Significance
Understanding what morphological and behavioral traits promote the success of diverse groups of organisms is a major goal of evolutionary biology. The ability to consume novel food sources has been linked to the spectacular radiation of herbivorous insects that eat terrestrial plants on Earth. Among the crustaceans, the arthropod group that dominates aquatic environments, relatively few major taxa have overcome the challenges of consuming primary producers (plants and macroalgae). However, lineages that include plant material in their diets support more species than their most closely related lineages. The results of our analyses support the hypothesis that a shift in diet promotes speciation in this diverse and ecologically important animal group.
Keywords: herbivory, crustaceans, diversification, speciation, arthropods
Abstract
About half of the world’s animal species are arthropods associated with plants, and the ability to consume plant material has been proposed to be an important trait associated with the spectacular diversification of terrestrial insects. We review the phylogenetic distribution of plant feeding in the Crustacea, the other major group of arthropods that commonly consume plants, to estimate how often plant feeding has arisen and to test whether this dietary transition is associated with higher species numbers in extant clades. We present evidence that at least 31 lineages of marine, freshwater, and terrestrial crustaceans (including 64 families and 185 genera) have independently overcome the challenges of consuming plant material. These plant-feeding clades are, on average, 21-fold more speciose than their sister taxa, indicating that a shift in diet is associated with increased net rates of diversification. In contrast to herbivorous insects, most crustaceans have very broad diets, and the increased richness of taxa that include plants in their diet likely results from access to a novel resource base rather than host-associated divergence.
The origin of novel traits that allow species to use a previously unexploited resource is widely used as an explanation for the high species richness in certain lineages of life on Earth. The hypothesis that these traits can be used to explain the variation in species richness among clades in the tree of life has been a major focus of evolutionary biology over the past decades (1, 2). Some of the most influential studies of trait-mediated diversification have focused on the morphological and behavioral innovations that allow access to a novel food resource and, in particular, the evolution of herbivory (3). Feeding on plants has long been considered an “evolutionary hurdle” (4) because of the low protein content of plant material relative to animal tissues and the presence of chemical and physical barriers to digesting plant material. Overcoming those hurdles was central to early hypotheses that plant feeding has promoted the high diversity seen in herbivorous insects (5). Formal tests of this hypothesis provided evidence that the shift to feeding on plant tissue is associated with higher species richness among the highly speciose orders of herbivorous insects (6) and with increased diversification in other organisms, including mammals (7) and dinosaurs (8).
The diversification of insects, in particular, once they began feeding on plant material, is thought to have given rise to much of the biological diversity on Earth today (9, 10), and there is an extensive literature that uses phylogenetic approaches to study the interaction between insect herbivores and their host plants (reviewed in refs. 11, 12). Increased diversity in the butterflies has been associated with shifts to feeding on more speciose plant groups (e.g., ref. 13) and to feeding on chemically distinct host plants (e.g., refs. 14 and 15). Among the beetles, the shift to feeding on angiosperms from the species-poor gymnosperms was associated with an increase in species richness by several orders of magnitude, leading Farrell (16) to title his study “Inordinate fondness: explained” in reference to J. B. S. Haldane’s well-known quote about the astounding diversity of beetles on Earth (17). More recent analyses, however, have found either that herbivory promotes insect diversification (18) or no evidence for herbivory per se promoting diversification among the beetles (19) and among all insects (3). Wiens et al. (20) showed that the degree to which herbivory can explain insect diversification rates varies among lineages, suggesting that the role of herbivory in promoting diversification will be best understood by the examination of a wide variety of plant-feeding taxa.
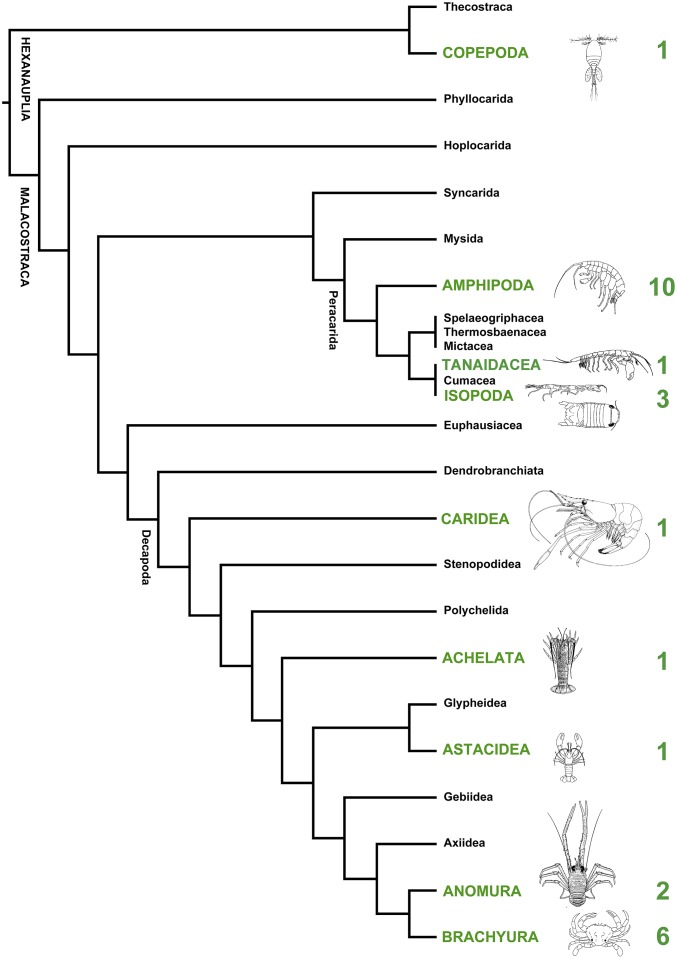
Here we test whether consuming live plant and macroalgal tissues is associated with higher species richness in the Crustacea, the other major group of arthropods that commonly consumes plant material (Fig. 1) and the lineage from which insects are derived (18). Crustaceans are abundant, species rich, and ecologically diverse in most aquatic systems (21). Herbivorous and omnivorous crustaceans play an important role in ecosystem functioning (22) and can affect the growth and abundance of primary producers on rocky intertidal shores (23), in kelp forests (24), seagrass beds (25), salt marshes (26), mangroves (27), freshwater wetlands (28), and in tropical forests on land (29). Herbivorous amphipods and isopods are particularly abundant on aquatic macrophytes and have been likened to insects because of their feeding mode, use of host plants as both food and shelter, and interactions with producer secondary metabolites (30). In common with herbivorous insects in terrestrial systems, herbivorous crustaceans in many aquatic systems are a major component of secondary production and are an important link to higher trophic levels (31). Crustacean herbivores did not arise until after the Devonian period, and Vermeij and Lindberg (32) conservatively estimated that herbivory arose independently three times in the isopods, five times in amphipods, and four times in brachyuran crabs. This repeated and independent evolution of plant feeding among the Crustacea gives us an opportunity to test whether plant feeding is associated with higher species richness in clades that now include plant material in their diets.
Fig. 1.
Crustaceans that feed on plants and macroalgae include (A) amphipods (Sunamphitoe femorata living in nests on the giant kelp Macrocystis pyrifera; image: I. Hinojosa), (B) isopods (Amphoroidea typa consuming the kelp Lessonia spicata), (C) crabs (Hemigrapsus crenulatus grazing on intertidal green algae), and (D) crayfish that feed on freshwater macrophytes (Pacifiastacus leniusculus; image: T. Renals).
