Highlights
-
•
Intravoxel incoherent motion may predict response to breast neoadjuvant treatment.
-
•
Histogram analysis amplifies IVIM by quantifying spatial heterogeneity.
-
•
Pseudodiffusvity (Dp) showed the highest potential for response prediction.
Keywords: Breast cancer, Diffusion weighted MRI, Intravoxel incoherent motion, Neoadjuvant treatment, Response evaluation criteria in solid tumors
Abstract
Objective
To examine the prognostic capabilities of intravoxel incoherent motion (IVIM) metrics and their ability to predict response to neoadjuvant treatment (NAT). Additionally, to observe changes in IVIM metrics between pre- and post-treatment MRI.
Methods
This IRB-approved, HIPAA-compliant retrospective study observed 31 breast cancer patients (32 lesions). Patients underwent standard bilateral breast MRI along with diffusion-weighted imaging before and after NAT. Six patients underwent an additional IVIM-MRI scan 12–14 weeks after initial scan and 2 cycles of treatment. In addition to apparent diffusion coefficients (ADC) from monoexponential decay, IVIM mean values (tissue diffusivity Dt, perfusion fraction fp, and pseudodiffusivity Dp) and histogram metrics were derived using a biexponential model. An additional filter identified voxels of highly vascular tumor tissue (VTT), excluding necrotic or normal tissue. Clinical data include histology of biopsy and clinical response to treatment through RECIST assessment. Comparisons of treatment response were made using Wilcoxon rank-sum tests.
Results
Average, kurtosis, and skewness of pseudodiffusion Dp significantly differentiated RECIST responders from nonresponders. ADC and Dt values generally increased (∼70%) and VTT% values generally decreased (∼20%) post-treatment.
Conclusion
Dp metrics showed prognostic capabilities; slow and heterogeneous pseudodiffusion offer poor prognosis. Baseline ADC/Dt parameters were not significant predictors of response. This work suggests that IVIM mean values and heterogeneity metrics may have prognostic value in the setting of breast cancer NAT.
1. Introduction
Diffusion weighted imaging (DWI) can be useful in characterizing cancerous tissue heterogeneity, motivating its broad adoption in oncologic management. While random water diffusion is found in all fluids, biological tissues can contribute to both active transport or pseudodiffusion effects to which DWI is sensitive; this is especially true with highly vascularized cancer tissue. Through DWI, biomarkers have been developed that are sensitive to microvascular flow via intravoxel incoherent motion (IVIM) analysis [1].
Using IVIM, one can quantify the tumor hypervascularity and hypercellularity, which has been shown in a range of breast cancer studies [2], [3], [4], [5], [6], [7], [8]. Furthermore, histogram analysis of the spatial distribution of IVIM parameters can provide additional markers (e.g. skewness and kurtosis) for characterization of the heterogeneity of the tumor microenvironment [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12].
While IVIM metrics can distinguish between benign and malignant breast lesions [2], [3], [5], [11], [13], there is also interest in determining whether these IVIM metrics can predict treatment response or segment patient populations based on degree of response to treatment. Neoadjuvant treatment (NAT), which has traditionally been used for locally advanced breast cancer [14], is increasingly used in operable breast cancer because it can be as effective as adjuvant chemotherapy. Additionally, it allows breast conservation in women who historically required mastectomy. Moreover, response to NAT has been shown to vary with breast cancer molecular subtype with the best possible outcome, pathological complete response (pCR), being associated with improved disease free survival [14], [15]. However, pCR rates after NAT are around 20–30% [15], [16], and broken down by molecular subtype, they range from less than 10% for Luminal A to 50–60% for Her2-positive, ER negative subtypes [17], [18]. Accordingly, NAT can be used as an in vivo test for chemo-sensitivity, thereby providing prognostic information with respect to long term response to treatment [16]. Therefore, there is motivation to better understand, segment, and develop prognoses for the patient population that does not report pCR.
A key advantage of an imaging marker of response to NAT is an early stratification between patients who have pCR, those who show response to NAT, and those who do not. Such a biomarker will provide utility in treatment planning as clinicians can assess the effectiveness of treatments early to avoid debilitating side effects, minimize treatment costs, and expedite alternative treatment protocols to increase overall survival. At present, pre- and post-NAT contrast-enhanced MRI (CE-MRI) are commonly used to evaluate response of a tumor to NAT [19]. However, there are limitations to CE-MRI as well as risks in using contrast agents for the minority of patients with impaired kidney function. Therefore, finding an alternative non-contrast imaging tool capable of predicting treatment response in the NAT setting is highly valued. Finally, beyond the highly desirable outcome of pCR in NAT, quantitative imaging can provide guidance on improved operability through tumor size reduction. Even when pCR is not achieved in the pre-surgical period, tumors can be dramatically downsized for excision and adjuvant treatment. The ability of quantitative imaging, such as IVIM, to stratify these partial responders is an area of great potential.
Conventional diffusion weighted imaging quantified by apparent diffusion coefficient (ADC) has an increasing track record in prognostic applications with a growing literature in the case of breast cancer [20], [21], [22]. The IVIM method has recently been applied to neoadjuvant response prediction in breast cancer studies after therapy [10], [23], considering mean values of IVIM metrics. To more extensively explore the prognostic utility of IVIM, in this study, we examine breast cancer patients using pre- and post-treatment imaging and compare the metrics from IVIM histogram analysis with treatment response.
2. Methods
2.1. Patients
This IRB approved, HIPAA-compliant retrospective study observed 31 breast cancer patients with 32 lesions (31 invasive ductal carcinoma and 1 invasive lobular carcinoma). All patients underwent two sets of MRI scans during April 2011 to March 2013 (‘pre-treatment scans’ and ‘post-treatment scans') accompanied by dose-dense adjuvant doxorubicin-based treatments (N = 24), taxol-based treatments (N = 9), and docetaxel-based treatments (N = 3). All patients had their first treatment within 30 days of their first MRI exam. Patients were diagnosed with breast cancer through stereotactic, US, or MRI core biopsy. Table 1 shows patients’ lesion characteristics and scanner magnetic field values. Final histopathology diagnosis was confirmed through histology, surgery, and clinical follow-up. Additionally, 6 of these patients underwent an extended post-treatment MRI scan with a multi b value diffusion-weighted imaging (DWI) approximately 16 weeks after the initiation of therapy (‘post-treatment IVIM scans’).
