Abstract
The aim of this study was to evaluate the role of inflammation and oxidative damage in hepatotoxicity of ethanol. Also we assessed protective effects of atorvastatin against ethanol-induced hepatotoxicity. In this study, the animals were divided into five groups: control, ethanol (10 mg/kg intraperitoneal (i.p.)), ethanol with atorvastatin (10, 20 mg/kg/day, i.p.) and ethanol-vitamin C group which received ethanol (10 mg/kg/day) plus vitamin C (200 mg/kg, i.p.) for 28 consecutive days. Then, the animals were euthanized and liver tissues were separated. Biochemical markers ALT and AST were measured. Moreover, glutathione (GSH) content, lipid peroxidation, protein carbonyl, nitric oxide and tumor necrosis factor-α (TNF-α) were evaluated. The administration of ethanol for 28 days resulted in an increase in liver damage, oxidative stress and inflammatory markers. The atorvastatin was able to prevent the ethanol-induced hepatotoxicity by decreasing the oxidative stress and inflammation processes. Our study showed the critical role of oxidative damage and inflammation in ethanol-induced hepatotoxicity that markedly was inhibited by administration of atorvastatin. Therefore, atorvastatin can be suggested for prevention of ethanol-induced hepatotoxicity.
Keywords: Atorvastatin, Ethanol, Hepatotoxicity, Oxidative stress, Inflammation
INTRODUCTION
Ethanol (ethyl alcohol) is a widely consumed organic solvent with various applications in industrial as solvent and in medical as antiseptic, antitussive, antidote, etc. Ethanol shows toxic effect both in acute overdose or in chronic consumption (1).
Chronic ethanol consumption could affect several organs of which liver is the primary target of ethanol toxicity. In fact, chronic ethanol abuse leads to hepatotoxicity that could develop steatosis, necrosis, and cirrhosis (2). The mechanisms involved in the hepatotoxicity of ethanol have not been well-understood, but previous studies have revealed the role of oxidative stress in the development of ethanol-induced hepatotoxicity (3,4,5,6). The most important pathway of ethanol metabolism is by alcohol dehydrogenase that in liver could induce generation of reactive oxygen species (ROS) and reduce the antioxidant level in liver tissue (3,4). Metabolism of ethanol through alcohol dehydrogenase, cytosolic xanthine and/or aldehyde oxidase, and also the mitochondrial respiratory chain could lead to reactive oxygen species (ROS) generation (3,7). The resulting oxidative stress can affect cellular components such as lipid, protein and DNA. Damage to lipid membrane and DNA due to oxidative stress could finally lead to cell death and tissue damage. On the other hand, alcohol-induced oxidative stress in liver tissue could promote hepatic inflammation (8). Another suggested mechanism for ethanol hepatotoxicity is the increased expression of inflammatory mediators such as TNF-α. In fact, increased production of inflammatory mediators e.g. TNF-α, lipid metabolites, as well as reactive oxygen intermediates have been shown in several models of alcohol related hepatotoxicity.
The production of TNF-α is the early step in many types of liver injury and activates the other inflammatory factors’ production that could lead to hepatocytes death in toxic situation. In patients with alcoholic hepatitis increased serum concentration of TNF-α has been reported (7). Several studies suggested using of antioxidants such as vitamins A or E (1,4), pentoxifylline (as an inhibitor of TNF-α), anti- TNF-α antibody and other cytokines (7), grape Vitis vinifera L. leaf as an antioxidant, glutathione (GSH), vitamin C (5), epigallocatechin-3-gallate (8), N-acetyl-L-cysteine (2), methanolic extract (ME) of Acorus calamus (9), aqueous extract of fenugreek (Trigonella foenum graecum) seeds (10), and quercetin (11) for attenuating of ethanol hepatotoxicity.
Statins inhibit HMG-CoA reductase (hydroxymethylglutaryl-CoA synthase), which is the rate-limiting enzyme of the mevalonate pathway of cholesterol synthesis, lowers cholesterol level (12). Atorvastatin is a most commonly and widely prescribed statin which showed antithrombotic, antiplatelet, anti-inflammatory, and antioxidative properties (13,14). Previous studies showed statins reduce lipid peroxidation and attenuate free radical damage and exhibit antioxidant activity against hydroxyl and proxy radicals. In addition, metabolites of atorvastatin reduce lipoprotein oxidation in a several oxidative systems (15). Also statins attenuate cisplatin-induced kidney damage via prevention of lipid peroxidation (16). Furthermore pretreatment with atorvastatin leads to significant decrease in TNF-α and myeloperoxidase activity that may result in decreased iNOS over expression and consequently lesser ROS and reactive nitrogen species (RNS) production (17). Therefore, present study aimed to determine if atorvastatin has a protective effect against ethanol hepatotoxicity via modulation of inflammatory and oxidative stress process.