In this study, we first provide a phylogenetic analysis of the distribution of plant feeding across all crustaceans and then use a sister-clade approach to test for shifts in species richness associated with plant feeding. We further test whether an evolutionary shift to plant feeding is associated with an increase in range size or geographic distribution. In general, niche breadth is positively associated with range size (33), and some explanatory models of insect diversification on higher plants assume that a shift to a broader range of diets will involve an increase in range size (34). The analysis of the geographic distributions of plant-feeding crustaceans and their sister clade also allows us to test whether any observed patterns in diversity are confounded by sampling localities (e.g., herbivores being more likely to be found in species-rich regions).
Results
Prevalence of Plant Feeding in the Crustacea.
Our review identified 185 genera from 64 families and five orders of marine, freshwater, and terrestrial Crustacea known to consume plant and macroalgal tissues (Table S1; detailed evidence for all genera is given in Dataset S1). Within each order, the number of plant-feeding taxa was a low proportion of the total number of genera and families (Table S1). Mapping the plant-feeding families onto available phylogenies (Fig. 2 and Fig. S1) provides a conservative estimate of 31 independent evolutionary transitions from a detritivorous and/or carnivorous diet to one that includes plant and macroalgal tissues (the most parsimonious hypothesized transitions are illustrated in Fig. S1).
Table S1.
The number of crustacean families and genera with evidence for feeding on live, multicellular macroalgae or vascular plants
| Order and suborder or infraorder | No. of families (%) | No. of genera (%) |
| Amphipoda | 22 (9) | 54 (3) |
| Gammaridae | 3 (7) | 4 (<1) |
| Senticaudata | 19 (18) | 50 (5) |
| Isopoda | 7 (5) | 22 (1) |
| Asellota | 1 (3) | 1 (<1) |
| Limnoriidea | 1 (33) | 3 (75) |
| Oniscoidea | 2 (5) | 2 (<1) |
| Sphaeromatidea | 1 (14) | 7 (5) |
| Valvifera | 2 (17) | 9 (9) |
| Tanaidacea | 1 (3) | 1 (<1) |
| Decapoda | 31 (15) | 103 (4) |
| Brachyura | 19 (17) | 79 (5) |
| Anomura | 6 (30) | 10 (4) |
| Achelata | 1 (30) | 1 (3) |
| Astacidea | 3 (50) | 10 (36) |
| Caridea | 2 (5) | 3 (<1) |
| Harpacticoida | 3 (5) | 5 (<1) |
| Total | 64 | 185 |
The full list of genera, types of plant material consumed, and types of evidence are given in Dataset S1.
Fig. 2.
Supertree of the Crustacea with major clades containing herbivores of macrophytes marked in green type and the minimum number of independent transitions to plant feeding (based on refs. 42, 74, and 75). The phylogenetic positions of transitions to plant feeding within these major crustacean taxa are illustrated in Fig. S1.
Contrast of Species Richness in Plant-Feeding and Sister Clades.
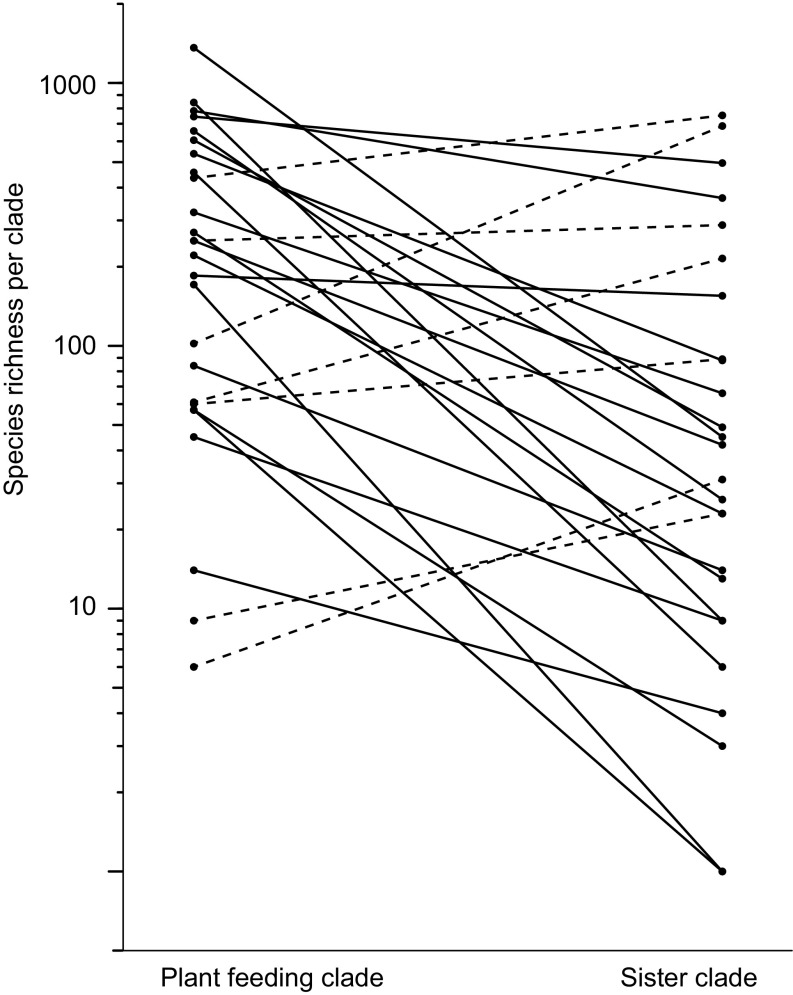
The species richness of plant-feeding clades was significantly greater than that of their sister clades, with the increase being 21-fold on average [mean ± SE: 21 ± 7.6, P = 0.005 with the diversity contrast test of Paradis (35), n = 26] (Fig. 3 and Table S2). Other statistical methods of comparing richness in sister contrasts gave similar results (Slowinski–Guyer test, χ2 = 103, P < 0.001; McConway–Sims test, χ2 = 50.4, P = 0.002). The higher richness in the plant-feeding clades remained when taxa known to feed only on macroalgae (i.e., that do not include angiosperms in their diet) were removed (P = 0.005, n = 17).
Fig. 3.
Contrasts of species richness in plant-feeding and sister clades (n = 26, P = 0.005). The solid lines are the 19 contrasts with more species in the plant-feeding clade; the dashed lines are the seven contrasts with fewer species in the plant-feeding clade.
Table S2.