Table 1.
Patient Lesion Characteristics. Numbers reported are N (%)a.
| All patients (N = 32) | Patients with ‘post-treatment IVIM scans' (N = 6) | |
|---|---|---|
| Type | ||
| IDC | 31 (96.9) | 6 (100) |
| ILC | 1 (3.1) | 0 (0) |
| Histology Grade | ||
| 2 | 2 (6.2) | 0 (0) |
| 3 | 28 (87.5) | 6 (100) |
| NA | 2 (6.2) | 0 (0) |
| Nuclear Grade | ||
| 2 | 8 (25) | 2 (33.3) |
| 3 | 18 (56.2) | 4 (66.7) |
| NA | 6 (18.8) | 0 (0) |
| Subtypes | ||
| HER2+ | 4 (12.5) | 1 (16.7) |
| Luminal-A | 12 (37.5) | 1 (16.7) |
| Luminal-B | 8 (25) | 3 (50) |
| Triple Negative | 8 (25) | 1 (16.7) |
| LVI | ||
| Negative | 7 (21.9) | 0 (0) |
| Positive | 24 (75) | 6 (100) |
| NA | 1 (3.1) | 0 (0) |
| Pathological Complete Response | ||
| pCR | 4 (14.5) | 0 (0) |
| No pCR | 28 (87.5) | 6 (100) |
| RECIST | ||
| CR or PR | 27 (84.4) | 5 (83.3) |
| PD or SD | 5 (15.6) | 1 (16.7) |
| Magnetic Field | ||
| 1.5T | 7 (21.9) | 2 (33.3) |
| 3T | 25 (78.1) | 4 (66.7) |
Abbreviations: IDC − Invasive Ductal Carcinoma, ILC − Invasive Lobular Carcinoma, SD/PD − Stable/Progressive Disease, PR/CR − Partial/Complete Response, LVI − Lymphovascular invasion, NA − Not Available.
2.2. MRI scans
All patients underwent two standard bilateral breast MRI (pre-/post-treatment) using contrast enhancement in a full body 1.5 or 3T MRI scanner system (Discovery MR450/750; GE Healthcare, Waukesha, WI) with either an 8 (3T) or 16 channel (1.5T) breast coil (Sentinelle Medical, Toronto, Canada), MR examinations included fat-suppressed T1- and T2-weighted imaging before and at three time points after the administration of intravenous contrast. All patients (n = 31) also underwent a multi b value DWI protocol ('pre-treatment IVIM scans') consisted of a single shot spin echo EPI sequence (TR/TE = 4000/85.3 ms; 4 averages; FOV = 28 × 28 to 36 × 36 cm2; slice thickness: 4–5 mm; acquired matrix: 128 × 128, interpolated to 256 × 256; 19–35 slices; and 10 b values, with b = 0, 30, 60, 90, 120, 250, 400, 600, 800, 1000 s/mm2), while 6 of 31 patients underwent a second multi b value DWI protocol ('post-treatment IVIM scans').
2.3. Image analysis
A breast radiologist (L.G..) identified lesions on anatomical post contrast T1 weighted images based on hyperintense signals from axial images, then region of interests (ROIs) were drawn on the DWI by another operator (G.Y.C.). Quantitative analysis of DWI was performed with custom analysis code (Igor Pro 6, Wavemetrics, Inc., Portland, USA) [2]. Images were first analyzed voxel-by-voxel using a monoexponential decay model with all b values to produce apparent diffusion coefficient (ADC) maps for all slices:
| (1) |
ADC maps, DWI images, and post-contrast T1-weighted images guided lesion segmentation for quantitative IVIM. ROIs were drawn on the largest single slice tumor area and analyzed for all b values. The cross-sectional area of the lesion as measured on DWI was recorded for each patient.
To distinguish signal attenuation due to microcirculation-induced spin dephasing from diffusion induced signal attenuation, a biexponential analysis was used to quantify microvascular and tissue structural properties. The signal decay curve was measured as a function of increasing b value and fitted to a biexponential equation:
| (2) |
where M0 is total reference magnetization, fp is perfusion fraction, Dp is pseudo-diffusivity, and Dt is tissue diffusivity. For all analyses, a “segmented” approach was employed by fitting the higher b value region (b > 200 s/mm2) to calculate IVIM parameters, fp and Dt, and then extracting Dp from a constrained fit to the entire b value set [2].
In addition, implementation of a vascular tumor tissue (VTT) filter was used for parametric map analysis [24]. Since voxel-wise IVIM analysis allows the delineation of viable tumor tissue within a typically heterogeneous texture of cellularity, necrosis, and normal tissue, one can apply a filter (VTT filter) that selectively chooses voxels that are most critical or relevant, i.e. highly vascular tissue that signifies the most aggressive regions of the cancer. This filter, based on the Aikake information criterion to test applicability of each voxel’s biexponential fit [24] provided the number of voxels of vascular tumor tissue, as a fraction of total lesion voxels. Finally, histogram analysis on all lesion voxels was performed to quantify tumor heterogeneity [2] with mean, maximum, minimum and heterogeneity metrics (standard deviation, skewness and kurtosis). The full workflow employed was described previously [2].
2.4. Clinical data
Data collected include histology of biopsy and surgical specimens to determine pathological complete response, (pCR). Another method of observing response was the RECIST criteria, where tumor size reduction was measured using pre- and post-treatment post-contrast T1 images [Complete response (CR) − disappearance of target lesion; partial response (PR) − at least 30% decrease in the sum of diameters of the target lesion, taking as reference the baseline sum of diameters; progressive disease (PD) − at least 20% increase in the sum of diameters of the target lesion; and stable disease (SD) − neither sufficient shrinkage for PR or sufficient enlargement for PD] [25]. One patient was excluded for lack of response data while 1 smaller lesion (<2 cm) was excluded. Table 1 shows patients’ prognostic factor data. IVIM biomarkers were tested for prognosis of response as defined by RECIST criteria.
