Abstract
Key message
Discriminatory co-expression of maize BBM and WUS transcriptional factor genes promoted somatic embryogenesis and efficient Agrobacterium -mediated transformation of recalcitrant maize inbred B73 and sorghum P898012 genotypes without use of a selectable marker gene.
Abstract
The use of morphogenic regulators to overcome barriers in plant transformation is a revolutionary breakthrough for basic plant science and crop applications. Current standard plant transformation systems are bottlenecks for genetic, genomic, and crop improvement studies. We investigated the differential use of co-expression of maize transcription factors BABY BOOM and WUSCHEL2 coupled with a desiccation inducible CRE/lox excision system to enable regeneration of stable transgenic recalcitrant maize inbred B73 and sorghum P898012 without a chemical selectable marker. The PHP78891 expression cassette contains CRE driven by the drought inducible maize RAB17M promoter with lox P sites which bracket the CRE, WUS, and BBM genes. A constitutive maize UBI M promoter directs a ZsGreen GFP expression cassette as a reporter outside of the excision sites and provides transient, transgenic, and developmental analysis. This was coupled with evidence for molecular integration and analysis of stable integration and desiccation inducible CRE-mediated excision. Agrobacterium-mediated transgenic introduction of this vector showed transient expression of GFP and induced somatic embryogenesis in maize B73 and sorghum P898012 explants. Subjection to desiccation stress in tissue culture enabled the excision of CRE, WUS, and BBM, leaving the UBI M::GFP cassette and allowing subsequent plant regeneration and GFP expression analysis. Stable GFP expression was observed in the early and late somatic embryos, young shoots, vegetative plant organs, and pollen. Transgene integration and expression of GFP positive T0 plants were also analyzed using PCR and Southern blots. Progeny segregation analysis of primary events confirmed correlation between functional GFP expression and presence of the GFP transgene in T1 plants generated from self pollinations, indicating good transgene inheritance. This study confirms and extends the use of morphogenic regulators to overcome transformation barriers.
Keywords: Maize, Sorghum, Morphogenic regulators, BABY BOOM, WUSCHEL2
Introduction
Cereal crops arguably feed the world and are grown in greater quantities on more land across more diverse ecosystems than any other crop (Borlaug 2002; FAOSTAT 2012). Over the past three decades, the ability to accomplish heritable genetic transformation of plants has been essential to basic science in plant biology and to myriad crop applications essential to worldwide agriculture and world food supply. Stable genetic transformation of the major cereal crops, such as specific varieties of maize, rice, wheat, and barley (Casas et al. 1993; Cheng et al. 1997; Dai et al. 2001; Gordon-Kamm et al. 1990; Hiei et al. 1997; Ishida et al. 1996; Ritala et al. 1994; Somers et al. 1992; Vasil et al. 1992; Zhao et al. 2000; Popelka and Altpeter 2003; Tingay et al. 1997) by standard technologies has been achieved, but major obstacles remain for genotypes important to genomic analyses and further crop applications. Now, the ability to conduct genome editing in plants (Altpeter et al. 2016; Svitashev et al. 2016) has created an increased need for more efficient and genotype-independent plant transgenic biology. To become more broadly significant, a pan-application systems approach to plant biology and genomics is now being actuated (Altpeter et al. 2016). However, these efforts have been stymied by technological limitations because of inherent features of standard plant transgenics. Plant transformation has been encumbered by several bottlenecks (Altpeter et al. 2016) including genotype and varietal dependence, explant sources and specific cell culture dependence, as well as laborious, expensive, and time-consuming technologies. This is especially true for the cereal crops. In addition, standard transformation biology involves the random stable insertion of a transgene into the genome with the use of a selectable marker transgene. The process to produce and analyze standard stable plant transgenics can involve years of effort, backcrossing into relevant germplasm, intensive analysis, and costly infrastructure. In addition, to apply the science commercially occupies massive resources and funds dedicated to regulatory approval. The need to overcome these obstacles is apparent (Altpeter et al. 2016).
These bottlenecks include numerous practical key factors for transformation of monocots whether Agrobacterium, biolistic, or protoplast mediated. These obstacles comprise extensive tissue culture genotype dependence, plant age and type of target tissue, types of vectors, promoters and other UTRs, preferred codon usage, reliance upon and type of selectable marker, Agrobacterium strains (when used), inoculation and co-cultivation conditions, and plant regeneration processes. Crucially, obviating these impediments involves achieving genotype and explant independence. Early studies suggested that embryogenic cultures or immature embryo explants are the most suitable and are commonly used for cereal crop transformation (Gordon-Kamm et al. 1990; Hiei et al. 1994; Ishida et al. 1996; Luppotto et al. 1999; Negrotto et al. 2000; Frame et al. 2002; Huang et al. 2004; Huang and Wei, 2005; Hiei et al. 2006; Vega et al. 2008). However, these techniques usually rely upon specific genotypes (such as Hi type II in maize, Bobwhite in wheat, Nipponbare in rice, etc.) to achieve successful transformation. Genotypes such as the maize inbred B73 and various sorghum cultivars remain either totally recalcitrant to transformation or transformation that is so inefficient as to be impractical. B73 is of particular interest as it provides the reference maize genome and has a significant history as an important genetic resource for breeding and genomic studies. While one report showed transformation of B73 via meristem culture, the transformation frequency was very low (Zhang et al. 2002) and this procedure has not become routine. Likewise, sorghum transformation by standard techniques is possible but at low frequencies and is also genotype and explant dependent.
Multiple molecular, biological, and developmental reports have shown that several morphogenic genes are involved in plant cell division, somatic embryogenesis, organogenesis, and plant regeneration. Expression of somatic embryogenesis receptor-like kinase1 (SERK1) (Schmidt et al. 1997), Leafy cotyledon1 (LEC1) and LEC2 (Harada 2001), Baby boom (BBM) (Boutilier et al. 2002), Maize ovule developmental protein 2 (ODP2) (Svitashev et al. 2016), Agamous-like15 (Harding et al. 2003), and WUSCHEL (WUS2) (Zuo et al. 2002; Bouchabke-Coussa et al. 2013) demonstrates morphogenic control of plant development. Recently, a technological breakthrough has been made by selectively and differentially co-expressing morphogenic regulators BBM and WUS2 genes, leading to successful direct somatic embryogenesis from various explant sources of several monocot crops including commercial maize inbred lines, sorghum, rice, and sugarcane (Lowe et al. 2016). Specific overexpression of morphogenic regulators has also recently been shown to improve monocot transformation in several commercial genotypes (Lowe et al. 2016) and facilitate gene editing (Svitashev et al. 2016).
BBM encodes an AP2/ERF transcription factor involved with root, seed, basal embryo, and shoot meristem development, and was identified first via subtractive hybridization in Brassica napus embryogenic microspore-derived cultures (Boutilier et al. 2002). Boutilier et al. showed that overexpression of BBM in transgenic Arabidopsis thaliana resulted in the development of ectopic somatic embryos. BBM overexpression in Populus tomentosa (Deng et al. 2009) and Theobroma cacao (Florez et al. 2015) has also been shown to promote somatic embryogenesis. The Pennisetum squamulatum apospory specific genomic region of PsASGR-BBML was shown to promote embryo formation without fertilization in a sexual tetraploid plant (Conner et al. 2015). More recently, in monocots, transgenic expression driven by a constitutively expressed BBM transcription factor was observed to confer its influence in a cell-autonomous manner (Lowe et al. 2016). These studies indicate that BBM may play a general role in the maintenance of meristematic stem cells in an undifferentiated state, and provide the basis for improved transformation.
WUS is a homeodomain-containing transcription factor, which has been shown to be required for stem cell specification in plants and is involved in early embryogenesis, organogenesis, and flowering (Laux et al. 1996). When ectopically expressed, WUS promotes somatic embryogenesis and enlarged meristems. The expression of WUS is restricted to a subset of meristematic cells subtending the stem cells during all stages of embryogenesis. This expression pattern may indicate that the WUS protein is involved with maintenance in cell fate by a diffusion gradient acting in a non-cell-autonomous fashion. Ectopic expression of WUS may facilitate morphogenic alterations in plant development.
Lowe et al. (2016) demonstrated that using a combination of BBM and WUS2 overexpression, they could overcome genotype dependence, and to some extent, explant dependence for transformation of selected genotypes of maize, sorghum, rice, and sugar cane. Their results showed a strong dependence on the promoters used to drive the morphogenic regulators BBM and WUS2. Using a strongly expressing promoter (Oleosin) in conjunction with WUS2 led to chimeric and often necrotic callus which regenerated only non-transformed plants. Using a combination strategy of pairing the strongly expressed maize Ubiquitin (UBI M) promoter (Christensen et al. 1992) with BBM and a weakly expressed nopaline synthase (NOS AT) promoter from Agrobacterium tumefaciens with WUS2 allowed for stable homogeneous embryogenic callus formation facilitated by phosphinothricin selection. This vector also contained CRE driven by the desiccation inducible RAB17M promoter with loxP sites bracketing the CRE, WUS, and BBM transgenes. CRE (Creates Recombination) is a well-studied approach to site-specific recombination involving the loxP sites (Odell et al. 1990). Desiccation-induced expression of CRE resulted in the excision of the CRE:WUS:BBM cassette allowing subsequent plant regeneration.
