Abstract
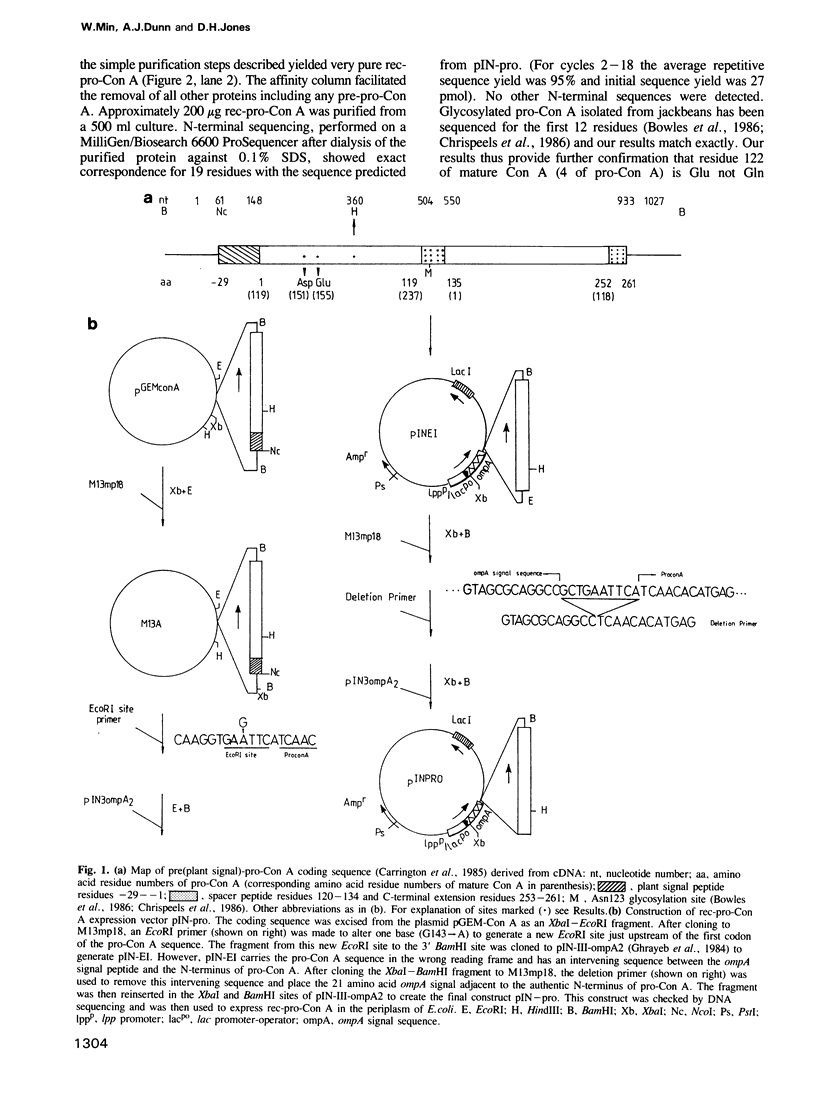
The complex post-translational processing of concanavalin A (Con A) in maturing jackbeans is unique because the non-glycosylated mature active protein is circularly permuted in primary sequence relative to its own inactive precursor (glycosylated pro-Con A) and to other legume lectins. We show here that non-glycosylated pro-Con A expressed in bacteria from recombinant cDNA (rec-pro-Con A) folds in vivo and in vitro to a stable form which is active without further processing. N-glycosylation alone must therefore be sufficient to inactivate pro-Con A--a novel role for glycosylation in regulating activity during protein maturation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles D. J., Marcus S. E., Pappin D. J., Findlay J. B., Eliopoulos E., Maycox P. R., Burgess J. Posttranslational processing of concanavalin A precursors in jackbean cotyledons. J Cell Biol. 1986 Apr;102(4):1284–1297. doi: 10.1083/jcb.102.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Pappin D. J. Traffic and assembly of concanavalin A. Trends Biochem Sci. 1988 Feb;13(2):60–64. doi: 10.1016/0968-0004(88)90030-8. [DOI] [PubMed] [Google Scholar]
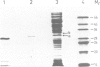
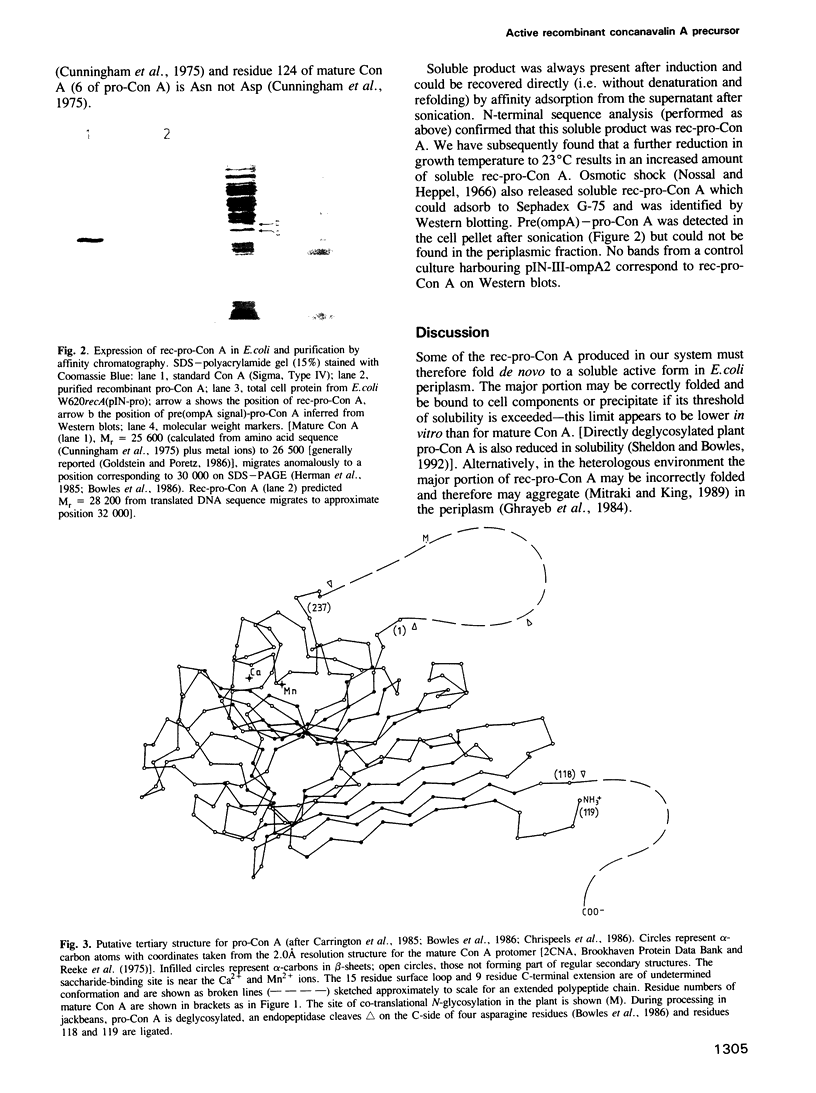
- Carrington D. M., Auffret A., Hanke D. E. Polypeptide ligation occurs during post-translational modification of concanavalin A. Nature. 1985 Jan 3;313(5997):64–67. doi: 10.1038/313064a0. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Hartl P. M., Sturm A., Faye L. Characterization of the endoplasmic reticulum-associated precursor of concanavalin A. Partial amino acid sequence and lectin activity. J Biol Chem. 1986 Aug 5;261(22):10021–10024. [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Hopp T. P., Edelman G. M. Favin versus concanavalin A: Circularly permuted amino acid sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3218–3222. doi: 10.1073/pnas.76.7.