Comparisons in species richness in crustacean clades that contain plant-feeding genera and their sister clades
| Plant-feeding clade | Sister clade | ||||
| Order | Clade | Species richness | Clade | Species richness | Ref. |
| Amphipoda | Ampithoidae | 185 | Corophiidae | 155 | 64 |
| Aoridae | 251 | Unciolidae | 42 | 64 | |
| Eophliantidae | 14 | Plioplateidae + Temnophliantidae | 4 | 63 | |
| Hyalidae + Najnidae + Hyalellidae + (Chiltonidae) | 251 | Talitridae | 288 | 63 | |
| Acanthogammaridae + Micruropodidae + (Typhlogammaridae) | 171 | Baikalogammaridae | 1 | 63 | |
| Gammaridae + Anisogammaridae + (Luciobliviidae) | 457 | Phreatogammaridae | 6 | 63 | |
| Crangonyctidae | 221 | Pseudocrangonyctidae | 23 | 63 | |
| Gammarellidae | 6 | Bathyporeiidae | 31 | 63 | |
| Calliopiidae +Pontogeneidae | 270 | Hornelliidae | 13 | 63 | |
| Nuuanuidae + Maeridae + (Melitdae) | 538 | Hadziidae | 88 | 63 | |
| Isopoda | Asselidae | 322 | Stenasselidae | 66 | 82 |
| Limnoriidae | 57 | Hadromastacidae | 3 | 65 | |
| Sphaeromatidae | 657 | Tecticipitidae + Ancinidae | 26 | 65 | |
| Tanaidacea | Tanaidae: Anatanaini | 45 | Tanaidae: Pancolini | 9 | 83 |
| Harpacticoida | Thalestridae: Thalestrinae | 84 | Thalestridae: Eudacytlopusinae | 14 | 84 |
| Decapoda | Palinuridae | 60 | Scyllaridae | 89 | 42 |
| Astacidae + Cambaridae + Parasticidae | 606 | Nephropidae | 49 | 42 | |
| Aeglidae | 61 | Lomisidae + Eumunididae + Kiwaidae + Chirostylidae | 215 | 67 | |
| Paguridae + Diogenidae + Coenobitidae + Lithodidae + Parapaguridae) + (Hapalogastridae) | 1362 | Pylochelidae | 45 | 67 | |
| Inachidae + Epialtidae + Majiidae | 843 | Corystidae | 9 | 68 | |
| Grapsidae + (Ocypodidae) + Gecarcinidae + Plagusiidae + Sesarmidae + Varunidae + Macrophthalmidae + Percnidae + (Mictyridae) + (Glypotograpsidae) + (Xenograpsidae) + (Cryptochiridae) | 783 | Dotillidae + Pinnotheridae | 365 | 68 | |
| Portunidae + Polybiidae + Menippidae (without Pseudocarcinus) | 435 | Calappidae + Parthenopidae + Dorippidae + Leucosiidae | 753 | 68 | |
| Xanthidae + Panopeidae | 745 | Pilumnidae + Galenidae + Tanaocheleidae + Goneplacidae | 496 | 68 | |
| Eriphiidae | 9 | Mathildellidae | 23 | 68 | |
| Trichodactylidae | 57 | Orithyiidae | 7 | 68 | |
| Hippolytidae | 102 | Alpheidae | 686 | 69 | |
The genera that have evidence for plant feeding within each family are listed in Dataset S1. The references are phylogenetic studies that identify the sister clades for each plant-feeding clade. The hypothesized transitions to plant feeding are illustrated in Fig. S1. Families in parentheses were not found in our review to include plants or macroalgae in their diets but are included in the plant-feeding clade as the most parsimonious explanation for the transition to plant feeding from detritivorous or predatory ancestors.
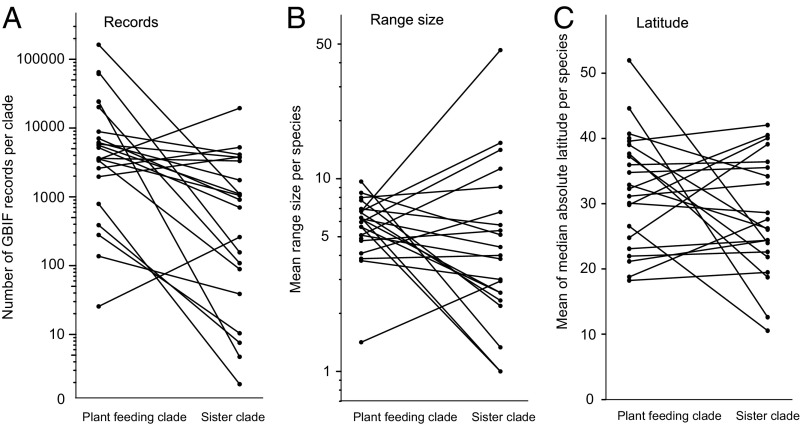
Although the plant-feeding clades were more frequently collected by biologists than their non–plant-feeding sister clades [more records per species in the Global Biodiversity Information Facility (GBIF) database, ratio t test, t = 4.97, P < 0.01] (Fig. 4A), the sister clades were very widely distributed (Fig. S2) and, on average, were very well represented in biological collections with a mean ± SE of 6,727 ± 1,827 records per clade in the Ocean Biogeographic Information System (OBIS) database and 2,364 ± 986 records per clade in the GBIF database. The diversity contrast test was robust when the seven contrasts that include the taxa most likely to be data deficient (having fewer than 100 records in the OBIS or GBIF databases, P = 0.023) were removed and when increasing numbers of contrasts were removed at random. The median probability of resampled diversity contrast tests did not exceed 0.05 until 12 randomly selected contrasts were removed from the total of 26 (Fig. S3). Thus we consider our result robust to the identity of any single contrast or to the possibility that any single sister clade was falsely classified as not plant feeding because of limited knowledge about its diet.
Fig. 4.
Contrasts of plant-feeding and sister clades in (A) the number of records, (B) mean range size per species, and (C) mean of the median absolute latitude (degrees from the equator) of species from occurrence records in the GBIF database. Range size is the number of 1° latitude × 1° longitude blocks in which presence is recorded.
Global Distributions of Plant-Feeding Clades.
We detected no association between plant feeding and range sizes, estimated from the number of 1° latitude × 1° longitude blocks in which each species within each clade had been recorded in the GBIF database (ratio t test, t = 0.51, P = 0.61) (Fig. 4B). This result remains unchanged when considering estimates of range size at larger spatial scales (i.e., the number of 5° × 5° and 10° × 10° cells) (all t tests, t < 1.2, P > 0.2). The larger number of species in the plant-feeding clades resulted in these clades having a larger global distribution, occupying more 1° × 1° cells in the GBIF database (ratio t test, t = 2.72, P = 0.01) (Fig. S4B).
In both hemispheres the latitudes from which the plant-feeding and their sister clades are found broadly overlap (Fig. 4B). With occurrence data from the GBIF database, we used the median of absolute latitude (i.e., degrees from the equator) for each species as a latitudinal midpoint, averaged these across all species within a clade, and used a standardized difference between these means (Hedge’s g) for each pair of plant-feeding and sister clades. Combining all differences in a meta-analysis provided an estimate of an overall difference that did not differ from zero (95% CI: −0.04 to 0.77). We thus consider it unlikely that our contrast of diversity between plant-feeding and their sister clades is confounded by any latitudinal patterns in the distribution of each clade type. For marine species, the plant-feeding and sister clades are widely distributed across biogeographic regions (Fig. S2), occurring on average in 8.3 of the 12 major realms defined by Spalding et al. (36). We detected no association between clade type (plant-feeding and their sister clades) and the number of contrasts within each biogeographic realm (χ2 = 4.23, df = 11, P = 0.96).
Discussion
Marine, freshwater, and terrestrial crustaceans have repeatedly overcome the challenges of consuming plant material, and those plant-feeding taxa are, on average, 21-fold more speciose than their sister taxa, supporting the hypothesis that a shift in diet is associated with increased diversification.
Prevalence of Plant Feeding in Crustaceans.
The phylogenetic distribution of plant-feeding crustacean taxa indicates that inclusion of plant material in the diet is a derived trait that has evolved multiple times from the ancestral diets of detritus and animal material. Our analyses with the latest available phylogenies suggest at least 31 independent evolutionary transitions from a detritivorous and/or carnivorous diet to a diet that incorporates live plant material, increasing the conservative estimate by Vermeij and Lindberg (32) of 12 times (three in the isopods, five in amphipods, and four in brachyuran crabs). Multiple independent transitions to plant-feeding diets also have been documented for other taxa, including insects (∼25 origins of phytophagy in ref. 3), molluscs (32, 37), birds (38), and fish (39).
