of side chains not related by symmetry (28). In both oligomers, individual strands hydrogen-bond to neighboring strands through alternating weak and strong interfaces (SI Appendix, Fig. S5A). The strong interface of the corkscrew is composed of nine interchain hydrogen bonds, and the weak interface is composed of seven hydrogen bonds: one intramain chain and six intermain chain hydrogen bonds. The β-strands in both oligomers have similar values of buried surface area (984 Å2 vs. 943 Å2) and shape complementarity (0.79 vs. 0.74) (SI Appendix, Table S2). The primary difference between the two architectures is that cylindrin is a closed barrel of defined stoichiometry (six strands), whereas the corkscrew, although highly curved, is not entirely closed and is likely to exist in a range of oligomeric sizes.
The role of the corkscrew in ALS is supported by a model accommodating full-length SOD1. We modeled the remainder of SOD1 around the corkscrew scaffold, keeping the tertiary structure of SOD1 intact everywhere except near the corkscrew and avoiding steric conflict (Fig. 1B). In our model, strands 2 and 3 detach from the native fold, exposing the corkscrew-forming residues 28–38. This local unfolding may be triggered by dissociation of the SOD1 dimer and metal depletion. Biochemical studies have noted that metal-depleted monomer is prevalent in patient tissues (29, 30) as well as in mouse models, and is an intermediate in the conversion of native holo-SOD1 to pathological aggregates (31). This model agrees with H/D exchange and with MD and MS studies showing that most familial SOD1 mutants have minimal change in their secondary structure and contain a partially unfolded β-barrel at physiological temperature with local unfolding in β-strand 3 (20, 31).
We probed the role of the corkscrew in SOD1-associated cytotoxicity by introducing mutations to disrupt corkscrew architecture. The absence of a bulky side chain at position 33 appears essential to form the concave inner surface of the corkscrew (Fig. 1C). Mutation of Gly33 to a larger residue, such as tryptophan (Fig. 1D) or valine, would introduce severe steric clashes with Val29 and Val31, destabilizing the corkscrew structure. Consistent with this observation, our all-atom MD simulations revealed that the corkscrew was less stable for W33 than for G33, regardless of whether the N terminus was proline or lysine (Fig. 1E and SI Appendix, Fig. S4C). Thus, if the corkscrew were a cytotoxic motif, we would expect G33 mutants to alleviate toxicity of SOD1 familial mutants.
Segment 28–38 Is Necessary and Sufficient for Toxicity.
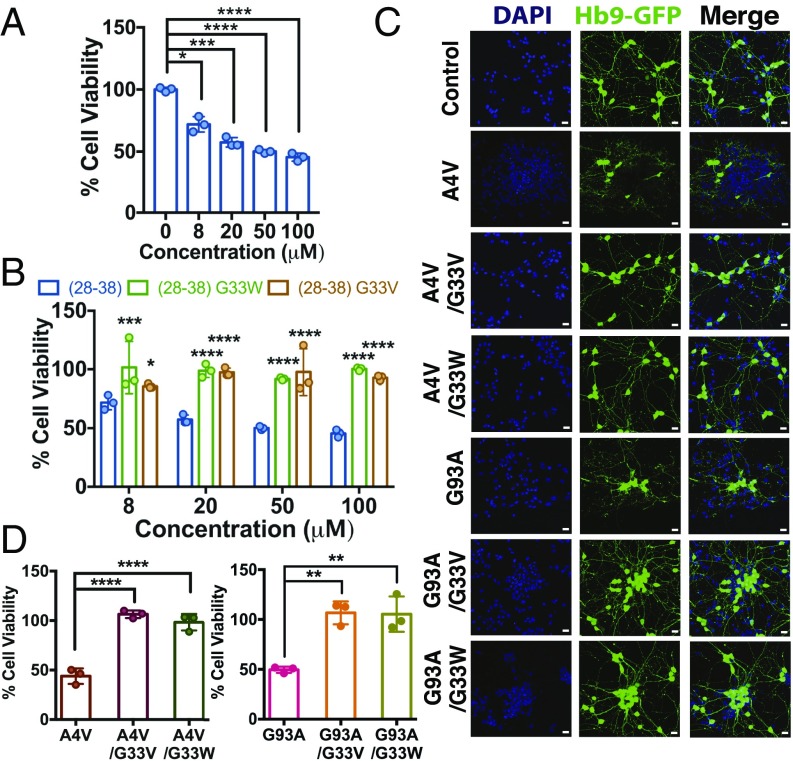
Corkscrew-disruptive mutations alleviated toxicity of segment 28–38. We assayed cytotoxicity by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction in embryonic stem-cell–derived motor neurons expressing GFP to facilitate visualization of neuron morphology (32). The corkscrew-forming segment was aggregated, applied to motor neurons, and incubated overnight. We found that viability was reduced by 60% compared with buffer-treated cells at physiological concentrations of 8–100 μM in a dose-dependent manner (Fig. 2A). In contrast, neither the segment harboring the corkscrew-disruptive G33W mutation (Fig. 2B) nor the less bulky G33V mutant (Fig. 2B) induced toxicity in cells at any concentration tested. The same trends were observed with the native 28–38 segment (P28) and the corresponding G33W and G33V mutations (SI Appendix, Fig. S7).