The present study aimed to confirm and extend the previous reports using morphogenic regulators to establish genetic transformation of B73 and sorghum P898012 via co-expression of maize WUS2 and BBM genes without the use of an exogenous selective agent. This study also shows increased frequency of transformation of recalcitrant varieties using this approach.
Materials and methods
Plant material
B73 and sorghum public genotype P898012 seeds were obtained from Germplasm Resources Information Network (GRIN: http://www.ars-grin.gov/). Seeds were planted in 3-gallon pots using Pro-mix soil and greenhouse grown at 28/21 °C, and a 16-h light/8-h dark photoperiod. Plants were self-pollinated, and maize ears or sorghum immature embryo explants were collected 9–11 days after pollination. Additional explants including young leaves from aseptically grown seedlings and whole imbibed seeds were also used.
Morphogenic vector design and Agrobacterium strains
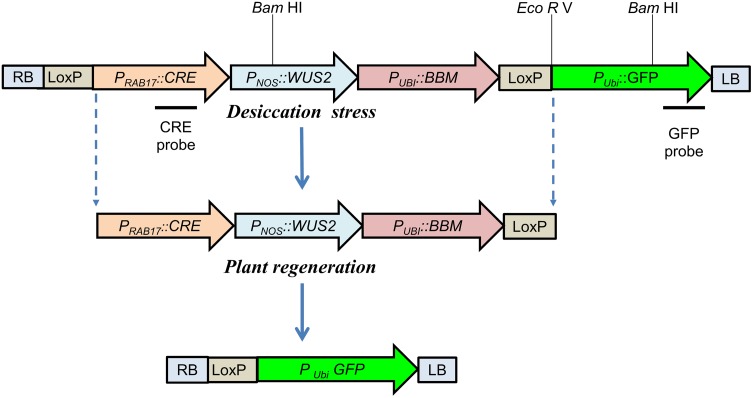
Figure 1 shows a diagrammatic representation of the PHP78891 vector. The PHP78891 vector comprises four expression cassettes: (1) RAB17M:CRE; (2) NOS At:WUS2; (3) UBIM:BBM; and (4) UBIM:GFP. The CRE:WUS2:BBM cassette is bracketed by lox P sites. One lox P site is flanked by the right Agrobacterium border and the other flanks the UBI M:GFP cassette within the left Agrobacterium border.
Fig. 1.
PHP78891 vector. PHP78891 vector comprises four expression cassettes: (1) a RAB17M: CRE; (2) a NOS At:WUS2; (3) UBI M:BBM; and (4) UBI M: GFP. The CRE:WUS2:BBM cassette is bracketed by lox P sites. One lox P site is flanked by Agrobacterium T-DNA right border (RB) and the other flanks the UBI M: GFP cassette within Agrobacterium T-DNA left border (LB)
Agrobacterium tumefaciens strains AGL1 and EHA 101 were used for maize and sorghum transformation, respectively. The AGL1-SVB strains harbor the PHP78891 binary vector as well as the super-binary vector pTOK233 (Hiei et al. 1994). The AGL1 strain without PHP78891 was used as a negative control for B73 experiments; the AGL1-SVB strain and AGL1 harboring just PHP78891 were used in comparative B73 transformation experiments. EHA 101 harboring the PHP78891 plasmid was used in all experiments on sorghum. PJLU13 was used as a negative control for GFP expression in sorghum and does not contain the lox P: CRE: WUS:BBM: lox P cassette. PJLU13 has a ubiquitinRice promoter driving expression of a GFP gene and a nopaline synthase termination signal. Another negative control was PYU2593 consisting of ubiquitinMaize promoter driving a GFP gene with a nopaline synthase termination signal.
Plant transformation
Plant transformation for maize and sorghum followed published protocols (Vega et al. 2008; Do et al. 2016 for maize and sorghum, respectively) with minor modifications.
Agrobacterium culture growth, inoculation, and co-cultivation
For both B73 and P898012 transformation, 5 days before co-cultivation, Agrobacterium AGL1 or EHA101 glycerol stocks were streaked onto YEP medium (5 g/L yeast extract, 10 g/L peptone, 5 g/L NaCl, 15 g/L agar, pH 7.0) plates with appropriate antibiotics and incubated at 28 °C in the dark for 3 days. Single colonies were selected and streaked onto fresh YEP plates with the same antibiotics and incubated for 2–3 days. One loop of bacterial culture was suspended in 5 mL of liquid infection medium (Zm-1 for maize, Sb-1 of sorghum) (Tables 1, 2, respectively). Inoculated cultures were shaken at 100 rpm for 4 h at room temperature. Cell density was adjusted to optical density (OD) = 0.35–0.40 at 550 nm using inoculation media. Isolated immature embryos (50–70 per tube) were washed with liquid medium, and replaced with Agrobacterium suspension (1 mL) and incubated for 2–5 min. After inoculation, embryos were poured on to the Zm-2 for maize or Sb-2 for sorghum medium plates (Tables 1, 2, respectively). Excess liquid medium was removed thoroughly from the co-cultivation plates, and the embryos were orientated adaxial (scutellar) side up and incubated at 20 °C (for maize) or 25 °C (for sorghum) in darkness for 3 days. Embryos were transferred to Zm-3 (for maize) or Sb-3 (for sorghum) resting medium (Tables 1, 2) and cultured for 7 days. B73 calli were subcultured to fresh Zm-4 medium (Table 1) biweekly whereas P898012 calli were subcultured every 10 days to fresh Sb-4 medium (Table 2). Neither the maize or sorghum media included a selective agent.
Table 1.
Medium compositions for maize B73 experiments
| Medium name and number | Medium name | Media components |
|---|---|---|
| Zm-1 | Inoculation | N6 salts 4.0 g/L (Chu et al. 1975), sucrose 68.5 g/L, glucose 36.0 g/L, l-proline 700 mg/L, 2,4-D 1.5 mg/L, MES 0.5 mg/L, pH 5.2, Eriksson’s vitamins mix (100×) 1 mL, thiamine HCL 1 mg/L and acetosyringone 1-mL from 100-mM concentration |
| Zm-2 | Co-cultivation | N6 salts 4.0 g/L, sucrose 30 g/L, l-proline 700 mg/L, 2,4-D 1.5 mg/L, MES 0.5 mg/L, pH 5.8, gelrite 3 g/L, Eriksson’s vitamins (100×) 1 ml, Myo-inositol 100 mg/L and acetosyringone 1 mL from 100- mM concentration |
| Zm-3 | Resting | N6 salts 4.0 g/L, sucrose 30 g/L, l-proline 700 mg/L, 2,4-D 1.5 mg/L, MES 0.5 mg/L, pH 5.8, gelrite 3 g/L, Eriksson’s vitamins (100×) 1 ml, SH vitamins (100×) 1 ml, Myo-inositol 100 mg/L, *cefotaxime 250-mg/L * prepared fresh and added after autoclave |
| Zm-4 | Somatic embryo development | MS salts (Murashige and Skoog 1962) 4 g/L, N6 salts macro (10×) 6 mL/L, B5 micro (10×) 6 mL/L, (ref.) sucrose 20 g/L, glucose 10 g/L, l-proline 700 mg/L, 2,4-D 0.5 mg/L, MES 0.5 mg/L, pH 5.8, gelrite 3 g/L, Eriksson’s vitamins (100×) 1 ml, SH vitamins (100×) 1 ml, Myo-inositol 100-mg/L, *cefotaxime 250 mg/L * prepared fresh and added after autoclave |
| Zm-5 | Desiccation | 2 Sterile, dry Whatman #70 mm filter paper sealed with parafilm |
| Zm-6 | Regeneration | MS salts 4.3 g/L, sucrose 40 g/L, pH 5.8, agar 8 g/L, MS vitamins (1000×) 5 mL/L, Myo-inositol 100-mg/L, *cefotaxime 250 mg/L * prepared fresh and added after autoclave |
| Zm-7 | Rooting | MS salts 4.3 g/L, sucrose 30 g/L, pH 5.8, agar 8 g/L, IBA 0.5 mg/L, MS vitamins (1000×) 5 mL/L, Myo-inositol 100 mg/L, *cefotaxime 250-mg/L* prepared fresh and added after autoclave |
Table 2.