MATERIALS AND METHODS
Animal's treatment
Male Wistar rats (200-250 g) were kept in an air-conditioned room with controlled temperature of 22 ± 2 °C and maintained on a 12:12 h light cycle with ad libitum feeding. All experimental procedures were conducted according to the ethical standards and protocols approved by the Committee of Animal Experimentation of Mazandaran University of Medical Sciences, Sari, Iran (Registration number: 1372). All efforts were made to minimize the number of animals and their suffering. Animals were randomly divided into five groups of six animals and the groups were as follows: control group, received normal saline; ethanol group, received ethanol 10 mg/kg, i.p.; treatment groups, received ethanol (10 mg/kg) plus atorvastatin at 10 and 20 mg/kg, i.p.; and positive control group, that received ethanol (10 mg/kg/day) plus vitamin C (200 mg/kg, i.p.). All treatments were continued for 28 consecutive days. Atorvastatin was injected 0.5 h after ethanol administration. All chemicals were dissolved in normal saline. Blood samples were collected from heart of animals and the serum was obtained by centrifugation. Serum ALT and AST were determined with commercial Kit (Pars Azmon, Iran). Liver tissue were excised and was homogenized in phosphate buffered saline, then centrifuged at 800× g for 10 min at 4 °C and maintained in -20 °C for oxidative stress markers assessment (18).
Total protein assay
Protein concentrations were determined through the Coomassie blue protein-binding as explained by Bradford (19).
Measurement of lipid peroxidation
Malondialdehyde (MDA) is the end product of lipid peroxidation. So the content of MDA was determined by thiobarbituric acid reactive substances expressed as the extent MDA of productions during an acid-heating reaction. Briefly, 0.25 mL phosphoric acid (0.05 M) was added to 0.2 mL of kidney tissue homogenate with the addition of 0.3 mL 0.2% thiobarbituric acid. All the samples were placed in a boiling water bath for 30 min. At the end, the tubes were shifted to an ice-bath and 0.4 mL n-butanol was added to each tube. Then, they were centrifuged at 3500 rpm for 10 min. The amount of MDA formed in each sample was assessed through measuring the absorbance of the supernatant at 532 nm with an ELISA reader (Tecan, Rainbow Thermo, Austria). Tetramethoxypropane was used as standard and MDA content was expressed as μM (20).
Measurement of protein carbonyl
Determination of protein carbonyl by spectrophotometric method, briefly 200 μL of kidney tissue is needed to homogenate. Samples are extracted in 500 μL of 20% (w/v) trichloroacetic acid (TCA). Then, samples placed at 4 °C for 15 min. The precipitates are exposed with 500 μL of 0.2% DNPH and 500 μL of 2 N HCl for control group, and samples were incubated at room temperature for 1 h with vortexing at 5-min intervals. Then proteins were precipitated by adding 55 μL of 100% TCA. The micro tubes were centrifuged and washed three times with 1000 μL of the ethanol-ethyl acetate mixture. And the micro tubes are dissolved in 200 μL of 6 M guanidine hydrochloride. The carbonyl content was determined by reading the absorbance at 365 nm (21).
Measurement of glutathione content
GSH content was determined by 5,5’-dithio-bis-[2-nitrobenzoic acid] (DTNB) as an indicator. Briefly 0.1 mL of liver tissue was added into 0.1 mol/L of phosphate buffers and 0.04% DTNB in a total volume of 3.0 mL (pH 7.4). Then developed yellow color was read at 412 nm using a spectrophotometer (UV-1601 PC, Shimadzu, Japan). GSH content was expressed as nM (18).
Measurement of nitric oxide
Nitric oxide was evaluated using commercial kits based on the Griess reagent. In this method, sulfanilic acid is quantitatively converted to a diazonium salt by reaction with nitrite in acid solution. The diazonium salt is then coupled to N-(1-naphthyl) ethylenediamine, forming an azo dye that can be spectrophotometrically quantitated based on its absorbance at 548 nm (22).