Fig. 2.
Corkscrew-forming segment 28–38 is necessary and sufficient for cytotoxicity. (A) Cell viability of motor neurons measured by an MTT reduction assay shows that the corkscrew segment (KVKVWGSIKGL) is toxic to primary motor neurons in a dose-dependent manner. Results are shown as mean ± SD (n = 3). Symbols represent individual values of triplicates, and bars represent average values. Statistical significance was analyzed using two-tailed t tests with Welch’s correction. (B) Corkscrew-forming segment (28–38) harboring single-point substitutions at Gly33 (G33V and G33W) is nontoxic to motor neurons. All peptide segments were prepared identically, and motor neurons were treated with different final concentrations. The statistical significance of G33V and G33W mutants was compared with segment 28–38 by two-way ANOVA. (C) Hb9-GFP–labeled motor neurons treated with 8 μM aggregated full-length familial mutants (A4V and G93A) lose neurites, but the corresponding corkscrew-disrupting mutants (G93A/G33V, G93A/G33W, A4V/G33V, and A4V/G33W) are nontoxic and neurons look healthy. (Scale bars, 20 μm.) (D) Cell viability measured by an MTT reduction assay confirming that the familial mutants A4V and G93A are toxic and that substitution of Gly33 with valine or tryptophan renders the protein nontoxic. Results are shown as mean ± SD (n = 3). Symbols represent individual values of triplicates, and bars represent average values. Statistical significance was analyzed by one-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Corkscrew-disruptive mutations also alleviated toxicity of full-length SOD1 familial mutants A4V and G93A. These proteins were recombinantly expressed, purified, and aggregated by demetallation and agitation at 37 °C for 12 h, which produced a mixture of fibrils and oligomers. Motor neurons were treated with aggregated proteins overnight and then assayed for cellular viability. A4V and G93A mutants were toxic to the cells at 8 μM, which looked drastically degenerated compared with buffer-treated cells (Fig. 2C) and demonstrated reduced viability (Fig. 2D). In contrast, A4V/G33V, A4V/G33W, G93A/G33V, and G93A/G33W proteins were nontoxic at the same concentration, and cellular morphologies were indistinguishable from the cellular morphologies of the buffer-treated cells. Together, these data suggest that the segment 28–38 is both necessary and sufficient for motor neuron toxicity.
Toxicity of Full-Length SOD1 Derives from Soluble Oligomers.
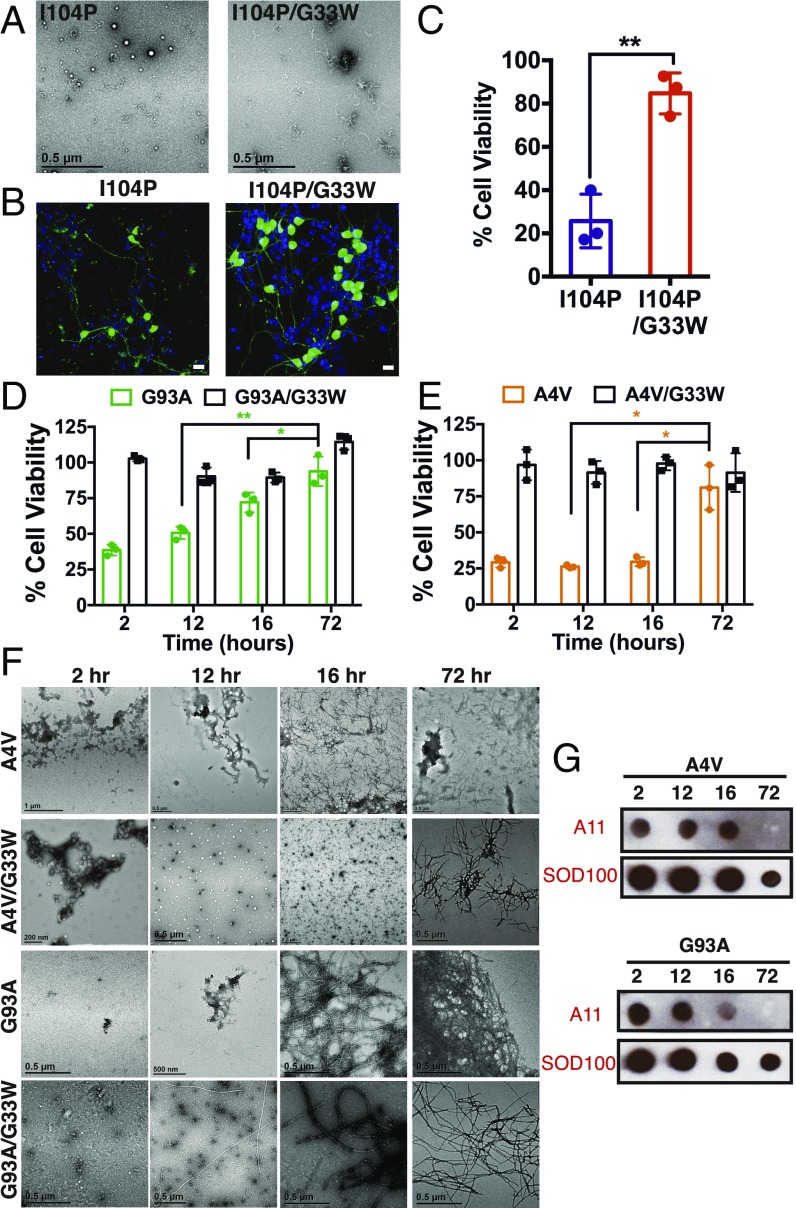