The concentration of plant feeding largely within three orders (of the more than 60 orders of extant Crustacea) is similar to the phylogenetic distribution of phytophagy in insects, in which it is found in relatively few orders (6). When considering all arthropods, the chelicerates and myriapods are primarily predators or scavengers, and within the crustacean clade [which includes insects (18)], consumption of multicellular primary producers is prominent only in the derived clades of the Hexapoda and Malacostraca. This paucity of plant-feeding clades supports the idea that the ability to consume plant material represents an evolutionary hurdle (4). The low proportion of major lineages that feed on macroalgae is not explained by lack of opportunity, with multicellular thalli present for ≈800 My and most marine herbivores (crustaceans, molluscs, echinoderms, and fish) arising only after the Paleozoic period (250 Mya) (32) but before the colonization of the sea by angiosperms ∼100 Mya. Similarly, multicellular plants were present on land from the Ordovician (470 Mya), whereas crustaceans did not colonize those habitats until much later [terrestrial amphipod, isopod, and crab fossils are known only from the Eocene (49 Mya) or later (40)]. Bousfield (41) estimated that most amphipod families had evolved by the Cretaceous, with both marine and freshwater groups presumed present in the Jurassic (150 Mya). Isopods and decapods are estimated to have diverged in the Ordovician–Silurian, although both groups do not attain the greatest diversity until the Mesozoic (42, 43). The actual timing of transitions to a diet including plant material is obviously more recent than the origin of the higher taxonomic groups considered here (44), so estimating the timing of these diet transitions will have to be inferred from time-calibrated phylogenies based on extant clades [as done recently for the amphipod family Gammaridae (45)].
Although there is an extensive literature examining the feeding biology of many crustaceans (Dataset S1), these reports are unevenly distributed among taxa, and the diets of most of the more than 67,000 species (46) are not known precisely (although much can be inferred from functional morphology). Uncertainty and variation in the diets of species within these clades and the possibility that diets of the sister clades are unknown have the potential to underestimate the number of times herbivory has arisen and either under- or overestimate the magnitude by which plant feeding has promoted diversity (3). There were relatively few families within our plant-feeding clades for which we found no evidence of plant feeding, but these families could have plant-, animal-, or detritus-based diets. Given our use of available phylogenies (many at the family level), there is certainly variation in feeding modes within the large groups we considered plant-feeding, both among species within the clade and within species, because many are omnivorous. We do not assume that every species within a plant-feeding clade includes plant material in its diet but conservatively estimate that there has been at least one transition to herbivory (or partial herbivory) in that clade.
Association Between Plant Feeding and Species Richness.
Twenty-six independent sister comparisons provided clear evidence for higher species richness in the plant-feeding clades, supporting the hypothesis that plant feeding is an important trait that has promoted net diversification rates (i.e., speciation rates minus extinction rates). Similar tests with replicated contrasts of clades have shown that diversification can be promoted with the evolution of morphological traits, e.g., floral nectar spurs (47), ornamental traits involved in sexual selection (e.g., ref. 48), and traits that facilitate species interactions, e.g., defense mutualisms in plants (49), in addition to traits associated with dietary innovations.
The repeated transitions to plant feeding indicate selective advantages from exploiting plant material as a food source. Adaptive hypotheses to explain plant feeding, recently reviewed by Sanchez and Trexler (50), include intake efficiency (the use of a sedentary resource, often as both habitat and food, limiting the costs associated with finding mobile animal prey), the ability to inhabit areas with a high biomass of primary producers but few animal prey, the high lipid concentrations in algae, and a lower likelihood of disease transmission from parasites in animal prey. Despite the nutritional differences between macroalgae and angiosperms, most (19 of 26) plant-feeding clades in our review included both in their diets, and the diversity contrast remained robust when those that fed only on macroalgae were removed.
Plant consumption may favor increased diversification because plants represent a new adaptive zone (an abundant and diverse resource that was previously unavailable to consumers), coevolution of specialized herbivores with their host plants, and a “parasitic” lifestyle in which herbivores of limited mobility are more likely to have subdivided populations because of patchy distributions of their hosts (11). Of these competing, although not necessarily mutually exclusive, hypotheses, we consider the higher richness in plant-feeding crustaceans to be most likely explained by these animals having access to a new adaptive zone with a more widespread resource base. This notion is consistent with the increased diversification in generalist mammalian herbivores accessing plant diets (7) and in three of four major clades of coral reef fish (Acanthuroidei, Chaetodontidae, Labridae, and Pomacentridae) when shifting to diets that include low-quality foods (algae, detritus, sponge, and corals) (39).
In stark contrast with herbivorous insects, plant-feeding crustaceans are almost all generalist consumers, able to consume material from many orders and several phyla of primary producers and also animal material and detritus when available (51, 52). Consequently, the mechanisms underlying host-associated divergence common in plant-feeding insects are unlikely to be important for plant-feeding crustaceans. The specialized and intimate associations between most insects and their hosts increases the likelihood of genetic linking between host use and mate choice and the likelihood that differences among populations will occur because of the patchy distribution of plants. Approximately half of speciation events among insects are estimated to be associated with shifts of specialized herbivores onto novel plant taxa (11, 34). However, the degree to which the initial transition to plant feeding has resulted in an increased diversification rate is unclear, with recent sister-clade comparisons failing to find any evidence for increased diversity among phytophagous beetles [eight contrasts (19)], and, for all insects, any effects of dietary ecology on species richness [26 contrasts (3)]. This finding contrasts with the positive association between plant feeding and species richness in the earlier influential study of Mitter et al. (6) using 13 sister contrasts across five orders of insects and with the more recent analyses of Wiens et al. (20) using phylogenetic generalized least-squares regression techniques.
The contrast of richness among sister clades is a powerful approach for testing hypotheses regarding diversification. It is important to note, however, that this approach is correlative (not causative), compares the net rate of cladogenesis (i.e., speciation minus extinction), and is sensitive to uncertainties in known sister relationships. We used recently available phylogenies for the Crustacea derived from both morphological and molecular data, but there is considerable uncertainty in the relationships among many higher taxa (44). With improved phylogenies that include both insects and the crustaceans from which they derived (18), there will be the opportunity to test further the role of feeding biology in the evolution of the Arthropoda. If the relationships among all taxa of interest are described within a single phylogeny, a series of more sophisticated analytical approaches to the sister-clade approach (including state-dependent speciation-minus-extinction models, e.g., ref. 53) or the more recent tests of Rabosky and Huang (54) could address the rates and timing of trait evolution.
Further differences between the plant–animal interactions of the largely aquatic crustaceans and the largely terrestrial insects also reduce the likelihood of host-associated divergence (51, 55) and may explain the lower global diversity of crustaceans as compared with insects. Given the central role of plant diversity in promoting insect diversity (13), the lower richness of aquatic primary producers [≈10,000 species of macroalgae in contrast to the ∼300,000 species of plants (55)] is likely to limit the opportunities of host-associated divergence. The higher structural and chemical diversity within angiosperms offers more opportunities for herbivores to specialize on plant parts (e.g., stems vs. leaves vs. seeds and internal vs. external feeding). The parasitic lifestyle common among insects, in which individual herbivores often live on a single plant, is comparatively rare among crustaceans, because crustacean life spans commonly exceed that of their algal diet. Few species feed internally within macrophyte tissues (56), and there are no parallels with the diverse lineages of insects with piercing and sucking mouthparts. Finally, with a higher potential for dispersal and gene flow in an aquatic medium, localized distributions that would promote divergence are less likely. Unlike holometabolous insects in which oviposition behavior frequently determines larval diets, most decapod crustaceans have dispersive larvae, and offspring habitats are decoupled from adult behavior [although brooding crustaceans (amphipods and isopods) could potentially place offspring on selected hosts (57)].