Medium compositions for sorghum P898012 experiments
| Medium name and number | Medium name | Media components |
|---|---|---|
| Sb-1 | Inoculation | MS salts 2.15 g/L, sucrose 68.5 g/L, glucose 36.0 g/L, 2, 4-D 1.5 mg/L, casamino acids 1 g/L, B5 vitamins (1000×) 1 ml, pH 5.2 and acetosyringone 1 ml from 100 mM concentration |
| Sb-2 | Co-cultivation | MS salts 2.15 g/L, sucrose 20 g/L, glucose 10 g/L, l-proline 700 mg/L, 2,4-D 2.0 mg/L, MES 500 mg/L, ascorbic acid 10 mg/L, B5 vitamins (1000×) 1 ml, pH 5.8, polyvinylpolypyrrolidone (PVPP) 10 g/L, Agar 8 g/L, and acetosyringone 1-ml from 100-mM concentration |
| Sb-3 | Resting | MS salts 4.33 g/L, sucrose 30 g/L, 2,4-D 2.0 mg/L, MES 500 mg/L, ascorbic acid 10 mg/L, asparagine 150 mg/L, coconut water 100 mL/L, B5 vitamins (1000×) 1 mL/L, pH 5.8, phytagel 2.5 g/L, PVPP 10 g/L, *carbenicillin 200 mg/L, *timentin 150-mg/L* prepared fresh and added after autoclave |
| S4 | Callus induction | MS salts 4.33 g/L, sucrose 30 g/L, MES 500 mg/L, 2,4-D 1.5 mg/L, B5 vitamins (1000×) 1 mL/L, pH 5.8, phytagel 2.5 g/L, PVPP 10 g/L, *carbenicillin 200 mg/L, *timentin 150-mg/L* prepared fresh and added after autoclave |
| Sb-5 | Desiccation | 2 Sterile, dry Whatman #70 mm filter paper sealed with parafilm |
| Sb-6 | Embryo Proliferation | MS salts 4.33 g/L, sucrose 30 g/L, MES 500 mg/L, 2,4-D 1.5 mg/L, kinetin 0.5 mg/L, B5 vitamins (1000×) 1 mL/L, pH 5.8, phytagel 2.5 g/L, PVPP 10 g/L, *carbenicillin 200 mg/L, *timentin 150 mg/L* prepared fresh and added after autoclave |
| Sb-7 | Regeneration | MS salts 4.33 g/L, sucrose 60 g/L, MES 500 mg/L, l-proline 700 mg/L, zeatin 0.5 mg/L, IAA 1 mg/L, ABA 0.025 mg/L, TDZ 0.1 mg/L, MS vitamin 5 mL/L (1000×), Myo-inositol 100 mg/L, pH 5.8, agar 8 g/L, PVPP 10 g/L, *carbenicillin 200 mg/L, *timentin 150 mg/L* prepared fresh and added after autoclave |
| Sb-8 | Rooting | MS salts 2.15 g/L, sucrose 30 g/L, NAA 0.25 mg/L, IBA 0.25 mg/L, MS vitamin (1000×) 1 mL/L, pH 5.8, phytagel 2.5 g/L, *carbenicillin 200 mg/L, *timentin 150-mg/L* prepared fresh and added after autoclave |
Somatic embryo development, desiccation, and regeneration
Transient expression was evaluated 3-day post-inoculation. Observable colonies of embryogenic cultures developed 5–10 weeks after inoculation. When embryogenic colonies were 0.5–1.0 cm in diameter, they were assayed for GFP expression, and transferred to petri dishes containing 2 sterile dry 3M Whatman filter papers. The petri dishes were sealed with parafilm and incubated at 25 °C in darkness. After 3 days, the desiccated cultures were transferred to regeneration medium (Table 2, Zm-6 for maize) or returned to resting medium (Table 2, Sb-3 for sorghum) for 1 week, followed by subculture to somatic embryo proliferation medium (Table 2, Sb-6 and then regeneration media (Table 2, Sb-7 for sorghum). Well-formed shoots were transferred to rooting media (Table 1, Zm-7 for maize) and (Table 2, Sb-8 for sorghum). Cultures were maintained at 25 ± 2 °C under a 16-h photoperiod for the regenerations stages. Plantlets with healthy roots were transferred to a greenhouse after 2–3 weeks of acclimatization.
Molecular analysis by PCR and southern blotting
Genomic DNA was isolated from leaf tissue using the method of Chen and Dellaporta (1994) with minor modifications. To confirm the presence of the GFP transgene (ZsGreen) and the absence of the CRE transgene in the T0 population, PCR assays were performed using the KAPA 3G Plant PCR kit (KAPA Biosystems) following the manufacturer’s instructions for PCR using purified DNA. The GFP amplicon is 598 bp and the primers were 5′-CCTGACCAAGGAGATGACCA-3′ (forward)/5′-GTCAGCTTGTGCTGGATGAA-3′ (reverse). The PCR assay for the presence/absence of the CRE transgene utilized the primers 5′-CAGTGAAGACCATCCAGCAA-3′ (forward)/5′-CCTAATGTCCTGGCACCTGT-3′ (reverse) resulting in a 227-bp amplified fragment. PCR conditions were 95 °C 3 min followed by 35 cycles of 95 °C 20 s/58 °C 15 s/72 °C 30 s, followed by a final extension at 72 °C 1 min. Products were visualized on a 1.2% agarose gel. In T1 lines, the presence/absence of the GFP (ZsGreen) and CRE transgenes was tested using the above primers in conjunction with KAPA 3G Plant PCR kit (KAPA Biosystems) following the manufacturer’s instructions for direct PCR from leaves. In that case, the PCR conditions were 95 °C 10 min followed by 35 cycles of 95 °C 30 s/57 °C 15 s/72 °C 30 s, followed by a final extension at 72 °C 1 min. Products were visualized on a 1.2% agarose gel. For Southern analysis, 15 µg of gDNA was cut overnight at 37 °C using either EcoRV or BamHI (New England Biolabs, Ipswitch, MA, USA), both of which cut only once inside the loxP to Agrobacterium LB interval found on the transformation plasmid PHP78891 (see Fig. 1). Southern transfer was performed following standard high-salt procedures; GFP and CRE probes were synthesized using the primers discussed above in conjunction with the PCR DIG Probe Synthesis Kit (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer’s instructions. The pre-hybridization, hybridization, post-hybridization washes, and chemiluminescent detection procedures were all performed according to the manufacturer’s instructions (Roche Applied Science, Indianapolis, IN, USA).
GFP fluorescence observations
GFP fluorescence observations were made using a Leica M205 FA stereomicroscope supplied with a DFC 7000T camera with GFP filter excitation wavelengths 470–540 nm, emission wavelengths 525–550 nm, and the magnification 10–40× with different filters suitable for the spectrum of 450–490 in excitation of GFP for maize or a Zeiss Discovery v20 with the magnification 10–40× and a GFP 470 filter for sorghum.
Results
Transient expression
Transient expression of GFP was observed 3–13-day post-inoculation in B73 and P898012 immature embryos. Transient expression analysis was conducted in B73 using Agrobacterium strain AGL1 with or without a super-binary vector. Figure 2a–f and Table 3 show results from three different transformation conditions for B73 immature embryos: (1) Agrobacterium AGL1 without a binary vector (Fig. 2a, b; negative control); (2) Agrobacterium AGL1 harboring PHP78891 (Fig. 2c, d); and (3) Agrobacterium AGL1 harboring PHP78891 containing a super-binary vector SBV (Fig. 2e, f). The frequency of transient transformation was analyzed and recorded by examining the GFP expression 3-day post-inoculation (Table 3; Fig. 2a–f). Transient expression efficiencies of B73 using PHP78891 combined with the super-binary vector were 98%, greater than that of the PHP78891 without the super-binary vector (68%), whereas no GFP expression was observed in experiments using AGL1 alone (Table 3; Fig. 2a–f). In addition, experiments using AGL1 with the super-binary vector showed the highest transient expression efficiency, with nearly all embryos showing strong GFP expression on all surfaces of the adaxial side of the scutellum and embryo margins (Fig. 2e, f). These results indicate that Agrobacterium AGL1 super-binary vector with PHP78891 was superior for delivery and hence was used in all subsequent experiments for stable transformation of B73.
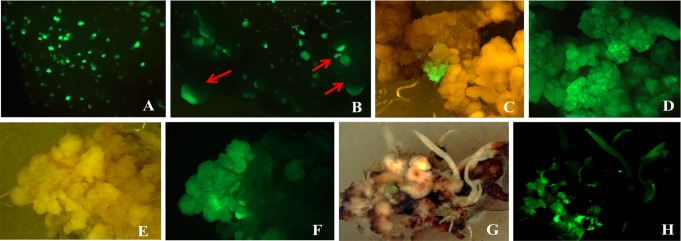
Fig. 2.
Transient expression of GFP in maize B73 using PHP78891. a AGL1/empty vector control brightfield image and b GFP fluorescence image (negative control). c AGL1/PHP78891 brightfield image and d GFP fluorescence image showing adaxial surface of the scutellum. e AGL1/PHP78891-SBV containing the super-binary vector brightfield image and f AGL1/PHP78891-SBV showing GFP foci on the surface of the scutellum cells. Micrographs taken 3 days post-inoculation
Table 3.
Transient GFP expression in maize B73 after 3 days of co-cultivation and embryogenic calli recovery with Agrobacterium AGL1, AGL1 with PHP78891, or AGL1 with PHP78891 and super-binary vector (All the GFP transient foci are developed into embryogenic callus or embryoids)
| Experiment | Agrobacterium and constructs | No. of immature embryos infected | GFP transient expression | % of transient expression | No. of embryogenic calli | % of embryogenic calli |
|---|---|---|---|---|---|---|
| 1 | AGL1 | 75 | 0 | 0 | 0 | 0 |
| 2 | AGL1 | 70 | 0 | 0 | 0 | 0 |
| 3 | AGL1 | 80 | 0 | 0 | 0 | 0 |
| 1 | AGL1 PHP78891 | 60 | 41 | 68.3 | 41 | 68.3 |
| 2 | AGL1 PHP78891 | 50 | 36 | 72 | 36 | 72 |
| 3 | AGL1 PHP78891 | 65 | 43 | 66.1 | 43 | 66.1 |
| 1 | AGL1 PHP78891-SBV | 55 | 53 | 96.3 | 53 | 96.3 |
| 2 | AGL1 PHP78891-SBV | 60 | 59 | 98.3 | 59 | 98.3 |
| 3 | AGL1 PHP78891-SBV | 80 | 78 | 97.5 | 78 | 97.5 |
Transient expression in sorghum P898012 was conducted using Agrobacterium strain EHA101 with or without PHP78891. Results were observed over nine experiments 3-day post-inoculation (Fig. 4a). The transient expression efficiency of P898012 using EHA101 with PHP78891 was an average of 170.3 GFP expressing units per embryo. These results indicate that the Agrobacterium strain EHA101 with PHP78891 is sufficient for delivery to support stable transformation experiments in sorghum P898012.