We asked if toxicity of full-length SOD1 derives from soluble oligomers or insoluble fibrils. We tested the cytotoxic effects of the non–fibril-forming mutant (I104P) (33). Even though it did not form any mature fibrils (Fig. 3A), it was toxic to motor neurons, and addition of the corkscrew-disrupting substitution, G33W, alleviated the cytotoxicity (Fig. 3 B and C). These results suggest that fibril formation is not essential for cytotoxicity.
Fig. 3.
Toxicity of full-length SOD1 derives from soluble oligomers. (A) Electron micrographs of a non–fibril-forming SOD1 mutant (I104P) and the corresponding double mutant (I104P/G33W) show some aggregates but no large fibrils. (B) Motor neurons treated with I104P lose neurites and have shrunken cell bodies (Left), but I104P/G33W-treated cells look healthy (Right). (Scale bars, 20 μm.) (C) Cell viability measured by an MTT reduction assay confirmed that I104P is toxic and I104P/G33W is nontoxic. Statistical significance was analyzed using a two-tailed t test with Welch’s correction. (D and E) Toxic properties of SOD1 mutants depend on the duration of aggregation. The A4V and G93A mutants aggregated for 12–16 h are toxic to motor neurons, whereas extended agitation for 72 h renders the proteins nontoxic. The corkscrew-disrupted proteins (A4V/G33W and G93A/G33W) are nontoxic irrespective of the duration of aggregation. Results are shown as mean ± SD (n = 3). Symbols represent individual values of triplicates, and bars represent average values. Statistical significance was analyzed by two-tailed t tests with Welch’s correction (*P < 0.05, **P < 0.01). (F) Representative electron micrographs of various preparations of the familial mutants A4V and G93A and the double mutants A4V/G33W and G93A/G33W. Some large aggregates can be seen at 2- to 16-h time points, but no fibrils can be seen, whereas all constructs show large fibril loads at 72 h. (G) Immunoblots of the familial mutants aggregated for different time points. Samples aggregated for 12–16 h are A11-positive, and both proteins lose A11 reactivity when aggregated for 72 h. SOD100 was used as a loading control.
To identify which species of SOD1 aggregate is toxic, we monitored the toxicity of various SOD1 mutants as their aggregates evolved over time. We found that both familial mutants (A4V and G93A) were toxic to cultured neurons when aggregated for 12–16 h but that extended aggregation for 72 h rendered the protein nontoxic (Fig. 3 D and E). A4V/G33W and G93A/G33W were nontoxic irrespective of the duration of aggregation. Negative-stain EM showed abundant fibrils in the samples aggregated for 72 h (Fig. 3F), and immunoblotting with the conformational oligomer-specific antibody (A11) suggested that samples aggregated for 72 h contained no oligomers (Fig. 3G). These results suggest that toxicity of aggregated SOD1 mutants derives from oligomers.