Measurement of TNF-α
Serum levels of tumor necrosis factor alpha (TNF-α) was determined using specific ELISA kits (R &D systems). Briefly, standards, control, and rat serum were pipetted into the wells pre-coated by a monoclonal antibody specific for rat TNF-α. After bonding of present TNF-α to the immobilized antibody, unbound substances were removed by washing. Then, an enzyme-linked polyclonal antibody specific for rat TNF-α was added to each well which followed by a washing step to remove any unbound antibody enzyme reagent. Finally, a substrate solution was added to the wells and the enzyme reaction yielded a blue product that turned yellow when the stop solution was added. Standard curves were constructed by using standard (recombinant rat TNF-α) and concentrations of the unknown sample were determined from the standard plots (23).
Statistical analysis
Results are presented as mean ± SD. All statistical analyses were performed using the SPSS software, version 14. Assays were performed in triplicate and the mean was used for the statistical analysis. Statistical analysis was determined using the one-way ANOVA test, followed by the post-hoc Tukey's test. Statistical significance was set at P < 0.05.
RESULTS
Effect of atorvastatin on biochemical markers
As shown in Table 1, ALT level significantly was increased in liver of ethanol-treated rats (P < 0.001), and markedly decreased after treatment with both doses of atorvastatin, respectively (P < 0.05, P < 0.001). Elevation of AST is an important marker in hepatotoxicity. As indicated in Table 1, AST level was markedly enhanced after ethanol administration in comparison with control group (P < 0.05). On the other hand, treatment with atorvastatin showed significant decrease in AST level compared to ethanol group (P < 0.05).
Table 1.
Effect of atorvastatin on ALT and AST levels in rats received ethanol.
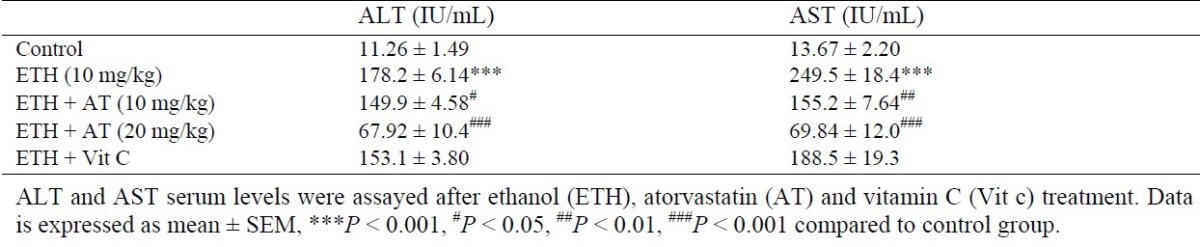
Effect of atorvastatin on lipid peroxidation
MDA is an important marker of oxidative damage to cellular lipids. As illustrated in Fig. 1, MDA level was significantly increased after administration of ethanol compared to the control group (P < 0.05). Moreover, treatment with both atorvastatin and Vit C significantly inhibited lipid peroxidation as compared to ethanol group (P < 0.05).
Fig. 1.
Effect of atorvastatin on malondialdehyde level in rats received ethanol. Ethanol (ETH), atorvastatin (AT) and vitamin C (Vit C). Data is expressed as mean ± SEM, **P < 0.01, #P < 0.05, ##P < 0.01 compared to control group.
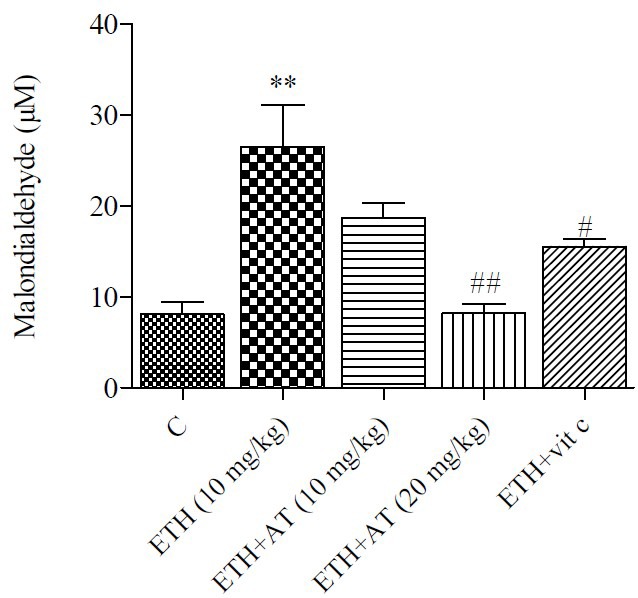
Effect of atorvastatin on protein carbonyl
Protein carbonyl which is an indicator of protein oxidation was increased in ethanol-treated group. As shown in Fig. 2, atorvastatin and Vit C treatment caused a significant decrease of protein carbonyl level compared to the ethanol group.