2.5. Statistical analysis
One subject contributed two observations due to bilateral breast cancer; all other subjects contributed only one observation. Differences in IVIM parameters by treatment response were tested using the Wilcoxon rank-sum test. Adjustment for multiple comparisons was made using the false discovery rate (FDR) method, which controls the expected proportion of null hypotheses that are incorrectly rejected. A p-value < 0.05 was considered statistically significant. P-values are shown both before and after adjustment for multiple comparisons. Statistical analyses were conducted in R software version 3.1.1 (R Core Development Team, Vienna, Austria).
3. Results
3.1. Patients
Mean age of all patients was 47.40 ± 8.84 years (50.18 ± 10.51 years for responders, 46.26 ± 11.66 years for nonresponders) with a range of 28–66 years. All analyzed lesions had a diameter that was greater than 2 cm on contrast-enhanced imaging and median (minimum, maximum) ROI size from DWI segmentation was 13.84 (3.43, 44.45) cm2. Additionally, all patients with pCR were considered responders according to RECIST criteria (all patients with RECIST criteria of CR were determined to be pCR); all other patients with other RECIST criteria were reported to have no pCR. A total of twenty four patients underwent a 3T MRI pre-treatment scan, while seven patients underwent a 1.5T MRI pre-treatment scan. Among the six patients who had both a pre- and post-treatment scan, two patients underwent a 1.5T MRI pre-treatment then a 3T MRI post-treatment scan while one patient had a pre-treatment 3T scan and a 1.5T post-treatment scan. The other three patients all received 3T MRI pre-/post-treatment scans.
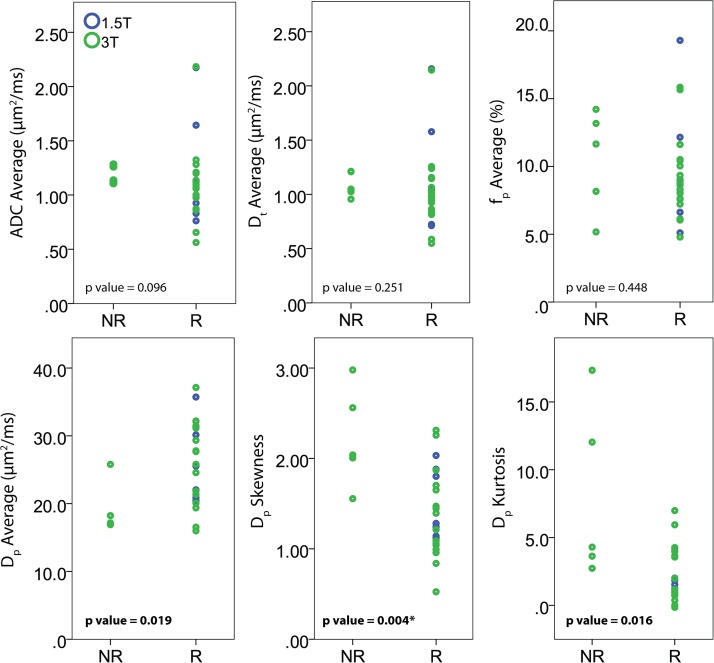
For the pre-treatment data, the median (minimum, maximum) value of average ADC found for all lesions was 1.10 (0.56, 2.18) μm2/ms, with 1.11 (0.56, 2.18) μm2/ms for all 3T results and 1.10 (0.76, 2.17) μm2/ms for all 1.5T results. The median (minimum, maximum) values of average Dt, fp, and Dp found for all lesions were 1.02 (0.55, 2.16) μm2/ms, 8.8 (4.8, 19.3) %, and 25.05 (15.99, 37.14) μm2/ms, respectively. The median (minimum, maximum) values for IVIM metrics stratified by field strength were Dt = 1.02 (0.55, 2.15) μm2/ms, fp = 8.9 (4.8, 15.8) %, and Dp = 25.52 (15.99, 37.14) μm2/ms for 3T, and Dt = 1.04 (0.71, 2.16) μm2/ms, fp = 8.7 (5.1, 19.3) %, and Dp = 22.04 (20.11, 35.73) μm2/ms for 1.5T. The values at different field strengths were similar (Fig. 1), and no significant differences were found between metrics obtained from different magnetic field strengths.
Fig. 1.
IVIM metrics in breast cancer patients stratified by field strength and RECIST response group. Unadjusted p-values for comparison of responders (R) to non-responders (NR) are shown.
3.2. Comparison of pre-treatment IVIM with clinical response to treatment
The median (minimum, maximum) values of average ADC for RECIST nonresponders versus responders to treatment was 1.14 (1.11, 1.28) μm2/ms versus 1.07 (0.56, 2.18) μm2/ms. For median (minimum, maximum) values of average Dt, fp, and Dp, the average values were 1.05 (0.96, 1.21) μm2/ms, 11.7 (5.2, 14.2)%, and 17.16 (16.9, 25.79) μm2/ms for nonresponders, while for responders they were 0.99 (0.55, 2.16) μm2/ms, 8.7 (4.8, 19.3)%, and 25.54 (15.99, 37.14) μm2/ms. The pre-treatment median (minimum, maximum) lesion size on DWI was 13.80 (3.43, 37.0) cm2 for RECIST responders and 23.98 (8.73, 44.45) cm2 for RECIST nonresponders; these values were not found to be significantly different. Table 2 shows ADC and IVIM average and histogram metric median values as well as VTT% and lesion size as a function of response to treatment. Average, skewness, and kurtosis of the Dp parameters significantly differentiated between RECIST responders and nonresponders to treatment before adjustment for multiple comparisons (Fig. 1). Furthermore, VTT% nearly significantly differentiated (p = 0.52) between RECIST responders and nonresponders (median values − 30.0% versus 40.7%, respectively) prior to adjustment for multiple comparisons. These significant findings also held true when selecting for patients who underwent 3T MRI scans. Once the results were adjusted using multiple comparisons, none of the findings were considered significant though Dp skewness merits further study. In addition, ADC and Dt had lower values for responders to treatment, but were not found to be significant response predictors.