With few crustaceans having specialized diets, the evolutionary transition to plant feeding is, in most cases, a shift to a broader diet that includes plants in addition to other material. Expanded diets are predicted to promote greater local abundance and a larger range size and then net diversification through a greater probability of allopatric speciation (58). Our analyses contrasted the general pattern of larger range sizes with wider resource use (33, 59), with no evidence for difference in range sizes between plant-feeding and sister clades. The links between niche breadth and range size are complicated by sampling issues and the phylogenetic independence among sampling units (60). Our sister contrasts provide tests of range size that control for phylogeny, but variation in sampling effort, whereby widespread taxa are more likely be the focus of studies that document diets, has the potential to influence overall patterns. Although our dataset cannot entirely exclude this explanation, the sister clades in our analyses were themselves mostly higher taxa (families and family groups) with very broad geographic distributions (Fig. S2) and in many cases were the subject of large numbers of ecological studies that document animal or detrital diets.
The higher richness of plant-feeding clades also could arise from geographic patterns in species richness if either plant-feeding or sister clades were sampled disproportionately from regions with high richness. We consider confounding by uneven sampling unlikely, because neither the occurrence of clades within biogeographic realms nor mean latitudes differed between plant-feeding and their sister clades (Fig. 4), and there are no clear gradients of increasing crustacean richness with decreasing latitude (61).
Conclusions
The repeated, independent transitions to a diet that includes plants and/or macroalgae are associated with higher species richness in a diverse selection of aquatic and terrestrial crustaceans and support the hypothesis that feeding on plants has promoted diversification among animal consumers. Although few studies have examined the role of evolutionary history in crustacean diets, existing studies do indicate a strong phylogenetic signal for feeding behavior and ecology in herbivorous crustaceans (52, 59, 62) and among other grazers of aquatic macrophytes [e.g., opisthobranch molluscs (37) and fish (39)]. Promising avenues of research will include tests of how phylogeny explains the composition of plant diets, tests of morphological changes associated with diet shifts, contrasts between marine and freshwater systems in which crustaceans coexist with insect herbivores, and tests for associations between herbivore richness and the diversity of available plants.
Materials and Methods
Review of Plant Feeding in the Crustacea.
From a literature search, we extracted a list of crustacean genera that include species known to feed on live, multicellular tissue from macroalgae and/or vascular plants [following the definition used by Mitter et al. (6) for plant-feeding insects and Vermeij and Lindberg (32) for marine herbivores]. The search terms and criteria for the evidence for plant feeding are detailed in the SI Text.
Contrast of Species Richness in Plant-Feeding and Sister Clades.
We tested whether plant feeding is associated with higher species richness in the Crustacea by contrasting the number of species in the clades that include plant-feeding genera with the number of species in their sister clades. Clades were predominantly families or groups of families, given the taxonomic resolution of the most recently published phylogenies for higher-level taxa among the amphipods (63, 64), isopods (65, 66), and decapods (42, 67–69). The methods for determining which taxa to include in each sister contrast are detailed in the SI Text. This sister-clade approach has been widely used (35) and does not require a detailed fossil record (which is poor for many groups of crustaceans) or a complete phylogeny for the entire group in focus (currently unavailable for the Crustacea at the taxonomic resolution required). The approach has the advantage of including the independent, replicated contrasts needed to test whether the repeated evolution of a trait is associated with changes in species richness and to control for differences in diversity expected for clades of varying age (70).
We contrasted the species richness in plant-feeding and sister clades with the likelihood ratio test developed by Paradis (35) in the package ‘ape’ in R (71). For amphipods and isopods, the currently accepted taxonomy and number of species in each clade were established from the World Amphipoda Database (marinespecies.org/amphipoda) and the World List of Marine Freshwater and Terrestrial Isopod Crustaceans (marinespecies.org/isopoda). For tanaids, copepods, and decapods, we used the World Register of Marine Species (WoRMS; marinespecies.org), with the exception of freshwater crayfish, whose species numbers were taken from refs. 72 and 73. To test how robust our results were to the identity of individual taxa included in the sister comparisons, we repeated the analyses (i) with only taxa that consumed angiosperms, (ii) excluding poorly studied taxa that are likely to have undescribed diets, and (iii) with individual taxa randomly removed (details of these resampling methods are given in the SI Text).
Global Distributions of Plant-Feeding Clades.
The global distributions of plant-feeding clades and their sister clades were contrasted (i) to test whether evolutionary shifts to plant feeding have facilitated increases in range size and (ii) to test the likelihood of our contrasts being confounded by possible regional differences in richness (latitude, biogeographic regions) or niche breadth [e.g., as varies with latitude in brachyuran crabs (59)]. Although declining species richness with increasing latitude is not the universal pattern among crustacean taxa (61), the potential exists for the plant-feeding clades to have been sampled disproportionately from regions with higher richness. We extracted the latitude and longitude of all available occurrence records for species within each clade from the GBIF (www.gbif.org/) and OBIS (www.iobis.org) databases. The methods for estimating range size and for contrasting range size, mean latitudes, and occurrence in biogeographic realms between plant-feeding and sister clades are detailed in the SI Text.
SI Materials and Methods
Review of Plant Feeding in the Crustacea.
Published examples of plant feeding in the Crustacea were found with a literature search in the ISI Web of Science (www.webofknowledge.com) and Google Scholar (https://scholar.google.com) with the following search terms: (Crustacea* or amphipod* or isopod* or crab* or lobster* or shrimp* or copepod*) and (graz* or herbivor*). These searches were supplemented with the reference lists of papers found, the authors’ own libraries, and published reviews of crustacean diets (52, 76–80). Evidence for plant feeding was derived from direct observations in the field, feeding assays in the laboratory, or examination of gut contents. Rarely, we included evidence from the analysis of stable isotopes when it unambiguously indicated macrophyte consumption. Omnivorous taxa are included, but only when gut contents were quantified and indicated that macroalgal or seagrass consumption was not accidental or incidental (using a threshold of 10% or more by volume or biomass). We excluded taxa that are known to feed only on detrital plant material or dead wood, because these consumers usually target decomposed material, and their nutrition is highly dependent on the fungi and bacteria associated with these resources. We excluded taxa that are known only to scrape epiphytes and biofilms from the surface of aquatic macrophytes, because this food resource is a complex mixture of bacteria, detritus, microalgae, and fungi.
Contrast of Species Richness in Plant-Feeding and Sister Clades.
Our review provided 26 independent contrasts of plant-feeding and sister clades. For each contrast, the families included in the plant-feeding and sister clades and the published phylogeny on which sister clades were allocated are detailed in Table S2 and illustrated in Fig. S1. Some plant-feeding clades include taxa nested within the clade for which our review found no evidence of plant feeding. We included these clades because doing so yielded the most parsimonious explanation for the transition to plant feeding from a detritivorous or predatory ancestor (i.e., the lowest number of transitions) (detailed in Fig. S1). Families for which there is good evidence for plant feeding but in which sister relationships are not known and families in which the sister clades included a mix of both taxa with evidence for plant feeding and taxa with no evidence for plant feeding are listed in our review of plant-feeding genera in Table S1 but were not included in the formal contrasts of species richness (Table S2).