Whereas the relevance of our work to SOD1-related ALS seems clear, the relevance to non–SOD1-related ALS is less clear. A few reports suggest that wild-type SOD1 (WT) aggregates in sporadic ALS (34–36) analogous to mutant SOD1 aggregates in familial ALS; however, it is unknown if WT and mutant SOD1 aggregate by the same mechanism or have the same toxicity. We compared the aggregation and cytotoxic properties of WT and mutant SOD1. We used Thioflavin T, a dye that fluoresces upon binding to fibrils. We found that the fibril-forming behavior of WT, G93A, and G33W constructs was similar (SI Appendix, Fig. S8). We then performed a motor neuron cytotoxicity assay with protein aggregates from several time points. WT or G93A protein samples that were allowed to aggregate for up to 12 h were toxic, whereas the same protein preparations that were aggregated for 16 h or longer exhibited no toxicity (SI Appendix, Fig. S8 A and B). G33W, which cannot form the corkscrew, was nontoxic regardless of the extent of fibril formation (SI Appendix, Fig. S8C). Although there is limited evidence of WT aggregation in non–SOD1-linked ALS cases, our results suggest that WT and mutant SOD1 share similar mechanisms of cytotoxicity that depend on self-assembly of residues 28–38; this exposure may be enhanced by structural perturbations induced by familial mutations.
Corkscrew Disruption Alleviates Defects in a Danio rerio (Zebrafish) ALS Model.
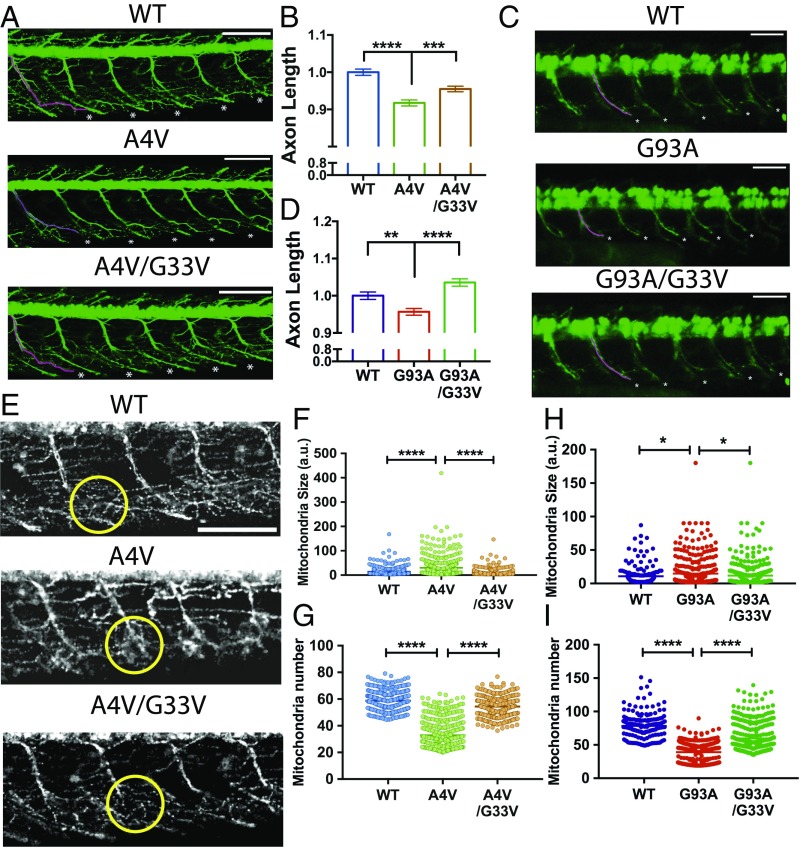
To determine whether corkscrew-disrupting mutations alleviate axonopathies caused by familial SOD1 mutants in vivo, we conducted experiments using a zebrafish model of ALS (37–40). We expressed A4V SOD1 with and without the corkscrew-disrupting substitution at Gly33 in a zebrafish TDL6 line in which the primary motor neurons are labeled with GFP and the mitochondria are labeled with dsRed (41). We analyzed the axons at 2 d postfertilization. The A4V mutation caused an 8% reduction in axon length (Fig. 4 A and B), as has been previously shown (39), but axon lengths of A4V/G33V-injected fish were significantly longer (Fig. 4 A and B). Additionally, we observed that 30% of A4V-injected fish were severely deformed and could not be imaged, suggesting that an acute phenotype is lethal (SI Appendix, Fig. S9C). In contrast, the WT and double-mutant–expressing fish did not display large mortality. We observed a similar phenotype upon expression of the G93A familial mutant; G93A-expressing zebrafish have 5% reduction in axon lengths, whereas axon lengths of G93A/G33V-expressing zebrafish were significantly longer (Fig. 4 C and D).
Fig. 4.