Fig. 4.
Transient and stable sorghum P898012 Transformation using PHP78891. a Transient expression EHA 101 PHP78891 3-day post-inoculation (GFP image); b Early stage somatic embryos (arrows) 13-day post-inoculation (GFP image). EHA 101 PHP78891 derived events after desiccation shows heterogeneous callus. c Brightfield and GFP image shows GFP positive embryogenic cluster within GFP negative organized callus. d Homogeneous GFP expressing embryogenic callus before desiccation (brightfield image); f corresponding GFP image showing heterogeneous callus after desiccation; g Heterogenous GFP expression in callus during plant regeneration (brightfield image); h corresponding GFP image
Stable transformation
GFP transient expression typically does not persist longer that 14–19 days in both B73 and P898012 experiments. Efforts to directly follow transient expression of GFP to stable colonies were unsuccessful. Somatic embryos appeared on the surface of adaxial surfaces on B73 and P898012 immature embryos during 7 days on resting medium (Table 1 Zm-3; Table 2 Sb-3, respectively). After 7 days on the resting media, the cultures were examined for GFP expression. Early developing embryos were observed as small and club-shaped, which occurred individually. The vector PHP78891-SBV resulted in higher frequencies of somatic embryo induction (98%) than that of PHP78891 alone (68%) in B73 (Table 3). This suggested that all calli showing GFP positive foci turned out to be embryogenic. After 7 days on resting medium, no somatic embryos were observed in the negative controls containing AGL1 without the binary vector. Somatic embryogenesis frequency appears to be correlated with higher transient GFP expression (see Fig. 2).
B73 cultures remained on resting medium for 4–7 weeks with subculturing every 2 weeks. Clusters of embryogenic calli formed between 4 and 7 weeks and were observed on various parts of explanted immature embryos. These calli exhibited small early stage somatic embryos and single-celled protrusions. The frequency of B73 embryogenic callus recovery without a selection agent using Agrobacterium AGL1, AGL1 with PHP78891, and AGL1 with PHP78891-SVB is shown in Table 3. AGL1 without the PHP78891 vector showed no somatic embryo response and no formation of embryogenic calli. The AGL1 with PHP78891 showed modest levels of embryo response and formation of embryogenic calli (66.1–72%). However, AGL1 with PHP78891-SVB in B73 showed high rates (over 96%) of embryo response and somatic embryogenic calli formation. Transformation frequency in B73 using AGL1 with PHP78891-SBV (Table 4) also showed a high number of embryogenic calli which survived the desiccation treatment and resulted in the regeneration of green shoots with an overall transformation frequency of 14.5–15%. Desiccation stress was applied 5–6-week post-inoculation. After desiccation treatment, callus that was not GFP positive appeared necrotic and did not typically survive subculture (Fig. 2a–d).
Table 4.
Transformation frequency in maize B73 using AGL1 with PHP78891-SBV
| Experiment | No. of immature embryos infected | Embryogenic calli | No. of calli surviving desiccation | No. of GFP positive calli | No. of GFP positive events that regenerated | Transformation frequency (%) |
|---|---|---|---|---|---|---|
| 1 | 55 | 53 | 32 | 29 | 8 | 14.5 |
| 2 | 60 | 59 | 29 | 25 | 9 | 15 |
| 3 | 80 | 78 | 70 | 63 | 12 | 15 |
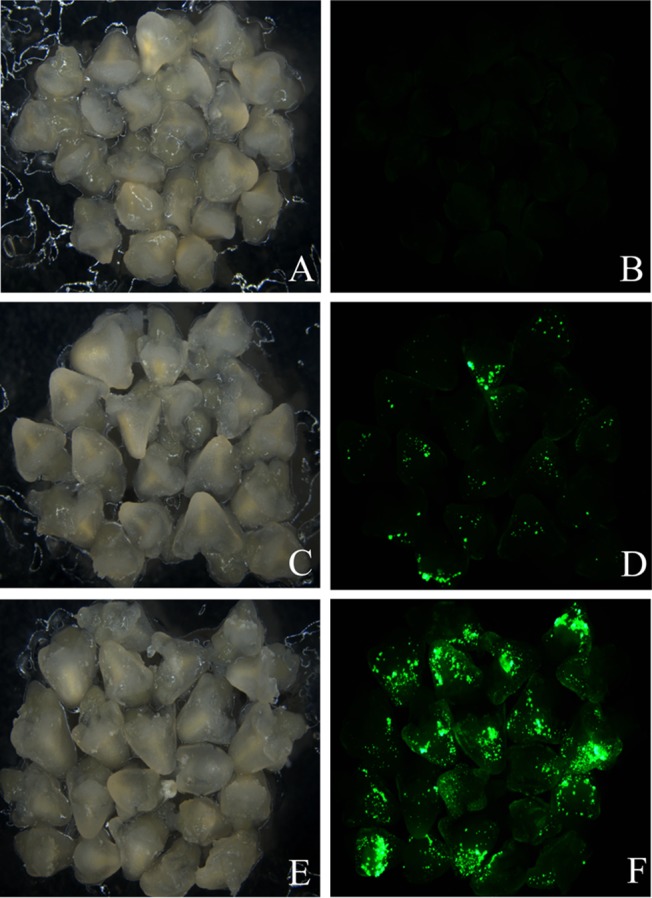
Similar results were observed in P898012 experiments. Transient expression after 3 days showed punctate single-celled GFP expression (Fig. 4a). After 13 days, these cultures, showed the presence of immature somatic embryos (Fig. 4b) in stages of development comparable to 4–7-day post-pollination zygotic embryos. These somatic embryos were not entirely GFP positive, indicative of transient expression. Under the conditions of this study, these somatic embryos did not mature, and GFP expression was eventually lost. After 4–7 weeks on resting media, GFP expression developed as small clusters on resting media (Fig. 4c, d). Representative frequency of embryogenic callus recovery with Agrobacterium EHA101 in P898012 (Table 5) shows that in comparison to the GFP expressing control without the CRE:WUS:BBM cassette (PJLU13 and PYU 2593 plasmids), embryos inoculated with PHP78891 produced GFP positive embryogenic calli at significantly higher frequencies, up to 54.54%.
Table 5.
Frequency of GFP expression of PHP78891 without selection using Agrobacterium EHA101 in sorghum P898012
| Experiment | Agrobacterium EHA101 with or without PHP78891 | No. of immature embryos infected | GFP positive embryogenic calli | % GFP expression |
|---|---|---|---|---|
| 1 | EHA101 PJLU13 | 11 | 0 | 0 |
| 2 | EHA 101 PYU 2593 | 55 | 0 | 0 |
| 3 | EHA 101 PYU 2593 | 46 | 0 | 0 |
| 4 | EHA101 PHP78891 | 11 | 6 | 54.54 |
| 5 | EHA101 PHP78891 | 180 | 28 | 15.56 |
| 6 | EHA101 PHP78891 | 128 | 30 | 23.44 |
| 7 | EHA101 PHP78891 | 52 | 10 | 19.23 |
These GFP positive calli were embryogenic (type II) and occurred within organized (type I) calli (Fig. 4). With repeated subculture every 2 weeks, these GFP positive clusters developed into homogenous proliferating somatic embryogenic calli for both maize (not shown) and sorghum (Fig. 4 for sorghum). After embryogenic calli were observed to be GFP positive and growing (5–6-week post-inoculation), selected colonies were placed onto sterile, dry filter paper to induce desiccation. After 3-day desiccation, the calli were dry, shrunken, and had a yellowish appearance. For B73, desiccated calli were subcultured to medium Zm-6 (Table 1). Similar to post-desiccation treatment of maize calli, sorghum did not show uniform GFP expression after desiccation stress (Fig. 4e, f). For P898012, desiccated cultures were returned to resting medium (Table 2, Sb-3) for 1 week and then subcultured to somatic embryo development medium (Table 2, Sb-4) for 2 weeks. The resulting proliferating calli showed a heterogeneous GFP expression after desiccation (Fig. 3a–d for maize and Fig. 4e, f for sorghum). In B73, the number and frequency of GFP positive calli was higher in those embryos inoculated with the AGL1 PHP78891-SVB (Fig. 3c, d) in comparison to embryos inoculated with AGL1 PHP78891 (Fig. 3a, b). These results are consistent with the transient expression results. All calli were then subcultured to regeneration media (Table 1, Zm-6 for maize; Table 2, Sb-6 for sorghum). Two weeks after subculture to regeneration media, many of the embryos had turned photosynthetically green and produced shoot buds, although some calli sectors or regenerating tissues had turned necrotic and did not survive. The regenerating shoot buds were then transferred to medium Zm-6 (Table 1) for maize or Sb-6 (Table 2) for sorghum for 2–3 additional weeks. After transfer to regeneration media, the calli continued to show a heterogeneous expression of GFP (Fig. 3e, f for maize; Fig. 4g, h for sorghum), but the individual plantlets regenerating from the calli appeared homogenous for GFP expression for both B73 and P898012.