To test whether the inclusion of plant material from angiosperms (terrestrial plants, seagrass, saltmarsh, and mangroves) could result in a different evolutionary outcome from the consumption of only macroalgal material, we repeated the diversity contrast test after excluding clades (n = 9) that consumed only macroalgae. With our crustacean clades being defined as plant feeding by the presence of published evidence of plant consumption (the evidence is summarized in Table S1), the potential exists for a biased sampling effect; that is, well-studied taxa are more likely to have exhaustively documented diets and thus to be classified as plant feeding, whereas lesser-known taxa are likely to have undescribed diets. This bias could occur if taxa occur in geographic regions more likely to be sampled by biologists. To test whether our results were affected by the inclusion of poorly known clades, we used the number of records in the OBIS database (available at www.iobis.org) for each marine taxon and in the GBIF database (available at www.gbif.org) for marine, terrestrial, and freshwater taxa as a proxy for the likelihood of taxa being encountered by biologists. We then repeated the contrast of species richness without the sister clades most likely to be data deficient (the six clades that had fewer than 100 records in either the OBIS or GBIF global databases). Furthermore, we tested the robustness of our results to the identity of any single contrast between plant-feeding and sister clades by removing increasing numbers of randomly chosen contrasts. We calculated the frequency distribution of P values from the likelihood ratio test after randomly dropping one contrast, repeated 1,000 times. We then repeated this calculation after dropping two contrasts, three contrasts, and more in turn.
Global Distributions of Plant-Feeding Clades.
Range size for the species in each clade was estimated by the number of 1° latitude × 1° longitude cells in which species were recorded, and the mean of these range sizes per clade was calculated. The mean range sizes of the plant-feeding and sister clades then were contrasted with a ratio t test in the package mratios in R. We then repeated this analysis with estimates of range size calculated as the number of 5° × 5° cells and 10° × 10° cells (estimates at larger scales are less likely to be affected by high sampling intensity within certain regions). The latitudinal distribution of plant-feeding and sister clades was contrasted by calculating the median of the absolute value of latitude for all species in a clade (i.e., median degrees from the equator) and then averaging these values. Plant-feeding and sister clades were then contrasted using a meta-analysis of the 26 contrasts, using the standardized difference in means for the plant-feeding and sister clade (Hedge’s g) and an intercept-only model in the package metafor in R (81). This method provided an estimate of the overall difference between groups, which was contrasted with zero (expected if plant-feeding and sister clades had similar latitudinal differences).
The biogeographic distribution of the marine clades was examined by counting the number of records from OBIS that fell within each of the 12 realms defined in Spalding et al. (36). The shape file for the ecoregions was downloaded (www.marineregions.org), and the number of coordinates within each realm was counted using the R package GISTools. The number of plant-feeding and sister clades per realm in the dataset was contrasted across the 12 realms with a two-way contingency table.
SI Results
Our review identified plant feeding in 64 families and 185 genera of marine, freshwater, and terrestrial Crustacea (Table S1). Although plant feeding occurs among many genera and families, the evidence for plant feeding is restricted to few higher level taxa, i.e., to only five orders (Amphipoda, Decapoda, Copepoda, Isopoda, and Tanaidacea) from the more than 60 orders of extant crustaceans. Plant feeding is best documented in the Amphipoda, in which species from many unrelated families consume macroalgae and seagrass in aquatic habitats, and in the Decapoda, in which true crabs, hermit crabs, lobsters, and crayfish frequently consume angiosperms and macroalgae in aquatic and terrestrial habitats. Among the Isopoda, plant-feeding species are abundant in coastal habitats but are restricted to very few families (particularly the Idoteidae and Sphaeromatidae).
The highest generic richness was among the decapods, with 103 genera from 31 families with plant feeding found in each of several major clades: the true crabs (Brachyura), hermit crabs and their allies (Anomura), spiny lobsters (Achelata), clawed lobsters (Astacidea), and shrimps (Caridea) (Table S1). Among the peracarids, feeding on macroalgal and plant material was found in 54 genera and 22 families of amphipods from two suborders, in 22 genera from seven families of isopods from three suborders, and rarely in the tanaidaceans (with only one report of seagrass seed consumption) (Table S1). Among the copepods, consumption of macroalgal tissue is limited to six genera from three families in the Harpacticoida, whose species are known to burrow into algal tissues (Table S1). For all groups, the percentage of families known to include plant-feeding genera is low (3–15%) relative to the total number of families within each of these orders whose species either feed on detrital or animal material or have unknown diets (Table S1).
The supporting evidence for the consumption of living, multicellular tissue from macroalgae or higher plants for each genus is detailed in Dataset S1.
Supplementary Material
Acknowledgments
We thank J. Fordyce, N. Whiteman, and anonymous reviewers for comments that have improved this article and G. Poore for advice on isopod phylogenies.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706399114/-/DCSupplemental.
References
- 1.Hunter JP. Key innovations and the ecology of macroevolution. Trends Ecol Evol. 1998;13:31–36. doi: 10.1016/s0169-5347(97)01273-1. [DOI] [PubMed] [Google Scholar]
- 2.Jezkova T, Wiens JJ. What explains patterns of diversification and richness among animal phyla? Am Nat. 2017;189:201–212. doi: 10.1086/690194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rainford JL, Mayhew PJ. Diet evolution and clade richness in hexapoda: A phylogenetic study of higher taxa. Am Nat. 2015;186:777–791. doi: 10.1086/683461. [DOI] [PubMed] [Google Scholar]
- 4.Strong DR, Lawton JH, Southwood R. Insects on Plants. Community Patterns and Mechanisms. Harvard Univ Press; Cambridge, MA: 1984. [Google Scholar]
- 5.Southwood TRE. The insect/plant relationship–An evolutionary perspective. Symposium of the Royal Entomological Society of London. 1973;6:3–25. [Google Scholar]
- 6.Mitter C, Farrell B, Wiegmann B. The phylogenetic study of adaptive zones: Has phytophagy promoted insect diversification? Am Nat. 1988;132:107–128. [Google Scholar]
- 7.Price SA, Hopkins SSB, Smith KK, Roth VL. Tempo of trophic evolution and its impact on mammalian diversification. Proc Natl Acad Sci USA. 2012;109:7008–7012. doi: 10.1073/pnas.1117133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett PM, Butler RJ, Nesbitt SJ. The roles of herbivory and omnivory in early dinosaur evolution. Earth Environ Sci Trans R Soc Edinb. 2010;101:383–396. [Google Scholar]
- 9.Ehrlich PR, Raven PH. Butterflies and plants: A study in coevolution. Evolution. 1964;18:586–608. [Google Scholar]
- 10.Turcotte MM, Davies TJ, Thomsen CJM, Johnson MTJ. Macroecological and macroevolutionary patterns of leaf herbivory across vascular plants. Proc Biol Sci. 2014;281:20140555. doi: 10.1098/rspb.2014.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler IS, Mitter C. The phylogenetic dimension of insect-plant interactions: A review of recent evidence. In: Tilmon K, editor. Specialization, Speciation, and Radiation: The Evolutionary Biology of Herbivorous Insects. University of California; Berkley: 2008. pp. 240–263. [Google Scholar]
- 12.Futuyma DJ, Agrawal AA. Macroevolution and the biological diversity of plants and herbivores. Proc Natl Acad Sci USA. 2009;106:18054–18061. doi: 10.1073/pnas.0904106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janz N, Nylin S, Wahlberg N. Diversity begets diversity: Host expansions and the diversification of plant-feeding insects. BMC Evol Biol. 2006;6:4. doi: 10.1186/1471-2148-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fordyce JA. Host shifts and evolutionary radiations of butterflies. Proc Biol Sci. 2010;277:3735–3743. doi: 10.1098/rspb.2010.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheat CW, et al. The genetic basis of a plant-insect coevolutionary key innovation. Proc Natl Acad Sci USA. 2007;104:20427–20431. doi: 10.1073/pnas.0706229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrell BD. “Inordinate fondness” explained: Why are there so many beetles? Science. 1998;281:555–559. doi: 10.1126/science.281.5376.555. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson GE. Homage to Santa Rosalia or why are there so many kinds of animals? Am Nat. 1959;93:145–159. [Google Scholar]
- 18.Regier JC, et al. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature. 2010;463:1079–1083. doi: 10.1038/nature08742. [DOI] [PubMed] [Google Scholar]
- 19.Hunt T, et al. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science. 2007;318:1913–1916. doi: 10.1126/science.1146954. [DOI] [PubMed] [Google Scholar]
- 20.Wiens JJ, Lapoint RT, Whiteman NK. Herbivory increases diversification across insect clades. Nat Commun. 2015;6:8370. doi: 10.1038/ncomms9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiel M, Watling L, editors. 2015. The Life Styles and Feeding Biology of the Crustacea, The Natural History of the Crustacea Series (Oxford Univ Press, Oxford) Vol 2.