Corkscrew-disrupting substitution of G33V alleviates axonopathies in a D. rerio (zebrafish) ALS model. (A) Micrographs of zebrafish embryos at 2 d postfertilization (dpf) show a reduction in axon lengths of A4V-expressing embryos, whereas corkscrew-disrupted A4V/G33V-expressing embryos have significantly longer axon lengths. The first axon is highlighted in pink for clarity, and axons measured for quantification are marked by an asterisk. (Scale bars, 100 μm.) (B) Quantification of axon lengths shows that A4V-expressing embryos have shorter axons than WT-expressing embryos. The corkscrew-disrupting substitution G33V alleviates the defect. Results are shown as mean ± SEM relative to WT for at least 72 embryos. Statistical significance was analyzed by one-way ANOVA. (C) Micrographs of zebrafish embryos at 2 dpf show a reduction in axon lengths of G93A-expressing embryos, whereas corkscrew-disrupted G93A/G33V-expressing embryos have significantly longer axons. The first axon is highlighted in pink for clarity, and axons measured for length are marked by an asterisk. (Scale bars, 100 μm.) (D) Quantifications of axon lengths show that G93A-expressing fish have shorter axons than WT-expressing fish. The corkscrew-disrupting substitution G33V alleviates the defect. Results are shown as mean ± SEM relative to WT for axons 12–16 of at least 73 embryos. Statistical significance was analyzed by one-way ANOVA. (E) Micrographs show A4V-expressing zebrafish have impaired mitochondria, which are clustered at the branch points (encircled) compared with WT. The mitochondrial network of A4V/G33V-expressing fish is similar to WT. (Scale bar, 100 μm.) (F and G) Quantitative analysis of the mitochondrial network shows A4V-expressing fish have a larger mitochondrial size [30.17 arbitrary units (a.u.)] and diffused clustering (fluorescence intensity of 32 a.u.) in the axons, indicative of defective fission, whereas A4V/G33V-expressing fish have healthy mitochondria (size of 10.57 a.u. and fluorescence intensity of 54 a.u.) similar to WT (size of 14.08 a.u. and fluorescence intensity of 58 a.u.). Symbols represent individual measurements made for each group. Statistical significance was analyzed by one-way ANOVA. (H and I) Quantitative analysis of the mitochondria network shows G93A-expressing fish have larger, more diffusely clustered mitochondria (size of 14.68 a.u. and fluorescence intensity of 39 a.u.), indicative of defective fission. The mitochondrial network of G93A/G33V-expressing fish (size of 11.68 a.u. and fluorescence intensity of 59 a.u.) is similar to WT (size of 10.78 a.u. and fluorescence intensity of 78 a.u.). Statistical significance was analyzed by one-way ANOVA. Symbols represent individual measurements for each group (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Defects in mitochondrial assembly and trafficking, along with vacuolation (42, 43) and abnormal clustering in neuronal processes (44–46), are established pathological phenotypes observed in transgenic mice, patient-derived cells, and other models. However, they have not been reported in any zebrafish model of ALS thus far. Therefore, we analyzed the mitochondrial morphology upon expression of SOD1 familial mutants. Expression of A4V mutant protein caused remarkable mitochondrial pathology characterized by abnormal diffused clustering at the branch points indicative of defective mitochondria (Fig. 4E), whereas A4V/G33V-expressing fish had a mitochondrial network similar to WT fish. These defects were quantified by measuring the size and fluorescence intensity of the mitochondria, confirming that A4V-expressing fish displayed enlarged mitochondria (Fig. 4F and SI Appendix, Fig. S9), which were fewer in number (Fig. 4G and SI Appendix, Fig. S9). We also observed similar defects in the G93A-expressing zebrafish (Fig. 4 H and I and SI Appendix, Fig. S9). Thus, disrupting the corkscrew segment alleviates ALS-linked axonopathies and mitochondrial defects in this in vivo model.
Discussion
Our experiments suggest that segment 28–38 of SOD1 is important for SOD1-mediated toxicity. The crystal structure of this segment revealed an oligomer composed of antiparallel, out-of-register β-strands, which assemble into a corkscrew-like structure. The G33V and G33W point mutants, which were designed to disrupt the observed oligomer, alleviated toxicity of both the isolated peptide and full-length SOD1. In a zebrafish model of ALS, G33V prevented axonopathies and mitochondrial defects, two characteristic features of ALS-linked pathology. Taken together, these results suggest that the corkscrew structure is critical for SOD1-mediated cytotoxicity.