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Wang J. L., Waxdal M. J., Edelman G. M. The covalent and three-dimensional structure of concanavalin A. II. Amino acid sequence of cyanogen bromide fragment F3. J Biol Chem. 1975 Feb 25;250(4):1503–1512. [PubMed] [Google Scholar]
- Ghrayeb J., Kimura H., Takahara M., Hsiung H., Masui Y., Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984 Oct;3(10):2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D. P. Circularly permuted proteins. Protein Eng. 1989 May;2(7):493–495. doi: 10.1093/protein/2.7.493. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Jr, Becker J. W., Edelman G. M. The covalent and three-dimensional structure of concanavalin A. IV. Atomic coordinates, hydrogen bonding, and quaternary structure. J Biol Chem. 1975 Feb 25;250(4):1525–1547. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectin biochemistry. New way of protein maturation. Nature. 1986 Sep 18;323(6085):203–204. doi: 10.1038/323203a0. [DOI] [PubMed] [Google Scholar]
- Sheldon P. S., Bowles D. J. The glycoprotein precursor of concanavalin A is converted to an active lectin by deglycosylation. EMBO J. 1992 Apr;11(4):1297–1301. doi: 10.1002/j.1460-2075.1992.tb05173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M. E., Carver J. P., Dunn R. J. Production of pea lectin in Escherichia coli. J Biol Chem. 1986 May 15;261(14):6141–6144. [PubMed] [Google Scholar]
- Wurtzel E. T., Movva N. R., Ross F. L., Inouye M. Two-step cloning of the Escherichia coli regulatory gene ompB, employing phage Mu. J Mol Appl Genet. 1981;1(1):61–69. [PubMed] [Google Scholar]
- Yamauchi D., Minamikawa T. Structure of the gene encoding concanavalin A from Canavalia gladiata and its expression in Escherichia coli cells. FEBS Lett. 1990 Jan 15;260(1):127–130. doi: 10.1016/0014-5793(90)80083-u. [DOI] [PubMed] [Google Scholar]