Fig. 2.
Effect of atorvastatin on protein carbonyl level in rats received ethanol. Ethanol (ETH), atorvastatin (AT) and vitamin C (Vit C). Data is expressed as mean ± SEM, ***P < 0.001, ##P < 0.01, ###P < 0.001 compared to control group.
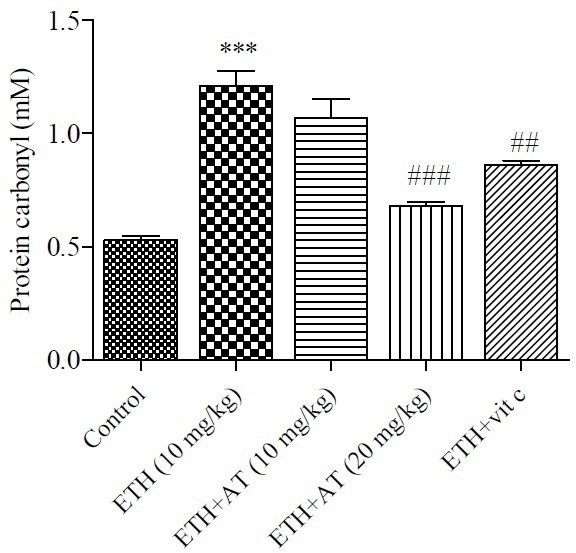
Effect of atorvastatin on glutathione content
The GSH levels (as the main intracellular antioxidant) diminished in ethanol group compared to control group. Treatment with atorvastatin showed an inhibition of GSH level in rats that significantly (P < 0.05) inhibited GSH oxidation in ethanol-treated group (Fig. 3).
Fig. 3.
Effect of atorvastatin on glutathion content in rats received ethanol. Ethanol (ETH), atorvastatin (AT) and vitamin C (Vit C). Data is expressed as mean ± SEM, **P < 0.01, #P < 0.05.
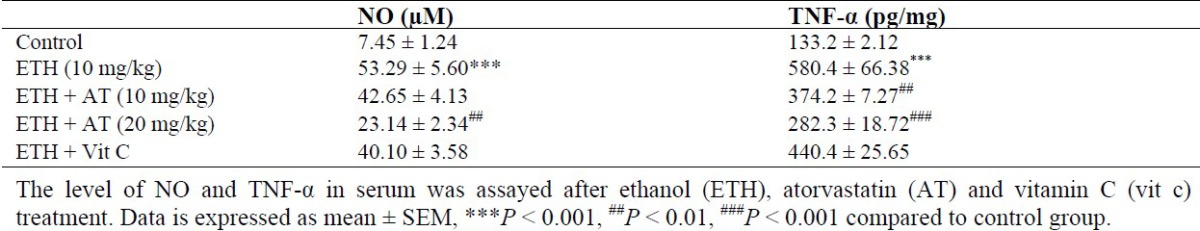
Effect of atorvastatin on nitric oxide and TNF-α
Increase of NO production has been reported in hepatotoxicity during the inflammatory process. Our results confirmed these findings. A significant increase in NO in serum was observed in ethanol-treated rats and significantly (P < 0.05) decreased by atorvastatin treatment at a dose of 20 mg/kg (Table 2). Indeed, TNF-α as an important inflammatory marker increased in ethanol-treated group in comparison with control group. As shown in Table 2, atorvastatin treatment significantly decreased TNF-α level in all doses compared to the ethanol group.
Table 2.
Effect of atorvastatin on inflammatory factors (NO and TNF-α) in rats received ethanol.
DISCUSSION
The focus of this study was to reveal the role of oxidative stress and inflammatory events in ethanol toxicity to rat liver and evaluation of the dose dependent effect of atorvastatin in attenuating ethanol induced hepatotoxicity via modulation of oxidative stress and inflammation.