- 22.Duffy JE, et al. Biodiversity mediates top-down control in eelgrass ecosystems: A global comparative-experimental approach. Ecol Lett. 2015;18:696–705. doi: 10.1111/ele.12448. [DOI] [PubMed] [Google Scholar]
- 23.Sousa WP. Experimental investigations of disturbance and ecological succession in a rocky intertidal algal community. Ecol Monogr. 1979;49:227–254. [Google Scholar]
- 24.Poore AGB, et al. Major consequences of minor damage: Impacts of small grazers on fast-growing kelps. Oecologia. 2014;174:789–801. doi: 10.1007/s00442-013-2795-4. [DOI] [PubMed] [Google Scholar]
- 25.Cook K, Vanderklift MA, Poore AGB. Strong effects of herbivorous amphipods on epiphyte biomass in a temperate seagrass meadow. Mar Ecol Prog Ser. 2011;442:263–269. [Google Scholar]
- 26.Alberti J, et al. Abiotic stress mediates top-down and bottom-up control in a Southwestern Atlantic salt marsh. Oecologia. 2010;163:181–191, and erratum (2011) 167:883. doi: 10.1007/s00442-009-1504-9. [DOI] [PubMed] [Google Scholar]
- 27.Cannicci S, et al. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat Bot. 2008;89:186–200. [Google Scholar]
- 28.Nyström P, Strand J. Grazing by a native and an exotic crayfish on aquatic macrophytes. Freshw Biol. 1996;36:673–682. [Google Scholar]
- 29.Green PT, O’Dowd DJ, Lake PS. Control of seedling recruitment by land crabs in rain forest on a remote oceanic island. Ecology. 1997;78:2474–2486. [Google Scholar]
- 30.Hay ME, Duffy JE, Pfister CA, Fenical W. Chemical defense against different marine herbivores: Are amphipods insect equivalents? Ecology. 1987;68:1567–1580. doi: 10.2307/1939849. [DOI] [PubMed] [Google Scholar]
- 31.Edgar GJ, Shaw C. The production and trophic ecology of shallow-water fish assemblages in southern Australia III. General relationships between sediments, seagrasses, invertebrates and fishes. J Exp Mar Biol Ecol. 1995;194:107–131. [Google Scholar]
- 32.Vermeij GJ, Lindberg DR. Delayed herbivory and the assembly of marine benthic ecosystems. Paleobiology. 2000;26:419–430. [Google Scholar]
- 33.Slatyer RA, Hirst M, Sexton JP. Niche breadth predicts geographical range size: A general ecological pattern. Ecol Lett. 2013;16:1104–1114. doi: 10.1111/ele.12140. [DOI] [PubMed] [Google Scholar]
- 34.Janz N. Ehrlich and Raven revisited: Mechanisms underlying codiversification of plants and enemies. Annu Rev Ecol Evol Syst. 2011;42:71–89. [Google Scholar]
- 35.Paradis E. Shift in diversification in sister-clade comparisons: A more powerful test. Evolution. 2012;66:288–295. doi: 10.1111/j.1558-5646.2011.01429.x. [DOI] [PubMed] [Google Scholar]
- 36.Spalding MD, et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. Bioscience. 2007;57:573–583. [Google Scholar]
- 37.Williams ST, Donald KM, Spencer HG, Nakano T. Molecular systematics of the marine gastropod families Trochidae and Calliostomatidae (Mollusca: Superfamily Trochoidea) Mol Phylogenet Evol. 2010;54:783–809. doi: 10.1016/j.ympev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Olsen AM. Exceptional avian herbivores: Multiple transitions toward herbivory in the bird order Anseriformes and its correlation with body mass. Ecol Evol. 2015;5:5016–5032. doi: 10.1002/ece3.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lobato FL, et al. Diet and diversification in the evolution of coral reef fishes. PLoS One. 2014;9:e102094. doi: 10.1371/journal.pone.0102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunlop JA, Scholtz G, Selden PA. Water-to-Land Transitions. Arthropod Biology and Evolution. Springer; Berlin: 2013. pp. 417–439. [Google Scholar]
- 41.Bousfield EL. An updated phyletic classification and palaeohistory of the Amphipoda. In: Schram FR, editor. Crustacean Phylogeny. Balkema; Rotterdam: 1983. pp. 257–277. [Google Scholar]
- 42.Bracken-Grissom HD, et al. The emergence of lobsters: Phylogenetic relationships, morphological evolution and divergence time comparisons of an ancient group (Decapoda: Achelata, Astacidea, Glypheidea, Polychelida) Syst Biol. 2014;63:457–479. doi: 10.1093/sysbio/syu008. [DOI] [PubMed] [Google Scholar]
- 43.Lins LSF, Ho SYW, Wilson GDF, Lo N. Evidence for Permo-Triassic colonization of the deep sea by isopods. Biol Lett. 2012;8:979–982. doi: 10.1098/rsbl.2012.0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ahyong ST, Crustacean evolution. The Natural History of the Crustacea: Evolution and Biogeography, eds Thiel M, Poore GCB (Oxford Univ Press, Oxford), in press.
- 45.Hou Z, Sket B. A review of Gammaridae (Crustacea: Amphipoda): The family extent, its evolutionary history, and taxonomic redefinition of genera. Zool J Linn Soc. 2016;176:323–348. [Google Scholar]
- 46.Ahyong ST, et al. Subphylum Crustacea Brünnich, 1772. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa. 2011;3148:165–191. doi: 10.11646/zootaxa.3703.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Hodges SA, Arnold ML. Spurring plant diversification: Are floral nectar spurs a key innovation? Proc R Soc Lond B Biol Sci. 1995;262:343–348. [Google Scholar]
- 48.Maia R, Rubenstein DR, Shawkey MD. Key ornamental innovations facilitate diversification in an avian radiation. Proc Natl Acad Sci USA. 2013;110:10687–10692. doi: 10.1073/pnas.1220784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber MG, Agrawal AA. Defense mutualisms enhance plant diversification. Proc Natl Acad Sci USA. 2014;111:16442–16447. doi: 10.1073/pnas.1413253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez JL, Trexler JC. The adaptive evolution of herbivory in freshwater systems. Ecosphere. 2016;7:e01414. [Google Scholar]
- 51.Hay ME, Steinberg PD. 1992. The chemical ecology of plant-herbivore interactions in marine versus terrestrial communities. Herbivores: Their Interaction with Secondary Metabolites. eds Rosenthal GA, Berenbaum MA (Academic, San Diego), Vol 2: Evolutionary and Ecological Processes, pp 371–413.