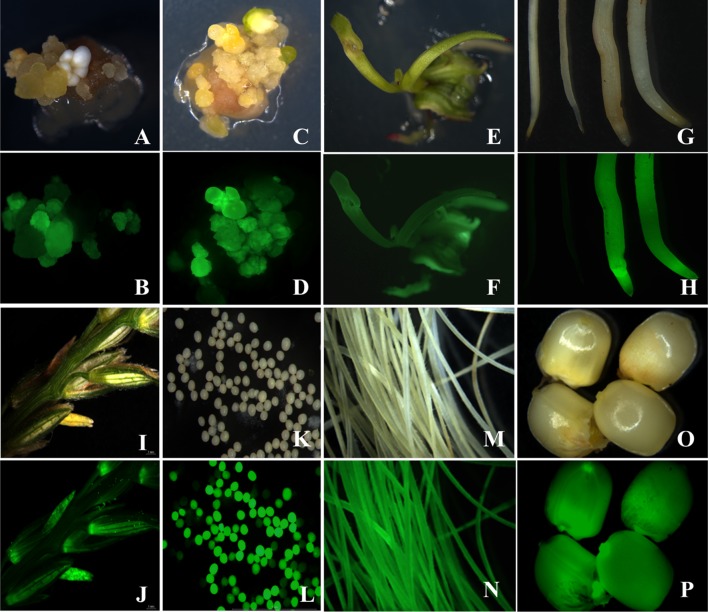
Fig. 3.
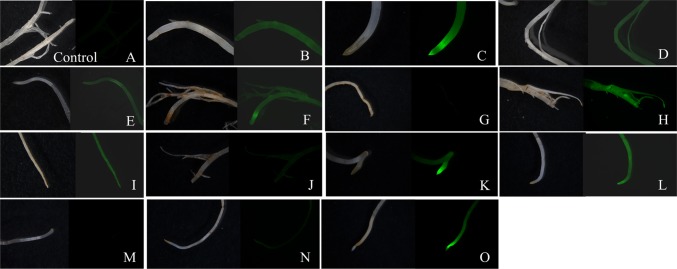
Expression of PHP78891 in T0 transgenics of maize B73. Maize B73 AGL1 PHP78891 calli after 3-day desiccation stress calli; comparable a (Brightfield image) and b (GFP image), AGL1 PHP78891-SBV after 3-day desiccation shows higher GFP sector expression frequencies; c (brightfield image) and d (GFP image), homogeneously GFP expressing shoot in regeneration after 3 days desiccation; e (Brightfield image) and f (GFP image), GFP positive roots (right) and wild type (left); g (Brightfield image) and h (GFP image), tassel from PHP7889-SBV event after anthesis with GFP positive anthers; i (Brightfield image) and j (GFP image), isolated pollen showing 1:1 segregation for GFP; k (Brightfield image) and l (GFP image), silk from regenerated plant; m (Brightfield image) and n (GFP image), kernels from regenerated plant; o (Brightfield image) and p (GFP image), kernels from regenerated plant
B73 plants regenerated from these events exhibited GFP expression in the T0 plants in vegetative tissues and floral structures, including young leaves (not shown), roots (Fig. 3g, h), tassel inflorescences and anthers (Fig. 3i, j), pollen (Fig. 3k, l), silks (Fig. 3m, n), and mature seeds (Fig. 3o, p). Pollen was segregating for 1:1 GFP expression. Other tissues including mature leaf surfaces, leaf vascular cell tissue, leaf margins with trichomes, tillers, and prop roots exhibited strong GFP expression as well. T0 P898012 plants also showed expression of GFP in vegetative and floral structures to maturity (data not shown).
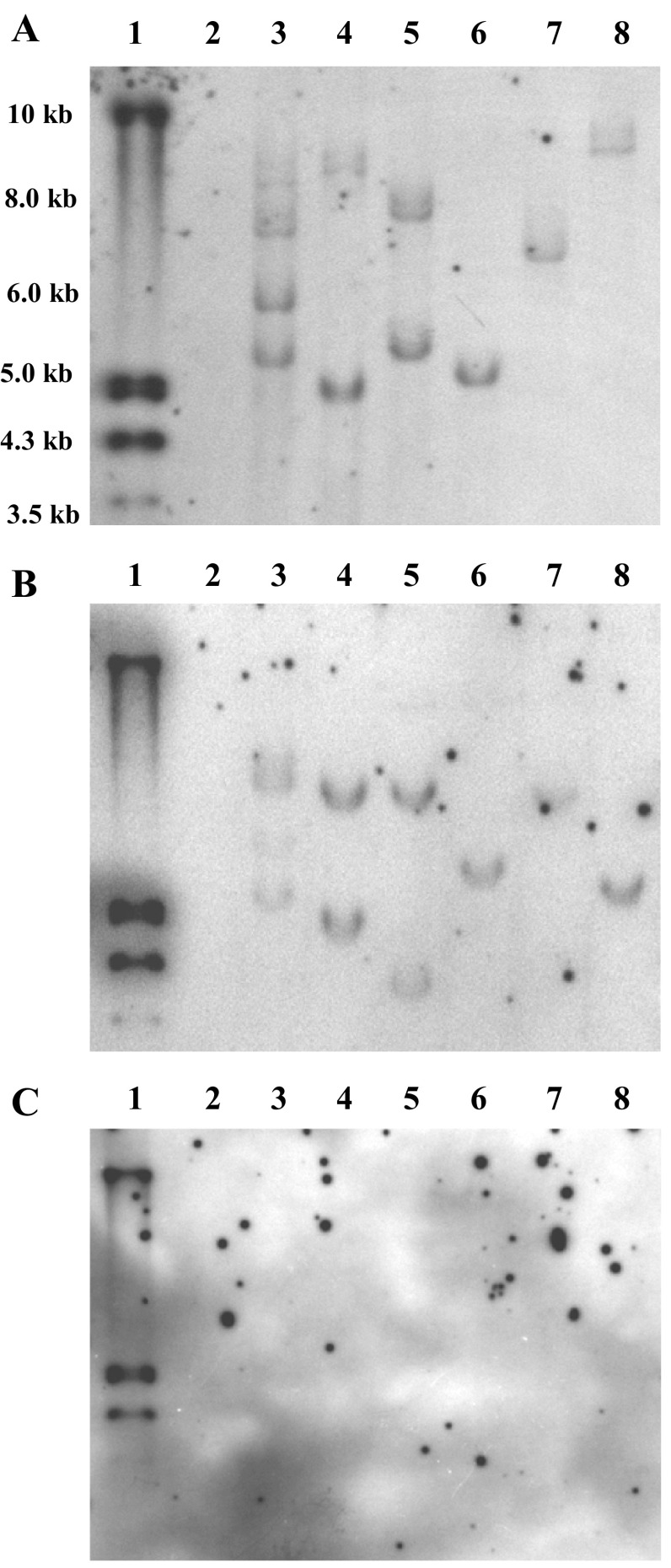
Results from molecular analysis confirmed that regenerated B73 and P898012 plants are transgenic. Figure 5 shows PCR amplification of GFP and CRE transgenes fragments. Transformed B73 plants show the presence of the GFP cassette but no amplification of the CRE amplicon. This result, in conjunction with successful regeneration of GFP positive plants, indicates the likely excision of the loxP-bound CRE:WUS:BBM cassette in the transformed plants. Southern blots confirmed these results (Fig. 6a–c). EcoRV cut once within 5′ region of the GFP cassette. Figure 6a shows PHP78891-transformed B73 events; M-1-6, M-1-8, MM-2-9, MM-4-1b, MM-4-1F, and MM-5-0; l-r, in lanes 3–8 (respectively). At least three of these events (MM-4-1b, MM-4-1F, and MM-5-0; l-r) show a single band when probed for GFP. BamHI cut twice within the cassette and when probed for GFP also showed the same expected insertions (Fig. 6b). When this blot was stripped and re-probed for CRE, these blots showed the absence of the CRE:WUS:BBM cassette (Fig. 6c). These results collectively confirmed the presence of the transgene and excision of loxP flanked cassettes, and that most of the transformants are low copy insertion events (Table 6).
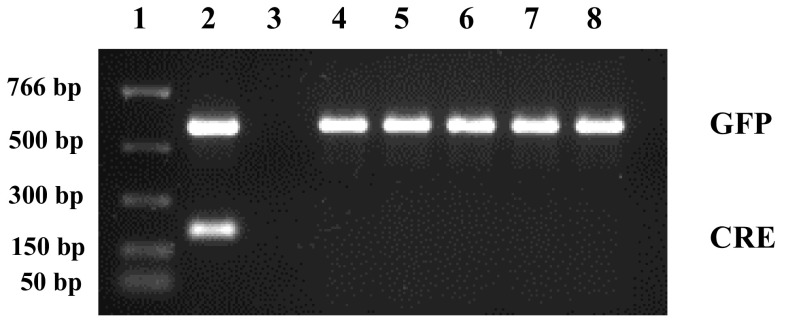
Fig. 5.
PCR amplification of ZsGreen and CRE fragments from maize B73 events. Lanes 1, 300 ng NE Biolabs PCR Marker; lane 2, positive control, amplification of ZsGreen (upper band, expected size 594 bp) and Mo CRE (lower band, expected size 227 bp; lane 3, B73 wild-type negative control; lanes 4–8, transformed plants, M-1-1, M-1-2, M-1-5, M-1-6, and M-1-8
Fig. 6.