The corkscrew structure explains its oligomeric state and suggests the identity of its potential interacting partners in the cell. The corkscrew is composed of a single twisted sheet rather than pairs of tightly mated sheets, as observed in 88 steric zipper structures published thus far. A clue about the identity of the corkscrew’s interacting partners in the cell is offered by examining the functions of its structural homologs. A search for corkscrew homologs in the Protein Data Bank using the DALI server (47) yielded matches with other highly twisted β-sheet proteins, such as membrane receptor proteins, enzymes, and bactericidal-permeability increasing (BPI) protein (SI Appendix, Fig. S10). The twisted sheet seen in the crystal structure of BPI has been shown to bind lipids and destabilize membranes (48). It is conceivable that the cleft seen in the corkscrew structure is important for cytotoxicity, potentially as a binding site for lipids. The cleft of corkscrew is accessible to lipids and small molecules. In contrast, cylindrin has no accessible cleft, which could explain its lower cytotoxicity relative to corkscrew.
Our results demonstrate that toxicity derives from the corkscrew oligomers rather than from fibrils (SI Appendix, Fig. S11). Previously, we have shown that out-of-register oligomers are likely off-pathway from in-register fibril formation due to the large energetic cost of rearrangement of out-of-register oligomers into in-register fibrils (49). Although we cannot ascertain whether the fibrils observed in our experiments are in-register, our results are consistent with this hypothesis. The corkscrew-disrupting mutations of G33V/G33W attenuate cytotoxicity but do not attenuate fibril formation. Cytotoxicity assays of the non–fibril-forming mutant (I104P) and the time-course assays with the familial mutants (A4V and G93A) suggest that toxicity is a property of soluble oligomers, and not of large insoluble fibrils. These findings for SOD1 align with the hypotheses proposed by others for amyloid-β and huntingtin that large insoluble aggregates are relatively inert deposits (50–53).
From a molecular perspective, it would be unlikely to find ALS-linked mutations in the 28–38 segment of SOD1, given its structural importance for mediating toxicity. Indeed, compared with other regions of SOD1, this segment contains few familial mutations, and no mutations are found in the core of this segment spanning residues 32–36 (19). Notably, familial mutants, including G37R and the rare mutants V29A and V31A, are found near the ends of this segment. From our crystal structure, we infer that all these mutations are compatible with the corkscrew structure, although it is unclear if they actively promote oligomer assembly.
In summary, we have identified an 11-residue segment in ALS-associated SOD1 that is necessary for its cytotoxicity. Our data support the hypothesis that SOD1 forms toxic oligomers composed of antiparallel, out-of-register β-sheet structures involving residues 28–38. This cytotoxic segment may be a target for developing structure-based ALS therapeutics.
Materials and Methods
Crystals of SOD1(28–38) with P28K substitution were grown by hanging drop vapor diffusion using VDX plates (Hampton Research). Lyophilized peptide at 98% purity (Genscript, Inc.) was dissolved to 50 mg/mL in 50 mM Tris⋅base buffer. The reservoir solution contained 0.2 M sodium citrate (pH 5) and 13% PEG 6000. All SOD1 constructs were expressed recombinantly in Escherichia coli. Hb9:eGFP mouse embryonic stem cells were maintained and differentiated into motor neurons as previously described (32). Aggregated protein preparations were added to cultured neurons at the given final concentration, and viability was measured by MTT reduction assay. Details are provided in SI Appendix, Materials and Methods.
All zebrafish (Danio rerio) were maintained in accordance with standard laboratory conditions (23). The University of California, Los Angeles Chancellor’s Animal Research Committee approved all experiments performed on zebrafish.
Supplementary Material
Acknowledgments
We thank Lisa Johnson, David Borchelt, and Joan Valentine for discussions; Hamilton Trinh, Michael Collazo, Duilio Cascio, and staff at Argonne Photon Source, Northeastern Collaborative Access Team beamline 24-ID-E. We thank Hynek Wichterle (Columbia University) for the gift of Hb9-eGFP embryonic stem cells. We are grateful for the support to D.S.E. from the Howard Hughes Medical Institute, Department of Energy, and a grant from the National Institutes of Health (NIH) (AG029430). B.G.N. was supported by the University of California, Los Angeles (UCLA) Broad Center of Regenerative Medicine and Stem Cell Research, the Rose Hills Foundation, and grants from the National Institute of Neurological Disorders and Stroke (NS072804), Muscular Dystrophy Association (92901), and the California Institute for Regenerative Medicine (CIRM) (RB1-01367 and RB5-07480). C.M.K. was supported by grants from CIRM (RT307678) and the National Institute of General Medical Sciences (GM61721). M.T.B. was supported by grants from the NIH (AG047116) and the National Science Foundation (CHE-1301032 and CHE-1565941). S.S. was supported by a Whitcome Pre-Doctoral fellowship; K.L.A. was supported by the UCLA Cellular and Molecular Biology Training program (Ruth L. Kirschstein NIH Grant GM007185), a UCLA-California Institute for Regenerative Medicine Training Grant, and a UCLA Graduate Division Dissertation Year Fellowship; R.N. supported by a Larry L. Hillblom Foundation Fellowship; and C.K.J. was supported by a Beckman Research Scholarship.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5DLI and 5IIW).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705091114/-/DCSupplemental.