Table 2.
Pre-treatment IVIM average and histogram metric median (min, max) values between RECIST responders and non-responders. Raw and adjusted p-values for Wilcoxon rank-sum test comparing responders to non-responders are given, with significant group differences in bold.
| Overall (N = 32) | Responders (N = 27) | Nonresponders (N = 5) | p | adj. p | ||
|---|---|---|---|---|---|---|
| ADC (μm2/ms) | Maximum | 2.16 (1.15, 3.00) | 2.20 (1.15, 3.00) | 2.13 (1.65, 2.93) | 0.938 | 0.938 |
| Minimum | 0.49 (0.01, 1.34) | 0.48 (0.01, 1.34) | 0.54 (0.05, 0.67) | 0.697 | 0.914 | |
| Kurtosis | 0.98 (−1.17, 4.90) | 0.97 (−1.17, 4.90) | 1.07 (−0.30, 4.83) | 0.815 | 0.938 | |
| Skewness | 0.73 (−1.33, 2.05) | 0.78 (−1.33, 2.05) | 0.21 (−0.72, 1.80) | 0.337 | 0.914 | |
| Average | 1.10 (0.56, 2.18) | 1.07 (0.56, 2.18) | 1.14 (1.11, 1.28) | 0.103 | 0.453 | |
| St. Dev. | 0.30 (0.10, 0.61) | 0.30 (0.10, 0.61) | 0.31 (0.14, 0.37) | 0.856 | 0.938 | |
| Dt (μm2/ms) | Maximum | 1.91 (1.12, 2.89) | 1.90 (1.12, 2.89) | 1.96 (1.64, 2.35) | 0.452 | 0.914 |
| Minimum | 0.42 (0.01, 1.37) | 0.4 (0.01, 1.37) | 0.48 (0.05, 0.62) | 0.938 | 0.938 | |
| Kurtosis | 0.76 (−1.08, 4.52) | 0.74 (−1.08, 4.43) | 1.01 (−0.42, 4.52) | 0.736 | 0.914 | |
| Skewness | 0.64 (−1.23, 1.94) | 0.78 (−1.23, 1.94) | 0.27 (−0.61, 1.36) | 0.659 | 0.914 | |
| Average | 1.02 (0.55, 2.16) | 0.99 (0.55, 2.16) | 1.05 (0.96, 1.21) | 0.287 | 0.914 | |
| St. Dev. | 0.26 (0.08, 0.59) | 0.25 (0.08, 0.59) | 0.26 (0.14, 0.35) | 0.938 | 0.938 | |
| fp | Maximum (%) | 29.8 (12.2, 83.1) | 29.6 (12.2, 83.1) | 32.5 (15.9, 69.2) | 0.622 | 0.914 |
| Kurtosis | 0.72 (−0.85, 23.56) | 0.62 (−0.85, 23.56) | 3.37 (−0.85, 5.61) | 0.697 | 0.914 | |
| Skewness | 0.96 (0.04, 4.54) | 0.96 (0.27, 4.54) | 1.73 (0.04, 1.88) | 0.622 | 0.914 | |
| Average (%) | 8.8 (4.8, 19.3) | 8.7 (4.8, 19.3) | 11.7 (5.2, 14.2) | 0.483 | 0.914 | |
| St. Dev. (%) | 5.6 (2.5, 17.0) | 5.2 (2.5, 17.0) | 7.2 (3.2, 11.4) | 0.586 | 0.914 | |
| Dp (μm2/ms) | Maximum | 96.43 (71.36, 99.94) | 96.42 (71.36, 99.8) | 97.42 (80.7, 99.94) | 0.659 | 0.914 |