- 52.Poore AGB, Hill NA, Sotka EE. Phylogenetic and geographic variation in host breadth and composition by herbivorous amphipods in the family Ampithoidae. Evolution. 2008;62:21–38. doi: 10.1111/j.1558-5646.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 53.FitzJohn RG, Maddison WP, Otto SP. Estimating trait-dependent speciation and extinction rates from incompletely resolved phylogenies. Syst Biol. 2009;58:595–611. doi: 10.1093/sysbio/syp067. [DOI] [PubMed] [Google Scholar]
- 54.Rabosky DL, Huang H. A robust semi-parametric test for detecting trait-dependent diversification. Syst Biol. 2016;65:181–193. doi: 10.1093/sysbio/syv066. [DOI] [PubMed] [Google Scholar]
- 55.Vermeij GJ. Plant defences on land and in water: Why are they so different? Ann Bot. 2016;117:1099–1109. doi: 10.1093/aob/mcw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mejaes BA, Poore AGB, Thiel M. 2015. Crustaceans inhabiting domiciles excavated from macrophytes and stone. The Life Styles and Feeding Biology of the Crustacea, The Natural History of the Crustacea Series, eds Thiel M, Watling L (Oxford Univ Press, Oxford), Vol 2, pp 118–144.
- 57.Poore AGB, Steinberg PD. Preference-performance relationships and effects of host plant choice in an herbivorous marine amphipod. Ecol Monogr. 1999;69:443–464. [Google Scholar]
- 58.Gaston KJ. The Structure and Dynamics of Geographic Ranges. Oxford Univ Press; Oxford: 2003. [Google Scholar]
- 59.Papacostas KJ, Freestone AL. Latitudinal gradient in niche breadth of brachyuran crabs. Glob Ecol Biogeogr. 2016;25:207–217. [Google Scholar]
- 60.Gaston KJ, Blackburn TM, Lawton JH. Interspecific abundance-range size relationships: An appraisal of mechanisms. J Anim Ecol. 1997;66:579–601. [Google Scholar]
- 61.Chaudhary C, Saeedi H, Costello MJ. Bimodality of latitudinal gradients in marine species richness. Trends Ecol Evol. 2016;31:670–676. doi: 10.1016/j.tree.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Best RJ, Stachowicz JJ. Phylogeny as a proxy for ecology in seagrass amphipods: Which traits are most conserved? PLoS One. 2013;8:e57550. doi: 10.1371/journal.pone.0057550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lowry JK, Myers AA. A phylogeny and classification of the Senticaudata subord. nov. Crustacea: Amphipoda) Zootaxa. 2013;3610:1–80. doi: 10.11646/zootaxa.3610.1.1. [DOI] [PubMed] [Google Scholar]
- 64.Myers AA, Lowry JK. A phylogeny and a new classification of the Corophiidea Leach, 1814 (Amphipoda) J Crustac Biol. 2003;23:443–485. [Google Scholar]
- 65.Brandt A, Poore GCB. Higher classification of the flabelliferan and related Isopoda based on a reappraisal of relationships. Invertebr Syst. 2003;17:893–923. [Google Scholar]
- 66.Poore GCB. Isopoda Valvifera: Diagnoses and relationships of the families. J Crustac Biol. 2001;21:205–230. [Google Scholar]
- 67.Bracken-Grissom HD, et al. A comprehensive and integrative reconstruction of evolutionary history for Anomura (Crustacea: Decapoda) BMC Evol Biol. 2013;13:128. doi: 10.1186/1471-2148-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsang LM, et al. Evolutionary history of true crabs (Crustacea: Decapoda: Brachyura) and the origin of freshwater crabs. Mol Biol Evol. 2014;31:1173–1187. doi: 10.1093/molbev/msu068. [DOI] [PubMed] [Google Scholar]
- 69.Li CP, De Grave S, Chan TY, Lei HC, Chu KH. Molecular systematics of caridean shrimps based on five nuclear genes: Implications for superfamily classification. Zool Anz. 2011;250:270–279. [Google Scholar]
- 70.Barraclough TG, Vogler AP, Harvey PH. Revealing the factors that promote speciation. Philos Trans R Soc Lond B Biol Sci. 1998;353:241–249. [Google Scholar]
- 71.Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 72.De Grave S, et al. A classification of living and fossil genera of decapod crustaceans. Raffles Bull Zool. 2009;Suppl 21:1–109. [Google Scholar]
- 73.Ahyong ST. 2014. Diversity and distribution of Australian freshwater crayfish with a check-list of the world Parastacidae and a key to the genera (Decapoda, Astacidea, Parastacoidea). Advances in Freshwater Decapod Systematics and Biology. Crustaceana Monographs 19, eds Yeo DCJ, Cumberlidge N, Klaus S (Brill, Leiden, The Netherlands), pp 245–271.
- 74.Richter S, Scholtz G. Phylogenetic analysis of the Malacostraca (Crustacea) J Zoological Syst Evol Res. 2001;39:113–136. [Google Scholar]
- 75.Oakley TH, Wolfe JM, Lindgren AR, Zaharoff AK. Phylotranscriptomics to bring the understudied into the fold: Monophyletic ostracoda, fossil placement, and pancrustacean phylogeny. Mol Biol Evol. 2013;30:215–233. doi: 10.1093/molbev/mss216. [DOI] [PubMed] [Google Scholar]
- 76.Lodge DM. Herbivory on freshwater macrophytes. Aquat Bot. 1991;41:195–224. [Google Scholar]
- 77.Wolcott DA, O’Connor NJ. Herbivory in crabs: Adaptations and ecological considerations. Am Zool. 1992;32:370–381. [Google Scholar]
- 78.Brawley SH. Mesoherbivores. In: John DM, Hawkins SJ, Price JH, editors. Plant-Animal Interactions in the Marine Benthos. Clarendon; Oxford: 1992. pp. 235–263. [Google Scholar]
- 79.Linton SM, Greenaway P. A review of feeding and nutrition of herbivorous land crabs: Adaptations to low quality plant diets. J Comp Physiol B. 2007;177:269–286. doi: 10.1007/s00360-006-0138-z. [DOI] [PubMed] [Google Scholar]
- 80.Jormalainen V. Grazers of macroalgae and higher plants. In: Thiel M, Watling L, editors. The Life Styles and Feeding Biology of the Crustacea, The Natural History of the Crustacea Series. Vol 2. Oxford Univ Press; Oxford: 2015. pp. 502–534. [Google Scholar]
- 81.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 82.Martin JW, Davis GE. Historical trends in crustacean systematics. Crustaceana. 2006;79:1347–1368. [Google Scholar]
- 83.Sotka EE, Bell T, Hughes LE, Lowry JK, Poore AGB. A molecular phylogeny of marine amphipods in the herbivorous family Ampithoidae. Zool Scr. 2017;46:85–95. [Google Scholar]
- 84.Willen E. Phylogeny of the Thalestridimorpha Lang, 1944 (Crustacea, Copepoda) Cuvillier; Göttingen, Germany: 2000. [Google Scholar]
- 85.Sieg J. Taxonomische monographie der Tanaidae Dana 1849 (Crustacea: Tanaidacea) Abh Senckenberg Natforsch Ges. 1980;537:1–267. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.