Southern blot of PHP78891 transformed maize B73 events using a DIG-labeled probe. a EcoRV restriction digest probed for GFP as shown in Fig. 1. b BamHI restriction digest probed for GFP, as shown in Fig. 1. c Stripped BamHI restriction digest shown in b, re-probed for CRE. Lanes 1, DIG-labeled molecular weight ladder III (Roche Diagnostics Corporation, IN, USA); lane 2, nontransformed maize B73; lanes 3–8, PHP78891-transformed B73 events, i.e., M-1-6, M-1-8, MM-2-9, MM-4-1b, MM-4-1F, and MM-5-0, respectively
Table 6.
Frequency of stable embryogenic callus recovery without selection using Agrobacterium EHA101 in sorghum P898012
| Experiment | Agrobacterium EHA101 with or without PHP78891 | No. of immature embryos infected | No. of desiccated embryogenic calli | No. of GFP positive events that regenerated | Transformation frequency (%) |
|---|---|---|---|---|---|
| 1 | PJLU13 | 11 | 0 | 0 | 0.0 |
| 2 | PYU 2593 | 55 | 0 | 0 | 0.0 |
| 3 | PYU 2593 | 46 | 0 | 0 | 0.0 |
| 4 | PHP78891 | 11 | 6 | 1 | 9.1 |
| 5 | PHP78891 | 180 | 28 | 5 | 2.8 |
| 6 | PHP78891 | 128 | 30 | 11 | 8.6 |
| 7 | PHP78891 | 52 | 10 | 6 | 11.5 |
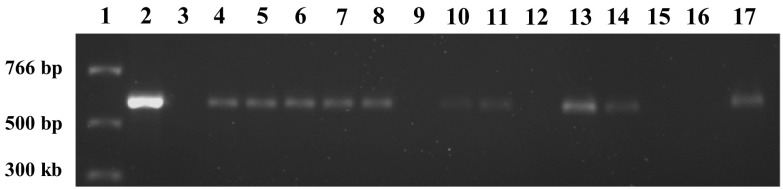
Inheritance and segregation of the transgene were investigated in T1 plants generated from self pollinations. PCR results confirmed that the hemizygous transgene was stably inherited in T1 plants as a single Mendelian trait (Fig. 7) which correlate with functional GFP expression (Fig. 8; Table 7). Representative PCR analysis of 14 T1 plants from the MM-4-1F line showed that nine tested positive for the transgene and four plants tested negative (Fig. 7). Functional GFP analysis confirmed these results with the same plants that were PCR positive for GFP expression and those that tested negative for PCR were also negative for GFP expression. The T1 plants in B–O (Fig. 8) correlated directly with the PCR lanes 3–17 in Fig. 7.
Fig. 7.
PCR of inheritance and segregation of the GFP transgene in T1 plants generated from self pollinations. Shown are PCR analysis of 14 T1 plants from the MM-4-1F line. Lane 1, molecular weight markers; lane 2, control reaction for GFP marker; lane 3, wild-type nontransformed control; lanes 4–17, T1 plants testing positive for the transgene in lanes 4, 5, 6, 7, 8, 10, 11, 13, 14, and 17; and, negative in lanes 9, 12, 15, and 16
Fig. 8.
Inheritance and segregation of the functional GFP transgene in T1 plants generated from self pollinations. Wild-type control a is negative for GFP expression. Roots from T1 plants from the MM-4-1F line b–o are shown in paired brightfield and GFP micrographs corresponding to the PCR results. The T1 plants in b–o correlate directly with lanes 3–17 in Fig. 7
Table 7.
Correlation between functional GFP expression and presence of the GFP transgene in T1 plants generated from self pollinations
| T0 parent line | T1 plant ID | GFP expression | PCR analysis |
|---|---|---|---|
| Control | A | Negative | Negative |
| B | Positive | Positive | |
| C | Positive | Positive | |
| D | Positive | Positive | |
| E | Positive | Positive | |
| F | Positive | Positive | |
| MM-4-1F | G | Negative | Negative |
| H | Positive | Positive | |
| I | Positive | Positive | |
| J | Negative | Negative | |
| K | Positive | Positive | |
| L | Positive | Positive | |
| M | Negative | Negative | |
| N | Negative | Negative | |
| O | Positive | Positive |
Discussion
Standard plant transformation is usually Agrobacterium, biolistics, or protoplast mediated, and is hampered by several technical bottlenecks (Altpeter et al. 2016) as well as long turnaround times to recover transgenic plants (9–18 months). The current developments in genomics and gene editing have amplified the need for transgenics (Altpeter et al. 2016) and have vastly increased the necessity for solutions to these obstacles. Recent reports (Lowe et al. 2016; Svitashev et al. 2016) using morphogenic regulators to mediate transformation offer a promising breakthrough solution to many of the obstacles which have encumbered standard plant transformation. Lowe et al. 2016 (personal communication, and Lowe SIVB Meetings 2016) reported use of this strategy on many commercial inbreds and using various explant sources including immature embryos, mature seeds, and mature leaves.
Most protocols for standard plant transformation rely entirely upon highly articulated tissue culture regimes for stable gene introduction and plant regeneration, which are genotype specific. These schemes all use specific genotypes which have been worked on for decades and focused on transformation, selection, and transformation responses. The use of morphogenic regulators to overcome barriers associated with these constraints has created a watershed advance for plant genome modification and agriculture. While the use of morphogenic regulators had been previously explored (Gordon-Kamm et al. 2002), differential overexpression of BABY BOOM and WUSCHEL2 driven by independent promoters was demonstrated to surmount many genotype obstacles associated with cell division and somatic embryogenesis during transformation (Lowe et al. 2016). Lowe et al. (2016) show dramatic increases in transformation frequencies through optimal expression of BBM and WUS and expanded breadth of transformation amenable genotypes. However, even though those studies broadened the range of transformable monocot lines, a genotype-independent transformation approach has remained yet to be achieved. For example, routine transformation of maize B73, despite its importance as a genetic model, has remained elusive.
Embryo formation in plants occurs via multiple mechanisms, such as zygotic embryogenesis, androgenesis, somatic embryogenesis, and apomixes (Mordhorst et al. 1997; Koltunow and Grossniklaus 2003). Several transcription factors are known to play a significant role in cell dedifferentiation and division and may control or induce somatic embryogenesis in plants. As a transcription factor, BBM has been identified as a marker for embryogenic cultures (Boutilier et al. 2002; Deng et al. 2009; Florez et al. 2015) and is likely involved with regulation of multiple genes involved with the early embryo development. WUS is known to be involved in the early embryogenesis (Laux et al. 1996) and as a homeodomain-containing transcription factor also probably regulates a battery of genes. The use of differentially expressed BBM and WUS has resulted in effecting ectopic somatic embryogenesis. Lowe et al. (2016) indicate that the transiently expressed WUS protein may be diffusible and capable to influence surrounding cells to be stimulated to divide and that this is consistent with the previous investigations on the WUS protein as a contributor to apical meristem organization (Mayer et al. 1998; Gallois et al. 2002; Yadav et al. 2011, 2013).
Our investigation indicates that transient expression of a CRE:WUS:BBM:GFP construct elicits transient GFP expression coincident with the early stages of somatic embryogenesis in B73 and P898012. However, following a period of transient expression, stable GFP positive transformed colonies did not appear until 4–7-week post-Agrobacterium inoculation. This indicates that the growth of cells from stably integrated events may not originate from transiently expressing cells or that maintained expression of morphogenic regulators requires significant time to elicit their effect. Once established, however, stable transformed the early embryogenic and GFP positive colonies appear within more organized compact (type I) callus. The embryogenicity and GFP expression in these events appears homogenous, indicating that they are probably not mosaic for insertion or expression. It is important to note that a selectable marker gene, such as PAT (phosphinothricin acetyltransferase), BAR (bialphos resistance), ALS (acetolactate synthase), HPT (hygromycin phosphotransferase), etc., was not used in this procedure. There are few substantiated reports of visual selection of transgenic plants without the use of a selection agent. Recently, Svitashev et al. (2016) showed the use of the maize transcription factors (maize ovule developmental protein 2 (ODP2) and maize WUS) in conjunction with a selectable and a visible fusion reporter gene marker. The selectable marker was a herbicide resistance transgene, maize-optimized phosphinothricin-N-acetyltransferase (MOPAT) translationally fused with the visual marker gene red fluorescent protein (DSRED). Transformants were generated using biolistic-mediated transformation followed by herbicide selection and plant regeneration. The GFP positive embryogenic calli in the present study could be maintained through continued subculture but would not regenerate. This observation suggests that expression of BBM and WUS is sufficient and necessary to induce somatic embryogenesis in B73 and P898012. Acting in this way, differential overexpression of morphogenic regulators acts as a type of developmental selectable marker, in that only those cells which are transformed and expressing the gene become embryogenic and arrested in that stage of development. Lowe et al. (2016) also reported the recovery of one inbred maize line without the use of a selectable marker. Thus, expression of BBM and WUS promotes recovery of stable transformants but prevents further development and plant regeneration. This observation was also intimated by Lowe et al. (2016) and necessitates the need for inducible expression of BBM and WUS or inducible excision via site-specific recombination.