References
- 1.Alzheimer A. Über einen eigenartigen schweren Erkrankungsprozeβ der Hirnrincle. Neurol Central. 1906;25:1134. German. [Google Scholar]
- 2.Baglioni S, et al. Prefibrillar amyloid aggregates could be generic toxins in higher organisms. J Neurosci. 2006;26:8160–8167. doi: 10.1523/JNEUROSCI.4809-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisele YS, et al. Targeting protein aggregation for the treatment of degenerative diseases. Nat Rev Drug Discov. 2015;14:759–780. doi: 10.1038/nrd4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawaya MR, et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature. 2007;447:453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle MD, et al. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat Struct Mol Biol. 2016;23:409–415. doi: 10.1038/nsmb.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wälti MA, et al. Atomic-resolution structure of a disease-relevant Aβ(1-42) amyloid fibril. Proc Natl Acad Sci USA. 2016;113:E4976–E4984. doi: 10.1073/pnas.1600749113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthelot K, Ta HP, Géan J, Lecomte S, Cullin C. In vivo and in vitro analyses of toxic mutants of HET-s: FTIR antiparallel signature correlates with amyloid toxicity. J Mol Biol. 2011;412:137–152. doi: 10.1016/j.jmb.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Celej MS, et al. Toxic prefibrillar α-synuclein amyloid oligomers adopt a distinctive antiparallel β-sheet structure. Biochem J. 2012;443:719–726. doi: 10.1042/BJ20111924. [DOI] [PubMed] [Google Scholar]
- 9.Sandberg A, et al. Stabilization of neurotoxic Alzheimer amyloid-beta oligomers by protein engineering. Proc Natl Acad Sci USA. 2010;107:15595–15600. doi: 10.1073/pnas.1001740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen DR, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 11.Sangwan S, Eisenberg DS. Perspective on SOD1 mediated toxicity in amyotrophic lateral sclerosis. Postepy Biochem. 2016;62:362–369. [PubMed] [Google Scholar]
- 12.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775, and erratum (1995) 269:149. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 13.Taylor JP, Brown RH, Jr, Cleveland DW. Decoding ALS: From genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruijn LI, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 15.Bruijn LI, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 16.Oztug Durer ZA, et al. Loss of metal ions, disulfide reduction and mutations related to familial ALS promote formation of amyloid-like aggregates from superoxide dismutase. PLoS One. 2009;4:e5004. doi: 10.1371/journal.pone.0005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zetterström P, et al. Soluble misfolded subfractions of mutant superoxide dismutase-1s are enriched in spinal cords throughout life in murine ALS models. Proc Natl Acad Sci USA. 2007;104:14157–14162. doi: 10.1073/pnas.0700477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park Y-N, et al. Huntingtin fragments and SOD1 mutants form soluble oligomers in the cell. PLoS One. 2012;7:e40329. doi: 10.1371/journal.pone.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright GSA, Antonyuk SV, Kershaw NM, Strange RW, Samar Hasnain S. Ligand binding and aggregation of pathogenic SOD1. Nat Commun. 2013;4:1758. doi: 10.1038/ncomms2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durazo A, et al. Metal-free superoxide dismutase-1 and three different amyotrophic lateral sclerosis variants share a similar partially unfolded beta-barrel at physiological temperature. J Biol Chem. 2009;284:34382–34389. doi: 10.1074/jbc.M109.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw BF, et al. Local unfolding in a destabilized, pathogenic variant of superoxide dismutase 1 observed with H/D exchange and mass spectrometry. J Biol Chem. 2006;281:18167–18176. doi: 10.1074/jbc.M600623200. [DOI] [PubMed] [Google Scholar]
- 22.Taylor DM, et al. Tryptophan 32 potentiates aggregation and cytotoxicity of a copper/zinc superoxide dismutase mutant associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2007;282:16329–16335. doi: 10.1074/jbc.M610119200. [DOI] [PubMed] [Google Scholar]
- 23.Do TD, et al. Amyloid β-protein C-terminal fragments: Formation of cylindrins and β-barrels. J Am Chem Soc. 2016;138:549–557. doi: 10.1021/jacs.5b09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, et al. Quaternary structure defines a large class of amyloid-β oligomers neutralized by sequestration. Cell Rep. 2015;11:1760–1771. doi: 10.1016/j.celrep.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerf E, et al. Antiparallel β-sheet: A signature structure of the oligomeric amyloid β-peptide. Biochem J. 2009;421:415–423. doi: 10.1042/BJ20090379. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer W, Grönwall C, Jonsson A, Ståhl S, Härd T. Stabilization of a beta-hairpin in monomeric Alzheimer’s amyloid-beta peptide inhibits amyloid formation. Proc Natl Acad Sci USA. 2008;105:5099–5104. doi: 10.1073/pnas.0711731105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laganowsky A, et al. Atomic view of a toxic amyloid small oligomer. Science. 2012;335:1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenberg DS, Sawaya MR. Structural studies of amyloid proteins at the molecular level. Annu Rev Biochem. 2017;86:69–95. doi: 10.1146/annurev-biochem-061516-045104. [DOI] [PubMed] [Google Scholar]