| Minimum | 0.66 (0.01, 3.07) | 0.80 (0.03, 3.07) | 0.27 (0.01, 2.16) | 0.186 | 0.690 | |
| Kurtosis | 2.37 (−0.15, 17.33) | 1.98 (−0.15, 6.99) | 4.29 (2.73, 17.33) | 0.021 | 0.181 | |
| Skewness | 1.42 (0.52, 2.98) | 1.24 (0.52, 2.31) | 2.04 (1.55, 2.98) | 0.005 | 0.143 | |
| Average | 25.05 (15.99, 37.14) | 25.54 (15.99, 37.14) | 17.16 (16.9, 25.79) | 0.018 | 0.181 | |
| St. Dev. | 17.04 (7.49, 23.44) | 16.82 (14.38, 23.44) | 19.24 (7.49, 19.71) | 0.622 | 0.914 | |
| VTT% | 35.0 (10.3, 72) | 30.0 (10.3, 64.8) | 40.7 (38.4, 72) | 0.052 | 0.335 | |
| Lesion Size (cm2) | 13.84 (3.43, 44.45) | 13.80 (3.43, 37.00) | 23.98 (8.73, 44.45) | 0.248 | 0.607 | |
3.3. Comparison of pre- and post-treatment IVIM
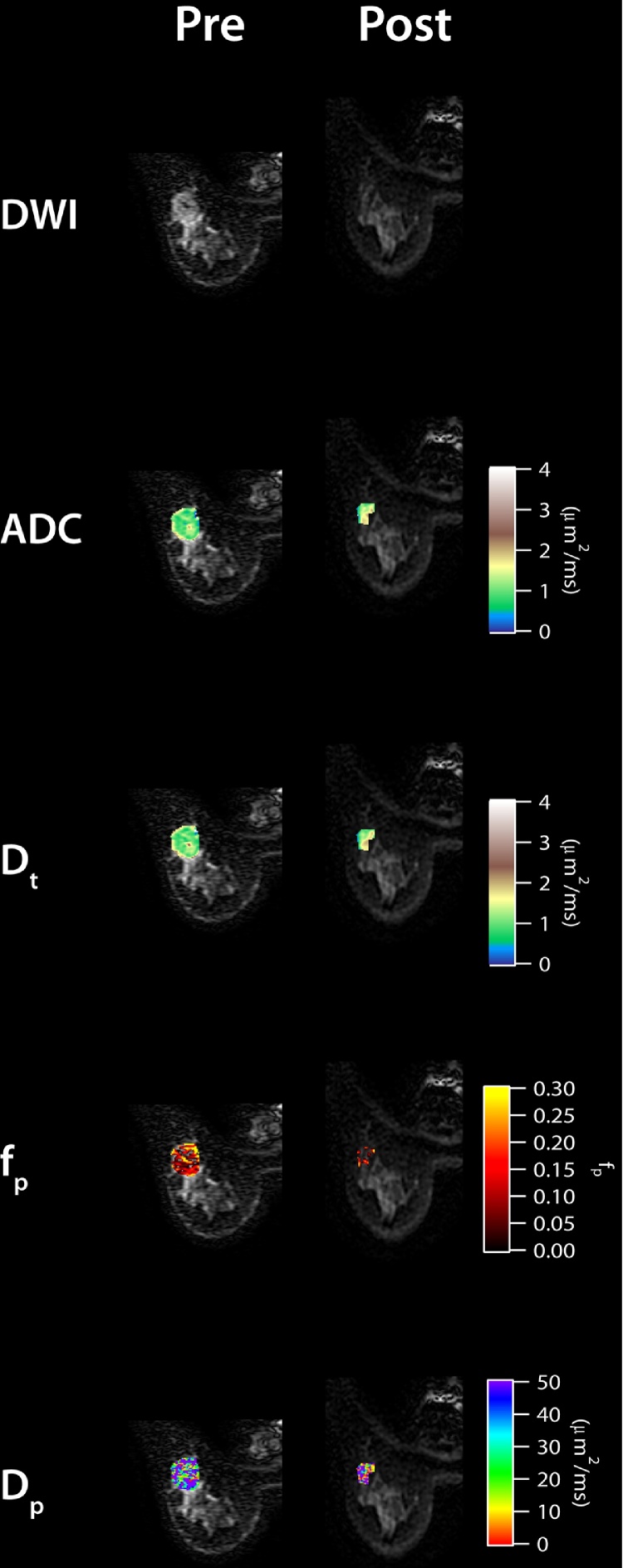
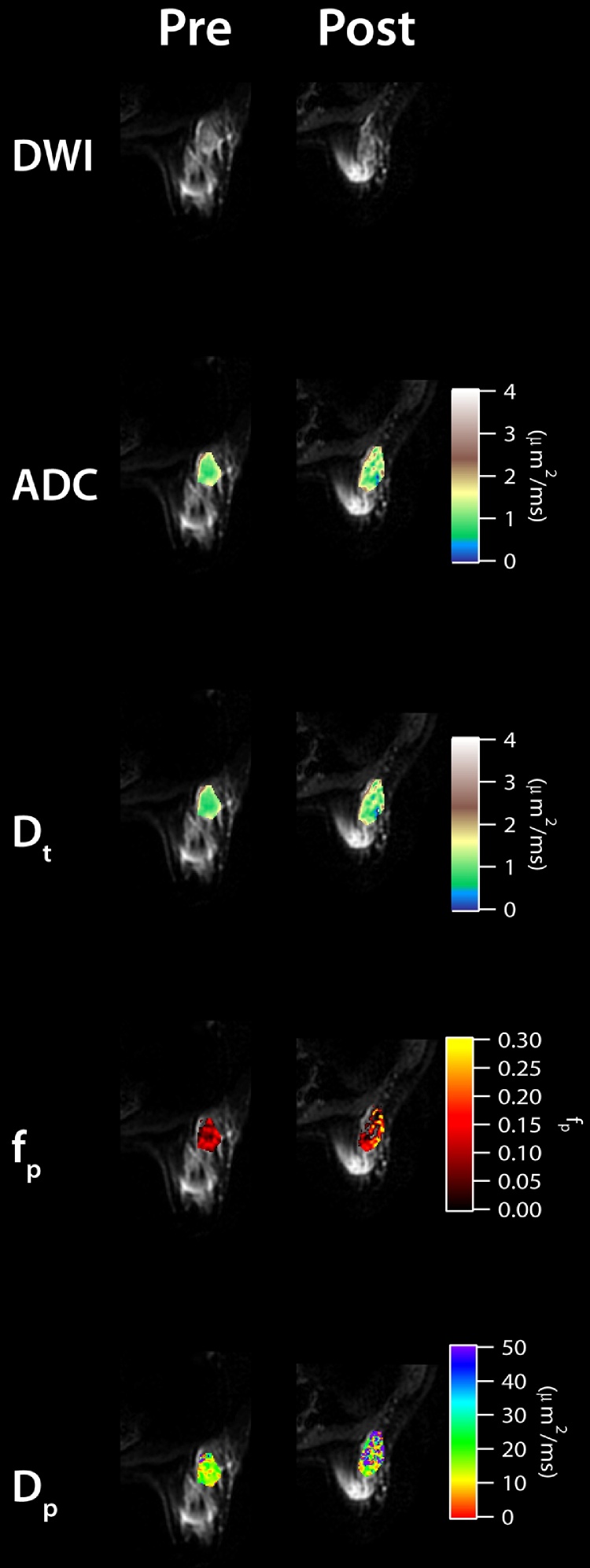
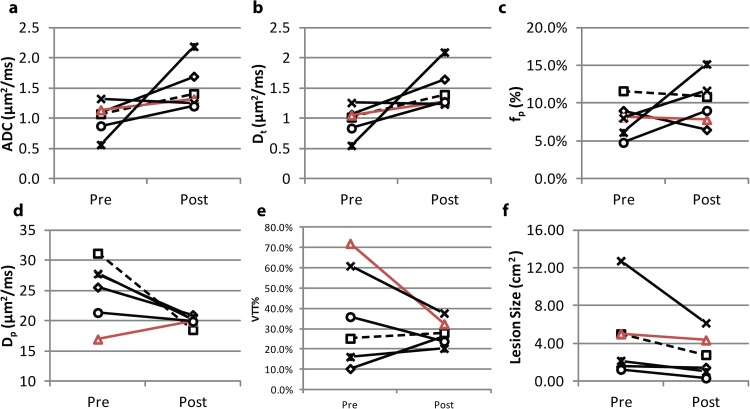
Next, we observe that the IVIM parameters changed when comparing between pre- and post-treatment MRI scans in six patients. Fig. 2 shows the parametric maps of pre- and post-treatment of a RECIST responding patient with invasive ductal carcinoma (IDC), while Fig. 3 shows the pre- and post-treatment maps of a RECIST nonresponding patient with IDC. Lesion size reduction is clearly observable in the responding patient while ADC and IVIM parameters also show noticeable differences with treatment (Fig. 2). ADC and Dt values show increases while fp and Dp are more heterogeneous in the post-treatment maps. In the nonresponding patient, the change in lesion size is not noticeable; however, some increased heterogeneity is seen in the post-treatment maps (Fig. 3). At the group level, ADC and Dt values increased (+70.4% and +74.1%, respectively) and VTT% values decreased post-treatment (−21.0%). Average tumor size reduction was −40.2%. Five dual scanned patients were responders while one patient was a nonresponder. Fig. 4 shows IVIM, VTT%, and lesion size values for each patient before and after treatment. Dp values generally decreased post-treatment (Fig. 4).