The CRE/lox site-specific recombination system of bacteriophage P1 has been used for various gene excision studies in transgenic plants (Dale and Ow 1990; Hoa et al. 2002; Srivastava et al. 1999). Desiccation induction of RAB17M: CRE expression can be used to remove the WUS2 and BBM transcriptional genes from the transgenic embryogenic callus before transfer to the regeneration medium. Desiccation stress was applied after visible GFP calli were readily observable (5–6-week post-inoculation). This indicates that the GFP calli were most likely the result of stable integration events. Following the desiccation treatments for both maize and sorghum, the calli were not uniform for GFP expression, indicating that either desiccation stress or induction of the RAB17M promoter was not uniform, loxP flanked cassette excision was not consistent or a combination of these factors. The CRE:BBM:WUS cassette is flanked by two directly repeated loxP sites. Induced CRE expression mediates excision of the cassette allowing plant regeneration. Before transfer to the regeneration medium, the embryos were desiccated for 3 days to eliminate the ectopic expression of WUS2 and BBM, to support normal plant regeneration. Without desiccation, we observed ectopic expression manifesting as secondary clusters of somatic embryos, folded leaves, and very short plantlets with thick abnormal root structures (data not shown).
The desiccation treatment of early embryogenic GFP positive calli likely induces the RAB17M promoter and expression of CRE resulting in the excision of the lox P: CRE:WUS:BBM: lox P cassette. The resulting calli were no longer homogeneous but exhibited large sectors of GFP embryogenic callus which were subsequently capable of regeneration. It is well known that CRE-mediated excision can be variable under some situations. Molecular analysis by both PCR and Southern blots confirmed the likely removal of the loxP:CRE:WUS:BBM: loxP cassette in regenerated plants. Furthermore, the Southern analysis show presumed single copy insertion events, indicating that these are probably not chimeric mosaics for loxP excision. This observation indicates that the regenerated plants from the current study are derived either from single-celled excision events or multi-celled, but homogenous excision events.
While Agrobacterium-mediated transformation is a common method for gene transfer to plants, transformation frequency differs widely among various Agrobacterium strains (Wu et al. 2003; Vega et al. 2008; Cho et al. 2014; Do et al. 2016; Zhang et al. 1999), and genotypes. For example in maize using EHA101, the efficiency reported for Hi II by Frame et al. (2002) was 5.5%, 2–6% in H99 (Sidorov et al. 2006), and 12–18% in Hi IIA X B F1 (Vega et al. 2008). Ishida et al. (1996) developed an Agrobacterium strain containing a super-binary vector with additional copies of virB, virC, and virG genes for transformation of immature embryos in maize A188. Their experiments showed a high frequency of transformation (5–50%), with all resulting plants being fertile. We have shown a higher frequency of both transient and stable transformation of B73 using the AGL1 strain containing the super-binary vector. Higher transformation frequency also correlated with higher plants recovered after excision of the CRE:WUS:BBM cassette. Wu et al. (2014) also reported a higher frequency of transformation in sorghum using the AGL1 strain containing the super-binary vector.
B73 represents a recalcitrant transformation genotype of genetic and genomic significance. Standard transformation techniques result in 0% transformation. We have shown that use of AGL1 strain containing the super-binary vector increases vector delivery. In conjunction with the overexpression of the morphogenic genes BBM and WUS, we were able to achieve significant frequencies of stable transformation of B73. Following desiccation, induced excision of the morphogenic genes and high rates of plant regeneration were achieved. This approach enables a significantly increased frequency of transformation from 0 to 15% for B73 and up to 6.2% for P898012 genotypes without the use of selection agents. Selectable marker independent transformation may contribute to overcoming transformation barriers and facilitate studies using gene editing functions. Our study confirms and extends the previous reports using morphogenic regulators to improve transformation (Lowe et al. 2016; Svitashev et al. 2016). In this study, we have established reliable transformation of the recalcitrant B73 and P898012 via co-expression of BBM and WUS. Using this approach, we also show increased frequency of transformation of these recalcitrant varieties. The observation of early stage somatic embryos 13 days after DNA delivery indicates that this response may be the result of transient expression of BBM and WUS prior to integration. We expect that these results will be further extended to include explant independence and exploitation of transient expression of the morphogenic regulators.
The ability to deploy gene editing functions, such a zinc finger nuclease (ZNF), transcription activator-like effector nucleases (TALENS), and more recently, clustered regularly interspaced short palindromic repeats (CRISPR), has dramatically increased the need for more efficient and genotype-independent plant transgenic biology. Svitashev et al. (2016) used biolistic delivery for introduction of ribonucleoprotein (RNP) complexes and the use of the maize transcription factors ODP2 and WUS, chemical and visual selection for the validation, and recovery of CRISPR/Cas9 target sequences. These studies indicate that the biology and technology for plant transformation is entering a new era. The ability to conduct gene editing, genetic modification, and plant transformation that is genotype-independent, Agrobacterium independent, DNA free (non-GMO), tissue culture free, high-throughput, high efficiency, and relatively rapid is of significant importance to basic plant biology and world agriculture. We expect that the current research in this area will achieve these goals.
Author contribution statement
ZZ and AK conceived and designed research; MM, KNV, and JH conducted research; MM, KNV, JH, ZZ, and AK analyzed results; MM, KNV, JH, ZZ, and AK wrote and revised the manuscript.
Acknowledgements
We thank Pioneer-DuPont for the PHP78891 construct. Acknowledgements also to Dr. Rongda Qu at North Carolina State University for the kind gift of the pJLU13 plasmid, and to Stephen Dellaporta at Yale University for the PYU 2593 which were used as controls. Thanks are also extended to Neng Wan for assistance in the UM greenhouse maize care. This project has been supported by the National Science Foundation Plant Genome Research Program (#1444478).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Muruganantham Mookkan, Kimberly Nelson-Vasilchik, and Joel Hague have contributed equally to this study.
Contributor Information
Zhanyuan J. Zhang, Phone: 573-882-6922, Email: zhangzh@missouri.edu
Albert P. Kausch, Phone: 401-874-7121, Email: apkausch@uri.edu
References
- Altpeter F, Springer NM, Bartley LE, Blechl A, Brutnell TP, et al. Advancing crop transformation in the era of genome editing. Plant Cell. 2016;28:1510–1520. doi: 10.1105/tpc.16.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug N. Feeding a world of 10 billion people: the miracle ahead. In Vitro Cell Dev Plant. 2002;38:221–228. doi: 10.1079/IVP2001279. [DOI] [Google Scholar]
- Bouchabke-Coussa O, Obellianne M, Linderme D, Montes E, Maia-Grondard A, Vilaine F, Pannetier C. Wuschel overexpression promotes somatic embryogenesis and induces organogenesis in cotton (Gossypium hirsutum L.) tissues cultured in vitro. Plant Cell Rep. 2013;32:675–686. doi: 10.1007/s00299-013-1402-9. [DOI] [PubMed] [Google Scholar]
- Boutilier K, Ovringa R, Sharma VK, Kieft H, Ouellet T, et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002;14:1737–1749. doi: 10.1105/tpc.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas AM, Kononowicz AK, Zehr UB, Tomes DT, Axtell JD, et al. Transgenic sorghum plants via microprojectile bombardment. Proc Natl Acad Sci USA. 1993;90:11212–11216. doi: 10.1073/pnas.90.23.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Dellaporta S. Urea-based plant DNA miniprep. In: Freeling M, Walbot V, editors. The maize handbook. New York: Springer; 1994. pp. 526–528. [Google Scholar]
- Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, et al. Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol. 1997;115:971–980. doi: 10.1104/pp.115.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M-J, Wu E, Kwan J, Yu M, Banh J, et al. Agrobacterium-mediated high-frequency transformation of an elite commercial maize (Zea mays L.) inbred line. Plant Cell Rep. 2014;33:1767–1777. doi: 10.1007/s00299-014-1656-x. [DOI] [PubMed] [Google Scholar]
- Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol. 1992;18:675–689. doi: 10.1007/BF00020010. [DOI] [PubMed] [Google Scholar]
- Chu CC, Wang CC, Sun CS, Hsu C, Yin KC, Chu CY, Bi FY. Establishment of an high-frequency medium for anther culture of rice, through comparative experiments on the nitrogen sources. Sci Sin. 1975;18:659–668. [Google Scholar]
- Conner JA, Mookkan M, Huo H, Chae K, Ozias-Akins P. A parthenogenesis gene of apomict origin elicits embryo formation from unfertilized eggs in a sexual plant. Proc Natl Acad Sci USA. 2015;112:11205–11210. doi: 10.1073/pnas.1505856112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Zheng P, Marmey P, Zhang S, Tian W, Chen S, Beachy RN, Fauquet C. Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Mol Breed. 2001;7:25–33. doi: 10.1023/A:1009687511633. [DOI] [Google Scholar]