The ethanol mediated liver damage was clearly demonstrated by marked elevation in serum level of liver enzymes. Several reports suggested the pivotal role of oxidative stress in ethanol mediated hepatotoxicity (1,4,24,25,26). ROS has deleterious effects on cellular macromolecules such as DNA, membrane lipids and proteins. So the measurement of lipid peroxidation, protein carbonyl or concentration of antioxidant capacity such as GSH content are good markers for studying the effect of ROS production (27). In this study, ethanol induced-oxidative stress was evident by decreased level of non-enzyme antioxidant such as GSH and also by increased level of lipid peroxidation and protein carbonyl.
It has been well shown that the oxidative damage induced by free radicals could be an important initiator in the pathogenesis of alcohol hepatotoxicity (6). Also, Nordman, et al. showed that free radical generation plays a main role in the ethanol toxicity in liver tissue (3). Previous studies have revealed that ethanol treatment results in the depletion of GSH content, decreases antioxidant activity and elevation of lipid peroxidation (6,7). Ethanol-induced ROS production leads to destructive peroxidation of cell membrane lipids that finally could lead to cell membrane disruption and necrosis (12). Thus, elevation of liver enzymes in serum accompanied with increased in oxidative stress markers indicates the role of ROS and oxidative stress in development of ethanol-induced liver injury. Recently, several studies linked ethanol-induced hepatotoxicity to the increased production of cytokines and inflammation (7). Inflammation can cause hepatotoxicity and development of hepatocytes death via the production of toxic by-products during inflammation process such as ROS and complement proteins (28). TNF-α is one of the most important inflammatory mediator produced in the initial step of several models of hepatotoxicity. Also, the role of nitric oxide in hepatotoxicity was confirmed by showing increased level of stable metabolites of nitric oxide including nitrites and nitrates (3,5,7,8,29). Indeed, nitric oxide in combination with oxygen radicals could form peroxynitrite that is known as the most potent oxidants that contribute in oxidative damage to cells and tissues.
The present study showed a significant increase in serum level of TNF-α and nitric oxide in ethanol-treated rats. On the other hand, both ROS and nitric oxide can stimulate up-regulation of NF-κB which is a transcription factor involved in controlling the TNF-α gene expression. Also, TNF-α can activate pro-apoptotic caspase downstream and lead to cell death via apoptosis or necrosis. Therefore, using agents with antioxidant or free radical scavenging properties could be a beneficial strategy for amelioration of ethanol- induced liver injury (30). Atorvastatin is a most commonly and widely prescribed statin which showed anti-thrombotic, anti-inflammatory, and anti-oxidative properties (12). In the present study, atorvastatin at dose of 20 mg/kg caused significant improvement in antioxidant system and suppression of inflammatory process and thus provides significant level of protection in ethanol-mediated liver damage by restoring the altered antioxidant defense system. The present finding revealed that atorvastatin significantly abrogated increased level of TNF-α and nitric oxide due to ethanol administration.
CONCLUSION
In view of the experimental results, it could be concluded that oxidative stress and inflammation may underlie the pathogenesis of ethanol–induced hepatotoxicity. Therefore, with respect to critical role of these mechanisms in promoting liver injury, atorvastatin could inhibit ethanol-induced hepatotoxicity via amelioration of oxidative stress and inflammation.
ACKNOWLEDGMENT
The content of this paper is extracted from the Pharm. D thesis (No. 509) submitted by Mahtab Mohammadbagheri which was financially supported by the Research Council of Mazandaran University of Medical Sciences, Sari, Iran.