Fig. 2.
Pre- and post-treatment IVIM maps for a RECIST responding patient (no pCR) with invasive ductal carcinoma.
Fig. 3.
Pre- and post-treatment IVIM maps for a RECIST nonresponding patient with invasive ductal carcinoma.
Fig. 4.
IVIM metrics (a-d), VTT% (e), and pre-/post-treatment size (cm2) (f) for the six patients with pre-/post-treatment scans. Red line (triangle symbol) indicates the patient who was labeled as a RECIST nonresponder (Fig. 3). The RECIST responding patient from Fig. 2 is indicated by the dashed line. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Comparisons of IVIM metrics with clinical response results showed that certain IVIM parameters were able to differentiate between RECIST responders and nonresponders. Among baseline measurements, Dp and VTT% were the most prognostic as high vascularity with slow and heterogeneous pseudodiffusion offering poor prognosis; in a related sense, decreases in Dp occurred in all dual-scanned responders. Moreover, the heterogeneity metrics of Dp showed the most interesting results and demonstrated the utility of using advanced metrics within IVIM analysis. The significant findings from histogram analysis show the potential to identify differences in tumor heterogeneity between responders and nonresponders. These Dp and VTT% results illustrate that the vascular entities from lesions may be most useful at predicting response to NAT, and characterization of heterogeneous distribution of blood volume may be an ideal predictor of response. Some recent cancer studies have noticed IVIM differences when comparing between responders and nonresponders [10], [26], showing that vascular, along with cellular, IVIM parameters can be predictive of response [10]. These observations are similar to the results found in this study. Beyond this trend, the expansion of IVIM analysis using histograms in this study can lend further support to better characterize the lesion vascularity.
It should be noted that breast diffusion MRI studies in the neoadjuvant setting used different ways to define response: four studies used pCR [10], [23], [27], [28] and three studies used RECIST [20], [21], [22]. In our study, we focused on RECIST to illustrate the potential of IVIM-MRI in predicting improved surgical operability of breast lesions. However, RECIST and pCR criteria did correlate in terms of those exhibiting complete response. Therefore, there remains strong motivation for having markers of response to NAT to improve treatment planning for surgery, increase operability, avoid debilitating side effects and expedite alternative treatments.
The IVIM parameters changes in patients who underwent pre- and post-treatment MRI scans may be due to the effects of doxorubicin, herceptin, or taxol-based NAT, which is known to change both the vascularity and cellularity of tumors based on their anti-angiogenic and cytotoxic effects [21], [29]. While the sample size (six patients), protocol variations (e.g. type of treatment, scan time after treatment, field strength, etc.), and infeasibility of inter-scan image registration (due to large morphologic changes) limited strong conclusions, general trends could still be observed between pre- and post-treatment scans.
Unlike previous studies [21], baseline values of ADC or Dt were not predictive of response in our cohort, although early increases in ADC and Dt values were observed to be largest in dual-scanned responders, suggesting a decline in cellularity. Baseline ADC trended towards significance, but in our cohort, this trend seems to have been dominated by vascular rather than cellular component as Dt showed no trends. Additionally, Che et al. [10] found baseline fp, mid-treatment fp and Dt, and pre/post changes to Dt and fp to predict response, while Bedair et al. [23] found baseline ADC, Dt, and stretched exponential distributed diffusion coefficient (DDC), as well as pre/post changes in ADC, DDC and fp to predict response. The lack of uniformity of protocol and patients in this study may have contributed to our different findings. However, this present study highlights the fact that both mean values and heterogeneity metrics from microvascular DWI parameters can have useful prognostic value in the setting of breast cancer.
This study was limited by several factors. First, patient population size was small, limiting statistical power and precluding multivariable analysis. Neither field strength nor neoadjuvant treatment protocol was controlled for in the enrollment paradigm; however, our initial observations showed very little bias based on these differences in field strength and studies have shown that protocols can be used to diminish the variations caused by differing field strength [29]. Future work will explore a standardized, uniform IVIM approach in the setting of breast cancer for more robust results. All of the significant findings did not persist when using multiple group comparison corrections; however, some findings, i.e. Dp skewness, showed results that are worthy of further study. Moreover, multiple comparison correction methods may often be too conservative for empirical research, especially in our case, when statistical tests are only performed where there is strong knowledge or understanding for expecting a certain result. While these corrections control for false positives, they do so at the potential expense of many more false negatives. Finally, there have been some concerns in regards to the low repeatability/reproducibility of certain IVIM parameters (fp, Dp) in some contexts [30] and we have not considered this aspect in this study.