- 29.Rakhit R, et al. An immunological epitope selective for pathological monomer-misfolded SOD1 in ALS. Nat Med. 2007;13:754–759. doi: 10.1038/nm1559. [DOI] [PubMed] [Google Scholar]
- 30.Liu H-N, et al. Targeting of monomer/misfolded SOD1 as a therapeutic strategy for amyotrophic lateral sclerosis. J Neurosci. 2012;32:8791–8799. doi: 10.1523/JNEUROSCI.5053-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentine JS, Hart PJ. Misfolded CuZnSOD and amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2003;100:3617–3622. doi: 10.1073/pnas.0730423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 33.Ivanova MI, et al. Aggregation-triggering segments of SOD1 fibril formation support a common pathway for familial and sporadic ALS. Proc Natl Acad Sci USA. 2014;111:197–201. doi: 10.1073/pnas.1320786110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruzman A, et al. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2007;104:12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forsberg K, et al. Novel antibodies reveal inclusions containing non-native SOD1 in sporadic ALS patients. PLoS One. 2010;5:e11552. doi: 10.1371/journal.pone.0011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guareschi S, et al. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc Natl Acad Sci USA. 2012;109:5074–5079. doi: 10.1073/pnas.1115402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramesh T, et al. A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease. Dis Model Mech. 2010;3:652–662. doi: 10.1242/dmm.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakowski SA, et al. Neuromuscular effects of G93A-SOD1 expression in zebrafish. Mol Neurodegener. 2012;7:44. doi: 10.1186/1750-1326-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Hoecke A, et al. EPHA4 is a disease modifier of amyotrophic lateral sclerosis in animal models and in humans. Nat Med. 2012;18:1418–1422. doi: 10.1038/nm.2901. [DOI] [PubMed] [Google Scholar]
- 40.Lemmens R, et al. Overexpression of mutant superoxide dismutase 1 causes a motor axonopathy in the zebrafish. Hum Mol Genet. 2007;16:2359–2365. doi: 10.1093/hmg/ddm193. [DOI] [PubMed] [Google Scholar]
- 41.Levesque MP, Krauss J, Koehler C, Boden C, Harris MP. New tools for the identification of developmentally regulated enhancer regions in embryonic and adult zebrafish. Zebrafish. 2013;10:21–29. doi: 10.1089/zeb.2012.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong PC, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 43.Shi P, Gal J, Kwinter DM, Liu X, Zhu H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2010;1802:45–51. doi: 10.1016/j.bbadis.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiskinis E, et al. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell. 2014;14:781–795. doi: 10.1016/j.stem.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vande Velde C, et al. Misfolded SOD1 associated with motor neuron mitochondria alters mitochondrial shape and distribution prior to clinical onset. PLoS One. 2011;6:e22031. doi: 10.1371/journal.pone.0022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magrané J, Cortez C, Gan W-B, Manfredi G. Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum Mol Genet. 2014;23:1413–1424. doi: 10.1093/hmg/ddt528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holm L, Rosenstrom P. Dali server: Conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545–W549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beamer LJ, Carroll SF, Eisenberg D. Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science. 1997;276:1861–1864. doi: 10.1126/science.276.5320.1861. [DOI] [PubMed] [Google Scholar]
- 49.Liu C, et al. Out-of-register β-sheets suggest a pathway to toxic amyloid aggregates. Proc Natl Acad Sci USA. 2012;109:20913–20918. doi: 10.1073/pnas.1218792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Treusch S, Cyr DM, Lindquist S. Amyloid deposits: Protection against toxic protein species? Cell Cycle. 2009;8:1668–1674. doi: 10.4161/cc.8.11.8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 52.Kuperstein I, et al. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martins IC, et al. Lipids revert inert Abeta amyloid fibrils to neurotoxic protofibrils that affect learning in mice. EMBO J. 2008;27:224–233. doi: 10.1038/sj.emboj.7601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.