REFERENCES
- 1.Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63(3):231–41. doi: 10.1016/0378-4274(92)90086-y. [DOI] [PubMed] [Google Scholar]
- 2.Ronis MJ, Butura A, Sampey BP, Shankar K, Prior RL, Korourian S, et al. Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic Biol Med. 2005;39(5):619–630. doi: 10.1016/j.freeradbiomed.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann R, Ribière C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med. 1992;12(3):219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- 4.Koch OR, Pani G, Borrello S, Colavitti R, Cravero A, Farrè S, et al. Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Mol Aspects Med. 2004;25(1):191–198. doi: 10.1016/j.mam.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Pari L, Suresh A. Effect of grape (Vitis vinifera L.) leaf extract on alcohol induced oxidative stress in rats. Food Chem Toxicol. 2008;46(5):1627–1634. doi: 10.1016/j.fct.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira CP, Da Costa Gayotto LC, Tatai C, Bina D, Ishimoto B, Janiszewski M, et al. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J Cell Mole Med. 2002;6(3):399–406. doi: 10.1111/j.1582-4934.2002.tb00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das SK, Vasudevan D. Alcohol-induced oxidative stress. Life Sci. 2007;81(3):177–187. doi: 10.1016/j.lfs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Yuan G, Gong Z, Zhou X, Zhangq P, Sun X, Li X. Epigallocatechin-3-gallate ameliorates alcohol-induced liver injury in rats. Int J Mol Sci. 2006;7(7):204–219. [Google Scholar]
- 9.Ilaiyaraja N, Khanum F. Amelioration of alcohol-induced hepatotoxicity and oxidative stress in rats by Acorus calamus. J Diet Suppl. 2011;8(4):331–345. doi: 10.3109/19390211.2011.615805. [DOI] [PubMed] [Google Scholar]
- 10.Thirunavukkarasu V, Anuradha C, Viswanathan P. Protective effect of fenugreek (Trigonella foenum graecum) seeds in experimental ethanol toxicity. Phytother Res. 2003;17(7):737–743. doi: 10.1002/ptr.1198. [DOI] [PubMed] [Google Scholar]
- 11.Molina MF, Sanchez-Reus I, Iglesias I, Benedi J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol Pharm Bull. 2003;26(10):1398–1402. doi: 10.1248/bpb.26.1398. [DOI] [PubMed] [Google Scholar]
- 12.Panonnummal R, Yarkey J, Dinoop DR. Are statins nephroprotective?:a dose dependent study in albino rats. Int J Pharm Pharm Sci. 2013;5(3):182–190. [Google Scholar]
- 13.Bösel J, Gandor F, Harms C, Synowitz M, Harms U, Djoufack PC, et al. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92(6):1386–1398. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- 14.Violi F, Carnevale R, Pastori D, Pignatelli P. Antioxidant and antiplatelet effects of atorvastatin by Nox2 inhibition. Trends Cardiovasc Med. 2014;24(4):142–148. doi: 10.1016/j.tcm.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Aviram M, Rosenblat M, Bisgaier CL, Newton RS. Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation. Atherosclerosis. 1998;138(2):271–280. doi: 10.1016/s0021-9150(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 16.Ýþeri S, Ercan F, Gedik N, Yüksel M, Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230(2):256–264. doi: 10.1016/j.tox.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 17.Mehany HA, Abo-youssef AM, Ahmed LA, Arafa E-SA, El-Latif HAA. Protective effect of vitamin E and atorvastatin against potassium dichromate-induced nephrotoxicity in rats. Beni-Suef University Journal of Basic and Applied Sciences. 2013;2(2):96–102. [Google Scholar]
- 18.Shokrzadeh M, Zamani E, Mehrzad M, Norian Y, Shaki F. Protective effects of propofol against methamphetamine-induced neurotoxicity. Toxicol Int. 2015;22(1):92–99. doi: 10.4103/0971-6580.172250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Hosseini JP, Shaki F, M Ghazi-Khansari M. Mechanisms of Arsenic (III) toxicity on isolated rat liver mitochondria. Res Pharm Sci. 2012;7(5):S155. [Google Scholar]
- 21.Reznick AZ, Packer L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. MethodsEnzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 22.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 23.Mahmoud AM, Germoush MO, Soliman AS. Berberine attenuates isoniazid-induced hepatotoxicity by modulating peroxisome proliferator-activated receptor gamma, oxidative stress and inflammation. Int J Pharm. 2014;10(8):451–460. [Google Scholar]
- 24.Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27(1):63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- 25.Song Z, Deaciuc I, Song M, Lee DYW, Liu Y, Ji X, et al. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin Exp Res. 2006;30(3):407–413. doi: 10.1111/j.1530-0277.2006.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaki F, Pourahmad J. Mitochondrial toxicity of depleted uranium: protection by beta-glucan. IJPR. 2012;12(1):131–140. [PMC free article] [PubMed] [Google Scholar]
- 27.Pourahmad J, Eskandari MR, Alavian G, Shaki F. Lysosomal membrane leakiness and metabolic biomethylation play key roles in methyl tertiary butyl ether-induced toxicity and detoxification. Toxicol Environ Chem. 2012;94(2):281–293. [Google Scholar]
- 28.Khanna P, Ong C, Bay BH, Baeg GH. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials. 2015;5(3):1163–80. doi: 10.3390/nano5031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122(7):2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czaja MJ, editor. Cell signaling in oxidative stress-induced liver injury. Seminars in liver disease. 2007;27(4):378–89. doi: 10.1055/s-2007-991514. [DOI] [PubMed] [Google Scholar]