- Dale EC, Ow DW. Intra- and intermolecular site-specific recombination in plant cells mediated by bacteriophage P1 recombinase. Gene. 1990;91:79–85. doi: 10.1016/0378-1119(90)90165-N. [DOI] [PubMed] [Google Scholar]
- Deng W, Luo K, Li Z, Yang Y. A novel method for induction of plant regeneration via somatic embryogenesis. Plant Sci. 2009;177:43–48. doi: 10.1016/j.plantsci.2009.03.009. [DOI] [Google Scholar]
- Do P, Lee HY, Mookkan M, Folk WR, Zhang Z. Rapid and efficient Agrobacterium-mediated transformation of sorghum (Sorghum bicolor) employing standard binary vectors and bar gene as a selectable marker. Plant Cell Rep. 2016;35:2065–2076. doi: 10.1007/s00299-016-2019-6. [DOI] [PubMed] [Google Scholar]
- FAOSTAT (2012). Available online: http://faostat.fao.org/site/567/default.aspx#ancor
- Florez SL, Erwin RL, Maximova SN, Guiltinan MJ, Curtis WR. Enhanced somatic embryogenesis in Theobroma cacao using the homologous BABY BOOM transcription factor. BMC Plant Biol. 2015;15:121. doi: 10.1186/s12870-015-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame BR, Shou H, Chikwamba RK, Zhang Z, Xiang C, et al. Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 2002;29:13–22. doi: 10.1104/pp.000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallois J-L, Woodward C, Reddy GV, Sablowski R. Combined SHOOT MERISTEMLESS and WUSCHEL trigger ectopic organogenesis in Arabidopsis. Development. 2002;129:3207–3217. doi: 10.1242/dev.129.13.3207. [DOI] [PubMed] [Google Scholar]
- Gordon-Kamm WJ, Spencer TM, Mangano ML, Adams TR, Daines RJ, et al. Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell. 1990;2:603–618. doi: 10.1105/tpc.2.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Kamm W, Dilkes BP, Lowe K, Hoerster G, Sun X, et al. Stimulation of the cell cycle and maize transformation by disruption of the plant retinoblastoma pathway. Proc Natl Acad Sci USA. 2002;99:11975–11980. doi: 10.1073/pnas.142409899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada JJ. Role of Arabidopsis LEAFY COTYLEDON genes in seed development. J Plant Physiol. 2001;158:405–409. doi: 10.1078/0176-1617-00351. [DOI] [Google Scholar]
- Harding EW, Tang W, Nichols KW, Fernandez DE, Perry SE. Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-LIKE15. Plant Physiol. 2003;133:653–663. doi: 10.1104/pp.103.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313X.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- Hiei Y, Komari T, Kubo T. Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol Biol. 1997;35:205–218. doi: 10.1023/A:1005847615493. [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ishida Y, Kasaoka K, Komari T. Improved frequency of transformation in rice and maize by treatment of immature embryos with centrifugation and heat prior to infection with Agrobacterium tumefaciens. Plant Cell Tissue Organ. 2006;87:233–243. doi: 10.1007/s11240-006-9157-4. [DOI] [Google Scholar]
- Hoa TTC, Bong BB, Huq E, Hodges TK. Cre-lox site-specific recombination controls the excision of a transgene from the rice genome. Theor Appl Genet. 2002;104:518–525. doi: 10.1007/s001220100748. [DOI] [PubMed] [Google Scholar]
- Huang X, Wei Z. Successful Agrobacterium-mediated genetic transformation of maize elite inbred lines. Plant Cell Tissue Organ. 2005;83:187–200. doi: 10.1007/s11240-005-5772-8. [DOI] [Google Scholar]
- Huang S, Gilbertson LA, Adams TH, Malloy KP, Reisenbigler EK, et al. Generation of marker free transgenics maize by regular two border Agrobacterium transformation vectors. Transgenic Res. 2004;13:451–461. doi: 10.1007/s11248-004-1453-3. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Satto H, Ohta S, Hiei Y, Komari T, Kumashiro T. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol. 1996;14:745–750. doi: 10.1038/nbt0696-745. [DOI] [PubMed] [Google Scholar]
- Koltunow AM, Grossniklaus U. Apomixis: a developmental perspective. Ann Rev Plant Bio. 2003;54:547–574. doi: 10.1146/annurev.arplant.54.110901.160842. [DOI] [PubMed] [Google Scholar]
- Laux T, Mayer KF, Berger J, Jurgens G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development. 1996;122:87–96. doi: 10.1242/dev.122.1.87. [DOI] [PubMed] [Google Scholar]
- Lowe K, Wu E, Wang N, et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell. 2016;28:1998–2015. doi: 10.1105/tpc.16.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppotto E, Reali A, Passera S, Chan MT. Maize inbred lines are susceptible to Agrobacterium tumefaciens-mediated transformation. Maydica. 1999;44:211–218. [Google Scholar]
- Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, et al. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95:805–815. doi: 10.1016/S0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- Mordhorst AP, Toonen MAJ, de Vries S. Plant embryogenesis. Crit Rev Plant Sci. 1997;16:535–576. doi: 10.1080/07352689709701959. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Negrotto D, Jolley M, Beer S, Wenck AR, Hansen G. The use of phosphomannose-isomerase as a selectable marker to recover transgenic maize plants (Zea mays L.) via Agrobacterium transformation. Plant Cell Rep. 2000;19:798–803. doi: 10.1007/s002999900187. [DOI] [PubMed] [Google Scholar]
- Odell J, Caimi P, Sauer B, Russell S. Site-directed recombination in the genome of transgenic tobacco. Mol Gen Genet. 1990;223:369–378. doi: 10.1007/BF00264442. [DOI] [PubMed] [Google Scholar]
- Popelka J, Altpeter F. Agrobacterium tumefaciens-mediated genetic transformation of rye (Secale cereale L.) Mol Biol. 2003;11:203–211. [Google Scholar]
- Ritala A, Aspegren K, Kurtén U, Salmenkallio-Marttila M, Mannonen L, Hannus R, Kauppinen V, Teeri T, Enari T-M. Fertile transgenic barley by particle bombardment of immature embryos. Plant Mol Biol. 1994;24:317–325. doi: 10.1007/BF00020170. [DOI] [PubMed] [Google Scholar]
- Schmidt EDL, Guzzo F, Toonen MAJ, de Vries SC. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124:2049–2062. doi: 10.1242/dev.124.10.2049. [DOI] [PubMed] [Google Scholar]
- Sidorov V, Gilbertson L, Addae P, Duncan D. Agrobacterium-mediated transformation of seedling-derived maize callus. Plant Cell Rep. 2006;25:320–328. doi: 10.1007/s00299-005-0058-5. [DOI] [PubMed] [Google Scholar]
- Somers DA, Rines HW, Gu W, Kaeppler HF, Bushnell WR. Fertile, transgenic oat plants. Nat Biotechnol. 1992;10:1589–1594. doi: 10.1038/nbt1292-1589. [DOI] [Google Scholar]
- Srivastava V, Anderson OD, Ow DW. Single-copy transgenic wheat generated through the resolution of complex integration patterns. Proc Natl Acad Sci USA. 1999;96:11117–11121. doi: 10.1073/pnas.96.20.11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitashev S, Schwartz C, Lenderts B, Young JK, Mark Cigan A. Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nat Commun. 2016;7:1–7. doi: 10.1038/ncomms13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingay S, McElroy D, Kalla R, Fieg S, Wang M, Thornton S, Brettell R. Agrobacterium tumefaciens-mediated barley transformation. Plant J. 1997;11:1369–1376. doi: 10.1046/j.1365-313X.1997.11061369.x. [DOI] [Google Scholar]
- Vasil V, Castillo AM, Fromm ME, Vasil IK. Herbicide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Nat Biotechnol. 1992;10:667–674. doi: 10.1038/nbt0692-667. [DOI] [Google Scholar]
- Vega JM, Yu W, Kennon AR, Chen X, Zhang ZJ. Improvement of Agrobacterium-mediated transformation in Hi-II maize (Zea mays) using standard binary vectors. Plant Cell Rep. 2008;27:297–305. doi: 10.1007/s00299-007-0463-z. [DOI] [PubMed] [Google Scholar]
- Wu H, Sparks C, Amoah B, Jones HD. Factors influencing successful Agrobacterium-mediated genetic transformation of wheat. Plant Cell Rep. 2003;21:659–668. doi: 10.1007/s00299-002-0564-7. [DOI] [PubMed] [Google Scholar]
- Wu E, Lenderts B, Glassman K, Berezowska-Kaniewska M, Chris-tensen H, Asmus T, Zhen S, Chu U, Cho M-J, Zhao Z-Y. Optimized Agrobacterium-mediated sorghum transformation protocol and molecular data of transgenic sorghum plants. In Vitro Cell Dev Plant. 2014;50:9–18. doi: 10.1007/s11627-013-9583-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Perales M, Gruel J, Girke T, Jönsson H, et al. WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev. 2011;25:2025–2030. doi: 10.1101/gad.17258511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Perales M, Gruel J, Ohno C, Heisler M, et al. Plant stem cell maintenance involves direct transcriptional repression of differentiation program. Mol Syst Biol. 2013;9:1–13. doi: 10.1038/msb.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xing A, Staswick P, Clemente TE. The use of glufosinate as a selective agent in Agrobacterium-mediated transformation of soybean. Plant Cell Tissue Organ Cult. 1999;56:37–46. doi: 10.1023/A:1006298622969. [DOI] [Google Scholar]
- Zhang S, Williams-Carrier R, Lemaux PG. Transformation of recalcitrant maize elite inbred lines using in vitro shoot meristematic cultures induced from germinated seedlings. Plant Cell Rep. 2002;21:263–270. doi: 10.1007/s00299-002-0513-5. [DOI] [Google Scholar]
- Zhao ZY, Cai T, Tagliani L, Miller M, Wang N, Pang H, Rudert M, Schroeder S, Hondred D, Seltzer J, Pierce D. Agrobacterium-mediated sorghum transformation. Plant Mol Biol. 2000;44:789–798. doi: 10.1023/A:1026507517182. [DOI] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Frugis G, Chua NH. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002;30:349–359. doi: 10.1046/j.1365-313X.2002.01289.x. [DOI] [PubMed] [Google Scholar]