In conclusion, this work represents a step forward in understanding the utility of diffusion MRI and IVIM. These tools can provide useful clinical, non-invasive biomarkers during cancer diagnosis and treatment. We demonstrate that several IVIM biomarkers show potential as predictors of treatment response to NAT. We also illustrate spatially dependent physiological changes that are observed after treatment. Further studies should be conducted to solidify the prognostic value of the IVIM biomarkers in longitudinal breast cancer studies for treatment and outcome monitoring.
Conflicts of interest
The authors declare that there no conflict of interest.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) Cancer Center Support Grant P30 CA08748 and by a grant from the Breast Cancer Research Foundation.
References
- 1.Le Bihan D., Breton E., Lallemand D., Aubin M.L., Vignaud J., Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 2.Cho G.Y., Moy L., Kim S.G., Baete S.H., Moccaldi M., Babb J.S., Sodickson D.K., Sigmund E.E. Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: comparison with malignant status, histological subtype, and molecular prognostic factors. Eur. Radiol. 2016;26(8):2547–2558. doi: 10.1007/s00330-015-4087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iima M., Yano K., Kataoka M., Umehana M., Murata K., Kanao S., Togashi K., Le Bihan D. Quantitative non-Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Invest. Radiol. 2015;50(4):205–211. doi: 10.1097/RLI.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 4.Suo S., Lin N., Wang H., Zhang L., Wang R., Zhang S., Hua J., Xu J. Intravoxel incoherent motion diffusion-weighted MR imaging of breast cancer at 3.0 tesla: comparison of different curve-fitting methods. J. Magn. Reson. Imaging: JMRI. 2015;42(2):362–370. doi: 10.1002/jmri.24799. [DOI] [PubMed] [Google Scholar]
- 5.Bokacheva L., Kaplan J.B., Giri D.D., Patil S., Gnanasigamani M., Nyman C.G., Deasy J.O., Morris E.A., Thakur S.B. Intravoxel incoherent motion diffusion-weighted MRI at 3.0 T differentiates malignant breast lesions from benign lesions and breast parenchyma. J. Magn. Reson. Imaging: JMRI. 2014;40(4):813–823. doi: 10.1002/jmri.24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigmund E.E., Cho G.Y., Kim S., Finn M., Moccaldi M., Jensen J.H., Sodickson D.K., Goldberg J.D., Formenti S., Moy L. Intravoxel incoherent motion imaging of tumor microenvironment in locally advanced breast cancer. Magn. Reson. Med. 2011;65(5):1437–1447. doi: 10.1002/mrm.22740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijkstra H., Dorrius M.D., Wielema M., Jaspers K., Pijnappel R.M., Oudkerk M., Sijens P.E. Semi-automated quantitative intravoxel incoherent motion analysis and its implementation in breast diffusion-weighted imaging. J. Magn. Reson. Imaging: JMRI. 2016;43(5):1122–1131. doi: 10.1002/jmri.25086. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y.J., Kim S.H., Kang B.J., Kang Y.J., Yoo H., Yoo J., Lee J., Son Y.H., Grimm R. Intravoxel incoherent motion (IVIM)-derived parameters in diffusion-weighted MRI: Associations with prognostic factors in invasive ductal carcinoma. J. Magn. Reson. Imaging: JMRI. 2017;45(5):1394–1406. doi: 10.1002/jmri.25514. [DOI] [PubMed] [Google Scholar]
- 9.Panek R., Borri M., Orton M., O'Flynn E., Morgan V., Giles S.L., deSouza N., Leach M.O., Schmidt M.A. Evaluation of diffusion models in breast cancer. Med. Phys. 2015;42(8):4833–4839. doi: 10.1118/1.4927255. [DOI] [PubMed] [Google Scholar]
- 10.Che S., Zhao X., Ou Y., Li J., Wang M., Wu B., Zhou C. Role of the intravoxel incoherent motion diffusion weighted imaging in the pre-treatment prediction and early response monitoring to neoadjuvant chemotherapy in locally advanced breast cancer. Medicine. 2016;95(4):e2420. doi: 10.1097/MD.0000000000002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C., Wang K., Chan Q., Liu Z., Zhang J., He H., Zhang S., Liang C. Intravoxel incoherent motion MR imaging for breast lesions: comparison and correlation with pharmacokinetic evaluation from dynamic contrast-enhanced MR imaging. Eur. Radiol. 2016;26(11):3888–3898. doi: 10.1007/s00330-016-4241-6. [DOI] [PubMed] [Google Scholar]
- 12.Ma D., Lu F., Zou X., Zhang H., Li Y., Zhang L., Chen L., Qin D., Wang B. Intravoxel incoherent motion diffusion-weighted imaging as an adjunct to dynamic contrast-enhanced MRI to improve accuracy of the differential diagnosis of benign and malignant breast lesions. Magn. Reson. Imaging. 2017;36:175–179. doi: 10.1016/j.mri.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q., Guo Y., Zhang J., Wang Z., Huang M., Zhang Y. Contribution of IVIM to conventional dynamic contrast-enhanced and diffusion-weighted MRI in differentiating benign from malignant Breast masses. Breast Care (Basel, Switzerland. 2016;11(4):254–258. doi: 10.1159/000447765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson A.M., Moulder-Thompson S.L. Neoadjuvant treatment of breast cancer. Ann. Oncol. 2012;23(Suppl 10):x231–x236. doi: 10.1093/annonc/mds324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colleoni M., Goldhirsch A. Neoadjuvant chemotherapy for breast cancer: any progress? Lancet Oncol. 2014;15(2):131–132. doi: 10.1016/S1470-2045(13)70584-9. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann M., von Minckwitz G., Mamounas E.P., Cameron D., Carey L.A., Cristofanilli M., Denkert C., Eiermann W., Gnant M., Harris J.R., Karn T., Liedtke C., Mauri D., Rouzier R., Ruckhaeberle E., Semiglazov V., Symmans W.F., Tutt A., Pusztai L. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann. Surg. Oncol. 2012;19(5):1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 17.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni L., Valagussa P., Swain S.M., Prowell T., Loibl S., Wickerham D.L., Bogaerts J., Baselga J., Perou C., Blumenthal G., Blohmer J., Mamounas E.P., Bergh J., Semiglazov V., Justice R., Eidtmann H., Paik S., Piccart M., Sridhara R., Fasching P.A., Slaets L., Tang S., Gerber B., Geyer C.E., Jr., Pazdur R., Ditsch N., Rastogi P., Eiermann W., von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 18.von Minckwitz G., Untch M., Blohmer J.U., Costa S.D., Eidtmann H., Fasching P.A., Gerber B., Eiermann W., Hilfrich J., Huober J., Jackisch C., Kaufmann M., Konecny G.E., Denkert C., Nekljudova V., Mehta K., Loibl S. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 19.Lobbes M.B., Prevos R., Smidt M., Tjan-Heijnen V.C., van Goethem M., Schipper R., Beets-Tan R.G., Wildberger J.E. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013;4(2):163–175. doi: 10.1007/s13244-013-0219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galban C.J., Ma B., Malyarenko D., Pickles M.D., Heist K., Henry N.L., Schott A.F., Neal C.H., Hylton N.M., Rehemtulla A., Johnson T.D., Meyer C.R., Chenevert T.L., Turnbull L.W., Ross B.D. Multi-site clinical evaluation of DW-MRI as a treatment response metric for breast cancer patients undergoing neoadjuvant chemotherapy. PLoS One. 2015;10(3):e0122151. doi: 10.1371/journal.pone.0122151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X.R., Cheng L.Q., Liu M., Zhang Y.J., Wang J.D., Zhang A.L., Song X., Li J., Zheng Y.Q., Liu L. DW-MRI ADC values can predict treatment response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. Med. Oncol. 2012;29(2):425–431. doi: 10.1007/s12032-011-9842-y. [DOI] [PubMed] [Google Scholar]
- 22.Sharma U., Danishad K.K., Seenu V., Jagannathan N.R. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22(1):104–113. doi: 10.1002/nbm.1245. [DOI] [PubMed] [Google Scholar]
- 23.Bedair R., Priest A.N., Patterson A.J., McLean M.A., Graves M.J., Manavaki R., Gill A.B., Abeyakoon O., Griffiths J.R., Gilbert F.J. Assessment of early treatment response to neoadjuvant chemotherapy in breast cancer using non-mono-exponential diffusion models: a feasibility study comparing the baseline and mid-treatment MRI examinations. Europeanradiology. 2017;27(7):2726–2736. doi: 10.1007/s00330-016-4630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S., Decarlo L., Cho G.Y., Jensen J.H., Sodickson D.K., Moy L., Formenti S., Schneider R.J., Goldberg J.D., Sigmund E.E. Interstitial fluid pressure correlates with intravoxel incoherent motion imaging metrics in a mouse mammary carcinoma model. NMR Biomed. 2012;25(5):787–794. doi: 10.1002/nbm.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., Rubinstein L., Shankar L., Dodd L., Kaplan R., Lacombe D., Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Nougaret S., Vargas H.A., Lakhman Y., Sudre R., Do R.K., Bibeau F., Azria D., Assenat E., Molinari N., Pierredon M.A., Rouanet P., Guiu B. Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-Section and volumetric analyses. Radiology. 2016;280(2):446–454. doi: 10.1148/radiol.2016150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bufi E., Belli P., Costantini M., Cipriani A., Di Matteo M., Bonatesta A., Franceschini G., Terribile D., Mule A., Nardone L., Bonomo L. Role of the apparent diffusion coefficient in the prediction of response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Clin. Breast Cancer. 2015;15(5):370–380. doi: 10.1016/j.clbc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Richard R., Thomassin I., Chapellier M., Scemama A., de Cremoux P., Varna M., Giacchetti S., Espie M., de Kerviler E., de Bazelaire C. Diffusion-weighted MRI in pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Eur. Radiol. 2013;23(9):2420–2431. doi: 10.1007/s00330-013-2850-x. [DOI] [PubMed] [Google Scholar]
- 29.Belli G., Busoni S., Ciccarone A., Coniglio A., Esposito M., Giannelli M., Mazzoni L.N., Nocetti L., Sghedoni R., Tarducci R., Zatelli G., Anoja R.A., Belmonte G., Bertolino N., Betti M., Biagini C., Ciarmatori A., Cretti F., Fabbri E., Fedeli L., Filice S., Fulcheri C.P., Gasperi C., Mangili P.A., Mazzocchi S., Meliado G., Morzenti S., Noferini L., Oberhofer N., Orsingher L., Paruccini N., Princigalli G., Quattrocchi M., Rinaldi A., Scelfo D., Freixas G.V., Tenori L., Zucca I., Luchinat C., Gori C., Gobbi G. M.R.I. Italian Association of Physics in Medicine Working Group on, Quality assurance multicenter comparison of different MR scanners for quantitative diffusion-weighted imaging. J. Magn. Reson. Imaging: JMRI. 2016;43(1):213–219. doi: 10.1002/jmri.24956. [DOI] [PubMed] [Google Scholar]
- 30.Jerome N.P., Miyazaki K., Collins D.J., Orton M.R., d'Arcy J.A., Wallace T., Moreno L., Pearson A.D., Marshall L.V., Carceller F., Leach M.O., Zacharoulis S., Koh D.M. Repeatability of derived parameters from histograms following non-Gaussian diffusion modelling of diffusion-weighted imaging in a paediatric oncological cohort. Eur. Radiol. 2017;27(1):345–353. doi: 10.1007/s00330-016-4318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]